Abstract
In the immunocompetent host, primary cytomegalovirus (CMV) infection is resolved by the immune response without causing overt disease. The viral genome, however, is not cleared but is maintained in a latent state that entails a risk of virus recurrence and consequent organ disease. By using murine CMV as a model, we have shown previously that multiple organs harbor latent CMV and that reactivation occurs with an incidence that is determined by the viral DNA load in the respective organ (M. J. Reddehase, M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U. H. Koszinowski. J. Exp. Med. 179:185–193, 1994). This predicts that a therapeutic intervention capable of limiting the load of latent viral genome should also reduce the risk of virus recurrence. Here we demonstrate the benefits and the limits of a preemptive CD8 T-cell immunotherapy of CMV infection in the immunocompromised bone marrow transplantation recipient. Antiviral CD8 T cells prevented CMV disease and accelerated the resolution of productive infection. The therapy also resulted in a lower load of latent CMV DNA in organs and consequently reduced the incidence of recurrence. The data thus provide a further supporting argument for clinical trials of preemptive cytoimmunotherapy of human CMV disease with CD8 T cells. However, CD8 T cells failed to clear the viral DNA. The therapy-susceptible portion of the DNA load differed between organs and was highest in the lungs. The existence of an invariant, therapy-resistant load suggests a role for immune system evasion mechanisms in the establishment of CMV latency.
Recurrence of productive infection by reactivation of latent viral genome in the immunocompromised host is a feature common to the members of the herpesvirus family (39; reviewed in reference 38). Specifically, in the case of human cytomegalovirus (CMV), the human herpesvirus type 5, primary as well as recurrent infection during the temporal immunodeficiency early after bone marrow (BM) transplantation (BMT) entails a risk of graft failure and severe organ manifestations of CMV disease (8, 44). Early findings by Quinnan et al. (24) have suggested a correlation between efficient reconstitution of the cellular immune response and the control of post-BMT CMV infection, and more recent clinical data have attributed this control to the reconstituted CD8 T cells (35). Accordingly, restoration of antiviral immunity in the critical phase before the reconstitution by BMT becomes effective should diminish the risk of CMV disease. Experimental research with the model of murine CMV infection has positively demonstrated the antiviral and protective efficacy of adoptively transferred acutely sensitized (31, 34) or memory (28) CD8 T cells recovered from immune donors as well as of short-term CD8 T-cell lines propagated in culture (32). These studies have been pivotal for clinical trials of a preemptive CD8 T-cell immunotherapy of post-BMT human CMV infection in patients (37, 43).
Infection of the BMT recipient can accidentally result from the transmission of infectious virus, however, productive infection is more commonly initiated by reactivation of latent CMV in either the transplant or the recipient’s own organs or, occasionally, both (11). For the murine model system, we have previously demonstrated the existence of multiple organ sites of CMV latency at which the latent viral DNA is retained after the resolution of productive primary infection and after clearance of the viral genome from hematopoietic leukocytic cells in BM and blood (27). In accordance with the wide distribution of the latent viral DNA, recurrence was found to occur focally in any of the organs, which led us to propose the concept of multifocal CMV latency and recurrence (27). Most importantly, the incidence of recurrence was found to correlate with the load of latent viral DNA in the respective tissue. Specifically, low virus dissemination and rapid control of infection in immunocompetent adult mice resulted in a low load and was associated with a low risk of recurrence, whereas the delayed control of infection in neonatal mice resulted in a high load and was associated with a high risk. Furthermore, there were also organ-specific differences. In accordance with the high incidence of interstitial CMV pneumonia after BMT, the lungs were identified as having a high load of latent CMV (2, 17).
It is apparent that antiviral CD8 T cells generated during primary infection as well as memory cells present during latency do not eradicate latently infected cells under physiological conditions, since latency would not exist if they did. However, it has been open to question whether adoptive transfer of antiviral CD8 effector cells could prevent the escape of virus into latency. We will show here that modulation of primary infection by experimental CD8 T-cell immunotherapy has indeed had an effect on the load of latent viral DNA in tissues. The effect of the therapy is of relevance, since the load of latent viral DNA can be kept below the threshold required for effective recurrence. Our data thus provide a further supporting argument for clinical trials of cytoimmunotherapy. Interestingly, however, the data also predict that no dosage of CD8 T cells will prevent the establishment of latency.
MATERIALS AND METHODS
BMT and concurrent CMV infection.
BMT was performed as a syngeneic BMT with female BALB/c (H-2d) mice used at the age of 8 weeks as BM donors and recipients. Hematoablative conditioning of the recipients was performed by total-body γ-irradiation with a single dose of 6 Gy from a 137Cs source (OB58; Buchler, Braunschweig, Germany). This irradiation is equivalent to a 50% lethal dose determined at day 30. Donor femoral and tibial BM cells (BMC) were obtained as described previously (22), and the indicated doses of BMC were injected intravenously (i.v.) into the tail vein of the recipients at ca. 6 h after the irradiation. Murine BM is practically devoid of mature T cells, and elimination of CD8 T cells by immunomagnetic sorting proved to have no influence on the reported data. Infection with 105 PFU of purified murine CMV Smith ATCC VR-194, purchased in 1981, was performed subcutaneously in the left hind footpad at ca. 2 h after BMT. According to a recent reevaluation of viral infectivity assays, a dose of 105 PFU of purified murine CMV is equivalent to 5 × 107 viral genomes and 1 × 107 infectious particles as measured by a reverse transcriptase (RT)-PCR-based focus expansion assay (17).
Generation of polyclonal antiviral CD8 T cells for immunotherapy.
The generation of a polyclonal short-term T-cell line, its in vivo antiviral activity, its in vitro cytolytic-T-lymphocyte (CTL) activity, and the molecular antiviral CTL specificities have been the subject of previous reports (29, 30, 32; reviewed in reference 16). In brief, immunocompetent BALB/c mice were sensitized by s.c. infection in the left hind footpad and lymphocytes were recovered on day 8 from the draining popliteal lymph node (LN) and were propagated in culture at a density of 5 × 104 cells per 0.2-ml round-bottom microtiter plate well in the presence of 10 U of recombinant interleukin-2 per culture. Importantly, to avoid antigen-specific selection in vitro, the primed LN lymphocytes were not restimulated with feeder cells and antigen. After 7 days of cultivation, CD4 T cells and, alternatively, CD8 T cells were eliminated by two rounds of treatment with anti-CD4 monoclonal antibody (MAb), clone GK-1.5, and complement and with anti-CD8 MAb, clone 19/178, and complement, respectively (32). The purity of the T-cell subsets was monitored by three-color cytofluorometric analysis of the expression of T-cell receptor α/β (clone H57-597; Pharmingen, San Diego, Calif.), CD4 (clone H129.19; GIBCO BRL, Eggenstein, Germany), and CD8 (clone 53-6.7; Becton Dickinson, San Jose, Calif.). Measurements were performed with a FACSort with CellQuest software (Becton Dickinson) for data processing. For preemptive immunotherapy, the indicated numbers of >98% pure T cells of either subset were injected i.v. into the tail vein of the BMT recipients simultaneously with the BMC.
