Abstract
Proximal femur fractures in the elderly are associated with significant loss of independence, mobility, and quality of life. This prospective study aimed to: (1) investigate gait biomechanics in intertrochanteric fracture (ITF) patients (A1 and A2 AO/OTA) managed via femoral nailing at 6 weeks and 6 months postoperative and how these compared with similarly aged elderly controls; and (2) investigate whether femoral offset shortening (FOS) and lateral lag screw protrusion (LSP) were associated with changes in gait biomechanics at postoperative time points. Hip radiographs and gait data were collected for 34 patients at 6 weeks and 6 months postoperatively. Gait data were also collected from similarly aged controls. FOS and LSP were measured from radiographs. Joint angles, external moments, and powers were calculated for the hip, knee, and ankle and compared between time points in ITF patients and healthy controls using statistical parametric mapping. The relationship between radiographic measures with gait speed, step length, peak hip abduction, and maximum hip abduction moment was assessed using a Pearson correlation. External hip adduction moments and hip power generation improved in the first 6 months postoperative, but differed significantly from healthy controls during single limb stance. LSP showed a moderate correlation with maximum hip abduction moment at 6 weeks postoperative (r = −0.469, p = 0.048). These results provide new detail on functional outcomes after ITF and potential mechanisms that functional deficiencies may stem from. Lag screw prominence may be an important factor in maintaining functional independence and minimizing the risk of secondary falls after ITF in the elderly.
Keywords: biomechanics, gait, hip, implant fixation, kinematics and kinetics
1. INTRODUCTION
Proximal femur fractures are one of the most prevalent injuries in the elderly, occurring in roughly 1.31 million people each year worldwide. 1 With changing demographics and a growing elderly population, the global incidence is rising, 2 and projected to reach 6.26 million people by 2050. 3 Effects of these fractures are pronounced and associated with declines in mobility, 4 quality of life, and increased mortality rates compared to age‐matched controls. 5 Previous research indicates up to 50% of patients lose the ability to function independently, 6 with approximately 40% of surviving patients after 1 year requiring walking frames. 4 Intertrochanteric fractures (ITF) account for roughly 50% of hip fractures, 7 and are commonly treated with femoral nails. 8 While facilitating fracture compression, femoral neck shortening is commonly reported postoperatively. 9 , 10 This has been correlated with lower patient‐reported functional outcome scores 11 and spatiotemporal gait parameters, 12 hypothesized to be due to reductions in the hip abductor moment arm. 13 Abductor weakness is then exhibited, due to a larger force requirement of the hip abductors to balance the pelvis through single limb support. 14 This weakness may clinically manifest as a Trendelenburg gait, observed as a contralateral pelvis drop during stance phase with trunk lean toward the ipsilateral side. 15
Following femoral nailing, lateral thigh pain over the greater trochanter is frequently reported, 16 stemming from prominence of the lag screw into soft tissue. Prominence may increase with fracture compression and shortening 17 causing increased pain, necessitating removal of the nail or lag screw. 18 Despite reoperation, implant removal is associated with additional complications, including increased risk of postoperative fracture. 19
After surgery, early weight bearing is suggested to improve recovery. 20 Reviews evaluating long‐term functional recovery report most recovery in walking ability and activities of daily living (ADLs) is achieved within 6 months 5 with some authors indicating balance may take up to 9 months to recover. Some authors report that most ADLs do not differ significantly between 4 months and 1 year postoperatively. 21 Although functional outcomes have previously been reported using patient‐reported outcomes measures 22 and spatiotemporal gait parameters, 12 there is no data describing changes in gait biomechanics after ITF in the elderly. 23 This data may allow us to quantify functional outcomes in more detail and allow for better estimations of patient mobility after treatment. Moreover, while femoral offset shortening (FOS) has been associated with lower functional outcome scores and lateral thigh pain after surgery, whether these measures are associated with differences in gait biomechanics are unclear. Specifically, external hip adduction moments, reflective of internal abduction moments including torques generated by the hip abductor muscles, 24 are of particular interest. With recent studies highlighting the effects of changes in hip geometry on postoperative gait patterns in total hip arthroplasty patients, 25 there is a need to explore whether femoral nailing after ITF influences gait biomechanics.
This paper aimed to: (1) identify changes in gait biomechanics after femoral nailing of ITFs between 6 weeks, and 6 months postoperatively; (2) identify differences in gait biomechanics between ITF patients and elderly controls; and (3) to investigate whether FOS and lateral lag screw protrusion (LSP) was correlated with contralateral pelvic drop, maximum external hip adduction moment, step length and gait speed from gait analysis. We first hypothesized that ITF patients would show improvements in joint angles, external moments, and power generation from 6 weeks to 6 months postoperative. Second, we hypothesized there would be differences between ITF patients and healthy controls at 6 months postoperative. Finally, we hypothesized that increased offset shortening and lateral LSP would be independently associated with increased contralateral pelvic drop and reduced external hip adduction moments.
2. METHODS
2.1. Participants and study design
This prospective study recruited a subset of patients from a large randomized controlled trial treating elderly ITF patients with a femoral nail. 26 In brief, elderly patients sustaining an ITF (A1 and A2 AO/OTA Fracture and Dislocation Classification) were randomized for treatment using a Gamma3 nail (Stryker; unlocked proximal lag screw), or Trigen Intertan nail (Smith and Nephew) with an unlocked or locked proximal lag screw. Patients were followed up at 6 weeks and 6 months postoperative where hip radiographs, pain score (visual analog scale [VAS]), and clinician‐assessed hip function score (Harris hip score [HHS]) were collected. Patients with an Abbreviated Mental Health Test Score (AMTS) ≥8, able to follow instruction by answering simple questions and able to walk independently either with or without a mobility aid were included in a subgroup described in the present study. For this subgroup, in addition to outcome measures previously detailed in our protocol paper, 26 3D gait data were collected at 6 weeks and 6 months postoperative. Elderly individuals over 60 years of age with no history of previous lower limb surgery were also recruited and 3D gait data were collected at a single time point as a reference group. The study protocol was approved by the Central Adelaide Local Health Network (HREC/17/RAH/433) and the study was registered on the Australia New Zealand Clinical Trials Registry (ANZCTR): ACTRN12618001431213. Written informed consent was obtained from all patients before enrollment.
