Abstract
Established in 2004, the Radiation and Nuclear Countermeasures Program (RNCP), within the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health has the central mission to advance medical countermeasure mitigators/therapeutics, and biomarkers and technologies to assess, triage, and inform medical management of patients experiencing acute radiation syndrome and/or the delayed effects of acute radiation exposure. The RNCP biodosimetry mission space encompasses: (1) basic research to elucidate novel approaches for rapid and accurate assessment of radiation exposure, (2) studies to support advanced development for US Food and Drug Administration (FDA) clearance of promising triage or treatment devices/approaches, (3) characterization of biomarkers and/or assays to determine degree of tissue or organ dose that can predict outcome of radiation injuries (i.e., organ failure, morbidity, and/or mortality), and (4) outreach efforts to facilitate interactions with researchers developing cutting edge biodosimetry approaches. Thus far, no biodosimetry device has been FDA cleared for use during a radiological/nuclear incident. At NIAID, advancement of radiation biomarkers and biodosimetry approaches is facilitated by a variety of funding mechanisms (grants, contracts, cooperative and interagency agreements, and Small Business Innovation Research awards), with the objective of advancing devices and assays toward clearance, as outlined in the FDA’s Radiation Biodosimetry Medical Countermeasure Devices Guidance. The ultimate goal of the RNCP biodosimetry program is to develop and establish accurate and reliable biodosimetry tools that will improve radiation preparedness and ultimately save lives during a radiological or nuclear incident.
Keywords: Biodosimetry assays, Resources, Mechanisms, Triage, Definitive dose, Predictive biodosimetry
Introduction
Radiation biodosimetry is defined as the estimation of received dose from past radiation exposure through observation of biologic variables or measurements [Gledhill and Mauro 1991] and encompasses devices that can conduct qualitative or quantitative assessments US Food and Drug Administration [2016]. Biological evidence of radiation exposure can be assayed across organs, cells, molecules, and/or biochemical levels. Given the terror attacks on September 11th, 2001, and the current geopolitical climate around the world, the importance of radiation biodosimetry cannot be underscored enough. A large-scale nuclear disaster will necessitate evaluation and clinical management of hundreds of thousands to millions of individuals [Waselenko et al., 2004; US Food and Drug Administration 2016; Garty et al., 2017]. Rapid and accurate radiation dose assessments are required to enable optimal triage, medical management, and predict health outcomes. In response to growing concerns about the ability of the US Government (USG) to mount a medical response to respond to such a disaster, several agencies were tasked with the mission to support research to develop biodosimetry approaches and strategies to diagnose and treat radiation injuries following a radiological/nuclear mass casualty public health emergency. The Radiation and Nuclear Countermeasures Program (RNCP) within the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), supports work across the entire spectrum of radiation research, including development of medical countermeasures (MCMs) to mitigate/treat injuries, decorporation strategies for radionuclides, and biodosimetry assays. With regard to drugs and biologics, the US Food and Drug Administration (FDA) pathway for MCM development using the “Animal Rule” [US Food and Drug Administration (FDA) 2015] has approved licensure of four products to treat hematopoietic complications resulting from radiation exposure: filgrastim (Neupogen®, 2015) [https://bit.ly/2ZJO9KH], pegfilgrastim (Neulasta®, 2015) [https://bit.ly/2U8OwdE], sargramostim (Leukine®, 2018) [https://bit.ly/2XYai6h], romiplostim (Nplate®, 2021) [https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125268s167lbl.pdf], and Udenyca (Coherus Bioscience, Inc) [https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761039s013lbl.pdf]. However, as of the writing of this manuscript, the FDA has not approved/cleared a radiation biodosimetry device or test for use in the event of a radiological or nuclear incident. The RNCP continues to facilitate biodosimetry-focused research at all stages, from basic research through advanced development targeting FDA clearance. This article provides an overview of the mission, mechanisms, and resources provided by the RNCP to the radiation research community for continued advancement of biodosimetry research.
NIH Goals for Radiation Biodosimetry
In 2005, a Blue-Ribbon Panel was convened by the NIH, to create a Strategic Plan and Research Agenda for MCMs against Radiological and Nuclear Threats [https://www.niaid.nih.gov/sites/default/files/radnucstrategicplan.pdf]. The following biodosimetry objectives were initially identified as:
Immediate Goals
Support rigorous quality assurance/quality control studies of current leading biodosimetry technologies to validate their use.
Increase the speed and efficiency of current assays to determine radiation doses received due to internal or external contamination with radioactive material.
Long-Term Goals
Develop new bioassays that can provide rapid and accurate radiation dose assessments, enabling optimal triage, medical management, or predictive outcome.
Develop biodosimetry tools and assays to evaluate radiation-related injuries and recovery processes of different physiological systems.
Develop and validate methods to estimate radiation dose and future risk following exposure to different sources of radioactive materials by various routes, including inhalation, ingestion, skin contact, or contamination of wounds.
With these goals as the starting point, the RNCP has identified end-uses for ideal biodosimetry tests: (1) field-deployable methods to assess ≥2 Gy exposure in humans, to differentiate exposed populations from the worried well and permit their triage [Sullivan et al., 2013], (2) laboratory-based, high throughput assays to determine definitive total- or partial-body radiation dose to the exposed individual, and (3) approaches to predict immediate and long-term consequences of radiation injury.
To achieve these goals, the RNCP has supported radiation biodosimetry research at different stages of development through a variety of funding initiatives, such as focused solicitations for single (U01) and multi-institutional (U19) cooperative agreements, pilot projects available through the NIAID-funded Centers for Medical Countermeasures against Radiation Consortium (CMCRC), standard R01, P01, and R21 research grant awards, Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grants, contracts (including a unique Product Development Services [PDS] award), and interagency agreements (IAAs). A major challenge to developing biodosimetry assays or devices for use in humans is the ethical infeasibility of conducting clinical studies in scenarios that appropriately mimic exposure to a large radiation dose during a large-scale radiation public health emergency. For biodosimetry test development, the FDA published a Radiation Biodosimetry Guidance to advise sponsors on requirements for assay or test clearance [US Food and Drug Administration 2016] [https://www.fda.gov/media/90385/download]. This guidance contrasts to the FDA regulations for approval of new drugs and licensure of biological products commonly known as the “Animal Rule” (21 CFR Parts 314.6000.650 for drugs and 21 CFR Parts 601.90-95 for biological products) (Federal Register Vol. 67, No. 105, 37988-37998, May 31, 2002). The biodosimetry guidance, which does not utilize the Animal Rule, requires the development of the biodosimetry signature or biomarkers in a large mammalian model that can be validated in clinical samples.
