Abstract
Genetic testing for persons with Parkinson's disease is becoming increasingly common. Significant gains have been made regarding genetic testing methods, and testing is becoming more readily available in clinical, research, and direct‐to‐consumer settings. Although the potential utility of clinical testing is expanding, there are currently no proven gene‐targeted therapies, but clinical trials are underway. Furthermore, genetic testing practices vary widely, as do knowledge and attitudes of relevant stakeholders. The specter of testing mandates financial, ethical, and physician engagement, and there is a need for guidelines to help navigate the myriad of challenges. However, to develop guidelines, gaps and controversies need to be clearly identified and analyzed. To this end, we first reviewed recent literature and subsequently identified gaps and controversies, some of which were partially addressed in the literature, but many of which are not well delineated or researched. Key gaps and controversies include: (1) Is genetic testing appropriate in symptomatic and asymptomatic individuals without medical actionability? (2) How, if at all, should testing vary based on ethnicity? (3) What are the long‐term outcomes of consumer‐ and research‐based genetic testing in presymptomatic PD? (4) What resources are needed for clinical genetic testing, and how is this impacted by models of care and cost‐benefit considerations? Addressing these issues will help facilitate the development of consensus and guidelines regarding the approach and access to genetic testing and counseling. This is also needed to guide a multidisciplinary approach that accounts for cultural, geographic, and socioeconomic factors in developing testing guidelines. © 2023 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease, genetic testing, genetic counseling, attitudes
Introduction
Significant advances have been made in understanding the genetic basis of Parkinson's disease (PD). 1 , 2 , 3 These advances have led to several clinical trials in genetic subtypes of PD, with additional trials in the pipeline. 4 Because the field has moved in the direction of subtyping persons with Parkinson's disease (PwP) based on their presumed disease biologic substrates, including individual‐level genetics, disclosure of genetic testing results to PwP in both research and clinical settings has become increasingly common. 5 , 6 , 7 Despite this paradigm shift, there are limited guidelines regarding who should be tested, which gene or genes should be examined, and how genetic counseling should be performed. Furthermore, knowledge of and attitudes 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 toward genetic testing vary widely among the stakeholders involved, including clinicians, researchers, genetic counselors, PwP, caregivers, and family members. There is also significant variability regarding access to testing resources. In this article we review the literature regarding the role of genetic testing and genetic counseling in PD and identify gaps that need to be filled and controversies that need to be addressed to successfully translate advances in PD genetics into real‐world clinical practice.
Methods
Protocol Development
The Task Force on Recommendations for Clinical Genetic Testing in Parkinson's Disease was developed with three primary objectives: (1) convene a panel of international experts to review the current state of the field in PD diagnostic genetic testing and counseling in various regions of the world; (2) review the ethical and social implications of genetic testing, counseling, and variable access to testing for those with PD; and (3) build consensus on the policies and recommendations for PD diagnostic genetic testing and counseling. 16 Members developed medical subject headings and other terms related to genetic testing in PD, which were used to formulate the following six questions (Table 1): (1) What are the current recommendations regarding indications for genetic testing in PD? (2) What are the genetic testing options for PwP and their families? (3) What are the different genes recommended for testing in different populations? (4) What are PwP's, their caregivers' and relatives', and clinicians' attitudes toward genetic testing? (5) What are PwP's experiences with receiving PD genetic testing results? (6) What genetic counseling services are offered and available for individuals undergoing genetic testing? Additional details regarding methodology can be found in Appendix S1, and all included articles and summaries are provided in Appendix S2.
TABLE 1.
Questions of interest addressed
| Research Question | Search Terms | Filters |
|---|---|---|
| What are the current recommendations regarding indications for genetic testing in PD and presymptomatic PD? | Parkinson* AND (“genetic test” OR “gene test” OR “genetic testing” OR “genetic screening” OR “genomic test” OR “mutation testing”) AND “english”[Language] | Human studies only, searches only title and abstracts |
| What are the genetic testing options for PwP and their families? | Parkinson* AND (“genetic test” OR “gene test” OR “genetic testing” OR “genetic screening” OR “genomic test” OR “mutation testing”) AND “english”[Language] | Human studies only, searches only title and abstracts |
| What are the different genes recommended for testing in different populations? | Parkinson* AND (“genetic test” OR “gene test” OR “genetic testing” OR “genetic screening” OR “genomic test” OR “mutation testing”) AND “english”[Language] | Human studies only, searches only title and abstracts |
| What are PwP's, their caregivers' and relatives', and clinicians' attitudes toward genetic testing? What are PwP's experiences with receiving PD genetic testing results? | Parkinson* AND (genetic* OR gene* OR genomic* OR mutation*) AND (attitude* OR “clinical practice”) AND “english”[Language] | Human studies only, searches only title and abstracts |
| What genetic counseling services are offered and available for individuals undergoing genetic testing? | Parkinson* AND (genetic* OR gene* OR genomic* OR mutation*) AND (counsel*) AND “english”[Language] | Human studies only, searches only title and abstracts |
Abbreviations: PD, Parkinson's disease; PwP, persons with Parkinson's disease.
The literature review did not yield complete answers to the questions posed, rather, it facilitated the identification of gaps in knowledge and controversies identified through authors' open commentary and discussion (Fig. 1). We summarize our findings from the literature review, gaps and controversies they raise, and subsequently, further discuss key gaps and controversies that need to be addressed.
FIG 1.
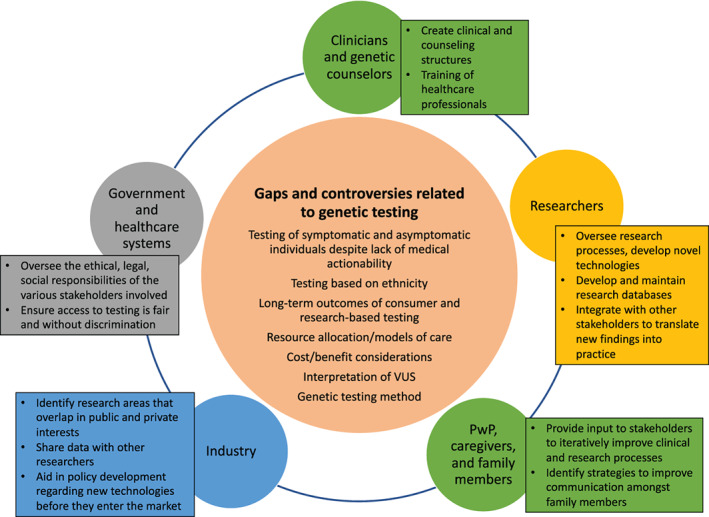
Illustration of the existing gaps and controversies related to genetic testing in Parkinson's disease and the relevant stakeholders that warrant engagement to address the highlighted issues. PwP, persons with Parkinson's disease; VUS, variants of unknown significance. [Color figure can be viewed at wileyonlinelibrary.com]
What We Know: Current Recommendations for Clinical Genetic Testing for PD
Diagnostic Testing in Symptomatic PD for Clinical Purposes
Various recommendations regarding whom and what to test for in the clinical setting were identified in the literature; however, original research supporting these recommendations is lacking. Recommendations are mostly based on expert opinion, predominantly neurologists, and some are now outdated, and coming mainly from North America and Europe. As reported for other related neurodegenerative disorders, genetic testing practices varied by region and healthcare provider. 17 Age of onset, ethnicity, and family history have typically been the considerations for offering PD genetic testing, as summarized in Table 2. 9 , 16 , 17 , 18 It has been suggested by some authors that “all patients with early‐onset PD (age <50 years), either sporadic or familial, are eligible for genetic testing.” 10 The European Federation of Neurological Societies/Movement Disorders Society–European Section (EFNS/MDS‐ES) Task Force recommends diagnostic genetic testing for PD on an individual basis considering family history and age of onset. High‐risk ethnic groups, such as the Ashkenazi Jewish and the North African Berber communities, may have a higher frequency of genetic forms of PD. 18 The EFNS/MDS‐ES Task Force concluded that studies that examine the utility of diagnostic gene testing studies are classified as Class III evidence (retrospective, ie, genetic testing performed in clinically characterized cohorts) and, therefore, diagnostic genetic testing for PD carries a Level B recommendation (ie, “probably effective”). 19 In addition to the EFNS/MDS‐ES guidelines, GeneReviews has published expert consensus regarding genetic testing for PD, which was updated in 2019. 20
TABLE 2.