2.2. Measurement of femoral offset
Femoral offset (FO) was defined as the perpendicular distance from the center of the femoral head to the longitudinal axis of the femoral shaft () (Figure 1A). FO was measured at baseline using intra‐operative image intensifier radiographs and at 6 weeks and 6 months using flat panel radiographs. Images were adjusted for magnification using the projected cylindrical diameter of the distal end of the femoral nail for the Intertan (11.5 mm) and proximal end for the Gamma (15.5 mm). Rotation correction was performed using the technique described and validated by Lechler et al. 27 Using this method, hip internal–external rotation and corresponding rotation correction factor (RCF) were calculated using the known caput‐collum‐diaphyseal and gamma angle () of the femoral nail, together with the projected CCD and gamma angle () of the femoral nail measured on radiographs. A rotation‐corrected femoral offset () was then calculated as the product of the measured projected femoral offset () and RCF. FOS was calculated as the change in between intra‐operative, 6‐week, and 6‐month time points. FOS was also expressed as a percentage of each patients original femoral offset measured from baseline intra‐operative image intensifier radiographs to indicate a relative FOS. Considering reported errors in accuracy of measured distances on digital radiographs of between 1.4 and 2.6 mm 28 and our patient data, measured increases in femoral offset of ≤4 mm between time points, were assumed to be due to patient alignment and the in‐plane resolution of radiographs, and considered to be 0. This was made under the assumption that increases in offset would only occur alongside radiological evidence of implant fixation failure. Measured increases in femoral offset of >4 mm were radiologically assessed for loss of implant fixation/impending cut‐out by an orthopedic surgeon and subsequently excluded from analysis.
Figure 1.
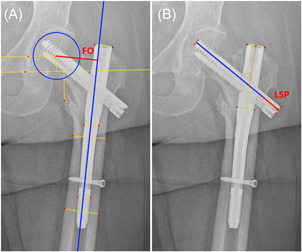
(A) Defining femoral offset measurements (indicated in red), changes between time points indicate amount of femoral offset shortening. (B) Defining lag screw lateral protrusion (indicated in red). [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Measurement of lateral lag screw protrusion
Lateral LSP was defined as the distance that the lateral end of the lag screw extended beyond the cortex of the femur along the axis of the lag screw (Figure 1B). To correct for projection errors, measurements of LSP were multiplied by a ratio of the true length of the lag screw and the projected length of the lag screw measured on radiographs. 29
2.4. Gait analysis
At each follow‐up, 45 retroreflective markers were placed at anatomical landmarks of the pelvis, lower limb, and torso, inclusive of rigid clusters placed on the thighs and lower legs. 30 A static trial was first captured with patient standing upright. Patients were instructed to walk at a self‐selected pace while walking trials were collected using a 10‐camera motion capture system (Vantage V5, Vicon Motion Systems Ltd., 100 Hz) and two force plates (AMTI Optima, 2000 Hz) measuring ground reaction forces (GRFs). Marker trajectories and GRFs were filtered with a zero‐lag second order low pass Butterworth filter at a cutoff frequency of 8 Hz. Patients unable to walk unaided walked with a custom‐built instrumented walker previously described. 31 In summary, vertical forces applied to each handle were recorded synchronously with motion capture trials. Instrumented walker loads were filtered with a zero‐lag fourth‐order Butterworth filter at a cutoff frequency of 10 Hz. For the elderly controls, the same marker setup and motion capture system were used, with participants instructed to walk at a self‐selected pace. Marker trajectories and GRFs were filtered in the same manner as the fracture patients.
2.5. Musculoskeletal modeling and data reduction
An OpenSim lower limb model 32 (gait2392) was scaled using an atlas‐based statistical shape modeling method (MAPClient) 33 by fitting principal components of an articulated shape model to landmarks collected from motion capture static trials and computing scale factors for each body segment. 34 Models were scaled using only body segment scale factors calculated from 6‐month static trials however, model mass was adjusted to the measured mass at a respective time point. This was under the assumption that there were negligible changes in skeletal segment length and size of body segments in the gait2392 model (torso, pelvis, femur, tibia, and foot) between 6 weeks and 6 months. However, changes in patient body mass between time points were accounted for, and body segment intertial parameters were adjusted using corrections previously reported by de Leva. 35 As an additional consideration, marker placement variability is a large source of error in motion capture research, given the subjective nature in relying on tactile and visual feedback for placement. 36 Therefore, using the statistical shape modeling approach for scaling models from a single time point, minimized modeling errors arising from variability in marker placements between time points. For patients who used the instrumented walker, left and right reaction forces and moments from the walker handles were applied at phantom joints placed at the acromion marker positions of the OpenSim model. 31
Inverse kinematics was used to reconstruct motion from marker data in captured walking trials. Joint angles for the hip, knee, and ankle and segment angle for the pelvis were calculated as Euler angles in accordance with the International Society of Biomechanics recommendations. 37 Joint angles from heel strike to following heel strike of the ipsilateral limb, corresponding with a complete gait cycle (stance and swing phases), were resampled to 101 points (time normalized) and expressed as a percentage of the gait cycle. Gait metrics of walking speed, step length, and maximum contralateral pelvic drop during stance were extracted as they reflect overall function and walking ability in this cohort. Presence of Trendelenburg gait was categorized as a contralateral pelvic drop of >4° during stance. 15 Joint kinematics and kinetics were reported in the sagittal and coronal plane for the hip, and sagittal plane for the knee and ankle. External joint moments were calculated using inverse dynamics using a recursive Newton–Euler approach and normalized to patient body mass at the respective time point. Joint moments from heel‐strike to toe‐off of the affected limb, corresponding with the stance phase of a gait cycle were resampled to 101 points (time normalized) and expressed as a percentage of stance phase. For each patient, maximum hip adduction moment during stance was extracted. Joint powers were calculated and normalized to patient body mass and expressed as a percentage of stance. Time normalization of joint angles across a gait cycle, and external moments and powers across stance phase, allowed for comparison of these outcomes across stance and swing phases of gait, at corresponding percentages of each phase.
2.6. Statistical analysis
Normality of radiographic and gait analysis data was assessed using a Shapiro–Wilk test and examination of Q–Q plots. Differences in radiographic measures of FOS and lateral protrusion of the lag screw were assessed between the three device groups using a one‐way ANOVA. Spatiotemporal gait outcomes were assessed within ITF patients using a paired t‐test and between ITF patients and the healthy cohort using an independent samples t‐test. Differences between time points in VAS pain scores and HHSs were assessed using a paired t‐test. To test the first hypothesis, a general linear model (GLM) implemented using statistical parametric mapping (SPM) was used to assess differences in joint kinematics and kinetics within ITF patients between 6 weeks and 6 months postoperative, across the time normalized gait cycle and stance phase, respectively. 38 Similarly, to test the second hypothesis, a GLM implemented using SPM was used to assess differences between groups in joint kinematics and kinetics between the fracture patients at each postoperative time point and the healthy elderly controls. Adjustments for gait speed were made by its inclusion into the GLM as a covariate, as suggested when comparing pathological individuals to healthy controls, 39 with a Bonferroni correction applied for multiple comparisons The spm(t)function describes the t‐statistic across each individual time node of the continuum. 38 Using random field theory, a critical threshold is calculated where only 5% (α = 0.05) of smooth random curves are expected to cross. 40 If at any point spm(t) crosses the critical threshold, a significant difference is indicated, and the null hypothesis of no significant difference rejected across the region of the suprathreshold continuum (suprathreshold clusters).
