Abstract
Background
Previous studies show the uptake of biannual ultrasound (US) surveillance in patients with cirrhosis is suboptimal. Here, our goal was to understand in broader terms how surveillance is being delivered to cirrhosis patients with cured hepatitis C in the UK.
Methods
Hepatitis C cirrhosis patients achieving a sustained viral response (SVR) to antiviral therapies were identified from the national Hepatitis‐C‐Research‐UK resource. Data on (i) liver/abdominal US examinations, (ii) HCC diagnoses, and (iii) HCC curative treatment were obtained through record‐linkage to national health registries. The rate of US uptake was calculated by dividing the number of US episodes by follow‐up time.
Results
A total of 1908 cirrhosis patients from 31 liver centres were followed for 3.8 (IQR: 3.4–4.9) years. Overall, 10 396 liver/abdominal USs were identified. The proportion with biannual US was 19% in the first 3 years after SVR and 9% for all follow‐up years. Higher uptake of biannual US was associated with attending a liver transplant centre; older age and cirrhosis decompensation. Funnel plot analysis indicated significant inter‐centre variability in biannual US uptake, with 6/29 centres outside control limits. Incident HCC occurred in 133 patients, of which 49/133 (37%) were treated with curative intent. The number of US episodes in the two years prior to HCC diagnosis was significantly associated with higher odds of curative‐intent treatment (aOR: 1.53; 95% CI: 1.12–2,09; p = .007).
Conclusions
This study provides novel data on the cascade of care for HCC in the UK. Our findings suggest biannual US is poorly targeted, inefficient and is not being delivered equitably to all patients.
Keywords: adherence, imaging, liver cancer, screening, ultrasonography
Key points.
HCC surveillance is inefficient (i.e., >10 000 scans performed in this cohort to treat only 49 HCC patients with curative‐intent).
There is inequity in how surveillance is implemented between liver centres.
Patients being prioritised for surveillance are not ideal candidates for curative‐intent treatment.
The number of ultrasound episodes received in the two years prior to HCC diagnosis is associated with greater odds of curative‐intent treatment.
1. INTRODUCTION
The number of hepatitis C virus (HCV)‐infected individuals achieving a sustained virological response (SVR) has increased rapidly since the introduction of direct‐acting antivirals (DAAs). 1 Achieving SVR is associated with diverse benefits 2 , 3 ; however, it does not completely eliminate the risk of hepatocellular carcinoma (HCC). Indeed, a recent meta‐analysis reported the incidence rate is 2.1 HCCs per 100 person years of follow‐up among cirrhosis patients following SVR. 4
HCC is a leading cause of cancer mortality worldwide, killing ~0.8 million people every year. 5 However, patients can have a favourable prognosis if treated with curative intent (i.e., via liver transplantation, surgical resection or ablation). Unfortunately, suitability for these treatment hinges on early HCC detection. Thus, because the majority of HCCs are not detected until an advanced stage, only about 4 patients in 10 go onto be treated with curative intent in the UK and other countries. 1 , 6
Clinical guidelines recommend individuals with cirrhosis should receive biannual ultrasound (US) of the liver/abdomen to maximise early HCC detection. 7 , 8 , 9 However, a recent systematic review reported that only 9.8% of cirrhosis patients receive biannual US surveillance, based mainly on data from North America. 10 At present, there is little detailed information regarding how surveillance is implemented in a real‐world cohort, particularly in a European setting. On this note, population‐based studies comprising a representative set of screening providers are crucial to build an accurate picture of surveillance practice as a whole. In the present study therefore, we used record‐linkage methods to integrate data on US exams, HCC incidence and curative HCC treatment, within a large multicentre cohort. Our goal was to study in detail how biannual US is implemented and patterned for HCV cirrhosis patients following SVR achievement.
2. METHODS
2.1. The HCVRUK resource
This study is underpinned by data from the Hepatitis C Research UK (HCVRUK) resource, a database of almost 12 000 patients with chronic HCV. 11 HCVRUK participants were recruited from 2012 to 2015 from more than 50 UK liver centres. Participants have been characterised in terms of a broad range of clinical, epidemiological, virological and treatment‐related factors, ascertained through clinical notes or through direct self‐report at study enrolment.
More recently, a subset of the cohort – all participants with a cirrhosis diagnosis – have been linked to nationwide registries held by NHS Digital (application number: NIC‐72626) This includes hospital episodes statistics (HES) data (e.g., admitted patient care database 12 ; diagnostic imaging dataset (DID) 13 and outpatient hospital admissions), mortality registrations and the NCRAS cancer registry. 14 Informed consent was obtained from all participants.
2.2. Study population: eligibility criteria
All HCVRUK participants diagnosed with liver cirrhosis who subsequently went on to achieve SVR through antiviral therapy were eligible for inclusion in the current analysis. Liver cirrhosis was defined as compensated or decompensated cirrhosis diagnosed during routine clinical investigation. In practice, diagnoses of cirrhosis were typically made following: liver biopsy; transient elastography; abdominal US; clinical examination; symptoms consistent with a decompensation episode; and routine liver function tests, according to clinical guidelines.
