Abstract
Previous studies have shown that in addition to its function in specific RNA encapsidation, the human immunodeficiency virus type 1 (HIV-1) nucleocapsid (NC) is required for efficient virus particle assembly. However, the mechanism by which NC facilitates the assembly process is not clearly established. Formally, NC could act by constraining the Pr55gag polyprotein into an assembly-competent conformation or by masking residues which block the assembly process. Alternatively, the capacity of NC to bind RNA or make interprotein contacts might affect particle assembly. To examine its role in the assembly process, we replaced the NC domain in Pr55gag with polypeptide domains of known function, and the chimeric proteins were analyzed for their abilities to direct the release of virus-like particles. Our results indicate that NC does not mask inhibitory domains and does not act passively, by simply providing a stable folded monomeric structure. However, replacement of NC by polypeptides which form interprotein contacts permitted efficient virus particle assembly and release, even when RNA was not detected in the particles. These results suggest that formation of interprotein contacts by NC is essential to the normal HIV-1 assembly process.
Human immunodeficiency virus type 1 (HIV-1) encodes three major genes, gag, pol, and env, which are commonly found in all mammalian retroviruses. It also encodes accessory genes whose protein products are important for regulation of its life cycle (6, 30, 35). However, of all the genes encoded by HIV-1, only the protein product of the gag gene has been found to be necessary and sufficient for the assembly of virus-like particles (11, 13, 17, 22, 32, 33). The HIV-1 Gag protein initially is expressed as a 55-kDa polyprotein precursor (Pr55gag), but during or shortly after particle release, Pr55gag ordinarily is cleaved by the viral protease (PR). The products of the protease action are the four major viral proteins matrix (MA), capsid (CA), nucleocapsid (NC), and p6, and the two spacer polypeptides p2 and p1, which represent sequences between CA and NC and between NC and p6, respectively (15, 19, 23, 30).
The HIV-1 nucleocapsid proteins have two Cys-X2-Cys-X4-His-X4-Cys (Cys-His) motifs, reminiscent of the zinc finger motifs found in many DNA binding proteins, and NC has been shown to facilitate the specific encapsidation of HIV-1 genomic RNAs. In addition to its encapsidation function, NC influences virus particle assembly (7, 10, 17, 21, 40). In particular, Gag proteins lacking the NC domain fail to assemble virus particles efficiently. Nevertheless, some chimeric Gag proteins which carry foreign sequences in place of NC have been shown to assemble and release virus particles at wild-type (wt) levels (2, 37, 40). Thus, it appears that in some circumstances, the role that NC plays in virus particle assembly can be replaced. To date, it is not clear how NC affects particle assembly, although several possibilities might be envisioned. One possibility is that deletion of NC unmasks inhibitory sequences in p2 or the C terminus of CA. Alternatively, NC may simply provide a stable monomeric folded structure which locks CA or other Gag domains into an assembly-competent conformation. Another possibility is that NC facilitates assembly by forming essential protein-protein contacts between neighbor Prgag molecules, as suggested in cross-linking studies (21). Finally, the assembly role of NC may stem from its RNA binding capabilities, a hypothesis supported by studies of Campbell and Vogt (5), which have shown that RNA facilitates the in vitro assembly of retroviral Gag proteins into higher-order structures.
To distinguish among possible mechanisms by which NC facilitates HIV-1 assembly, we replaced NC with polypeptides having known structural characteristics and examined particle assembly directed by these chimeric proteins. Using this approach, we have found that NC does not play a passive role in HIV-1 assembly as either a mask to assembly inhibitor domains or a nonspecific, stably folded structure. Rather, sequences known to form strong interprotein contacts were observed to enhance assembly, suggesting a similar role for the NC domain itself. With several assembly-competent chimeric proteins, we detected no particle-associated RNAs. These results suggest that while RNA may be essential to virus assembly in the context of the wt Pr55gag protein, it is dispensable for formation of virus-like particles from chimeric proteins.
MATERIALS AND METHODS
Recombinant DNA constructs.
The NC mutants used in this study are based on the wt parental construct HIVgpt (27, 36, 38). In HIVgpt, viral sequences derive from HIV-1 strain HXB2, and the env gene has been replaced by the Escherichia coli drug resistance guanosine phosphoribosytransferase (gpt) gene (25), transcribed from the simian virus 40 early promoter. Mutations are numbered according to the HIV HXB2 proviral sequence. The constructs 2498T, TARK, TAM, and ApoTE have been described previously (21, 40). Briefly, 2498T is a PR− version of wt HIVgpt, which produces wt Gag proteins but no pol open reading frame (ORF) protein products upon transfection into Cos7 cells. TARK, TAM, and ApoTE are Gag C-terminal truncation mutants. In TARK, the gag ORF is terminated within the NC region, six residues before the first zinc finger motif; in TAM, the gag ORF terminates at the junction of p2 and NC; in ApoTE, the gag ORF terminates midway through p2. In the constructs PstTARK, PstTAM, and PstApoTE, portions of CA, P2, and NC were duplicated. For PstTARK, this was achieved by fusing the C-terminal portion of the TARK construct gag coding region (from the PstI site at nucleotide [nt] 1419) to the nt 2096 BglII site of wt HIVgpt. The nucleotide junction sequence at the fusion site is nt 2096 5′ AG ATC CCC GGG TAC CGA GCT CGA ATT CAT CGA TCC TCT AGA GTC GAT CGA CCT GCA GAA TGG GAT 3′ nt 1432, where the normal gag gene sequences are in plain font, linker sequences are in boldface, and the duplicated CA sequences derived from TARK are in italics. PstTAM and PstApoTE were created similarly by using sequences from TAM or ApoTE in place of TARK. The junction sequences at the fusion sites for PstTAM and PstApoTE are identical as those in PstTARK, but the ORFs terminate sooner, at the C terminus and the middle of the duplicated p2 regions, respectively.
For the constructs UPRT and HGXPRT, the NC region was replaced with Toxoplasma gondii monomeric enzymes uracil phosphoribosyltransferase (UPRT) and hypoxanthine-xanthine-guanine phosphoribosyltransferase (HXGPRT), which catalyze the phosphoribosylation of pyrimidine and purine bases to the nucleotide level (8, 9, 31). For constructs, UPRT and HXGPRT sequences (8, 9) (kindly provided by Buddy Ullman) were inserted at the p2 region of gag. The junction sequences for both constructs are nt 1899 5′ ACA AAT TCC TGC AGC CCT ATG 3′, where the HIV-1 sequences are in plain font, the linker sequences are in boldface, and the UPRT or HGXPRT ATG start codons are in italics; UPRT and HGPRT use their own stop codons to terminate ORFs.