To test the third hypothesis, the relationship between FO shortening from baseline to 6 months, and gait speed, step length, maximum external hip adduction moment, and amount of contralateral pelvic drop at 6‐month follow‐up was assessed using a Pearson correlation (r). Associations were also tested between FO changes from 6 weeks to 6 months with the amount of change in gait outcomes between 6‐week and 6‐month follow‐ups. Associations between lateral LSP measured at 6 weeks and 6 months, with each of the gait analysis measures at the same respective time point were also assessed using a Pearson correlation. Significance levels were set at α = 0.05.
3. RESULTS
Sixty‐one patients managed with a femoral nail were recruited (Figure 2), of which 34 were followed up at 6 months (Table 1). Thirty‐one patients were able to be followed up at both time points of 6 weeks and 6 months postoperative and included in the kinematic analysis. For 2 patients, there were invalid force plate data, and 4 with invalid load cell data from the instrumented walker, thus 25 patients were included in the kinetic analysis. At 6 weeks postoperative, the mean VAS pain score was 3.4 ± 2.3 and HHS was 63.3± 15.3. At 6 months postoperative, the mean VAS pain score was 2.3 ± 2.3 and HHS was 77.6 ± 14.6 (Table 2). For healthy controls, 12 elderly individuals were available for 3D gait data collection (Table 3), from which kinematic and kinetic gait analysis was conducted.
Figure 2.
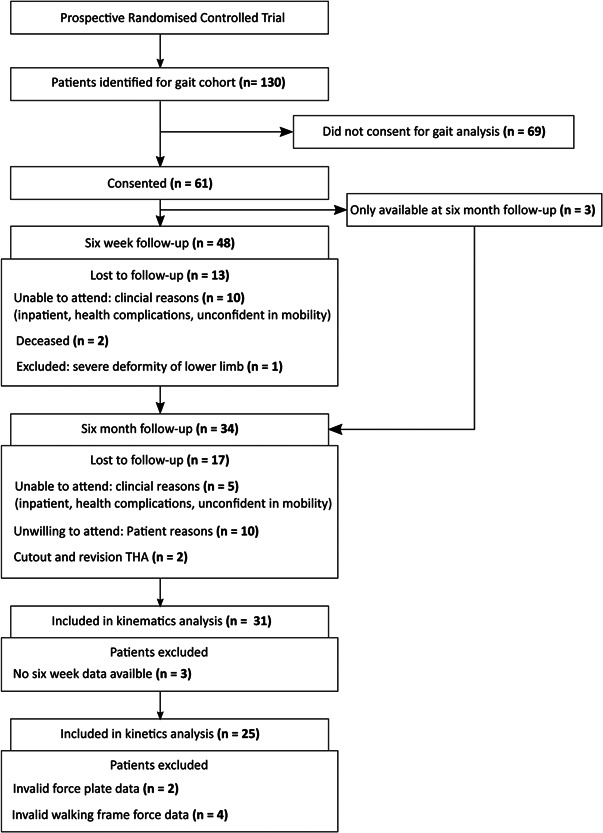
Patient recruitment and data flowchart for the fracture cohort.
Table 1.
Demographic characteristics of included patients
| Characteristic | Total |
|---|---|
| Fracture cohort | |
| Gender | 34 |
| Male | 15 |
| Female | 19 |
| Age (mean, range) | 80.4 (57–95) |
| Fracture side | |
| Left | 17 |
| Right | 17 |
| Healthy cohort | |
| Gender | 12 |
| Male | 6 |
| Female | 6 |
| Age (mean, range) | 70.8.4 (61–84) |
Table 2.
Mean pain VAS score and Harris hip score with standard deviation at 6 weeks (n = 23) and 6 months (n = 30) postoperative
| Postoperative time point | Six weeks | Six months | p Value* |
|---|---|---|---|
| VAS (/10) | 3.3 (2.3) | 2.3 (2.2) | 0.081 |
| HHS (/100) | 63.2 (15.0) | 77.7 (14.3) | <0.001† |
p Values from paired t‐test.
Significant at p < 0.05.
Table 3.
Mean spatiotemporal outcomes with standard deviation for fracture patients at 6 weeks (n = 31) and 6 months (n = 34) postoperative and healthy elderly control (n = 12)
| p Value* | ||||||
|---|---|---|---|---|---|---|
| Six weeks | Six months | Healthy elderly cohort | Six weeks to 6 months | Six weeks healthy | Six months healthy | |
| Gait speed (m/s) | 0.38 (0.21) | 0.56 (0.22) | 1.06 (0.19) | p < 0.001 | p < 0.001† | p < 0.001† |
| Step length (m) | 0.39 (0.08) | 0.44 (0.09) | 0.63 (0.08) | p < 0.001 | p < 0.001† | p < 0.001† |
| Stance/swing ratio | 2.58 (1.00) | 2.21 (1.08) | 1.58 (0.17) | p = 0.002 | p < 0.001† | p = 0.054 |
p Values from paired t‐test in comparisons between fracture patients and independent samples t‐test in comparisons between fracture patients and healthy elderly reference.
Significant at p < 0.05.
3.1. Radiographic measurements
Out of 34 patients available at 6 months, an increase in femoral offset of 6 mm was measured in one patient at the 6‐month time point with impending cutout visible on radiographs. This was considered fixation failure and excluded from analysis of radiographic measures. One patient did not have a 6‐month radiograph taken. Thus, radiographic measures were analyzed from 32 (n = 32) patients at 6 months, and 30 (n = 30) at 6‐week time points. Measures of FOS and lateral protrusion of the lag screw did not differ between femoral nail device groups in patients analyzed at 6 weeks (p = 0.951 and p = 0.115, respectively) and 6 months (p = 0.560 and p = 0.658, respectively). Thus, the pooled group of patients was analyzed in this study. At 6 weeks postoperative, there was an average decrease in femoral offset of 2.5 mm (range 0–9.7 mm), and 3.1 mm (range 0–16.6 mm) from baseline to 6 months postoperative. Between 6 weeks and 6 months, there was an average decrease in femoral offset of 1.0 mm (0–7.0 mm). As a percentage of baseline femoral offset measured from image intensifier radiographs, offset shortening was 5.9% (range 0%–16.3%) at 6 weeks, and 7.2% (range 0%–21.7%) at 6 months. Between 6 weeks and 6 months, offset shortening as a percentage of the original femoral offset was 2.2% (range 0%–16.4%) in patients available at both time points. Average lateral LSP was 5.8 mm (range 0–16.3 mm) at 6 weeks and 6.2 mm (range 0–16.3 mm) at 6 months.