RNCP/NIAID Research Continuum: Processes, Mechanisms, and Resources
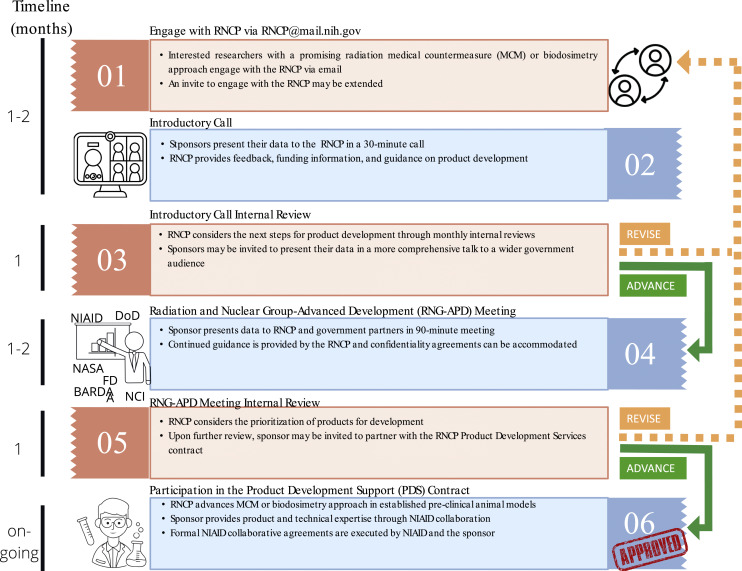
As the RNCP identifies technologies and products, the team engages with academics and companies in early to mid-stage development to learn about new areas in radiation research, offer guidance, incorporate products into the RNCP development pipeline, and provide information on funding opportunities to advance radiation research (Fig. 1). Since the RNCP has a specific product development end goal, the funding portfolio is weighted toward funding mechanisms that have substantial programmatic oversight, with a small number of traditional granting mechanisms. In addition, the RNCP has taken an active approach to provide information about opportunities for junior scientists that aligns with the NIH’s Diversity, Equity, Inclusion, and Accessibility goals (Report 116-450 on H.R. 7614: Diversity at NIH Working Group and Strategic Plan [https://www.federalregister.gov/documents/2021/06/30/2021-14127/diversity-equity-inclusion-and-accessibility-in-the-federal-workforce] and President Biden’s Executive Order 14035) [https://www.congress.gov/congressional-report/116th-congress/house-report/450].
Fig. 1.
RNCP outreach and engagement pipeline.
Processes
Outreach and Engagement by the RNCP
The RNCP is a well-established program that straddles basic and translational science with the main goal of advancing mitigators and biodosimetry approaches for FDA licensure. As mentioned previously, this work is accomplished via several grant/contract mechanisms, one of which provides the RNCP with a unique ability to be at the forefront of developing product/technology-enabling investigation new drug studies in collaboration with the sponsor through the PDS and IAA. To engage with the community at large, the RNCP has developed a sponsor engagement pipeline that is adapted to fit the needs of each sponsor. Here, a sponsor is defined as a person or entity – academic institution, individual researcher or pharmaceutical company, private organizations, or government stakeholders – that seek to engage with the RNCP. The RNCP delineates the step-wise interactions that serve as an information exchange platform that can lead to: (1) introducing the RNCP and other USG partners to new technologies, products, and initiatives, (2) demonstrating the needs and gaps in radiation biology, (3) learning about the program’s mission and goals, (4) hearing about any RNCP or USG funding opportunities, and (5) being considered to be a collaborator to test a sponsor’s product or technology in the RNCP’s preclinical animal radiation models (Fig. 1).
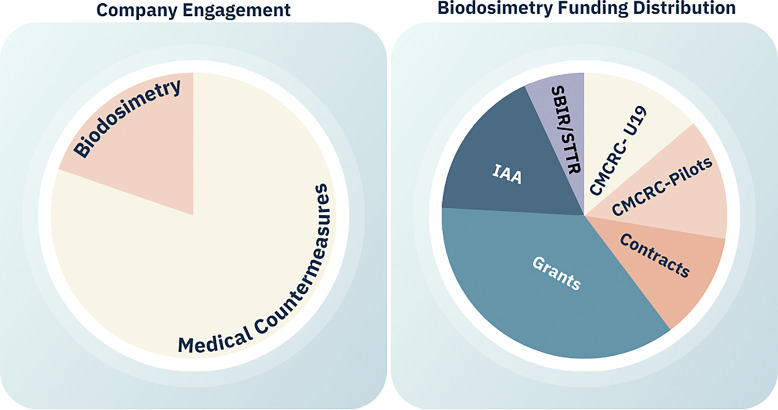
From the group’s establishment in 2004 through 2022, the RNCP engaged with more than 330 sponsors and tested over 550 products/technology through the PDS and IAA testing mechanisms. Since 2004, the RNCP has engaged with more than 65 groups focusing on biodosimetry alone through these pathways. Furthermore, this area of science has been well supported via other funding mechanisms such as the CMCRCs many multi-solicitation awards, CMCRC pilot projects, contracts, cooperative agreements and grants, IAAs, and SBIR/STTR. MCM development has advanced more rapidly than biodosimetry (Fig. 2); however, the program is working to identify ways to advance promising biodosimetry approaches by continuing to prioritize them through biomarker and biodosimetry U01 consortium, contracts, and engaging with sponsors to consult on circumventing challenges such as access to large animal sample acquisition and many others. For example, through the PDS, the RNCP was able to generate biological samples from irradiated nonhuman primates (NHPs), which were made available to 12 portfolio awardees for biomarker analyses (described in detail later).
Fig. 2.
RNCP engagement and Biodosimetry portfolio from 2004 to present.
Initial engagement starts with the communications between the sponsor and RNCP staff, indicating interest (Fig. 1). Once talks have been established, the sponsor will be invited to an introductory (intro) call where a brief exchange of information takes place. The sponsor provides information on their technology and answers key questions such as intellectual property; the approach, validation, and verification studies; plans for commercialization; and regulatory strategies. The RNCP provides scientific feedback, information on minimal requirements for biomarkers and biodosimetry medical devices, and funding information. If the technology/approach is promising, the RNCP may extend a second invite to present in a more comprehensive meeting called the Radiation and Nuclear Group-Advanced Product Development (RNG-APD) meeting. In the RNG-APD meeting, the sponsor will have access to feedback from radiation-focused, USG funding partners.