In other constructs, NC regions were replaced with wt or mutant leucine zipper domains from human CREB protein (20). In the construct wtzip, the wt CREB leucine zipper domain, from CREB residue 284 to its C terminus, was fused to gag; the juncture sequence is HIV-1 nt 1899 5′ ACA AAT TCC TGC AGC CCG GGG GAT CGA GAG TGT CGT 3′, where HIV-1 sequences are in plain font, the linker sequences are in boldface, and the sequences derived from the human CREB protein encompassing the leucine zipper domain, starting from amino acid residue 284, are in italics. The constructs Ezip and Kzip have the same juncture sequences as wtzip. However, in Ezip, Arg300, Gln307, Ile312, Lys319, and Leu321 were mutated to Glu, while in Kzip, Glu298, Arg300, Glu305, Gln307, Ile312, Glu314, and Leu321 were mutated to Lys and Asn308 was mutated to His (20).
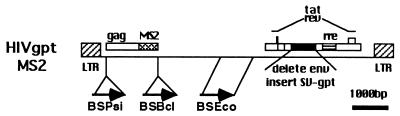
In a separate construct, the E. coli bacteriophage MS2 coat protein coding region (1, 24) (kindly provided by Marvin Wickens) was used to replace the HIV-1 NC domain. Two similar constructs were made with different junction sequences. For MS2BglII, the junction sequence is HIV-1 nt 1899 5′ ACA AAT TCC TGC AGC CCG GGG GAT CCG CGG GGT ACT GAG AGA CAG GCT AAT TTT TTA GGG AAG ATC CAT ATG GCT TCT AAC TTT ACT 3′, where the HIV-1 sequences are in plain font, the linker sequences are in boldface, and the sequences derived from the bacteriophage MS2 coat protein starting from its first amino acid residue are in italics. For MS2Sma, the junction sequence is HIV-1 nt 1899 5′ ACA AAT TCC TGC AGC CCG GGG ATC CAT ATG GCT TCT AAC TTT ACT 3′. The bacteriophage MS2 coat protein has been shown to bind to a short hairpin in its genomic RNA (1). For testing possible RNA encapsidation, a short EcoRI fragment, gaatt ccggc tagaa ctagt ggatc ccccg ggcag cttgc atgcc tgcag gtcga ctcta gaaaa catga ggatc accca tgtct gcagg tcgac tctag aaaac atgag gatca cccat gtctg caggt cgact ctaga ggatc ggaat tc, containing two such hairpins was gratefully received from Marvin Wickens and inserted at different sites along the MS2BglII proviral genome. As a control, an EcoRI fragment from HIV nt 4648 to 5743 from MS2BglII was deleted to make the construct MS2ΔEco. The MS2 binding site then was inserted at this EcoRI site, creating MS2BSEco. In MS2BSPsi, the EcoRI binding site fragment (see above) was inserted into the compatible ApoI site at HIV nt 757 of MS2BglII. Finally, in MS2BSBcl, the binding site fragment ends were converted to BamHI sites (ggatc ccccg ggcag cttgc atgcc tgcag gtcga ctcta gaaaa catga ggatc accca tgtct gcagg tcgac tctag aaaac atgag gatca cccat gtctg caggt cgact ctaga ggatc ggaat tcctgcagcccgggggatcc; sequences derived from the original EcoRI fragment are in plain font, and the modifying sequences are in boldface) and inserted into the MS2BglII nt 2429 BclI site.
Cell culture.
Cos7 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum and penicillin plus streptomycin. For calcium phosphate transfections, 20 to 30% confluent Cos7 cells on 10-cm-diameter plates were transfected as described previously (12, 36, 37, 38). Medium supernatants and cells were collected at 72 h posttransfection.
Gag protein analysis.
Detailed procedures for virus release assays have been described elsewhere (40). Briefly, at 72 h posttransfection, medium supernatants were collected and centrifuged at 4°C for 10 min at 1,000 × g to remove cell debris. Cell-free supernatants then were centrifuged through 2-ml 20% sucrose cushions to pellet virus particles. Cells were washed twice with 10 ml of ice-cold phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 1.47 mM KH2PO4, 8.05 mM NaHPO4 [pH 7.4]) and then pelleted at 4°C for 10 min at 1,000 × g. The cell pellets were lysed and collected by 10 min of microcentrifugation at 13,700 × g. Aliquots of virus pellet resuspensions and cell lysates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) along with an internal control recombinant HIV CA standard (21) for Gag protein quantitation purposes. After SDS-PAGE and electroblotting onto nitrocellulose filters, Gag proteins were immunodetected with mouse anti-HIV CA monoclonal antibody from hybridoma cell line Hy183 (made by Bruce Chesebro and obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health) as the primary antibody and an alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G as the secondary antibody. HIV Gag proteins immunodetected on the nitrocellulose membranes were quantitated by using DeskScan II 2.0 Alias and NIH Image 1.59/fat software, and levels were normalized to those for the internal control recombinant HIV CA.
Detailed procedures for sucrose density gradient fractionations can be found in previous publications (14, 16, 36, 40). In short, 72 h posttransfection, supernatants were collected from transfected Cos7 cells and centrifuged to remove cell debris. Cell-free supernatant material was pelleted by centrifugation through 20% sucrose cushions, resuspended in 200 μl of phosphate-buffered saline, mixed with internal control Moloney murine leukemia virus (M-MuLV), and layered onto linear 20 to 60% sucrose gradients in SW50.1 polyallomer tubes. Gradients were centrifuged at 4°C for 24 h at 240,000 × g (equilibrium for particles of 3S or greater). After centrifugation, 400-μl fractions were collected from the top to the bottom of the gradients. Each fraction was aliquoted for measurement of density and of HIV and M-MuLV Gag protein levels.
RNA analysis.
Viral and cellular RNA samples were isolated and detected by previous methods (40). After transfections, aliquots of virus resuspensions were used for protein analysis, while the remainder of the virus preparations were used for viral RNA isolations by multiple phenol-chloroform extractions and ethanol precipitation. Total cellular RNAs were prepared by guanidium thiocyanate-cesium chloride equilibrium centrifugation and were quantitated spectrophotometrically.
