Abstract
Aims
To examine association of liver‐expressed antimicrobial peptide 2 (LEAP2), an endogenous ghrelin antagonist with anorexiant effects, to key cardiometabolic risk factors in people with overweight and obesity.
Methods
In this cross‐sectional study, we sought to identify associations between LEAP2 levels and cardiometabolic risk factors, including body composition (dual X‐ray absorptiometry), insulin and glucose metabolism (oral and intravenous glucose tolerance tests and hyperinsulinaemic‐euglycaemic clamps), plasma lipids and inflammation markers (ELISA and multiplex assays).
Results
In 65 participants with overweight or obesity (63.1% male, mean age 31.3 ± 8.5 years), LEAP2 levels were associated with total body fat, but not with body mass index or waist‐hip ratio in both univariable and age‐ and sex‐adjusted models (P < 0.05). Higher LEAP2 level was also positively associated with higher insulin secretion in univariable (P = 0.047) and multivariable models adjusted for age, sex and body fat (P = 0.03), but not with fasting glucose levels (P ≥ 0.05). Higher LEAP2 levels were associated insulin resistance (P = 0.07) after adjustment for age and sex, but the association disappeared after an additional adjustment for body fat (P = 0.2). There was an inverse association between LEAP2 levels and nuclear factor kappa‐B (NFκB) activity in the peripheral blood mononuclear cells in age‐, sex‐ and body fat‐adjusted models (P = 0.04). There were no associations with cardiovascular risk factors (lipids, blood pressure) or other inflammation markers.
Conclusions
These results provide important insights into the association between LEAP2 and cardiometabolic health in a high‐risk population of individuals with overweight and obesity. This is a first report of an association between LEAP2 and insulin secretion, insulin sensitivity and NFκB activity. LEAP2 may represent an important potential therapeutic target to promote insulin secretion in people with type 2 diabetes and obesity.
Keywords: inflammation, insulin resistance, insulin secretion, obesity, overweight, metabolic syndrome, diabetes
1. INTRODUCTION
In the last two decades, the number of individuals living with obesity has tripled. 1 Overweight and obesity are important risk factors for several cancers, cardiovascular disease and type 2 diabetes and are associated with increased all‐cause mortality. 2 There is a range of mechanisms by which overweight and obesity are thought to cause chronic diseases, but insulin resistance, hyperglycaemia, chronic low‐grade inflammation and oxidative stress are the most well understood. 3 , 4
Lifestyle modification with calorie‐restrictive diets and exercise programmes are the mainstay of treatment for obesity and related chronic diseases. 5 , 6 However, while diet and exercise programmes promote weight loss, adherence to these interventions is poor, with the majority of individuals either failing to decrease their body mass index (BMI), or rapidly regaining weight, often exceeding baseline weight. 7 , 8 Lifestyle modifications can be supplemented with medications, which act through a number of pathways to facilitate weight loss. While these medications promote weight loss, particularly in conjunction with lifestyle interventions, their effects are hampered by undesirable side effects. Given these challenges, there has been significant interest in identifying novel means to promote weight loss in people living with overweight and obesity.
An emergent area of interest for developing anti‐obesity agents is the modulation of appetite through pharmacological intervention. In humans, appetite is regulated by a number of neurological, endocrine and paracrine mechanisms, most of which are regulated by the hypothalamus and brainstem. 9 One of the most influential regulators of appetite is the “hunger hormone” ghrelin, which increases in periods of negative energy balance and leads to increased appetite. However, it also has a host of additional central and peripheral actions, including in learning, reward seeking, sleep‐wake cycles and glucose metabolism. 10 Inhibition of the ghrelin system may be a target for weight loss. 11 However, plasma ghrelin concentrations are suppressed during obesity, and obesity is associated with reduced ghrelin efficacy. 12 Although targeting ghrelin or its receptor directly may not deliver beneficial outcomes for weight loss, other potential factors regulating the ghrelin system may be important. Recently, liver‐expressed antimicrobial peptide 2 (LEAP2) has emerged as a potent endogenous inhibitor of ghrelin signalling through competitive inhibition of growth hormone secretagogue receptor 1a (GHS‐R1a). 13 As indicated by its name, LEAP2 was originally identified as a peptide expressed by the liver with a role in immune defence, as it had dose‐dependent antimicrobial activity in some model microbes. 14 However, it has now been shown to be expressed strongly by the small intestine, particularly the jejunum, 13 and it mirrors the activity of ghrelin, that is, it decreases in states of negative energy balance, such as fasting, and increases with periods of positive energy balance, such as obesity. Indeed, it is thought that LEAP2 underlies the reduced actions of ghrelin in obesity 15 by reducing the activity appetite‐stimulating neuropeptide Y neurons in the hypothalamus. 16 The means by which LEAP2 may act on the central nervous system are currently unclear as it is not yet known whether it can cross the blood‐brain barrier, however, LEAP2 mRNA has been shown to be expressed in the brains of rats. 17 Recent studies showed that LEAP2 reduced ad libitum food intake and improved postprandial glucose tolerance in healthy men, 18 supporting a potential role in diabetes in adults 19 and children. 20 LEAP2 may therefore be a promising anti‐obesity target. 21 However, there remain many questions regarding its actions in vivo, particularly surrounding its actions on risk factors associated with overweight and obesity, such as insulin sensitivity, secretion and glucose metabolism, cardiometabolic variables, as well as inflammation and oxidative stress.
