Abstract
Objectives
The aim of this study was to repurpose a drug for the treatment of bipolar depression.
Methods
A gene expression signature representing the overall transcriptomic effects of a cocktail of drugs widely prescribed to treat bipolar disorder was generated using human neuronal‐like (NT2‐N) cells. A compound library of 960 approved, off‐patent drugs were then screened to identify those drugs that affect transcription most similar to the effects of the bipolar depression drug cocktail. For mechanistic studies, peripheral blood mononuclear cells were obtained from a healthy subject and reprogrammed into induced pluripotent stem cells, which were then differentiated into co‐cultured neurons and astrocytes. Efficacy studies were conducted in two animal models of depressive‐like behaviours (Flinders Sensitive Line rats and social isolation with chronic restraint stress rats).
Results
The screen identified trimetazidine as a potential drug for repurposing. Trimetazidine alters metabolic processes to increase ATP production, which is thought to be deficient in bipolar depression. We showed that trimetazidine increased mitochondrial respiration in cultured human neuronal‐like cells. Transcriptomic analysis in induced pluripotent stem cell‐derived neuron/astrocyte co‐cultures suggested additional mechanisms of action via the focal adhesion and MAPK signalling pathways. In two different rodent models of depressive‐like behaviours, trimetazidine exhibited antidepressant‐like activity with reduced anhedonia and reduced immobility in the forced swim test.
Conclusion
Collectively our data support the repurposing of trimetazidine for the treatment of bipolar depression.
Keywords: bipolar depression, drug repurposing, gene expression signature, trimetazidine
1. INTRODUCTION
Many medications for BD have substantial tolerability burdens, and do not adequately alleviate symptoms for many people with BD, particularly depression 1 that leaves individuals severely incapacitated. Undesirable side effects also lead to high levels of treatment non‐adherence. The first line treatment for BD is a mood stabiliser such as lithium, 2 however many patients either do not respond or discontinue due to side effects. Symptoms of mania can be treated with antipsychotics and/or mood stabilisers, 2 however these medications do not effectively address bipolar depression, neurocognitive deficits and quality of life. 3 No significant progress has been made for decades in pharmacological treatments for BD, and polypharmacy is the norm. 4 Faced with a lack of optimal treatments and limited biological knowledge, it is essential to use novel approaches to identify new therapeutics for BD, especially those that display novel target engagement.
The heterogeneity of BD, in combination with its complex and poorly understood neurobiology, makes it unsuitable for traditional drug discovery approaches that target a single protein thought to play a role in the aetiology and/or pathophysiology of the disease. Here we used a novel approach to drug discovery for BD to bypass this impasse, validated by our group for type 2 diabetes 5 , 6 , 7 and by others in oncology. 8 The process involves the use of next‐generation sequencing in human neuronal‐like (NT2‐N) cells to identify a gene expression signature (GES) that highlights a small number of genes whose expression levels best define the overall biological effects of a treatment or disease.
The aim of the study was to identify drugs that may be repurposed to treat BD using a GES‐based screen and then test the best candidate on depression‐like behaviours using rat models. We first created a GES for the effects of a combination of drugs commonly prescribed to treat BD, and then used the GES to screen a library of off‐patent drugs to identify those drugs that most closely mimic the effects of the BD drug combination.
2. MATERIALS AND METHODS
2.1. Ethics
Procedures were approved by the Barwon Health Human Research Ethics Committee (Project 17.205), Deakin University Human Research Ethics Committee (Project 2018‐240), North‐West University AnimCare Animal Research Ethics Committee (DoH reg. no. AREC‐130913‐015, Approval Number: NWU‐00578‐19‐A5), or Florey Animal Ethics Committee (Approval Number 20‐042‐FINMH). All procedures were concordant with the relevant national guidelines for research.
2.2. GES cell culture and next generation sequencing (NGS)
Ntera2 human teratocarcinoma cells (NT2) were cultured and differentiated as previously described. 9 Following differentiation, the neuronal‐like cells (NT2‐N) were treated with the “BD drug cocktail” (2.5 mM lithium chloride, 0.5 mM valproate, 50 μM lamotrigine and 50 μM quetiapine; Sigma‐Aldrich, Castle Hill, Australia) or vehicle control (0.2% DMSO; Sigma‐Aldrich) for 24 h (n = 20 per group). Total RNA was extracted, quantified and quality controlled as previously described. 9 RNAseq was performed as previously described. 9
2.3. Generation of GES
NGS data was interrogated to identify the optimal set of genes (GES) that best predicts the differences between the BD drug cocktail‐treated and vehicle‐treated control cells as we have previously described. 5 Given a set of 20 replicates per treatment, the predictive set of genes was limited to <8 to guarantee sufficient statistical estimation of joint predictors. A GES of <8 genes was also preferred in view of its subsequent requirement—a manageable number of genes that can be measured for compound library screening.
Briefly, we used diagonal linear discriminant analysis (DLDA) and the signal‐to‐noise ratio (SNR) statistic on genes with evidence of differential expression between the BD drug cocktail and vehicle‐treated cells (p < 0.01, t‐tests). DLDA was then performed with a forward stepwise variable selection to identify the minimal set of genes that best discriminate the BD drug cocktail treatment group from the vehicle treatment group. The DLDA‐selected genes were ranked using the SNR statistic according to their discriminating ability. SPSS (IBM, NY, USA) was then used to reduce signature genes to a small subset that was discriminating and displayed divergent expression profiles.