Antisense 183-base 32P-labeled probes for RNase protections were prepared from Blue HX 680-831 by in vitro transcription using T3 polymerase as described before (38). For protection assays, probes were hybridized to aliquots of the viral and cellular RNA samples, which were mixed with carrier Saccharomyces cerevisiae RNA. Hybridizations, RNase digestions, electrophoresis, and detection of protected RNA bands were done by published methods (38). Protected bands on X-ray films and Gag protein signals from corresponding Western blots were processed by DeskScan II 2.0 Alias and NIH Image 1.59/fat software for quantitation as previously outlined (39).
RESULTS
Release of gag mutants and chimeras.
Besides its function in the specific encapsidation of the viral genomic RNA, the HIV-1 nucleocapsid protein appears to influence the assembly phase of the virus life cycle (3, 4, 7, 10, 17, 21, 39, 40). In particular, deletions or major mutations in NC or p2 have been shown to inhibit virus particle assembly (40). However, little is known about the mechanisms by which NC exert its effects on assembly. To investigate what role(s) NC might play during assembly, we replaced it with polypeptides with known structural characteristics. Our assumption (see Discussion) was that the requirements for particle assembly by chimeric Prgag proteins be similar to those for wt Pr55gag. Thus, it might be possible to infer NC function from analysis of chimeras.
Initially, we tested the hypothesis that deletion or mutation of the gag NC coding region exposes regions of p2 or CA that inhibit virus particle assembly and/or release. As a positive control for these experiments, we used 2498T, a PR− construct which efficiently (44, 77) (Fig. 1a) produces unprocessed immature virus particles. Other control constructs were NC deletions ApoTE and TAM, which have been shown to be release defective, and the partial NC deletion construct TARK, which assembles and releases virus-like particles but less efficiently than 2498T (77) (Fig. 1a). Our experimental constructs PstApoTE, PstTAM, and PstTARK all have wt gag sequences through NC and into p1. However, these constructs have C-terminal gag sequence duplications such that they terminate as follows: PstApoTE after a partial duplication of CA and p2; PstTAM after CA and a complete duplication of p2; and PstTARK after a duplication of CA, p2, and 11 residues of NC. Our rationale was that the duplicated C-terminal CA and p2 sequences of PstApoTE and PstTAM ought to block virus particle release from cells if they were inhibitory.
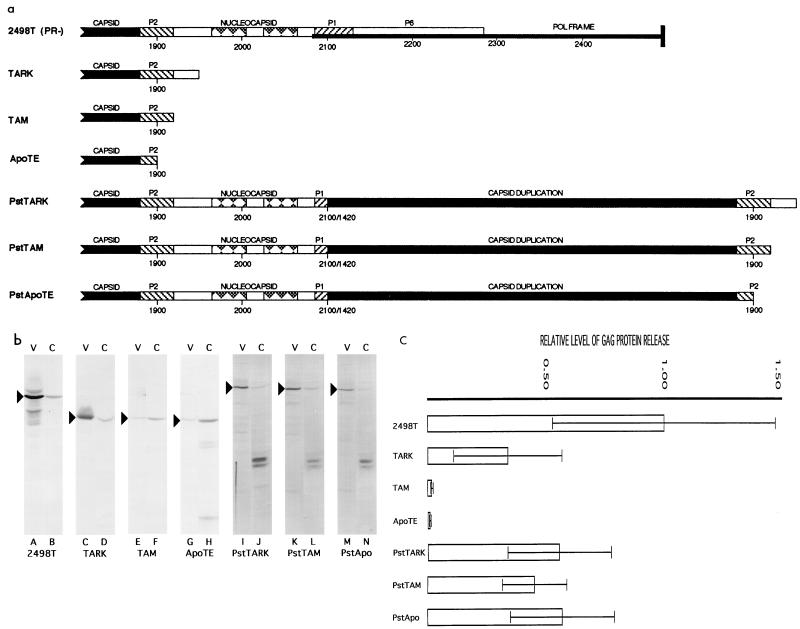
FIG. 1.
(a) HIV Gag truncation and capsid duplications. All constructs used in this study are based on the parental construct HIVgpt (30, 36, 38), which has the viral sequences of HIV HXB2. Both HIVgpt and its Pr− version 2498T have been described in previous publications (21, 40). Since NC deletion and substitution mutants are all Pr−, they are compared with 2498T. 2498T is diagrammed to show the C-terminal portion of the gag gene and the beginning of the pol gene. Only the C termini of CA, p2, NC, p1, and p6 of the gag ORF are shown: CA, black; p2 and p1, diagonal bars; NC, white with Cys-His motifs indicated as diamonds; p6, white HIV-1 proviral nucleotide numbers are designated. 2498T expresses the wt Pr55gag polyprotein but not pol gene products due to a termination codon inserted on the pol frame at the HindII site at nt 2498 (21, 40). NC deletion mutants TARK, TAM, and ApoTE have translation terminators in the gag gene, causing the gag ORF to terminate 11 residues after (TARK), precisely at (TAM), or 5 residues before (ApoTE) the junction of p2 and NC. For PstTARK, PstTAM, and PstApoTE, sequences from TARK, TAM, and ApoTE, starting from the PstI site at nt 1419 in CA and ending beyond the gag coding sequences of these constructs, were joined to the wt gag sequence at the nt 2096 BglII site in the p1 coding region of HIVgpt through a short linker sequence. Consequently, these constructs encode duplications of most of CA plus part of p2 (PstApoTE), all of p2 (PstTAM), or all of p2 plus 11 residues of NC. The precise junction sequences of these constructs are provided in Materials and Methods. (b) Medium supernatant (V) and cell (C) samples were collected 72 h after transfections of Cos7 cells with the indicated constructs. Particles were pelleted from cell-free medium samples, and half of the resuspended pellets were separated by SDS-PAGE. Cell pellets were lysed and centrifuged to remove debris, and 1/20 of the cell lysate samples were fractionated by SDS-PAGE. After electrophoresis, Gag proteins were electroblotted onto nitrocellulose filters and immunodetected with a mouse anti-p24 monoclonal antibody from hybridoma cell line Hy183 as the primary antibody. Precursor Gag proteins, identified by antibody reactivity and comparison of gel migration mobilities to known standards, are indicated with arrowheads. Sizes for the 2498T, TARK, TAM, and ApoTE proteins were as observed previously (40), while PstApoTE, PstTAM, and PstTARK migrated at calculated sizes of 68.0, 68.7, and 69.5 kDa, respectively, consistent with predicted values (67 to 69 kDa). Note that the lower-molecular-weight doublet bands observed in lanes H, J, L, and N are cellular cross-reactive bands which occasionally show up with this antibody. (c) Gag proteins in matched cell and media supernatant samples from several experiments were detected as for panel B and quantitated using the programs DeskScan II 2.0 Alias and NIH Image 1.59/fat. Gag protein levels were normalized to those of a bacterially expressed HIV CA protein standard run on each gel, and ratios of the total Gag protein levels in the media versus cells were calculated. The ratios of the Gag truncation and capsid duplication constructs were normalized to that of 2498T. Thus, the values of the ratios indicate relative levels of Gag protein release. Note that standard deviations are shown with the mean values and are calculated from the following numbers of independent transfections: 2498T, 13; TARK, 5; TAM, 4; ApoTE, 2; PstTARK, 3; PstTAM, 5; and PstApoTE, 3.
