Abstract
Endometriosis is a serious, chronic disorder where endometrial tissue grows outside the uterus, causing severe pelvic pain and infertility. It affects 11% of women. Endometriosis is a multifactorial disorder of unclear etiology, although retrograde menstruation plays a major role. It has a genetic component with over 40 genetic risk factors mapped, although their mechanism of action is still emerging. New evidence suggests a role for retrograde menstruation of endometrial stem/progenitor cells, now that identifying markers of these cells are available. Recent lineage tracing and tissue clearing microscopy and 3D reconstruction has provided new understanding of endometrial glandular structure, particularly the horizontal orientation and interconnection of basalis glands. New sequencing technologies, particularly whole genome DNA sequencing are revealing somatic mutations, including in cancer driver genes, in normal and eutopic endometrium of patients with endometriosis, as well as ectopic endometriotic lesions. Methylome sequencing is offering insight into the regulation of genes and the role of the environmental factors. Single cell RNA sequencing reveals the transcriptome of individual endometrial cells, shedding new light on the diversity and range of cellular subpopulations of the major cell types present in the endometrium and in endometriotic lesions. New endometrial epithelial organoid cultures replicating glandular epithelium are providing tractable models for studying endometriosis. Organoids derived from menstrual fluid offer a non‐invasive source of endometrial tissue and a new avenue for testing drugs and developing personalized medicine for treating endometriosis. These new approaches are rapidly advancing our understanding of endometriosis etiology.
Keywords: endometrial stem/progenitor cells, endometriosis, genetic variants, retrograde menstruation, somatic mutations
INTRODUCTION
Endometriosis and disease heterogeneity
Endometriosis is defined as the presence of endometrial‐like tissue outside of the uterine cavity. Endometriotic lesions can form on the peritoneal surface of body organs, including the bowel, ovaries, uterus, bladder, on the body wall lining the peritoneal cavity and deep infiltrating lesions form in the pouch of Douglas. Endometriosis lesions cause a multitude of symptoms; chronic pelvic pain, bowel and bladder dysfunction, painful sex. Approximately 30%–50% 1 , 2 of patients will be diagnosed with infertility and approximately 50% will suffer from anxiety or depression. 3
Lesions form close to blood vessels, ensuring their survival, and become highly innervated contributing to the chronic pelvic pain experienced by many sufferers. Persistent inflammation in the peritoneal cavity resulting from repetitive deposition of menstrual tissue and active breakdown of established lesions, also contributes to pain and allows lesions to persist in the peritoneal cavity.
Endometriosis research, diagnosis, treatment and management is complicated by the heterogeneity of the disease. 4 The number or location of lesions does not correlate with symptoms 5 and for some patients, the disease is discovered when they undergo explorative laparoscopy for unexplained infertility. Lesions appear in different forms as they progress from new clear/white, to red, then black lesions 6 , 7 and finally a white scar reflecting a loss of endometrial glands and stroma and increased collagen deposition. 8 However, macroscopically similar lesions have different behaviors and can cause different symptoms. 4 Lesion morphology is also heterogenous. In a study of superficial lesions, inter‐ and intra‐patient variability of gland profiles and stroma was independent of menstrual cycle stage 9 with the authors suggesting that the different gland profiles may reflect the lesions' responses to steroid hormones. This is supported by another study where deep infiltrating lesions exhibited high variability of estrogen receptor alpha and progesterone within glands, and between lesions and patients. 10 This may explain why patients have variable responses to hormonal therapies, highlighting the need to personalize therapy for each patient.
Theories of endometriosis etiology
Retrograde menstruation
Sampson 11 was the first to hypothesize that retrograde menstruation may cause endometriosis (Figure 1). According to his theory, menstrual blood refluxes backwards through the Fallopian tubes into the pelvic cavity, whereby menstrual tissue fragments attach to peritoneal organs and develop lesions. Retrograde menstruation is found in over 90% of menstruating patients during gynecological surgery 12 and endometrial stem/progenitor cells have been isolated from peritoneal fluid 13 suggesting a potential mechanism for their survival and differentiation in endometriosis patients 14 (see below). Young people with oblique vaginal septum syndrome, cervical atresia, and other obstructive genital tract malformations are more likely to have endometriosis, 15 , 16 supporting a role for refluxed menstrual fluid reaching the peritoneal cavity. The asymmetrical anatomical distribution of superficial endometriosis lesions, with a greater proportion located on the right‐hand side of the peritoneal cavity due to clockwise peritoneal currents, also supports retrograde menstruation. 17 , 18 While 90% of menstruators may experience retrograde menstruation, the overall prevalence of endometriosis approximates 11%, 19 indicating additional factors are involved in endometriosis pathogenesis.
FIGURE 1.
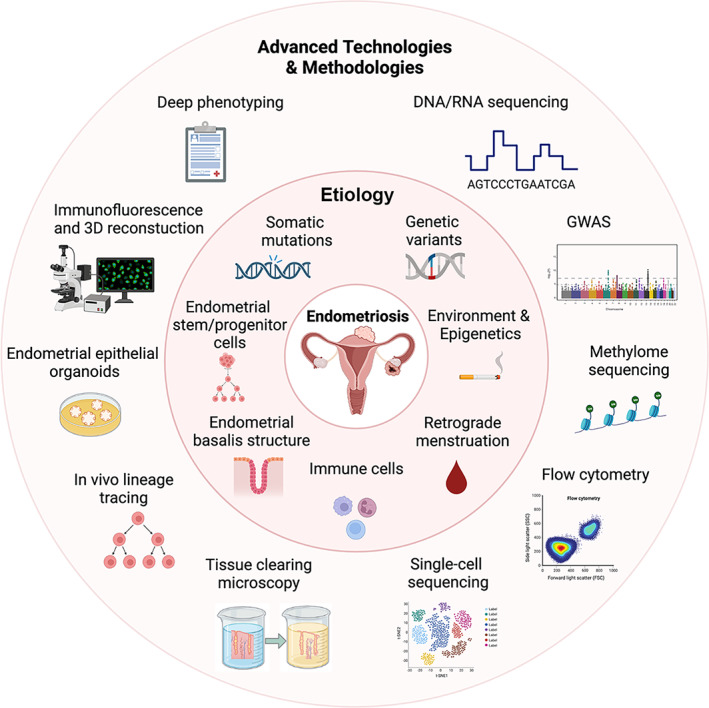
Schematic showing that advanced technologies and methodologies are enabling the generation of new concepts on the etiology of endometriosis.
Additional etiologies of endometriosis
Sampson's theory fails to explain the etiology of endometriosis in males, 20 and females with Mayer–Rokitansky–Küster–Hauser syndrome. 21 , 22 , 23 The Mullerian remnants hypothesis implies that endometrial‐like tissue in the peritoneal cavity are derived from primitive endometrial cells misplaced during embryonic development. 24 The coelomic metaplasia hypothesis suggests that the coelomic epithelium, which gives rise to Mullerian duct epithelium, the precursor cells of the endometrium, is the source of cells seeded during development. They lie dormant until exposure to endogenous hormones at menarche. 25 Multiple origins may contribute to endometriosis, especially given the disease heterogeneity. However, evidence from clinical and molecular studies, especially recent observations on patterns of somatic mutations in endometrium and lesions 26 , 27 , 28 provides strong support for retrograde menstruation as the most common source of cells for endometriosis lesions. 29 , 30
Endometriosis is multifactorial in etiology, with genetics and environment each contributing approximately 50%. 30 The cells of origin of endometriosis lesions are likely stem/progenitor cells as only these cells are clonogenic and have the ability to initiate new growths of endometrial tissue in ectopic sites. 31 Clonogenic endometrial cells are found in specific niches, mainly, but not exclusively, in the basalis endometrium.
NEW CONCEPTS ON THE ETIOLOGY OF ENDOMETRIOSIS
Structural features of the endometrial basalis
The endometrium undergoes over 400 menstrual cycles during a woman's reproductive lifespan. Basic histological techniques first described post‐menstrual endometrial epithelium migrating from the stumps of the basalis glands of menstruating endometrium. 32 , 33 These findings were confirmed by scanning electron microscopy 34 and hysteroscopy. 35 Markee highlighted vascular changes and the speed of luminal epithelial repair by transplanting the endometrium into the eye of rhesus monkeys. 36 More recently the structure of the basalis layer was revealed by lineage tracing of mitochondrial DNA mutations and 3D reconstruction of fixed tissue (Figure 1). This study identified complex interconnected horizontal basalis glands as the origin of non‐branching, single, vertical functionalis glands. 37 The authors also found evidence of a multipotent epithelial stem/progenitor cell that regenerated the basalis and functionalis glandular lineages. Others have identified somatic mutations in basalis epithelial cells that suggest endometrial glands arise from a single ancestral cell. 28 Tissue clearing microscopy, immunofluorescence and 3D reconstruction confirmed the horizontal rhizome‐like glandular network (Figure 1) 38 and showed the spatiotemporal dynamics of glands in human endometrium. 39 They also showed that multiple vertical glands originate from a single ancestral clone in a horizontal segment of a basalis gland, and that the vertical glands diversify by acquiring additional mutations. This horizontal rhizome‐like structure of endometrial basalis glands may safeguard them from enzymatic destruction during menstruation thereby protecting the epithelial progenitor cell niche.
