Abstract
Motivation to seek social interactions is inherent to all social species. For instance, even with risk of disease transmission in a recent pandemic, humans sought out frequent in-person social interactions. In other social animals, socialization can be prioritized even over water or food consumption. Zebra finches, Taeniopygia guttata, are highly gregarious songbirds widely used in behavioural and physiological research. Songbirds, like humans, are vocal learners during development, which rely on intense auditory learning. Aside from supporting song learning, auditory learning further supports individual identification, mate choice and outcome associations in songbirds. To study auditory learning in a laboratory setting, studies often employ operant paradigms with food restriction and reinforcement and require complete social isolation, which can result in stress and other unintended physiological consequences for social species. Thus, in this work, we designed an operant behavioural method leveraging the sociality of zebra finches for goal-directed behaviours. Our approach relies on visual social reinforcement, without depriving the animals of food or social contact. Using this task, we found that visual social reinforcement was a strong motivational drive for operant behaviour. Motivation was sensitive to familiarity towards the stimulus animal and higher when engaging with a familiar versus a novel individual. We further show that this tool can be used to assess auditory discrimination learning using either songs or synthetic pure tones as stimuli. As birds gained experience in the task, they developed a strategy to maximize reward acquisition in spite of receiving more punishment, i.e. liberal response bias. Our operant paradigm provides an alternative to tasks using food reinforcement and could be applied to a variety of highly social species, such as rodents and nonhuman primates.
Keywords: auditory discrimination, auditory learning, avian, Go/No-go, operant conditioning, sociality
Songbirds are valuable models for understanding vocal learning, which comprises a set of traits that enable the memorization and then replication of sounds for communication (Mello, 2014; Petkov & Jarvis, 2012). Vocal learning and production are thus inherently social behaviours and have evolved exclusively in social species, such as songbirds and humans (Jarvis, 2019). The initial stages of vocal learning involve intense auditory learning and sensorimotor feedback (Brainard & Doupe, 2002). After mastering song production, mature songbirds continue to rely on auditory learning throughout life for individual identification, mate choice and associations between sounds and outcomes/objects (Gentner et al., 2000; Macedo-Lima & Remage-Healey, 2020; Verzijden et al., 2012). Zebra finches, Taeniopygia guttata, for instance, are highly social, gregarious, socially monogamous songbirds (Zann, 1996) widely used in behavioural and physiological research in a laboratory setting. In zebra finches, in which only males produce song, females nevertheless use auditory learning during development to guide song perception and preference in adulthood (Hauber et al., 2010).
A primary way to study auditory learning under laboratory conditions is to use behavioural apparatuses that can automatically couple sound stimuli with contingent outcomes. Operant paradigms are widely used tools for the study of songbird behaviour in the laboratory and are instrumental for the study of motivated behaviours (Becker, 2005). The majority of operant tasks involving songbirds employ food reinforcement (Benney & Braaten, 2000; Chen & ten Cate, 2015; Cynx & Nottebohm, 1992; Furest Cataldo et al., 2023; Gentner & Margoliash, 2003; Gess et al., 2011; Sahu et al., 2022; Schneider & Woolley, 2013; but see Tokarev & Tchernichovski, 2014). One study used a similar approach with water deprivation (Lim et al., 2016). To encourage motivation, food/water reinforcement paradigms commonly require resource deprivation for many hours before testing and thus may cause distress to the studied subject (Astheimer et al., 1991). To this point, some studies with songbirds have adapted food reinforcement paradigms to last for the entire daylight period and mitigate effects of food deprivation (Roach et al., 2017; Sahu et al., 2022). In this context, an operant tool geared towards shorter sessions might be desired, to better accommodate acute manipulations such as pharmacological treatments or neural data collection.
Tasks that employ food/water deprivation are often accompanied by complete social isolation for experimental precision, which might also lead to ancillary physiological changes in focal animals. For social species, imposed social isolation induces an adaptive physiological state in response to the contrast between an individual’s preferred social condition (i.e. in a group) versus its current condition (alone) (Cacioppo et al., 2015). In zebra finches, social deprivation impacts juvenile song learning and adult behaviour, gene expression, hormone levels and neurogenesis (Banerjee & Adkins-Regan, 2011; Carouso-Peck & Goldstein, 2019; George et al., 2020; Lipkind et al., 2002; Remage-Healey et al., 2003). Nevertheless, social isolation has been traditionally employed in behavioural experiments in neuroscience and psychology because it eliminates distractors and potential interference by nonfocal animals. Thus, when studies with highly social animals in captivity maintain naturalistic social contact, this can support normal physiological, hormonal, neural and behavioural profiles.
With those aspects in mind, we set out to devise an operant behavioural method to leverage the social gregariousness of zebra finches as a motivated behaviour. Our approach relies on visual social reinforcement, without depriving the animals of food, water or complete social contact. We predicted that visual social reinforcement would be particularly motivating for zebra finches, because not only are they highly gregarious but they have also evolved ‘distance calls’ triggered by visual separation from their mates (Elie & Theunissen, 2016; Vignal et al., 2004, 2008; Vignal & Mathevon, 2011; Zann, 1996). Using this tool, we examined whether familiarity with the stimulus animal underlies motivation to perform operant behaviour and whether social reinforcement is sufficient to guide auditory learning of different auditory stimulus categories (conspecific songs or pure tones) in a Go/No-go task. We found that visual social reinforcement was a strong motivational drive for adult zebra finches of both sexes to engage in operant behaviour, which can then be leveraged to study auditory learning and discrimination. Birds’ motivation to gain social reinforcement was higher when engaging with a familiar individual than with a novel individual. We further show that this social motivation was a sufficient reinforcer to assess auditory discrimination learning using conspecific songs or synthetic pure tones, akin to previous protocols that used food or water as reinforcers (Bell et al., 2015; Gess et al., 2011; Lim et al., 2016; Schneider & Woolley, 2013).
Our work describes a low-cost operant behavioural paradigm that can be used as a tool to assess auditory discrimination, learning and social motivation. We present this paradigm as an alternative to tasks using food reinforcement that can be applied to a variety of highly social species like the zebra finch.
METHODS
Ethical Note
All procedures were in accordance with the Institutional Animal Care and Use Committee at the University of Massachusetts Amherst (Protocol number 2019–0058). Zebra finches were bred and raised by conspecifics in our aviary at the University of Massachusetts Amherst (14:10 h light:dark cycle). Animals in large flight aviaries were provided ad libitum food and water and with enrichment including nesting material, natural perches and hanging swings, egg food supplement (Quiko) and water baths twice a week. Animals in experimental cages were provided ad libitum food and water and with natural perches and egg food supplement.
Animals
Across all experiments, 20 adult (>90 days posthatch) zebra finches were used as focal birds for two experiments detailed below. All birds were known to not have been actively breeding and had been housed in two adjacent single-sex cages (physically separated but acoustically and visually in contact) before they were randomly selected for the experiments. Selected birds were placed in small cages (30.48 × 22.86 × 40.64 cm) with an opposite-sex individual inside a sound attenuation chamber (Eckel Industries, inner dimensions 60.96 × 60.96 × 81.28 cm) for ~1–2 weeks. For experiment 1, which examined the effects of familiarity on operant task motivation (see details below), five males and three females were trained and used as focal birds. Two of these males had been involved in a previous pharmacological study and had been implanted with bilateral intracerebral cannulae targeting the caudomedial nidopallium (secondary auditory region). Data from these animals for this experiment were collected at least 2 weeks after any pharmacological manipulation (at least 1 week following vehicle treatments). These same two males also participated in experiment 2 (see below), but their data for this experiment preceded the pharmacological manipulations via cannula.