To assess assembly and release levels, cell lysate and virus-like particle-associated Gag protein levels were measured after transfection of Cos7 cells with experimental and control constructs. As expected, the 2498T Gag protein was detected in cells (Fig. 1b, lane B) and was released well from the cells (lane A). In contrast, and as observed previously, the ApoTE and TAM proteins did not direct release of virus-like particles efficiently (lanes E to H). Also expected were results with TARK (lanes C and D), showing Prgag release at levels higher than those of ApoTE and TAM but lower than that of 2498T. When the duplication proteins PstApoTE, PstTAM, and PstTARK were tested in the same assay, all three appeared to be released at reasonably high efficiencies (lanes I to N). For quantitative purposes, experiments were repeated several times, cellular and particle-associated Gag protein levels were determined, and release levels were compiled (Fig. 1c). As illustrated, 2498T was released well from cells, ApoTE and TAM were released poorly, and TARK was released less efficiently than 2498T. All duplication constructs released virus-like particles at considerably higher levels than ApoTE and TAM and 45 to 58% as well as 2498T (Fig. 1c). These results do not support the notion that the free C termini of p2 and CA actively inhibit particle assembly by ApoTE and TAM proteins.
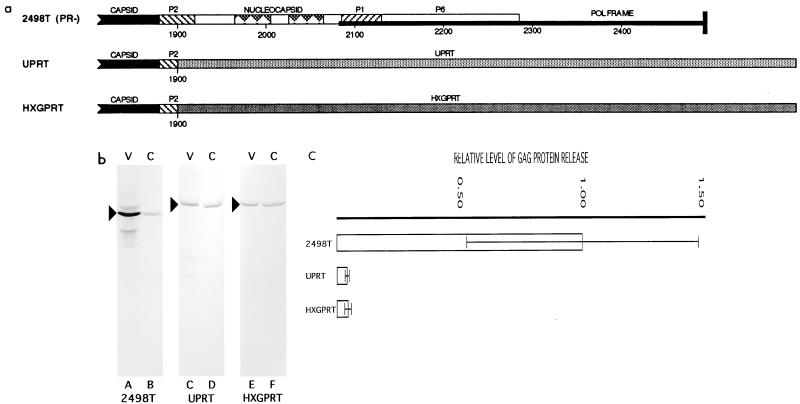
While the above-described experiments suggest that CA or p2 residues in NC deletion mutants do not actively inhibit assembly, NC might be required simply because it nonspecifically restricts p2 and/or CA into an assembly-competent conformation. To test this possibility, NC was replaced with monomeric proteins which form well-defined structures (8, 9, 31). The protein sequences used to replace NC were UPRT and HXGPRT enzymes from T. gondii (8, 9) (Fig. 2a). UPRT and HXGPRT are 26- to 27-kD polypeptides, and UPRT behaves as a monomer in solution (8), while HXGPRT is a monomer at concentrations lower than 4 μM but can form weak dimers with a dissociation constant of 40 μM (9, 31). As shown in Fig. 2b, lanes D and F, chimeric Gag proteins which have the HIV-1 NC regions replaced by UPRT and HXGPRT were expressed well in transfected Cos7 cells. When protein samples from the transfected Cos7 cell lysates were assayed for UPRT and HXGPRT enzymatic activities (8, 9, 31), we observed specific activities of approximately 0.16 and 0.14 nmol/min/μg for UPRT and HXGPRT, respectively (data not shown). These specific activities correspond to 36 and 0.2% of the activities observed for the purified enzymes, suggesting that at least the UPRT enzyme domain retained an intact folded structure. However, while 2498T proteins were released efficiently from cells (compare lanes A and B), the UPRT and HXGPRT versions were not (lanes C and E). Quantitation of several independent transfections (Fig. 2c) showed that release levels of UPRT and HXGPRT were only 4 to 5% of 2498T levels and only marginally higher than levels for the negative control ApoTE and TAM constructs. These data suggest that NC does not enhance virus assembly simply because it nonspecifically constrains CA or p2.
FIG. 2.
(a) Monomeric enzyme substitutions. 2498T is diagrammed in Fig. 1a. The constructs UPRT and HGPRT were constructed from the coding regions of the UPRT and HXGPRT enzymes from T. gondii (8, 9, 31). Starting from their first methionine residues, the enzyme coding regions were fused to gag at nt 1899 in the p2 region through a short linker. The precise sequences of the fusion sites of these constructs are provided in Materials and Methods. (b) Particle-associated medium supernatant (V) and cell (C) samples from Cos7 cells transiently transfected with the indicated constructs were prepared, electrophoresed, and electroblotted, and Prgag proteins (arrowheads) were detected as described for Fig. 1b. (c) Gag proteins in matched cell and medium supernatant samples from several transfections were detected, quantitated, and normalized as for Fig. 1c. Ratios of the total Gag protein levels in the particle-associated medium supernatant versus cell samples were calculated and normalized to that of 2498T. Values shown thus indicate relative levels of Gag protein release. Standard deviations are shown with the mean values and derive from 13 independent transfections for 2498T and 3 independent transfections for both UPRT and HXGPRT.