3.2. Gait analysis
3.2.1. Spatiotemporal outcomes
Average spatiotemporal outcomes at 6 weeks and 6 months are shown in Table 3. Significant increases in gait speed (mean increase 0.18 m/s, [95% CI, 0.12–0.23 m/s]; p ≤ 0.001), step length (mean increase 0.05 m, [95% CI, 0.03–0.07 m]; p ≤ 0.001) and reductions in stance/swing ratio (mean reduction 0.37, [95% CI: 0.15–0.60]; p= 0.002) were identified between time points. Pearson correlation showed a weak to moderate positive correlations 41 between gait speed and HHS outcomes at 6 weeks (r = 0.534, p = 0.009) and 6 months (r = 0.372, p = 0.043).
3.2.2. Joint kinematics
Adjusting for walking speed in ITF patients between postoperative time points, significant increases in hip flexion and knee flexion between the two time points were identified within the gait cycle (Figure 3A).
Figure 3.
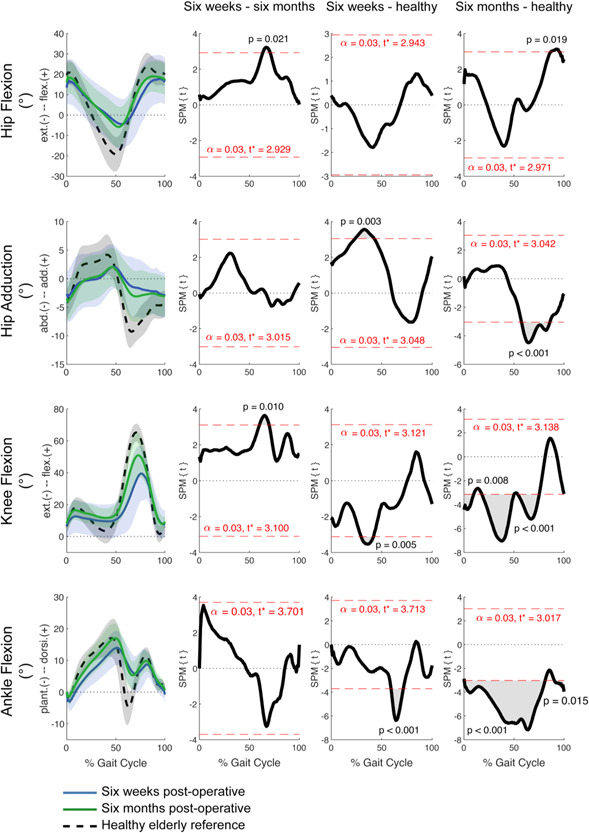
Mean joint kinematics and standard deviation for ITF patients at each time point and healthy elderly controls. General linear model t‐statistic SPM(t) for comparisons of ITF patients between 6 weeks and 6 months postoperative and with healthy controls. Bonferroni adjustments for multiple comparisons made with inclusion of gait speed as a covariate. ITF, intertrochanteric fracture; SPM, statistical parametric mapping. [Color figure can be viewed at wileyonlinelibrary.com]
In comparisons between ITF patients at 6 weeks and 6 months postoperative, suprathreshold clusters were identified for the hip and knee in the sagittal plane (Figure 3). At the hip, there were increases in flexion during initial to midswing (~65%–75% GC; Figure 3). Increases in knee flexion were also identified through initial and midswing (~60%–75% GC; Figure 3). No differences in hip adduction and ankle flexion were observed in ITF patients between time points. At 6 weeks, the amount of contralateral pelvic drop during stance averaged 2° (range 0°–4°), with three patients demonstrating a positive Trendelenburg sign. At 6 months, there was an average contralateral pelvic drop during stance of 2° (range 0°–6°), with two patients demonstrating a positive Trendelenburg sign, one of which demonstrated a positive sign at 6 weeks.
Comparing ITF patients at 6 weeks postoperative to the healthy cohort and adjusting for walking speed, there were significant deficiencies in hip adduction between midstance and terminal stance (Figure 3). Significant differences were observed in knee extension at midstance (~30%–45% GC; Figure 3), and ankle plantarflexion from initial to midswing (~60%–75% GC) (Figure 3) with ITF patients falling short of elderly controls.
ITF patients at 6 months postoperative displayed hip, knee, and ankle kinematics that fell significantly short of the healthy controls (Figure 3). At the hip, there were deficiencies in flexion during terminal swing (~85%–95% GC; Figure 3), and abduction throughout swing (~65%–90% GC; Figure 3). Knee extension also fell significantly short of controls throughout stance and early swing (~0%–75% GC; Figure 3), along with ankle flexion throughout a majority of the gait cycle (Figure 3).
3.2.3. External joint moments
For comparisons within the fracture patients between 6 weeks and 6 months, increases in external joint moments were identified at the hip, knee, and ankle throughout stance (Figure 4).
Figure 4.
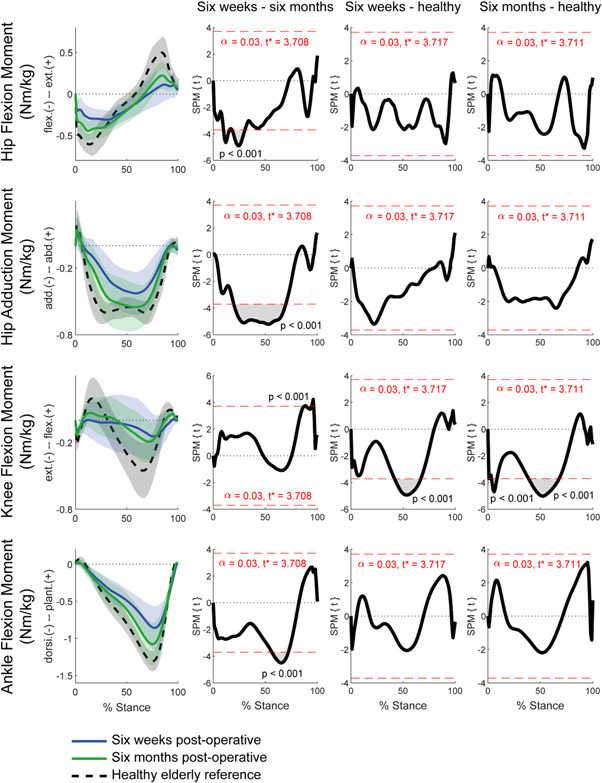
Mean external joint moments and standard deviation for ITF patients at each time point and healthy elderly controls. General linear model t‐statistic SPM(t) for comparisons of ITF patients between 6 weeks and 6 months postoperative and with healthy controls. Bonferroni adjustments for multiple comparisons made with inclusion of gait speed as a covariate. ITF, intertrochanteric fracture; SPM, statistical parametric mapping. [Color figure can be viewed at wileyonlinelibrary.com]
As depicted by the suprathreshold clusters (Figure 4), hip flexion moments increased during loading response (up to 30% stance), while hip adduction moments increased throughout single limb stance (~30%–70% stance). Knee flexion moments increased during terminal stance and pre‐swing (~85%–100% stance) along with ankle dorsiflexion moments during midstance and progression of the torso over the supporting limb (~50%–75% stance).