For experiment 2, which examined auditory learning (see details below), 12 males and two females completed Go/No-go training (including 2 males also involved in experiment 1). The experimental timeline is illustrated in Fig. 1a.
Figure 1.

Training stages in the social reward operant task. (a) Experimental timeline. After 1–2 weeks of cohousing and isolation from other birds, animals were separated in adjacent cages and experiments began. (b) Introduction. The glass was automatically turned transparent for 6 s every 30–60 s intervals, granting visual access between birds. Sessions lasted 4 h and were repeated for two consecutive days. (c) After the introduction stage, animals were trained to trigger an infrared beam break switch to activate the glass. An LED light signalled when the switch was active. When the beam break was triggered, the glass turned transparent for 6 s. This stage of training was repeated until birds reached the motivation threshold (see Methods). Data from this stage were used in experiment 1. (d) After shaping, some birds proceeded to the auditory discrimination Go/No-go procedure. Birds initiated all trials by triggering the switch and playback of a Go or No-go trial, with pGo and pNo-go probabilities, respectively. Solid arrows indicate trajectories that yielded consequences (reward or punishment); dotted arrows indicate trajectories that did not yield consequences (except for a 6 s timeout). Possibilities after a Go trial are indicated by blue arrows and those after a No-go trial by red arrows. Birds had 2 s to respond after the stimulus was played. Reward consisted of activation of the polarized glass and visual access to the other bird for 6 s, while punishment consisted of a loud burst of white noise and the switch being inoperant for 16 s. Lack of response to stimuli did not yield consequences. Illustration depicts a male as the focal subject, but females were also used in our experiments. Data from this stage were used in experiment 2. Modified with permission from Macedo-Lima and Remage-Healey (2020).
Two males in this study were briefly involved in concurrent pharmacological studies and received saline orally 1 h before the task (Macedo-Lima & Remage-Healey, 2020). Part of their behavioural data has been published as a control for pharmacological treatments. In the current work, the learning curves for these two individuals were indistinguishable from those of animals not treated with saline and were analysed jointly. The data for these two individuals are reported in the Results for transparency.
Apparatus
The behavioural set-up has been described briefly in a previous study (Macedo-Lima & Remage-Healey, 2020) and is expanded here in detail in the Supplementary material. The set-up consisted of two adjacent cages separated by a sheet of polarized glass (Smart Tint, 50.8 × 50.8 cm), placed inside a sound attenuation chamber. Behavioural automation code was custom-made in Python for control of Raspberry Pi computers (Raspberry Pi Foundation, Cambridge, U.K.). The script was designed to run daily and without the need for constant experimenter input or monitoring. Raspberry Pis were controlled via other computers through a wi-fi router (Secure Shell connection). Raspberry Pis controlled the sheet of polarized glass through a relay board (SainSmart 8-channel relay module), an amplifier (Pyle Home PCA2 2×40 Watts) feeding into a speaker (Sony SRS-XB12), an infrared beam break sensor (5 mm or 3 mm; Adafruit) and a red light-emitting diode, LED (Fig. 1). Python code for controlling the set-up inside the chamber can be found on the Github repository (github.com/HealeyLab/RPi_behavioral_automation).
Behavioural training stages consisted of modified versions of the stages described in Gess et al. (2011). After removing a focal and nonfocal (i.e. stimulus) bird from the aviary, they were housed in the same cage inside a sound attenuation chamber for 1–2 weeks, then separated and placed in adjacent cages divided by a sheet of opaque polarized glass that blocked visual (but not auditory) contact between birds. The focal animal’s cage contained an infrared beam break sensor mounted on a semi-opaque (sanded) piece of acrylic with dust shields made from PVC pipe pieces positioned at 7 cm height and 2 cm away from a perch. A red LED was positioned behind the acrylic piece such that light through the acrylic appeared as a diffuse halo. Sessions took place 7 days/week, started 2–3 h after lights-on and lasted 4 h (except where otherwise noted). This schedule was designed to accommodate acute pharmacological manipulations and acute data collection, since starting 2–3 h after lights-on allows time for drug administration. Additionally, this time serves as a buffer for animals to hydrate and feed before the experiment. The period of ~17–18 h of visual isolation was designed to increase motivation to obtain visual contact during the window of data collection. Details of the training stages are described in detail below.
Behavioural Training
Introduction
To introduce birds to the function of the polarized glass, the glass turned transparent for 6 s at pseudorandom intervals between 30 s and 60 s (Fig. 1b). This training stage lasted for 4 h and was repeated for two consecutive days.
Shaping
Next, birds were trained to operate the switch (Fig. 1c; 2–17 days). On the first day of shaping, the switch was baited with egg food supplement (Quiko Exotic) attached to a small piece of red, transparent or white laboratory tape (red LED always gave a red hue to the target). When birds pecked at the tape, the infrared beam would be triggered and the glass would turn transparent for 6 s. Each time the red LED turned on, it would indicate that the switch was active, and when it was turned off, that the switch was inoperable (i.e. when the glass was transparent and when the training session was over).
Performance files generated by the software were constantly monitored at this stage. If the number of activations was higher than ~30, the tape was removed and shaping continued. After 4 h of training, if the activation count was not higher than 100, shaping was extended for an additional 7–8 h (until lights-off), and the same procedure was used the next day. Shaping was repeated daily until the following two criteria were met on two consecutive days: (1) consistently triggering the beam without the baited tape (more than 50 activations) and (2) doing so during a 4 h trial. If (1) was achieved during an extended 7–8 h trial, a 4 h trial (without baited tape) window was used the following day. After two consecutive days of achieving the criteria above, birds could be tested in the social motivation task or trained in the Go/No-go task. All animals trained in this protocol passed these criteria, with two exceptions that developed a habit of activating the switch by flying through the sensor instead of pecking at it and were removed from the study.
Experiment 1: Effects of Familiarity on Operant Task Motivation
Zebra finches form social bonds and recognize individuals (D’Amelio et al., 2017; Elie & Theunissen, 2018). We examined whether familiarity and sex of the subject and the stimulus would influence the motivation to obtain social reinforcement by measuring the number of times the bird would activate the switch to make the glass transparent. After removal from group housing, a pair of opposite-sex birds was housed in the same cage for ~1–2 weeks before the experiment. Thus, the nonfocal birds in this pair were considered the ‘familiar animals’ hereon. Eight birds (3 females) performed daily shaping protocol for at least 2 days (2–7 days) with the familiar animal as stimulus, which was always of the opposite sex. Only data from the last day were analysed here. Ten minutes before testing started on the following day, the familiar animal was removed and a novel stimulus animal of either the same or opposite sex was placed in the reward (stimulus) cage. Presentation order of the novel animals’ sex was pseudorandom and counterbalanced by focal animal sex. After testing ended (4 h later), the novel stimulus animal was removed and the familiar animal was placed back in the reward cage, but birds had no visual contact until the following day. Testing with another novel stimulus animal only took place following at least 1 day of testing with the familiar animal so that exposure to novel stimulus animals was always preceded by training days with the familiar animal.
On testing days with the familiar animal, the stimulus animal was disturbed/handled 10 min before the task to emulate the handling effects experienced by the novel stimulus animals.
Novel stimulus individuals were removed from the aviary for the duration of the session (~4 h). They were only used once as reinforcers (nonfocal) and were returned to the aviary after experiments. Adult novel stimulus animals of both sexes were randomly selected from our single-sex cages. Juvenile individuals (20–30 days posthatch) were randomly selected from breeding flight cages and were sexually nondimorphic (fully dark bill, grey cheek patches, no zebra chest pattern). The presentation order of novel stimulus animals was controlled for in our statistical model.