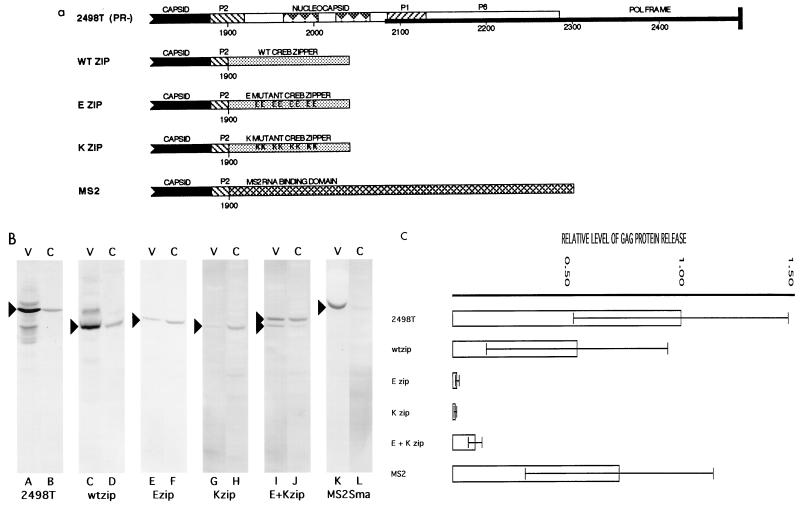
To test whether it might function as an active assembly domain by making interprotein contacts, NC was replaced by proteins with known abilities to form protein-protein interactions. One such construct was wtzip, in which NC was replaced by 44 residues comprising the wt leucine zipper domain of human CREB DNA binding protein (20) (Fig. 3a). Two control constructs were the mutant zipper constructs Ezip and Kzip (Fig. 3a), which form homodimers inefficiently but readily form leucine zipper heterodimers (20). As shown in Fig. 3b (lanes C and D), the wtzip construct directs release of virus-like particles similar to that seen for the positive control 2498T (lanes A and B), and quantitation of independent transfections showed wtzip release levels to be over half of that of the control (Fig. 3b). In contrast with wtzip, we observed that the mutant Ezip and Kzip chimeric proteins did not direct particle release efficiently (Fig. 3b and c). Because we found that the Kzip chimeric protein consistently showed a higher mobility than the wtzip or Ezip proteins in SDS-polyacrylamide gels, it was possible to distinguish the Kzip and Ezip chimeras in cotransfections. Interestingly, when the Kzip and Ezip constructs were cotransfected into Cos7 cells, virus-like particles were released from cells at higher levels than with either construct alone. As shown in Fig. 3b, cotransfection of Ezip and Kzip constructs into cells (lane J) resulted in a relative increase in Ezip protein release and a marked increase in Kzip protein release (lane I). Quantitation (Fig. 3c) showed that chimeric protein release was increased over fivefold in cotransfections relative to individual transfections. While cotransfection release levels were only 18% that of wtzip release levels, part of this difference may be attributable to differences in cotransfection efficiencies, difference in Ezip and Kzip chimeric protein expression levels, and/or reduced dimerization of mutant versus wt proteins. Taken together, our results with wt and mutant zipper chimeras strongly indicated that the assembly function of HIV-1 NC can be replaced by polypeptides which form interprotein contacts.
FIG. 3.
(a) Oligomerization domain substitutions. 2498T is described and diagrammed as in Fig. 1a. In the construct wtzip, the wt leucine zipper domain from the human CREB protein (20) starting from amino acid residue 284 was fused to HIV-1 nt 1899 in the p2 region of gag through a short linker sequence. The constructs Ezip and Kzip were created similarly to wtzip but possess zipper region mutations: in Ezip, Arg300, Gln307, Ile312, Lys319, and Leu321 were converted to Glu; in Kzip, Glu298, Arg300, Glu305, Gln307, Ile312, Glu314, and Leu321 were converted to Lys and Asn308 was converted to His (20). In the construct MS2, the bacteriophage MS2 coat protein (1, 18, 19, 24, 26, 28, 29, 34), starting from its first amino acid residue, was fused to HIV-1 nt 1899 through a short linker sequence. Precise sequences of the fusion sites of these constructs are given in Materials and Methods. (b) Gag proteins in particle-associated media supernatant (V) and cell (C) samples from Cos7 cells transfected with the indicated constructs were detected as described for Fig. 1b. Note that for single-construct transfections, 16-μg DNA samples were used, while Ezip-Kzip cotransfections used 8 μg of each plasmid construct. (c) Gag proteins in matched cell and medium supernatant samples from several transfections were detected, quantitated, and normalized as for Fig. 1c. Ratios of the total Gag protein levels in the media versus cells were calculated and normalized to that of 2498T. Standard deviations and mean values derive from the following numbers of independent transfections: 2498T, 13; wtzip, 8; Ezip, 5; Kzip, 4; Ezip plus Kzip, 5; and MS2, 2.
As an additional test, we replaced NC with the E. coli bacteriophage MS2 coat protein (24) (Fig. 3a), which functions as multimer (24). Ordinarily, this protein binds and encapsidates the bacteriophage RNA and also acts as translational repressor of the phage replicase by binding to an RNA hairpin structure in the phage RNA genome (1, 18, 26, 28, 29, 34). Since the MS2 coat protein tolerates N-terminal fusions, we reasoned that it might function in place of NC. As shown in Fig. 3b (lanes K and L), cells transfected with MS2 constructs release high levels of virus-like particles—nearly comparable to 2498T release levels (Fig. 3c). These results substantiate the hypothesis that NC assembly domain can be replaced by protein domains known to make interprotein contacts.
Characterization of wtzip and MS2 virus-like particles.
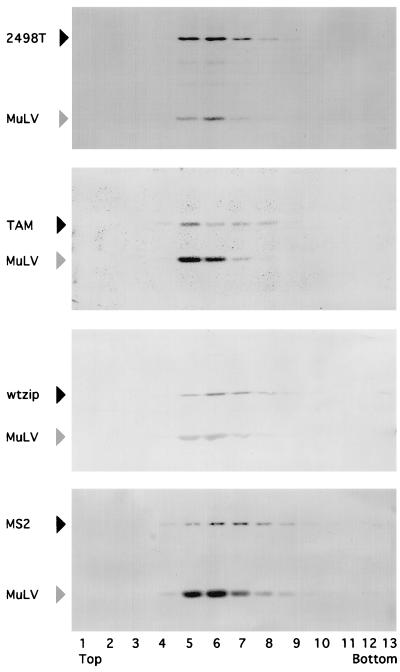
Previous work has implied a correlation concerning the presence of the NC domain and assembly of tightly packed virus-like particles which have characteristic densities (16, 40). To assay the densities of well-released NC substitution mutants, virus particles were pelleted from cell-free supernatants from transfected Cos7 cells and mixed with internal control M-MuLV, and then sedimented by equilibrium centrifugation through linear 20 to 60% sucrose gradients. Fractions were collected from the top to the bottom of the gradients after centrifugation, and each fraction was aliquoted for measurement of HIV and M-MuLV Gag protein levels by SDS-PAGE and immunoblotting. As shown in Fig. 4 and as seen previously (40), 2498T particles are slightly more dense than the internal control M-MuLV particles, suggesting that they are tightly packed; in contrast, the NC deletion mutant TAM came to equilibrium at a density approximately that of the M-MuLV control. The chimeric Gag proteins wtzip and MS2 (Fig. 4) show sedimentation patterns similar to that of 2498T, suggesting that these domains help mediate tight packing of Gag proteins within virus particles.