When comparing ITF patients to the healthy cohort, significant deficiencies in knee flexion moments were identified during midstance (~40% to −70% stance) at 6 weeks and 6 months postoperative (Figure 4).
3.2.4. Joint powers
In the fracture patients, increases in hip power generation and increases in knee power generation and absorption between 6 weeks and 6 months were identified (Figure 5). Significant increases in ankle power absorption were also identified between the two time points.
Figure 5.
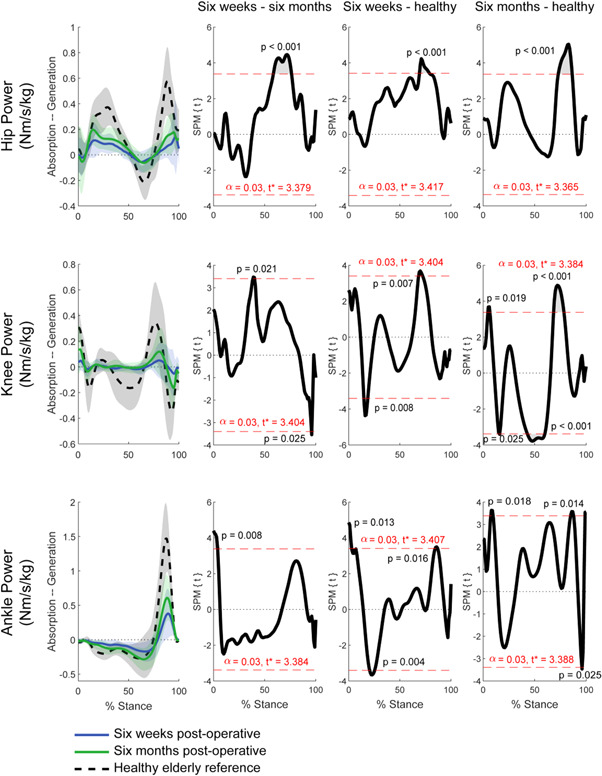
Mean joint powers and standard deviation for ITF patients at each time point and healthy elderly controls. General linear model t‐statistic SPM(t) for comparisons of ITF patients between 6 weeks and 6 months postoperative and with healthy controls. Bonferroni adjustments for multiple comparisons made with inclusion of gait speed as a covariate. ITF, intertrochanteric fracture; SPM, statistical parametric mapping. [Color figure can be viewed at wileyonlinelibrary.com]
Suprathreshold clusters were identified for joint powers at the hip, knee, and ankle between 6 weeks and 6 months in ITF patients (Figure 5). There were increases in hip power generation during midstance and terminal stance of single limb support (~60%–80% stance). At the knee, two suprathreshold clusters were identified describing increases in power generation during mid‐stance, and increased power absorption towards terminal stance to pre‐swing (~90% to ~95% stance). At the ankle, power absorption increased during loading response of the ipsilateral foot (~0%–10% stance). At 6 weeks postoperative, power generation at the hip and knee of ITF patient, fell short of healthy controls during terminal stance (~70%–80% stance). At the ankle, less power absorption during early loading response (~0%–5% stance) and power generation toward terminal stance (~80%–90% stance) were observed. At 6 months postoperative, deficiencies were identified in similar regions, between ITF patients and healthy controls. Specifically, hip power generation was significantly less than healthy controls during terminal stance of single limb support (~70%–80% stance). Additionally, ankle power absorption fell short of healthy controls during loading response (~5%–10% stance) and during terminal stance and pre‐swing (~90%–100% stance).
3.3. Correlations between radiographic measures and gait analysis outcomes
From baseline to 6 months postoperative, there was no association between amount of FOS and gait speed, step length, maximum hip abduction, or maximum hip adduction moment (Table 4). Similarly, there was no correlation between amount of FOS and change in gait measures between 6 weeks and 6 months. The magnitude of lateral LSP showed a moderate negative correlation 41 with maximum external hip adduction moment at 6 weeks postoperative (r = −0.469, p= 0.048; Figure 6).
Table 4.
Pearson correlations between amount of lateral lag screw protrusion and femoral offset shortening with gait analyses outcomes at 6 weeks and 6 months postoperative
| Gait speed | Step length | Contralateral Pelvic Drop | Max hip adduction moment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Correlation r | p Value* | n | Correlation r | p Value* | n | Correlation r | p Value* | n | Correlation r | p Value* | |
| Δ Femoral offset (baseline – 6 months) | 32 | −0.183 | 0.951 | 32 | −0.237 | 0.764 | 32 | 0.036 | 1.690 | 30 | 0.002 | 1.690 |
| Δ Femoral offset (6 weeks to 6 months) | 29 | −0.227 | 0.948 | 29 | −0.032 | 1.736 | 29 | −0.025 | 1.736 | 25 | −0.240 | 0.948 |
| Δ Relative femoral offset (baseline – 6 months) | 32 | −0.247 | 0.516 | 32 | −0.299 | 0.388 | 32 | −0.056 | 1.520 | 30 | 0.036 | 1.520 |
| Δ Relative femoral offset (6 weeks to 6 months) | 29 | −0.231 | 0.912 | 29 | −0.028 | 1.774 | 29 | −0.019 | 1.774 | 25 | −0.244 | 0.912 |
| Lag screw protrusion (6 weeks) | 30 | −0.087 | 0.646 | 30 | −0.234 | 0.639 | 30 | 0.203 | 0.639 | 28 | −0.469 | 0.048† |
| Lag screw protrusion (6 months) | 32 | −0.034 | 0.853 | 32 | −0.208 | 0.759 | 32 | 0.178 | 0.759 | 30 | −0.400 | 0.112 |
Note: n: number of participants.
p Values corrected with Holm–Bonferroni adjustment.