2.4. Compound screen
NT2‐N cells were treated with drugs (10 μM for 24 h) from the Prestwick library comprising 960 approved, off‐patent drugs, most of which have long and well‐known clinical safety histories and diverse mechanisms of action. Each plate also contained 4 wells of positive controls (drug combination) and 4 wells of negative controls (vehicle).
RNA was extracted, real‐time PCR was performed and normalised as described previously, 9 using primers in Table S1. The GES gene expression in control samples was checked. Treatment was repeated if any gene was expressed at >2 standard deviations outside the mean for the whole screen.
The mean gene expression value for each compound was divided by the mean of the vehicle‐treated cells, and this value was used to generate an intraplate score for each compound by dividing the effect of the compound on gene expression relative to the vehicle‐treated cells by the effect of the BD drug combination relative to the vehicle. For each compound, the values of 1 minus the absolute value of the intraplate scores for each of the three genes in the GES were summed to generate an overall “similarity score” (how similar the overall effects were relative to the BD drug cocktail effects), and the compounds ranked accordingly. The net result was a ranked list of compounds in order of those that most closely resemble the effects of the BD drug combination on the GES.
2.5. Induced pluripotent stem cell (iPSC) generation
Blood samples were collected in EDTA tubes from a healthy adult (male, 49 years‐old, Caucasian) and peripheral blood mononuclear cells (PBMCs) were isolated from total blood cells using Lymphoprep (StemCell Technologies) in SepMate™‐15 tubes (StemCell Technologies).
PBMCs were used to generate iPSCs using Cytotune™‐iPS 2.0 Sendai Reprograming kit (ThermoFisher Scientific) at the Murdoch Children's Research Institute using a methodology described previously. 10 The absence of mycoplasma contamination in the iPSC line was confirmed by PCR.
2.6. iPSC differentiation into neurons and astrocytes co‐culture
A culture of deep‐ and upper‐layer excitatory neurons and astrocytes, which form functional cortical networks, was generated from iPSCs as previously described with modifications. 11 , 12 Neural induction of stem cells was achieved by dual inhibition of the SMAD signalling pathway. Details of the iPSC and cortical networks' characterisation are described in Table S2.
2.7. Effects of trimetazidine in cortical networks
Cells were seeded (100 K/well) into 24‐well plates and treated with 0.1, 1, 10 or 100 μM trimetazidine (Sigma) or vehicle control (0.01% water). After 24 h, cells were harvested and NGS was performed as described previously. Genes with trends of up‐ or down‐regulation across increased trimetazidine doses were identified using the R package IsoGene. 13 The likelihood ratio test for monotonic trend was calculated based on permutations (n = 100). p‐values were adjusted for multiple testing using Benjamini‐Hochberg correction.
To find potential pathways regulated by trimetazidine, genes with significant dose–response analysis results (adjusted p‐value<0.05) were submitted to the functional annotation tool of the database for annotation, visualization and integrated discovery (DAVID). 14
2.8. Flinders sensitive line rat study
Flinders Sensitive Line (FSL) rats are a widely used genetic animal model of depression. 15 FSL rats were bred and housed at the vivarium (SAVC reg: FR15/13458) of the North‐West University, South Africa. Male rats were group‐housed (3–4 rats/cage) from post‐natal day (PND)40, with corncob bedding changed weekly and temperature maintained at 22 ± 1°C in a relative humidity of 55% ± 10%. A 12 h light/dark cycle was followed with food provided ad libitum. Body weight was measured daily. Rats were randomly assigned into different test groups: Vehicle control, 10 mg/kg/d or 20 mg/kg/d trimetazidine (n = 12‐13/group), for which the doses were based on an earlier behavioural pharmacology study with trimetazidine in animals. 16 Trimetazidine was administered in drinking water over a 28‐day period, commencing on PND40. Behavioural tests were performed as previously described in the following order: sucrose preference test on PND60, open field tests on PNDs 66 and 67, forced swim test 17 on PND67, and elevated plus maze on PND68. 18 Rats were humanely killed, and the hippocampi dissected and immediately immersed in isolation buffer (pH 7.2) for respiration analysis.
2.9. Cellular bioenergetics and mitochondrial function analysis
NT2‐N cells were seeded (80 K/well) into 24‐well Seahorse V7 plates and treated with 0.1 or 1 μM trimetazidine or vehicle control (n = 4) for 24 h. The oxygen consumption rate (OCR) was assessed using a Seahorse XFe Flux Analyser (Seahorse Bioscience) as previously described. 19 Data was normalised to total protein using the Pierce BCA Protein Assay (ThermoFisher).
For respiration analyses from rat hippocampus, tissue was sampled using a 1 mM punch biopsy needle and placed in a Seahorse Islet capture plate in Kreb Henseleit Buffer (KHB) followed by capture screens before the plate was incubated in a non‐CO2 incubator for 10 min at 30°C. Respiration was measured for two 2 min periods, separated by a 2 min mix period, after injection of malate 5 mM, glutamate 1 mM and succinate 5 mM. 5 mM ADP (pH 7.4) was then injected and respiration was measured again. Data were normalised to total protein within each sample and the maximal respiratory capacity was established as the peak respiration values obtained following ADP injection.