Mechanisms for Research and Development at the RNCP
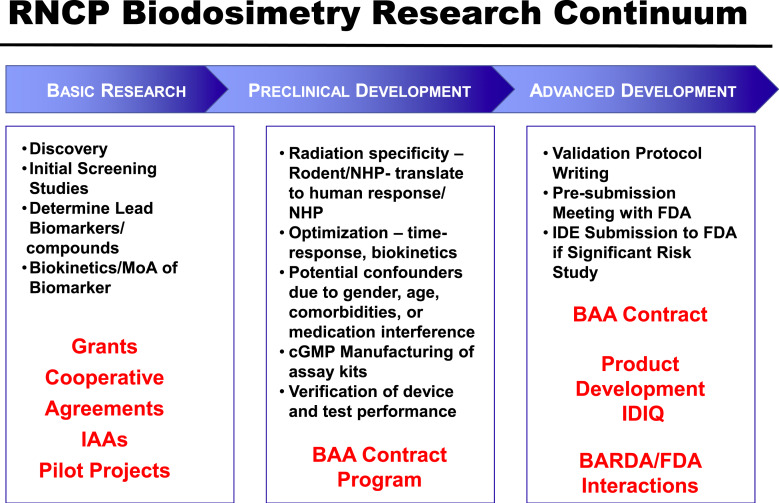
To realize the RNCP mission, the program supports basic, translational, and applied research. This effort includes development, use, and expansion of core facilities needed to test and characterize biodosimetry assays for regulatory approval. The program also provides training and educational materials in radiobiology research (Fig. 3).
Fig. 3.
RNCP funding mechanisms to support biodosimetry advancement along the entire research continuum.
Discovery Stage Studies
Several funding mechanisms are used to support highly innovative very early research that utilize approaches such as in vitro systems, rodent models, and ex vivo biosamples to address biodosimetry needs. The CMCRC Opportunities Fund Management Core (OFMC) provides peer-reviewed pilot awards for high-risk, high-reward projects in program-wide areas of interest. These awards allow for foreign involvement and have made international biodosimetry collaborative efforts possible with the Bundesamt für Strahlenschutz, Germany [2020], Public Health England [Cruz-Garcia et al., 2021], and Health Canada [Wang et al., 2019]. Through these and other early stage awards discussed, a variety of novel biomarkers are being explored for their use in predicting acute and late effects of radiation exposure. Since the inception of the RNCP, more than 50 biodosimetry pilot grants have been funded.
Through IAAs with the National Cancer Institute (NCI), FDA, and the Armed Forces Radiobiology Research Institute (AFRRI), the RNCP has funded early biodosimetry research in several areas that include automation of cytogenetic dicentric and micronuclei assays, identification of biomarkers of partial-body exposures, health assessment of individuals exposed in utero during the Chernobyl incident, circulating RNA signatures for assessing radiation dose [Aryankalayil et al., 2021], proteomics [Sproull et al., 2022a], and the use of telomeres as markers of radiation exposure. The RNCP has also funded R01 grant awards to advance the area of predictive biodosimetry (RFA-AI-11-033). The goal of this R01 solicitation was to identify, evaluate, and characterize biomarkers of radiation injury to specific organs and tissues of physiological systems and to link these markers to relevant clinical outcomes such as organ failure, other major morbidity, and mortality. This funding mechanism allowed for identification of functional approaches such as novel imaging modalities for the lung, biomarkers of delayed effects of acute radiation exposure, and development of miRNA, proteomics, and metabolomics platforms. This funding stream has already resulted in several publications [Drané et al., 2017; Fendler et al., 2017], and patents [https://patents.google.com/patent/WO2016130572A2/un]. In addition to high-quality reporting, several funded investigators successfully competed for follow-on cooperative agreement awards and have been able to gain additional support from other USG agencies to advance their biodosimetry signatures toward FDA consideration.
Early to Mid-Stage Research
At this stage in development, the biodosimetry signature is generally well-defined and the research will focus on supporting end-use application. Here, biokinetics of the signature, impact of the radiation quality, radiation dose and dose rate effects, total- versus partial-body exposures, and selection of appropriate biosamples can be investigated. Early studies in clinical samples as well as preliminary translational studies in NHPs are also supported through several funding mechanisms. These approaches include assessment of circulating cell depletion kinetics [Goans et al., 2001; Waselenko et al., 2004; Azizova et al., 2008], DNA damage (cytogenetics) [Garty et al., 2010], gamma-H2AX [Turner et al., 2019], and micronucleus assays [Repin et al., 2020], telomere length measurements [McKenna et al., 2019], fluorescence in situ hybridization [Tucker 2001]; and different “omics” approaches in radiation biodosimetry, including proteomics [Lee et al., 2018], genomics [Ghandhi et al., 2015], metabolomics [Laiakis et al., 2017], lipidomics [Pannkuk et al., 2017], and transcriptomics [Menon et al., 2016]. Further, the program has supported development of assays for detection of radiation-induced changes in free DNA or RNA in circulating blood [Zhang et al., 2010], panels of reactive oxygen or nitrogen species [Wei et al., 2019], inflammasomes, and “cytokine storms” specific to radiation exposure [Zhang et al., 2012].
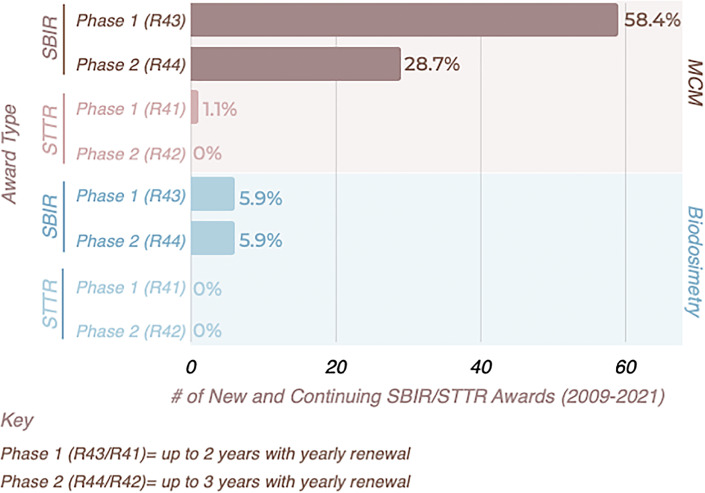
SBIR/STTR Program
Two awards available to mid-stage radiation researchers are the NIAID SBIR and STTR programs that annually award more than USD 120 million to assist small US businesses to engage in research and development with the potential for commercialization and for the benefit of public health. These programs are one of the largest sources of technology financing within the USA. SBIR awards are made to small businesses, while the STTR program has the unique feature of requiring applicant organizations to formally collaborate with a research institution, such as an academic laboratory. The RNCP has formally participated in this program since 2009, supporting research through past RNCP-specific program announcements, and currently through the PHS 2022-2 omnibus solicitation for SBIR/STTR [https://grants.nih.gov/grants/guide/pa-files/PA-22-176.html/https://grants.nih.gov/grants/guide/pa-files/PA-22-178.html] grant applications. To date, ∼12% of SBIR/STTR grants issued by the RNCP have been exclusively for development of biodosimetry devices. Funded grants include phase I awards (R41 [STTR] or R43 [SBIR]) for mid-stage research and phase II awards (R42 [STTR] or R44 [SBIR]) for more advanced research activities (Fig. 4). Funding capacity for the RNCP SBIR/STTR awards is capped under an approved waiver [https://seed.nih.gov/sites/default/files/HHS_Topics_for_Budget_Waivers.pdf] that allows for a maximum of USD 300,000 per year for up to 2 years for a phase I award, and up to USD 1,000,000 per year for up to 3 years for a phase II award. As this represents an important mechanism to support advancement of promising approaches, contact with a RNCP program officer is advised to obtain additional information and guidance prior to applying to one of these solicitations.