To evaluate the potential role of LEAP2 in obesity and related cardiometabolic risks, we sought to identify its association with important risk factors of diabetes and cardiovascular disease. We hypothesized that LEAP2 would be positively associated with adiposity, insulin resistance, dyslipidaemia and inflammation.
2. METHODS
2.1. Design
To identify associations between LEAP2 and indicators of cardiometabolic disease, we conducted a cross‐sectional analysis of baseline data for a previous clinical trial of non‐diabetic adults with overweight or obesity (BMI ≥25 and ≥30, respectively). 29 Participants were community‐dwelling adults, aged 18 to 60 years from Melbourne, Australia. Participants were excluded if they were taking medications, vitamins, or supplements, or were current smokers or heavy alcohol users. Participants were also ineligible to participate if they had evidence or history of diabetes or cardiovascular, central nervous system, gastrointestinal, endocrine, hematological, respiratory, or kidney diseases as well as psychiatric disorders. Pregnant, lactating, or peri‐ or postmenopausal women, those diagnosed with cancer in the preceding 5 years, or with acute inflammation were also excluded.
2.2. Outcome measures
2.2.1. LEAP2 measurement
LEAP2 was measured in baseline, fasted human plasma samples using a previously validated 15 LEAP2 38‐77 (Human/LEAP2 37‐76 [Mouse]) enzyme‐linked immunosorbent assay kit (#EK‐075040; Phoenix Pharmaceuticals Inc, Burlingame, California). Whole blood was collected through peripheral venipuncture into collection tubes coated with EDTA (BD Biosciences, San Jose, California), before being centrifuged, and the plasma was collected via sterile pipette and stored at −80°C prior to analysis.
2.3. Anthropometric, clinical, and biochemical measurements
The protocol and methodology used in the study have been described comprehensively elsewhere. 30 Briefly, body composition including total body fat was determined by dual‐energy X‐ray absorptiometry. Weight and height were measured by a calibrated scale and standing stadiometer and used to calculate BMI as weight (kg) divided by height (m). 2 Waist and hip measurements were assessed in line with World Health Organization guidelines. 31 Blood pressure, heart rate, pulse pressure and mean arterial pressure were measured using an Omron digital blood pressure monitor. All participants underwent a 75‐g oral glucose tolerance test (OGTT), performed after a 12‐hour overnight fast to assess fasting and 2‐hour post‐load blood glucose concentrations. Insulin and glucose variables were also measured during intravenous glucose tolerance tests (IVGTT) after a 12‐hour overnight fast. First, fasting blood samples were collected by venepuncture, before participants underwent a 30‐minute IVGTT with 50 mL 50% glucose delivered over 3 minutes, and blood insulin and glucose measurements taken at 3, 4, 5, 6, 8, 10, 15, 20, 25 and 30 minutes. The IVGTT data were used to calculate area under the curve (AUC), for first‐phase (3 to 5 minutes), second‐phase (10 to 30 minutes) and total glucose levels via the trapezoidal method. Insulin sensitivity was assessed by “gold‐standard” hyperinsulinaemic‐euglycaemic clamps, with M‐values calculated. A 9‐mU/kg bolus insulin injection was used to initiate the procedure, followed by a 40 mU · m−2 · min−1 infusion for at least 120 minutes. A variable glucose infusion was adjusted every 5 minutes to maintain euglycaemia at a concentration of 5 nmol/L for ≥30 minutes.
Commercial immunoassays were used to assess total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and triglycerides. Plasma high‐sensitivity C‐reactive protein was measured by highly sensitive near‐infrared particle immunoassays on Synchron LX analyzers (Beckman Coulter Inc., Australia). Serum levels of inflammatory markers, including interleukin (IL)‐1β, IL‐6, IL‐8, IL‐10 and monocyte chemoattractant protein‐1 (MCP‐1), as well as adipokines (leptin, resistin, adiponectin and adipsin) were measured using commercial multi‐analyte assays (LEGENDplex™; Biolegend, San Diego, California) according to the manufacturer's instructions on a BD™ LSR II flow cytometer. The concentration of nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NFκB) in the peripheral blood mononuclear cells was measured by the TransAM NFκB DNA‐binding activity assay (Active Motif, Carlsbad, California) to identify transcription factor activation, as described previously. 29
2.4. Statistics
Data are presented as mean ± standard deviation (SD) or median (interquartile range) for normal and non‐normal distributions, respectively. Visual inspections of histograms were used to determine distributions, and skewed variables were log‐transformed where appropriate, otherwise non‐parametric tests were used. Independent Students t‐tests were used to determine differences by sex or diabetes history, while differences by ethnicity were determined using one‐way analysis of variance. Pearson and Spearman correlations were used to assess univariable correlations between LEAP2 concentrations and outcomes of interest with normal or non‐normal distributions, respectively. Multivariable regression using general linear models was used to examine relationships between LEAP2 concentrations and anthropometric, glycaemic and cardiovascular/ inflammation variables, with adjustment for predetermined, clinically relevant factors including age, sex and adiposity (% body fat). All data analyses were conducted using STATA/SE V.15.0 (College Station, Texas) and P values < 0.05 were taken to indicate statistical significance.