Experiment 2: Auditory Association Learning (Go/No-go) Task
After shaping (described above), birds were trained in an auditory Go/No-go task (Fig. 1d). When a bird triggered the infrared beam to initiate a trial, one of two sounds was pseudorandomly played from the speaker. One sound was assigned as ‘Go’ and associated with positive reinforcement while another sound was assigned as ‘No-go’ and paired with punishment. If the bird pecked again within a 2 s interval, it would receive a consequence as follows. In a Go trial, the glass would turn transparent for 6 s, resulting in a ‘hit’; in a No-go trial, a 2 s burst of white noise would be played and a 16 s inactivation (timeout) period would follow, resulting in a ‘false alarm’. If the bird did not respond to the sound within 2 s, a 6 s inactivation period would follow, regardless of the type of sound, resulting in a ‘correct rejection’ (No-go trial) or a ‘miss’ (Go trial).
Go/No-go training always started with Go trial probability (pGo) of 90%. If birds responded to trials (hit or false alarm) more than 50 times in one daily session, pGo was reduced for the following day to 75%, then to 50% on the third day. Two males in the tone-learning group were exceptions: one subject was advanced from 90% to 50% (skipping 75% pGo); another subject’s motivation dropped precipitously when pGo was changed from 90% to 75% (from 200–400 trials to 30–40 trials), so we reinstated the 90% pGo until motivation stabilized. Because of the high variability in the number of sessions required for birds to advance to the training with 50% pGo, we opted to not evaluate learning speed in this study.
In pilot experiments, most birds’ performance reached an asymptote between 70% and 80% correct, so we employed a learning criterion of 70% correct on two consecutive days (modified from Gess et al., 2011). The sound stimuli used in the Go/No-go task were either two unfamiliar zebra finch songs or two pure tones and are described below.
About 60% of the birds that we attempted to train on the Go/No-go protocol did not maintain satisfactory motivation levels (>50 trials per day) throughout training and were removed from the study.
Daily sessions with fewer than 20 trials were excluded from the analyses. In total, seven sessions from four different birds (3 days, 2 days, 1 day and 1 day, respectively) were removed. Five out of these seven sessions occurred when animals were still in the procedural learning stage (with pGo > 50%).
Sound Stimuli
For the tone discrimination learning task, 2 s pure tones were generated in Adobe Audition 2014. A fade-in/fade-out filter was applied to the first and last 100 ms of the tones (Fig. 2). Digital sound level was attenuated to –25 dB re 100% and amplified to ~60–70 dB SPL in the behavioural booths, measured with a sound level meter (RadioShack) at the focal animal’s perch near the infrared switch. A 2 s white noise burst was generated in the same software, but digital sound level was attenuated to –5 dB re 100%, which, after the same amplification factor used for tones, resulted in a sound pressure level of ~90 dB SPL in the behavioural booths.
Figure 2.

Stimuli used in Go/No-go auditory discrimination learning task. Birds learned to discriminate between two previously unheard conspecific songs (song discrimination; left) or two pure tones of varying frequencies, 200 Hz apart (tone discrimination; right). Songs were always flanked by zebra finch choruses to standardize stimulus duration (see Methods).
The lower and upper limits of tone frequencies were selected based on the zebra finch audibility curve (Okanoya & Dooling, 1987), which lies within the range of most zebra finch natural vocalizations (Elie & Theunissen, 2016). Tones were drawn from a set of 21 pure tones ranging from 2 kHz to 4 kHz in 100 Hz intervals (2, 2.1, 2.2, …, 3.8, 3.9, 4 kHz). At the start of training, two tones that were 200 Hz apart were randomly selected. The choice of a 200 Hz interval was arbitrary but intended to make for relatively easy discrimination, as determined in a pharmacological study (Macedo-Lima & Remage-Healey, 2020).
We evaluated the effect of repeated instances of tone discrimination learning on learning strategies by changing the tone stimuli after birds reached learning criterion (>70% correct on two consecutive days). We define a ‘learning block’ as the bird’s performance from the first day of testing with a new pair of tones until they reach criterion. Once birds achieved learning criterion, a new pair of tones was randomly selected and birds had to relearn the arbitrary association. The logic for selecting stimuli was as follows: for the first training block with the first tone pair, a pair of pure tones, 200 Hz apart, was randomly selected and the association between the lower or higher tone and either a Go or No-go trial was randomized (e.g. lower tone with No-go trials) for each bird. After reaching learning criterion, a new pair of tones, 200 Hz apart, was randomly selected and the relationship between relative frequency and trial type was switched (e.g. the lower tone previously associated with No-go trials became associated with Go trials). This switch in both absolute and relative frequencies associated with trial type requires that animals switch from the previous association rule and yields a new learning curve (Macedo-Lima & Remage-Healey, 2020).
For the song discrimination learning task, two 2 s songs were selected from a public online database of zebra finch songs recorded in several laboratories in the U.S.A. (Gardner, 2011). Therefore, the songs were unfamiliar to the subjects in our colony. Each song contained two motifs (sequence of elements or syllables that repeat) and introductory notes (Sossinka & Böhner, 1980). A 0.25 s flanking chorus noise was added to the beginning and end of each song to standardize onset and offset cues (Schneider & Woolley, 2013). Otherwise, these cues could facilitate behavioural discrimination differently depending on the song used. The flanking chorus was a mixture of songs built with a separate sample of seven conspecific and unfamiliar songs. Each song stimulus was 2.5 s long (2 s song + 0.5 s flanking chorus). Stimuli were high-pass filtered at 250 Hz and the digital sound level was normalized to 0 dB re 100% (maximum), then amplified when played to reach approximately 63 dB SLP using a sound level meter (Extech, A-weighted) inside the sound attenuation booth. All sound edits were performed in Adobe Audition 2014. For each bird, one song was as assigned as a Go stimulus and the other as a No-go stimulus in a counterbalanced way.
Data Analysis and Software
Behavioural data were analysed and plotted using custom-made Python and R scripts. For experiment 1, familiar stimulus data (i.e. number of activations) were taken from the day immediately preceding testing with novel stimuli (opposite or same sex counterbalanced by focal subject’s sex). Data for each group were recorded from 4 h of testing collected in 1 day. Data were analysed by a mixed-effects generalized linear model followed by ANOVA (GLM/ANOVA), employing stimulus familiarity and focal sex as fixed factors, and focal identity (ID) as a random effect factor. Due to statistical power constraints and our unbalanced design (i.e. familiar individuals were always of opposite sex), focal and stimulus sex effects were analysed in a separate GLM/ANOVA employing focal sex and stimulus sex as fixed factors, and focal ID as a random effect factor. We tested the effects of repeated presentations of the familiar stimulus in a GLM/ANOVA including focal sex as fixed effect, stimulus presentation order as a covariate and focal ID as a random effect. Finally, we tested the effect of juvenile stimuli with a GLM/ANOVA including stimulus identity (juvenile versus familiar) as a fixed effect and focal ID as a random effect.
As in other studies using Go/No-go paradigms with food reinforcement, zebra finches tested under the present social paradigm adopted different strategies for learning Go versus No-go trials. For example, birds commonly exhibit high liberal response bias (indiscriminate responses) at the beginning of training, yielding high success in Go trials and low success in No-go trials (Anand & Nealen, 2019; Gess et al., 2011). Thus, for experiment 2, whole-day performance was analysed by % correct, hit rate (only Go trials) and false alarm rate (only No-go trials) using the following formulas: % correct = 100 × number of hits + number of rejections/number of all trials; hit rate = 100 × number of hits/number of Go trials; hit rate = 100 × number of hits/number of Go trials; false alarm rate = 100 × number of false alarms/number of No-go trials. Additionally, response bias (c) was calculated as c = −0.5 × (Z(hit rate) + Z(false alarm rate)), where Z is the inverse-normal cumulative distribution value at the probabilities. Positive response bias values are interpreted as conservative (indiscriminate nonresponses), while negative values are interpreted as liberal (indiscriminate responses) (Macmillan & Creelman, 1990).