Fig. 4.
RNCP grants funded by the NIAID Small Business Innovation Research (SBIR)/Small Business Technology Transfer (STTR) Program.
CMCRC U19 Cooperative Agreements
The CMCRC program supports multidisciplinary approaches that span a spectrum of research from basic through translational research to develop new medical products to assess, diagnose, mitigate, and/or treat the short- and long-term consequences of radiation exposure after a radiological or nuclear event. This research program was originally established by NIAID in 2005 [RFA-AI-04-045] and renewed in 2010 [RFA-AI-09-036], 2015 [RFA-AI-14-055], and 2020 [RFA-AI-19-012]. Under the current award (2021-2025), the CMCRC biodosimetry research center consists of a partnership between Columbia University and Georgetown University. Since 2005, this center has been continuously supported to explore three different biodosimetry approaches, including high throughput, fully automated cytogenetics, gene expression, and metabolomics. Each approach has a different capability in terms of throughput, time-to-result, dose reconstruction, radiation exposure type identification, and individual radiosensitivity prediction. A goal of this center is to develop a variety of large-scale, radiation exposure scenario models to better understand differential responses of predictive biomarkers and to optimally use them to identify and characterize individual exposure levels. A second goal is to develop high-throughput biodosimetry approaches for individualized prediction of radiation injury, from photon, neutron, and photon/neutron mixed-field irradiation and from different radiation dose rates. Lastly, while model systems (e.g., ex vivo human exposure or in vivo animal exposure) are used to explore functional genomic and metabolomic biodosimetry/biomarker approaches, the end goal is to identify and optimize their integrated usage for translation to real-life, large-scale, human radiation exposure scenarios.
Unique radiation quality exposures under development, beyond X-ray/gamma high-dose rate models, include ultra-high-dose rate [Pannkuk et al., 2022], neutron [Xu et al., 2015; Laiakis et al., 2021; Wang et al., 2021], and long-term, low, and variable-dose-rate exposures [Garty et al., 2020; Pannkuk et al., 2021]. Because an investigation new drug may produce a significant neutron component during prompt radiation exposure, a broad energy spectrum neutron irradiator was created to simulate these exposure conditions. For anticipated long-term radiation exposures, 137Cs is used, since it is the most biologically important agent released in many accidental (or malicious) radiation incidents. It can enter the food chain and be consumed, or, if present in the environment (e.g., from fallout), can provide external irradiation over prolonged times. In either case, due to the high penetration of the energetic γ rays emitted by 137Cs, an individual would be exposed to a low dose rate, uniform, whole-body irradiation. The variable dose-rate external 137Cs irradiator device developed by Columbia University scientists allows modeling of these complex exposures, providing exposures at dose rates of 0.1–1.2 Gy/day by repurposing discarded brachytherapy seeds from radiation therapy clinics [Garty et al., 2020]. These platforms can be used to irradiate ex vivo blood or mice at varying doses. Using these approaches, researchers have generated gene expression signatures under a variety of exposure conditions. The team plans to test their performance for continuous dose reconstruction and refine them for a variety of complex exposure scenarios [Turner et al., 2019; Shuryak et al., 2020].
At Georgetown University, metabolite signatures resulting from radiation exposures are being identified using liquid chromatography time of flight mass spectrometry (LC-TOF-MS) fingerprinting methods to identify individual exposure to various radiation sources, dose, and dose rates [Laiakis et al., 2021; Pannkuk et al. 2021, 2022]. Overall, the goal is to translate these reference signatures to distinguish between exposed and nonexposed individuals, and, in particular, between those exposed to high doses that need urgent medical attention and individuals with a lower dose or no exposure that will not need immediate treatment.
Radiation Biodosimetry Assay and Devices U01 Cooperative Agreement Consortium
A cooperative agreement RFA, awarded in 2019, was released with the goal of advancing studies for additional mid-stage development of biodosimetry approaches, and with the intent to transition studies to more advanced development under a Broad Agency Announcement (BAA) funded by NIAID or BARDA. A further intention of this consortium is to assist researchers in advancing their work toward an initial meeting with the FDA to discuss regulatory strategies. The research topics in these awards cover the development and validation of rapid, reliable, inexpensive, and easy-to-use techniques/assays and devices for use in all segments of the civilian population including geriatric, pediatric, and immunocompromised individuals. Also included is the exploration of biokinetics for promising biomarkers that are quantifiable at 24 h or later post-exposure (i.e., days to weeks or months after irradiation) to allow repeated assays over time. These approaches should accurately predict acute and/or delayed radiation injuries to one or more organs and/or tissues of physiological systems. The ideal radiation biomarker will be measurable in a noninvasive or minimally invasive way (e.g., finger stick, urine, saliva, or skin scraping, as opposed to spinal fluid, biopsy, or complex imaging) to allow for repeated assays over time, be sensitive to incremental differences in radiation exposure, and be specific over a wide range of radiation qualities. In addition to several publications in high-impact journals [Rogers et al., 2020; Lacombe and Zenhausern 2022], investigators have gone on to receive follow-on funding from NIAID and BARDA for advanced development of many of the approaches funded through this consortium [https://www.medicalcountermeasures.gov/newsroom/2022/asell/]. Another unique aspect of RNCP-supported science is the provision of seed money to encourage collaborations between members of the consortium, an option that this group has utilized to create multiple strong partnerships between laboratories.
Mid- to Advanced-Stage Development
Several contract BAAs have been released by RNCP with the intent of funding mid- to advanced-stage research and development through support of translational aspects of biodosimetry signatures. Activities supported under these contracts include bridging studies between NHP and clinical samples, testing of potential confounders of age, sex, medical interference, or comorbidities in clinical samples, as well as manufacturing of Current Good Manufacturing Practice reagent and supply kits, verification of device and test performance, and creation of validation protocols. Interactions with the FDA are included as milestones for these awards, and as part of their regulatory development approach, the offeror must prepare a pre-submission briefing package and meet with the FDA within the period of performance of the contract. In addition, several areas of scientific inquiry that were initially supported by the RNCP and championed by industry were successfully transitioned to BARDA for follow-on support (e.g., Northrup Grumman, Asell LLC, and Dxterity).