FIG. 4.
Sucrose density gradient fractionation of virus and virus-like particles. Virus pellets prepared from cell-free supernatants from transfected Cos7 cells were resuspended in PBS, mixed with mouse M-MuLV suspensions, and layered on top of the linear 20 to 60% sucrose gradients. Gradients were centrifuged for 24 h at 240,000 × g such that particles with a sedimentation coefficient of 3S or greater would come to equilibrium. After centrifugation, a total of 13 fractions were collected from the top to bottom. Each fraction was monitored for density, and HIV-1 and M-MuLV Gag proteins (black and gray arrowheads) were visualized after SDS-PAGE by immunoblotting.
Although wt zipper portion of CREB protein has no known RNA binding function, it was of interest to ascertain whether spliced or full-length viral RNA might be incorporated into wtzip particles. Similarly, because the MS2 coat protein is known to bind RNA, it also was of interest to assay the RNA content of MS2 chimeric particles. In this regard, since the MS2 coat protein preferentially binds a target RNA hairpin structure, we introduced this element into the MS2BglII construct at three different sites, creating the constructs MS2BSPsi, MS2BSBcl, and MS2BSEco (Fig. 5). For detecting viral RNAs in cells and virus-like particles produced from Cos7 cell transfections, an antisense RNA probe that crosses the HIV-1 major splice donor site was used, so that full-length and spliced viral RNAs could be detected simultaneously (39, 40). Using this probe and RNA samples from cells and virus-like particles, we followed previous protocols for quantitation of spliced and full-length viral RNAs and for normalization of virus yields by Gag protein quantitation (39, 40) (see Materials and Methods).
FIG. 5.
MS2 binding site constructs. The bacteriophage MS2 coat protein has been shown to bind to a short hairpin in its genomic RNA (1). For testing possible RNA encapsidation, a short EcoRI fragment containing two such hairpins was inserted at different sites along the MS2BglII (see Materials and Methods and Fig. 3a) proviral genome. As a control, an EcoRI fragment from HIV nt 4648 to 5743 from MS2BglII was deleted to make the construct MS2ΔEco. The EcoRI fragment containing the MS2 binding sites then was inserted at this EcoRI site, creating MS2BSEco. In MS2BSPsi, the EcoRI binding site fragment was inserted into the compatible ApoI site at HIV nt 757 of MS2BglII. Finally, in MS2BSBcl, the binding site fragment ends were converted to BamHI sites and inserted into the MS2BglII nt 2429 BclI site. Precise sequences of the junction sites of these constructs are given in Materials and Methods.
Genomic and spliced RNA signals from cell and virus samples used for Fig. 6 and corresponding Gag protein levels from virus particles detected by Western blotting were quantitated by using DeskScan II 2.0 Alias and NIH Image 1.59/fat software. From these data, for each construct (wtzip, MS2Bg1IIBSRI, and MS2BglIIΔRI) we calculated the following ratios: (i) total levels of viral spliced and unspliced RNAs in particle samples/cell sample and (ii) total particle-associated viral RNA signals/particle Gag protein signals. Calculated ratios normalized to the 2498T ratio (set at 100) were <1.0 for all mutants.
FIG. 6.
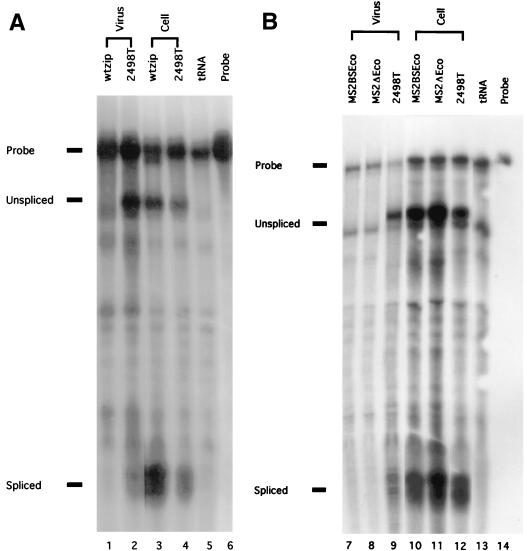
Genomic and spliced RNA levels in cells and virus particles of wtzip and MS2 HIV-1 constructs. Aliquots of cellular and viral RNA samples prepared from transfected cells and virus pellets, as described in Materials and Methods, were mixed with 10 μg of yeast tRNA, ethanol precipitated, dried, and hybridized to an HIV-1 antisense probe of 183 nt (lanes 6 and 14), which crosses the HIV-1 major splice donor site. Following the RNase protection described in Materials and Methods, the probe is capable of detecting both spliced viral transcripts at a fragment size of 63 to 64 nt and unspliced, genomic RNAs at a protected fragment size of 150 nt. Results from mock reactions using yeast tRNA samples alone were used (lanes 5 and 13). Note that viral RNA signals were normalized for total Gag protein content to yield encapsidation efficiencies as indicated in Results.
As shown in Fig. 6, genomic and spliced viral RNAs are readily detected in both wtzip- and 2498T-transfected cells (lanes 3 and 4). Additionally, 2498T specifically encapsidates large levels of genomic RNA and low levels of spliced RNAs into virus particles (lane 2; see above). In contrast, wtzip does not appear to encapsidate either genomic or spliced RNAs (lane 1; see above). Similar results were observed when NC was replaced by the MS2 coat protein (lanes 7 to 14). Once again, 2498T was observed to encapsidate high levels of full-length viral RNAs and lower levels of spliced RNAs (lanes 9 and 12). In contrast, the virus-like particles formed by MS2 chimeric proteins failed to package RNAs transcribed from the MS2ΔEco construct (lanes 8 and 11; see above). Even insertion of two MS2 RNA hairpin binding sites at the EcoRI site in MS2BSEco, the BclI site in MS2BSBcl, or the HIV-1 5′ noncoding region in MS2BSPsi (Fig. 5) failed to facilitate detectable RNA encapsidation into virus-like particles produced by MS2 chimeric proteins (lanes 7 and 10; data not shown for MS2BSBcl and MS2BSPsi). While it is possible that nonviral RNAs were encapsidated into wtzip or MS2 particles, these results are consistent with the interpretation that the assembly function of the HIV-1 nucleocapsid protein can be replaced by a polypeptide which does not bind RNA (see Discussion).