Current recommendations regarding specific gene testing for Parkinson's disease based on the available literature 10 , 19
| Gene | Inheritance | Recommendation |
|---|---|---|
| SNCA | Dominant inheritance | Test for point mutations and gene multiplications only in families with multiple affected members in more than one generation with early‐ or late‐onset PD |
| LRRK2 | Dominant inheritance | Test for known pathogenic variants in patients with a clinical picture of typical PD and a positive family history |
| Sporadic pattern | Test for known LRRK2 founder mutations in the appropriate populations (ie, with known high mutation frequencies) | |
| GBA1 | Sporadic, recessive, or dominant pattern | Test in patients with typical PD limited to the known founder mutations of established pathogenic role in the appropriate populations (ie, with known high mutation frequencies) |
| PRKN, PINK1, DJ‐1 | Recessive inheritance | Test in patients with typical PD, particularly when the disease onset is <50 years of age |
| Sporadic pattern | Test when onset is very early and if consanguinity is present in the family (<40 years) | |
| ATP13A2, PLA2G6, FBXO7, DNAJC6, SYNJ1, VPS13C, PTRHD1 | Sporadic pattern or recessive inheritance |
Test when onset is very early (<40 years) if no mutation in PRKN, PINK1, and DJ‐1 genes is found Phenotype is atypical |
Abbreviation: PD, Parkinson's disease.
Diagnostic testing is complicated by the incomplete penetrance of variants in several key genes linked to PD and this is an important consideration when testing PwP. Further, even with a specific gene, such as variants in glucocerebrosidase (GCase; GBA1), there can be a range in expression and penetrance. For instance, variants in GBA1 have been classified as severe or mild, depending on their effect on the GCase enzyme, and vary in their effect on developing PD. GCase enzyme activity is also affected by whether individuals are homozygous or heterozygous variant carriers. Penetrance may be as high as 29.7% by age 80 years 21 , 22 depending on the variant type and population studied. In another study, risk for PD associated with GBA1 was 10% at 60 years, 16% at 70 years, and 19% at 80 years of age. 23
Presymptomatic Testing in Unaffected Individuals at Risk for Disease
Historically, presymptomatic testing for PD has been controversial because of the variable penetrance of some of the major PD gene variants and because no disease‐modifying therapeutics are currently available. 21 Specific guidelines for presymptomatic testing in PD have not been established. There is an opportunity to learn from experience with other neurodegenerative conditions, such as Huntington's disease (HD), a monogenic disorder with near‐complete penetrance, and for which extensive guidelines exist. 24 However, considerations related to presymptomatic testing for PD are highly complex and different, and they are outside the scope of this current effort.
Current Genetic Testing Options for PD
A variety of methods have been used to perform genetic testing on cohorts of PwP from different ethnic backgrounds in both clinical and research settings. 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Sanger sequencing was commonly used; however, next‐generation sequencing (NGS), including deletion/duplication analyses, is becoming increasingly common for accurate, efficient, affordable, and rapid testing of genes and pathogenic variants associated with PD. 45
In clinical and research settings, multigene panels using NGS are widely available in many countries, although mostly high‐income areas, 73 and they tend to be an efficient way to test for PD genetic variants given the difficulty in assessing the likely genetic form through clinical evaluation. 46 Additional testing may be warranted to determine copy number variants, which might not be captured by NGS and may not be part of current routine analysis. These include separate deletion/duplication analysis. 5 , 46 Care must be taken to determine whether GBA1 is included within the testing panel and includes full sequencing, because coverage for the range of variants can vary among laboratories. 47 Testing beyond the analysis of select known pathogenic variants can result in the identification of variants of unknown significance (VUSs) requiring expert interpretation and follow‐up, and it may not yield definitive results regarding pathogenicity. Information about specific testing options is available at the Genetic Testing Registry (https://www.ncbi.nlm.nih.gov/gtr/). In the future, as technology continues to improve, whole‐genome sequencing (WGS) is likely to become the preferred approach for genetic testing in PD.
Regarding cost, in some countries such as Canada, testing is covered under the healthcare system. In the United States and many other countries, insurance may not typically cover the cost of diagnostic PD genetic testing, and testing may not be available in lower‐income countries. 73 However, in the research setting, several ongoing studies offer free genetic testing with or without genetic counseling (Table 3), 5 although such testing is restricted to a limited number of sites outside of the United States, Canada, and Europe. Both research and direct‐to‐consumer (DTC) testing may be differently regulated than clinical testing. For example, in the United States, clinical laboratories are government regulated and must have Clinical Laboratory Improvement Amendments certification, whereas DTC labs are not required to be certified.
TABLE 3.
Major research projects offering genetic testing and/or genetic counseling for qualifying people with Parkinson's disease and/or their relatives 5
| PD Research Project | Contact Details |
|---|---|
| PDGENEration, Parkinson's Foundation | genetics@parkinson.org, http://www.parkinson.org/PDGENEration |
| ClinicalTrials.gov Identifier: NCT04057794 | |
| Parkinson's Progression Markers Initiative, The Michael J. Fox Foundation | https://www.ppmi‐info.org/contact‐us/ |
| Fox Insight substudy, The Michael J. Fox Foundation | info@foxinsight.org |
| Rostock International Parkinson's Disease study, Centogene | ClinicalTrials.gov Identifier: NCT03866603 |
Note: Details on the scope of each program can be found on the respective websites.
Abbreviation: PD, Parkinson's disease.
Current State of Clinical Genetic Counseling for PD
Genetic counseling for PD can occur in a variety of settings for various indications, including prenatal, pediatric, or adult populations. Content of sessions will vary depending on the indication and the provider involved. Possessing adequate knowledge and having time to provide genetic counseling has been recognized as key to the provision of quality genetic testing for patients with neurodegenerative conditions such as PD. 17 Although genetic counselors are specialized in providing this type of care, they may not be available or used at all sites, requiring that clinicians assume this role, which may be the case in many countries. In a survey of movement disorder specialists from North America, 8 there was evidence that providers were unprepared and uncomfortable integrating PD genetics information into routine patient care. Furthermore, the study found that genetic testing, and therefore genetic counseling, is not routinely performed for PD. 8 However, this is a single study limited to North America and thus may not be generalizable worldwide. Clinician training varies greatly by region and country, and thus clinician comfort level with genetic counseling may vary accordingly.
Recently, experience has been rapidly gained through multiple large PD research studies offering genetic testing and counseling to thousands of individuals with PD and those at risk (Parkinson's Progression Markers Initiative [PPMI], 48 PD GENEration (ClinicalTrials.gov: NCT04994015), Rostock International Parkinson's Disease [ROPAD] study 49 ), and neurologists, research genetic counselors, and movement disorder specialists have published expert opinions on performing PD genetic counseling for those with and without manifest disease. 7 , 20 In addition, experience with disclosure of LRRK2 research results was recently published, which may be instructive for clinical care. 50 Remote genetic counseling provided by PD genetic counselors versus genetic counseling provided by local clinical sites regarding GBA1 variant status (and others) was recently compared as part of the PD GENEration pilot study (ClinicalTrials.gov: NCT04994015), with the results of the study currently pending. Counseling for GBA1 is particularly complicated given penetrance variability, as discussed earlier, and potential association with Gaucher disease (GD) depending on the variant type. Literature from the HD and the dementia fields 51 may provide insights into conditions such as PD, amyotrophic lateral sclerosis (ALS), and frontotemporal dementia, which may have complex patterns of inheritance in many instances. 17 Experts generally agree that genetic testing for complex disorders such as PD should be accompanied by pretest and posttest genetic counseling. 5 , 16 , 52 , 53
Novel ways to deliver genetic counseling that differ from a traditional model are increasingly being considered because of the shortage of genetic counselors. 54 Already, neurologists are being trained to provide some level of genetic counseling as PD genetic testing becomes more widely performed. 8 , 45 When possible, however, it is recommended that clinicians should refer patients to genetic counselors and/or medical geneticists for further discussion when cases become complex (outside of their level of comfort), involve prenatal testing, or are predictive in nature. 5 , 20 General guidelines for PD genetic counseling are emerging, although most are United States‐centric, 20 and there is a need to consider how practices should be adapted according to healthcare system and/or geographical region.
Current Clinician and Patient Attitudes Toward and Knowledge of Genetic Testing
Surveys of clinicians and patients have provided insights into the attitudes toward and knowledge of genetic testing for PD. In a 2019 survey of movement disorders specialists from 146 Parkinson Study Group sites in the United States (n = 131) and Canada (n = 15), only 17% of respondents said they would not offer genetic testing. 8 Still, around 87% of participants reported referring fewer than 10 patients for genetic testing in the 12 months before completing the survey. The most cited reason for not referring for genetic testing included lack of insurance coverage/cost to the patient.
There is a significant need to increase knowledge among healthcare providers and patients regarding genetic testing in PD. Among the Parkinson Study Group clinicians surveyed, 8 nearly all respondents correctly answered the basic knowledge questions, but responders were not confident regarding their genetic knowledge. When respondents were asked to rate their confidence in genetic knowledge as it pertains to PD using a Likert scale from 0 to 100, the mean score was 47.6 (SD, 26.3), indicating low confidence. Notably, PD‐specific questions regarding the inheritance and penetrance of GBA1 and LRRK2 variants were answered correctly by only 60% of clinicians.