2.3. Study population: exclusion criteria
Eligible patients were excluded for (i) pre‐SVR liver transplant, (ii) a pre‐SVR HCC diagnosis, (iii) missing identifiers for record linkage, and (iv) <12 months of follow‐up after SVR achievement.
2.4. Ultrasound data
Data on abdominal/liver US examinations performed after SVR achievement were ascertained through NHS digital data. Two specific registries were used to capture imaging events: First, the diagnostic imaging dataset (DID), which provides patient‐level information on radiology scans performed in NHS England for diagnostic purposes. DID data are derived from local radiology information systems, which are collated by clinical commissioning groups, and submitted monthly to NHS Digital. 13 Modality and body site of radiology scans are indicated through SNOMED‐CT codes. In this study, we selected US procedures performed specifically on either the liver or the abdomen using the SNOMED‐CT codes listed in Table S1. Data from the DID were then supplemented with the HES outpatient data, which provides information on outpatient hospital visits attended in NHS England. For this database, abdominal/liver US events were identified using the U082 OPCS4 code (Table S1). At the time of analysis, both the out‐patient dataset and the DID were complete until 31 March 2020. In a sensitivity analysis, we also included magnetic resonance imaging (MRI) and computed‐tomography (CT) scans of the liver and/or abdomen to see what impact this had on surveillance uptake.
Please note, medical indications for imaging procedures are not recorded in these data registries and so were unavailable in this study.
2.5. Study follow‐up period
For each patient, the follow‐up period began at the date of SVR, defined as 12 weeks after treatment completion (i.e., SVR12). However, if a patient achieved SVR before enrolment into HCVRUK study, then we commenced follow‐up time at the date of study enrolment to avoid immortal time bias.
Follow‐up ended at the earliest of (i) date of liver transplant (if at all); (ii) diagnosis of HCC (if at all); (iii) date of death (if at all); or (iv) the study completion date of 31st December 2019.
Information on liver transplantation, HCC diagnosis and date of death was ascertained through NHS digital registries. The specific code sets used to identify these events are indicated in Table S1.
2.6. Ultrasound event vs ultrasound episode
We distinguished between US events (i.e., single liver/abdominal US) and US episodes (i.e., a cluster of US events relating to a single US episode). Thus, where patients had multiple US events within a 90‐day period, these were collapsed into a single US episode, with the earliest scan date retained. This step was to avoid overestimating uptake in patients with a single US episode entailing immediate follow‐up scans. Sensitivity analyses were performed exploring the impact of using a longer (150 days) and shorter time window (30 days) than 90 days.
2.7. Primary outcome event
The primary outcome event was biannual US, defined as a rate of ≥2.0 US per year. The rate of US uptake was calculated by dividing the total number of US episodes by the total follow‐up time per patient. Patients without biannual US were separated into three groups:
Annual US: rate of ≥1.0 but <2.0 US episodes per year
Infrequent US: rate of <1.0 but >0 US episodes per year.
No US: zero US episodes during follow‐up.
In sensitivity analyses, more lenient definitions of biannual US were considered: (a) > 1.85 USs per year (1 scan per 6.5 months); and (b) 1.71 US per year (1 US per 7 months).
2.8. Study covariates
Study covariates were ascertained from two sources. First, information recorded directly on the HCVRUK clinical databases; second, hospital admission records occurring prior to SVR achievement (based on the hospital episodes statistics Admitted Patient Care dataset 12 ).
Study covariates ascertained from the HCVRUK clinical database were age; gender; ethnicity (Caucasian and non‐Caucasian/unknown); decompensated cirrhosis; risk of HCC; and attending a liver transplant centre at HCVRUK recruitment. Baseline decompensation was defined as a decompensation event (ascites, bleeding varices or encephalopathy) before SVR. All dates of decompensation episodes were ascertained from the HCVRUK database. HCC risk at baseline was estimated using the aMAP score, 15 which is calculated from information on age, gender, albumin, bilirubin and platelet count. The aMAP risk score was chosen because a previous validation analysis indicated that it had better discrimination and calibration in this patient group than rival HCC risk scores. 16 When calculating aMAP, albumin, bilirubin and platelet count values were determined from test results performed up to a year before treatment initiation. If more than one test was performed during this window, then the mean value was used. These methods were used for consistency with previous studies and because antiviral therapy can cause temporary changes in laboratory tests that may not reflect long‐term risk profile.
Two covariates were derived from a patient's hospital admission history. These were (a) previous substance use‐related hospital admission; and (b) previous alcohol use‐related hospital admission. Three levels were considered for both variables: (1) no previous admission; (2) non‐recent admission (defined as more than 3 years prior to SVR); and (3) recent admission (defined as less than three years prior to SVR). The ICD codes used to identify these events are provided in Table S1.