DISCUSSION
Previous studies have shown that NC is essential to assembly of mammalian or avian retroviruses (7, 10, 17, 21, 40). Although it is clear that deletions of NC inhibit virus assembly, previous studies have not completely elucidated the mechanism(s) by which NC influences assembly. Cross-linking studies have shown that HIV-1 NC residues on adjacent Prgag molecules are in close proximity (21), and in vitro assembly experiments have implicated RNA as an accessory in the wt HIV assembly pathway (5). However, our approach has been to assess the abilities of polypeptides to substitute for the assembly function of NC in vivo and to infer NC’s role from the known characteristics of the replacement sequences. This method certainly can be used to examine how protein sequences can enhance virus-like particle assembly or release in the context of the amino-terminal portions of the HIV-1 Prgag protein (MA-CA). However, it should be obvious that inferences regarding NC function are dependent on the number of possible ways a polypeptide might influence particle assembly or release.
Subject to the above-specified qualification, our results support the hypothesis that NC is an active assembly domain (2), which can be replaced by heterologous domains which form interprotein contacts. Duplication of CA and p2 at the C terminus of Gag did not inhibit virus assembly (Fig. 1), suggesting that NC does not simply mask sequences which are toxic to virus assembly. Replacement of NC by monomeric proteins UPRT and HGPRT did not facilitate virus-like particle release (Fig. 2), which suggests that NC does not act passively, by forming a stable structure that restricts p2 or CA in an assembly-competent conformation. However, the MS2 coat protein and the wt CREB zipper domain both form interprotein contacts, and replacing NC with either of them increased release levels relative to the NC deletion proteins ApoTE and TAM (Fig. 3). Additionally, replacement of NC with mutant Ezip or Kzip leucine zippers, which are incapable of forming homodimers, did not facilitate assembly of virus-like particles. In contrast, in cotransfection experiments, which permit the formation of heterodimers, release levels were increased (Fig. 3).
The wt human CREB zipper domain is not known to bind RNA. Thus, when it replaced NC, it was not surprising that no viral genomic or spliced RNA appeared to be encapsidated into the wtzip virus particles (Fig. 6 and data in Results). Although the bacteriophage MS2 coat protein can bind to its own RNA genome at a specific hairpin structure (1, 18, 26, 28, 29, 34), in the HIV-1 Gag fusion proteins, it did not appear to bind spliced or unspliced HIV-1 viral RNA sequences, even when MS2 binding sites were present in the viral genomes (Fig. 6 and data in Results). Although we have not tested assembly of cellular RNAs into virus particles, if spliced viral RNA encapsidation is indicative of nonspecific RNA incorporation, we would expect little RNA in these particles. This implies that NC may be replaced by domain which does not need to bind RNA (40). While evidence suggests that fusion proteins of Gag with MS2 coat protein and wt zipper domain do not need to bind RNA for assembly purposes, the studies of Campbell and Vogt suggest that loss of the NC RNA binding function correlates with a decreased efficiency of assembly in vitro (5). It is possible that NC interprotein contacts are mediated indirectly by RNA; alternatively, RNA might be required for assembly in the context of NC. Insofar as specific RNA encapsidation is concerned, it is clear that the wt Pr55gag protein can efficiently and specifically encapsidate genomic-length HIV-1RNA (40) (Fig. 6 and data in Results). In contrast, even in the presence of its binding sequence at different sites in the HIV-1 genome, the Gag-MS2 chimera (MS2) did not appear to bring viral RNA into virus particles (Fig. 6, data in Results, and data not shown). What might be the reasons for this lack of RNA binding? Possibly, the MS2 coat protein when fused to Gag is nonfunctional for RNA binding. Alternatively, the RNA binding site may not be folded appropriately or might be masked by cellular or viral factors. Yet another explanation is that the viral RNAs may not localize to the assembly sites of the chimeric proteins. Investigation of these and other possibilities may provide further insights to HIV-1 assembly and encapsidation mechanisms.
ACKNOWLEDGMENTS
We thank Jason McDermott, Marylene Mougel, and Sonya Karanjia for help and advice throughout the course of this work. The anti-M-MuLV CA monoclonal antibody was a gift from Bruce Chesebro, who also made the anti-HIV CA Hy183 hybridoma cell line that was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Two molecular clones encoding the UPRT and HXGPRT proteins used in this study were generously provided by Buddy Ullman, and Darrick Carter kindly performed enzyme assays. The clones encoding the wt and mutant E and K leucine zippers were the gifts of Richard Goodman, Marc Loriaux, and Jim Lundblatt, and the bacteriophage MS2 coat protein and binding site clones were generously provided by Marvin Wickens.
This work was supported by grant 2RO1 CA47088-07 from the National Cancer Institute.