2.10. Social isolation with chronic restraint stress rat model
To assess the effects of 30 mg/kg trimetazidine on depression‐like behaviours, adolescent rats received isolation combined with daily restraint stress. This model was chosen because it is widely used at this age, 20 well‐established as mildly stressful to change the hypothalamus‐pituitary–adrenal axis response, 21 and causes depression‐like behaviours that can be rescued with experimental manipulation/s. 22 , 23 Male Sprague–Dawley rats were weaned and group‐housed with littermates until PND 35, then weighed and moved to single‐housing in open‐top cages (60 × 40 × 26 cm) on a 12:12 h light: dark cycle (lights on:07:00). Standard chow and water were provided ad libitum. Rats were weighed every 4 days. From PND 37, each rat received restraint stress for 30 min per day for 10 days. At PND 47, rats were randomly allocated to vehicle or trimetazidine (30 mg/kg dissolved in saline with 1% DMSO) intervention administered intraperitoneally daily for 14d. At PND 59, all rats were tested in a large open field (1 × 1 meter). Rats were placed in the central zone of the arena and allowed to move freely for 10 min. At PND 60, rats were assessed in Porsolt's forced swim test, with 10 min pre‐swim learning phase in a clear perspex cylinder (10 litres) containing tepid water (23–25°C) followed by an identical 5 min test the next day (PND 61). 24 This second session was video recorded for manual scoring for immobility, a subset of which was validated with automated and unbiased analysis of total immobility time using ForcedSwimScan (CleverSys Inc.). Trimetazidine injection occurred 5 h after behavioural tests on PND 59 and 60 (last 2 days of trimetazidine intervention) to avoid any potential acute behavioural responses to drug administration.
2.11. Statistical analysis
For mitochondrial respiration analyses, data were checked for normality of distribution using Kolmogorov–Smirnov tests. Because data were normally distributed and group variances were homogeneous (Levene's test), group mean differences were assessed using one‐way ANOVAs with post hoc LSD tests.
For the behavioural assessments in FSL rats, a one‐way ANOVA or Kruskal‐Wallis test was used to analyse group differences between treatment groups in the sucrose preference, open field and forced swim tests. A two‐way ANOVA was used to compare time spent in the closed and open arms of the elevated plus maze. A repeated‐measures ANOVA was used (with sphericity assumed in all instances) for bodyweight analyses. In all instances, analyses were followed by the Bonferroni post hoc test. For social isolation with chronic restraint stress rat model, t‐tests were used for each measure, except for bodyweight analyses that used repeated‐measures ANOVA.
3. RESULTS
The overall features of the NGS dataset obtained from the NT2‐N cells treated with a combination of BD drugs are described elsewhere. 9 A GES was generated which best describes the overall biological effects of the drug cocktail in NT2‐N cells (Figure 1A). The GES consists of 3 genes: ANXA2 and FBN1, whose expression was decreased after drug treatment, and TPP3, which was increased. Together, the expression of these three genes predicted which treatment group the cells were in with a power of >99%. The differential expression of the GES genes was confirmed by RT‐PCR (Figure 1B). The effects of the drug cocktail on these genes were not dominated by one of the drugs but were representative of the overall effects of the 4 drugs in combination (Figure 1C), representing a net therapeutic effect.
FIGURE 1.

(A) Gene expression signature that best describes the overall biological effects of the bipolar disorder drug cocktail in NT2‐N cells. (B) Confirmation of the differential expression of the GES genes by RT‐PCR. (C) Confirmation of the overall effects of the four drugs on the expression of the signature genes individually and in combination (*p < 0.05; ***p < 0.001 compared to vehicle).
In total, 960 drugs were screened for their effects on the expression of the GES genes in NT2‐N cells and this data was used to calculate a similarity score for each drug, with a lower similarity score indicating more similar effects to the BD drug cocktail. The screening results were filtered to exclude drugs that were not approved for use in humans, had been withdrawn or received a black box warning. The “hit” drugs from the screen are shown in Table 1, along with their effects on the expression of the GES genes and overall GES similarity scores.
TABLE 1.
The top drugs from the gene expression screen (effects on expression of the GES genes and overall GES similarity scores).