2.9. Factors associated with biannual US
Logistic regression was used to identify factors associated with receiving biannual US. Candidate predictors assessed in univariable and multivariable models included age (per ten‐year increase); sex (male vs. female); ethnicity (Caucasian vs. non‐Caucasian/unknown); previous alcohol‐related hospital admission(s) (recent, and not recent vs. no); previous drug‐related hospital admission (recent, and not recent vs. no); decompensated cirrhosis (yes vs. no); and attendance at a liver transplant centre (yes vs. no). Duration of follow‐up can also affect biannual screening uptake, as it is easier to be adherent over a two‐year duration vs. a four‐year duration, for example. Thus, duration of follow‐up was also included as a covariate, which functioned as a type of offset in the model.
2.10. Variability in biannual US across individual clinics
Funnel plots were constructed to assess variation in biannual US uptake between individual centres. A funnel plot comprises a series of data points, one for each liver centre represented in our cohort. 17 The vertical position of each data point reflects the crude proportion of patients who received biannual US at the centre in question; the horizontal position reflects the clinic's sample size. In the absence of inter‐clinic heterogeneity, all data points should move towards convergence as sample size increases. The binomial distribution was used to generate 95% and 99% control limits; centres outside these limits can be considered to exhibit atypical uptake that is unlikely to reflect sampling error.
2.11. HCC incidence rate
HCC cases were defined as a cancer, mortality or an inpatient hospital admission for HCC (ICD10: C22.0). The incidence rate of HCC was calculated by dividing the number of incident events by the study follow‐up period.
2.12. Association between US uptake and curative HCC treatment
For individuals with HCC, we calculated the number of US episodes performed in the two years prior to their HCC diagnosis. We then determined the association between the number of US episodes in that period and the odds of being treated for HCC with curative intent.
Curative‐intent treatment for HCC was defined as ablation, resection or liver transplantation, according to clinical guidelines. 8 OPCS4 codes in the HES admitted patient care dataset were used to identify these instances of curative‐intent treatment (Table S1).
A logistic regression model was fitted to identify factors associated with curative treatment. In addition to the number of US episodes. Other covariates included in this model were age at SVR, gender, and decompensated cirrhosis at SVR.
2.13. Validation
Internal and external validation approaches was performed to assess if NHS digital data can reliably measure US uptake in cirrhosis patients.
Two types of internal validation were carried out. First, the average time interval between consecutive US scans was calculated to assess consistency with the screening interval recommended in clinical guidelines. Second, we assessed the timing of imaging examinations in patients who developed HCC. Our expectation was that there would be a spike in imaging procedures performed on/around the date of HCC diagnosis.
For external validation, we collected information on liver/abdominal US directly from liver centres for a subset of patients. These data were used to assess agreement between US uptake inferred from NHS digital data versus US inferred directly from liver centres. For more information, see Appendix A.
3. RESULTS
3.1. Derivation of the study population
A total of 2550 patients met our study inclusion criteria. We then excluded individuals if they had a pre‐SVR liver transplant (n = 250, 11%); had a pre‐SVR diagnosis of HCC (n = 127; 6%); were missing identifiers for record linkage (n = 133; 6%); or had less than 12 months follow‐up (n = 132, 6%). Thus, our final study population comprised the remaining 1908 patients (Figure 1).
FIGURE 1.
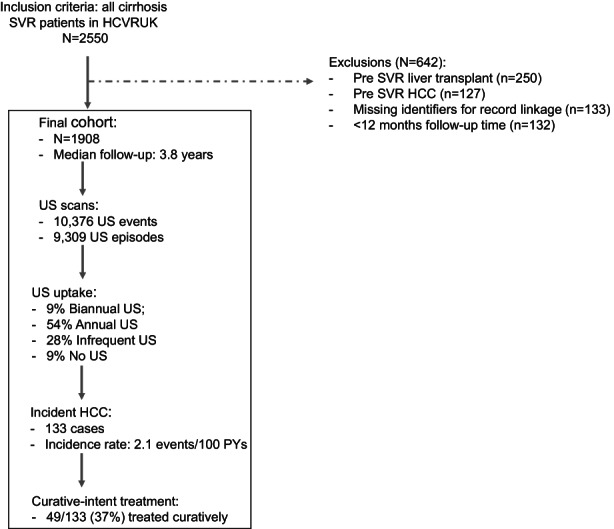
HCC care cascade.
3.2. Characteristics of study population
The study population were recruited from 31 liver centres covering all major geographical regions in England (Figure S1). Individuals were mostly middle‐aged (mean age: 55.0), male (74%) and Caucasian (81%). One‐fifth had a previous episode of decompensated cirrhosis (19.9%). The proportion with a recent hospital admission for substance use and alcohol use was 24% and 12% respectively. The median aMAP score at SVR achievement was 61.3, equivalent to a predicted 3‐year HCC probability of ~4.1%. Of note, aMAP score was missing for 575/1908 (30.1%) participants (see Table 1).
TABLE 1.