Endometrial stem/progenitor cells
Human endometrial stem/progenitor cells were first identified as clonogenic cells, 40 which demonstrated adult stem cell properties of self‐renewal, differentiation and high proliferative capacity in in vitro functional assays. 41 Both epithelial progenitor cells and mesenchymal stem cells (eMSC) were identified. Surface markers were discovered for both stem/progenitor cell types that enriched for the respective clonogenic cells and also demonstrated the above classic stem cell properties (Figure 2). Specifically, co‐expression of PDGFRβ and CD146 isolated a small population of eMSC, demonstrating their pericyte identity by immunofluorescence. 44 A single perivascular marker, SUSD2 (formerly W5C5), also purifies clonogenic eMSC, 45 which reconstitute stromal tissue in vivo. Unbiased gene expression profiling of highly purified endometrial epithelial cells, comparing pre‐menopausal and post‐menopausal endometrium, found 11 differentially upregulated surface markers in post‐menopausal cells. 46 The most consistent was CDH2, which encodes for N‐cadherin (Figure 2). Magnetic bead‐sorted N‐cadherin+ endometrial epithelial cells were enriched in clonogenic cells compared to N‐cadherin− cells, and demonstrated adult stem cell properties in functional in vitro assays. N‐cadherin+ epithelial cells were located in the deepest gland profiles in the rhizome‐like glands of the basalis, directly adjacent to the myometrium. The surface marker, SSEA‐1 identifies basalis epithelium (Figure 2) and has the progenitor properties of longer telomeres, telomerase activity and differentiation in in vitro assays. 47 These markers highlighted a potential cellular hierarchy in human endometrium (Figure 2) with the most primitive N‐cadherin+ progenitors located in the bases of the horizontal glands and the SSEA‐1+ cells proximal to the N‐cadherin+ cells. 46 SSEA‐1+ cells are located at the ill‐defined basalis‐functionalis junction indicating they would migrate from the gland stumps during menstruation to rapidly resurface the denuded endometrial surface to become the new luminal epithelium. 42 Two transcription factors, nuclear AXIN2 48 and nuclear SOX9 47 also selectively mark basalis epithelium (Figure 2), although recent spatial transcriptomics also found SOX9 in the functionalis of proliferative stage endometrium. 49 These markers of human endometrial stem/progenitor cells have enabled their quantification in endometrial tissues and body fluids. 13
FIGURE 2.
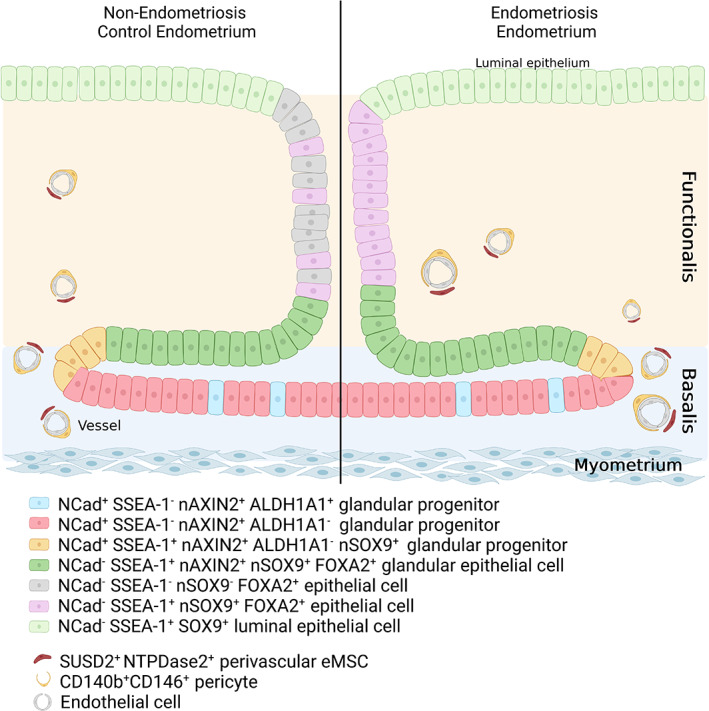
Schematic showing the location of endometrial stem/progenitor cells in the basalis, functionalis and luminal epithelium of human endometrium based on specific surface markers of these cells demonstrating adult stem cell activity. Note the horizontal gland structure in the basalis gland from which emanates the vertical gland in the functionalis. It is likely that the NCAD−SSEA‐1+nSOX9+basalis epithelial cells re‐epithelialize the raw endometrial surface during menstruation to become the luminal epithelial cells. In endometriosis, most functionalis glandular epithelial cells are SSEA‐1+nSOX9+ (purple, right hand side), in contrast to normal endometrium, where they are only occasionally found in the functionalis (purple, left hand side). CD140b, PDGFRβ; eMSC, endometrial mesenchymal stem cells; NCAD, N‐cadherin; NTPDase2, nucleoside triphosphate diphosphohydrolase‐2 Source: Adapted from Salamonsen et al. 42 and Cousins et al. 43
Sampson's theory of endometriosis has now been extended to include endometrial stem/progenitor cells (Figure 1) which have been found in menstrual fluid as N‐Cadherin+ cells and SUSD2+ eMSC. 13 Importantly, these cells were found in peritoneal fluid on day 2–3 of menstruation in people with endometriosis, but also in a small proportion of people without the disease. Endometrial stem/progenitor cells were occasionally found in peritoneal fluid of non‐menstruating people with endometriosis, but not in controls, suggesting persistence of stem/progenitor cells with potential to initiate lesions. Clonogenic cells were also found in menstrual and peritoneal fluids of menstruating people, but not in peripheral blood, indicating their source is endometrium rather than bone marrow. 13 SSEA‐1+ epithelial cells were found abnormally in the glands of functionalis endometrium of people with endometriosis, 50 suggesting they will be shed in large numbers in menstrual fluid where they may gain access to peritoneal cavity during menstruation. More recently, SSEA‐1+ cells were found in menstrual fluid. 51 This raises the question of whether luminal epithelial SSEA‐1+SOX9+ cells retain progenitor cell activity as they also reach the peritoneal cavity during menstruation. Together, these findings suggest that genetic and environmental endometriosis risk factors may differentially affect retrogradely shed endometrial epithelial progenitor cells, the likely cells of origin of endometriosis lesions, to promote the establishment of lesions in women with endometriosis. Clonogenic stomal cells have been identified in endometriosis lesions. 52 Another source of endometrial stem/progenitor cells in peritoneal fluid during menstruation could be any shed from established superficial endometriotic lesions. Thus, susceptibility to forming lesions in women with endometriosis may be the presence of endometriosis risk genes, somatic mutations and/or environmentally‐mediated epigenetic changes in the stem/progenitor cells that provide a competitive advantage for lesion initiation.
Upon reaching the pelvic cavity, clonogenic endometrial stem/progenitor cells need to adhere to the mesothelial lining and/or invade beneath the peritoneum to establish an endometriotic lesion. 53 Since N‐cadherin+ epithelial progenitors express nuclear ERα, 46 it is expected they would respond to rising estrogen levels during the proliferative stage of subsequent menstrual cycles to generate the ectopic glands in developing endometriotic lesions. Some SSEA‐1+ basalis epithelial cells show nuclear ERα in 3D cultures, 47 suggesting they may also directly respond to estrogen to proliferate and generate lesions. In contrast, the ERα− SUSD2+ eMSC 54 rely on ERα‐expressing niche cells (endothelial, perivascular or stromal) in eutopic and ectopic endometrium to respond to estrogen. eMSC signal in a paracrine manner to endothelial cells by releasing of angiogenic growth factors 55 and promoting angiogenesis required for lesion growth.
In stromal endometriosis, SUSD2+ eMSC in menstrual fragments containing niche cells may attach and initiate stromal endometriosis lesions when shed into the pelvic cavity. Here they may proliferate and differentiate into endometrial stromal cells to form endometriotic lesions, as we have shown previously in mouse transplantation studies. 45 This is supported by gene profiling studies demonstrating that CD146+PDGFRβ+ eMSC from women with endometriosis spontaneously differentiate into stromal cells in vitro and pass on a decidualization defect to their cellular progeny, not present in normal eMSC or stromal cells. 56 , 57 Single cell RNA sequencing (scRNAseq) of fresh eutopic endometrium, peritoneal lesions and adjacent peritoneal tissue showed many SUSD2 + cells with a perivascular, angiogenic and immunomodulatory gene expression profile indicating their key role in peritoneal lesion progression. 58 However, scRNAseq studies on concurrent fresh menstrual and peritoneal fluid samples are needed to confirm that eMSC are the cell of origin to establish endometriotic stromal lesions in vivo.
Retrograde endometrial waves to transport cells of origin of endometriosis into the pelvic cavity
The Müllerian Duct‐derived inner myometrium, comprising circular smooth muscle cells, undergoes abnormal caudal‐fundal‐directed contractions producing endometrial waves that transport endometrial tissue fragments into the pelvic cavity of menstruating women with endometriosis with greater frequency than control women. 59 , 60 , 61 This uterine dysperistalsis implicated in abnormal retrograde menstruation, has previously been assessed by transvaginal ultrasound videos or transport of radioactive particles to determine direction of uterine contractions, 62 , 63 however these approaches are subjective and laborious. Recently introduced electrohysterography used to monitor pregnancy, 64 provides a new more quantitative and less invasive technique that could be adapted for determining the role of abnormal endometrial waves associated with retrograde menstruation in women with endometriosis. 65
Endometrial‐mesenchymal‐transition and mesenchymal‐epithelial‐transition
Epithelial‐mesenchymal‐transition (EMT), the process where epithelial cells slowly lose their epithelial phenotype and gain a mesenchymal phenotype, has been implicated in progression but not establishment of endometriosis. Several studies comparing eutopic endometrium from control and endometriosis patients revealed only subtle differences in EMT specific‐pathway markers, suggesting that EMT may not be involved in disease initiation, 66 reviewed by Konrad et al.
Increased protein expression of EMT pathway markers TWIST, 67 SNAIL, 68 , 69 SLUG 70 and ZEB1 71 and mesenchymal marker N‐cadherin 72 were observed concurrently with decrease expression of the epithelial marker, E‐cadherin, in ectopic lesions compared to patient matched eutopic endometrium. 70 Interestingly, E cadherin expression was higher in deep infiltrating lesions compared to ovarian and peritoneal lesions, 72 in keeping with the hypothesis that mesenchymal‐epithelial‐transition (MET) occurs in deep infiltrating endometriosis. 72 MET has also been implicated in the progression of red lesions to black lesions via epithelial cell differentiation. 73 , 74 Cell–cell contact markers, Claudins 1 and 4 have decreased protein expression in lesions 75 but other Claudins are unchanged, suggesting that cell–cell contacts remain intact in ectopic lesions and that only partial EMT occurs. While location of lesions was assessed in many studies, the stage of disease was not, therefore it is difficult to ascertain whether partial EMT may be completed as disease progresses or whether the epithelial cells maintain a partial EMT state throughout the life of a lesion. Approximately 50% of endometriotic lesions contain glandular epithelium when examined histologically 76 but, due to limitations with longitudinal studies of lesions, it is unknown whether a menstrual fragment needs an epithelial compartment to survive and grow, or whether the glandular epithelial compartment has undergone EMT to support the growth of the lesion.