2.5. Study approval
All participants provided written informed consent to participate in the analysis. The study was performed in accordance with the requirements of the declaration of Helsinki and was approved by the Monash University Human Research Ethics Committee (ref. CF13/3874‐2 013 001 988).
3. RESULTS
3.1. Sample characteristics
Participant characteristics are presented in Table 1. Sixty‐five participants (24 female and 41 male) were included in the analysis. The mean age and BMI of participants was 31.3 ± 8.5 years and 31.5 ± 5.2 kg/m2, respectively, with 30 participants (46%) classified as overweight (BMI 25 to 29.9 kg/m2) and 35 (54%) classified as obese (BMI ≥30 kg/m2). LEAP2 concentrations did not differ by sex, ethnicity, or history of diabetes (any‐degree or first‐degree relative) and were not correlated with age (all P > 0.1).
TABLE 1.
Sample characteristics
| Variable | Mean ± SD or median (IQR) |
|---|---|
| Demographic and anthropometric measures | |
| Age, years | 31.3 ± 8.5 |
| Sex: M/F, n (%) | 41 (63.1) / 24 (36.9) |
| Body mass index, kg/m2 | 30.2 (27.8, 33.3) |
| Waist‐hip ratio | 0.9 (0.9, 1.0) |
| Body fat, % | 40.1 ± 8.7 |
| Fat mass, kg | 36.0 ± 11.0 |
| Fat free mass, kg | 53.4 ± 12.4 |
| Glycaemic measures | |
| Fasting blood glucose, mmol/L | 4.6 ± 0.5 |
| 2‐hour post OGTT blood glucose, mmol/L | 5.6 ± 1.6 |
| Insulin sensitivity, M; mg/kg/min | 6.6 ± 2.8 |
| Fasting insulin by IVGTT, mlU/L | 8.9 (6.7, 12.8) |
| Total insulin AUC by IVGTT, mlU/L | 1615.7 (1078.2, 2478.6) |
| First‐phase insulin AUC by IVGTT, mlU/L | 294.5 (185.2, 491.2) |
| Second‐phase insulin AUC by IVGTT, mlU/L | 1058.4 (672.1, 1854.0) |
| Cardiovascular measures | |
| Systolic blood pressure, mmHg | 121.1 ± 12.5 |
| Diastolic blood pressure, mmHg | 80.3 ± 8.6 |
| Heart rate, bpm | 75.3 ± 11.7 |
| Pulse pressure, mmHg | 40.8 ± 9.9 |
| Mean arterial pressure, mmHg | 93.9 ± 8.9 |
| Inflammation markers and adipokines | |
| High‐sensitivity C‐reactive protein, mg/L | 1.7 (0.9, 4.4) |
| Interleukin‐1β, pg/mL | 26.8 ± 23.2 |
| Nuclear factor kappa‐B, pg/ug protein | 44.5 ± 28.6 |
| Adiponectin, ng/mL | 4749.4 (2348.1, 11 243.4) |
| Adipsin, ng/mL | 1058.2 ± 914.7 |
| Leptin, ng/mL | 2.8 (1.5, 15.8) |
| Resistin, ng/mL | 0.4 (0.3, 1.2) |
| LEAP2, ng/mL | 21.04 (8.30) |
Note: Data presented as mean ± SD or median (IQR) for non‐normally distributed variables, unless otherwise indicated. OGTT, oral glucose tolerance test; IVGTT, intravenous glucose tolerance test.
3.2. Associations with anthropometric measures
There were no associations between LEAP2 concentrations and any anthropometric measures including BMI, total body fat, fat mass or fat‐free mass (all P > 0.1; Table 2). After adjustment for age and sex, there was a positive association between LEAP2 and % body fat (P = 0.049), but relationships with the other anthropometric measures remained nonsignificant (Model 1, Table 3).
TABLE 2.