From the PwP perspective, attitudes regarding genetic testing vary by the population surveyed and by type of testing proposed. For instance, in a study of PwP living in Australia, support was higher for diagnostic testing (97%) versus predictive (78%) or prenatal (58%) testing. 13 In contrast, when responses of US participants were compared with those in Singapore, 85% to 92% of US‐based PwP had a positive attitude toward the potential medical benefits of genetic testing, compared with 32% to 42% of PwP in Singapore, highlighting that attitudes about genetic testing are likely different among cultures. 15 In North America, multiple studies have documented a strong interest in genetic testing and genetic counseling by PwP, their relatives, and caregivers. 6 , 7 , 45 Among PwP, knowledge of PD genetics is highly variable, with a mean percent of correct responses ranging from 37% 12 to 73% depending on the study and population examined. 9 , 11 , 12 , 13 , 55 , 56 , 57 , 58 , 59 Although cultural, religious, and educational factors impact patient experiences and attitudes regarding genetic testing, the lack of preventive and disease‐modifying therapies also likely impact decision making. Data are lacking regarding patient and clinician attitudes toward and knowledge of genetic testing for PD from lower‐income countries.
The specific gene being examined (ie, GBA1) may also play a critical role in patient attitudes toward genetic testing. As noted, the relationship between GBA1, PD, and GD is a complex one and may pose its own unique challenges. A survey of PwP and caregivers revealed a desire for information about GD that may have an impact on their or their family's health. 12 In a study of partners who had screened negative for GBA1 variants, the majority (87%) felt that everyone should be informed before carrier screening regarding the relationship between GD and PD. 55 Specifically, for those with GD, most patients thought that discussion of PD should occur at the time of GD diagnosis and should come from their healthcare provider. 56
Experience with Genetic Testing and Genetic Counseling in Patients and Their Relatives
Little is known about the genetic counseling experience specifically for PD, although this has been documented for other neurodegenerative conditions. 17 , 24 , 51 , 60 , 61 Only one study thus far has examined PD genetic testing and genetic counseling outcomes in PwP and relatives at risk. 7 Participants from a large North American population were surveyed after receiving genetic counseling, and they reported high satisfaction and no significant adverse sequelae. However, the population was highly homogenous and educated, and testing was targeted to only two variants, warranting further studies in more diverse, global populations. 7
Gaps and Controversies
Controversy: Clinical Testing of Symptomatic and Asymptomatic Individuals Without Medical Actionability
We define medical actionability as the availability of a proven clinical intervention or a change in clinical decision making (ie, whether to perform a procedure). From the clinician perspective, absence of disease‐modifying therapies and the variable penetrance of most known variants currently limit the usefulness of genetic diagnostic testing for PD in clinical practice 62 (lack of medical actionability). At the present time, no clinical trials have demonstrated the clear benefit of an intervention based on a genetic subtype of PD, although clinical trials aimed at targeting genetic subtypes of PD have offered genetic testing as part of the screening process. Thinking among clinicians and researchers may be shifting, with the idea that genetic information gleaned from testing may provide useful information to patients and their caregivers relating to genotype–phenotype correlations, disease prognosis, treatment options (ie, reconsideration of deep brain stimulation surgery for those with GBA1 variants 63 ), consequences for children, and for some, reproductive planning. 6
From the PwP perspective, there is increasing recognition that PwP may experience personal utility from knowing this information, whether it is to simply obtain more information about the condition, help answer questions regarding causation, inform family members, or contribute to research. 7 Increasingly, genetic testing, whether for those with disease or without, but at risk, is viewed as an individual choice and a personal decision. Personal and not just medical utility is increasingly being considered by the PD community as justification for testing, especially in those countries that have sponsored testing programs. PwP and family members in some parts of the world have access to PD genetic testing through DTC services, even though practitioners may believe in limiting testing to specific cases of PD.
It is interesting to note that in a systematic review of diagnostic testing for ALS and frontotemporal dementia, seven studies from five countries found that more than half of surveyed patients and relatives were not aware of the availability of genetic testing and, thus, not likely informed by their practitioners. When informed, 83% thought testing should be offered to all patients. 17 A survey of movement disorder specialists felt “patients do not want genetic testing,” although this does not match patient survey responses where a significant interest is reported. 8 Regardless of disagreements over who should be tested and under what conditions, most experts agree that individuals interested in predictive testing or those with a low likelihood of receiving abnormal results from testing (ie, low risk) should at least be referred for genetic counseling. 16 , 45 In the near future, data from large studies such as PD GENEration (ClinicalTrials.gov: NCT04994015), ROPAD, 49 and other international studies may shed light on the utility and impact of widespread genetic testing and genetic counseling for PD.
Controversy: Clinical Testing Based on Ethnicity
Because the yield of a test, and thus the benefit‐to‐cost ratio, will be higher in those populations with a greater frequency of variants, it has been suggested that testing is potentially more beneficial in specific populations. Studies that use comprehensive PD panels in specific ethnic populations could be performed, indicating which genes are present in these populations and which are not. One such example is the ROPAD Study. 49 In theory, such studies could help design panels that are tailored to specific ethnic/genetic backgrounds. At the present time, few such studies exist and may not be feasible given rapidly expanding globalization. Furthermore, the ethical implications of testing based solely on an individual's ethnic background need to be considered. Currently available studies tested for the presence of selected variants in specific ethnic groups. 15 , 64 , 65 , 66 , 67 Based on high frequency of pathogenic variants in the selected genes, we can conclude that these genes such as LRRK2 should be included in panels designed for North African or Ashkenazi Jewish populations. 64 However, given the lack of published data on the prevalence of rarer variants in these populations, narrow panels may miss variant carriers. Guidelines, such as those from the EFNS/MDS‐ES Task Force, are available, 19 but the field has advanced significantly since that time. We suggest that there is a critical need to regularly update specific guidelines, with input from stakeholders, that guide genetic testing for PD. At the present time, there is no standard genetic panel or guidelines for genetic testing that are applicable to all patients with PD. Efforts to fill this gap are underway as evidenced by programs such as PD GENEration. 123 Such programs may inform testing so it can be tailored to the needs of the individual country, region, or ethnic population, if necessary. As genetic testing methods advance, WGS is likely to become the preferred approach for testing in PD, which will limit the utility of less comprehensive panels, although WGS will come with its own set of unique challenges.
Gap: Long‐Term Outcomes of Consumer‐ and Research‐Based Genetic Testing in Presymptomatic PD
As discussed earlier, presymptomatic PD testing is controversial but remains available through DTC testing in which the physician is circumvented. Not infrequently, PwP and family members may consult with their clinical providers after having completed DTC genetic testing. Increasingly, people can obtain their raw genetic data via DTC or research testing. This raises ethical and legal issues that remain to be fully explored. 27 Although presymptomatic testing may be discouraged by some, it has been performed in the research setting for years through studies such as PPMI. 48 If genetic testing results are given to participants in such settings, genetic counseling is imperative. However, the long‐term ramifications of disclosing results in these settings are not fully understood, although preliminary work in PD and more extensive research in other specialties suggest less harm than anticipated. 7 In general, more research and follow‐up are needed to document short‐ and long‐term sequelae of genetic testing in both affected and unaffected individuals, regardless of test results.
Gap: Resource Allocation, Models of Care, and Cost‐Benefit Considerations for Clinical Genetic Testing
Although genetic testing is currently available, there exists a need to develop multidisciplinary genetic testing programs that are person centered, flexible, and readily accessible to the PD community to allow PwP and their family members to make informed, personalized medical decisions. This is supported by work done with genetic testing programs for HD and ALS. 68 Team members may include a new generation of movement disorder specialists trained in neurogenetics, genetic counselors, geneticists, social workers, and psychologists. Using this multidisciplinary team‐based approach, clinicians could integrate medical and family histories into genetic discussions, and PwP would gain an understanding of disease causation, transmission, penetrance, expressivity, potential VUSs, and potential therapeutic options. This approach would not be “one size fits all,” because cultural beliefs, religious values, cost considerations, and other differences in health systems and practices of different countries would need to be considered in customizing these programs. 13 , 14 , 70 Ultimately, genetic testing should be offered respecting patient autonomy and following an informed decision, recognizing that family issues and support will be important to address. Clinician guidelines and checklists for PD genetic discussions with minimum talking points could be helpful in this regard.