Endometriosis is an estrogen‐dependent disease. Since estrogen induces EMT in other diseases including ovarian cancer and breast cancer 77 , 78 , 79 it is likely to drive EMT in endometriosis. Estrogen increases ZEB1 promoter activity and mRNA expression in Ishikawa cells in vitro, 80 which had downstream effects on mRNA expression of E‐cadherin (decreased) and vimentin (increased). In another study using primary endometrial epithelial cells, treatment with β‐estradiol led to a decrease in E cadherin protein expression and an increase in migratory and invasive properties. 69 In the same study, treatment with an estrogen receptor antagonist ICI increased E cadherin expression and decreased vimentin and Snail mRNA expression, 69 reversing EMT.
While estrogen may drive EMT in endometriosis, progesterone resistance also plays a part in disease pathogenesis. A recent study has shown that EMT may contribute to the downregulation of progesterone receptors (PR) in lesions, making them less responsive to progestin therapy. 81 In this study, knockdown of SNAI1 and SNAI2 in endometriosis cell lines resulted in an increase in PR expression 81 indicating that PR resistance may be driven via EMT.
Immune cells
Immune cells, from both the innate and adaptive immune responses, play a role in endometriosis (Figure 1), both those shed in eutopic endometrium and those in the peritoneal environment are thought to contribute to disease pathogenesis.
The innate immune response in endometriosis
scRNAseq of eutopic endometrium revealed 13 transcriptomically distinct immune cell subtypes, 82 5 from a macrophage cluster and 8 from a lymphocyte cluster. Monocyte and macrophage enrichment scores were elevated in mid secretory endometrium in endometriosis patients compared to controls. 82 This supports an earlier study where immunohistochemistry of eutopic endometrium showed that CD68+ macrophages were increased in the endometrium of endometriosis compared to control patients. 83 This coincides with an increase in eutopic endometrial monocyte chemoattractant protein‐1 (MCP‐1) expression in endometriosis patients. 84 The importance of eutopic endometrial‐derived macrophages to lesion establishment and progression was demonstrated in small animal models. 85 , 86 Depletion of eutopic endometrial macrophages by doxycycline in a donor‐recipient mouse model resulted in fewer lesion‐derived macrophages and smaller lesions. 86
Mass cytometry of peritoneal fluid from endometriosis patients reveals 40 distinct immune cell types 87 which were stratified by disease stage. Macrophages exhibited an increase in both pro‐inflammatory (CD64/CD40) and anti‐inflammatory (CD163/CD206) markers compared to patient matched peripheral blood, indicating alternative activation of macrophages. This supported an earlier study where scRNAseq of peritoneal fluid from endometriosis patients identified seven subsets of macrophages, 88 in which both pro‐inflammatory and pro‐repair genes were expressed, highlighting the heterogeneity of macrophages and their potential dual roles in the pathogenesis of endometriosis.
Adaptive immune response in endometriosis
Mass cytometry also indicated that T cell activation was increased in the peritoneal fluid of patients with endometriosis patients compared with controls. 87 CD25highFoxp3+ regulatory T cells (Tregs) were increased in peritoneal fluid in endometriosis, 89 and positively correlated with increases in peritoneal cytokines, IL‐10 and TGFB1. Both cytokines regulate Fibrinogen‐like protein 2 (FGL2) expression, and peritoneal fluid Tregs of endometriosis patients also have increased FGL2 expression. 90 FGL2 drives macrophage polarization toward the pro‐repair phenotype highlighting a potential positive feedback loop between T cells and macrophages that drives endometriosis progression. NK cell cytoxicity is decreased in the peritoneal fluid of endometriosis patients 91 irrespective of disease stage, indicating a mechanism by which menstrual fragments may survive in the peritoneal cavity.
Altered genomic programs
Genetic risk factors
Twin studies have estimated the heritability of endometriosis at 0.47–0.51 92 , 93 indicating that genetic factors contribute to 50% of the variation in disease risk. Genome‐wide association studies (GWASs) investigating the association between common germline genetic variants and endometriosis have provided strong evidence for the contribution of many genetic variants across the genome 30 , 94 , 95 , 96 , 97 (Figure 1). The most recent published endometriosis GWA meta‐analysis 97 identified 19 independent signals in 14 genomic loci associated with the disease. 97 Candidate genes in the risk regions have been linked to hormonal regulation (ESR1, FSHB, GREB1) and cell adhesion and proliferation (CDC42, CDKN2BAS, VEZT, FGD6). The estimated proportion of variance in endometriosis risk captured by common single nucleotide polymorphisms (SNPs) across the genome (SNP‐based heritability) was 26%. 96 , 98 When restricted to more severe forms of the disease (rAFS Stage III/IV) the variance captured increases to 34%. 96 Similarly, studies report larger genetic effects and a larger genetic burden for individual risk factors in more severe disease, 99 with lead GWAS SNPs estimated to capture 5.19% 97 of the variance in stage III/IV disease compared to 1.75% in overall disease risk. 97 Identification of candidate causal genes in endometriosis risk loci relies on subsequent functional annotation of variants in these regions.
Somatic mutations
Evidence is emerging that endometriotic lesions display a mutational burden higher than expected in normal tissue through the acquisition of mutations as cells age. 28 Untargeted sequencing approaches have identified mutations across the genome, some being cancer driver genes (Table 1), 26 , 28 , 108 suggesting they may provide an advantage in cell attachment, growth or survival thereby contributing to endometriosis pathogenesis (Figure 1). A profile of mutated cancer driver genes in endometriotic lesions is beginning to emerge (Table 1).
TABLE 1.
Somatic mutations in cancer driver genes reported in endometriotic lesions and human endometrium.
| Gene | SUP | OMA | DIE | Endometrium | References |
|---|---|---|---|---|---|
| AKT1 | Yes | 169 | |||
| ARHGAP35 | Yes | Yes | 28, 110 | ||
| ARID1A | Yes | Yes | Yes | No | 26, 108, 169, 170, 171 |
| ARID5B | Yes | 110 | |||
| ATM | Yes | 110 | |||
| ATRX | Yes | Yes | 111 | ||
| BRAF | No | Yes | 110, 171 | ||
| CARD10 | Yes | 172 | |||
| CARD11 | Yes | 172 | |||
| CDH4 | Yes | 110 | |||
| CDKN1B | Yes | 110 | |||
| CREBBP | Yes | 110 | |||
| CSMD3 | Yes | 28 | |||
| CTCF | Yes | 173 | |||
| CTNNB1 | Yes | 108 | |||
| DNAH7 | Yes | 111 | |||
| EGFR | Yes | 110 | |||
| ERBB2 | Yes | Yes | Yes | 108, 110 | |
| ERBB3 | Yes | 110 | |||
| ERK1 | No | 171 | |||
| ERK2 | No | 171 | |||
| ERRB2 | Yes | 169 | |||
| FAT1 | Yes | 110 | |||
| FBN2 | Yes | 28 | |||
| FBXW7 | Yes | Yes | 28, 110 | ||
| FGFR2 | Yes | 110, 169 | |||
| FOXA2 | Yes | 110 | |||
| FRG1 | Yes | 28 | |||
| HEATR1 | Yes | No | 28 | ||
| HRAS | No | Yes | 110, 171 | ||
| KIAA1109 | Yes | 28 | |||
| KMT2C | Yes | 110 | |||
| KMT2D | Yes | 110 | |||
| KRAS | Yes | Yes | Yes | Yes | 26, 28, 108, 110, 111, 169, 171, 174, 175 |
| MUC6 | Yes | 28 | |||
| NF1 | Yes | 110 | |||
| NOTCH2 | Yes | 110 | |||
| NRAS | No | Yes | 169, 171 | ||
| PIK3CA | Yes | Yes | Yes | Yes | 26, 28, 108, 110, 169, 171 |
| PIK3R1 | Yes | Yes | 28, 110 | ||
| PLCG1 | Yes | 110 | |||
| PLXNB2 | Yes | Yes | 28 | ||
| PPP2R1A | Yes | Yes | Yes | 26, 28, 110, 171 | |
| PRDM1 | Yes | 110 | |||
| PTEN | No | Yes | Yes | 108, 110, 169, 171 | |
| PTPN13 | Yes | 28 | |||
| RRAS | Yes | 110 | |||
| RYR1 | Yes | Yes | 111 | ||
| SMAD2 | Yes | 110 | |||
| SPOP | Yes | 110 | |||
| STAG2 | Yes | 110 | |||
| TASR31 | Yes | 28 | |||
| TP53 | Yes | 110 | |||
| TRERF1 | Yes | 176 | |||
| TTN | Yes | 28 | |||
| ZFHX3 | Yes | 110 |
Note: Yes indicates mutation has been detected in at least one patient in the referenced study. No indicates no mutation has been identified and specifically reported in the referenced study. Blanks represent not specifically mentioned.
Abbreviations: DIE, deeply infiltrating endometriosis; OMA, endometrioma; SUP, superficial peritoneal endometriosis.
The suite of mutations appears relatively consistent across lesion subtypes, despite slight variations. The genes most commonly mutated in endometrioma (OMA) include KRAS, PIK3CA and TTN 28 whereas KRAS and PTEN are more common in deep infiltrating endometriosis (DIE) 26 , 108 (Table 1). Current evidence suggests a similar profile will exist in superficial (SUP) lesions. 108 Significantly, separation of epithelial and stromal compartments revealed that mutations are restricted to the epithelial glands, 109 suggesting that the influence of acquired genetic mutations on endometriosis pathogenesis is mediated by the epithelial cells directly or through their interaction with surrounding cells and microenvironment.