Correlations between LEAP2 concentrations and cardiometabolic variables
| Variable | Correlations | |
|---|---|---|
| r | P | |
| Demographic and anthropometric measures | ||
| Age (years) | 0.15 | 0.2 |
| Body mass index a (kg/m2) | 0.04 | 0.7 |
| Waist‐hip ratio b | 0.08 | 0.5 |
| Body fat (%) | 0.10 | 0.4 |
| Fat mass (kg) | 0.14 | 0.3 |
| Fat free mass (kg) | 0.03 | 0.8 |
| Glycaemic measures | ||
| Fasting blood glucose (mmol/L) | −0.01 | 0.9 |
| 2‐hour post OGTT blood glucose (mmol/L) | 0.21 | 0.09 |
| Insulin sensitivity (M; mg/kg/min) | −0.22 | 0.07 |
| Fasting insulin by IVGTT a (mlU/L) | 0.39 | 0.001 |
| Total insulin AUC by IVGTT a (mlU/L) | 0.25 | 0.047 |
| First phase insulin AUC by IVGTT a (mlU/L) | 0.28 | 0.02 |
| Second phase insulin AUC by IVGTT a (mlU/L) | 0.25 | 0.04 |
| Cardiovascular measures | ||
| Systolic blood pressure (mmHg) | 0.22 | 0.08 |
| Diastolic blood pressure (mmHg) | 0.29 | 0.02 |
| Heart rate (bpm) | 0.08 | 0.5 |
| Pulse pressure (mmHg) | 0.02 | 0.9 |
| Mean arterial pressure (mmHg) | 0.29 | 0.02 |
| Inflammation markers and Adipokines | ||
| C‐reactive protein b (mg/L) | 0.13 | 0.3 |
| Interleukin‐1β (pg/mL) | −0.22 | 0.07 |
| Nuclear factor kappa‐B (pg/μg protein) | −0.20 | 0.1 |
| Adiponectin a (ng/mL) | −0.10 | 0.4 |
| Adipsin (ng/mL) | 0.04 | 0.8 |
| Leptin a (ng/mL) | 0.03 | 0.8 |
| Resistin b (ng/mL) | 0.02 | 0.8 |
Note: Data were analysed using Pearson correlations unless otherwise indicated. Bold denotes statistical significance.
Abbreviations: AUC, area under the curve; HDL, high‐density lipoprotein cholesterol; IVGTT, intravenous glucose tolerance test; LDL, low‐density lipoprotein cholesterol; OGTT, oral glucose tolerance test.
Non‐normal data which were log‐transformed prior to Pearson correlation analysis.
Data were analysed using nonparametric Spearman correlation tests.
TABLE 3.
Associations between LEAP2 and cardiometabolic outcomes after adjustment for covariates
| Dependent variable | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Coefficient | R2 | P | Coefficient | R2 | P | |
| Anthropometric measures | ||||||
| Body mass index a (kg/m2) | 0.04 (−0.1, 0.1) | 0.07 | 0.5 | – | ||
| Waist‐hip ratio a | −0.1 (−0.3, 0.7) | 0.06 | 0.2 | – | ||
| Body fat (%) | 0.2 (0.001, 0.3) | 0.60 | 0.049 | – | ||
| Fat mass (kg) | 0.3 (−0.1, 0.6) | 0.16 | 0.1 | – | ||
| Fat free mass (kg) | −0.01 (−0.3, 0.3) | 0.49 | 0.9 | – | ||
| Glycaemic measures | ||||||
| Fasting blood glucose (mmol/L) | −0.001 (−0.02,0.01) | 0.08 | 0.9 | −0.001 (−0.02, 0.02) | 0.08 | 0.9 |
| 2‐hour blood glucose post‐OGTT (mmol/L) | 0.04 (−0.01, 0.09) | 0.07 | 0.1 | 0.04 (−0.02, 0.09) | 0.06 | 0.2 |
| Insulin sensitivity (M; mg/kg/min) | −0.1 (−0.2, −0.003) | 0.10 | 0.04 | −0.05 (−0.1,0.03) | 0.26 | 0.2 |
| Fasting insulin by IVGTT a (mlU/L) | 0.6 (0.3, 1.0) | 0.22 | <0.001 | 0.6 (0.3, 1.0) | 0.23 | 0.001 |