A major barrier to achieving such a multidisciplinary model is the paucity of geneticists and genetic counseling resources (at least in the United States), and this will have an impact on the ability to implement programs based on a paradigm of providing in‐person pretest and posttest genetic counseling, as taken from HD‐related guidelines. Because PD is a common disease, these resource considerations will be important challenges to implementing genetic testing paired with counseling in clinical practice. In addition, these challenges raise the need to develop educational initiatives and programs on PD genetics, involving healthcare providers, movement disorder experts, and genetic counselors. Research regarding patient and family attitudes toward testing for PD support the recommendation that diagnostic testing should be at least discussed with patients as an option, where training and resources allow this consideration. Because offering PD genetic testing varies among clinicians, more targeted education and consensus guidelines will be needed to address this potential barrier to patients and relatives receiving testing. A categorical recommendation to not test individuals, including family members, as suggested by some authors, should be approached with caution because this could be perceived as limiting person autonomy and paternalistic in nature. Rather, in these cases, formal genetic counseling could be offered to explore the needs and expectations of patients and their relatives. In looking to the future, the availability of technological innovations, such as telemedicine, novel models of genetic service delivery, and social media, may allow for the widespread acceptance and implementation of such comprehensive programs. 54 , 71
These issues are likely to become increasingly complex as genetic testing methods advance and WGS is likely to become the preferred approach for testing in PD. The expected increased availability of WGS comes with exciting opportunities but also significant challenges. One potential benefit is that WGS is a comprehensive test that enables rapid integration of new genetic findings into molecular diagnostics, allowing for data to be reanalyzed as more information becomes available. 72 Thus new variants may be determined, and information regarding prior VUSs may be clarified. The challenge such technology poses lies in understanding who is ultimately responsible for such reanalysis and reinterpretation of data. Patients will need to be informed of the process for reinitiation and reinterpretation of data. Furthermore, this process may, at least initially, not be reimbursed by insurance and may not be an automated process, but this is rapidly evolving. For instance, in May 2020, the Australian Medicare Benefits Schedule added a provision for the reimbursement of reanalysis testing in relation to “genetic testing for childhood syndromes,” 73 and other countries are likely to follow suit for a variety of indications that will expand over time.
Conclusions
Current published data provided limited guidance on genetic testing and counseling standards for PD. At the present time, genetic testing is performed clinically in limited settings and on a case‐by‐case basis, with wide regional differences. From a research perspective, genetic testing is performed to (1) aid in improving subtyping based on genotype–phenotype correlations and (2) provide PwP with an opportunity to participate in clinical trials based on their genetic status. 18 As we have learned from the COVID‐19 pandemic, a major challenge exists in ensuring that there is equal access to such research opportunities regardless of income, geography, sex, and ethnicity. Indeed, most of the data discussed comes from mainly White non‐Hispanic populations, and although efforts have expanded to Latin America (LARGE‐PD study), 122 studies are needed in more diverse populations. Non‐White populations, many of whom may come from lower‐ or middle‐income countries, may have unique barriers to accessing genetic testing, counseling, and research opportunities. The Global Parkinson's Genetics Program (GP2) is an example of a collaborative effort to overcome such barriers. 123 Programs such as GP2 may allow for examination of genetic variants across different regions/populations, and guidelines will be important to manage the data generated and to return results to participants effectively.
For genetic testing to become clinically relevant and ubiquitous, improved PD models for drug discovery and screening are needed, which will allow for discovery of disease‐modifying treatments and acceptance of gene‐specific therapies. 27 , 33 , 70 , 76 , 77 , 78 In the meantime, in some parts of the world, there is strong consumer interest driving more widespread PD testing, which will require new considerations of whom, how, and why to test, as well as how PD genetic testing will be provided consistently, with quality and equity.
Due to the anticipated increase in PD genetic testing, models for providing genetic services, including standards of practice, and educational initiatives, targeting PwP, family members, and clinicians, will need to be developed. Tools and resources will be crucial to support informed, personalized decisions. Additional research and recommendations are needed to address some of the remaining major controversies, such as genetic testing and clinical actionability versus patient autonomy and the right to know, cost‐benefit considerations and equal access to testing, representation of all populations, and harnessing and adapting to technological changes in the field.
Author Roles
Design, execution, and analysis: G.P., L.C., J.S., J.V., R.N.A., M.M., C.M.S., S.B., V.B., S.J.C., T.F., E.G., A.H., N.H., T.L., K.M., D.M., I.N., A.T., D.R., M.S., A.S., O.S., N.E.M., T.S., R.S.‐P., and C.K. Writing of initial draft: G.P. Editing of final version of the manuscript: G.P., L.C., J.S., J.V., R.N.A., M.M., C.M.S., S.B., V.B., S.J.C., T.F., E.G., A.H., N.H., T.L., K.M., D.M., I.N., A.T., D.R., M.S., A.S., O.S., N.E.M., T.S., R.S.‐P., and C.K.
Financial Disclosures of All Authors (for the Preceding 12 Months)
Gian Pal—Stock ownership in medically related fields: Baudax Bio. Consultancies: Kyowa Kirin. Expert Testimony: yes. Employment: Rutgers‐Robert Wood Johnson Medical School. Honoraria: Guidepoint. Grants: National Institutes of Health.
Lola Cook—Grants: funding received from The Michael J. Fox Foundation (MJFF) and Parkinson's Foundation are used to support salaries.
Jeanine Schulze—Grants: funding received from The MJFF and Parkinson's Foundation are used to support salaries.
Jennifer Verbrugge—Grants: funding received from The MJFF and Parkinson's Foundation are used to support salaries.
Roy N. Alcalay—Consultancies: Sanofi, Takeda, Gain Therapeutics, and Parkinson's Foundation. Grants: The MJFF, Parkinson's Foundation, Biogen (to institution), and National Institutes of Health.
Marcelo Merello—Consultancies: Abbott and Corpomedica Arg. Advisory Boards: Abbott and St. Jude. Employment: MDS‐Wiley (editor). Honoraria:Viguera Eds. Royalties: Random House Oxford University Press. Grants: Safra Foundation and FONCYT.
Carolyn M. Sue—Employment: University of Sydney and Northern Sydney Health District.
Partnerships. Royalties: Oxford University Press. Patents: PCT/AU2015/000194. Grants: National Health and Medical Research Council of Australia, Medical Research Futures Fund, Aligning Science Across Parkinson's (ASAP).
Soraya Bardien—Grants: South African Medical Research Council (Self‐Initiated Research Grant) and the National Research Foundation of South Africa (grant 129249).
Vincenzo Bonifati—Employment: Erasmus MC Rotterdam, the Netherlands. Honoraria: The International Parkinson and Movement Disorder Society, as Chair of the Int. Congress Scientific Program Committee 2019–2021; Elsevier Ltd, as co‐Editor‐in‐Chief of Parkinsonism & Related Disorders. Grants: Alzheimer Nederland; Stichting Parkinson Fonds (the Netherlands).
Sun Ju Chung—Nothing to report.
Tatiana Foroud—Advisory Boards: Mayo ADRC, Mt. Sinai ADRC, Penn ADRC, and NIAGADS advisor. Employment: Indiana University School of Medicine. Contracts: The MJFF and Parkinson's Foundation. Honoraria: none that are independent of Advisory Board–provided honoraria. Grants: National Institutes of Health, NCAA/DoD.
Emilia Gatto—Consultancies: Bago Argentina. Advisory Boards: Bago Argentina. Honoraria: Roche, Bago, UCB, and Biopas. Grants: Novartis.
Anne Hall—Honoraria: Parkinson's Foundation (Steering Committee member, PDGENEration Study). Other: FDA Patient Engagement Collaborative member (funded through CTTI and Duke University).
Nobutaka Hattori—Stock ownership in medically related fields: PARKINSON Laboratories Co. Ltd (Equity stock (8%)). Consultancies: Sumitomo Pharma, Takeda Pharmaceutical, Kyowa Kirin, PARKINSON Laboratories Co., Ltd, and Teijin Pharma. Advisory Boards: Sumitomo Pharma, Takeda Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, and Teijin Pharma. Employment: Juntendo University and RIKEN Center for Brain Science. Honoraria: Sumitomo Pharma, Takeda Pharmaceutical, Kyowa Kirin, AbbVie GK, Otsuka Pharmaceutical, Novartis Pharma, Ono Pharmaceutical, Eisai, Teijin Pharma, Daiichi Sankyo Co. FP pharma. Grants: Japan Society for the Promotion of Science, Japan Agency for Medical Research and Development, Japan Science and Technology Agency, Health Labour Sciences Research Grant, International Parkinson and Movement Disorder Society, and MJFF.
Tim Lynch—Employment: UCD/MMUH/IEHG. Honoraria: Clonmel Healthcare. Grants: Health Research Board, MJFF, Irish Institute of Clinical Neuroscience.
Karen Marder—Advisory Boards: CHDI, HSG, Parkinson's Foundation, and Novartis. Grants: National Institutes of Health, MJFF, HDSA, LBDA. Other: Springer.
Deborah Mascalzoni—Grants: Deutsche Forschungsgemeinschaft (FOR2488); the Department of Innovation, Research and Universities of the Province of South Tyrol; and the Innovative Medicines Initiative project FACILITATE.
Ivana Novakovic—Nothing to report.
Avner Thaler—Employment: Tel‐Aviv Medical Center. Grants: MJFF.
Deborah Raymond—Grants: National Institutes of Health Grant 1 U01 NS107016‐01A1 and MJFF (PPMI).
Mehri Salari—Nothing to report.
Ali Shalash—Nothing to report.
Oksana Suchowersky—Advisory Boards: AbbVie and Sunovion. Employment: University of Alberta. Royalties: UpToDate and Springer. Grants: Wave Life Sciences, Roche, and University Hospital Foundation.
Niccolo E. Mencacci—Advisory Boards: PDEGENERATION Steering Committee. Employment: Northwestern University. Honoraria: Parkinson's Foundation and Aligning Science Across Parkinson's Global Parkinson's Genetics Program (ASAP GP2). Grants: Parkinson's Foundation.