| Fold change in expression | ||||||||
|---|---|---|---|---|---|---|---|---|
| Similarity Score | Name | Class | Indication | Mechanism of action | Comment | ANXA2 | FBN1 | TPPP3 |
| 0 | BD drug combination | (Positive control) | 0.51 +/−0.01 | 0.65 +/−0.02 | 1.45 +/−0.04 | |||
| 0.95 | Levodopa | Dopamine precursor | Parkinson's | Increases dopamine synthesis | Side effects | 0.75 | 0.63 | 1.17 |
| 1.04 | Hexamethonium dibromide dihydrate | Anticholinergic | Hypertension | nicotinic cholinergic antagonist | Does not cross BBB | 0.87 | 0.68 | 1.22 |
| 1.12 | Clofazimine | DNA binding | Leprosy | Antibacterial | 0.67 | 0.69 | 1.20 | |
| 1.17 | Isoxicam | NSAID | Inflammation | Anti‐inflammatory | Not novel | 0.72 | 0.78 | 1.16 |
| 1.28 | Harmane hydrochloride | MAO inhibitor | N/A | Neurotoxin, endogenous ligand for benzodiazepine receptor | Neurotoxic | 0.56 | 0.68 | 1.09 |
| 1.30 | Carteolol hydrochloride | Beta‐blocker | Glaucoma | Non‐selective beta‐adrenergic receptor antagonist; serotonin receptor antagonist | Does not cross BBB | 0.63 | 0.66 | 0.97 |
| 1.34 | Maprotiline hydrochloride | Antidepressant | Depression | Norepinephrine reuptake inhibitor | Not novel | 0.61 | 0.80 | 1.02 |
| 1.41 | Famotidine | Antihistamine | Peptic ulcer | Histamine H2 receptor antagonist | 0.75 | 0.84 | 1.17 | |
| 1.43 | Pentolinium bitartrate | Antihypertensive | Hypertensive crisis | Nicotinic acetylcholine receptor antagonist | Unacceptable side effect profile | 0.69 | 0.86 | 1.21 |
| 1.46 | Mebendazole | Antiparasitic | Infection | Microtubule synthesis inhibitor | Antiparasitic | 0.81 | 0.79 | 1.23 |
| 1.49 | Clebopride maleate | Antiemetic | Gastrointestinal disorders | Dopamine antagonist | Extrapyramidal side effects | 0.70 | 0.66 | 0.99 |
| 1.49 | Cefoperazone dihydrate | Cephalosporin antibiotic | Infection | Bacterial cell wall synthesis inhibitor | Does not cross BBB | 0.63 | 0.71 | 0.87 |
| 1.50 | Protriptyline hydrochloride | Antidepressant | Depression/ | Norepinephrine and serotonin reuptake inhibitor | Not novel | 0.65 | 0.71 | 1.07 |
| ADHD | ||||||||
| 1.50 | Glafenine hydrochloride | NSAID | Inflammation | Anti‐inflammatory | Not novel | 0.74 | 0.74 | 1.06 |
| 1.52 | Pirenperone | Antipsychotic | N/A | Selective antagonist of the serotonin 5‐HT2 receptors | Not novel | 1.06 | 0.74 | 1.25 |
| 1.54 | Risperidone | Antipsychotic | Schizophrenia/Bipolar disorder | Dopamine antagonist and serotonin antagonist | Not novel | 0.78 | 0.89 | 1.19 |
| 1.55 | Trimetazidine dihydrochloride | Anti‐ischaemic | Angina pectoris | Fatty acid beta‐oxidation inhibitor | 0.61 | 0.58 | 0.98 | |
| 1.56 | Pyrimethamine | Aminopyrimidine | Infections | Folate metabolism blocker | Antiparasitic | 0.67 | 0.58 | 0.96 |
| 1.57 | Benzamil hydrochloride | Sodium channel blocker | Cystic fibrosis | Inhibits renal sodium reabsorption | Does not cross BBB | 0.84 | 0.82 | 1.23 |
Trimetazidine was identified as a drug of interest for further investigation because it has an excellent safety profile, is known to cross the blood‐brain barrier and has not previously been used to treat neuropsychiatric disorders. Given that trimetazidine has been shown to act on mitochondrial function, we wanted to determine whether trimetazidine had a similar mechanism of action in neurons as in cardiomyocytes. Cells treated with trimetazidine displayed increased oxygen consumption indicative of improved mitochondrial function (Figure 2).
FIGURE 2.

Oxygen consumption rate in NT2‐N cells after treatment with trimetazidine (*p = 0.041 compared to vehicle).
NGS was also used to investigate the transcriptional effects of trimetazidine in iPSC‐derived cortical networks, a more biologically relevant human pre‐clinical model. Isogene analysis identified several genes with evidence for dose‐dependent regulation by trimetazidine, and these genes were enriched for several pathways (Table S3), including focal adhesion and MAPK signalling. Further analysis revealed that genes in both the focal adhesion and MAPK signalling pathways showed overall evidence of downregulation as the dose of trimetazidine increased. Within the focal adhesion pathway, there were notable groups of structural genes that showed evidence of dose‐dependent downregulation by trimetazidine, including collagens, laminins and filamins (Figure S1).
In FSL rats, treatment with 10 or 20 mg/kg/d trimetazidine for 28 days had no effect on bodyweight or general locomotor activity (Figure 3A), yet tended to reduce immobility in the forced swim test (by 8%, p = 0.11; Figure 3B), increase time spent in the open arms and decrease time spent in the closed arms of the EPM, consistent with an anxiolytic effect (p ≤ 0.0005; Figure 3C) and reduced anhedonia as indicated by increased sucrose preference at the higher dose (p = 0.021; Figure 3D). Trimetazidine at 20 mg/kg/d significantly increased respiratory capacity in the hippocampus of rats compared to the vehicle control group (p = 0.001; Figure 3E).
FIGURE 3.