Significant at p < 0.05.
Figure 6.
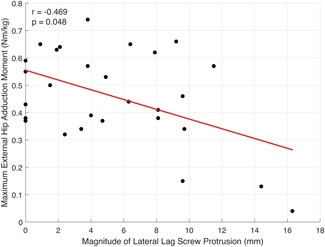
Scatter plot with line of best fit (red) illustrating the Pearson correlation between the magnitude of lateral lag screw protrusion and the maximum hip abduction moment at 6 weeks postoperative [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This study aimed to identify changes in gait biomechanics between 6 weeks and 6 months postoperatively in elderly ITF patients, and how these differ from elderly controls. We also aimed to investigate whether FOS and lateral LSP were correlated with outcomes from gait analysis. From our results, spatiotemporal parameters of gait speed, step length, and stance/swing ratio increased significantly between 6 weeks and 6 months postoperative indicating overall improvement in walking ability. However, slower gait speeds by an average of 0.50 m/s (61.4%), and larger step lengths by an average of 0.19 m (35.5%) were identified in ITF patients at 6 months postoperative, in comparison to healthy controls. Gait speed in older adults above 60 years has been reported to serve as a significant predictor of adverse outcomes including severe disability, cognitive impairment, falls, and mortality. 42 Population‐level studies in older adults report gait speeds of between 0.92 and 1.13 m/s with inclusion of individuals with pathology. 43 Furthermore, a cutoff of 0.8 m/s has been used to predict adverse health outcomes including secondary falls, 42 which the patients in our study at 6 months postoperative fall below.
Considering the age and vulnerability of this cohort, the literature indicates that postoperative functional goals are to achieve similar levels of independence and ambulation as at pre‐fracture. 44 Previous studies assessing functional independence have reported declines in functional outcome scores and ADLs, 45 however changes in gait biomechanics after ITF have been unexplored. These data provide more detail on functional outcomes and potential mechanisms that functional deficiencies stem from. Our results showed overall improvements in hip, knee, and ankle biomechanics between 6 weeks and 6 months postoperative in ITF patients primarily in single limb stance. Specifically, increases in external hip adduction moment and hip power generation during progression of the torso over the supporting limb, where forward propulsion is generated, were identified. When compared to elderly controls, there were significant deficiencies in hip abduction, ankle plantarflexion as well as hip and ankle power generation, particularly during the propulsive phase of single limb stance. In the elderly, decreases in hip power generation, 46 peak hip abduction, and ankle dorsiflexion 47 have been associated with increased fall risk. Studies also indicated that increased ankle plantarflexion and power generation are important mechanisms in increasing gait speed, 48 which may contribute to reduced gait speeds observed in fracture patients. Thus, it could be suggested that minimizing declines in hip ROM and power generation is important in maintaining functional independence after ITF.
Our analysis of radiographic measures showed no correlation between FOS at 6 months postoperative with gait speed, step length, contralateral pelvic drop, or external hip adduction moments. Similarly, there was no correlation between FOS and changes in the aforementioned gait measures between 6 weeks and 6 months postoperative. While some authors have reported associations between increased FOS and decreased spatiotemporal gait parameters, 12 others have found no association between offset shortening and functional outcome, suggesting that violation of the abductor's muscles negates potential biomechanical advantages of larger femoral offsets. 49 This may explain our findings, with generally poor abductor function and rigid angular pelvic motions exhibited in our patient group. Rigid motions may explain by generally poor physical function common in elderly hip fracture patients, 50 resulting from age‐related changes in pelvis and lower limb motions. 51
Our results showed a moderate correlation between the magnitude of lateral LSP at 6 weeks postoperative with reduced external hip adduction moments. However, this was not significant at 6 months. Hip abduction moments have previously been reported to be an important factor in postural stability control in the mediolateral direction, 52 and used as a predictor of future falls in older adults. 53 Thus, the amount of LSP may be a more important factor than FOS in the management of ITFs in minimizing secondary fall risk and declines in functional independence.
4.1. Limitations
When interpreting these results, several limitations must be acknowledged. First, a limited number of patients were analyzed arising from the complex patient demographic reflected in their age and comorbidities. This presented challenges in attendance of follow‐ups and number of patients analyzed. Second, the cohort analyzed in this study included patients managed with either one of two femoral nails of different designs. While nail variability was not a factor in comparisons among ITF patients, the effect of nail variability was considered for comparisons between ITF patients and the healthy cohort. For patients analyzed, there was no difference in FOS and protrusion of the lag screw between femoral nail groups. Based on these results, we analyzed the pooled group of patients to investigate overall changes in gait biomechanics and associations between these radiographic measures and gait outcomes. However, we cannot rule out that outcomes may vary with the type of femoral nail used and parameters inherent to femoral nail design. Additionally, the ITF subgroup included patients who walked with and without a walking frame (Supporting Information). Although previous research has shown the use of a walker to affect posture of the torso and increase in upper limb joint moments, lower limb joint biomechanics of walker‐assisted gait in the elderly has been unexplored. 23 In our cohort, only four patients required a walker at 6 months. With generally frail and rigid lower limb motion of this cohort, 51 and current literature, 23 it is plausible that the most discernible differences in gait mechanics occur at the torso and upper limbs. Considering similar lower limb gait mechanics during stance at 6 weeks and being mindful of overstratification of data into smaller groups and risking bias, 54 the use of a walking frame was not accounted for in this analysis.
Limitations of anatomical measurements from plain film radiographs must be considered when interpreting these results. With authors reporting errors in the accuracy of measured distances on digital radiographs of between 1.4 and 2.6 mm for long distances, 28 there is a degree of uncertainty in these measures. Future work should further explore the effects of femoral nail positioning and geometry of the bone‐implant construct on gait biomechanics in ITF patients.
5. CONCLUSION
Patients showed significant improvements in gait biomechanics in the first 6 months after surgery. However, were considerably short of elderly controls in measures that have been associated with increased fall risk. Lag screw prominence may be an important factor in minimizing secondary fall risk and maintaining independence after ITF.