RNCP Resources
The PDS Contract
Access to NHP samples taken at designated times after irradiation are critical for biomarker assay development, yet obtaining these kinds of samples is often challenging, given the high costs and limited availability of animals. NIAID is in a unique position of collaborating with various researchers and can therefore supply these kinds of biological samples, while assuring confidentiality for organizations (to ensure patent position or exclusively publish). For example, in a 2018 NIAID supported study, a variety of biological samples were obtained from rhesus macaques (Macaca mulatta) exposed to 4 Gy of total body irradiation (4 males, 4 females) or age-matched, sham-irradiated controls (1 male, 1 female). Samples were collected pre-irradiation and at various times following exposure, after which time they were processed, stored, and shipped to 12 USG-supported investigators, both nationally and internationally. The sample types collected included blood, plasma, serum, urine, feces, and saliva. This effort led to the publication of two manuscripts with several others in preparation. In one study, Laiakis et al. identified radiation-specific responses of several biomarkers, primarily amino acids and nucleotides, in the saliva of irradiated NHPs [Laiakis et al., 2019]. Some of these markers were increased in the first days following radiation exposure while others had latent increases. Using a SomaScan assay system (SomaLogic, Boulder, CO), Sproull et al. used NHP plasma provided by the RNCP, along with similar samples from irradiated mice, minipigs, and patients undergoing myeloablative combined chemo- and radiation therapy, to identify candidate protein biomarkers and functional protein families that indicate responses to tissue damage [Sproull et al., 2022b].
Additional publications and advancements in biodosimetry are expected from data generated from evaluating samples obtained from this study. To examine the long-term effects of these radiation exposures and potentially follow the progression of injury and biomarkers of injury, the monkeys (who all survived, due to the nonlethal radiation dose that was selected for the study) were shipped to the Wake Forest University School of Medicine, to be enrolled into the RNCP-funded NHP Radiation Survivor Cohort, which is discussed in more detail [https://school.wakehealth.edu/research/labs/j-mark-cline-lab/radiation-late-effects-cohort]. This biomarker study has allowed researchers to examine the immediate time course of various endpoints and continue to monitor the same animals for late complications. Future sample collection studies under consideration could use animals that receive a higher level of exposure to determine whether observed changes are radiation dose dependent.
The sections above describe the development of products that could be used to identify patients exposed to ionizing radiation and who should be treated. Biomarkers from samples obtained noninvasively could also be used to track injury and recovery in patients or in animals used to model radiation exposure or to test MCMs. In another study funded through the PDS contract, rhesus macaques were exposed to 12 Gy of irradiation with 2.5% of the bone marrow shielded and the progression of radiation-induced, multi-organ injury was examined. In this study, injury was defined using various hematological and clinical measurements as well as histological evaluation. These outcomes were linked to proteomic and metabolomic measurements obtained from plasma and tissues samples [Huang et al., 2020a; Kumar et al., 2021]. Alterations to circulating metabolites and proteins were compared to alterations in the heart [Zalesak-Kravec et al., 2021], jejunum [Huang et al., 2020b], lung [Huang et al., 2020a, 2020b, 2021a, 2021b], liver, and kidney [Cohen et al., 2020]. The researchers found various consensus metabolomic biomarkers associated with multi-organ injury. The analysis of plasma showed activation of the acute response pathway, which is consistent with the response to inflammation, as well as pathways associated with lipid dysregulation [Yu et al., 2021]. Other nutrient signaling pathways were also dysregulated in the irradiated NHPs.
NHP Radiation Survivor Cohort
Since establishment of the NHP Radiation Survivor Cohort in 2007, this NIAID supported program has evolved into a valuable resource for the entire radiation research community. At any given time, the cohort housed at Wake Forest University is home to ∼200 rhesus macaques (with the recent initiation of a cynomolgus macaque colony) that survived exposure to total body irradiation doses ranging up to 9.5 Gy (including unirradiated, age-matched controls). Both male and female NHPs reside in the colony, with most animals exposed as juveniles. These precious animals are critical to the search for biomarkers of radiation injury and provide unique insight into the natural history of an irradiated animal model that closely simulates human responses. To date, multiple comorbidities – some anticipated (e.g., cancers) and others surprising (e.g., diabetes, immune blind spots) – have been observed in these animals, and numerous publications document these and other radiation exposure outcomes [Robbins et al., 2011; Kavanagh et al., 2015; DeBo et al., 2016; Hanbury et al., 2016; Andrews et al. 2017, 2020; Caudell et al., 2019; Michalson et al., 2019; Bacarella et al., 2020; Thakur et al., 2021].
An important feature of the NHP cohort at Wake Forest is their availability to researchers to continue using them for exploratory studies. Researchers who are already investigating acute radiation responses can use their existing data and utilize innovative strategies and modeling to predict late radiation complications. Further corroboration of these prediction models can be done with aging irradiated NHPs, as they are followed through in-life studies in the cohort. Upon the completion of the 2018 biomarker collection study sponsored by the RNCP and mentioned above, all NHPs were transferred to Wake Forest, where they continue to be available for follow-on studies as well as routine wellness assessments and diagnostics (Table 1).
Table 1.
Recurring assessments in the Wake Forest Radiation Late Effects Cohort
| Procedure | Frequency | J | F | M | A | M | J | J | A | S | O | N | D | Animals assessed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health and welfare observations | Twice daily | • | • | • | • | • | • | • | • | • | • | • | • | 200/year |
| Cognitive testing | Rotating subset | • | • | • | • | • | • | • | • | • | • | • | • | 16/year |
| Urine collection | Annually | • | • | • | • | 200/year | ||||||||
| Body composition (DEXA) | Annually | • | • | • | • | 200/year | ||||||||
| CT scan lung | Annually | • | • | • | • | 200/year | ||||||||
| Bronchoalveolar lavage | ||||||||||||||
| Hemoglobin A1c | Annually | • | • | • | • | 200/year | ||||||||
| CT scan whole body | Annually | • | • | • | • | 200/year | ||||||||
| MRI brain | Once for all then q 3yrs | • | • | • | 80/year | |||||||||
| Echocardiography (subset) | Annually | • | • | 30/year | ||||||||||
| Bone marrow sampling | Once for all then q 3yrs | • | • | • | 80/year | |||||||||
| Abdominal | Annually | • | • | • | 200/year | |||||||||
| Ultrasound | ||||||||||||||
| Eye exams | Annually | • | • | • | 200/year | |||||||||
| Physical exam* | Annually | • | • | • | 200/year | |||||||||
| Hematology/clinical chemistry/blood bank | 3x/year | • | • | • | 600/year | |||||||||
| Immune profile – flow cytometry | Annually | • | • | • | • | 200/year | ||||||||
| TB test | 3x/year | • | • | • | 600/year | |||||||||
| Hormonal profile | Once on arrival | 300 | ||||||||||||
| Exome sequencing | Once for all | 300 |
*In addition to as-needed veterinary care 365 days/year. Table courtesy of Dr. George Schaaf, Wake Forest SOM.