Effects of TMZ on body weight (A), depressive‐like (B), anxiety (C) and anhedonia (D) behaviours in Flinders Sensitive Line rats. (A) Flinders Sensitive Line (FSL) rats gained weight (p < 0.05), independent of chronic treatment with vehicle, 10 or 20 mg/kg/d trimetazidine (p = 0.60). (B) Chronic treatment with 10 or 20 mg/kg/d trimetazidine for 28 days tended to reduce mean time spent immobile in Flinders Sensitive Line (FSL) rats in the 5 min forced swim test (p = 0.11). (C) In FSL rats, mean time spent in the open arms and closed arms in an elevated plus maze (*p < 0.05 significant different between arms). (D) In FSL rats, mean percentage sucrose preference compared to vehicle (*p = 0.021). (E) Maximal respiratory capacity in hippocampus after treatment with trimetazidine (n = 6; *p = 0.001 compared to vehicle).
Given the tendency for reduced immobility in the forced swim test in FSL rats, we conducted a follow‐up study using the social isolation with chronic restraint stress rat model of depressive‐like behaviour using a higher dose of trimetazidine (30 mg/kg/d). This study showed no effect of trimetazidine on bodyweight (p = 0.34) or locomotion (p = 0.58) (Figure 4A,B). However, trimetazidine significantly reduced immobility in the forced swim test (p = 0.03), consistent with an antidepressant‐like effect (Figure 4C).
FIGURE 4.
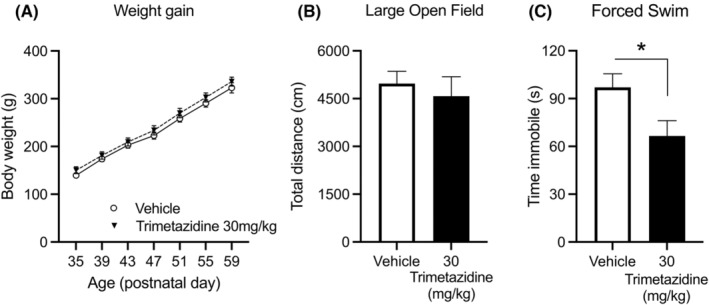
Effects of TMZ on body weight (A), anxiety (B) and depressive‐like in isolation and physical restraint rats treated with 30 mg/kg/d trimetazidine for 30 days. (A) Sprague–Dawley rats exposed to isolation and physical restraint significant gained weight (p < 0.0001) independent of treatment with 30 mg/kg/d trimetazidine or vehicle (Treatment: p = 0.34 and Treatment x Age interaction: p = 0.93). (B) Trimetazidine treatment had no effect on locomotion (p = 0.58). (C) Chronic treatment with 30 mg/kg/d trimetazidine for 14 days in isolation and physical restraint rats; mean time spent immobile in rats in the 5 min forced swim test (*p = 0.03).
4. DISCUSSION
A combination of transcriptomics, drug screening and in vitro and in vivo mechanistic studies identified trimetazidine as a potential drug to treat BD depression. Trimetazidine acts primarily on mitochondrial capacity, function and substrate utilisation, increasing the efficiency of ATP production, which is likely to be beneficial in BD depression. This is concordant with extant theoretical models which see mitochondrial function at the core of the disorder; decreased in depression and increased in mania with regulatory failure at the heart of the disorder. 25 We also showed effects on focal adhesion and MAPK signalling pathways, both of which could plausibly contribute to improvements in depressive symptoms. Finally, we showed anxiolytic and antidepressant‐like properties of trimetazidine in two different animal models of depressive‐like behaviour, providing evidence to support the repurposing of trimetazidine for the treatment of BD depression.
The genes comprising the GES were: (1) ANXA2 (Annexin 2), which encodes a calcium‐dependent phospholipid‐binding protein involved in the regulation of cellular growth and signal transduction pathways. ANXA2 had increased gene expression in PBMCs of patients with BD (p < 0.001). 26 (2) FBN1 (Fibrillin 1), encoding an extracellular matrix protein which may be a structural component of the blood–brain barrier. 27 Genetic variation in FBN1 was associated with BD in a Norwegian sample (GWAS study; adj. p < 0.001, odds ratios 0.59–0.61). 28 (3) TPPP3 (Tubulin Polymerization Promoting Protein Family Member 3), which encodes a tubulin binding protein with microtubule stabilising activity and may play a role in cell proliferation and mitosis. 29 TPPP3 has not been previously associated with BD.
The drug screen highlighted agents with known effects in the disorder such as antidepressants (maprotiline, protriptyline; both used for treating BD depression) and antipsychotics (pirenpirone, risperidone) already in use for treating mania, as well as non‐steroidal anti‐inflammatory agents (isoxicam, glafenine) which are already under investigation for such disorders.
Trimetazidine is a cytoprotective anti‐ischaemic agent used to treat stable angina pectoris. It is a selective inhibitor of 3‐ketoacyl‐CoA thiolase (the final step in beta‐oxidation of fatty acids), which results in impaired fatty acid uptake and oxidation, 30 which in turn results in enhanced myocardial glucose oxidation, a more efficient way to make ATP. 31 It is currently being tested for the treatment of hypertension, coronary artery disease, heart failure and hepatocellular carcinoma, and several studies/meta‐analyses have shown an overall decrease in all‐cause mortality in participants taking trimetazidine. 32 , 33
Mechanistic studies in cells (mainly cardiomyocytes), animal models and human participants have shown that trimetazidine improves mitochondrial function, 34 , 35 lowers oxidative stress 36 , 37 and has anti‐inflammatory properties. 38 , 39 All these mechanisms are currently proposed to be targets for the treatment of BD. We showed that trimetazidine has similar metabolic effects in neuronal cells, with an increase in OCR following treatment, which is consistent with previous effects seen in cardiomyocytes. This effect in cell culture translated to an improved maximal respiratory capacity in the hippocampus of rats. Therefore, the beneficial metabolic effects of trimetazidine may also be seen in the brain, and this increase in metabolic efficiency may be useful for improving symptoms in the depressive phase of BD.