| Total insulin AUC by IVGTT a (mlU/L) | 0.5 (0.1, 0.9) | 0.10 | 0.03 | 0.5 (0.1, 1.0) | 0.11 | 0.03 |
| First‐phase insulin AUC by IVGTT a (mlU/L) | 0.8 (0.2, 1.4) | 0.10 | 0.02 | 0.8 (0.2, 1.4) | 0.11 | 0.01 |
| Second‐phase insulin AUC by IVGTT a (mlU/L) | 0.5 (0.07, 0.9) | 0.11 | 0.02 | 0.5 (0.04, 0.9) | 0.12 | 0.03 |
| Cardiovascular and inflammation markers | ||||||
| SBP (mmHg) | 0.2 (−0.1, 0.5) | 0.28 | 0.2 | 0.2 (−0.02, 0.5) | 0.30 | 0.4 |
| DBP (mmHg) | 0.3 (0.02, 0.5) | 0.14 | 0.04 | 0.2 (−0.2, 0.5) | 0.19 | 0.07 |
| Heart rate (bpm) | 0.1 (−0.2, 0.5) | 0.02 | 0.4 | 0.1 (−0.3, 0.5) | 0.05 | 0.7 |
| Pulse pressure (mmHg) | −0.06 (−0.3, 0.2) | 0.30 | 0.6 | −0.08 (−0.3, 0.2) | 0.28 | 0.6 |
| Mean arterial pressure (mmHg) | 0.3 (−0.003, 0.5) | 0.18 | 0.05 | 0.2 (−0.05, 0.5) | 0.24 | 0.1 |
| C‐reactive protein a (mg/L) | 0.3 (−0.4, 1.0) | 0.03 | 0.4 | 0.2 (−0.5, 1.0) | 0.08 | 0.6 |
| Interleukin‐1β (pg/ml) | −0.5 (−1.1, 0.2) | 0.18 | 0.2 | −0.4 (−1.1, 0.3) | 0.19 | 0.3 |
| Nuclear factor kappa‐B (pg/μg protein) | −1.0 (−1.6, −0.2) | 0.05 | 0.1 | −1.0 (−1.9, −0.04) | 0.12 | 0.04 |
indicates non‐normally distributed variables where data were log‐transformed prior to analysis. Model 1 is adjusted for age and sex; Model 2 is adjusted for age, sex and total body fat. Bold denotes statistical significance.
3.3. Associations with glycaemic measures
In univariable analyses, LEAP2 concentrations were positively associated with fasting insulin (Figure 1A; P = 0.001), total insulin secretion (Figure 1B, P = 0.047), and first‐ (Figure 1C; P = 0.02) and second‐phase (Figure 1D; P = 0.04) insulin secretion AUC, determined by IVGTT (Table 2). These associations remained significant in multivariable models adjusted for age and sex and with additional adjustment for body fat (all P < 0.05; Table 3).
FIGURE 1.
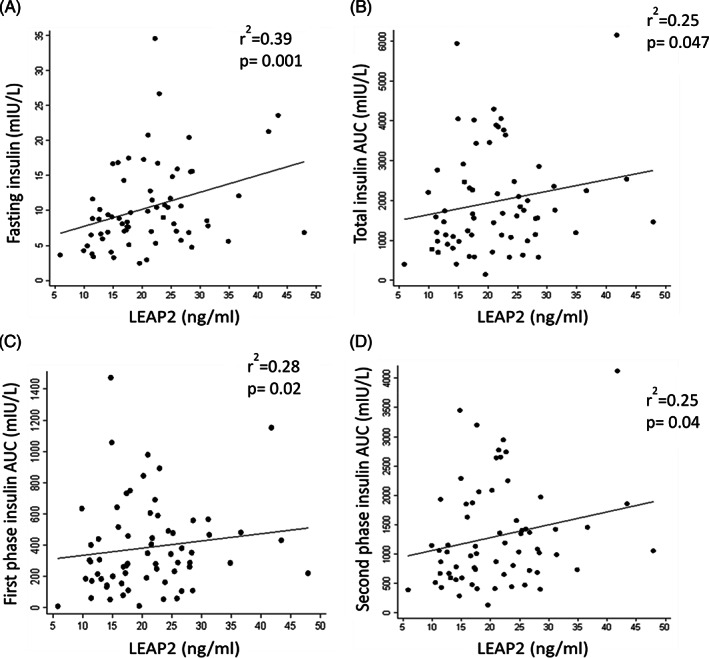
Associations between liver‐expressed antimicrobial peptide 2 (LEAP2) concentrations and fasting insulin (A); total insulin area under the curve (AUC) (B); first‐phase insulin AUC (C); and second‐phase insulin AUC (D).
There was a trend for a negative association between LEAP2 concentrations and insulin sensitivity (P = 0.07; Table 2), which became significant after adjusting for age and sex (P = 0.04; Model 1, Table 3) or age, sex and BMI (P = 0.037), but not in the model adjusted for age, sex and body fat (P = 0.2; Model 2, Table 3). There were no relationships with fasting or 2‐hour post‐OGTT glucose levels in univariable or multivariable analyses. In examining insulin secretion from IVGTT data, a stepwise regression analysis of age, sex, fat mass and waist‐hip ratio resulted in only LEAP2 being included in the final model, with a 1% increase in LEAP2 concentration resulting in a 0.26% increase in total insulin AUC (P = 0.034), a 0.3% increase in first‐phase AUC (P = 0.016) and a 0.27% increase in second‐phase insulin AUC (P = 0.032; Table 4).
TABLE 4.