Mutational profiles of epithelial glands display heterogeneity. Examination of individual epithelial glands both within endometriotic lesions, and individual glands from different lesions in the same patient identified variations in their mutational profile. 28 In endometriotic glands of one subject, similar PIK3CA mutations were observed across six glands, whereas different and distinct mutations were found in the ovaries of another patient, 28 indicating different lesions harbor distinct mutation profiles and may be populated by a unique set of cells. Clonality analysis suggested a selective advantage existed in some glands. 28 The contribution of mutations to endometriosis pathogenesis may be regulated by the mutation and from when and where it was acquired.
Recently epithelial cells of the normal endometrium were shown to harbor an elevated mutational burden, ranging from 209 to 2833 base substitutions per woman, 110 many in cancer driver genes (Table 1). In an individual, the mutational profile of the endometrium is similar although not identical to endometriotic lesions, suggesting glands are the source of the initial mutations and that lesions acquire additional mutations, particularly in cancer driver genes when matched DIE lesions were compared to eutopic endometrium. 111
Only a few of many endometrial glands can be examined experimentally, and they also show significant heterogeneity in mutational profiles within the same patient. 110 Three‐dimensional profiling and tissue clearing techniques (Figure 1) found that vertical functionalis glands with matching mutational profiles occupy endometrial regions up to 4.7 mm2, which originated from a common section of a horizontal rhizome‐like gland in the basalis. 39 Thus, endometrial glands in distal regions are populated by progeny of common epithelial progenitor cells. Endometrial stem/progenitor cells in the rhizome‐like structures of basalis endometrium that acquire somatic mutations in cancer driver genes may confer a selective advantage for their survival, attachment and ability to establish lesions if they reach the peritoneal cavity via retrograde menstruation, thereby contributing to endometriosis pathogenesis. A number of questions, remain: how and when these mutations are acquired, how are they influenced by the surrounding environment, do they achieve a selective advantage and achieve clonality and how do they avoid a progression to malignancy?
Environmental contribution to endometriosis and potential links to mutation
Somatic single‐nucleotide variants, cytogenetic aneuploidy and structural chromosomal variants are strongly correlated with age, 110 , 112 suggesting both time and environment contributions to accumulation of DNA variations (Figure 1). The rate of mutation acquisition differs among cells, tissue and individuals and is primarily associated with relative exposures to environmental factors, inherited deficits in DNA‐repair systems and acquired genetic and epigenetic abnormalities. 113 Environmental damage can be organ and cell‐specific and induced by lifestyle factors including ultraviolet light, ionizing radiation, tobacco smoke, chemotherapeutic drugs, and exposure to environmental toxins. 113
Environmental and lifestyle factors have been investigated in endometriosis etiology. Smoking, 114 alcohol consumption 115 and dietary choices do not show robust associations with endometriosis, although some evidence suggests excess red meat intake 116 , 117 and a protective effect of phytoestrogen intake. 118 , 119 Nor does there appear robust evidence for an association between environmental pollutants and endometriosis. Dioxins and polychlorinated biphenyls showed no association, 120 , 121 , 122 , 123 with limited and contradictory evidence for bisphenol A, 124 , 125 phthalates 125 , 126 and parabens. 127 , 128
Despite current estimates suggesting a 49% environmental contribution to endometriosis risk, there is currently limited data supporting specific environmental exposures or lifestyle factors increasing the risk of endometriosis, and less suggesting they influence the induction of mutations in endometrial cells. Most studies are limited by sample size and variations in environmental conditions experienced by the different populations and are yet to directly assess whether environmental exposures increase the mutational burden in relevant cell types. The emerging evidence for elevated cancer driver mutations present in benign endometriosis, and their clear association with age and experience suggest this could represent a rich source of enquiry for endometriosis pathogenesis. The increasing availability of deeply phenotyped patient cohorts (Figure 1) and complex 3D in vitro models (Figure 1) now provides opportunities to explore environmental and lifestyle contributions to endometriosis through a direct influence on the DNA of endometrial cells.
Genetic regulation of gene expression
Studies investigating changes in gene expression associated with endometriosis have identified large differences in gene expression between eutopic endometrium and ectopic endometriosis lesions. 129 , 130 , 131 Deregulated genes in endometriotic lesions were enriched in PI3K‐AKT, WNT and MAPK signaling, oxidative stress and focal adhesion pathways and included several genes from the IGF/IGFBP and MMP families. Whether or not the dysregulation of these pathways occurs as a cause or consequence of disease remains to be determined. Differences in the expression of candidate endometriosis susceptibility genes have also been identified between eutopic endometrium from patients with and without endometriosis 132 , 133 however, these genes do not replicate in larger genome‐wide studies. 134 , 135 , 136
Statistical approaches have identified putative causal relationships between genetic variants, expression of genes and risk of endometriosis, through the integration of summary statistics from GWAS and expression quantitative trait loci (eQTLs) 137 (Figure 1). Such approaches identified that variants regulating the expression of genes involved in cell adhesion and proliferation, LINC00339, VEZT, FGD6, and CDC42, in endometrium and blood, also increase risk of endometriosis. 134 , 135 , 138 Functional annotation of variants on chr12q22, regulating expression of VEZT and FGD6, revealed that the causal variant likely resides in a bidirectional promoter for these two genes and has also been associated with risk of epithelial ovarian cancer. 139 Functional studies investigating the interaction between genetic variants on chr1p36.12 and nearby genes suggested that endometriosis risk variants interact with the promoters of LINC00339, CDC42 and WNT4 and the risk allele is associated with increased expression of CDC42 in blood cells. 138 , 140 Expression of genes with critical roles in hormonal regulation have also been implicated in endometriosis. Variants on chromosome 6 near ESR1 have been associated with endometriosis and various other reproductive traits and diseases. While no evidence of risk variants regulating expression of genes in this region has been reported, genes in the region are highly correlated with the expression of ESR1 and PGR suggesting genetic variants in the region could impact co‐regulation of these hormone receptor genes. 141 Risk variants located in another estrogen responsive gene, GREB1, have been associated with transcriptional splicing of GREB1 in ovarian tissue. 142 Large‐scale eQTL and sQTL studies 142 , 143 provide strong evidence for tissue and cell‐type specific genetic effects on gene expression and splicing, highlighting the need to investigate genetic effects in disease relevant tissues and cell‐types to better understand how genetic risk factors regulate genes and increase endometriosis risk.
Epigenetic modifications
Epigenetic modification refers to changes in gene activity that do not arise from changes in DNA sequence, but rather are due to behaviors and environmental exposures (Figure 1). DNA methylation (DNAm) is one of the most common modifications measured in disease studies. Methylation studies in human endometrium and endometriosis have identified differences at DNAm sites across the genome between endometriotic and normal endometrial tissue 136 and stromal cells. 144 Differentially methylated sites were mapped to genes and pathways implicated in the pathology of endometriosis and decidualization including HOX gene clusters, nuclear receptor genes, the GATA family of transcription factors, 144 WNT signaling, angiogenesis, cadherin signaling, and gonadotropin‐releasing‐hormone‐receptor pathways. 136 Hypomethylation and overexpression of GATA6 in ectopic endometrial stromal cells restricts the ability of cells to decidualise and has been linked to the transformation of endometrial stromal cells into endometriotic‐like cells that produce estrogen. 145 Changes in methylation profiles across the menstrual cycle in eutopic endometrium from patients with endometriosis have also been reported however, these changes fail to replicate between studies likely due to small sample sizes and limited power to detect subtle differences. 136 , 146 , 147 Epigenetic signals capture variation in disease however, it can be challenging to distinguish between cause and consequence of disease. Epigenetic profiles vary widely between tissues and cell‐types, 148 as such cell‐type specific epigenetic effects associated with endometriosis may be relevant to disease etiology and pathogenesis.
Evidence for putative causal effects of methylation on endometriosis can be discerned from genetics. Genetic effects on methylation in human endometrium have been identified in the form of methylation quantitative trait loci (mQTLs). Variants regulating methylation in endometrium have been associated with reproductive traits and diseases including a variant regulating methylation in GREB1 and endometriosis. 147
Bacterial contamination hypothesis
The association between endometriosis and chronic inflammation may be explained by the “bacterial contamination hypothesis” where bacterial endotoxin, that is, lipopolysaccharide or LPS induce the pro‐inflammatory environment via the LPS/TLR4 cascade in the pelvis. 149 Cultured menstrual blood is more highly contaminated with Escherichia coli from women with endometriosis than those without. Furthermore, higher levels of endotoxin are found in menstrual and peritoneal fluid in women with endometriosis. 150 The increased endotoxin in the pelvic area of women with endometriosis may result from migration of E. coli from the vagina to the uterine cavity via the menstrual blood, or from the E. coli and endotoxin arising from the gut and translocating via enterocytes into the pelvic cavity. 150 The growth of endometriotic lesions is stimulated by TLR4‐mediated inflammation induced by E. coli. 150 Higher levels of prostaglandin E2 in menstrual fluid of women with endometriosis also enhances the growth of E. coli in vitro 151 and increased microbial colonization and endometritis is seen in the uterus of women with endometriosis. 152 Analysis of bacterial ribosomal RNA genes revealed an increased bacterial subclinical infection risk in women with endometriosis compared to those without. 153 Potential treatment strategies for controlling bacterial colonization include antibiotic treatment where a single dose of the antibiotic Levofloxacin decreases some bacterial genera in the endometrium of women with and without endometriosis. 100 Whether or not these bacterial genera are contributing to the cause or more likely the progression of endometriosis is yet to be determined. While there is evidence of a dysregulated gut or reproductive tract microbiome in women with endometriosis, there is little consensus regarding the particular microbiota that may lead to endometriotic lesion growth. 101 Additionally, some bacteria promote endometriosis and others potentially protect against it highlighting the need for further investigations into this area. 102
INFLUENCE OF NEW TECHNOLOGIES ON UNDERSTANDING ENDOMETRIOSIS PATHOGENESIS
Organoids and multicellular organoids
The development of 3D organoid cell culture models using patient‐derived cells allows the investigation of in vivo mechanisms in a pre‐clinical setting (Figure 1). Endometrial epithelial organoids (EEO) have been established from human and mouse endometrial epithelia and comprise ciliated and unciliated, proliferating, secretory and stem/progenitor epithelial cells. 103 , 104 , 105 , 106 EEO reflect the cycling human endometrium by responding to hormones of the menstrual cycle including estrogen and progesterone, as evident in their gene and protein expression, and cellular changes. 103 , 104 , 105 , 106 EEO retain a “memory” of the donor as shown in organoids derived from endometriosis and endometrial cancer patients. 107 Indeed, EEO from ectopic and eutopic endometriosis samples express epithelial cell markers and steroid hormone receptors, preserving endometrial glandular structure and cell characteristics. Endometriosis‐derived organoids enable comprehensive investigations into the development and pathogenesis of endometriosis. Findings from stage IV endometriosis‐derived organoids suggest involvement of cancer driver genes in the development of endometriosis. 107 Lesion formation can be modeled by implanting ectopic endometriosis‐derived organoids in the peritoneal cavity of mice. 107 The role of epigenetics in the development of endometriosis has been investigated using organoids derived from ectopic and eutopic endometrium from endometriosis patients. 154 Recently, menstrual fluid organoids have been established which reflect the same properties as those derived from endometrial tissue. 155 , 156 The efficiency and non‐invasive method for obtaining endometrial cells from menstrual fluid presents a promising avenue for personalized medicine and tailoring drug treatments for individual endometriosis patients.