GLM (‘glmmTMB’ R package) (Brooks et al., 2017) models were fitted with fixed slopes and random intercepts, and residuals were inspected and tested using the ‘DHARMa’ R package (Hartig, 2021). If Gaussian family GLM model calculation failed (e.g. nonconvergence problem) or if residuals were not normally distributed, we employed the following steps: (1) unit transformations (multiplication/division by factor of 10); (2) square-root transformation; (3) log transformation. We reran the GLM calculation at each step and, if it succeeded and the residuals were normally distributed, then no further steps were taken. Otherwise, we simplified the models and repeated steps 1–3. We evaluated GLM statistical significance using ANOVA (‘car’ R package) (John & Weisberg, 2019). We performed post hoc Tukey pairwise tests using the ‘emmeans’ R package (Lenth, 2021) after ‘regridding’ (i.e. backtransforming to original scale, if transformations were performed). Effects were considered statistically significant when P < 0.05. For conciseness, in some tests with multiple effects or comparisons, we listed all significant results, then summarized nonsignificant results by stating the next lowest P-valued test as the lowest bound for the other tests (e.g. all other: χ21 < 2.099, P > 0.147).
RESULTS
Experiment 1: Effects of Familiarity on Operant Task Motivation
Familiarity with the stimulus subject increases motivation
To test whether social motivation was dependent on familiarity between focal and stimulus subjects, we exposed focal birds to familiar and novel adult stimulus individuals (Fig. 3a, b). GLM/ANOVA results showed a significant main effect of familiarity, such that familiar stimuli elicited more trials than novel stimuli, regardless of the focal animal’s sex (familiarity factor: χ21 = 8.772, P = 0.003; all other factors: χ21 < 0.158, N = 8, P > 0.691; Fig. 3a). Trials elicited with the familiar stimuli were stable across all interleaved exposures and independent of the sex of the focal animal (GLM/ANOVA: presentation order factor: χ21 = 1.765, P = 0.184; sex factor: χ21 = 0.227, P = 0.634; sex*presentation order: χ21 = 0.099, P = 0.753; ‘Familiar’ bars in Fig. 3a).
Figure 3.
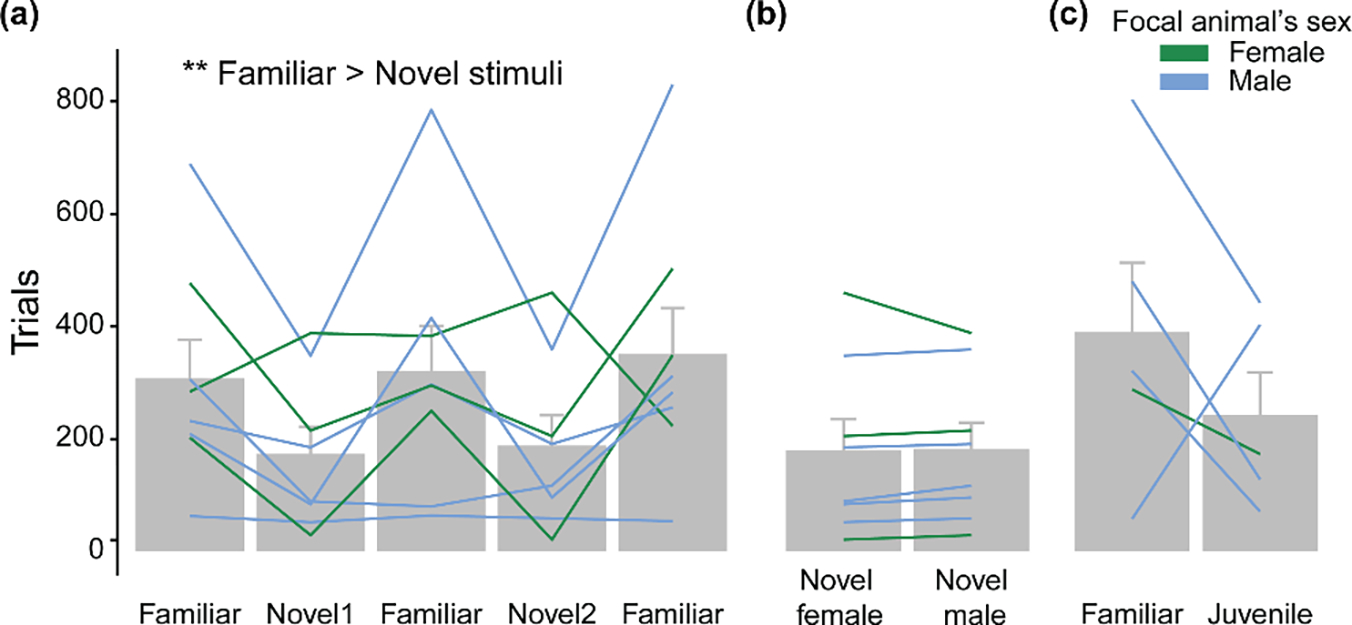
(a) Focal animals of both sexes (N = 8; 3 females, 5 males) were presented to familiar opposite-sex individuals and to novel individuals of both sexes in the adjacent cage separated by polarized glass on alternating days. (b) Same data as in (a) for novel subjects but grouped by sex. (c) A subset of animals (4 males, 1 female) were also exposed to sexually nondimorphic juveniles (<30 days posthatch). Each line represents data from one individual. Asterisks indicate GLM/ANOVA statistical significance for main effect. **P < 0.01.
Because of our incomplete design (i.e. absence of familiar same-sex stimuli), we tested the effects of sex in a separate model including only the novel stimulus animals. No effects of stimulus or focal animal sex were detected (all χ21 < 2.099, P > 0.147; Fig. 3b), suggesting that focal animals did not favour visual contact with either sex of unfamiliar animals.
In a subset of the focal animals (4 males, 1 female), we also compared the motivation to gain visual access to the familiar individual with the motivation towards a novel sexually nondimorphic juvenile (<30 days posthatch; Fig. 3c). A GLM/ANOVA did not detect significant differences in stimulus identity (χ21 = 1.573, P = 0.210), despite all animals except for one male having elicited fewer trials towards the juveniles than towards the familiar opposite-sex individual.
In summary, zebra finches of both sexes were more motivated to elicit visual social contact with familiar individuals of the opposite sex when compared to novel individuals of either sex. This suggests that familiarity with the social partner was a key driver of motivation in our task.
Experiment 2: Auditory Association Learning (Go/No-go) Task
Social reward can be leveraged to drive auditory learning
After focal animals were experienced in operating the switch to obtain reinforcement, a subset of animals was trained to discriminate sounds in our auditory association learning task with visual social reward. They were divided into two groups: song discrimination or tone discrimination (Fig. 2). They were trained to perform a Go/No-go task using either two conspecific songs or two pure tones 200 Hz apart (see Methods). First, birds were introduced to the Go/No-go task with a procedural learning stage, in which they learned to trigger the infrared switch twice: once to elicit the sound and once to respond to it. During this stage we employed a higher probability (90% followed by 75%) of Go trials being triggered (pGo; see Methods) to prevent decays in motivation. pGo was progressively decreased until reaching 50% once birds started responding to >50 trials in a single session. The period of higher pGo ranged between 1 day and 13 days (median = 5 days). After pGo was lowered to 50%, both male and female birds successfully reached criterion between 6 days and 30 days (median = 14 days). The total duration of the training, including the procedural learning stage, ranged between 9 days and 35 days (median = 19.5 days).