We further investigated the mechanism(s) of action of trimetazidine in co‐cultured neurons and astrocytes through transcriptomic analysis. Genes affected by trimetazidine were enriched for the focal adhesion and MAPK signalling pathways, both of which appeared to be down‐regulated following treatment. Focal adhesions are specialised structures that form at cell‐extracellular matrix contact points and are involved in cell shape and motility, as well as receptor‐mediated signalling. Focal adhesion is critical for neurite outgrowth and axon pathfinding, and likely plays a key role in neuronal plasticity, 40 , 41 with paxilin appearing to play a central role. Among the focal adhesion genes that were downregulated, some were collagen isoforms. Overall, collagens had a strong dose‐dependent decrease in expression, which is of interest because several collagen isoforms have been identified as genetic risk factors for BD. 42 , 43
The MAPK signalling pathway, which regulates many processes including cell proliferation, differentiation and migration, was also transcriptionally downregulated by trimetazidine. This pathway is well known to contribute to inflammation and neuroinflammation, 44 which is a key factor in BD pathophysiology. 45 , 46 , 47 Indeed, the MAPK signalling pathway has been associated with BD in GWAS, transcriptomic and methylation studies 37 , 39 and MAPK signalling is increased in lymphocytes from patients with BD compared with healthy controls. 48 , 49 Furthermore, genetic predictors of treatment outcome in patients with BD were enriched for genes involved in the MAPK signalling pathway. 50 Collectively, our data suggest that in addition to the predicted metabolic effects of trimetazidine, this drug may also have beneficial effects on BD symptoms by regulating the focal adhesion and MAPK signalling pathways, and identify these pathways as new targets for the treatment of BD.
From a psychotropic viewpoint, a recent animal study first demonstrated the anxiolytic properties of trimetazidine. 16 The current study confirms and extends this, describing the broad psychotropic actions of trimetazidine, including anxiolytic, antidepressant and hedonic actions, in validated animal models of depression. This study focused on bipolar depression, but given that the drug cocktail used included mood stabilisers and drugs used to treat mania, and the screen highlighted both antidepressants and antipsychotics, future investigations should include testing the effects of trimetazidine in models of mania. In both FSL rats and rats subjected to restraint stress trimetazidine exhibited anxiolytic effects, consistent with previously published work. 16 Trimetazidine also reduced anhedonia and tended to reduce immobility in the forced swim test at the highest dose in FSL rats, which is suggestive of antidepressant‐like properties. To investigate the latter further, we treated socially isolated rats subjected to restraint stress using a higher dose of trimetazidine and found evidence of antidepressant activity (reduced immobility in the forced swim test). Collectively these data indicate that trimetazidine has both anxiolytic and antidepressant‐like activity in rodent models of depressive behaviour and supports the repurposing of trimetazidine for the treatment of BD depression.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Supporting information
Figure S1.
Tables S1–S3.
ACKNOWLEDGEMENTS
The authors thank the Murdoch Children's Research Institute iPSC Core Facility for their assistance with iPSCs. The NWU authors acknowledge Cor Bester, Kobus Venter and Juandré Snyman for overseeing the wellbeing of the animals, and Dr Geoffrey de Brouwer for ensuring the experimental work could continue during the National COVID‐19 lockdown period. This project was supported by the National Health and Medical Research Council (NHMRC) Project Grant (GNT1078928) and the NHMRC Centre of Excellence (GNT1153607). MB is a NHMRC Senior Principal Research Fellow (1156072). There are no competing financial interests in relation to this work. Open access publishing facilitated by Deakin University, as part of the Wiley ‐ Deakin University agreement via the Council of Australian University Librarians.