While endometrial epithelial organoids have many benefits in recreating a 3D in vivo environment, endometriosis is a multicellular disease involving interactions between epithelial cells, stromal fibroblasts, extracellular matrix, immune cells, vasculature and nerve cells. Endometrial stromal cells from women with endometriosis exhibit disordered decidualization, a process essential for establishing a receptive endometrial environment for embryo implantation. 157 , 158 , 159 As such, incorporating these cells into a 3D model of endometrial epithelial organoids would provide greater insight into the effect of endometriosis on both the eutopic and ectopic endometrium. Recent advances have been made in establishing an extracellular matrix and suitable media conducive to both stromal and epithelial growth in co‐culture. 160 , 161 , 162 Multicellular 3D culture systems are needed to appropriately model in vivo endometriosis conditions in vitro.
Single cell RNA sequencing
The initial application of single‐cell RNA‐seq to endometriosis focused on generating cell atlases of spatiotemporal time points of the endometrium (Figure 1). One study assessing individual samples from successive days of the menstrual cycle identified four major transformations of the endometrium during the menstrual cycle and the interplay of cell types mediating these transitions. 163 Subsequently, analysis has focused on the maternal‐fetal interface and the cell types present. This resulted in the identification of two perivascular cells, distinguished by different MCAM expression concentrations 164 and three stromal cell subsets, two of which expressed markers similar to decidualized stromal cells identified earlier, and three NK cells subsets suggesting an immune modulatory component at this interface. A similar analysis reported two distinct endometrial fibroblasts, smooth muscle and endothelial cells, epithelial and two distinct NK cells with 48.7% of the decidual sample containing cells with high ECM expression and decidualized fibroblasts displaying two distinct differentiation trajectories. 165 , 166
The identification of these cells and the measurement of their individual gene expression profiles in multicellular organs provides the opportunity to chart which cells are present and determine whether their relative proportions change in individual patients or correlate with clinical presentations. Additionally, the characterization of their distinct transcriptomic profiles provides the opportunity to identify whether subtle variations within cell types occur that are indicative of pathogenic processes. Together the data generated by in‐depth single‐cell sequencing provides the powerful and unique opportunity to chart all disease‐relevant cells within the analyzed tissue and their potential individual contributions to endometriosis etiology.
While this powerful technique is generating an atlas of the endometrium in its varying spatio‐temporal presentations, it is yet to be comprehensively utilized in the discovery of variations related to endometriosis. Leveraging single‐cell RNA‐seq data from the endometrium of patients without endometriosis, bulk RNA‐seq data from patients with and without endometriosis, and cellular deconvolution showed an increased enrichment of epithelial and endothelial cells, plasmacytoid dendritic cells and monocytes in the endometrium of patients with endometriosis during the mid‐secretory stage. 82 However, this study was limited by sample size and the accuracy of cell deconvolution methods from bulk RNA‐seq data. Recently, a study using 19 individuals identified a mesenchymal cell signature derived through altered differentiation that was more likely present in women with endometriosis. 167 These cells showed changes in growth profiles, were characterized by high expression of matrix metalloproteinases (MMP) MMP3 and MMP10, and may have a role in endometriosis etiology. A similar fibroblast signature characterized by MMP3 and MMP10 was observed in menstrual fluid of patients with endometriosis, 168 suggesting these altered fibroblasts may be maintained during menstruation and may represent a possible non‐invasive diagnosis signature.
IMPLICATIONS FOR DIAGNOSIS AND NEW THERAPIES
The bringing together of recent major advances in technologies available to investigate endometriosis; next generation sequencing, new analytical methods, endometrial stem/progenitor cell identities and organoids has major implications for finding a non‐invasive diagnostic test desperately needed for endometriosis. Menstrual fluid provides non‐invasive sampling of endometrial tissue for somatic mutations, endometriosis risk genes, endometrial stem/progenitor cells and endometrial proteins as potential biomarkers for diagnosis. Menstrual fluid‐derived and endometrial tissue organoids provide a platform for modeling molecular mechanisms involved in endometriosis. As the pathogenesis of endometriosis is revealed in future studies, these organoids can be used for drug screening and provision of personalized medicine to patients with endometriosis. New concepts on the etiology of endometriosis are emerging, including the “cells of origin” and their transport into the pelvic cavity, genetic risk factors, epigenetic modifications and somatic mutations, environmental factors such as bacterial contamination and EMT. Combining this knowledge with new high powered molecular and cellular technologies will likely provide new avenues for diagnosis and treatment of endometriosis based on the etiology of endometriosis as it becomes revealed.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this article.
ACKNOWLEDGMENTS
This work was supported by the United States Department of Defense, through Congressionally Directed Medical Research Program under Award No. EO1 W81XWH1910364 (to Caroline E. Gargett and Grant W. Montgomery supporting Harriet C. Fitzgerald). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Caroline E. Gargett and Grant W. Montgomery are supported by a National Health and Medical Research Council of Australia Investigator Grants (1173882 and 1177194, respectively). This work is also supported by the Victorian Government's Operational Infrastructure Support Program. The authors wish to thank Dr. Thomas Tapmeier for the Tissue Clearing Microscopy icon in Figure 1. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Cousins FL, McKinnon BD, Mortlock S, Fitzgerald HC, Zhang C, Montgomery GW, et al. New concepts on the etiology of endometriosis. J Obstet Gynaecol Res. 2023;49(4):1090–1105. 10.1111/jog.15549
All authors contributed equally to this study.
Funding information National Health and Medical Research Council, Grant/Award Numbers: 1173882, 1177194; U.S. Department of Defense, Grant/Award Number: W81XWH1910364/PR180827
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Counseller VS, Crenshaw JL Jr. A clinical and surgical review of endometriosis. Am J Obstet Gynecol. 1951;62:930–42. [DOI] [PubMed] [Google Scholar]
- 2. Winkel CA. Evaluation and management of women with endometriosis. Obstet Gynecol. 2003;102:397–408. [DOI] [PubMed] [Google Scholar]
- 3. Missmer SA, Tu F, Soliman AM, et al. Impact of endometriosis on women's life decisions and goal attainment: a cross‐sectional survey of members of an online patient community. BMJ Open. 2022;12:e052765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Heterogeneity of endometriosis lesions requires individualisation of diagnosis and treatment and a different approach to research and evidence based medicine. Facts Views Vis Obgyn. 2019;11:57–61. [PMC free article] [PubMed] [Google Scholar]
- 5. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22:266–71. [DOI] [PubMed] [Google Scholar]
- 6. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68:585–96. [DOI] [PubMed] [Google Scholar]
- 7. Redwine DB. Age‐related evolution in color appearance of endometriosis. Fertil Steril. 1987;48:1062–3. [PubMed] [Google Scholar]
- 8. Köhler G, Lorenz G. Zur Korrelation von endoskopischem und histologischem Bild der Endometriose. Endometriose. 1991;4:56–60. [Google Scholar]
- 9. Colgrave EM, Bittinger S, Healey M, et al. Superficial peritoneal endometriotic lesions are histologically diverse and rarely demonstrate menstrual cycle synchronicity with matched eutopic endometrium. Hum Reprod. 2020;35:2701–14. [DOI] [PubMed] [Google Scholar]
- 10. Brichant G, Nervo P, Albert A, Munaut C, Foidart J‐M, Nisolle M. Heterogeneity of estrogen receptor α and progesterone receptor distribution in lesions of deep infiltrating endometriosis of untreated women or during exposure to various hormonal treatments. Gynecol Endocrinol. 2018;34:651–5. [DOI] [PubMed] [Google Scholar]
- 11. Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:442–69. [Google Scholar]
- 12. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–4. [PubMed] [Google Scholar]
- 13. Masuda H, Schwab KE, Filby CE, et al. Endometrial stem/progenitor cells in menstrual blood and peritoneal fluid of women with and without endometriosis. Reprod Biomed Online. 2021;43:3–13. [DOI] [PubMed] [Google Scholar]
- 14. Ulukus M. Stem cells in endometrium and endometriosis. Womens Health. 2015;11:587–95. [DOI] [PubMed] [Google Scholar]
- 15. Miyazaki Y, Orisaka M, Nishino C, Onuma T, Kurokawa T, Yoshida Y. Herlyn–Werner–Wunderlich syndrome with cervical atresia complicated by ovarian endometrioma: a case report. J Obstet Gynaecol Res. 2020;46:347–51. [DOI] [PubMed] [Google Scholar]
- 16. Tong J, Zhu L, Chen N, Lang J. Endometriosis in association with Herlyn–Werner–Wunderlich syndrome. Fertil Steril. 2014;102:790–4. [DOI] [PubMed] [Google Scholar]
- 17. Vercellini P, Abbiati A, Vigano P, et al. Asymmetry in distribution of diaphragmatic endometriotic lesions: evidence in favour of the menstrual reflux theory. Hum Reprod. 2007;22:2359–67. [DOI] [PubMed] [Google Scholar]
- 18. Vercellini P, Aimi G, De Giorgi O, Maddalena S, Carinelli S, Crosignani PG. Is cystic ovarian endometriosis an asymmetric disease? Br J Obstet Gynaecol. 1998;105:1018–21. [DOI] [PubMed] [Google Scholar]