Dosimetry Harmonization Efforts
It has been recognized for many years that a standardized and well-defined approach to radiation dosimetry should be adopted within the radiation biology community [Desrosiers et al., 2013]. However, although the need has been recognized, realization of an effort to achieve this goal has proven more challenging. It is critical for all biomarker identification studies to confirm the radiation dose delivered and intended, which is especially important in doses ranging from 6 to 9 Gy in animal models (doses that are believed to simulate the pivot point between survival and death in humans, estimated to be between 2 and 4 Gy). In these cases, the steepness of the radiation dose response curve, coupled with inaccurate dosimetry, can lead to incorrect assessments of biomarker evolution at different radiation doses.
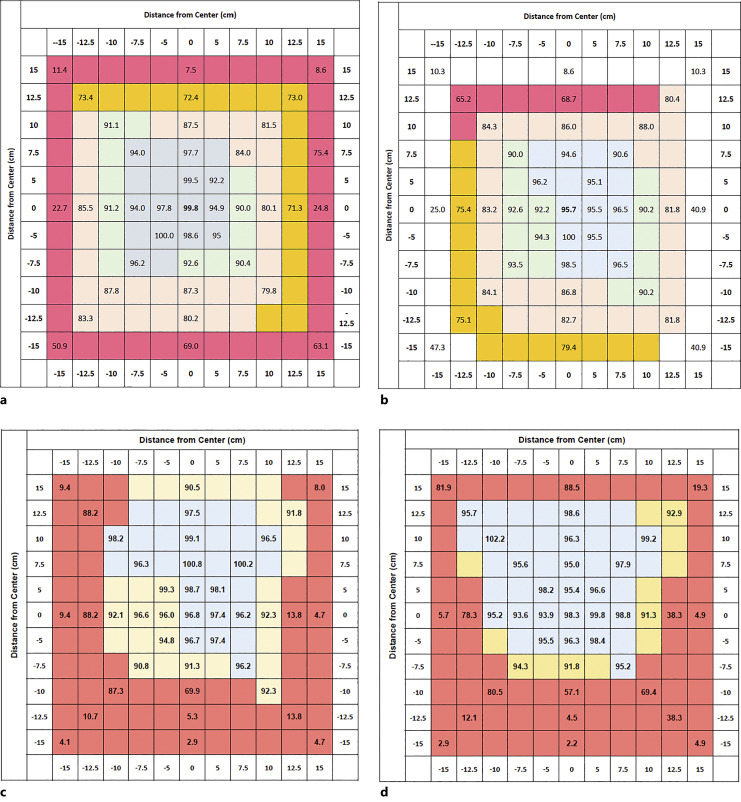
To ensure accurate and reproducible study results, a need to standardize dosimetry assessments within and among institutions conducting radiobiology research was identified. Centralized oversight in radiation biology dosimetry standards was essentially nonexistent at the time that the RNCP began to explore the possibility of implementing a program, and wide-ranging efforts to implement such an approach have not previously been undertaken in the USA. Although past efforts were made by the RNCP to establish standardized dosimetry monitoring within limited groups of radiation researchers, such as those implemented by the NIAID CMCRC Physics Core and through prior PDS awardees at the University of Maryland School of Medicine, these efforts were restricted to funded participants of these multi-project U19 and/or contract research programs. Therefore, to increase the impact of these fledgling dosimetry harmonization efforts, NIAID made a concerted effort to broaden the scope of its dosimetry program to span the entire RNCP-funded research portfolio. This effort was implemented through a contract solicitation titled “RNCP-Wide Dosimetry Guidance & Monitoring of Sources and Irradiation Protocols,” which sought proposals from offerors with the facilities, expertise, and capabilities to develop a centralized RNCP-led strategy for dosimetry harmonization that specifically applied to projects across the RNCP portfolio. In June of 2020, a contract was awarded to the University of Wisconsin (Madison) Medical Radiation Research Center, which includes an Accredited Dosimetry Calibration Laboratory [https://uwmrrc.wisc.edu/?q=content/about-uwmrrc]. This contract provides NIST-traceable standardized dosimetry monitoring across the RNCP portfolio with a goal of achieving delivered doses of within 5% of a defined target dose (4 Gy). Since inception, the contract has provided dosimetry assessment for both radionuclide-based, orthovoltage cabinet-type radiobiology X-ray irradiators, and 4MV LINAC photon beams. An example of the dramatic effect that changes in output energy, fluence, and filtration can have on the dose distribution at the target platform surface is illustrated (Fig. 5). To date, this dosimetry contract has enabled surveying of more than 30 irradiators, with additional machines added annually. The outcomes of this effort indicate a continued need for a centralized and standardized well-defined approach to radiation dosimetry for preclinical radiation biology studies. This effort may also support improved reporting of irradiation conditions in the literature to help promote replicable exposure conditions between biodosimetry experiments conducted at individual institutions, as well as to harmonize these conditions among institutions.
Fig. 5.
Representative dosimetry maps demonstrating effect on dose distribution from energy output, fluence, and filtration. a and b are images of dose distribution maps from the same cabinet style X-ray irradiator delivering a 4 Gy dose under two different energy output settings and with two different amounts of beam filtration, while c and d are dosimetry maps from a second cabinet style X-ray unit delivering the same dose but under different energy and beam conditions.
Conclusion
The need for radiation biodosimetry approaches has been consistent for decades – to rapidly and accurately assess exposure and inform medical interventions for affected individuals. The urgency, however, has greatly increased, given current geopolitical conditions, as well as novel and wide-spread medical, scientific, civilian, and industrial applications of radiation and radiological materials. Ultimately, to prepare the nation to effectively respond to a public health emergency, it is essential to have effective approaches to diagnose and assess the biological impact of intentional or accidental acute high-dose radiation exposures. These global events continue to drive the RNCP mission to fund and partner with other USG agencies to (e.g., BARDA, NCI, FDA) advance appropriate biodosimetry tests to support medical treatment decisions made in response to an emergency. These efforts include organizing workshops to identify gaps in the science, provide funding to appropriately address any deficiencies, and publishing biodosimetry-specific meeting reports and special journal issues. Together, the RNCP is committed to providing multiple triage approaches and effective treatment strategies to enhance the USG public health emergency response toolkit.
Acknowledgments
The opinions contained herein are the private views of the authors and are not necessarily those of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
This research received no external funding.
Author Contributions
All authors contributed to conceptualization, literature review, original draft preparation, editing, and review of this manuscript.
Funding Statement
This research received no external funding.