Bortolasci CC, Kidnapillai S, Spolding B, et al. Use of a gene expression signature to identify trimetazidine for repurposing to treat bipolar depression. Bipolar Disord. 2023;25:661‐670. doi: 10.1111/bdi.13319
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bauer MS, Mitchner L. What is a "mood stabilizer"? An Evidence‐Based Response. Am J Psychiatry. 2004;161(1):3‐18. [DOI] [PubMed] [Google Scholar]
- 3. Morton E, Murray G, Michalak EE, et al. Quality of life in bipolar disorder: towards a dynamic understanding. Psychol Med. 2018;48(7):1111‐1118. [DOI] [PubMed] [Google Scholar]
- 4. Sachs GS, Peters AT, Sylvia L, Grunze H. Polypharmacy and bipolar disorder: what's personality got to do with it? Int J Neuropsychopharmacol. 2014;17(7):1053‐1061. [DOI] [PubMed] [Google Scholar]
- 5. Konstantopoulos N, Foletta VC, Segal DH, et al. A gene expression signature for insulin resistance. Physiol Genomics. 2011;43(3):110‐120. [DOI] [PubMed] [Google Scholar]
- 6. Konstantopoulos N, Molero JC, McGee SL, et al. Methazolamide is a new hepatic insulin sensitizer that lowers blood glucose in vivo. Diabetes. 2012;61(8):2146‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simpson RW, Nicholson GC, Proietto J, et al. Efficacy and safety of oral methazolamide in patients with type 2 diabetes: a 24‐week, placebo‐controlled, double‐blind study. Diabetes Care. 2014;37(11):3121‐3123. [DOI] [PubMed] [Google Scholar]
- 8. Stegmaier K, Ross KN, Colavito SA, O'Malley S, Stockwell BR, Golub TR. Gene expression‐based high‐throughput screening(GE‐HTS) and application to leukemia differentiation. Nat Genet. 2004;36(3):257‐263. [DOI] [PubMed] [Google Scholar]
- 9. Kidnapillai S, Bortolasci CC, Udawela M, et al. The use of a gene expression signature and connectivity map to repurpose drugs for bipolar disorder. World J Biol Psychiatry. 2020;21(10):775‐783. [DOI] [PubMed] [Google Scholar]
- 10. Vlahos K, Sourris K, Mayberry R, et al. Generation of iPSC lines from peripheral blood mononuclear cells from 5 healthy adults. Stem Cell Res. 2019;34:101380. [DOI] [PubMed] [Google Scholar]
- 11. Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012;7(10):1836‐1846. [DOI] [PubMed] [Google Scholar]
- 12. Shi Y, Kirwan P, Smith J, Robinson HPC, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15(3):477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pramana S, Lin D, Haldermans P, et al. IsoGene: an R package for analyzing dose‐response studies in microarray experiments. R J. 2010;2:5‐11. [Google Scholar]
- 14. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44‐57. [DOI] [PubMed] [Google Scholar]
- 15. Overstreet DH, Wegener G. The flinders sensitive line rat model of depression‐25 years and still producing. Pharmacol Rev. 2013;65(1):143‐155. [DOI] [PubMed] [Google Scholar]
- 16. Kolik LG, Nadorova AV, Stolyaruk VN, Miroshkina IA, Tsorin IB, Kryzhanovskii SA. Anxiolytic properties of trimetazidine in experimental models of increased anxiety. Bull Exp Biol Med. 2017;162(5):643‐646. [DOI] [PubMed] [Google Scholar]
- 17. Steyn SF, Harvey BH, Brink CB. Pre‐pubertal, low‐intensity exercise does not require concomitant venlafaxine to induce robust, late‐life antidepressant effects in Flinders sensitive line rats. Eur J Neurosci. 2020;52(8):3979‐3994. [DOI] [PubMed] [Google Scholar]
- 18. Regenass W, Möller M, Harvey BH. Studies into the anxiolytic actions of agomelatine in social isolation reared rats: role of corticosterone and sex. J Psychopharmacol. 2018;32(2):134‐145. [DOI] [PubMed] [Google Scholar]
- 19. Gaur V, Connor T, Sanigorski A, et al. Disruption of the class IIa HDAC corepressor complex increases energy expenditure and lipid oxidation. Cell Rep. 2016;16(11):2802‐2810. [DOI] [PubMed] [Google Scholar]
- 20. Burke AR, McCormick CM, Pellis SM, Lukkes JL. Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci Biobehav Rev. 2017;76:280‐300. [DOI] [PubMed] [Google Scholar]
- 21. Perry CJ, Campbell EJ, Drummond KD, Lum JS, Kim JH. Sex differences in the neurochemistry of frontal cortex: impact of early life stress. J Neurochem. 2021;157(4):963‐981. [DOI] [PubMed] [Google Scholar]
- 22. Zhou Y, Yan M, Pan R, et al. Radix polygalae extract exerts antidepressant effects in behavioral despair mice and chronic restraint stress‐induced rats probably by promoting autophagy and inhibiting neuroinflammation. J Ethnopharmacol. 2021;265:113317. [DOI] [PubMed] [Google Scholar]
- 23. Ghalandari‐Shamami M, Nourizade S, Yousefi B, Vafaei AA, Pakdel R, Rashidy‐Pour A. Beneficial effects of physical activity and crocin against adolescent stress induced anxiety or depressive‐like symptoms and dendritic morphology remodeling in prefrontal cortex in adult male rats. Neurochem Res. 2019;44(4):917‐929. [DOI] [PubMed] [Google Scholar]
- 24. Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant‐like activity in rodents. Nat Protoc. 2012;7(6):1009‐1014. [DOI] [PubMed] [Google Scholar]
- 25. Morris G, Walder K, McGee SL, et al. A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev. 2017;74:1‐20. [DOI] [PubMed] [Google Scholar]
- 26. Zhang L, Su TP, Choi K, et al. P11 (S100A10) as a potential biomarker of psychiatric patients at risk of suicide. J Psychiatr Res. 2011;45(4):435‐441. [DOI] [PubMed] [Google Scholar]