We did not find differences between birds performing tone and song discrimination in trials to reach criterion (GLM/ANOVA: χ21 = 1.090, P = 0.297; Fig. 4b). This suggests that that the discrimination difficulty of the stimuli used in the two discrimination tasks was comparable.
Figure 4.

(a) Daily % correct trajectories of song (N = 6; 2 females) and tone (N = 8; all males) discrimination groups. Each line represents a subject. Symbols indicate the final day of training, when birds achieved the learning criterion, i.e. 2 consecutive days above 70% correct. Grey shading indicates an initial period of higher probability of Go trials to encourage birds’ motivation to participate in the task during the procedural learning period. During this period, birds had to learn to trigger the infrared switch once to trigger the sound and again to respond to the sound. Dashed lines indicate chance level (black) and the learning criterion (green) during discrimination training when pGo = 50%. Performance during tone and song discrimination trials for (b) trials to reach the learning criterion, (c) average trials per day and (d) >70% correct (dashed green line), (e) hit rate, (f) false alarm rate and (g) response bias on the last day of training.
Decades of previous work have found that songbirds spontaneously perform operant tasks that elicit playback of conspecific song, suggesting that conspecific song might have inherently rewarding properties (Clayton, 1988; Collins, 1999; Fujii et al., 2021; Kato et al., 2010; Miller, 1979; Riebel, 2000; Stevenson, 1967). If song playback was rewarding in our task, then we would expect to see higher motivation to elicit trials in the song discrimination group. However, we found no differences in the average trials triggered per day between the two groups (χ21 = 0.031, P = 0.861; Fig. 4c), which suggests that the nature of the auditory stimulus did not impact motivation to elicit social reinforcement.
We compared final performance between the two groups once the learning criterion was reached. We did not find differences between song and tone discrimination learning in any performance parameter after learning, including % correct, hit rates, false alarm rates and response bias (all χ21 < 1.744, P > 0.187; Fig. 4d–g). Therefore, zebra finches of both sexes successfully learned to discriminate auditory stimuli using social reinforcement. Thus, either songs or tones can be employed in this protocol with comparable results in learning rates and postlearning performance.
Liberal response bias emerges with task experience
In the above experiment, the tone discrimination task used pure tones with a 200 Hz gap between them. Then, any pair of tones in the range of frequencies we selected should provide a similar discrimination challenge to birds. Under this assumption, we tested whether multiple rounds of tone discrimination learning would affect strategies used by zebra finches in the Go/No-go task with social reinforcement. We monitored four male birds’ performance across three consecutive training blocks of tone discrimination learning, with a new randomly selected pair of tones at each block (see Methods; Fig. 5a). The association between which tone had higher frequency and the trial type was reversed for each block (e.g. block 1: 2.2 kHz=Go and 2 kHz=No-go → block 2: 1.6 kHz=Go and 1.8 kHz=No-go → block 3: 2.8 kHz=Go and 2.6 kHz=No-go).
Figure 5.

(a) Daily % correct trajectories of four male birds (coloured lines in a–e) performing three learning blocks (b1, b2, b3) of discrimination learning between two tones, 200 Hz apart. When learning criterion was achieved (>70% correct on two consecutive days), absolute frequencies were changed and the relationship between relative frequency and trial type (e.g. higher tone = Go; lower tone = No-go) was reversed (see Methods). Block 1 performance was preceded by a period of procedural learning (grey shading) with higher probability of Go trial (pGo > 50%; see Methods). Dashed lines indicate chance level (black) and the 70% correct learning criterion (green) during discrimination training when pGo = 50%. Data points surrounded by a black circle are from subjects handled and treated daily with saline orally 1 h before the task during block 3 for a concurrent pharmacological study (see Methods). Performance on the last day of training for (b) % correct across the three blocks (dashed line indicates 70% learning criterion), (c) hit rate and (d) false alarm rates across blocks 1–3. Asterisks represent results from Tukey post hoc tests (see Results for details): **P < 0.01; ***P < 0.001.
We found that birds achieved similar proficiency levels among training blocks (% correct; GLM/ANOVA: χ21 = 0.976, P = 0.614; Fig. 5b). However, a GLM/ANOVA detected a significant effect of training block on hit rates (χ21 = 10.707, P = 0.005; Fig. 5c) and false alarm rates (χ21 = 18.940, P < 0.001; Fig. 5d) on the last day of training. Post hoc Tukey tests revealed that last day hit rates increased from block 1 to block 3 (t7 = 3.253, P = 0.03; all other blocks: t7 < 1.936, P > 0.197), but so did false alarm rates (t7 = 4.350, P = 0.008; all other blocks: t7 < 2.279, P > 0.125). Therefore, despite similar overall performance (% correct) across training blocks at the end of training, performance in Go trials versus No-go trials progressively diverged with experience, as birds favoured acquiring more rewards (higher hit rates) at the expense of receiving more punishments (higher false alarm rates).
This progressive increase in both hit rate and false alarm rate suggests that birds developed a more liberal response bias with experience in the task. To test this, we calculated response bias (c; see Methods) on the last day of each training block. A GLM/ANOVA detected a significant effect of training block on c (χ21 = 21.986, P < 0.001). Post hoc Tukey tests revealed a significant decrease in c from block 1 to block 3 (t7 = 4.682, P = 0.006; all other blocks: t7 < 2.567, P > 0.084). More negative c values can be interpreted as more liberal response bias. Therefore, as birds became more experienced with repeated tone discrimination training, their response bias became progressively more liberal.
DISCUSSION
Motivation to seek social interactions is inherent to all social species. For humans, even when facing the risk of disease transmission and monetary fines during the COVID-19 pandemic, avoidable social interactions were still highly sought after (Farboodi et al., 2021). In other social animals, such as donkeys, Equus asinus, socialization is often prioritized over water or food consumption after physical exertion (Swann, 2006). European starlings, Sturnus vulgaris, are motivated to work to gain access to images of conspecifics (Perret et al., 2015), and zebra finches spend more time with large versus small groups when given the choice (Goodson et al., 2009). Therefore, social species will seek out social interactions even when there is a cost.
Many studies using operant conditioning paradigms for songbirds have relied on food reinforcement (Bell et al., 2015; Benney & Braaten, 2000; Chen & ten Cate, 2015; Cynx & Nottebohm, 1992; Gentner & Margoliash, 2003; Gess et al., 2011; Schneider & Woolley, 2013; but see Tokarev & Tchernichovski, 2014), which often requires not only food but complete social deprivation. Our work describes a low-cost behavioural tool for operant conditioning training in a songbird species that does not require food deprivation or complete social isolation. Our training protocol runs daily and automatically, requiring little human intervention. In this task, we observed that animals remained highly motivated to gain social reward even after many weeks of daily training. Thus, in addition to studying social motivation, we leveraged this paradigm to assess auditory learning and discrimination, akin to previous protocols that employed food as a reinforcer (Bell et al., 2015; Gess et al., 2011; Schneider & Woolley, 2013). Because animals in this task are not deprived of food, water or complete social contact, we propose that it can be used as a viable alternative paradigm to tasks requiring food and social deprivation in highly social species such as the zebra finch.
In socially monogamous species, once a breeding pair is formed, individuals preferentially attend to their mate’s social cues (Curtis et al., 2001; D’Amelio, Trost, et al., 2017; Williams et al., 1992). Zebra finches in particular develop preferences to their mate’s calls (Hernandez et al., 2016; Vignal et al., 2004) and songs (Woolley & Doupe, 2008). In experiment 1, we found that birds of both sexes were more motivated to gain visual access to familiar over novel individuals. It is possible that isolating individuals in the same cage for 1 week or more before our task, or the repeated daily visual engagement, resulted in pair bonding (Kingsbury & Goodson, 2014; Silcox & Evans, 1982). Because we did not directly evaluate pair bonding in our birds, we can only conclude that familiarity and/or pair bonding enhanced social motivation in our task.