In vivo depletion of T-cell subsets.
On days 7 and 14 after BMT, the reconstituting immune system was depleted of CD4 T cells or, alternatively, of CD8 T cells by i.v. injection of 1 mg of purified MAb anti-CD4, clone YTS 191.1, or anti-CD8, clone YTS 169.4, respectively (4).
Assays of viral infectivity and verification of latent infection. (i) Acute infection.
Virus titers in organs after BMT and infection were measured from organ homogenates by a plaque assay on permissive mouse embryo fibroblasts (MEF) by the previously described technique of centrifugal enhancement of infectivity (17). Titers are expressed as PFU* to indicate the enhancement and are given as PFU* per organ. Usually, a log10 titer determination started with a 1/100 aliquot of an organ homogenate, which defines the detection limit as 100 PFU* per organ.
(ii) Latent infection.
Criteria for the definition of CMV latency in organs were discussed in detail recently (2, 17). Absence of infectivity, e.g., in the lungs, was verified by testing the homogenate of the left lung and the postcaval lobe in total by the RT-PCR-based focus expansion assay, an assay shown to detect infectivity in 5 genomes of purified murine CMV (17). This defines the detection limit as ca. 0.01 PFU or as ca. 0.2 PFU*. In addition to a negative result in this assay, chronic viral productivity at unknown remote sites was excluded by the absence of viral DNA from the blood. Mice were bled from the tail vein every second month and were considered latently infected only after clearance of viral DNA from the blood, as judged by a PCR specific for exon 4 of the viral ie1 gene (2, 17).
(iii) Recurrent infection.
Recurrence was induced in latently infected mice by γ-irradiation with a single dose of 6.5 Gy. Viral infectivity was measured on day 14 in organ homogenates by the RT-PCR-based focus expansion assay (17). In brief, mouse organs or, specifically, the five lobes of the perfused lungs were homogenized separately, and aliquots equaling 1/18 of the whole lungs were used to infect MEF under the influence of a 1,000 × g centrifugal force. After 72 h of focus formation in the indicator cultures, poly(A)+ RNA was isolated and a 100-ng aliquot was subjected to an RT-PCR specific for exon 3/4 of the ie1 gene. Amplification products (a 15-μl aliquot thereof) were analyzed by agarose gel electrophoresis (2% [wt/vol] agarose), Southern blotting, and hybridization with a γ-32P-end-labeled oligonucleotide probe directed against the splice junction. RT-PCR specific for hypoxanthine phosphoribosyltransferase (HPRT) transcripts was used as a control for RNA purification efficacy (17).
Quantitation of latent viral DNA in organs.
Pieces of tissue (specifically, the superior lobe, the middle lobe, and the inferior lobe of the perfused lungs) were dissected aseptically and transferred immediately to liquid nitrogen for storage. Total DNA was isolated by standard procedures of proteinase K digestion, phenol-chloroform-isoamyl alcohol extraction, and precipitation with ethanol. To reduce the viscosity, DNA was sonicated with a cup ultrasonicator (Branson Ultrasonics, Danbury, Conn.). The total amount of DNA was measured by determining the optical density at 260 nm. The number of viral copies was quantitated by performing serial dilutions of DNA, followed by a PCR specific for a 363-bp sequence within exon 4 of the immediate-early (IE) gene ie1 of murine CMV (14) as described previously (2) or by a PCR specific for a 510-bp sequence within the continuous open reading frame of the gB gene (25, 26). In the gB-PCR, oligonucleotides 5′-84250–84269 and 5′-84759–84740 served as the forward and reverse primers, respectively, and oligonucleotide 5′-84450–84469 served as the probe (26; GenBank accession no. MCU68299 [complete genome]). Plasmids pIE111 (21) or genomic murine CMV DNA derived from purified virions (17) were supplemented with organ DNA from uninfected mice and were titrated as standards. PCRs were performed in an MWG Biotech OmniGene thermocycler (Hybaid Ltd., Teddington, England) in either 0.5-ml safe lock reaction tubes (Eppendorf, Hamburg, Germany) or conically welled polycarbonate 96-well (0.17 ml) microplates (Omniplate 96; Hybaid Ltd.). The reactions were performed in a volume of 50 μl containing 15 mM Tris-HCl (pH 8.4), 60 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 20% (vol/vol) glycerol, 1 mM each deoxynucleoside triphosphate, 25 pmol of each primer, and 1.5 U of Taq DNA polymerase (Eurobio, Raunheim, Germany). Amplification was performed in 30 cycles with denaturation at 95°C for 120 s in the first cycle and at 96°C for 30 s in the remaining cycles, primer annealing at 58°C for 60 s, and primer extension at 72°C for 60 s that was extended to 300 s in the last cycle. For monitoring the comparable DNA content of test samples, a 702-bp fragment of the single copy gene encoding tumor necrosis factor alpha TNF-α (40, 42) was amplified by PCR with oligonucleotides 5′-n5859–5880 and 5′-n6560–6540 as forward and reverse primers, respectively and with oligonucleotide 5′-n6159–6183 serving as the probe (GenBank accession no. M38296). PCR buffers and conditions were as described above, except that the primer-annealing temperature was 55°C. For the tube system, amplification products (20-μl aliquots) were analyzed by agarose gel electrophoresis (1.2% [wt/vol] agarose) and ethidium bromide staining followed by Southern blotting. Specific signals were visualized by autoradiography after hybridization with the respective γ-32P-end-labeled internal oligonucleotide probe. For the microplate system, amplification products (20 μl) were vacuum dot blotted onto nylon membrane by using the Minifold dot blot manifold device (Schleicher & Schuell, Keene, N.H.) and hybridized accordingly. The radioactivity per dot was measured with a digital phosphorimaging system (Fujifilm bioimaging system BAS 2500; Fuji, Tokyo, Japan) and is expressed as phosphostimulated luminescence units. Data analysis was performed with Tina 2.10 software (Raytest, Straubenhardt, Germany).