- 27. Van der Donckt C, Roth L, Vanhoutte G, et al. Fibrillin‐1 impairment enhances blood‐brain barrier permeability and xanthoma formation in brains of apolipoprotein E‐deficient mice. Neuroscience. 2015;295:11‐22. [DOI] [PubMed] [Google Scholar]
- 28. Djurovic S, Gustafsson O, Mattingsdal M, et al. A genome‐wide association study of bipolar disorder in Norwegian individuals, followed by replication in Icelandic sample. J Affect Disord. 2010;126(1–2):312‐316. [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Xu Y, Ye K, et al. Knockdown of tubulin polymerization promoting protein family member 3 suppresses proliferation and induces apoptosis in non‐small‐cell lung cancer. J Cancer. 2016;7(10):1189‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dézsi CA. Trimetazidine in practice: review of the clinical and experimental evidence. Am J Ther. 2016;23(3):e871‐e879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopatin YM, Rosano GM, Fragasso G, et al. Rationale and benefits of trimetazidine by acting on cardiac metabolism in heart failure. Int J Cardiol. 2016;203:909‐915. [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Lu Y, Jiang H, et al. Additional use of trimetazidine in patients with chronic heart failure: a meta‐analysis. J Am Coll Cardiol. 2012;59(10):913‐922. [DOI] [PubMed] [Google Scholar]
- 33. Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta‐analysis of randomised controlled trials in heart failure. Heart. 2011;97(4):278‐286. [DOI] [PubMed] [Google Scholar]
- 34. Dehina L, Vaillant F, Tabib A, et al. Trimetazidine demonstrated cardioprotective effects through mitochondrial pathway in a model of acute coronary ischemia. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(3):205‐215. [DOI] [PubMed] [Google Scholar]
- 35. Kuzmicic J, Parra V, Verdejo HE, et al. Trimetazidine prevents palmitate‐induced mitochondrial fission and dysfunction in cultured cardiomyocytes. Biochem Pharmacol. 2014;91(3):323‐336. [DOI] [PubMed] [Google Scholar]
- 36. Shao L, Ma A, Figtree G, Zhang P. Combination therapy with coenzyme Q10 and trimetazidine in patients with acute viral myocarditis. J Cardiovasc Pharmacol. 2016;68(2):150‐154. [DOI] [PubMed] [Google Scholar]
- 37. Wu Q, Qi B, Liu Y, et al. Mechanisms underlying protective effects of trimetazidine on endothelial progenitor cells biological functions against H2O2‐induced injury: involvement of antioxidation and Akt/eNOS signaling pathways. Eur J Pharmacol. 2013;707(1–3):87‐94. [DOI] [PubMed] [Google Scholar]
- 38. Hao J, Du H, Li WW, et al. Effects of atorvastatin combined with trimetazidine on myocardial injury and inflammatory mediator in unstable angina patients during perioperative of percutaneous coronary intervention. Eur Rev Med Pharmacol Sci. 2015;19(23):4642‐4646. [PubMed] [Google Scholar]
- 39. Chen J, Lai J, Yang L, et al. Trimetazidine prevents macrophage‐mediated septic myocardial dysfunction via activation of the histone deacetylase sirtuin 1. Br J Pharmacol. 2016;173(3):545‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ivankovic‐Dikic I, Grönroos E, Blaukat A, Barth BU, Dikic I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol. 2000;2(9):574‐581. [DOI] [PubMed] [Google Scholar]
- 41. Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin‐mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9(10):1274‐1283. [DOI] [PubMed] [Google Scholar]
- 42. Lydall GJ, Bass NJ, McQuillin A, et al. Confirmation of prior evidence of genetic susceptibility to alcoholism in a genome‐wide association study of comorbid alcoholism and bipolar disorder. Psychiatr Genet. 2011;21(6):294‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shih WL, Kao CF, Chuang LC, Kuo PH. Incorporating information of microRNAs into pathway analysis in a genome‐wide association study of bipolar disorder. Front Genet. 2012;3:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kheiri G, Dolatshahi M, Rahmani F, Rezaei N. Role of p38/MAPKs in Alzheimer's disease: implications for amyloid beta toxicity targeted therapy. Rev Neurosci. 2018;30(1):9‐30. [DOI] [PubMed] [Google Scholar]
- 45. Berk M, Vieta E, Dean OM. Anti‐inflammatory treatment of bipolar depression: promise and disappointment. Lancet Psychiatry. 2020;7(6):467‐468. [DOI] [PubMed] [Google Scholar]
- 46. Brunoni AR, Supasitthumrong T, Teixeira AL, et al. Differences in the immune‐inflammatory profiles of unipolar and bipolar depression. J Affect Disord. 2020;262:8‐15. [DOI] [PubMed] [Google Scholar]
- 47. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804‐817. [DOI] [PubMed] [Google Scholar]
- 48. Wieck A, Grassi‐Oliveira R, do Prado CH, et al. Differential neuroendocrine and immune responses to acute psychosocial stress in women with type 1 bipolar disorder. Brain Behav Immun. 2013;34:47‐55. [DOI] [PubMed] [Google Scholar]
- 49. do Prado CH, Rizzo LB, Wieck A, et al. Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology. 2013;38(5):667‐676. [DOI] [PubMed] [Google Scholar]
- 50. Fabbri C, Serretti A. Genetics of long‐term treatment outcome in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:17‐24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Tables S1–S3.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