We note that our paradigm does not isolate birds acoustically. While advantageous from the animal welfare perspective, this raises the possibility of acoustic interference by the nonfocal birds. Familiarity and pair bonding result in more vocal exchanges (D’Amelio, Klumb, et al., 2017; D’Amelio, Trost, et al., 2017), so it is possible that, in experiment 1, familiar birds communicated more during the task. While we cannot rule out that more vocal communication elicited higher motivation from the focal bird to elicit visual contact, our interpretation that familiarity increased motivation to obtain visual contact with the stimulus bird is not incompatible with the ability of birds to communicate vocally throughout the task.
In experiment 1, we hand-chased/handled the familiar nonfocal subjects briefly 10 min before the task. This treatment approximated, but was not equivalent to, what novel subjects experienced, i.e. hand-chasing/handling plus relocation to a new cage. Therefore, it is possible that the effects we observed here were the result of the novel nonfocal birds’ behaviour in response to relocation stress. For example, nonfocal birds may vocalize less when they are relocated to a new cage, which might in turn affect the focal bird’s motivation to obtain visual contact. We do not have video/audio recordings from these sessions, so we cannot rule out this possibility.
We could not evaluate the complete interaction between familiarity and sex, because we lacked a familiar same-sex experimental group. It is well established that zebra finches of both sexes can form strong pair bonds with same-sex individuals (Elie et al., 2011; Tomaszycki & Zatirka, 2014). Therefore, we predict that the effect of familiarity in our experiments was not specific to birds of opposite sex, and that same-sex familiar individuals should elicit comparable motivation from focal birds.
Decades of previous work have demonstrated that songbirds are highly motivated to perform operant tasks that elicit conspecific song playback as an unconditioned stimulus and that they show a highly sophisticated discrimination capacity of different song types. For example, socially isolated female zebra finches elicited hundreds of song playbacks daily and showed preferences for familiar over novel songs (Clayton, 1988; Miller, 1979; Riebel, 2000), for complex songs over simple songs (Collins, 1999) and for their mate’s song over a nonmate’s song (Clayton, 1988). Socially isolated male zebra finches also prefer their father’s song over novel songs (Clayton, 1988). Similar findings were reported in Bengalese finches, Lonchura striata (Kato et al., 2010), but preference wanes in males after the critical period for song learning closes (Fujii et al., 2021). In experiment 2, we did not observe differences in self-initiated trials/day between song or tone discrimination groups. This suggests that the nature of the stimulus playback itself (songs versus synthetic tones) did not influence the motivation to elicit trials. It is possible that using song playback as a conditioned stimulus, such as for our Go/No-go task, in order to obtain a secondary reinforcement overrides the reinforcing qualities of conspecific song.
In our tone discrimination learning task, birds developed a liberal response bias strategy as they became more familiarized with the task (i.e. after multiple blocks of learning with different tones). This suggests that experience with the task progressively changed the salience of the stimuli, either social rewards became more salient or punishment became less aversive. Reward/punishment salience ratio (i.e. how good the reward is versus how bad the punishment is) is likely to affect response bias. Harsher punishments typically push performance towards more conservative response bias, and the opposite effect can be elicited with more appealing rewards (Bowen et al., 2020). Therefore, in an appetitive Go/No-go task, liberal response bias can occur if misses (failing to obtain the reward) are perceived as more costly than false alarms (receiving punishment) (Jones et al., 2015). Our results are consistent with the interpretation that, with longer time in training, the perceived salience of the punishment gets progressively lower, while the salience of social rewards might remain constant, which could explain the emergence of the liberal response bias we observed. Future studies could manipulate the salience of the reward or the punishment to manipulate response bias. For example, to reduce reward salience, studies could use only novel individuals as rewards and increase punishment salience by using longer timeouts, longer lights-out periods or air puffs.
In experiment 2, it is possible that vocal interference by the nonfocal bird affected how focal birds responded to the auditory stimuli. We analysed audio recordings from a limited sample of training sessions and did not observe evidence that nonfocal birds were signalling meaningful information about stimulus type. Rather, nonfocal birds called indiscriminately (predominantly ‘tet/stack’ calls) regardless of whether the stimulus was associated with a Go trial or a No-go trial (personal observations). Nevertheless, we cannot rule out that some of the variability we observed in our paradigm could be attributed to vocal interference. Designing a behavioural apparatus with better control over the acoustic contact between birds (e.g. complete acoustic isolation except for periods of visual contact) could help mitigate these concerns but might be more financially costly.
The subjective experience of rewards can be dissected into two main components: ‘liking’ (valence/hedonic value) and ‘wanting’ (incentive to seek). These components are thought to be regulated by mostly different brain regions and physiological states (Berridge et al., 2009). Comparing with other studies that used food rewards, our findings could reflect how different reward modalities are processed in the brain. Relevant brain circuits might differ between the two paradigms, such that food deprivation/seeking engage hunger brain circuits (Sternson et al., 2013), while social deprivation/seeking engages the social behaviour network (Hu et al., 2021). In experiment 1, it was possible to obtain a behavioural readout of the hedonic value of social rewards, such that zebra finches were more motivated to seek familiar/pair-bonded individuals over novel individuals. Future work could test the hedonic value of other types of stimulus animals, such as parents, offspring, siblings or groups versus single birds. Therefore, our paradigm could be an interesting tool to assess the hedonic value of social stimuli and the brain circuitries involved.
In conclusion, in this work we describe a low-cost behavioural tool to assess social motivation and auditory discrimination learning in zebra finches. Using this tool, we show that zebra finches of both sexes are highly motivated to work to obtain visual access to a conspecific, even when they risk punishment in an auditory discrimination task. We show that motivation is dependent on familiarity with the stimulus animal and is salient enough to drive auditory learning when birds need to discriminate between natural stimuli (songs) or between pure tones to obtain reward. Finally, we show that when birds become more experienced with the task, they adopt a strategy to maximize receiving social rewards in spite of receiving more punishment. This increase in liberal response bias might be attributed to a progressive devaluing of punishment with task experience.
Supplementary Material
Highlights.
We used a novel social reward-based operant task in zebra finches.
Birds were more motivated to obtain access to familiar over novel conspecifics.
Social motivation reinforced auditory discrimination learning in a Go/No-go task.
Birds with more experience in the Go/No-go task developed liberal response bias.
Acknowledgments
We are thankful to Joseph Bergan, Jeffrey Podos and Karine Fenelon and to current and former members of the Healey Lab at the University of Massachusetts, especially Amanda Krentzel, Catherine de-Bournonville, Christina Moschetto, Daniel Pollak, Daniel Vahaba, Garrett Scarpa, Hannah Boyd, Jeremy Spool, Katie Schroeder and Maaya Ikeda for valuable input on this project. Finally, we thank two anonymous referees for crucial feedback on this manuscript. This work was supported by the United States National Institutes of Health (R01NS082179) and U.S. National Science Foundation (IOS1354906); M.M.L. was a CAPES-Brazil Fellow (13640/13–5).
Footnotes
Declaration of Interest
We declare no conflicting interests.
Supplementary Material
Supplementary material associated with this article is available, in the online version, at https://doi.org/
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The data supporting this article has been uploaded to a public repository (https://github.com/HealeyLab/Macedo-Limaetal2023).