- 19. Rowlands IJ, Abbott JA, Montgomery GW, Hockey R, Rogers P, Mishra GD. Prevalence and incidence of endometriosis in Australian women: a data linkage cohort study. BJOG. 2021;128:657–65. [DOI] [PubMed] [Google Scholar]
- 20. Rei C, Williams T, Feloney M. Endometriosis in a man as a rare source of abdominal pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2018;2018:2083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konrad L, Dietze R, Kudipudi PK, Horne F, Meinhold‐Heerlein I. Endometriosis in MRKH cases as a proof for the coelomic metaplasia hypothesis? Reproduction. 2019;158:R41–7. [DOI] [PubMed] [Google Scholar]
- 22. Troncon JK, Zani AC, Vieira AD, Poli‐Neto OB, Nogueira AA, Rosa ESJC. Endometriosis in a patient with Mayer–Rokitansky–Kuster–Hauser syndrome. Case Rep Obstet Gynecol. 2014;2014:376231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan L, Zhao X, Qin X. MRKH syndrome with endometriosis: case report and literature review. Eur J Obstet Gynecol Reprod Biol. 2011;159:231–2. [DOI] [PubMed] [Google Scholar]
- 24. Pitot MA, Bookwalter CA, Dudiak KM. Mullerian duct anomalies coincident with endometriosis: a review. Abdom Radiol (NY). 2020;45:1723–40. [DOI] [PubMed] [Google Scholar]
- 25. Gruenwald P. Origin of endometriosis from the mesenchyme of the celomic walls. Am J Obstet Gynecol. 1942;44:470–4. [Google Scholar]
- 26. Anglesio MS, Papadopoulos N, Ayhan A, et al. Cancer‐associated mutations in endometriosis without cancer. N Engl J Med. 2017;376:1835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Praetorius TH, Leonova A, Lac V, et al. Molecular analysis suggests oligoclonality and metastasis of endometriosis lesions across anatomically defined subtypes. Fertil Steril. 2022;118:524–34. [DOI] [PubMed] [Google Scholar]
- 28. Suda K, Nakaoka H, Yoshihara K, et al. Clonal expansion and diversification of cancer‐associated mutations in endometriosis and Normal endometrium. Cell Rep. 2018;24:1777–89. [DOI] [PubMed] [Google Scholar]
- 29. Bulun SE. Endometriosis caused by retrograde menstruation: now demonstrated by DNA evidence. Fertil Steril. 2022;118:535–6. [DOI] [PubMed] [Google Scholar]
- 30. Montgomery GW, Mortlock S, Giudice LC. Should genetics now be considered the pre‐eminent etiologic factor in endometriosis? J Minim Invasive Gynecol. 2020;27:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87–101. [DOI] [PubMed] [Google Scholar]
- 32. Ludwig H, Metzger H, Frauli M. Endometrial tissue remodelling and regeneration. In: D'Arcangues C, Fraser IS, Newton JR, Odlind V, editors. Contraception and mechanisms of endometrial bleeding. Cambridge: Cambridge University Press; 1990. p. 441–66. [Google Scholar]
- 33. Novak E, Linde RWT. The endometrium of the menstruating uterus. JAMA. 1924;83:900–6. [Google Scholar]
- 34. Ferenczy A. Studies on the cytodynamics of human endometrial regeneration. II. Transmission electron microscopy and histochemistry. Am J Obstet Gynecol. 1976;124:582–95. [DOI] [PubMed] [Google Scholar]
- 35. Garry R, Hart R, Karthigasu KA, Burke C. A re‐appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum Reprod. 2009;24:1393–401. [DOI] [PubMed] [Google Scholar]
- 36. Christiaens GCJE. Markee: menstruation in intraocular endometrial transplants in the rhesus monkey. Eur J Obstet Gynecol Reprod Biol. 1982;14:63–5. [DOI] [PubMed] [Google Scholar]
- 37. Tempest N, Jansen M, Baker AM, et al. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J Pathol. 2020;251:440–51. [DOI] [PubMed] [Google Scholar]
- 38. Yamaguchi M, Yoshihara K, Suda K, et al. Three‐dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamaguchi M, Nakaoka H, Suda K, et al. Spatiotemporal dynamics of clonal selection and diversification in normal endometrial epithelium. Nat Commun. 2022;13:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–50. [DOI] [PubMed] [Google Scholar]
- 41. Gargett CE, Schwab KE, Zillwood RM, Nguyen HPT, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salamonsen LA, Hutchison JC, Gargett CE. Cyclical endometrial repair and regeneration. Development. 2021;148(17):dev199577. [DOI] [PubMed] [Google Scholar]
- 43. Cousins FL, Filby CE, Gargett CE. Endometrial stem/progenitor cells—their role in endometrial repair and regeneration. Front Reprod Health. 2022;3:811537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwab KE, Gargett CE. Co‐expression of two perivascular cell markers isolates mesenchymal stem‐like cells from human endometrium. Hum Reprod. 2007;22:2903–11. [DOI] [PubMed] [Google Scholar]
- 45. Masuda H, Anwar SS, Buhring HJ, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem‐like cells. Cell Transplant. 2012;21:2201–14. [DOI] [PubMed] [Google Scholar]
- 46. Nguyen HPT, Xiao L, Deane JA, et al. N‐cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod. 2017;32:2254–68. [DOI] [PubMed] [Google Scholar]
- 47. Valentijn AJ, Palial K, Al‐Lamee H, et al. SSEA‐1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod. 2013;28:2695–708. [DOI] [PubMed] [Google Scholar]
- 48. Nguyen HP, Sprung CN, Gargett CE. Differential expression of wnt signaling molecules between pre‐ and postmenopausal endometrial epithelial cells suggests a population of putative epithelial stem/progenitor cells reside in the basalis layer. Endocrinol. 2012;153:2870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garcia‐Alonso L, Handfield LF, Roberts K, et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet. 2021;53:1698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hapangama DK, Drury J, Da Silva L, et al. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis‐like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum Reprod. 2019;34:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wyatt KA, Filby CE, Davies‐Tuck ML, Suke SG, Evans J, Gargett CE. Menstrual fluid endometrial stem/progenitor cell and supernatant protein content: cyclical variation and indicative range. Hum Reprod. 2021;36:2215–29. [DOI] [PubMed] [Google Scholar]
- 52. Chan RW, Ng EH, Yeung WS. Identification of cells with colony‐forming activity, self‐renewal capacity, and multipotency in ovarian endometriosis. Am J Pathol. 2011;178:2832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Occult microscopic endometriosis: undetectable by laparoscopy in normal peritoneum. Hum Reprod. 2013;29:462–72. [DOI] [PubMed] [Google Scholar]
- 54. Ulrich D, Tan KS, Schwab K, et al. Mesenchymal stem/stromal cells in postmenopausal endometrium. Hum Reprod. 2014;29:1895–905. [DOI] [PubMed] [Google Scholar]
- 55. Gurung S, Williams S, Deane JA, Werkmeister JA, Gargett CE. The transcriptome of human endometrial mesenchymal stem cells under TGFbetaR inhibition reveals improved potential for cell‐based therapies. Front Cell Dev Biol. 2018;6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barragan F, Irwin JC, Balayan S, et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod. 2016;94:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gargett CE, Gurung S. Endometrial mesenchymal stem/stromal cells, their fibroblast progeny in endometriosis, and more. Biol Reprod. 2016;94:129. [DOI] [PubMed] [Google Scholar]
- 58. Tan Y, Flynn WF, Sivajothi S, et al. Single‐cell analysis of endometriosis reveals a coordinated transcriptional programme driving immunotolerance and angiogenesis across eutopic and ectopic tissues. Nat Cell Biol. 2022;24:1306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Exacoustos C, Luciano D, Corbett B, et al. The uterine junctional zone: a 3‐dimensional ultrasound study of patients with endometriosis. Am J Obstet Gynecol. 2013;209(248):e241–7. [DOI] [PubMed] [Google Scholar]
- 60. Naftalin J, Jurkovic D. The endometrial–myometrial junction: a fresh look at a busy crossing. Ultrasound Obstet Gynecol. 2009;34:1–11. [DOI] [PubMed] [Google Scholar]
- 61. Salamanca A, Beltrán E. Subendometrial contractility in menstrual phase visualized by transvaginal sonography in patients with endometriosis. Fertil Steril. 1995;64:193–5. [PubMed] [Google Scholar]
- 62. Bulletti C, de Ziegler D. Uterine contractility and embryo implantation. Curr Opin Obstet Gynecol. 2006;18:473–84. [DOI] [PubMed] [Google Scholar]
- 63. Leyendecker G, Kunz G, Wildt L, Beil D, Deininger H. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Hum Reprod. 1996;11:1542–51. [DOI] [PubMed] [Google Scholar]
- 64. Sammali F, Kuijsters NPM, Huang Y, et al. Dedicated ultrasound speckle tracking for quantitative analysis of uterine motion outside pregnancy. IEEE Trans Ultrason Ferroelectr Freq Control. 2019;66:581–90. [DOI] [PubMed] [Google Scholar]
- 65. Filby CE, Rombauts L, Montgomery GW, Giudice LC, Gargett CE. Cellular origins of endometriosis: towards novel diagnostics and therapeutics. Semin Reprod Med. 2020;38:201–15. [DOI] [PubMed] [Google Scholar]
- 66. Konrad L, Dietze R, Riaz MA, et al. Epithelial–mesenchymal transition in endometriosis‐when does it happen? J Clin Med. 2020;9:1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li J, Ma J, Fei X, Zhang T, Zhou J, Lin J. Roles of cell migration and invasion mediated by twist in endometriosis. J Obstet Gynaecol Res. 2019;45:1488–96. [DOI] [PubMed] [Google Scholar]
- 68. Cai X, Shen M, Liu X, Guo SW. Reduced expression of eukaryotic translation initiation factor 3 subunit e and its possible involvement in the epithelial‐mesenchymal transition in endometriosis. Reprod Sci. 2018;25:102–9. [DOI] [PubMed] [Google Scholar]
- 69. Xiong W, Zhang L, Liu H, et al. E(2)‐mediated EMT by activation of β‐catenin/Snail signalling during the development of ovarian endometriosis. J Cell Mol Med. 2019;23:8035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Proestling K, Birner P, Gamperl S, et al. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod Biol Endocrinol. 2015;13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Furuya M, Masuda H, Hara K, et al. ZEB1 expression is a potential indicator of invasive endometriosis. Acta Obstet Gynecol Scand. 2017;96:1128–35. [DOI] [PubMed] [Google Scholar]