Characteristics of study population.
| Characteristic | n (col %) | |
|---|---|---|
| baseline | ||
| Age, mean (sd) | 55.0 (sd: 9.2) | |
| Gender | Female | 506 (26.5) |
| Male | 1402 (73.5) | |
| Ethnicity | White | 1542 (80.8) |
| non‐White | 366 (19.2) | |
| Decompensated cirrhosis, n (col%) | No | 1529 (80.1) |
| Yes | 379 (19.9) | |
| Follow‐up at transplant clinic | No | 1105 (57.9) |
| Yes | 803 (42.1) | |
| Alcohol hospital admission, n (col%) | No | 1450 (76.0) |
| Past | 227 (11.9) | |
| Recent | 231 (12.1) | |
| Substance misuse hospital admission, n (col%) | No | 1102 (57.8) |
| Past | 348 (18.2) | |
| Recent | 458 (24.0) | |
| IFN free therapy | No | 472 (24.7) |
| Yes | 1436 (75.2) | |
| Year SVR achievement, median (IQR) | 2015 (IQR: 2014–2016) | |
| aMAP score at SVR, median (IQR) | 61.3 (IQR: 55.9–65.9) | |
| Follow‐up | ||
| Median duration of follow‐up (years) | 3.8 (IQR: 3.3–4.9) | |
| Total number of ultrasound scans | 10 376 | |
| Number of incidents HCCs | 133 | |
Note: amap indicates risk of HCC at SVR achievement. A value of 61.3 suggests 3‐year HCC probability of 4.1%. However, amap score at this time point was missing for 575 (30.1%) of participants.
The median duration of follow‐up was 3.8 years per patient, ranging from 1 to 8 years. Most patients achieved SVR between 2014 and 2016.
3.3. Uptake of biannual US
In total, there were 10 376 scans observed during follow‐up translating into 9309 screening episodes (Figure 1). The proportion who received biannual US was 8.8% (n = 168). Otherwise, 54% (n = 1035) received annual surveillance; 28% (n = 536) received infrequent surveillance; and 9% (n = 169) received no surveillance (Figure 2).
FIGURE 2.
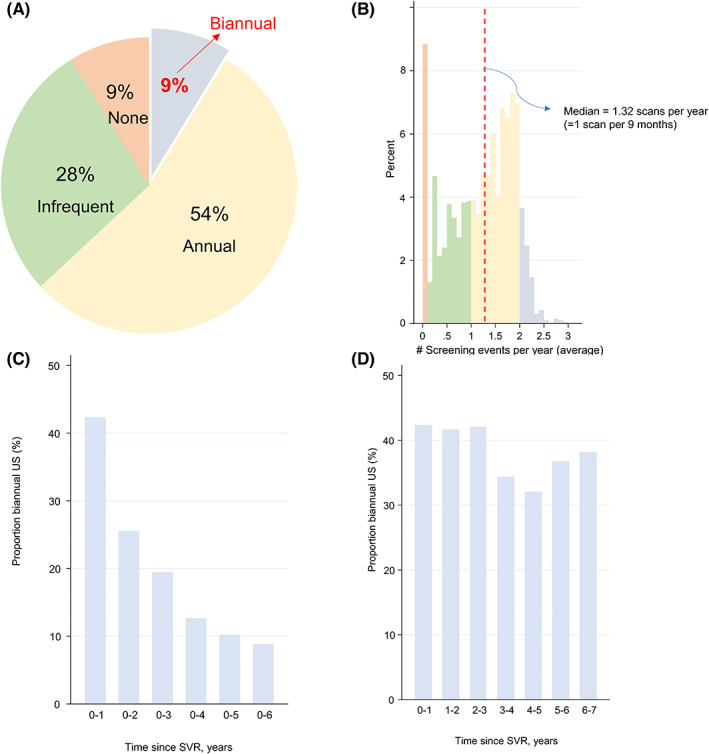
Summary of US uptake: (A) uptake category; (B) distribution of uptake rate; (C)uptake by duration Follow‐up; (D) uptake within 1‐year time bands.
The proportion with biannual surveillance increased to 19.7% and 29.0% when defined as ≥1 scan per 6.5 months and ≥1 scan per 7 months respectively. It was also sensitive to the window period used to define contiguous scans (i.e., 15.7% with a shorter 30‐day interval versus 4.0% for a longer 150‐day window) and increased too if CT and MRI scans were included to 12.4% (Figure S2).
Biannual uptake was highest over shorter time periods (i.e., 42% in the first year following SVR versus 19% in the first 3 years after SVR). This is because it is easier to be adherent to biannual US over a shorter time period than a longer one. However, within 1 year time bands, the proportion with biannual US was relatively constant with time, albeit highest in the first 3 years after SVR (Figure 2).
3.4. Factors associated with biannual US
Three main factors were associated with biannual US in multivariate regression analysis (Table 2). First, older patients were more likely to receive biannual US than younger patients (aOR per 10‐year increase: 1.41; 95% CI: 1.17–1.71; p < .001). Second, patients with decompensated cirrhosis had greater odds versus patients with compensated cirrhosis (aOR: 1.64; 95% CI: 1.10–2.43; p = .02). Third attendance at a liver transplant centre was associated with greater uptake (aOR: 3.41; 95% CI: 2.40–4.83; p < .001).
TABLE 2.