References
- Anand K, & Nealen PM (2019). Differential Go and NoGo learning within an auditory discrimination task. Animal Behavior and Cognition, 6(2), 141–157. 10.26451/abc.06.02.05.2019 [DOI] [Google Scholar]
- Astheimer LB, Buttemer WA, & Wingfield JC (1991). Interactions of corticosterone with feeding, activity and metabolism in passerine birds of corticosterone with interactions and activity in passerine metabolism birds. Ornis Scandinavica, 23(3), 355–365. [Google Scholar]
- Banerjee SB, & Adkins-Regan E (2011). Effect of isolation and conspecific presence in a novel environment on corticosterone concentrations in a social avian species, the zebra finch (Taeniopygia guttata). Hormones and Behavior, 60(3), 233–238. 10.1016/j.yhbeh.2011.05.011 [DOI] [PubMed] [Google Scholar]
- Becker JB (2005). Rapid effects of estradiol on motivated behaviors. In Gaillard R-C, Kordon C, & Christen Y (Eds.), Hormones and the brain (pp. 155–172). Springer-Verlag. [Google Scholar]
- Bell BA, Phan ML, & Vicario DS (2015). Neural responses in songbird forebrain reflect learning rates, acquired salience, and stimulus novelty after auditory discrimination training. Journal of Neurophysiology, 113(5), 1480–1492. 10.1152/jn.00611.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benney KS, & Braaten RF (2000). Auditory scene analysis in estrildid finches (Taeniopygia guttata and Lonchura striata domestica): A species advantage for detection of conspecific song. Journal of Comparative Psychology, 114(2), 174–182. 10.1037/0735-7036.114.2.174 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW. (2009). Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73. 10.1016/j.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen HJ, Marchesi ML, & Kensinger EA (2020). Reward motivation influences response bias on a recognition memory task. Cognition, 203, Article 104337. 10.1016/j.cognition.2020.104337 [DOI] [PubMed] [Google Scholar]
- Brainard MS, & Doupe AJ (2002). What songbirds teach us about learning. Nature, 417(6886), 351–358. 10.1038/417351a [DOI] [PubMed] [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, & Bolker BM (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal, 9(2), 378–400. 10.32614/RJ-2017-066 [DOI] [Google Scholar]
- Cacioppo JT, Cacioppo S, Cole SW, Capitanio JP, Goossens L, & Boomsma DI (2015). Loneliness across phylogeny and a call for comparative studies and animal models. Perspectives on Psychological Science, 10(2), 202–212. 10.1177/1745691614564876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carouso-Peck S, & Goldstein MH (2019). Female social feedback reveals non-imitative mechanisms of vocal learning in zebra finches. Current Biology, 29(4), 631–636. 10.1016/j.cub.2018.12.026 [DOI] [PubMed] [Google Scholar]
- Chen J, & ten Cate C (2015). Zebra finches can use positional and transitional cues to distinguish vocal element strings. Behavioural Processes, 117, 29–34. 10.1016/j.beproc.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Clayton NS (1988). Song discrimination learning in zebra finches. Animal Behaviour, 36(4), 1016–1024. 10.1016/S0003-3472(88)80061-7 [DOI] [Google Scholar]
- Collins SA (1999). Is female preference for male repertoires due to sensory bias? Proceedings of the Royal Society of London, Series B: Biological Sciences, 266(1435), 2309–2314. 10.1098/rspb.1999.0924 [DOI] [Google Scholar]
- Curtis JT, Liu Y, & Wang Z (2001). Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster). Brain Research, 901(1–2), 167–174. 10.1016/S0006-8993(01)02343-5 [DOI] [PubMed] [Google Scholar]
- Cynx J, & Nottebohm F (1992). Role of gender, season, and familiarity in discrimination of conspecific song by zebra finches (Taeniopygia guttata). Proceedings of the National Academy of Sciences of the United States of America, 89(4), 1368–1371. 10.1073/pnas.89.4.1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio PB, Klumb M, Adreani MN, Gahr ML, & ter Maat A (2017). Individual recognition of opposite sex vocalizations in the zebra finch. Scientific Reports, 7(1), Article 5579. 10.1038/s41598-017-05982-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio PB, Trost L, & ter Maat A (2017). Vocal exchanges during pair formation and maintenance in the zebra finch (Taeniopygia guttata). Frontiers in Zoology, 14(1), Article 13. 10.1186/s12983-017-0197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elie JE, Mathevon N, & Vignal C (2011). Same-sex pair-bonds are equivalent to male–female bonds in a life-long socially monogamous songbird. Behavioral Ecology and Sociobiology, 65(12), 2197–2208. 10.1007/s00265-011-1228-9 [DOI] [Google Scholar]
- Elie JE, & Theunissen FE (2016). The vocal repertoire of the domesticated zebra finch: A data-driven approach to decipher the information-bearing acoustic features of communication signals. Animal Cognition, 19(2), 285–315. 10.1007/s10071-015-0933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elie JE, & Theunissen FE (2018). Zebra finches identify individuals using vocal signatures unique to each call type. Nature Communications, 9(1), Article 4026. 10.1038/s41467-018-06394-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farboodi M, Jarosch G, & Shimer R (2021). Internal and external effects of social distancing in a pandemic. Journal of Economic Theory, 196, Article 105293. 10.1016/j.jet.2021.105293 [DOI] [Google Scholar]
- Fujii TG, Ikebuchi M, & Okanoya K (2021). Sex differences in the development and expression of a preference for familiar vocal signals in songbirds. PLoS One, 16(1), Article e0243811. 10.1371/journal.pone.0243811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furest Cataldo B, Yang L, Cabezas B, Ovetsky J, & Vicario DS (2023). Novel sound exposure drives dynamic changes in auditory lateralization that are associated with perceptual learning in zebra finches. Communications Biology, 6(1), Article 1205. 10.1038/s42003-023-05567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, & Ball GF (2000). Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. Journal of Neurobiology, 42(1), 117–133. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, & Margoliash D (2003). Neuronal populations and single cells representing learned auditory objects. Nature, 424(6949), 669–674. 10.1038/nature01731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JM, Bell ZW, Condliffe D, Dohrer K, Abaurrea T, Spencer K, Leitão A, Gahr M, Hurd PJ, & Clayton DF (2020). Acute social isolation alters neurogenomic state in songbird forebrain. Proceedings of the National Academy of Sciences of the United States of America, 117(38), 23311–23316. 10.1073/pnas.1820841116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gess A, Schneider DM, Vyas A, & Woolley SMN (2011). Automated auditory recognition training and testing. Animal Behaviour, 82(2), 285–293. 10.1016/j.anbehav.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, & Kingsbury MA (2009). Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science, 325(5942), 862–866. 10.1126/science.1174929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig F (2021). DHARMa: Residual Diagnostics For Hierarchical (Multi-Level / Mixed) Regression Models (R package version 0.4.3) [Computer software]. https://florianhartig.github.io/DHARMa/ [Google Scholar]
- Hauber ME, Campbell DLM, & Woolley SMN (2010). The functional role and female perception of male song in zebra finches. Emu - Austral Ornithology, 110(3), 209–218. 10.1071/MU10003 [DOI] [Google Scholar]
- Hernandez AM, Perez EC, Mulard H, Mathevon N, & Vignal C (2016). Mate call as reward: Acoustic communication signals can acquire positive reinforcing values during adulthood in female zebra finches (Taeniopygia guttata). Journal of Comparative Psychology, 130(1), 36–43. 10.1037/a0040027 [DOI] [PubMed] [Google Scholar]