RESULTS
Endogenously reconstituted and exogenously restored antiviral CD8 T cells both protect against lethal CMV disease after BMT.
After hematoablative treatment of 8-week-old BALB/c mice with 6 Gy of γ-irradiation, murine CMV infection prevents endogenous BM reconstitution and causes lethal multiorgan disease combined with BM aplasia, referred to as CMV aplastic anemia (19, 22) (Fig. 1, top). Reconstitution of the BM by syngeneic BMT with 105 BMC is not sufficient to prevent lethal CMV disease but results in a slight delay of mortality (Fig. 1, top), which is due to an immediate but only temporary cessation of granulocytopenia and thrombocytopenia (not shown). Under these conditions, restoration of immunity by adoptive transfer of antiviral CD8 effector T cells is protective whereas adoptive transfer of sensitized CD4 T cells has no effect on the course of disease (Fig. 1, top). The antigen specificity of the antiviral CD8 T cells had been the subject of previous reports (for a review, see reference 16). Since the CD8 T cells were derived from primed lymph node cells without in vitro restimulation by viral antigens, they are thought to closely represent the authentic specificity repertoire. The polyclonal population comprises CTL clones specific for infected cells in all stages of the viral replicative cycle (29), with a high proportion of IE-phase-specific CTL (30).
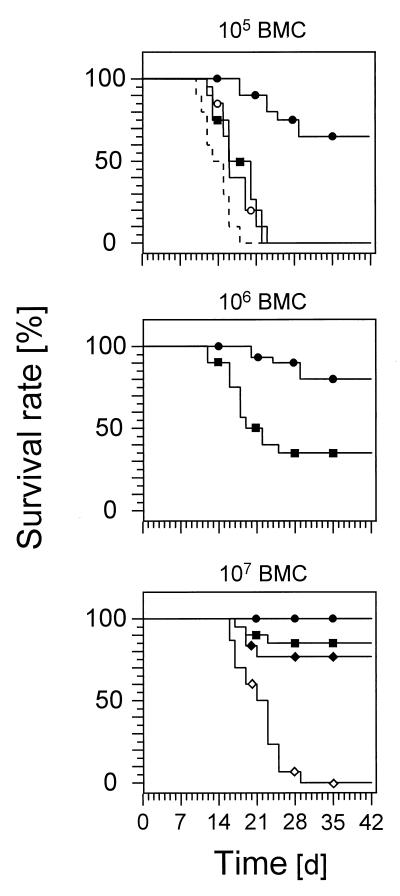
FIG. 1.
CD8 T cells are essential for the control of CMV infection after BMT. Shown are Kaplan-Meier survival plots for groups of 20 mice, giving the survival rates (ordinate) as a function of time (abscissa) after syngeneic BMT, performed with the indicated doses of BMC, followed by intraplantar infection with murine CMV. Note that in the absence of infection, survival rates after hematoablative γ-ray treatment with 6 Gy ranged from 40 to 60% in groups without BMT and were 100% after reconstitution with any of the indicated doses of BMC (not shown). The dashed line indicates the control group given hematoablative γ-ray treatment with 6 Gy followed by infection but no BMT. In all other experimental groups, the recipients received BMC and were infected. The presence and absence of CD8 T cells are indicated by solid and open symbols, respectively. Solid squares indicate groups of recipients reconstituted by BMT with the indicated doses of BMC. Solid circles indicate immunotherapy by adoptive transfer of 106 CD8 T cells. Open circles indicate adoptive transfer of 106 CD4 T cells. Solid diamonds indicate in vivo depletion of CD4 T cells. Open diamonds indicate in vivo depletion of CD8 T cells.
Interestingly, upon increasing the number of BMC in the BMT, survival times and the final survival rates improved, and, specifically, after reconstitution with 107 BMC, an additional adoptive transfer of CD8 T cells appeared to be unnecessary, as judged just by the criterion of survival (Fig. 1, middle and bottom). This raised the question whether protection by CD8 T-cell immunotherapy and protection by high-dose BMT operated by the same mechanism. By cytofluorometric analysis of the reconstituting immune system, it was found that T cells reappear in the recipients in week 3 after BMT but remain absent in thymectomized, uninfected BMT recipients (data not shown). This indicated that all the T cells were derived from hematolymphopoietic reconstitution. The protective effect of high-dose BMT was completely abrogated by selective in vivo depletion of the reconstituting CD8 T cells, whereas the selective in vivo depletion of reconstituting CD4 T cells had no effect on the survival rate (Fig. 1, bottom). In conclusion, the reconstitution of CD8 T cells is decisive for the control of CMV infection after BMT, and a preemptive CD8 T-cell immunotherapy can substitute for inefficient endogenous reconstitution.
CD8 T-cell immunotherapy accelerates the resolution of acute primary CMV infection.
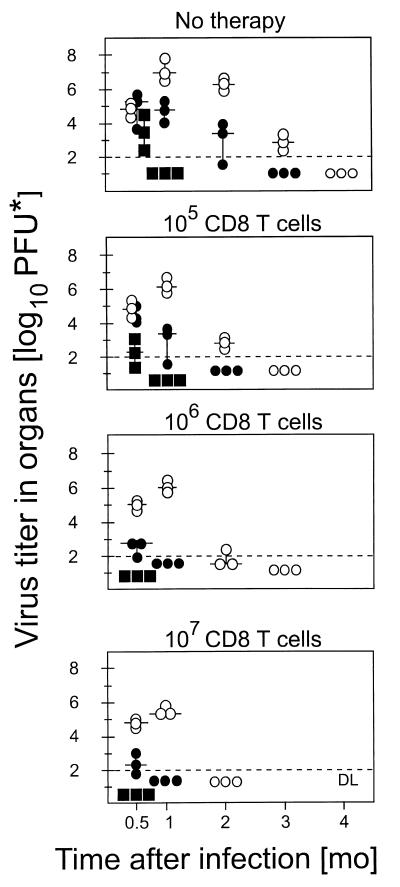
After BMT performed with 107 BMC, a beneficial effect of preemptive CD8 T-cell immunotherapy was no longer apparent with regard to survival (Fig. 1, bottom). More detailed information on the course of CMV infection in the surviving recipients is provided by measuring the extent of virus replication in organs (Fig. 2). Endogenous reconstitution of CD8 T cells requires up to 4 months to resolve virus replication in the major target organs affected by florid CMV infection, with a typical succession of rapid clearance of productive infection in the adrenal glands, delayed clearance in the lungs, and slow clearance in the salivary glands, which represent the preferred site of chronic CMV replication (Fig. 2, top). Therapy with increasing doses of CD8 T cells proved to be beneficial in that it significantly reduced the extent and duration of virus replication in vital organs, such as the lungs and the adrenal glands. The phase of chronic, asymptomatic CMV replication in the salivary glands was shortened by 2 months, which is of relevance with regard to the risk of virus transmission after BMT. In conclusion, preemptive CD8 T-cell immunotherapy modulates the course of primary infection by increasing the efficacy of antiviral control in quantitative and in kinetic terms.