- 72. Matsuzaki S, Darcha C. Epithelial to mesenchymal transition‐like and mesenchymal to epithelial transition‐like processes might be involved in the pathogenesis of pelvic endometriosis. Hum Reprod. 2012;27:712–21. [DOI] [PubMed] [Google Scholar]
- 73. Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR‐200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moon RT, Kohn AD, Ferrari GVD, Kaykas A. WNT and β‐catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. [DOI] [PubMed] [Google Scholar]
- 75. Gaetje R, Holtrich U, Engels K, et al. Differential expression of claudins in human endometrium and endometriosis. Gynecol Endocrinol. 2008;24:442–9. [DOI] [PubMed] [Google Scholar]
- 76. Cabe AG, Estrada AD, Hoyt T, et al. Endogenous fluorescence of hemosiderin in endometriosis to improve clinical detection. Transl Med Commun. 2019;4:9. [Google Scholar]
- 77. Ding JX, Feng YJ, Yao LQ, Yu M, Jin HY, Yin LH. The reinforcement of invasion in epithelial ovarian cancer cells by 17 beta‐Estradiol is associated with up‐regulation of Snail. Gynecol Oncol. 2006;103:623–30. [DOI] [PubMed] [Google Scholar]
- 78. Huang Y, Fernandez SV, Goodwin S, et al. Epithelial to mesenchymal transition in human breast epithelial cells transformed by 17beta‐estradiol. Cancer Res. 2007;67:11147–57. [DOI] [PubMed] [Google Scholar]
- 79. Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates Snail and slug in the down‐regulation of E‐cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol Endocrinol. 2008;22:2085–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu RF, Chen ZX, Zhou WD, et al. High expression of ZEB1 in endometriosis and its role in 17β‐estradiol‐induced epithelial‐mesenchymal transition. Int J Clin Exp Pathol. 2018;11:4744–58. [PMC free article] [PubMed] [Google Scholar]
- 81. Ma L, Andrieu T, McKinnon B, et al. Epithelial‐to‐mesenchymal transition contributes to the downregulation of progesterone receptor expression in endometriosis lesions. J Steroid Biochem Mol Biol. 2021;212:105943. [DOI] [PubMed] [Google Scholar]
- 82. Bunis DG, Wang W, Vallvé‐Juanico J, et al. Whole‐tissue deconvolution and scRNAseq analysis identify altered endometrial cellular compositions and functionality associated with endometriosis. Front Immunol. 2021;12:788315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. 2009;24:325–32. [DOI] [PubMed] [Google Scholar]
- 84. Tan X, Lang J, Liu D. Expression of monocyte chemotacticp protein‐1 in the eutopic endometrium of women with endometriosis. Zhonghua Fu Chan Ke Za Zhi. 2001;36:89–91. [PubMed] [Google Scholar]
- 85. Haber E, Danenberg HD, Koroukhov N, Ron‐El R, Golomb G, Schachter M. Peritoneal macrophage depletion by liposomal bisphosphonate attenuates endometriosis in the rat model. Hum Reprod. 2009;24:398–407. [DOI] [PubMed] [Google Scholar]
- 86. Hogg C, Panir K, Dhami P, et al. Macrophages inhibit and enhance endometriosis depending on their origin. PNAS. 2021;118:e2013776118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guo M, Bafligil C, Tapmeier T, et al. Mass cytometry analysis reveals a distinct immune environment in peritoneal fluid in endometriosis: a characterisation study. BMC Med. 2020;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zou G, Wang J, Xu X, et al. Cell subtypes and immune dysfunction in peritoneal fluid of endometriosis revealed by single‐cell RNA‐sequencing. Cell Biosci. 2021;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Olkowska‐Truchanowicz J, Sztokfisz‐Ignasiak A, Zwierzchowska A, et al. Endometriotic peritoneal fluid stimulates recruitment of CD4+ CD25high FOXP3+ Treg cells. J Clin Med. 2021;10:3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hou XX, Wang XQ, Zhou WJ, Li DJ. Regulatory T cells induce polarization of pro‐repair macrophages by secreting sFGL2 into the endometriotic milieu. Commun Biol. 2021;4:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oosterlynck DJ, Meuleman C, Waer M, Vandeputte M, Koninckx PR. The natural killer activity of peritoneal fluid lymphocytes is decreased in women with endometriosis. Fertil Steril. 1992;58:290–5. [DOI] [PubMed] [Google Scholar]
- 92. Saha R, Pettersson HJ, Svedberg P, et al. Heritability of endometriosis. Fertil Steril. 2015;104:947–52. [DOI] [PubMed] [Google Scholar]
- 93. Treloar SA, O'Connor DT, O'Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71:701–10. [DOI] [PubMed] [Google Scholar]
- 94. Albertsen HM, Chettier R, Farrington P, Ward K. Genome‐wide association study link novel loci to endometriosis. PLoS One. 2013;8:e58257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nyholt DR, Low S‐K, Anderson CA, et al. Genome‐wide association meta‐analysis identifies new endometriosis risk loci. Nat Genet. 2012;44:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Painter JN, Anderson CA, Nyholt DR, et al. Genome‐wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011;43:51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sapkota Y, Steinthorsdottir V, Morris AP, et al. Meta‐analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee SH, Harold D, Nyholt DR, et al. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer's disease, multiple sclerosis and endometriosis. Hum Mol Genet. 2012;22:832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sapkota Y, Attia J, Gordon SD, et al. Genetic burden associated with varying degrees of disease severity in endometriosis. Mol Hum Reprod. 2015;21:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Khan KN, Fujishita A, Muto H, et al. Levofloxacin or gonadotropin releasing hormone agonist treatment decreases intrauterine microbial colonization in human endometriosis. Eur J Obstet Gynecol Reprod Biol. 2021;264:103–16. [DOI] [PubMed] [Google Scholar]
- 101. Salliss ME, Farland LV, Mahnert ND, Herbst‐Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 2021;28:92–131. [DOI] [PubMed] [Google Scholar]
- 102. Talwar C, Singh V, Kommagani R. The gut microbiota: a double‐edged sword in endometriosis. Biol Reprod. 2022;107:881–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boretto M, Cox B, Noben M, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long‐term expandability. Development. 2017;144:1775–86. [DOI] [PubMed] [Google Scholar]
- 104. Fitzgerald HC, Dhakal P, Behura SK, Schust DJ, Spencer TE. Self‐renewing endometrial epithelial organoids of the human uterus. PNAS. 2019;116:23132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Haider S, Gamperl M, Burkard TR, et al. Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinol. 2019;160:2282–97. [DOI] [PubMed] [Google Scholar]
- 106. Turco MY, Gardner L, Hughes J, et al. Long‐term, hormone‐responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19:568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Boretto M, Maenhoudt N, Luo X, et al. Patient‐derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21:1041–51. [DOI] [PubMed] [Google Scholar]
- 108. Lac V, Verhoef L, Aguirre‐Hernandez R, et al. Iatrogenic endometriosis harbors somatic cancer‐driver mutations. Hum Reprod. 2019;34:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noe M, Ayhan A, Wang TL, Shih IM. Independent development of endometrial epithelium and stroma within the same endometriosis. J Pathol. 2018;245:365–9. [DOI] [PubMed] [Google Scholar]
- 110. Moore L, Leongamornlert D, Coorens THH, et al. The mutational landscape of normal human endometrial epithelium. Nature. 2020;580:640–6. [DOI] [PubMed] [Google Scholar]
- 111. Koppolu A, Maksym RB, Paskal W, et al. Epithelial cells of deep infiltrating endometriosis harbor mutations in cancer driver genes. Cells. 2021;10(4):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci. 2011;18:229–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mustjoki S, Young NS. Somatic mutations in “benign” disease. N Engl J Med. 2021;384:2039–52. [DOI] [PubMed] [Google Scholar]
- 114. Bravi F, Parazzini F, Cipriani S, et al. Tobacco smoking and risk of endometriosis: a systematic review and meta‐analysis. BMJ Open. 2014;4:e006325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hemmert R, Schliep KC, Willis S, et al. Modifiable life style factors and risk for incident endometriosis. Paediatr Perinat Epidemiol. 2019;33:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Parazzini F, Chiaffarino F, Surace M, et al. Selected food intake and risk of endometriosis. Hum Reprod. 2004;19:1755–9. [DOI] [PubMed] [Google Scholar]
- 117. Yamamoto A, Harris HR, Vitonis AF, Chavarro JE, Missmer SA. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. 2018;219:178.e171–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Youseflu S, Jahanian Sadatmahalleh SH, Mottaghi A, Kazemnejad A. Dietary phytoestrogen intake and the risk of endometriosis in Iranian women: a case‐control study. Int J Fertil Steril. 2020;13:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tsuchiya M, Miura T, Hanaoka T, et al. Effect of soy isoflavones on endometriosis: interaction with estrogen receptor 2 gene polymorphism. Epidemiology. 2007;18:402–8. [DOI] [PubMed] [Google Scholar]
- 120. Louis GM, Weiner JM, Whitcomb BW, et al. Environmental PCB exposure and risk of endometriosis. Hum Reprod. 2005;20:279–85. [DOI] [PubMed] [Google Scholar]
- 121. Pauwels A, Schepens PJ, D'Hooghe T, et al. The risk of endometriosis and exposure to dioxins and polychlorinated biphenyls: a case–control study of infertile women. Hum Reprod. 2001;16:2050–5. [DOI] [PubMed] [Google Scholar]
- 122. Lebel G, Dodin S, Ayotte P, Marcoux S, Ferron LA, Dewailly E. Organochlorine exposure and the risk of endometriosis. Fertil Steril. 1998;69:221–8. [DOI] [PubMed] [Google Scholar]
- 123. Niskar AS, Needham LL, Rubin C, et al. Serum dioxins, polychlorinated biphenyls, and endometriosis: a case–control study in Atlanta. Chemosphere. 2009;74:944–9. [DOI] [PubMed] [Google Scholar]