Factors associated with biannual screening uptake.
| Characteristic | Uptake, % | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| OR | p | aOR | p | |||
| Age, per 10‐year increase | NA | 1.57 (1.31–1.87) | <.001 | 1.41 (1.17–1.71) | <.001 | |
| Gender | Female | 9.3 | REF (1.00) | – | REF (1.00) | – |
| Male | 8.6 | 0.92 (0.65–1.31) | .65 | 1.08 (0.74–1.57) | .70 | |
| Ethnicity | White | 8.4 | REF (1.00) | – | REF (1.00) | – |
| Non‐White | 10.4 | 1.26 (0.86–1.84) | .24 | 1.09 (0.73–1.64) | .67 | |
| Decompensated cirrhosis | No | 7.9 | REF (1.00) | – | REF (1.00) | – |
| Yes | 12.4 | 1.65 (1.15–2.36) | .006 | 1.64 (1.10–2.43) | .02 | |
| Alcohol hospital admission | No | 9.7 | REF (1.00) | – | REF (1.00) | – |
| Past | 4.8 | 0.48 (0.25–0.90) | .02 | 0.51 (0.26–1.00) | .05 | |
| Recent | 7.4 | 0.74 (0.44–1.25) | .27 | 0.81 (0.44–1.50) | .51 | |
| Substance misuse hospital admission | No | 10.1 | REF (1.00) | – | REF (1.00) | – |
| Past | 8.0 | 0.78 (0.51–1.20) | .26 | 1.05 (0.66–1.67) | .84 | |
| Recent | 6.3 | 0.60 (0.40–0.92) | .02 | 0.69 (0.42–1.12) | .13 | |
| Follow‐up at transplant clinic | No | 4.7 | REF (1.00) | – | REF (1.00) | – |
| Yes | 14.4 | 3.42 (2.43–4.81) | <.001 | 3.41 (2.40–4.83) | <.001 | |
| Follow‐up duration, per 1‐year increase | NA | 0.73 (0.64–0.82) | <.001 | 0.72 (0.63–0.82) | <.001 | |
Note: Statistically significant associations are highlighted in grey.
Individuals with a past alcohol‐related hospital admission were less likely to receive biannual US versus those without (aOR: 0.51; 95% CI: 0.26–1.00; p = .05). There was also a trend towards reduced uptake in individuals with a recent hospital admission for substance abuse, albeit this did not reach statistical significance (aOR: 0.69; 95% CI: 0.42–1.12; p = .13). Gender and ethnicity were not associated with biannual surveillance. All associations remained broadly similar in sensitivity analyses (Table S2).
In a post hoc analysis, the association between attending a transplant centre and receiving biannual US did not attenuate after adjusting for HCC risk (i.e., via aMAP score) (Table S3). This analysis also suggested individuals with a higher risk of HCC were more likely to receive biannual US (aOR per 1 unit increase in aMAP: 1.06; 95% CI: 1.03–1.09; p < .001).
3.5. Variability in biannual US between liver centres
The crude proportion of patients who received biannual uptake varied from 0% to 18% by liver centre. Funnel plots indicated statistically significant heterogeneity in biannual uptake between individual centres (Figure 3). Two centres with poor validation data were omitted from this plot (see Appendix A for further details). Of the 29 centres remaining, 6 (21%) were outside the 95% control limits (i.e., 4 centres above and 2 centres below). Inter‐centre variability was even more pronounced in the first 3 years after SVR, where 10/29 (34%) centres were outside control limits (Figure S3).
FIGURE 3.
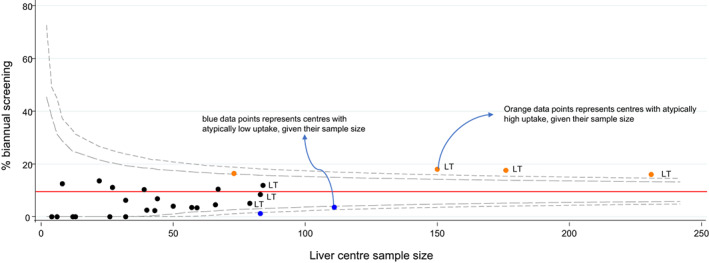
Funnel plots indicating the proportion of patients receiving biannual surveillance by liver centre. Liver centres are represented by circular data points. Liver transplant centres are marked “LT”. The red horizontal line is the average uptake for all the data points represented in the plot. The grey dashed line refers to the 95% and 99% control limits, calculated using the exact method. Data points for two centres (centre “S” and “K”) were omitted from this plot. Please see appendix A for further details.
3.6. Relationship between biannual screening and curative HCC treatment
133 incident cases of HCC were observed during follow‐up. The HCC incidence rate was 2.2 events per 100 person years (95% CI: 1.8–2.6). Of the 133 incident HCC cases observed, 37% (n = 49) were treated for HCC with curative intent. The proportion treated with curative intent increased roughly stepwise with number of US episodes received in the two years prior to HCC diagnosis; that is 8%, 17%, 44% 39% 46% and 56% in patients with 0, 1, 2, 3, 4 and 5 US episodes respectively (p = .06) (Figure 4).
FIGURE 4.