FIG. 2.
CD8 T-cell immunotherapy modulates the course of primary CMV infection. Throughout, BMT was performed with 107 BMC and was followed by intraplantar infection (corresponding to Fig. 1, bottom). The group that did not receive immunotherapy served as a reference showing the normal course of infection during reconstitution after BMT (top). In the remaining three groups, immunotherapy was performed by adoptive transfer of the indicated doses of CD8 T cells. Virus titers in organs (ordinate) were monitored as a function of time after BMT and infection (abscissa). The dashed line gives the detection limit of the infectivity assay, which was 100 PFU* per organ. Individual titers are depicted for three mice per time point. The median value is marked by a short horizontal bar. Cases in which virus titers were uniformly negative for an organ are depicted only on the first occasion, but the titers then remained negative throughout the kinetics. Symbols: open circles, salivary glands; solid circles, lungs; solid squares, adrenal glands.
Verification of the establishment of CMV latency after resolution of acute infection.
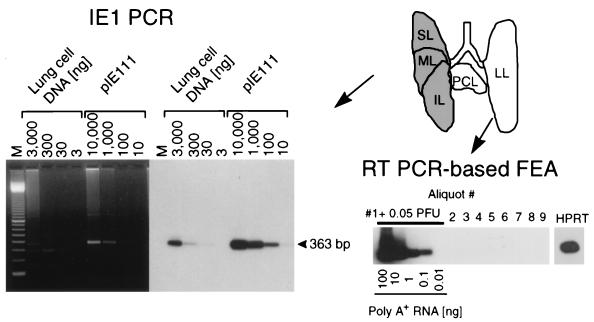
Latent infection implies that the viral genome is retained while infectious virus is absent. The discrimination between molecular latency and persistent infection below the detection limit of infectivity assays has been a long-debated problem. The recently described RT-PCR-based focus expansion assay (17) can detect infectivity with the utmost possible sensitivity. This assay was therefore used to verify the absence of infectivity in organs. It is important to note that at the time of analysis 12 months after BMT and infection, viral DNA was absent from blood. This finding excludes hematogenous dissemination of virus from an unknown remote site of persistent productive infection (detailed in reference 17; not shown here). Infectious virus was then no longer detectable in any organ tested, including the salivary glands, the spleen, and the adrenal glands (data not shown). Since the lungs represent a major organ site of CMV latency and recurrence (2, 17, 27), we focus here on the lungs for documenting the data for the group that did not receive immunotherapy (Fig. 3). For each individual mouse included in the latency analysis, the left lung and the postcaval lobe were used for the infectivity assay and the remaining three lobes were used to verify the presence of latent viral DNA. The total homogenate of the left lung and the postcaval lobe was distributed to nine indicator cultures for the RT-PCR-based focus expansion assay. Aliquot 1 was supplemented with 0.05 PFU of purified murine CMV as a positive control. This low dose of infectious virus was clearly detected by the assay, whereas aliquots 2 through 9 were negative (Fig. 3, lower right). Total DNA was isolated from the superior, middle, and inferior lobes, and a 363-bp sequence within exon 4 of the murine CMV ie1 gene was amplified by PCR. The lung cell DNA and the IE1 plasmid pIE111 that contains gene ie1 (14, 21) were titrated to titer determination in parallel (Fig. 3, left). The specific internal probe hybridized exclusively to an amplification product of the correct size. The assay detected 10 copies of the control plasmid and revealed the presence of the sequence within a lower limit of 30 ng of the lung cell DNA. In conclusion, viral DNA was present in the lungs in the absence of infectivity.
FIG. 3.
Verification of CMV latency in the lungs. The analysis is shown for the group with no CD8 T-cell therapy. (Top right) Scheme of the lobular anatomy of the lungs in ventral view is depicted at the upper right. For each individual mouse included in a latency analysis performed 12 months after infection, the left lung (LL) and postcaval lobe (PCL) were used to verify the absence of infectious virus by the RT-PCR-based focus expansion assay (FEA) whereas the superior lobe (SL), middle lobe (ML), and inferior lobe (IL) were used to detect the presence of latent viral DNA. (Bottom right) Nine 2-ml aliquots of the homogenate of the LL and PCL were tested for the presence of infectivity in cultures of permissive cells by the RT-PCR-based focus expansion assay. As a positive control, aliquot 1 was supplemented with 0.05 PFU of purified murine CMV. Poly(A)+ RNA derived from this culture was serially diluted as indicated, whereas for each of the remaining cultures, 100 ng of poly(A)+ RNA was subjected to ie1 exon 3/4-specific RT-PCR, leading to an amplification product of 188 bp. The autoradiograph was obtained after hybridization with a γ-32P-end-labeled oligonucleotide probe directed against the splice junction. For culture 9, the presence of RNA was verified by an RT-PCR specific for the HPRT housekeeping gene transcript. (Left) DNA isolated from the SL, ML, and IL was subjected to an ie1 exon 4-specific PCR. Plasmid pIE111 was added to pulmonary DNA from uninfected BMT recipients (10,000 copies in 3 μg) and was titrated in parallel. Amplification products were analyzed by gel electrophoresis. Lane M contains the 100-bp size marker kit. An ethidium bromide-stained gel is shown on the left, and the corresponding Southern blot autoradiograph obtained after hybridization with a γ-32P-end-labeled internal oligonucleotide probe is shown on the right.
Preemptive CD8 T-cell immunotherapy limits the load of latent viral DNA in the lungs.