- 124. Upson K, Sathyanarayana S, De Roos AJ, Koch HM, Scholes D, Holt VL. A population‐based case–control study of urinary bisphenol A concentrations and risk of endometriosis. Hum Reprod. 2014;29:2457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Buck Louis GM, Peterson CM, Chen Z, et al. Bisphenol A and phthalates and endometriosis: the endometriosis: natural history, diagnosis and outcomes study. Fertil Steril. 2013;100:162–9. e161–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cobellis L, Latini G, De Felice C, et al. High plasma concentrations of di‐(2‐ethylhexyl)‐phthalate in women with endometriosis. Hum Reprod. 2003;18:1512–5. [DOI] [PubMed] [Google Scholar]
- 127. Peinado FM, Ocón‐Hernández O, Iribarne‐Durán LM, et al. Cosmetic and personal care product use, urinary levels of parabens and benzophenones, and risk of endometriosis: results from the EndEA study. Environ Res. 2021;196:110342. [DOI] [PubMed] [Google Scholar]
- 128. Pollack AZ, Krall JR, Kannan K, Buck Louis GM. Adipose to serum ratio and mixtures of persistent organic pollutants in relation to endometriosis: findings from the ENDO study. Environ Res. 2021;195:110732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Honda H, Barrueto FF, Gogusev J, Im DD, Morin PJ. Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium. Possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod Biol Endocrinol. 2008;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Meola J, Rosa e Silva JC, Dentillo DB, et al. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertil Steril. 2010;93:1750–73. [DOI] [PubMed] [Google Scholar]
- 131. Wu Y, Kajdacsy‐Balla A, Strawn E, et al. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147:232–46. [DOI] [PubMed] [Google Scholar]
- 132. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. [DOI] [PubMed] [Google Scholar]
- 133. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–31. [DOI] [PubMed] [Google Scholar]
- 134. Fung JN, Mortlock S, Girling JE, et al. Genetic regulation of disease risk and endometrial gene expression highlights potential target genes for endometriosis and polycystic ovarian syndrome. Sci Rep. 2018;8:11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mortlock S, Kendarsari RI, Fung JN, et al. Tissue specific regulation of transcription in endometrium and association with disease. Hum Reprod. 2020;35:377–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rahmioglu N, Drong AW, Lockstone H, et al. Variability of genome‐wide DNA methylation and mRNA expression profiles in reproductive and endocrine disease related tissues. Epigenetics. 2017;12:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mortlock S, McKinnon B, Montgomery GW. Genetic regulation of transcription in the endometrium in health and disease. Front Reprod Health. 2022;3:795464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Powell JE, Fung JN, Shakhbazov K, et al. Endometriosis risk alleles at 1p36.12 act through inverse regulation of CDC42 and LINC00339. Hum Mol Genet. 2016;25:5046–58. [DOI] [PubMed] [Google Scholar]
- 139. Mortlock S, Corona RI, Kho PF, et al. A multi‐level investigation of the genetic relationship between endometriosis and ovarian cancer histotypes. Cell Rep Med. 2022;3(3):100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kho PF, Mortlock S, Amant F, et al. Genetic analyses of gynecological disease identify genetic relationships between uterine fibroids and endometrial cancer, and a novel endometrial cancer genetic risk region at the WNT4 1p36.12 locus. Hum Genet. 2021;140:1353–65. [DOI] [PubMed] [Google Scholar]
- 141. Marla S, Mortlock S, Houshdaran S, et al. Genetic risk factors for endometriosis near estrogen receptor 1 and coexpression of genes in this region in endometrium. Mol Hum Reprod. 2021;27:gaaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. The GTEx consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yazar S, Alquicira‐Hernandez J, Wing K, et al. Single‐cell eQTL mapping identifies cell type specific genetic control of autoimmune disease. Science. 2022;376:eabf3041. [DOI] [PubMed] [Google Scholar]
- 144. Dyson MT, Roqueiro D, Monsivais D, et al. Genome‐wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10:e1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bernardi LA, Dyson MT, Tokunaga H, et al. The essential role of GATA6 in the activation of estrogen synthesis in endometriosis. Reprod Sci. 2019;26:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Houshdaran S, Nezhat CR, Vo KC, Zelenko Z, Irwin JC, Giudice LC. Aberrant endometrial DNA methylome and associated gene expression in women with endometriosis. Biol Reprod. 2016;95:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mortlock S, Restuadi R, Levien R, et al. Genetic regulation of methylation in human endometrium and blood and gene targets for reproductive diseases. Clin Epigenetics. 2019;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Moss J, Magenheim J, Neiman D, et al. Comprehensive human cell‐type methylation atlas reveals origins of circulating cell‐free DNA in health and disease. Nat Commun. 2018;9:5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Khan KN, Fujishita A, Hiraki K, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. 2018;17:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Khan KN, Kitajima M, Hiraki K, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. 2010;94:2860–2863.e2863. [DOI] [PubMed] [Google Scholar]
- 151. Khan KN, Kitajima M, Yamaguchi N, et al. Role of prostaglandin E2 in bacterial growth in women with endometriosis. Hum Reprod. 2012;27:3417–24. [DOI] [PubMed] [Google Scholar]
- 152. Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Intra‐uterine microbial colonization and occurrence of endometritis in women with endometriosis. Hum Reprod. 2014;29:2446–56. [DOI] [PubMed] [Google Scholar]
- 153. Khan KN, Fujishita A, Masumoto H, et al. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:69–75. [DOI] [PubMed] [Google Scholar]
- 154. Esfandiari F, Favaedi R, Heidari‐Khoei H, et al. Insight into epigenetics of human endometriosis organoids: DNA methylation analysis of HOX genes and their cofactors. Fertil Steril. 2021;115:125–37. [DOI] [PubMed] [Google Scholar]
- 155. Cindrova‐Davies T, Zhao X, Elder K, et al. Menstrual flow as a non‐invasive source of endometrial organoids. Commun Biol. 2021;4:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Filby CE, Wyatt KA, Mortlock S, et al. Comparison of organoids from menstrual fluid and hormone‐treated endometrium: novel tools for gynecological research. J Pers Med. 2021;11:1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Aghajanova L, Horcajadas JA, Weeks JL, et al. The protein kinase a pathway‐regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151:1341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen‐activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′‐cyclic adenosine 5′‐monophosphate inhibition of cyclin D1. Endocrinology. 2009;150:4701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cook CD, Hill AS, Guo M, et al. Local remodeling of synthetic extracellular matrix microenvironments by co‐cultured endometrial epithelial and stromal cells enables long‐term dynamic physiological function. Integr Biol (Camb). 2017;9:271–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rawlings TM, Makwana K, Taylor DM, et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife. 2021;10:e69603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wiwatpanit T, Murphy AR, Lu Z, et al. Scaffold‐free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2020;105:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wang W, Vilella F, Alama P, et al. Single‐cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat Med. 2020;26:1644–53. [DOI] [PubMed] [Google Scholar]
- 164. Vento‐Tormo R, Efremova M, Botting RA, et al. Single‐cell reconstruction of the early maternal–fetal interface in humans. Nature. 2018;563:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Queckbörner S, von Grothusen C, Boggavarapu NR, Francis RM, Davies LC, Gemzell‐Danielsson K. Stromal heterogeneity in the human proliferative endometrium—a single‐cell RNA sequencing study. J Pers Med. 2021;11(6):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Suryawanshi H, Morozov P, Straus A, et al. A single‐cell survey of the human first‐trimester placenta and decidua. Sci Adv. 2018;4:eaau4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. McKinnon BD, Lukowski SW, Mortlock S, et al. Altered differentiation of endometrial mesenchymal stromal fibroblasts is associated with endometriosis susceptibility. Commun Biol. 2022;5:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shih AJ, Adelson RP, Vashistha H, et al. Single‐cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med. 2022;20:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lac V, Nazeran TM, Tessier‐Cloutier B, et al. Oncogenic mutations in histologically normal endometrium: the new normal? J Pathol. 2019;249(2):173–81. [DOI] [PubMed] [Google Scholar]
- 170. Wiegand KC, Shah SP, Al‐Agha OM, et al. ARID1A mutations in endometriosis‐associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zou Y, Zhou JY, Guo JB, et al. The presence of KRAS, PPP2R1A and ARID1A mutations in 101 Chinese samples with ovarian endometriosis. Mutat Res. 2018;809:1–5. [DOI] [PubMed] [Google Scholar]
- 172. Zou Y, Zhou JY, Wang F, et al. Analysis of CARD10 and CARD11 somatic mutations in patients with ovarian endometriosis. Oncol Lett. 2018;16(1):491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Guo J, Cao B, Xu X, Wu F, Zhu B. Novel CTCF mutations in Chinese patients with ovarian endometriosis. Mol Med Rep. 2018;18(1):1031–6. [DOI] [PubMed] [Google Scholar]
- 174. Yachida N, Yoshihara K, Suda K, et al. Biological significance of KRAS mutant allele expression in ovarian endometriosis. Cancer Sci. 2021;112(5):2020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kim MS, Yoo NJ, Hwang H, Kim MR, Lee SH. Absence of KRAS hotspot mutations in endometriosis of Korean patients. Histopathology. 2018;73(2):357–60. [DOI] [PubMed] [Google Scholar]
- 176. Cao B, Zeng Y, Wu F, et al. Novel TRERF1 mutations in Chinese patients with ovarian endometriosis. Mol Med Rep. 2018;17(4):5435–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