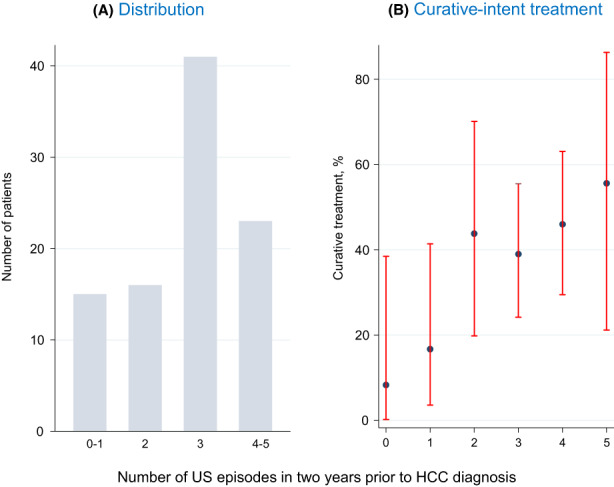
Ultrasound episodes two years prior to HCC diagnosis (N = 133).
In multivariate analysis, the odds of curative intent treatment increased by 53% for each additional US episode performed (aOR: 1.53; 95% CI: 1.12–2.09; p = .007). Conversely, older age (aOR per 10‐year increase: 0.49; 95% CI: 0.28–0.83; p = .008) and decompensated cirrhosis at SVR (aOR: 0.28; 95% CI: 0.11–0.71; p = .007) were associated with lower odds of curative treatment (Table 3; Figure 5).
TABLE 3.
Factors associated with receiving curative HCC therapies (N = 133).
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR | p | aOR | p | ||
| # USs in previous 2 years (odds for each additional US performed) | 1.52 (1.14–2.03) | .004 | 1.53 (1.12–2.09) | .007 | |
| Age, per 10‐year increase | 0.62 (0.38–0.99) | .047 | 0.49 (0.28–0.83) | .008 | |
| Gender | Female | REF (1.00) | – | REF (1.00) | – |
| Male | 1.82 (0.74–4.47) | .19 | 1.40 (0.53–3.72) | .50 | |
| Decompensated cirrhosis | No | REF (1.00) | – | REF (1.00) | – |
| Yes | 0.32 (0.14–0.73) | .007 | 0.28 (0.11–0.71) | .007 | |
Note: Statistically significant associations are highlighted in grey.
FIGURE 5.
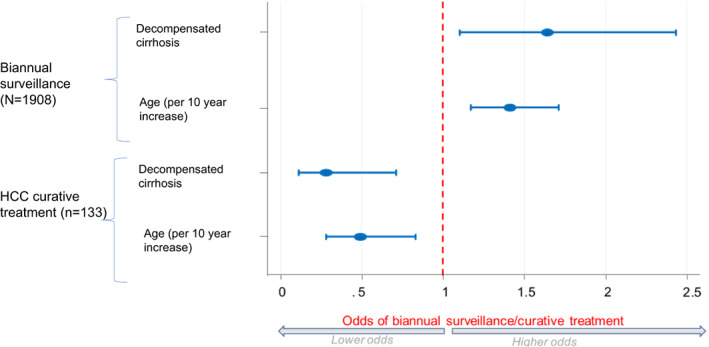
Older age and decompensated cirrhosis are associated with higher odds of biannual surveillance, but lower odds of HCC curative treatment.
In a post hoc subgroup analysis, higher aMAP score at SVR was associated with lower odds of curative‐intent treatment, albeit the association did not reach statistical significance (aOR per 1 unit increase in aMAP: 0.93; 95% CI: 0.986–1.01; p = .086).
3.7. Validation
The median time interval between successive screening events was 182–189 days (Figure S4). The date of HCC diagnosis coincided with a peak in the number of imaging procedures performed (Figure S5). Overall, we show NHS digital had adequate validity for measuring US uptake in cirrhosis patients. Detailed information can be found in Appendix A.
4. DISCUSSION
This study describes the delivery of biannual US screening in a large multi‐centre cohort of patients with cured hepatitis C in the UK. Our analysis raises several important and novel points regarding the implementation of HCC surveillance in the UK. First, the identification of >10 000 US scans suggests appreciable resources are in fact being deployed towards early HCC detection in this population. Nevertheless, few patients are receiving biannual US as recommended in guidelines 9 – that is only 19% in the first three year after SVR achievement and 9% during all years of follow‐up. Second, the odds of biannual screening were ~ 3 times greater for patients attending a liver transplant centre. This suggests that rather than being delivered equally to all eligible patients, US uptake is influenced by arbitrary factors such as the type of liver clinic one is attending. In a similar vein, funnel plot analyses showed significant variability in uptake of biannual US between individual liver centres. To the best of our knowledge, this is a novel finding that raises important questions regarding equity of access. Third, our study describes the HCC care cascade in unique detail, from surveillance of at‐risk patients through to receipt of curative‐intent treatment. Our findings highlight the low efficiency of biannual US. For example, 10 376 US scans were performed in this cohort, to ultimately treat only 49 patients with curative intent. It should be pointed out that not all US scans are performed exclusively for HCC surveillance (e.g., US is also used to detect mild ascites in patients with cirrhosis 18 ); nevertheless, even with this in mind, the yield is still very low. Our data suggest biannual US is not currently being targeted in the most appropriate way. Indeed, we show there is a disparity between individuals who are currently being prioritised for biannual US versus those who are good candidates for curative‐intent treatment. For example, on the one hand, older patients were more likely to receive biannual US, but on the other hand, they were less likely to be treated with curative intent if they did develop HCC (Figure 5). The same pattern applied to decompensated disease and may extrapolate to HCC risk in general. These observations caution that focusing screening on higher risk patients may not necessarily translate into more patients being treated for HCC with curative intent. This has implications for the current debate around individualised HCC surveillance. 19 , 20 , 21 , 22 Fourth, our study suggests the more US scans you receive in the two years prior to HCC, the greater your odds of receiving curative‐intent treatment are. This supports the fundamental premise of biannual US and reinforces the potential benefits for patients. However, further work is needed to articulate the net benefit of surveillance to patients and clinicians in terms of years‐of‐life‐gained, and how this may vary for different patient groups (e.g., older patients). This information is crucial to support shared decision making. 23 Without a randomised controlled trial, this may be best established using decision modelling methods. 24 , 25 Finally, we demonstrate the validity of the NHS England DID for quantifying uptake of biannual US in patients with cirrhosis. Future studies may consider linking the DID to broader datasets such as the clinical practice research datalink. 26 In this way, one could repeat this study for a much larger set of patients with a range of cirrhosis beyond just hepatitis C.