REFERENCES
- 1.Bardwell V J, Wickens M. Purification of RNA and RNA-protein complexes by an R17 coat protein affinity method. Nucleic Acids Res. 1990;18:6587–6594. doi: 10.1093/nar/18.22.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett R P, Nelle T D, Wills J W. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1993;67:6487–6498. doi: 10.1128/jvi.67.11.6487-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz R D, Ohagen A, Hoglung S, Goff S P. Retroviral nucleocapsid domains mediate the specific recognition of genomic viral RNAs by chimeric Gag polyproteins during RNA packaging in vivo. J Virol. 1995;69:6445–6456. doi: 10.1128/jvi.69.10.6445-6456.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz R D, Luban J, Goff S P. Specific binding of human immunodeficiency virus type 1 gag polyprotein and nucleocapsid protein to viral RNAs detected by RNA mobility shift assays. J Virol. 1993;67:7190–7200. doi: 10.1128/jvi.67.12.7190-7200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell S, Vogt V. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cann, A. J., and J. Karn. 1989. Molecular biology of HIV-1: new insights into the virus life cycle. AIDS 3(Suppl. 1):S19–S34. [PubMed]
- 7.Carriere C, Gay B, Chazal N, Morin N, Boulanger P. Sequence requirements for encapsidation of deletion mutants and chimeras of human immunodeficiency virus type-1 Gag precursor into retrovirus-like particles. J Virol. 1995;69:2366–2377. doi: 10.1128/jvi.69.4.2366-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter, D., R. G. K. Donald, D. Roos, and B. Ullman. Expression, purification, and characterization of uracil phosphoribosyltransferase from Toxoplasma gondii. Mol. Biochem. Parasitol., in press. [DOI] [PubMed]
- 9.Donald R G K, Carter D, Ullman B, Roos D S. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- 10.Dorfman T, Luban J, Goff S P, Haseltine W A, Gottlinger H G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gheysen D, Jacobs E, de Foresta F, Thiriart D, Francotte M, Thines D, De Wilde M. Assembly and release of HIV-1 precursor pr55gag virus-like particles from recombinant baculovirus-infected cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 12.Graham R, van der Eb A. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 13.Haffar O, Garrigues J, Travis B, Moran P, Zarling J, Hu S-L. Human immunodeficiency virus-like, nonreplicating Gag-Env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990;64:2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen M, Jelinek L, Whiting S, Barklis E. Transport and assembly of Gag proteins into Moloney murine leukemia virus. J Virol. 1990;64:5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson L E, Bowers M A, Sowder II R C, Serabyn S A, Johnson D G, Bess J W, Jr, Arthur L O, Bryant D K, Fenselau C. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processing, and complete amino acid sequences. J Virol. 1992;66:1856–1865. doi: 10.1128/jvi.66.4.1856-1865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones T, Blaug G, Hansen M, Barklis E. Assembly of Gag–β-galactosidase proteins into retrovirus particles. J Virol. 1990;64:2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krausslich H G, Facke M, Heuser A M, Konvalinka J, Zentgraf H. The spacer peptide between human immunodeficiency virus capsid and nucleocapsid proteins is essential for ordered assembly and viral infectivity. J Virol. 1995;69:3407–3419. doi: 10.1128/jvi.69.6.3407-3419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeCuyer K A, Behlen L S, Uhlenbeck O C. Mutants of the bacteriophage MS2 coat protein that alter its cooperative binding to RNA. Biochemistry. 1995;34:10600–10606. doi: 10.1021/bi00033a035. [DOI] [PubMed] [Google Scholar]
- 19.Leis J, Baltimore D, Bishop J B, Coffin J, Fleissner E, Goff S P, Oroszlan S, Robinson H, Skalka A M, Temin H M, Vogt V. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988;62:1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loriaux M M, Rehfuss R P, Brennan R G, Goodman R H. Engineered leucine zippers show that hemiphosphorylated CREB complexes are transcriptionally active. Proc Natl Acad Sci USA. 1993;90:9046–9050. doi: 10.1073/pnas.90.19.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott J, Farrell L, Ross R, Barklis E. Structural analysis of human immunodeficiency virus type 1 Gag protein interactions, using cysteine-specific reagents. J Virol. 1996;70:5106–5114. doi: 10.1128/jvi.70.8.5106-5114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mergener K, Facke M, Welker R, Brinkmann V, Gelderblom H R, Krausslich H G. Analysis of HIV particle formation using transient expression of subviral constructs in mammalian cells. Virology. 1992;186:25–39. doi: 10.1016/0042-6822(92)90058-w. [DOI] [PubMed] [Google Scholar]
- 23.Mervis R J, Ahmad N, Lillehoj E P, Raum M G, Salazar F H R, Chan H W, Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988;62:3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min Jou W, Haegeman G, Ysebaert M, Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972;237:82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- 25.Mulligan R C, Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci USA. 1981;78:2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni C Z, Syed R, Kodandapani R, Wickersham J, Peabody D S, Ely K R. Crystal structure of the MS2 coat protein dimer: implications for RNA binding and virus assembly. Structure. 1995;3:255–263. doi: 10.1016/S0969-2126(01)00156-3. [DOI] [PubMed] [Google Scholar]
- 27.Page K A, Landau N R, Littman D R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peabody D S. The RNA binding site of bacteriophage MS2 coat protein. EMBO J. 1993;12:595–600. doi: 10.1002/j.1460-2075.1993.tb05691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peabody D S, Lim F. Complementation of RNA binding site mutations in MS2 coat protein heterodimers. Nuclei Acids Res. 1996;24:2352–2359. doi: 10.1093/nar/24.12.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratner L, Haseltine W, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, Ivanoff L, Petteway S R, Jr, Pearson M L, Lautenberger J A, Papas T S, Ghrayeb J, Chang N T, Gallo R C, Wong-Staal F. Complete nucleotide sequences of the AIDS virus, HTLV-III. Nature. 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher M A, Carter D, Roos D S, Ullman B. Crystal structures of Toxoplasma gondii HGXPRTase reveal the catalytic role of a long flexible loop. Nat Struct Biol. 1996;3:881–887. doi: 10.1038/nsb1096-881. [DOI] [PubMed] [Google Scholar]
- 32.Shioda T, Shibuta H. Production of human immunodeficiency virus (HIV)-like particles from cells infected with recombinant vaccinia viruses carrying the gag gene of HIV. Virology. 1990;175:139–148. doi: 10.1016/0042-6822(90)90194-v. [DOI] [PubMed] [Google Scholar]
- 33.Smith A J, Cho M-I, Hammarskjold M-L, Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into virus-like particles. J Virol. 1990;64:2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valegard K, Murray J B, Stockley P G, Stonehouse N J, Liljas L. Crystal structure of an RNA bacteriophage coat protein-operator complex. Nature. 1994;371:623–626. doi: 10.1038/371623a0. [DOI] [PubMed] [Google Scholar]
- 35.Wain-Hobson S, Sonigo P, Danos O, Cole S, Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985;40:9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- 36.Wang C-T, Barklis E. Assembly, processing, and infectivity of human immunodeficiency virus type 1 Gag mutants. J Virol. 1993;67:4264–4273. doi: 10.1128/jvi.67.7.4264-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C-T, Stegeman-Olsen J, Zhang Y, Barklis E. Assembly of HIV Gag-β-galactosidase fusion proteins into virus particles. Virology. 1994;200:524–534. doi: 10.1006/viro.1994.1215. [DOI] [PubMed] [Google Scholar]
- 38.Wang C-T, Zhang Y, McDermott J, Barklis E. Conditional infectivity of a human immunodeficiency virus matrix domain deletion mutant. J Virol. 1993;67:7067–7076. doi: 10.1128/jvi.67.12.7067-7076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Barklis E. Nucleocapsid protein effects on the specificity of retrovirus RNA encapsidation. J Virol. 1995;69:5716–5722. doi: 10.1128/jvi.69.9.5716-5722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Barklis E. Effects of nucleocapsid mutations on human immunodeficiency virus assembly and RNA encapsidation. J Virol. 1997;71:6765–6776. doi: 10.1128/jvi.71.9.6765-6776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]