Stepwise regression analysis: Covariates considered but not included in the model with P value entry parameter of 0.05 and removal parameter of 0.1: age, sex, waist‐hip ratio, fat mass
| Variable | Standardized coefficient (95% CI) | P value |
|---|---|---|
| Total insulin AUC a | 0.266 (0.038, 0.912) | 0.034* |
| First‐phase insulin AUC a | 0.300 (0.139, 1.315) | 0.016* |
| Second‐phase insulin AUC a | 0.269 (0.042, 0.877) | 0.032* |
Note: Included variables: LEAP2 concentration.
Abbreviations: AUC, area under the curve; CI, confidence interval.
Variable log‐transformed; therefore, coefficient interpretation is multiplicative.
3.4. Associations with cardiovascular measures and inflammation markers
LEAP2 concentrations were positively associated with diastolic blood pressure (P = 0.02) but the association with systolic blood pressure did not reach statistical significance (P = 0.08; Table 2). LEAP2 concentrations also correlated positively with mean arterial pressure (r = 0.29, P = 0.02) but not with pulse pressure, heart rate, or any of the plasma lipids (Table 2). The relationship with diastolic blood pressure remained significant after adjustment for age and sex (P = 0.04) but was attenuated after additional adjustment for body fat (P = 0.07). Similarly, the relationship with mean arterial pressure was no longer significant after adjustment for age and sex, or after additional adjustment for body fat (P = 0.1; Table 2).
There was no relationship between LEAP2 concentrations and NFκB activity in the peripheral blood mononuclear cells on univariable analysis or in the age‐ and sex‐adjusted model; however, LEAP2 was negatively associated with NFκB activity in the model adjusted for age, sex and body fat (P = 0.04; Table 3). There were no associations with any of the other inflammation markers or adipokines measured, including after adjustment for age, sex or body fat.
4. DISCUSSION
The results of this study demonstrated novel relationships between LEAP2 and key cardiometabolic risk factors, including insulin secretion and sensitivity, diastolic blood pressure and NFκB activity. Although the relationships between LEAP2 and insulin secretion, diastolic blood pressure and NFκB activity were independent of obesity, the relationship between LEAP2 concentrations and insulin resistance disappeared after adjustment for body fat, suggesting that this relationship is mediated by obesity. Although there were no associations between LEAP2 and weight, BMI or waist‐hip ratio, there was a relationship with body fat in the age‐ and sex‐adjusted models. This may suggest that, rather than being purely regulated by energy balance, adiposity may be a stronger driver of LEAP2 levels, independently of age and sex. This is supported by animal data showing that LEAP2 concentrations decreased fat mass, 16 however, our data are the first evidence of this relationship in humans. One recent study identified an insulinotropic action of a LEAP2 peptide in vitro, however, this did not translate into interventional studies. 22 This novel relationship between LEAP2 and insulin secretion has important implications for its potential as a therapeutic agent in the management of type 2 diabetes and metabolic syndrome.
We reported a novel association between LEAP2 and fasting insulin and insulin secretion, which are independent of adiposity. LEAP2 levels were associated with insulin secretion, even after adjustment for body fat, which suggests an effect independent from the effect of LEAP2 on obesity. This is controversial in the context of ghrelin, with inconsistent evidence that it suppresses glucose‐stimulated insulin secretion. 23 Both ghrelin and LEAP2 are known to act on GHS‐R1a, which is also found in mouse and human pancreatic islets, providing a potential mechanism for their effect. 23 , 24 Given that ghrelin decreases glucose‐mediated insulin secretion via this pathway, LEAP2 may act via this pathway to oppose the action of ghrelin and facilitate insulin secretion. GHS‐R1a is likely to be a critical mechanism, since GHS‐R1a knockout mice do not show a LEAP2‐mediated glucose‐lowering effect. 18 Others have suggested that LEAP2 has unknown alternative receptors, showing anorexic effects on GHS‐R1a knockout mice. 25 Another plausible mechanism for the action of LEAP2 centres on growth hormone (GH). Recently, it has been shown that LEAP2 administration suppresses GH concentrations in humans. 18 GH is typically considered to have an antagonistic relationship with insulin, decreasing sensitivity in peripheral tissues and causing hyperinsulinaemia. However, our current study does not support a role for LEAP in decreasing GH concentrations, as this would predict a decrease in insulin concentrations. We suggest the increased insulin secretion shown in this study is presumably independent from GH signalling. The notion of LEAP2 as a modulator of insulin‐glucose metabolism is complicated and, although LEAP2 was associated with insulin sensitivity measured by gold‐standard hyperinsulinaemic‐euglycaemic clamp after an adjustment for age and sex, this association disappeared after additional adjustment for body fat. This suggests that the effect of LEAP2 on insulin resistance is mediated through adiposity and strengthens the case for a mechanism of increased insulin secretion, rather than sensitivity. Moreover, the lack of association between LEAP2 and glucose concentrations supports this idea, but this could also be complicated by hepatic glucose output, which was not assessed in this study. Interestingly, the relationship was not described in previous studies of patients living with type 2 diabetes, in which LEAP2 was not associated with insulin levels or resistance, although it was associated with glycated haemoglobin (HbA1c). 19 However, in our study we used a gold‐standard hyperinsulinaemic‐euglycaemic glucose clamp for measurement of insulin sensitivity, which is likely responsible for the difference in the results between our study and the study by Li et al. 19 This means, combined with its anti‐obesity effects, LEAP2 may be uniquely suited for the management of type 2 diabetes, targeting multiple facets of disease.