- Hu RK, Zuo Y, Ly T, Wang J, Meera P, Wu YE, & Hong W (2021). An amygdala-to-hypothalamus circuit for social reward. Nature Neuroscience, 24(6), 831–842. 10.1038/s41593-021-00828-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED (2019). Evolution of vocal learning and spoken language. Science, 366(6461), 50–54. 10.1126/science.aax0287 [DOI] [PubMed] [Google Scholar]
- John F, & Weisberg S (2019). An R companion to applied regression (3rd ed.). Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [Google Scholar]
- Jones PR, Moore DR, Shub DE, & Amitay S (2015). The role of response bias in perceptual learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 41(5), 1456–1470. 10.1037/xlm0000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Hasegawa T, & Okanoya K (2010). Song preference of female Bengalese finches as measured by operant conditioning. Journal of Ethology, 28(3), 447–453. 10.1007/s10164-010-0203-7 [DOI] [Google Scholar]
- Kingsbury MA, & Goodson JL (2014). Pair bond formation is impaired by VPAC receptor antagonism in the socially monogamous zebra finch. Behavioural Brain Research, 272, 264–268. 10.1016/j.bbr.2014.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R (2021). emmeans: Estimated marginal means, aka least-squares means (R package Version 1.6.3) [Computer software]. https://CRAN.R-project.org/package=emmeans [Google Scholar]
- Lim Y, Lagoy R, Shinn-Cunningham BG, & Gardner TJ (2016). Transformation of temporal sequences in the zebra finch auditory system. eLife, 5, Article e18205. 10.7554/eLife.18205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, & Barnea A (2002). Social change affects the survival of new neurons in the forebrain of adult songbirds. Behavioural Brain Research, 133(1), 31–43. 10.1016/S0166-4328(01)00416-8 [DOI] [PubMed] [Google Scholar]
- Macedo-Lima M, & Remage-Healey L (2020). Auditory learning in an operant task with social reinforcement is dependent on neuroestrogen synthesis in the male songbird auditory cortex. Hormones and Behavior, 121, Article 104713. 10.1016/j.yhbeh.2020.104713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, & Creelman CD (1990). Response bias: Characteristics of detection theory, threshold theory, and ‘nonparametric’ indexes. Psychological Bulletin, 107(3), 401–413. 10.1037/0033-2909.107.3.401 [DOI] [Google Scholar]
- Mello CV (2014). The zebra finch, Taeniopygia guttata: An avian model for investigating the neurobiological basis of vocal learning. Cold Spring Harbor Protocols, 2014(12), 1237–1242. 10.1101/pdb.emo084574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB (1979). Long-term recognition of father’s song by female zebra finches. Nature, 280(5721), 389–391. 10.1038/280389a0 [DOI] [Google Scholar]
- Okanoya K, & Dooling RJ (1987). Hearing in passerine and psittacine birds: A comparative study of absolute and masked auditory thresholds. Journal of Comparative Psychology, 101(1), 7–15. 10.1037/0735-7036.101.1.7 [DOI] [PubMed] [Google Scholar]
- Perret A, Henry L, Coulon M, Caudal JP, Richard JP, Cousillas H, Hausberger M, & George I (2015). Social visual contact, a primary ‘drive’ for social animals? Animal Cognition, 18(3), 657–666. 10.1007/s10071-015-0834-8 [DOI] [PubMed] [Google Scholar]
- Petkov CI, & Jarvis ED (2012). Birds, primates, and spoken language origins: Behavioral phenotypes and neurobiological substrates. Frontiers in Evolutionary Neuroscience, 4, Article 12. 10.3389/fnevo.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Adkins-Regan E, & Romero LM (2003). Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Hormones and Behavior, 43(1), 108–114. 10.1016/S0018-506X(02)00012-0 [DOI] [PubMed] [Google Scholar]
- Riebel K (2000). Early exposure leads to repeatable preferences for male song in female zebra finches. Proceedings of the Royal Society of London, Series B: Biological Sciences, 267(1461), 2553–2558. 10.1098/rspb.2000.1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebel K, & Slater PJB (1998). Testing female chaffinch song preferences by operant conditioning. Animal Behaviour, 56(6), 1443–1453. 10.1006/anbe.1998.0933 [DOI] [PubMed] [Google Scholar]
- Roach SP, Mennill DJ, & Phillmore LS (2017). Operant discrimination of relative frequency ratios in black-capped chickadee song. Animal Cognition, 20(5), 961–973. 10.1007/s10071-017-1115-5 [DOI] [PubMed] [Google Scholar]
- Sahu PK, Montenegro C, Lambert CT, Oprea A, Deimeke MJ, Rennie V, Smeltz SML, Benowicz TJ, Patel D, Phillmore LS, & Sturdy CB (2022). Effect of feed-time duration on discrimination of vocalizations in a go/no-go operant paradigm. Behavioural Processes, 203, Article 104777. 10.1016/j.beproc.2022.104777 [DOI] [PubMed] [Google Scholar]
- Schneider DM, & Woolley SMN (2013). Sparse and background-invariant coding of vocalizations in auditory scenes. Neuron, 79(1), 141–152. 10.1016/j.neuron.2013.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcox AP, & Evans SM (1982). Factors affecting the formation and maintenance of pair bonds in the zebra finch, Taeniopygia guttata. Animal Behaviour, 30(4), 1237–1243. 10.1016/S0003-3472(82)80216-9 [DOI] [Google Scholar]
- Sossinka R, & Böhner J (1980). Song types in the zebra finch Poephila guttata castanotis. Zeitschrift für Tierpsychologie, 53(2), 123–132. 10.1111/j.1439-0310.1980.tb01044.x [DOI] [Google Scholar]
- Sternson SM, Nicholas Betley J, & Cao ZFH (2013). Neural circuits and motivational processes for hunger. Current Opinion in Neurobiology, 23(3), 353–360. 10.1016/j.conb.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JG (1967). Reinforcing effects of chaffinch song. Animal Behaviour, 15(4), 427–432. 10.1016/0003-3472(67)90040-1 [DOI] [PubMed] [Google Scholar]
- Swann WJ (2006). Improving the welfare of working equine animals in developing countries. Applied Animal Behaviour Science, 100(1–2), 148–151. 10.1016/j.applanim.2006.04.001 [DOI] [Google Scholar]
- Tokarev K, & Tchernichovski O (2014). A novel paradigm for auditory discrimination training with social reinforcement in songbirds. bioRxiv. 10.1101/004176 [DOI] [Google Scholar]
- Tomaszycki ML, & Zatirka BP (2014). Same-sex partner preference in zebra finches: Pairing flexibility and choice. Archives of Sexual Behavior, 43(8), 1469–1475. 10.1007/s10508-014-0377-0 [DOI] [PubMed] [Google Scholar]
- Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW, & Svensson EI (2012). The impact of learning on sexual selection and speciation. Trends in Ecology & Evolution, 27(9), 511–519. 10.1016/j.tree.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Vignal C, & Mathevon N (2011). Effect of acoustic cue modifications on evoked vocal response to calls in zebra finches (Taeniopygia guttata). Journal of Comparative Psychology, 125(2), 150–161. 10.1037/a0020865 [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N, & Mottin S (2004). Audience drives male songbird response to partner’s voice. Nature, 430, 448–451. 10.1038/nature02645 [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N, & Mottin S (2008). Mate recognition by female zebra finch: Analysis of individuality in male call and first investigations on female decoding process. Behavioural Processes, 77(2), 191–198. 10.1016/j.beproc.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, & Carter CS (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Hormones and Behavior, 26(3), 339–349. 10.1016/0018-506X(92)90004-F [DOI] [PubMed] [Google Scholar]
- Woolley SC, & Doupe AJ (2008). Social context-induced song variation affects female behavior and gene expression. PLoS Biology, 6(3), 0525–0537. 10.1371/journal.pbio.0060062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann R (1996). The zebra finch: A synthesis of field and laboratory studies. Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article has been uploaded to a public repository (https://github.com/HealeyLab/Macedo-Limaetal2023).