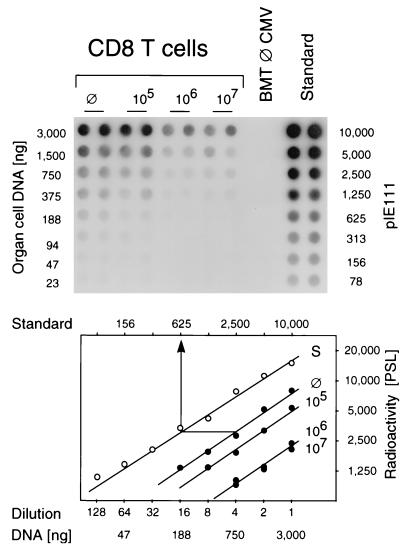
We have shown in a previous report that the extent of virus dissemination in the acute phase of infection determines the load of latent viral genome in organs (27). Accordingly, the modulation of the acute infection by CD8 T cells (Fig. 2) should affect the viral DNA load measured many months later. We therefore quantitated the viral DNA in latently infected lungs 12 months after BMT, infection, and immunotherapy, by PCR specific for exon 4 of the ie1 gene. Since the radioactive internal probe hybridized to a single, specific band of 363 bp (Fig. 3), it was possible to do the quantitation in a dot blot system followed by phosphorimaging. It is worth emphasizing that there was no signal after PCR with DNA derived from the lungs of age-matched uninfected BMT recipients. Plasmid pIE111 added to this control DNA served as a positive standard for the PCR (Fig. 4, top). The amount of cellular DNA in all test groups was monitored by a PCR specific for the gene that encodes TNF-α (data not shown). In essence, the autoradiograph of the plate shows that the amount of latent viral DNA is smaller in the groups given CD8 T-cell therapy. The copy numbers of the viral sequence in lung cell DNA were then estimated from the linear portions of the log-log plots of dilution versus radioactivity (Fig. 4, bottom). Based on the fact that 6 μg of DNA represents the DNA content of 106 diploid mammalian cells, the latent viral DNA load in the lungs of mice given no immunotherapy was thus calculated to be 625 viral genomes per 750 ng of lung cell DNA (an example is given in Fig. 4, bottom), or ca. 5,000 viral genomes per 106 lung cells. By a similar calculation, the load was found to be 3,000 and 1,000 viral genomes per 106 lung cells after immunotherapy with 105 and 106 CD8 T cells, respectively. Notably, a further increase in the number of CD8 T cells had no effect.
FIG. 4.
Quantitation of latent CMV DNA in the lungs after CD8 T-cell immunotherapy. Lung cell DNA pooled at 12 months after BMT and infection from the superior lobe, middle lobe, and inferior lobe of five mice per experimental group was serially diluted in duplicate in log2 steps and subjected to ie1 gene exon 4-specific PCR in a microplate format. (Top) Autoradiograph obtained after dot blotting of the amplification products and hybridization with a γ-32P-end-labeled internal oligonucleotide probe. ⊘, Group with no CD8 T-cell therapy; BMT ⊘ CMV, Uninfected BMT recipients; Standard, plasmid pIE111 added to pulmonary DNA from uninfected BMT recipients. (Bottom) Computed phosphorimaging results of the same blot. Log-log plots of radioactivity (mean of duplicates) measured as phosphostimulated luminescence (PSL) units (ordinate) versus the dilutions (abscissa) are shown. The lower and upper rules relate the dilutions to the amount of lung cell DNA and to the number of plasmids in the standard (S, open circles), respectively.
Determination of the latent CMV DNA load is independent of the detected gene.
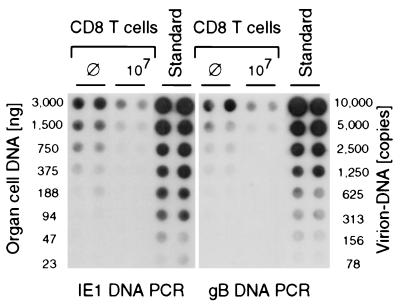
For a viral genome of 230,278 bp (26), it is difficult to prove the physical and functional integrity of the low-copy latent DNA. Determination of the load may therefore include an unknown proportion of defective genomes. So far, our determination of the load by PCR was based on the presence of a 363-bp sequence within exon 4 of the regulatory ie1 gene, representing positions 180,551 to 180,913 at the HindIII K/L fragment junction (14, 26). One might speculatively object that cells may have maintained the regulatory IE region but might have discarded the rest of the CMV genome and hence may be incapable of reactivating to productive infection. To exclude this possibility, we have chosen a sequence located ca. 100 kbp away, namely, a 510-bp sequence within the gB gene, representing positions 84250 to 84759 within the HindIII D fragment (25, 26). The latent CMV DNA load in the salivary glands was then determined for the extreme cases, namely, the group given no therapy and the group given maximal therapy, by comparing IE1-specific and gB-specific amplification (Fig. 5). Purified virion DNA, in which both genes are present in a single copy, was chosen to provide an identical standard for both PCRs. The latent DNA contained both regions of the CMV genome with identical copy numbers, and there was no indication that the therapy-susceptible and the therapy-resistant fractions of the load would differ in this respect.
FIG. 5.
Determination of the latent CMV DNA load by comparative amplification of sequences from distant regions of the viral genome. For salivary gland cell DNA of the infected groups with no therapy (⊘) and with high-dose therapy (107 CD8 T cells), latent viral DNA was quantitated by PCR amplification of an ie1 gene sequence (left panel) and a gB gene sequence (right panel), with virion DNA serving as a common standard for both PCRs. Shown are the autoradiographs obtained after dot blotting of the amplification products and hybridization with the respective γ-32P-end-labeled internal oligonucleotide probes. The loads were calculated, as in Fig. 4, from the linear portions of the log-log plots of radioactivity (mean of duplicates) versus the DNA dilutions (not depicted). For the groups given no therapy and given therapy, the loads were determined to be ca. 3,200 and 1,250 copies per 106 cells, respectively, regardless of whether the calculation was based on ie1 gene-specific amplification or on gB gene-specific amplification.
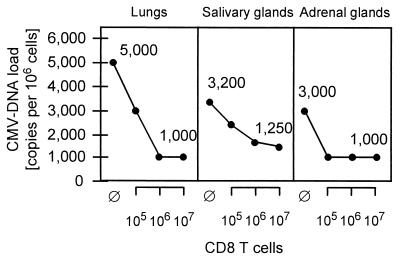
Organ-specific differences in the load of latent CMV DNA and in the increment of CD8 T-cell immunotherapy.
The load of latent viral DNA was also determined for the adrenal glands (data not shown). All the results, expressed as copy number per 106 cells, are compiled in Fig. 6. In the group with no CD8 T-cell therapy, the highest load was determined for the lungs (5,000 copies), followed by the salivary glands (3,200 copies) and the adrenal glands (3,000 copies). As indicated by the slope of the graphs, the efficacy of CD8 T-cell immunotherapy differs between organs and, specifically, the establishment of latency in the salivary glands is less accessible to control by antiviral CD8 T cells. Notably, after therapy, the loads in the lungs and the adrenal glands and, by extrapolation of the graph, the load in the salivary glands did not fall below a constant value of ca. 1,000 copies per 106 cells. Apparently, this is not a problem of dosage but indicates a principal limit, that is, a fraction of latent DNA which resists CD8 T-cell therapy.