Our study has several limitations that merit discussion. First, we did not have any information on the medical indication for the imaging procedures included. Many of the scans considered may not meet a strict definition of HCC surveillance. For example, they may have been performed in response to symptoms (e.g., weight loss or changes in liver blood test values), or with more than one medical indication in mind (e.g., detecting mild ascites in addition to checking for focal lesions on the liver 18 ). Nevertheless, the aim of this study was to assess adherence to clinical guidelines, which simply recommend biannual US checks of the liver/abdomen for focal HCC lesions. 7 , 8 , 9 In this sense, it does not matter if the US was prompted by symptoms or if it was carried out with an additional objective in mind – as long as the US provided an opportunity to detect HCC. Second, we did have any data on BCLC stage at HCC diagnosis and thus had to rely on curative treatment as a marker of early HCC detection. Third, a patient's liver centre was defined as the liver centre overseeing their care at the time of enrolment into HCVRUK. However, this may not necessarily be the same centre overseeing care at the time of SVR achievement. Thus, there may be some misclassification with respect to this variable. In addition, we did not exclude patients with advanced liver cirrhosis (i.e., Child‐Pugh C) that were not on the waiting list for liver transplantation at the time of SVR achievement. Such patients would not be eligible for HCC screening as per EASL guidelines. 8 However, they represent a relatively small patient subgroup and so their inclusion is unlikely to have biased our results. Another caveat to note is that most patients in this study were treated for HCV in specialist care settings. More recently however, there has been a shift towards treating patients in the community where it is even more difficult to engage patients in HCC surveillance. In this respect, the low surveillance uptake observed in this study may even be on the optimistic side. Another limitation is that some participants in this cohort may have emigrated – in which case, the imaging and outcome data will not be reliable. Further, we did not have information on social factors – such as deprivation, household income or education – which may influence uptake of biannual US. Finally, we did not have recourse to detailed information about individual liver centres (e.g., staffing, number of dedicated hepatologists), to permit a more thorough investigation into inter‐centre variability.
Overall, this study provides important insight into how HCC surveillance is being delivered in the UK. Our findings argue for greater standardisation in delivery and arguably a need to monitor HCC surveillance routinely at a national level.
FUNDING INFORMATION
HCVRUK was established through a grant from Medical Research Foundation (Grant ID: C0365). The Medical Research Foundation also supported this study via a viral hepatitis fellowship awarded to HI (Grant ID: C0825). The STOP‐HCV study was funded by the Medical Research Council, United Kingdom (Grant ID: MR/K01532X/1). This work has also been supported by the Deliver study, funded by Cancer Research UK (Grant ID: C30358/A29725). The funders had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
CONFLICT OF INTEREST STATEMENT
There are no relevant conflicts of interest to report.
Supporting information
Data S1
ACKNOWLEDGEMENTS
Foremost, we would like to thank the participants of the HCVRUK study themselves. We would also like to acknowledge the Principal Investigators of sites/clinics included in this study. These were as follows: Gelson W (Addenbrookes Hospital, Cambridge); MacDonald D (Royal Free, London); Richardson P (Royal Liverpool University Hospital); Ryder S (Queen's Medical Centre, Nottingham); Aldersley M (St James's University Hospital, Leeds); McPherson S (Freeman Hospital, Newcastle); Verma S (Royal Sussex County Hospital, Brighton); Priest M (Gartnavel General Hospital, Glasgow); Barnes E (John Radcliffe Hospital, Oxford); Ustianowski A (North Manchester General Hospital); Leen C (Western General Hospital, Edinburgh); Forton D (St George's Hospital, London); Gordon F (Bristol Royal Infirmary); Lawson A (Royal Derby Hospital); Wiselka M (Leicester Royal Infirmary); Aspinall R (Queen Alexandra Hospital, Portsmouth); Khakoo S (Southampton General Hospital); Ch'ng C (Singleton Hospital, Swansea); Dillon J (Ninewells Hospital, Dundee); Shirley English (Aberdeen Royal Infirmary); Corless L. (Hull Royal Infirmary); Moreea S (Bradford Teaching Hospitals NHS Foundation Trust); Foster G (The Royal London Hospital, Whitechapel); Barclay S (Glasgow Royal Infirmary); Gorard D (Wycombe Hospital); Rye K (Royal Shrewsbury Hospital); O'Donohue J (University Hospital Lewisham); Healy B (University Hospital of Wales, Cardiff); Foxton M (Chelsea and Westminster Hospital); Shields P (Royal Preston Hospital); Shorrock C (Blackpool Victoria Hospital); Prince M (Manchester Royal Infirmary); Kennedy N. (Monklands Hospital); Gera A (Queen Elizabeth Hospital Woolwich).