Tanya Simuni—Consultancies: 4D Pharma, Acadia, AcureX, AskBio, Amneal, Blue Rock Therapeutics, Caraway, Critical Path for Parkinson's Consortium, Denali, MJFF, Neuroderm, Roche, Sanofi, Sinopia, Sunovion, Takeda, UCB, Vanqua Bio, and Voyager. Advisory Boards: 4D Pharma, Acadia, AcureX, AskBio, Amneal, Denali, Neuroderm, Roche, Sanofi, Sunovian, and UCB. Employment: Northwestern University. Grants: Biogen, Roche, Neuroderm, Sanofi, Amneal, Prevail, UCB, National Institute of Neurological Disorders and Stroke, MJFF, Parkinson's Foundation.
Rachel Saunders‐Pullman—Employment: Icahn School of Medicine at Mount Sinai. Grants: National Institutes of Health, National Institute of Neurological Disorders and Stroke Grants U01 NS107016 (related, but no conflict) and P20NS123220, New York State Empire Clinical Research Investigator Program, and the MJFF.
Christine Klein—Consultancies: Centogene and Lundbeck. Advisory Boards: Retromer Therapeutics. Employment: University of Lübeck and University Hospital Schleswig‐Holstein. Honoraria: Desitin. Royalties: Oxford University Press. Grants: German Research Foundation, BMBF, and MJFF.
Supporting information
Appendix S1. Methods and search strategy.
Appendix S2. Included articles and summaries.
Acknowledgments
We acknowledge Sandra Videmsky for her work in coordinating this effort.
Relevant conflicts of interest/financial disclosures: R.N.A. has received consultation fees from Sanofi, Takeda, and Gain Therapeutics; his institution received research grants from Biogen. S.B. was supported by the South African Medical Research Council. E.G. has contracts with Roche for clinical trials and CHDI foundation for Enroll‐HD study. N.E.M. has received honorarium from the Parkinson's Foundation to serve in the steering committee of the PD GENEration study. R.S.‐P. was supported by National Institute of Neurological Disorders and Stroke Grants U01 NS107016 (related, but no conflict) and P20NS123220 and the NY Empire Clinical Research Investigator Program. G.P., L.C., J.S., J.V., M.M., C.M.S., V.B., S.J.C., T.F., A.H., N.H., T.L., K.M., D.M., I.N., A.T., D.R., M.S., A.S., O.S., T.S., and C.K. have nothing to report.
Funding agencies: G.P. was supported by the National Institute of Neurological Disorders and Stroke Grant K23‐NS097625‐05. S.B. was supported by the South African Medical Research Council (Self‐Initiated Research Grant) and the National Research Foundation of South Africa (Grant 129249). N.E.M. was supported by the Parkinson's Foundation and Aligning Science Across Parkinson's (ASAP; Global Parkinson's Genetics Program [GP2]). C.K. was supported by the DFG (FOR 2488), The Michael J. Fox Foundation, and ASAP (GP2). C.M.S. was supported by the National Health and Medical Research Council, Medical Research Future Fund, and the ASAP Collaborative Research Network. R.S.‐P. was supported by National Institute of Neurological Disorders and Stroke Grants U01 NS107016 (related, but no conflict) and P20NS123220 and the Bigglesworth Family Foundation.
Full financial disclosures and author roles may be found in the online version of this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Kasten M, Hartmann C, Hampf J, et al. Genotype‐phenotype relations for the Parkinson's disease genes Parkin, PINK1, DJ1: MDSGene systematic review. Mov Disord. 2018;33(5):730–741. 10.1002/mds.27352 [DOI] [PubMed] [Google Scholar]
- 2. Wittke C, Petkovic S, Dobricic V, et al. Genotype‐phenotype relations for the atypical parkinsonism genes: MDSGene systematic review. Mov Disord. 2021;36(7):1499–1510. 10.1002/mds.28517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trinh J, Zeldenrust FMJ, Huang J, et al. Genotype‐phenotype relations for the Parkinson's disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord. 2018;33(12):1857–1870. 10.1002/mds.27527 [DOI] [PubMed] [Google Scholar]
- 4. Prasuhn J, Brüggemann N. Genotype‐driven therapeutic developments in Parkinson's disease. Mol Med. 2021;27(1):42. 10.1186/s10020-021-00281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cook L, Schulze J, Naito A, Alcalay RN. The role of genetic testing for Parkinson's disease. Curr Neurol Neurosci Rep. 2021;21(4):17. 10.1007/s11910-021-01100-7 [DOI] [PubMed] [Google Scholar]
- 6. den Heijer JM, van Hilten JJ, Kievit AJA, Bonifati V, Groeneveld GJ. Experience in genetic counseling for GBA1 variants in Parkinson's disease. Mov Disord Clin Pract. 2021;8(1):33–36. 10.1002/mdc3.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verbrugge J, Cook L, Miller M, et al. Outcomes of genetic test disclosure and genetic counseling in a large Parkinson's disease research study. J Genet Couns. 2021;30(3):755–765. 10.1002/jgc4.1366 [DOI] [PubMed] [Google Scholar]
- 8. Alcalay RN, Kehoe C, Shorr E, et al. Genetic testing for Parkinson disease: current practice, knowledge, and attitudes among US and Canadian movement disorders specialists. Genet Med. 2020;22(3):574–580. 10.1038/s41436-019-0684-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maloney KA, Alaeddin DS, von Coelln R, Dixon S, Shulman LM, Schrader K, Guan Y. Parkinson's disease: Patients' knowledge, attitudes, and interest in genetic counseling. J Genet Couns. 2018;27(5):1200–1209. 10.1007/s10897-018-0239-3 [DOI] [PubMed] [Google Scholar]
- 10. Di Fonzo A, Monfrini E, Erro R. Genetics of movement disorders and the practicing clinician; who and what to test for? Curr Neurol Neurosci Rep. 2018;18(7):37. 10.1007/s11910-018-0847-1 [DOI] [PubMed] [Google Scholar]
- 11. Falcone DC, Wood EM, Xie SX, Siderowf A, Van Deerlin VM. Genetic testing and Parkinson disease: assessment of patient knowledge, attitudes, and interest. J Genet Couns. 2011;20(4):384–395. 10.1007/s10897-011-9362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sakanaka K, Waters CH, Levy OA, Louis ED, Chung WK, Marder KS, Alcalay RN. Knowledge of and interest in genetic results among Parkinson disease patients and caregivers. J Genet Couns. 2014;23(1):114–120. 10.1007/s10897-013-9618-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scuffham TM, McInerny‐Leo A, Ng SK, Mellick G. Knowledge and attitudes towards genetic testing in those affected with Parkinson's disease. J Community Genet. 2014;5(2):167–177. 10.1007/s12687-013-0168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shkedi‐Rafid S, Ofer‐Bialer G, Meiner V, Calderon‐Margalit R. Clinicians' attitudes toward general screening of the Ashkenazi‐Jewish population for prevalent founder BRCA1/2 and LRRK2 mutations. Public Health Genomics. 2013;16(4):174–183. 10.1159/000351592 [DOI] [PubMed] [Google Scholar]
- 15. Tan EK, Lee J, Hunter C, Shinawi L, Fook‐Chong S, Jankovic J. Comparing knowledge and attitudes towards genetic testing in Parkinson's disease in an American and Asian population. J Neurol Sci. 2007;252(2):113–120. 10.1016/j.jns.2006.10.016 [DOI] [PubMed] [Google Scholar]
- 16. Task Force on Recommendations for Clinical Genetic Testing in Parkinson's Disease.