FIG. 6.
Effect of CD8 T-cell immunotherapy on the load of latent CMV DNA in different organs. The viral DNA loads in the indicated organs are expressed as viral copies per 106 cells of the respective tissues. ⊘, Control group given no CD8 T-cell immunotherapy.
Effect of CD8 T-cell immunotherapy on the risk of CMV recurrence.
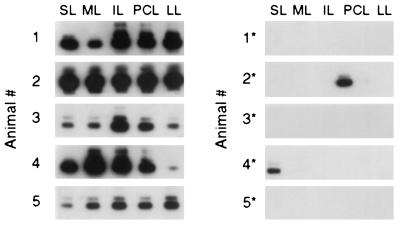
The establishment of CMV latency after resolution of primary infection entails a risk of virus recurrence and consequent recrudescent disease. By comparing incidences of CMV recurrence in mice infected either as neonates or as adults, we previously documented a positive correlation between the latent CMV DNA load and the risk of recurrence (27). Accordingly, down-modulation of the load of latent CMV by CD8 T cells should reduce the incidence of recurrence. However, this postulate is not as clear as it might appear. So far, the data have revealed a therapy-susceptible fraction of the load, which was 4,000 copies per 106 cells in the lungs and 2,000 copies per 106 cells in salivary glands and adrenal glands, and an invariant therapy-resistant fraction of 1,000 copies per 106 cells in any of the three organs tested. At present, we do not know whether the viral DNA detected by PCR represents functional genomes or, if so, whether this then applies to both fractions of viral DNA load. In the extreme assumption, only the resistant fraction might contain latent genome capable of reactivation. In this case, CD8 T-cell immunotherapy would result in a reduced amount of retained viral DNA but without any beneficial consequence with respect to virus reactivation. To test this objection, we determined the incidence of recurrence after immunoablative treatment for the latently infected group given no therapy in comparison to the group that had received 107 CD8 T cells. The lungs were chosen for the readout, because this is the organ with the highest absolute load as well as the highest load difference between the groups. In the group given no therapy and with a consequent high load, recurrence was detected within only 14 days in all five mice tested and, with some variance in quantity, in all five lobes of the lungs. This indicated a high frequency of productive reactivation events after the ablation of immune control. In contrast, in the group given therapy and with consequently low load, recurrence occurred in only two out of five mice and only in a single lobe in each (Fig. 7). Even though the incidence of recurrence was low, the few positive cases demonstrate that the therapy-resistant latent viral DNA included functional viral genomes capable of productive reactivation.
FIG. 7.
Effect of CD8 T-cell immunotherapy on the incidence of recurrence. Latently infected mice of the group with no therapy (left) (mice 1 to 5) and the group with immunotherapy by 107 CD8 T cells (right) (mice 1∗ to 5∗) were subjected to immunoablative γ-ray treatment with 6.5 Gy. Recurrence of viral infectivity was measured 14 days later in separate lobes of the lungs (see the scheme in Fig. 3) by an RT-PCR-based focus expansion assay. For each lobe, the result of one culture infected with one 2-ml aliquot of the respective homogenate is depicted. The ie1 exon 3/4-specific RT-PCR was performed with 100 ng of poly(A)+ RNA.
In conclusion, CD8 T-cell immunotherapy of acute CMV infection can reduce the risk of virus recurrence from latency.
DISCUSSION
The importance of latent tissue reservoirs and of the viral genome load as a predictor for progression from an asymptomatic to an acute state of infection is a topic of increasing interest, in particular in AIDS research (3). For herpes simplex virus reactivation from latency, Roizman and Sears have proposed a decisive role for the copy number of the latent viral DNA (39). Experimental evidence was provided by our own work on murine CMV latency (27). Mice infected as neonates underwent a prolonged productive primary infection resulting in a high load of latent viral DNA in multiple organs. By contrast, mice infected as immunocompetent adults rapidly cleared the productive primary infection and retained only a low load of latent viral DNA. Most importantly, high and low load correlated with high and low risk of recurrent infection, respectively. The same principle was revealed by the organ-specific load. Organs with a high load, such as the lungs (2), proved to be favored sites of CMV recurrence (2, 27). In the present study, we asked whether the latent CMV DNA load could be manipulated by a therapeutic intervention for reducing the risk of recurrent CMV infection in an experimental setting of BMT.
Encouraged by the promising results with the murine model, a preemptive CD8 T-cell immunotherapy of CMV disease is in clinical trials (37, 43; reviewed in reference 36) and has so far proved to be successful in terms of reducing the incidence of CMV disease after BMT. The benefit of the therapy is difficult to verify for the individual patient, because it remains unknown whether the individual would have developed CMV disease in the absence of therapy. The uncertainty is because not all high-risk patients reactivate CMV after BMT and because even after diagnosed CMV infection, the incidence of CMV disease is only ca. 50%. This raises the question whether preemptive immunotherapy is medically indicated.
Our present work has recalled previous data by demonstrating that a preemptive CD8 T-cell immunotherapy prevents lethal CMV disease in recipients suffering from an inefficient hematolymphopoietic CD8 T-cell reconstitution. Sensitized CD4 T cells did not substitute for CD8 T cells. Likewise, in mice with efficient endogenous reconstitution, harmless CMV infection progressed to lethal CMV disease after selective in vivo depletion of reconstituting CD8 T cells, whereas depletion of reconstituting CD4 T cells did not interfere with the control of infection. This is an important finding, since it appears to contradict previous work on the efficient control of murine CMV infection in mice undergoing long-term depletion of either CD4 T cells (12) or CD8 T cells (13). Apparently, the mechanism by which CD4 T cells acquire antiviral function in the long-term absence of the CD8 subset cannot develop in the short period during hematolymphopoietic reconstitution after BMT. Graft-versus-host disease prophylaxis by depletion of CD8 T cells is therefore not indicated during a diagnosed CMV infection. This conclusion is in line with clinical experience made after an in vivo-ex vivo T-cell depletion for allogeneic BMT (10).