References
- Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, et al. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res. 2017;187:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RN, Bloomer EG, Olson JD, Hanbury DB, Dugan GO, Whitlow CT, et al. Non-human primates receiving high-dose total-body irradiation are at risk of developing cerebrovascular injury years postirradiation. Radiat Res. 2020;194:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryankalayil MJ, Martello S, Bylicky MA, Chopra S, May JM, Shankardass A, et al. Analysis of lncRNA-miRNA-mRNA expression pattern in heart tissue after total body radiation in a mouse model. J Transl Med. 2021;19:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizova TV, Osovets SV, Day RD, Druzhinina MB, Sumina MV, Pesternikova VS, et al. Predictability of acute radiation injury severity. Health Phys. 2008;94:255–63. [DOI] [PubMed] [Google Scholar]
- Bacarella N, Ruggiero A, Davis AT, Uberseder B, Davis MA, Bracy DP, et al. Whole body irradiation induces diabetes and adipose insulin resistance in nonhuman primates. Int J Radiat Oncol Biol Phys. 2020;106:878–86. [DOI] [PubMed] [Google Scholar]
- Caudell DL, Michalson KT, Andrews RN, Snow WW, Bourland JD, DeBo RJ, et al. Transcriptional profiling of non-human primate lymphoid organ responses to total-body irradiation. Radiat Res. 2019;192(1):40–52. 10.1667/RR15100.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EP, Farese AM, Parker GA, Kane MA, MacVittie TJ. Lack of cellular inflammation in a non-human primate model of radiation nephropathy. Health Phys. 2020;119(5):588–93. 10.1097/HP.0000000000001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Garcia L, Badie C, Anbalagan S, Moquet J, Gothard L, O’Brien G, et al. An ionising radiation-induced specific transcriptional signature of inflammation-associated genes in whole blood from radiotherapy patients: a pilot study. Radiat Oncol. 2021;16(1):83. 10.1186/s13014-021-01807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBo RJ, Lees CJ, Dugan GO, Caudell DL, Michalson KT, Hanbury DB, et al. Late effects of total-body gamma irradiation on cardiac structure and function in male rhesus macaques. Radiat Res. 2016;186(1):55–64. 10.1667/RR14357.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers M, DeWerd L, Deye J, Lindsay P, Murphy MK, Mitch M, et al. The importance of dosimetry standardization in radiobiology. J Res Natl Inst Stand Technol. 2013;118:403–18. 10.6028/jres.118.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drané P, Brault ME, Cui G, Meghani K, Chaubey S, Detappe A, et al. TIRR regulates 53BP1 by masking its histone methyl-lysine binding function. Nature. 2017;543(7644):211–6. 10.1038/nature21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler W, Malachowska B, Meghani K, Konstantinopoulos PA, Guha C, Singh VK, et al. Evolutionarily conserved serum microRNAs predict radiation-induced fatality in nonhuman primates. Sci Transl Med. 2017;9(379):eaal2408. 10.1126/scitranslmed.aal2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, et al. The RABIT: a rapid automated biodosimetry tool for radiological triage. Health Phys. 2010;98(2):209–17. 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty G, Amundson S, Laiakis E, Fornace A, Brenner D. High throughput biodosimetry methods: radiobiology and methods public textbook. Pittsburgh, PA: Centers for Medical Countermeasures against Radiation, National Institute of Allergy and Infectious Disease, University of Pittsburgh; 2017. p. 1–18. [Google Scholar]
- Garty G, Xu Y, Johnson GW, Smilenov LB, Joseph SK, Pujol-Canadell M, et al. VADER: a variable dose-rate external (137)Cs irradiator for internal emitter and low dose rate studies. Sci Rep. 2020;10(1):19899. 10.1038/s41598-020-76941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandhi SA, Smilenov LB, Elliston CD, Chowdhury M, Amundson SA. Radiation dose-rate effects on gene expression for human biodosimetry. BMC Med Genomics. 2015;8:22. 10.1186/s12920-015-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill BL, Mauro F. New Horizons in Biological Dosimetry. Wiley-Liss; 1991. [Google Scholar]
- Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment in criticality accidents. Health Phys. 2001;81(4):446–9. 10.1097/00004032-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Hanbury DB, Peiffer AM, Dugan G, Andrews RN, Cline JM. Long-term cognitive functioning in single-dose total-body gamma-irradiated rhesus monkeys (Macaca mulatta). Radiat Res. 2016;186(5):447–54. 10.1667/RR14430.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu J, Liu T, Defnet AE, Zalesak S, Farese AM, et al. Proteomics of non-human primate plasma after partial-body radiation with minimal bone marrow sparing. Health Phys. 2020a;119(5):621–32. 10.1097/HP.0000000000001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu J, Liu T, Tudor G, Defnet AE, Zalesak S, et al. Proteomic evaluation of the natural history of the acute radiation syndrome of the gastrointestinal tract in a non-human primate model of partial-body irradiation with minimal bone marrow sparing includes dysregulation of the retinoid pathway. Health Phys. 2020b;119(5):604–20. 10.1097/HP.0000000000001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu J, Farese AM, MacVittie TJ, Kane MA. Acute proteomic changes in non-human primate kidney after partial-body radiation with minimal bone marrow sparing. Health Phys. 2021a;121(4):345–51. 10.1097/HP.0000000000001475. [DOI] [PubMed] [Google Scholar]
- Huang W, Yu J, Liu T, Defnet AE, Zalesak-Kravec S, Farese AM, et al. Acute proteomic changes in lung after radiation: toward identifying initiating events of delayed effects of acute radiation exposure in non-human primate after partial body irradiation with minimal bone marrow sparing. Health Phys. 2021b;121(4):384–94. 10.1097/HP.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Dendinger MD, Davis AT, Register TC, DeBo R, Dugan G, et al. Type 2 diabetes is a delayed late effect of whole-body irradiation in nonhuman primates. Radiat Res. 2015;183(4):398–406. 10.1667/RR13916.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wang P, Farese AM, MacVittie TJ, Kane MA. Metabolomics of multiorgan radiation injury in non-human primate model reveals system-wide metabolic perturbations. Health Phys. 2021;121(4):395–405. 10.1097/HP.0000000000001472. [DOI] [PubMed] [Google Scholar]