- 17. Crook A, Jacobs C, Newton‐John T, O'Shea R, McEwen A. Genetic counseling and testing practices for late‐onset neurodegenerative disease: a systematic review. Journal of Neurology. 2022;269(2):676–692. 10.1007/s00415-021-10461-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Payne K, Walls B, Wojcieszek J. Approach to assessment of Parkinson disease with emphasis on genetic testing. Med Clin North Am. 2019;103(6):1055–1075. 10.1016/j.mcna.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 19. Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS‐ES/ENS [corrected] recommendations for the diagnosis of Parkinson's disease. Eur J Neurol. 2013;20(1):16–34. 10.1111/ene.12022 [DOI] [PubMed] [Google Scholar]
- 20. Cook Shukla L, Schulze J, Farlow J, Pankratz ND, Wojcieszek J, Foroud T. Parkinson disease overview. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews® [Internet]. Seattle (WA): University of Washington; 1993. –2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1223/ [Google Scholar]
- 21. Anheim M, Elbaz A, Lesage S, et al. Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology 2012;78(6):417–420. 10.1212/WNL.0b013e318245f476 [DOI] [PubMed] [Google Scholar]
- 22. Rana HQ, Balwani M, Bier L, Alcalay RN. Age‐specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med. 2013;15(2):146–149. 10.1038/gim.2012.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balestrino R, Tunesi S, Tesei S, Lopiano L, Zecchinelli AL, Goldwurm S. Penetrance of glucocerebrosidase (GBA) mutations in Parkinson's disease: a kin cohort study. Mov Disord. 2020;35(11):2111–2114. 10.1002/mds.28200 [DOI] [PubMed] [Google Scholar]
- 24. MacLeod R, Tibben A, Frontali M, Evers‐Kiebooms G, Jones A, Martinez‐Descales A, Roos RA. Recommendations for the predictive genetic test in Huntington's disease. Clin Genet. 2013;83(3):221–231. 10.1111/j.1399-0004.2012.01900.x [DOI] [PubMed] [Google Scholar]
- 25. Bertoli‐Avella AM, Giroud‐Benitez JL, Akyol A, et al. Novel parkin mutations detected in patients with early‐onset Parkinson's disease. Mov Disord. 2005;20(4):424–431. 10.1002/mds.20343 [DOI] [PubMed] [Google Scholar]
- 26. Di Fonzo A, Tassorelli C, De Mari M, et al. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson's disease. Eur J Hum Genet. 2006;14(3):322–331. 10.1038/sj.ejhg.5201539 [DOI] [PubMed] [Google Scholar]
- 27. Klein C, Schlossmacher MG. The genetics of Parkinson disease: implications for neurological care. Nat Clin Pract Neurol. 2006;2(3):136–146. 10.1038/ncpneuro0126 [DOI] [PubMed] [Google Scholar]
- 28. Goldwurm S, Zini M, Mariani L, et al. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counseling in Parkinson disease. Neurology 2007;68(14):1141–1143. 10.1212/01.wnl.0000254483.19854.ef [DOI] [PubMed] [Google Scholar]
- 29. Doehring A, Kirchhof A, Lötsch J. Genetic diagnostics of functional variants of the human dopamine D2 receptor gene. Psychiatr Genet. 2009;19(5):259–268. 10.1097/YPG.0b013e32832d0941 [DOI] [PubMed] [Google Scholar]
- 30. Bardien S, Lesage S, Brice A, Carr J. Genetic characteristics of leucine‐rich repeat kinase 2 (LRRK2) associated Parkinson's disease. Parkinsonism Relat Disord. 2011;17(7):501–508. 10.1016/j.parkreldis.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 31. Grünewald A, Kasten M, Ziegler A, Klein C. Next‐generation phenotyping using the parkin example: time to catch up with genetics. JAMA Neurol. 2013;70(9):1186–1191. 10.1001/jamaneurol.2013.488 [DOI] [PubMed] [Google Scholar]
- 32. Monroy‐Jaramillo N, Guerrero‐Camacho JL, Rodríguez‐Violante M, Boll‐Woehrlen MC, Yescas‐Gómez P, Alonso‐Vilatela ME, López‐López M. Genetic mutations in early‐onset Parkinson's disease Mexican patients: molecular testing implications. Am J Med Genet, Part B 2014;165b(3):235–244. 10.1002/ajmg.b.32228 [DOI] [PubMed] [Google Scholar]
- 33. Gasser T. Usefulness of genetic testing in PD and PD trials: a balanced review. J Parkinsons Dis. 2015;5(2):209–215. 10.3233/jpd-140507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewthwaite AJ, Lambert TD, Rolfe EB, et al. Novel GCH1 variant in Dopa‐responsive dystonia and Parkinson's disease. Parkinsonism Relat Disord 2015;21(4):394–397. 10.1016/j.parkreldis.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gorostidi A, Martí‐Massó JF, Bergareche A, Rodríguez‐Oroz MC, López de Munain A, Ruiz‐Martínez J. Genetic mutation analysis of Parkinson's disease patients using multigene next‐generation sequencing panels. Mol Diagn Ther. 2016;20(5):481–491. 10.1007/s40291-016-0216-1 [DOI] [PubMed] [Google Scholar]
- 36. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain. 2017;140(7):1959–1976. 10.1093/brain/awx118 [DOI] [PubMed] [Google Scholar]
- 37. Blauwendraat C, Faghri F, Pihlstrom L, et al. NeuroChip, an updated version of the NeuroX genotyping platform to rapidly screen for variants associated with neurological diseases. Neurobiol Aging. 2017;57:247.e9. 10.1016/j.neurobiolaging.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Landoulsi Z, Benromdhan S, Ben Djebara M, et al. Using KASP technique to screen LRRK2 G2019S mutation in a large Tunisian cohort. BMC Med Genet. 2017;18(1):70. 10.1186/s12881-017-0432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bentley SR, Bortnick S, Guella I, et al. Pipeline to gene discovery—analysing familial parkinsonism in the Queensland Parkinson's project. Parkinsonism Relat Disord. 2018;49:34–41. 10.1016/j.parkreldis.2017.12.033 [DOI] [PubMed] [Google Scholar]
- 40. Montaut S, Tranchant C, Drouot N, et al. Assessment of a targeted gene panel for identification of genes associated with movement disorders. JAMA Neurol. 2018;75(10):1234–1245. 10.1001/jamaneurol.2018.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Illés A, Csabán D, Grosz Z, et al. The role of genetic testing in the clinical practice and research of early‐onset parkinsonian disorders in a Hungarian cohort: increasing challenge in genetic counselling, improving chances in stratification for clinical trials. Front Genet. 2019;10:1061. 10.3389/fgene.2019.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Djarmati A, Guzvić M, Grünewald A, et al. Rapid and reliable detection of exon rearrangements in various movement disorders genes by multiplex ligation‐dependent probe amplification. Mov Disord. 2007;22(12):1708–1714. 10.1002/mds.21370 [DOI] [PubMed] [Google Scholar]
- 43. Douglas MR, Lewthwaite AJ, Nicholl DJ. Genetics of Parkinson's disease and parkinsonism. Expert Rev Neurother. 2007;7(6):657–666. 10.1586/14737175.7.6.657 [DOI] [PubMed] [Google Scholar]
- 44. Kay DM, Bird TD, Zabetian CP, et al. Validity and utility of a LRRK2 G2019S mutation test for the diagnosis of Parkinson's disease. Genet Test. 2006;10(3):221–227. 10.1089/gte.2006.10.221 [DOI] [PubMed] [Google Scholar]
- 45. Olgiati S, Quadri M, Bonifati V. Genetics of movement disorders in the next‐generation sequencing era. Mov Disord. 2016;31(4):458–470. 10.1002/mds.26521 [DOI] [PubMed] [Google Scholar]
- 46. Cook L, Schulze J, Verbrugge J, et al. The commercial genetic testing landscape for Parkinson's disease. Parkinsonism Relat Disord. 2021;92:107–111. 10.1016/j.parkreldis.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cook L, Schulze J, Kopil C, et al. Genetic testing for Parkinson disease: are we ready? Neurol Clin Pract. 2021;11(1):69–77. 10.1212/CPJ.0000000000000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parkinson Progression Marker I . The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95(4):629–635. 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Skrahina V, Gaber H, Vollstedt EJ, et al. The Rostock International Parkinson's disease (ROPAD) study: protocol and initial findings. Mov Disord. 2021;36(4):1005–1010. 10.1002/mds.28416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pont‐Sunyer C, Bressman S, Raymond D, Glickman A, Tolosa E, Saunders‐Pullman R. Disclosure of research results in genetic studies of Parkinson's disease caused by LRRK2 mutations. Mov Disord. 2015;30(7):904–908. 10.1002/mds.26250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. America HDSo . Genetic Testing Protocol for Huntington's Disease; 2016.