We also showed, for the first time, an inverse association between LEAP2 and NFκB, however, the potential mechanisms underpinning this relationship are unclear. It is difficult to know whether this is a factor of the cardiometabolic effects of LEAP2 influencing inflammatory and oxidative stress pathways, or whether it is a direct action on circulating leukocytes. LEAP2 is known to play a role in pathogen defence, being expressed by monocytes. 26 Additionally, ghrelin has been shown to cause NFκB activation in human lymphocytes, 27 which may explain why a purported ghrelin antagonist would lead to reductions in signalling. In addition, NFκB has repeatedly been associated with insulin sensitivity, 28 as treatments which inhibit signalling reverse insulin resistance. The inverse association may indicate that LEAP2 regulates immune function; however, this requires further investigation.
One important limitation of our study was the inability to measure plasma ghrelin concentrations due to sample degradation. Therefore, our conclusions are based solely on plasma LEAP2 and not the relationship between plasma ghrelin and LEAP2. In addition, the cross‐sectional design precludes causality and the sample comprised only healthy adults with overweight and obesity. Hence, the direction of the observed relationships cannot be determined and results are not generalizable to other populations including lean individuals or those with pre‐existing conditions.
In conclusion, this study provides evidence of relationships between LEAP2 and glucose‐insulin metabolism, lending support to future research into its use as a potential anti‐obesity and antidiabetic agent. Importantly for its therapeutic translation as an insulin secretagogue, it appears to act by directly increasing insulin output, rather than modulating sensitivity. Should LEAP2 have additional benefits to insulin secretion and inflammation, as well as its established effects on weight loss, it could represent a powerful means to combat obesity and type 2 diabetes. Further interventional preclinical studies and clinical trials are required to explore the effect of direct administration of the hormone, to guide future therapeutic development, as well as evaluate the potential for off‐target effects. Additionally, studies exploring the interaction between LEAP2, pancreatic insulin secretion, and adipose tissue are required to reveal its role in these intertwined physiologies.
AUTHOR CONTRIBUTIONS
Conceptualization: Romana Stark, Zane B. Andrews, Barbora de Courten. Formal Analysis: Jack Feehan, Aya Mousa. Investigation: Romana Stark, Aya Mousa. Resources: Zane B. Andrews, Barbora de Courten. Writing—original draft: Jack Feehan. Writing—review and editing: Jack Feehan, Romana Stark, Aya Mousa, Zane B. Andrews, Barbora de Courten. Visualization: Jack Feehan. Supervision: Zane B. Andrews, Barbora de Courten. Project Administration: Barbora de Courten. Funding acquisition: Zane B. Andrews, Barbora de Courten.
FUNDING INFORMATION
National Health and Medical Research Council (NHMRC).
CONFLICTS OF INTEREST
There are no conflicts of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14968.
ACKNOWLEDGMENTS
This research was supported by the National Health and Medical Research Council (NHMRC; grant application 1047897 to B.d.C. for the current trial). A.M. is supported by a biomedical research fellowship provided by the NHMRC of Australia.
Stark R, Feehan J, Mousa A, Andrews ZB, de Courten B. Liver‐expressed antimicrobial peptide 2 is associated with improved pancreatic insulin secretion in adults with overweight and obesity. Diabetes Obes Metab. 2023;25(5):1213‐1220. doi: 10.1111/dom.14968
R.S. and J.F. are co‐first authors, with order determined via random draw. Z.B.A. and B.D.C. are co‐senior authors.
Trial registration: Clinicaltrials.gov ‐ NCT02112721.
Funding information National Health and Medical Research Council
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Figshare at 10.6084/m9.figshare.21737717.