AUTHOR CONTRIBUTIONS
Mark Rickman and Dominic Thewlis designed the study. Arjun Sivakumar developed and carried out the data collection, musculoskeletal modeling, and analyses of results. Arjun Sivakumar took the lead in writing the manuscript. All authors provided feedback and contributed to shaping the research, analysis, and manuscript.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors acknowledge Kieran Bennet for his contributions to the development of the instrumented walking frame and modeling workflow used in this study. This study is funded through an investigator‐initiated grant by Smith & Nephew Inc Orthopaedic Division (SN). DT received fellowship funding from the NHMRC (CDF ID: 1126229). Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Sivakumar A, Rickman M, Thewlis D. Gait biomechanics after proximal femoral nailing of intertrochanteric fractures. J Orthop Res. 2023;41:862‐874. 10.1002/jor.25427
REFERENCES
- 1. Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15(11):897‐902. 10.1007/s00198-004-1627-0 [DOI] [PubMed] [Google Scholar]
- 2. Lakstein D, Hendel D, Haimovich Y, Feldbrin Z. Changes in the pattern of fractures of the hip in patients 60 years of age and older between 2001 and 2010: a radiological review. Bone Joint J. 2013;95‐b(9):1250‐1254. 10.1302/0301-620x.95b9.31752 [DOI] [PubMed] [Google Scholar]
- 3. Cooper C, Campion G, Melton LJ IIIrd. Hip fractures in the elderly: a world‐wide projection. Osteoporos Int. 1992;2(6):285‐289. 10.1007/bf01623184 [DOI] [PubMed] [Google Scholar]
- 4. Kammerlander C, Gosch M, Kammerlander‐Knauer U, Luger TJ, Blauth M, Roth T. Long‐term functional outcome in geriatric hip fracture patients. Arch Orthop Trauma Surg. 2011;131(10):1435‐1444. 10.1007/s00402-011-1313-6 [DOI] [PubMed] [Google Scholar]
- 5. Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long‐term disability outcomes following hip fracture. BMC Geriatr. 2016;16(1):158. 10.1186/s12877-016-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takahashi A, Naruse H, Kitade I, et al. Functional outcomes after the treatment of hip fracture. PLoS One. 2020;15(7):e0236652. 10.1371/journal.pone.0236652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adeyemi A, Delhougne G. Incidence and economic burden of intertrochanteric fracture: a Medicare claims database analysis. JBJS Open Access. 2019;4(1):0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anglen JO, Weinstein JN, American Board of Orthopaedic Surgery Research Committee . Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700‐707. [DOI] [PubMed] [Google Scholar]
- 9. Gilat R, Lubovsky O, Atoun E, Debi R, Cohen O, Weil YA. Proximal femoral shortening after cephalomedullary nail insertion for intertrochanteric fractures. J Orthop Trauma. 2017;31(6):311‐315. 10.1097/bot.0000000000000835 [DOI] [PubMed] [Google Scholar]
- 10. Ciufo DJ, Ketz JP. Proximal femoral shortening and varus collapse after fixation of “stable” pertrochanteric femur fractures. J Orthop Trauma. 2021;35(2):87‐91. [DOI] [PubMed] [Google Scholar]
- 11. Zlowodzki M, Brink O, Switzer J, et al. The effect of shortening and varus collapse of the femoral neck on function after fixation of intracapsular fracture of the hip. J Bone Joint Surg Br. 2008;90‐B(11):1487‐1494. 10.1302/0301-620x.90b11.20582 [DOI] [PubMed] [Google Scholar]
- 12. Gausden EB, Sin D, Levack AE, et al. Gait analysis after intertrochanteric hip fracture: does shortening result in gait impairment. J Orthop Trauma. 2018;32(11):554‐558. 10.1097/BOT.0000000000001283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnston RC, Brand RA, Crowninshield RD. Reconstruction of the hip. A mathematical approach to determine optimum geometric relationships. J Bone Joint Surg Am. 1979;61(5):639‐652. [PubMed] [Google Scholar]
- 14. Neumann DA. Biomechanical analysis of selected principles of hip joint protection. Arthritis Care Res. 1989;2(4):146‐155. 10.1002/anr.1790020409 [DOI] [PubMed] [Google Scholar]
- 15. Bailey R, Selfe J, Richards J. The role of the Trendelenburg Test in the examination of gait. Phys Ther Rev. 2009;14(3):190‐197. 10.1179/174328809X452836 [DOI] [Google Scholar]
- 16. Nherera L, Trueman P, Horner A, Watson T, Johnstone AJ. Comparison of a twin interlocking derotation and compression screw cephalomedullary nail (InterTAN) with a single screw derotation cephalomedullary nail (proximal femoral nail antirotation): a systematic review and meta‐analysis for intertrochanteric fractures. J Orthop Surg Res. 2018;13(1):46. 10.1186/s13018-018-0749-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koval KJ. Intramedullary nailing of proximal femur fractures. Am J Orthop. 2007;36(4 Suppl):S4‐S7. [PubMed] [Google Scholar]
- 18. Hesse B, Gächter A. Complications following the treatment of trochanteric fractures with the gamma nail. Arch Orthop Trauma Surg. 2004;124(10):692‐698. 10.1007/s00402-004-0744-8 [DOI] [PubMed] [Google Scholar]
- 19. Rosen M, Kasik C, Swords M. Management of lateral thigh pain following cephalomedullary nail: a technical note. Spartan Med Res J. 2020;5(1):12931. 10.51894/001c.12931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koval KJ, Friend KD, Aharonoff GB, Zuckerman JD. Weight bearing after hip fracture: a prospective series of 596 geriatric hip fracture patients. J Orthop Trauma. 1996;10(8):526‐530. [DOI] [PubMed] [Google Scholar]
- 21. Heikkinen T, Jalovaara P. Four or twelve months' follow‐up in the evaluation of functional outcome after hip fracture surgery. Scand J Surg. 2005;94(1):59‐66. 10.1177/145749690509400115 [DOI] [PubMed] [Google Scholar]
- 22. de Joode SGCJ, Kalmet PHS, Fiddelers AAA, Poeze M, Blokhuis TJ. Long‐term functional outcome after a low‐energy hip fracture in elderly patients. J Orthop Traumatol. 2019;20(1):20. 10.1186/s10195-019-0529-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mundt M, Batista JP, Markert B, Bollheimer C, Laurentius T. Walking with rollator: a systematic review of gait parameters in older persons. Eur Rev Aging Phys Act. 2019;16:15. 10.1186/s11556-019-0222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sloot LH, van der Krogt MM. Interpreting joint moments and powers in gait. Handbook of Human Motion. Springer International Publishing; 2018:625‐643. [Google Scholar]