In BMT recipients in which hematolymphopoietic reconstitution of endogenous CD8 T cells sufficed for preventing lethal CMV disease, a preemptive supplementary CD8 T-cell immunotherapy was not without benefit. Specifically, the therapy accelerated the resolution of acute infection and limited the establishment of latency, as reflected by a lower load of latent CMV DNA in the organs. In consequence, the therapy also reduced the incidence of CMV recurrence. Demonstrating the correlation between the viral genome load and recurrence is not feasible for human CMV latency in patients, since this would require organ biopsies in virtually healthy individuals followed by experimental immunosuppression. Clearly, this question is a case for the murine model. The results documented here provide a supporting argument in favor of a preemptive CD8 T-cell immunotherapy in BMT patients who are at risk of a CMV infection.
Having shown the benefits of CD8 T-cell immunotherapy, the model also revealed the limits. The data indicate that CD8 T cells cannot prevent the establishment of CMV latency. At first glance, this conclusion is not at all new. Previous attempts to prevent CMV latency with CD8 T cells have also failed (1). However, it remained unknown whether the failure in clearing the viral DNA was a trivial problem of dosage or revealed a fundamental limit. This question has now been answered. In three quite different organs, namely, the lungs, the salivary glands, and the adrenal glands, the latent virus DNA load was susceptible to preemptive therapy, with the notable exception of a therapy-resistant fraction of ca. 1,000 genomes per 106 tissue cells. Specifically, for the lungs, 106 CD8 T cells reduced the load from 5,000 to 1,000 copies but a 10-fold increase in the number of CD8 T cells did not further improve the therapeutic effect. Likewise, in the adrenal glands, a load of 1,000 genomes was reached with only 105 CD8 T cells, but a 100-fold increase in the dose was ineffectual. Finally, the salivary glands proved in general to be less susceptible to control by CD8 T cells, which is a known fact that has been discussed repeatedly (12, 18, 27, 34). Nevertheless, the load in the salivary glands was eventually controlled by high doses of CD8 T cells. Notably, the extrapolation to >107 CD8 T cells also gives an estimate of 1,000 copies per 106 tissue cells for the therapy-resistant load. In conclusion, the establishment of latency is not prevented, because there apparently exists a site of latency that evades recognition by CD8 T cells.
We now have to propose two qualitatively distinct sites of CMV latency: site I is susceptible to control by CD8 T cells and accounts for the load differences among different organs. Notably, the ranking of lungs > salivary glands > adrenal glands (Fig. 6) is in good agreement with previous data obtained with different models of murine CMV latency and with a different route of infection (27), which suggests an organ-specific component. In contrast, site II is resistant to control by CD8 T cells and accounts for a load that is invariant among organs. At present, one can only speculate on the cellular nature of the two sites. We could discuss different cell types as well as different stages of one cell type. For clarity, it must be emphasized that both sites evade recognition by CD8 T cells once latency is established. The difference dates back to a stage before latency. One possibility is that some cells do present antigenic peptides and hence are susceptible to control by CD8 T cells before they escape into latency, while other cells never present antigenic peptides. There is indeed overwhelming evidence for a molecular immune system evasion by human as well as murine CMV. Both viruses have evolved multiple molecular strategies for preventing the presentation of antigenic peptides (for a review, see reference 9). The redundancy in this function suggests an important role of immune system evasion in the biology of CMVs. Since acute infection is effectively controlled by CD8 T cells, it is not unreasonable to speculate that the physiological role of immune system evasion is in latency rather than in productive infection. Interference with antigen presentation is a function mainly of early genes of murine CMV (5, 6, 41). In permissive cell types, the viral replicative cycle will proceed to virus production and cell death. If cell types exist in which a switch to latency can occur in the early phase of viral gene expression, the respective cells are supposed to be susceptible to lysis by CD8 T cells specific for the immunodominant immediate-early nonapeptide (7, 30, 33) before they reach the early phase. This could explain the therapy-susceptible site I latency. For the therapy-resistant site II latency, we propose a cell type that retains viral DNA without viral gene expression or a cell type that evades immune system control because of a constitutive incapability in presenting antigens to CD8 T cells. To explain virus recurrence, all postulated sites of latency must be facultatively permissive for productive infection. Remarkably, the load in site II latency is a constant value for different organs that comprise different tissues, suggesting a ubiquitous cell type of even tissue distribution. This would exclude an organ-specific parenchymal cell and point to a “stromal” cell. Latency in stromal cell types has been suggested previously from indirect evidence (20, 23), but the low load of the CMV genome during latency has so far precluded an unequivocal identification of the cell type. Hematopoietic progenitor cells and their leukocyte progeny have also been proposed as candidates for CMV latency (15). Our data do not argue against this. However, it must be emphasized that our study refers to latency after clearance of the viral genome from BM and blood (17). Hematopoietic progenitors and circulating leukocytes, including blood monocytes, are therefore not the carrier cells of the latent viral genome detected here, whereas resident histiocytes have still to be considered. Clearly, the search for the latently infected cell type(s) in CMV infection is far from its end.
Instead of proposing two sites of extraleukocytic CMV latency, one could argue that CD8 T cells might control the hematogenic spread of the virus from the plantar site of inoculation to the target organs rather than controlling the establishment of latency within the organs. This objection is not supported by the data. After therapy with a high dose of CD8 T cells, productive infection still disseminated to the salivary glands and lungs whereas the titer in the adrenal glands was below the level of detection. Nevertheless, the therapy-resistant load was the same for all three organs. Along the same line of argument, the therapy-resistant load was reached in the adrenal glands after therapy with only 105 CD8 T cells, although productive infection was then still detectable in the adrenal glands. Likewise, if we compare the salivary glands and lungs, the prolonged and high virus productivity in the salivary glands did not result in an accordingly high load. Since productive CMV infection is cytolytic for fully permissive cells in situ (19), the cells that account for the virus titer measured during acute infection are distinct from those that retain the latent viral genome. This explains the lack of correlation between virus productivity and the load of latent viral DNA (1, 27).
In conclusion, CMV latency still keeps secrets to be addressed in future research. The data presented here have revealed the first evidence of two types of latency that differ with respect to immune system evasion properties in the early stage of their establishment. This finding is of theoretical and practical importance, since a significant portion of the latent virus load and consequent risk of recurrent infection can be prevented by antiviral CD8 T cells. The model of murine CMV infection thus provides a supportive argument for a preemptive CD8 T-cell immunotherapy of human CMV disease.
ACKNOWLEDGMENTS
This work was supported by a grant to M.J.R. by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (BMBF), Collaborative Research Project on CMV, individual project 01KI 9319/7; and by the Deutsche Forschungsgemeinschaft, project RE 712/3-2.