- Lacombe J, Zenhausern F. Effect of mechanical forces on cellular response to radiation. Radiother Oncol. 2022;176:187–98. 10.1016/j.radonc.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Pannkuk EL, Chauthe SK, Wang YW, Lian M, Mak TD, et al. A serum small molecule biosignature of radiation exposure from total body irradiated patients. J Proteome Res. 2017;16(10):3805–15. 10.1021/acs.jproteome.7b00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Nishita D, Bujold K, Jayatilake MM, Bakke J, Gahagen J, et al. Salivary metabolomics of total body irradiated nonhuman primates reveals long-term normal tissue responses to radiation. Int J Radiat Oncol Biol Phys. 2019;105(4):843–51. 10.1016/j.ijrobp.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Canadell MP, Grilj V, Harken AD, Garty GY, Brenner DJ, et al. Small molecule responses to sequential irradiation with neutrons and photons for biodosimetry applications: an initial assessment. Radiat Res. 2021;196(5):468–77. 10.1667/RADE-20-00032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Pujol Canadell M, Shuryak I, Perrier JR, Taveras M, Patel P, et al. Candidate protein markers for radiation biodosimetry in the hematopoietically humanized mouse model. Sci Rep. 2018;8(1):13557. 10.1038/s41598-018-31740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Robinson E, Taylor L, Tompkins C, Cornforth MN, Simon SL, et al. Chromosome translocations, inversions and telomere length for retrospective biodosimetry on exposed U.S. atomic veterans. Radiat Res. 191:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon N, Rogers CJ, Lukaszewicz AI, Axtelle J, Yadav M, Song F, et al. Detection of acute radiation sickness: a feasibility study in non-human primates circulating miRNAs for triage in radiological events. PLoS One. 2016;11(12):e0167333. 10.1371/journal.pone.0167333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalson KT, Macintyre AN, Sempowski GD, Bourland JD, Howard TD, Hawkins GA, et al. Monocyte polarization is altered by total-body irradiation in male rhesus macaques: implications for delayed effects of acute radiation exposure. Radiat Res. 2019;192(2):121–34. 10.1667/RR15310.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Singh VK, Fornace AJ. Lipidomic signatures of nonhuman primates with radiation-induced hematopoietic syndrome. Sci Rep. 2017;7(1):9777. 10.1038/s41598-017-10299-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Girgis M, Garty GY, Morton SR, Pujol-Canadell M, et al. Biofluid metabolomics of mice exposed to external low-dose rate radiation in a novel irradiation system, the variable dose-rate external (137)Cs irradiator. J Proteome Res. 2021;20(11):5145–55. 10.1021/acs.jproteome.1c00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Garty G, Bansal S, Ponnaiya B, Wu X, et al. Biofluid metabolomics and lipidomics of mice exposed to external very high-dose rate radiation. Metabolites. 2022;12(6):520. 10.3390/metabo12060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repin M, Pampou S, Brenner DJ, Garty G. The use of a centrifuge-free RABiT-II system for high-throughput micronucleus analysis. J Radiat Res. 2020;61(1):68–72. 10.1093/jrr/rrz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins ME, Bourland JD, Cline JM, Wheeler KT, Deadwyler SA. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat Res. 2011;175(4):519–25. 10.1667/RR2497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CJ, Lukaszewicz AI, Yamada-Hanff J, Micewicz ED, Ratikan JA, Starbird MA, et al. Identification of miRNA signatures associated with radiation-induced late lung injury in mice. PLoS One. 2020;15(5):e0232411. 10.1371/journal.pone.0232411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuryak I, Ghandhi SA, Turner HC, Weber W, Melo D, Amundson SA, et al. Dose and dose-rate effects in a mouse model of internal exposure from 137Cs. Part 2: integration of gamma-H2AX and gene expression biomarkers for retrospective radiation biodosimetry. Radiat Res. 2020;196(5):491–500. 10.1667/RADE-20-00042.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproull M, Kawai T, Krauze A, Shankavaram U, Camphausen K. Prediction of total-body and partial-body exposures to radiation using plasma proteomic expression profiles. Radiat Res. 2022a;198(6):573–81. 10.1667/RADE-22-00074.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproull M, Nishita D, Chang P, Moroni M, Citrin D, Shankavaram U, et al. Comparison of proteomic expression profiles after radiation exposure across four different species. Radiat Res. 2022b;197(4):315–23. 10.1667/RADE-21-00182.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Prasanna PG, Grace MB, Wathen LK, Wallace RL, Koerner JF, et al. Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations. Health Phys. 2013;105(6):540–54. 10.1097/HP.0b013e31829cf221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur P, DeBo R, Dugan GO, Bourland JD, Michalson KT, Olson JD, et al. Clinicopathologic and transcriptomic analysis of radiation-induced lung injury in nonhuman primates. Int J Radiat Oncol Biol Phys. 2021;111(1):249–59. 10.1016/j.ijrobp.2021.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JD. Fish cytogenetics and the future of radiation biodosimetry. Radiat Prot Dosimetry. 2001;97(1):55–60. 10.1093/oxfordjournals.rpd.a006638. [DOI] [PubMed] [Google Scholar]
- Turner HC, Lee Y, Weber W, Melo D, Kowell A, Ghandhi SA, et al. Effect of dose and dose rate on temporal γ-H2AX kinetics in mouse blood and spleen mononuclear cells in vivo following Cesium-137 administration. BMC Mol Cell Biol. 2019;20(1):13. 10.1186/s12860-019-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA) . Product development under the animal rule. Guidance for Industry; 2015. [Google Scholar]
- US Food and Drug Administration . Radiation biodosimetry medical countermeasure devices: guidance for industry and food and drug administration staff. Center for devices and radiological health. Rockville, MD: US Food and Drug Administration.; 2016. [Google Scholar]
- Wang Q, Rodrigues MA, Repin M, Pampou S, Beaton-Green LA, Perrier J, et al. Automated triage radiation biodosimetry: integrating imaging flow cytometry with high-throughput robotics to perform the cytokinesis-block micronucleus assay. Radiat Res. 2019;191(4):342–51. 10.1667/RR15243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lee Y, Pujol-Canadell M, Perrier JR, Smilenov L, Harken A, et al. Cytogenetic damage of human lymphocytes in humanized mice exposed to neutrons and X rays 24 h after exposure. Cytogenet Genome Res. 2021;161(6–7):352–61. 10.1159/000516529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. Ann Intern Med. 2004;140(12):1037–51. 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- Wei J, Wang B, Wang H, Meng L, Zhao Q, Li X, et al. : Radiation-induced normal tissue damage: oxidative stress and epigenetic mechanisms. Oxid Med Cell Longev. 2019, 2019:3010342. 10.1155/2019/3010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Randers-Pehrson G, Turner HC, Marino SA, Geard CR, Brenner DJ, et al. Accelerator-based biological irradiation facility simulating neutron exposure from an improvised nuclear device. Radiat Res. 2015;184(4):404–10. 10.1667/RR14036.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Huang W, Liu T, Defnet AE, Zalesak-Kravec S, Farese AM, et al. Effect of radiation on the essential nutrient homeostasis and signaling of retinoids in a non-human primate model with minimal bone marrow sparing. Health Phys. 2021;121(4):406–18. 10.1097/HP.0000000000001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesak-Kravec S, Huang W, Wang P, Yu J, Liu T, Defnet AE, et al. Multi-omic analysis of non-human primate heart after partial-body radiation with minimal bone marrow sparing. Health Phys. 2021;121(4):352–71. 10.1097/HP.0000000000001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang M, Yang S, Cao Y, Bingrong Zhang S, Yin L, et al. A new biodosimetric method: branched DNA-based quantitative detection of B1 DNA in mouse plasma. Br J Radiol. 2010;83(992):694–701. 10.1259/bjr/49886569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yin L, Zhang K, Sun W, Yang S, Zhang B, et al. Response patterns of cytokines/chemokines in two murine strains after irradiation. Cytokine. 2012;58(2):169–77. 10.1016/j.cyto.2011.12.023. [DOI] [PubMed] [Google Scholar]