- 52. Alcalay RN, Caccappolo E, Mejia‐Santana H, et al. Frequency of known mutations in early‐onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67(9):1116–1122. 10.1001/archneurol.2010.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buhat DM, Tan EK. Genetic testing of LRRK2 in Parkinson's disease: is there a clinical role? Parkinsonism Relat Disord. 2014;20(Suppl 1):S54–S56. 10.1016/S1353-8020(13)70015-4 [DOI] [PubMed] [Google Scholar]
- 54. Hallquist MLG, Tricou EP, Ormond KE, et al. Application of a framework to guide genetic testing communication across clinical indications. Genome Med 2021;13(1):71. 10.1186/s13073-021-00887-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mulhern M, Bier L, Alcalay RN, Balwani M. Patients' opinions on genetic counseling on the increased risk of Parkinson disease among Gaucher disease carriers. J Genet Couns. 2018;27(3):675–680. 10.1007/s10897-017-0161-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zaretsky L, Zeid N, Naik H, Alcalay RN, Balwani M. Knowledge and attitudes of Parkinson's disease risk in the Gaucher population. J Genet Couns. 2020;29(1):105–111. 10.1002/jgc4.1185 [DOI] [PubMed] [Google Scholar]
- 57. Fraint A, Ouyang B, Metman LV, Jones C, Hall DA, Marder K, Pal G. Patient knowledge and attitudes towards genetic testing in Parkinson's disease subjects with deep brain stimulation. Parkinsons Dis. 2019;2019:3494609. 10.1155/2019/3494609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Giladi N, Mirelman A, Thaler A, Bar‐Shira A, Gurevich T, Orr‐Urtreger A. Fighting the risk of developing Parkinson's disease; clinical counseling for first degree relatives of patients with Parkinson's disease. J Neurol Sci. 2011;310(1–2):17–20. 10.1016/j.jns.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 59. Gupte M, Alcalay RN, Mejia‐Santana H, et al. Interest in genetic testing in Ashkenazi Jewish Parkinson's disease patients and their unaffected relatives. J Genet Couns. 2015;24(2):238–246. 10.1007/s10897-014-9756-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eno CC, Barton SK, Dorrani N, Cederbaum SD, Deignan JL, Grody WW. Confidential genetic testing and electronic health records: a survey of current practices among Huntington disease testing centers. Mol Genet Genomic Med. 2020;8(1):e1026. 10.1002/mgg3.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Migliore S, Jankovic J, Squitieri F. Genetic counseling in Huntington's disease: potential new challenges on horizon? Front Neurol. 2019;10:453. 10.3389/fneur.2019.00453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Crosiers D, Theuns J, Cras P, Van Broeckhoven C. Parkinson disease: insights in clinical, genetic and pathological features of monogenic disease subtypes. J Chem Neuroanat. 2011;42(2):131–141. 10.1016/j.jchemneu.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 63. Pal G, Mangone G, Hill EJ, et al. Parkinson disease and subthalamic nucleus deep brain stimulation: cognitive effects in GBA mutation carriers. Ann Neurol. 2022;91(3):424–435. 10.1002/ana.26302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Change N, Mercier G, Lucotte G. Genetic screening of the G2019S mutation of the LRRK2 gene in Southwest European, North African, and Sephardic Jewish subjects. Genet Test. 2008;12(3):333–339. 10.1089/gte.2007.0098 [DOI] [PubMed] [Google Scholar]
- 65. De Rosa A, De Michele G, Guacci A, et al. Genetic screening for the LRRK2 R1441C and G2019S mutations in Parkinsonian patients from Campania. J Parkinsons Dis. 2014;4(1):123–128. 10.3233/JPD-130312 [DOI] [PubMed] [Google Scholar]
- 66. Deng H, Le W, Huang M, Xie W, Pan T, Jankovic J. Genetic analysis of LRRK2 P755L variant in Caucasian patients with Parkinson's disease. Neurosci Lett. 2007;419(2):104–107. 10.1016/j.neulet.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 67. Funalot B, Nichols WC, Perez‐Tur J, Mercier G, Lucotte G. Genetic screening for two LRRK2 mutations in French patients with idiopathic Parkinson's disease. Genet Test. 2006;10(4):290–293. 10.1089/gte.2006.10.290 [DOI] [PubMed] [Google Scholar]
- 68. Crook A, Jacobs C, Newton‐John T, Richardson E, McEwen A. Patient and relative experiences and decision‐making about genetic testing and counseling for familial ALS and FTD: a systematic scoping review. Alzheimer Dis Assoc Disord 2021;35(4):374–385. 10.1097/wad.0000000000000458 [DOI] [PubMed] [Google Scholar]
- 69. Tomiyama H, Li Y, Yoshino H, Mizuno Y, Kubo S, Toda T, Hattori N. Mutation analysis for DJ‐1 in sporadic and familial parkinsonism: screening strategy in parkinsonism. Neurosci Lett. 2009;455(3):159–161. 10.1016/j.neulet.2009.03.033 [DOI] [PubMed] [Google Scholar]
- 70. Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2(1):a008888. 10.1101/cshperspect.a008888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Verbrugge J, Rumbaugh M, Cook L, et al. The promise and pitfalls of Facebook advertising: a genetic Counselor's perspective. J Genet Couns. 2018;27(2):326–328. 10.1007/s10897-017-0207-3 [DOI] [PubMed] [Google Scholar]
- 72. Schormair B, Kemlink D, Mollenhauer B, et al. Diagnostic exome sequencing in early‐onset Parkinson's disease confirms VPS13C as a rare cause of autosomal‐recessive Parkinson's disease. Clin Genet. 2018;93(3):603–612. 10.1111/cge.13124 [DOI] [PubMed] [Google Scholar]
- 73. Robertson AJ, Tan NB, Spurdle AB, Metke‐Jimenez A, Sullivan C, Waddell N. Re‐analysis of genomic data: An overview of the mechanisms and complexities of clinical adoption. Genet Med. 2022;24(4):798–810. 10.1016/j.gim.2021.12.011 [DOI] [PubMed] [Google Scholar]
- 74. Wu YR, Lin HY, Chen CM, et al. Genetic testing in spinocerebellar ataxia in Taiwan: expansions of trinucleotide repeats in SCA8 and SCA17 are associated with typical Parkinson's disease. Clin Genet. 2004;65(3):209–214. 10.1111/j.0009-9163.2004.00213.x [DOI] [PubMed] [Google Scholar]
- 75. Hamid E, Ayele BA, Massi DG, et al. Availability of therapies and Services for Parkinson's disease in Africa: a continent‐wide survey. Mov Disord 2021;36(10):2393–2407. 10.1002/mds.28669 [DOI] [PubMed] [Google Scholar]
- 76. Domingo A, Klein C. Genetics of Parkinson disease. Handb Clin Neurol. 2018;147:211–227. 10.1016/B978-0-444-63233-3.00014-2 [DOI] [PubMed] [Google Scholar]
- 77. Lubbe SJ, Escott‐Price V, Gibbs JR, et al. Additional rare variant analysis in Parkinson's disease cases with and without known pathogenic mutations: evidence for oligogenic inheritance. Hum Mol Genet. 2016;25(24):5483–5489. 10.1093/hmg/ddw348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kumar KR, Djarmati‐Westenberger A, Grünewald A. Genetics of Parkinson's disease. Semin Neurol. 2011;31(5):433–440. 10.1055/s-0031-1299782 [DOI] [PubMed] [Google Scholar]
- 79. Gatto EM, Walker RH, Gonzalez C, et al. Worldwide barriers to genetic testing for movement disorders. Eur J Neurol. 2021;28(6):1901–1909. 10.1111/ene.14826 [DOI] [PubMed] [Google Scholar]
- 80. Check Hayden E. The rise and fall and rise again of 23andMe. Nature 2017;550(7675):174–177. 10.1038/550174a [DOI] [PubMed] [Google Scholar]
- 81. Monfrini E, Di Fonzo A. Leucine‐rich repeat kinase (LRRK2) genetics and Parkinson's disease. Adv Neurobiol. 2017;14:3–30. 10.1007/978-3-319-49969-7_1 [DOI] [PubMed] [Google Scholar]
- 82. Mullin S, Schapira A. The genetics of Parkinson's disease. Br Med Bull. 2015;114(1):39–52. 10.1093/bmb/ldv022 [DOI] [PubMed] [Google Scholar]
- 83. Scarciolla O, Brancati F, Valente EM, et al. Multiplex ligation‐dependent probe amplification assay for simultaneous detection of Parkinson's disease gene rearrangements. Mov Disord. 2007;22(15):2274–2278. 10.1002/mds.21532 [DOI] [PubMed] [Google Scholar]
- 84. Schroeder C, Walter M, Berg D, et al. High‐throughput homogeneous mass cleave assay technology for the diagnosis of autosomal recessive Parkinson's disease. J Mol Diagn. 2008;10(3):217–224. 10.2353/jmoldx.2008.070100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sterckx S, Cockbain J, Howard H, Huys I, Borry P. “Trust is not something you can reclaim easily”: patenting in the field of direct‐to‐consumer genetic testing. Genet Med 2013;15(5):382–387. 10.1038/gim.2012.143 [DOI] [PubMed] [Google Scholar]
- 86. Sun ZF, Zhang YH, Guo JF, et al. Genetic diagnosis of two dopa‐responsive dystonia families by exome sequencing. PLoS One. 2014;9(9):e106388. 10.1371/journal.pone.0106388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dobričić V, Tomić A, Branković V, et al. GCH1 mutations are common in Serbian patients with dystonia‐parkinsonism: challenging previously reported prevalence rates of DOPA‐responsive dystonia. Parkinsonism Relat Disord. 2017;45:81–84. 10.1016/j.parkreldis.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 88. Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2‐associated Parkinson's disease: a case‐control study. Lancet Neurol. 2008;7(7):583–590. 10.1016/S1474-4422(08)70117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Klein C, Hattori N, Marras C. MDSGene: closing data gaps in genotype‐phenotype correlations of monogenic Parkinson's disease. J Parkinsons Dis. 2018;8(s1):S25–S30. 10.3233/JPD-181505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klein C. Implications of genetics on the diagnosis and care of patients with Parkinson disease. Arch Neurol. 2006;63(3):328–334. 10.1001/archneur.63.3.328 [DOI] [PubMed] [Google Scholar]