Hamill V, Gelson W, MacDonald D, et al. Delivery of biannual ultrasound surveillance for individuals with cirrhosis and cured hepatitis C in the UK. Liver Int. 2023;43:917‐927. doi: 10.1111/liv.15528
Handling Editor: Alejandro Forner
REFERENCES
- 1. Innes H, McDonald SA, Hamill V, et al. Declining incidence of hepatitis C related hepatocellular carcinoma in the era of interferon free therapies: a population‐based cohort study. Liver Int. 2021;42:561‐574. doi: 10.1111/liv.15143 [DOI] [PubMed] [Google Scholar]
- 2. Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct‐acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453‐1464. [DOI] [PubMed] [Google Scholar]
- 3. McDonald SA, Pollock KG, Barclay ST, et al. Real‐world impact following initiation of interferon‐free hepatitis C regimens on liver‐related outcomes and all‐cause mortality among patients with compensated cirrhosis. J Viral Hepat. 2020;27:270‐280. [DOI] [PubMed] [Google Scholar]
- 4. Lockart I, Yeo MGH, Hajarizadeh B, Dore GJ, Danta M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta‐analysis. Hepatology. 2022;76:139‐154. doi: 10.1002/hep.32341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-31 [DOI] [PubMed] [Google Scholar]
- 6. Cross TJS, Villanueva A, Shetty S, et al. A national survey of the provision of ultrasound surveillance for the detection of hepatocellular carcinoma. Frontline Gastroenterol. 2016;7:82‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heimbach JK, Kuilk LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358‐380. [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182‐236. [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellent . Cirrhosis in over 16 s: Assessment and Management. NICE Guideline; 2016. Available at: https://www.nice.org.uk/guidance/ng50 [Google Scholar]
- 10. Wolf E, Rich NE, Marreo JA, Parikh ND, Singal AG. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta‐analysis. Hepatology. 2021;73:713‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLauchlan J, Innes H, Dillon JF, et al. Cohort profile: the hepatitis C virus (HCV) research UK clinical database and biobank. Int J Epidemiol. 2017;46:1391‐1391h. doi: 10.1093/ije/dyw362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data resource profile: hospital episode statistics admitted patient care (HES APC). Int J Epidemiol. 2017;46:1093‐1093i. doi: 10.1093/ije/dyx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NHS . Diagnostic Imaging Dataset . Accessed: February 2022. https://www.england.nhs.uk/statistics/statistical‐work‐areas/diagnostic‐imaging‐dataset/
- 14. Henson KE, Elliss‐Brookes L, Coupland VH, et al. Data resource profile: National Cancer Registration dataset in England. Int J Epidemiol. 2020;49:16‐16h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368‐1378. [DOI] [PubMed] [Google Scholar]
- 16. Innes H, Jepsen P, McDonald S, et al. Performance of models to predict hepatocellular carcinoma risk among UK patients with cirrhosis and cured hepatitis C infection. JHEP Rep. 2021;3:100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med. 2005;24:1185‐1202. [DOI] [PubMed] [Google Scholar]
- 18. Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujiwara N, Friedman SL, Goosens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157:56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021;74:458‐465. [DOI] [PubMed] [Google Scholar]
- 22. Nahon P, Quang EV, Ganne‐Carrie N. Stratification of hepatocellular carcinoma risk following HCV eradication or HBV control. J Clin Med. 2021;10:353. doi: 10.3390/jcm10020353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singal AG, Volk ML, Rakoski MO, et al. Patient involvement in healthcare is associated with higher rates of surveillance for hepatocellular carcinoma. J Clin Gastroenterol. 2011;45:727‐732. [DOI] [PubMed] [Google Scholar]
- 24. Taylor EJ, Jones RL, Guthrie JA, Rowe IA. Modelling the benefit and harms of surveillance for hepatocellular carcinoma: information to support informed choices. Hepatology. 2017;66:1546‐1555. [DOI] [PubMed] [Google Scholar]
- 25. Mueller PP, Chen Q, Ayer T, et al. Duration and cost‐effectiveness of hepatocellular carcinoma surveillance in hepatitis C patients after viral eradication. J Hepatol. 2022;77:55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