- 25. Esbjörnsson AC, Kiernan S, Mattsson L, Flivik G. Geometrical restoration during total hip arthroplasty is related to change in gait pattern – a study based on computed tomography and three‐dimensional gait analysis. BMC Musculoskelet Disord. 2021;22(1):369. 10.1186/s12891-021-04226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sivakumar A, Thewlis D, Ladurner A, Edwards S, Rickman M. Proximal Femoral Nail Unlocked versus Locked (ProFNUL): a protocol for a multicentre, parallel‐armed randomised controlled trial for the effect of femoral nail mode of lag screw locking and screw configuration in the treatment of intertrochanteric femur fractures. BMJ Open. 2020;10(2):e032640. 10.1136/bmjopen-2019-032640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lechler P, Frink M, Gulati A, et al. The influence of hip rotation on femoral offset in plain radiographs. Acta Orthop. 2014;85(4):389‐395. 10.3109/17453674.2014.931196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fowler JR, Ilyas AM. The accuracy of digital radiography in orthopaedic applications. Clin Orthop Relat Res. 2011;469(6):1781‐1784. 10.1007/s11999-010-1628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serrano R, Blair JA, Watson DT, et al. Cephalomedullary nail fixation of intertrochanteric femur fractures: are two proximal screws better than one? J Orthop Trauma. 2017;31(11):577‐582. [DOI] [PubMed] [Google Scholar]
- 30. Cappozzo A, Catani F, Leardini A, Benedetti MG, Della Croce U. Position and orientation in space of bones during movement: experimental artefacts. Clin Biomech. 1996;11(2):90‐100. 10.1016/0268-0033(95)00046-1 [DOI] [PubMed] [Google Scholar]
- 31. Sivakumar A, Bennett K, Rickman M, Thewlis D. An instrumented walker in three‐dimensional gait analysis: improving musculoskeletal estimates in the lower limb mobility impaired. Gait Posture. 2022;93:142‐145. 10.1016/j.gaitpost.2022.01.023 [DOI] [PubMed] [Google Scholar]
- 32. Delp SL, Anderson FC, Arnold AS, et al. OpenSim: open‐source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54(11):1940‐1950. 10.1109/tbme.2007.901024 [DOI] [PubMed] [Google Scholar]
- 33. Zhang J, Sorby H, Clement J, eds. The MAP Client: user‐friendly musculoskeletal modelling workflows. Biomedical Simulation. Springer International Publishing; 2014. [Google Scholar]
- 34. Bahl JS, Zhang J, Killen BA, et al. Statistical shape modelling versus linear scaling: effects on predictions of hip joint centre location and muscle moment arms in people with hip osteoarthritis. J Biomech. 2019;85:164‐172. 10.1016/j.jbiomech.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 35. de Leva P. Adjustments to Zatsiorsky‐Seluyanov's segment inertia parameters. J Biomech. 1996;29(9):1223‐1230. 10.1016/0021-9290(95)00178-6 [DOI] [PubMed] [Google Scholar]
- 36. Chambers C, Goode B. Variability in gait measurements across multiple sites. Gait Posture. 1996;4:167. [Google Scholar]
- 37. Wu G, Siegler S, Allard P, et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—part I: ankle, hip, and spine. J Biomech. 2002;35(4):543‐548. 10.1016/S0021-9290(01)00222-6 [DOI] [PubMed] [Google Scholar]
- 38. Pataky TC, Vanrenterghem J, Robinson MA. Zero‐ vs. one‐dimensional, parametric vs. non‐parametric, and confidence interval vs. hypothesis testing procedures in one‐dimensional biomechanical trajectory analysis. J Biomech. 2015;48(7):1277‐1285. 10.1016/j.jbiomech.2015.02.051 [DOI] [PubMed] [Google Scholar]
- 39. Fukuchi CA, Fukuchi RK, Duarte M. Effects of walking speed on gait biomechanics in healthy participants: a systematic review and meta‐analysis. Syst Rev. 2019;8(1):153. 10.1186/s13643-019-1063-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adler RJ, Taylor JE. Random Fields and Geometry. Springer; 2007. [Google Scholar]
- 41. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763‐1768. [DOI] [PubMed] [Google Scholar]
- 42. Abellan Van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community‐dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881‐889. 10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 43. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50‐58. 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palm H. Hip fracture: the choice of surgery. In: Falaschi P, Marsh D, eds. Orthogeriatrics: The Management of Older Patients with Fragility Fractures. Springer International Publishing; 2021:125‐141. [PubMed] [Google Scholar]
- 45. Ganz SB, Peterson MGE, Russo PW, Guccione A. Functional recovery after hip fracture in the subacute setting. HSS J. 2007;3(1):50‐57. 10.1007/s11420-006-9022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perry MC, Carville SF, Smith ICH, Rutherford OM, Newham DJ. Strength, power output and symmetry of leg muscles: effect of age and history of falling. Eur J Appl Physiol. 2007;100(5):553‐561. 10.1007/s00421-006-0247-0 [DOI] [PubMed] [Google Scholar]
- 47. Chiacchiero M, Dresely B, Silva U, DeLosReyes R, Vorik B. The relationship between range of movement, flexibility, and balance in the elderly. Top Geriatr Rehabil. 2010;26(2):148‐155. [Google Scholar]
- 48. Graf A, Judge JO, Õunpuu S, Thelen DG. The effect of walking speed on lower‐extremity joint powers among elderly adults who exhibit low physical performance. Arch Phys Med Rehabil. 2005;86(11):2177‐2183. 10.1016/j.apmr.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 49. Reindl R, Harvey EJ, Berry GK, Rahme E. Intramedullary versus extramedullary fixation for unstable intertrochanteric fractures: a prospective randomized controlled trial. J Bone Joint Surg Am. 2015;97(23):1905‐1912. [DOI] [PubMed] [Google Scholar]
- 50. Sanders D, Bryant D, Tieszer C, et al. A multicenter randomized control trial comparing a novel intramedullary device (InterTAN) versus conventional treatment (sliding hip screw) of geriatric hip fractures. J Orthop Trauma. 2017;31(1):1‐8. 10.1097/bot.0000000000000713 [DOI] [PubMed] [Google Scholar]
- 51. Gimmon Y, Riemer R, Rashed H, et al. Age‐related differences in pelvic and trunk motion and gait adaptability at different walking speeds. J Electromyography Kinesiol. 2015;25(5):791‐799. 10.1016/j.jelekin.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 52. Porto JM, Freire Júnior RC, Bocarde L, et al. Contribution of hip abductor–adductor muscles on static and dynamic balance of community‐dwelling older adults. Aging Clin Exp Res. 2019;31(5):621‐627. 10.1007/s40520-018-1025-7 [DOI] [PubMed] [Google Scholar]
- 53. Hilliard MJ, Martinez KM, Janssen I, et al. Lateral balance factors predict future falls in community‐living older adults. Arch Phys Med Rehabil. 2008;89(9):1708‐1713. 10.1016/j.apmr.2008.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Stavola BL, Cox DR. On the consequences of overstratification. Biometrika. 2008;95(4):992‐996. 10.1093/biomet/asn039 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