- 91. Klein C, Djarmati A. Parkinson disease: genetic testing in Parkinson disease‐who should be assessed? Nat Rev Neurol. 2011;7(1):7–9. 10.1038/nrneurol.2010.197 [DOI] [PubMed] [Google Scholar]
- 92. Wider C, Foroud T, Wszolek ZK. Clinical implications of gene discovery in Parkinson's disease and parkinsonism. Mov Disord. 2010;25(S1):S15–S20. 10.1002/mds.22723 [DOI] [PubMed] [Google Scholar]
- 93. Williams ES, Barrett MJ, Dhamija R, Toran L, Chambers C, Mahadevan MS, Golden WL. Phase determination using chromosomal microarray and fluorescence in situ hybridization in a patient with early onset Parkinson disease and two deletions in PRKN. Mol Genet Genomic Med. 2018;6(3):457–462. 10.1002/mgg3.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Belarbi S, Hecham N, Lesage S, et al. LRRK2 G2019S mutation in Parkinson's disease: a neuropsychological and neuropsychiatric study in a large Algerian cohort. Parkinsonism Relat Disord. 2010;16(10):676–679. 10.1016/j.parkreldis.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 95. Cao L, Zhang T, Zheng L, et al. The GIGYF2 variants are not associated with Parkinson's disease in the mainland Chinese population. Parkinsonism Relat Disord. 2010;16(4):294–297. 10.1016/j.parkreldis.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 96. De Rosa A, Criscuolo C, Mancini P, et al. Genetic screening for LRRK2 gene G2019S mutation in Parkinson's disease patients from southern Italy. Parkinsonism Relat Disord. 2009;15(3):242–244. 10.1016/j.parkreldis.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 97. Fahmy E, Rabah A, Sharaf S, Helmy H, Kamal A. Interleukin‐18 promoter polymorphisms and idiopathic Parkinson disease: an Egyptian study. Acta Neurol Belg. 2019;119(2):219–224. 10.1007/s13760-018-0927-7 [DOI] [PubMed] [Google Scholar]
- 98. Furtado S, Farrer M, Tsuboi Y, et al. SCA‐2 presenting as parkinsonism in an Alberta family: clinical, genetic, and PET findings. Neurology. 2002;59(10):1625–1627. 10.1212/01.wnl.0000035625.19871.dc [DOI] [PubMed] [Google Scholar]
- 99. Grimes DA, Racacho L, Han F, Panisset M, Bulman DE. LRRK2 screening in a Canadian Parkinson's disease cohort. Can J Neurol Sci. 2007;34(3):336–338. 10.1017/s0317167100006788 [DOI] [PubMed] [Google Scholar]
- 100. Hedrich K, Hagenah J, Djarmati A, et al. Clinical spectrum of homozygous and heterozygous PINK1 mutations in a large German family with Parkinson disease: role of a single hit? Arch Neurol. 2006;63(6):833–838. 10.1001/archneur.63.6.833 [DOI] [PubMed] [Google Scholar]
- 101. Inzelberg R, Hassin‐Baer S, Jankovic J. Genetic movement disorders in patients of Jewish ancestry. JAMA Neurol. 2014;71(12):1567–1572. 10.1001/jamaneurol.2014.1364 [DOI] [PubMed] [Google Scholar]
- 102. Jankovic MZ, Kresojevic ND, Dobricic VS, Markovic VV, Petrovic IN, Novakovic IV, Kostic VS. Identification of novel variants in LRRK2 gene in patients with Parkinson's disease in Serbian population. J Neurol Sci. 2015;353(1–2):59–62. 10.1016/j.jns.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 103. Jankovic MZ, Dobricic V, Kresojevic N, et al. Identification of mutations in the PARK2 gene in Serbian patients with Parkinson's disease. J Neurol Sci. 2018;393:27–30. 10.1016/j.jns.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 104. Kessler C, Atasu B, Hanagasi H, et al. Role of LRRK2 and SNCA in autosomal dominant Parkinson's disease in Turkey. Parkinsonism Relat Disord. 2018;48:34–39. 10.1016/j.parkreldis.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 105. Mo X, Liu D, Li W, et al. Genetic screening for mutations in the Nrdp1 gene in Parkinson disease patients in a Chinese population. Parkinsonism Relat Disord. 2010;16(3):222–224. 10.1016/j.parkreldis.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 106. Papapetropoulos S, Adi N, Shehadeh L, Bishopric N, Singer C, Argyriou AA, Chroni E. Is the G2019S LRRK2 mutation common in all southern European populations? J Clin Neurosci. 2008;15(9):1027–1030. 10.1016/j.jocn.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 107. Peeraully T, Tan EK. Genetic variants in sporadic Parkinson's disease: east vs west. Parkinsonism Relat Disord 2012;18:S63–S65. 10.1016/s1353-8020(11)70021-9 [DOI] [PubMed] [Google Scholar]
- 108. Peng F, Sun YM, Chen C, et al. The heterozygous R1441C mutation of leucine‐rich repeat kinase 2 gene in a Chinese patient with Parkinson disease: a five‐year follow‐up and literatures review. J Neurol Sci. 2017;373:23–26. 10.1016/j.jns.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 109. Ruiz‐Martinez J, Gorostidi A, Ibanez B, et al. Penetrance in Parkinson's disease related to the LRRK2 R1441G mutation in the Basque country (Spain). Mov Disord. 2010;25(14):2340–2345. 10.1002/mds.23278 [DOI] [PubMed] [Google Scholar]
- 110. Shi C, Li F, Yang J, et al. DNAJC6 mutations are not common causes of early onset Parkinson's disease in Chinese Han population. Neurosci Lett. 2016;634:60–62. 10.1016/j.neulet.2016.09.044 [DOI] [PubMed] [Google Scholar]
- 111. Squillaro T, Cambi F, Ciacci G, et al. Frequency of the LRRK2 G2019S mutation in Italian patients affected by Parkinson's disease. J Hum Genet. 2007;52(3):201–204. 10.1007/s10038-006-0105-2 [DOI] [PubMed] [Google Scholar]
- 112. Tan EK, Shen H, Tan LC, et al. The G2019S LRRK2 mutation is uncommon in an Asian cohort of Parkinson's disease patients. Neurosci Lett. 2005;384(3):327–329. 10.1016/j.neulet.2005.04.103 [DOI] [PubMed] [Google Scholar]
- 113. Vijayan B, Gopala S, Kishore A. LRRK2 G2019S mutation does not contribute to Parkinson's disease in South India. Neurol India 2011;59(2):157–160. 10.4103/0028-3886.79125 [DOI] [PubMed] [Google Scholar]
- 114. Vinish M, Prabhakar S, Khullar M, Verma I, Anand A. Genetic screening reveals high frequency of PARK2 mutations and reduced Parkin expression conferring risk for parkinsonism in north West India. J Neurol Neurosurg Psychiatry 2010;81(2):166–170. 10.1136/jnnp.2008.157255 [DOI] [PubMed] [Google Scholar]
- 115. Wang Y, Wu JJ, Liu FT, et al. Olfaction in Parkin carriers in Chinese patients with Parkinson disease. Brain Behav. 2017;7(5):e00680. 10.1002/brb3.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Williams‐Gray CH, Goris A, Foltynie T, et al. Prevalence of the LRRK2 G2019S mutation in a UK community based idiopathic Parkinson's disease cohort. J Neurol Neurosurg Psychiatry. 2006;77(5):665–667. 10.1136/jnnp.2005.085019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Williams‐Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA. The CamPaIGN study of Parkinson's disease: 10‐year outlook in an incident population‐based cohort. J Neurol Neurosurg Psychiatry 2013;84(11):1258–1264. 10.1136/jnnp-2013-305277 [DOI] [PubMed] [Google Scholar]
- 118. Zhang L, Quadri M, Guedes LC, et al. Comprehensive LRRK2 and GBA screening in Portuguese patients with Parkinson's disease: identification of a new family with the LRRK2 p.Arg1441His mutation and novel missense variants. Parkinsonism Relat Disord. 2013;19(10):897–900. 10.1016/j.parkreldis.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 119. Zheng Y, Pei Z, Liu Y, et al. Cognitive impairments in LRRK2‐related Parkinson's disease: a study in Chinese individuals. Behav Neurol. 2015;2015:621873. 10.1155/2015/621873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bates M. Direct‐to‐consumer genetic testing: is the public ready for simple, At‐home DNA tests to detect disease risk? IEEE Pulse. 2018;9(6):11–14. 10.1109/mpul.2018.2869315 [DOI] [PubMed] [Google Scholar]
- 121. Bordelon YM, Smith M. Movement disorders in pregnancy. Semin Neurol. 2007;27(5):467–475. 10.1055/s-2007-991128 [DOI] [PubMed] [Google Scholar]
- 122. Steinbart EJ, Smith CO, Poorkaj P, Bird TD. Impact of DNA testing for early‐onset familial Alzheimer disease and frontotemporal dementia. Arch Neurol. 2001;58(11):1828–1831. 10.1001/archneur.58.11.1828 [DOI] [PubMed] [Google Scholar]
- 123. Global Parkinson's Genetics Program . GP2: the global Parkinson's genetics program. Mov Disord. 2021;36(4):842–851. 10.1002/mds.28494 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Methods and search strategy.
Appendix S2. Included articles and summaries.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