REFERENCES
- 1.Balthesen M, Dreher L, Lucin P, Reddehase M J. The establishment of cytomegalovirus latency in organs is not linked to local virus production during primary infection. J Gen Virol. 1994;75:2329–2336. doi: 10.1099/0022-1317-75-9-2329. [DOI] [PubMed] [Google Scholar]
- 2.Balthesen M, Messerle M, Reddehase M J. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun T-W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y-H, Brookmeyer R, Zeiger M A, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature (London) 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 4.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:548–550. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 5.Del Val M, Hengel H, Häcker H, Hartlaub U, Ruppert T, Lucin P, Koszinowski U H. Cytomegalovirus prevents antigen presentation by blocking the transport of peptide-loaded major histocompatibility complex class I molecules into the medial-Golgi compartment. J Exp Med. 1992;176:729–738. doi: 10.1084/jem.176.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Val M, Münch K, Reddehase M J, Koszinowski U H. Presentation of CMV immediate-early antigen to cytolytic T lymphocytes is selectively prevented by viral genes expressed in the early phase. Cell. 1989;58:305–315. doi: 10.1016/0092-8674(89)90845-3. [DOI] [PubMed] [Google Scholar]
- 7.Del Val M, Schlicht H-J, Volkmer H, Messerle M, Reddehase M J, Koszinowski U H. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J Virol. 1991;65:3641–3646. doi: 10.1128/jvi.65.7.3641-3646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman S J, Zaia J A. Treatment and prevention of cytomegalovirus pneumonia after bone marrow transplantation: where do we stand? Blood. 1994;83:2392–2398. [PubMed] [Google Scholar]
- 9.Hengel H, Koszinowski U H. Interference with antigen processing by viruses. Curr Opin Immunol. 1997;9:470–476. doi: 10.1016/s0952-7915(97)80097-0. [DOI] [PubMed] [Google Scholar]
- 10.Hertenstein B, Hampl W, Bunjes D, Wiesneth M, Duncker C, Koszinowski U H, Heimpel H, Arnold R, Mertens T. In vivo/ex vivo T cell depletion for GvHD prophylaxis influences onset and course of active cytomegalovirus infection and disease after BMT. Bone Marrow Transplant. 1995;15:387–393. [PubMed] [Google Scholar]
- 11.Ho, M. 1991. Observations from transplantation contributing to the understanding of pathogenesis of CMV infection. Transplant. Proc. 23(Suppl. 3):104–109. [PubMed]
- 12.Jonjic S, Mutter W, Weiland F, Reddehase M J, Koszinowski U H. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989;169:1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski U H. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol. 1990;64:5457–5464. doi: 10.1128/jvi.64.11.5457-5464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keil G M, Ebeling-Keil A, Koszinowski U H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987;61:1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo K, Xu J, Mocarski E S. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koszinowski U H, Del Val M, Reddehase M J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 17.Kurz S, Steffens H-P, Mayer A, Harris J R, Reddehase M J. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71:2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski U H. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer A, Podlech J, Kurz S, Steffens H-P, Maiberger S, Thalmeier K, Angele P, Dreher L, Reddehase M J. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of essential stromal hemopoietin genes. J Virol. 1997;71:4589–4598. doi: 10.1128/jvi.71.6.4589-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercer J A, Wiley C A, Spector D H. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J Virol. 1988;62:987–997. doi: 10.1128/jvi.62.3.987-997.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messerle M, Bühler B, Keil G M, Koszinowski U H. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J Virol. 1992;66:27–36. doi: 10.1128/jvi.66.1.27-36.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutter W, Reddehase M J, Busch F W, Bühring H-J, Koszinowski U H. Failure in generating hemopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med. 1988;167:1645–1658. doi: 10.1084/jem.167.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pomeroy C, Hilleren P J, Jordan M C. Latent murine cytomegalovirus DNA in splenic stromal cells of mice. J Virol. 1991;65:3330–3334. doi: 10.1128/jvi.65.6.3330-3334.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinnan G V, Kirmani N, Rook A H, Manischewitz J, Jackson L, Moreschi G, Santos G W, Saral R, Burns W H. HLA-restricted T lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307:7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 25.Rapp M, Messerle M, Bühler B, Tannheimer M, Keil G M, Koszinowski U H. Identification of the murine cytomegalovirus glycoprotein B gene and its expression by recombinant vaccinia virus. J Virol. 1992;66:4399–4406. doi: 10.1128/jvi.66.7.4399-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddehase M J, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski U H. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med. 1994;179:185–193. doi: 10.1084/jem.179.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddehase M J, Jonjic S, Weiland F, Mutter W, Koszinowski U H. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J Virol. 1988;62:1061–1065. doi: 10.1128/jvi.62.3.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddehase M J, Keil G M, Koszinowski U H. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984;14:56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- 30.Reddehase M J, Koszinowski U H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature (London) 1984;312:369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- 31.Reddehase M J, Mutter W, Koszinowski U H. In vivo application of recombinant interleukin 2 in the immunotherapy of established cytomegalovirus infection. J Exp Med. 1987;165:650–656. doi: 10.1084/jem.165.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddehase M J, Mutter W, Münch K, Bühring H-J, Koszinowski U H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddehase M J, Rothbard J B, Koszinowski U H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature (London) 1989;337:651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- 34.Reddehase M J, Weiland F, Münch K, Jonjic S, Lüske A, Koszinowski U H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 36.Riddell S R, Greenberg P D. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;13:545–586. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 37.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 38.Roizman B. Herpesviridae. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2221–2230. [Google Scholar]
- 39.Roizman B, Sears A E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- 40.Shakhov A N, Nedospasov S A. Molecular cloning of genes coding for tumor necrosis factor. Complete nucleotide sequence of the genome copy of TNF-alpha in mice. Bioorg Khim. 1987;13:701–705. [PubMed] [Google Scholar]
- 41.Thäle R, Szepan U, Hengel H, Geginat G, Lucin P, Koszinowski U H. Identification of the mouse cytomegalovirus genomic region affecting major histocompatibility complex class I molecule transport. J Virol. 1995;69:6098–6105. doi: 10.1128/jvi.69.10.6098-6105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilcek J, Lee T H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991;266:7313–7316. [PubMed] [Google Scholar]
- 43.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 44.Winston D J, Ho W G, Champlin R E. Cytomegalovirus infections after bone marrow transplantation. Rev Infect Dis. 1990;12:S776–S792. doi: 10.1093/clinids/12.supplement_7.s776. [DOI] [PubMed] [Google Scholar]