REFERENCES
- 1. World Health Organization . Obesity and overweight; 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga MJM. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121‐135. [DOI] [PubMed] [Google Scholar]
- 4. Cercato C, Fonseca FJD. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman D, Brukner P. Chapter 8 ‐ exercise across the lifespan: exercise and obesity. In: Feehan J, Tripodi N, Apostolopoulos V, eds. Exercise to Prevent and Manage Chronic Disease across the Lifespan. Cambridge, MA: Academic Press; 2022:97‐115. [Google Scholar]
- 6. Koliaki C, Spinos T, Spinou Μ, Brinia Μ‐E, Mitsopoulou D, Katsilambros N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare. 2018;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodríguez‐Rodríguez E, Aparicio A, Aranceta‐Bartrina J, et al. Low adherence to dietary guidelines in Spain, especially in the overweight/obese population: The ANIBES Study. J Am Coll Nutr. 2017;36(4):240‐247. [DOI] [PubMed] [Google Scholar]
- 8. Rivera‐Torres S, Fahey TD, Rivera MAJG. Adherence to exercise programs in older adults: informative report. Gerontol Geriatr Med. 2019;5:2333721418823604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perry B, Wang Y. Appetite regulation and weight control: the role of gut hormones. Nutr Diabetes. 2012;2(1):e26‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Müller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Ghrelin. 2015;4(6):437‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schalla MA, Stengel AJC. Pharmacological modulation of ghrelin to induce weight loss: successes and challenges. Curr Diab Rep. 2019;19(10):1‐11. [DOI] [PubMed] [Google Scholar]
- 12. Zigman JM, Bouret SG, Andrews ZB. Obesity impairs the action of the neuroendocrine ghrelin system. Trends Endocrinol Metab. 2016;27(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ge X, Yang H, Bednarek MA, et al. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 2018;27(2):461‐469. e6. [DOI] [PubMed] [Google Scholar]
- 14. Krause A, Sillard R, Kleemeier B, et al. Isolation and biochemical characterization of LEAP‐2, a novel blood peptide expressed in the liver. Protein Sci. 2003;12(1):143‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shankar K, Metzger NP, Singh O, et al. LEAP2 deletion in mice enhances ghrelin's actions as an orexigen and growth hormone secretagogue. Mol Metab. 2021;53:101327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mani BK, Puzziferri N, He Z, et al. LEAP2 changes with body mass and food intake in humans and mice. J Clin Invest. 2019;129(9):3909‐3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Islam MN, Mita Y, Maruyama K, et al. Liver‐expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J Endocrinol. 2020;244(1):13‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hagemann CA, Jensen MS, Holm S, et al. LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell Rep Med. 2022;3(4):100582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Huang P, Xiong J, et al. Serum levels of ghrelin and LEAP2 in patients with type 2 diabetes mellitus: correlation with circulating glucose and lipids. Endocr Connect. 2022;11(5):e220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fittipaldi AS, Hernández J, Castrogiovanni D, et al. Plasma levels of ghrelin, des‐acyl ghrelin and LEAP2 in children with obesity: correlation with age and insulin resistance. Eur J Endocrinol. 2020;182(2):165‐175. [DOI] [PubMed] [Google Scholar]
- 21. Gupta D, Ogden SB, Shankar K, Varshney S, Zigman JM. A LEAP 2 conclusions? Targeting the ghrelin system to treat obesity and diabetes. Mol metab. 2021;46:101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hagemann CA, Zhang C, Hansen HH, et al. Identification and metabolic profiling of a novel human gut‐derived LEAP2 fragment. J Clin Endocrinol Metabol. 2020;106(2):e966‐e981. [DOI] [PubMed] [Google Scholar]
- 23. Gray SM, Page LC, Tong J. Ghrelin regulation of glucose metabolism. J Neuroendocrinol. 2019;31(7):e12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta D, Dowsett GK, Mani BK, et al. High coexpression of the ghrelin and LEAP2 receptor GHSR with pancreatic polypeptide in mouse and human islets. Endocrinology. 2021;162(10):bqab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Islam MN, Zhang W, Sakai K, et al. Liver‐expressed antimicrobial peptide 2 functions independently of growth hormone secretagogue receptor in calorie‐restricted mice. Peptides. 2022;151:170763. [DOI] [PubMed] [Google Scholar]
- 26. Howard A, Townes C, Milona P, Nile CJ, Michailidis G, Hall J. Expression and functional analyses of liver expressed antimicrobial peptide‐2 (LEAP‐2) variant forms in human tissues. Cell Immunol. 2010;261(2):128‐133. [DOI] [PubMed] [Google Scholar]
- 27. Sung EZ, Da Silva NF, Goodyear SJ, McTernan PG, Arasaradnam RP, Nwokolo CU. Ghrelin promotes nuclear factor kappa‐B activation in a human B‐lymphocyte cell line. Mol Biol Rep. 2011;38(8):4833‐4838. [DOI] [PubMed] [Google Scholar]
- 28. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mousa A, Naderpoor N, de Courten MP, et al. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D–deficient, overweight or obese adults: a randomized placebo‐controlled trial. Am J Clin Nutr. 2017;105(6):1372‐1381. [DOI] [PubMed] [Google Scholar]
- 30. de Courten B, Mousa A, Naderpoor N, Teede H, de Courten MP, Scragg R. Vitamin D supplementation for the prevention of type 2 diabetes in overweight adults: study protocol for a randomized controlled trial. Trials. 2015;16(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization . Waist circumference and waist‐hip ratio: report of a WHO expert consultation. Geneva: World Health Organization; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Figshare at 10.6084/m9.figshare.21737717.


