Abstract
Aims
The aim of this study was to determine if single items in the quality of life questionnaire short form 36 (SF36) were associated with cardiovascular events in patients with type 2 diabetes mellitus (T2DM).
Methods
In 756 T2DM patients (260 women) from the CARDIPP study, nine questions from the domains vitality and well‐being in SF36 were analysed. Patients, 55–66 years, were recruited in 2005–2008 and followed up until 31 December 2018 for the incidence of major adverse cardiovascular events (MACE), that is, myocardial infarction, stroke or cardiovascular death.
Results
Median follow‐up time: 11.6 years, during which 119 (16%) MACE occurred. The SF36 items: ‘seldom full of pep’ (HR 1.2, 95% CI: 1.1–1.4, p = 0.006), ‘seldom a lot of energy’ (HR 1.3, 95%CI: 1.1–1.5, p < 0.001), ‘worn out’ (HR 1.2, 95%CI: 1.0–1.4, p = 0.020) and ‘seldom happy’ (HR 1.2, 95%CI: 1.0–1.4, p = 0.012) were independent risk factors for MACE in separate models, as well as male sex, diabetes duration, HbA1c, sagittal abdominal diameter and aortic pulse wave velocity. The variables ‘seldom full of pep’ and ‘seldom a lot of energy’ remained associated with MACE when conducting separate analyses for sexes. Only ‘seldom a lot of energy’ remained associated with MACE when all items from SF‐36 were comprised in the same model.
Conclusions
One single question regarding energy levels from SF36 may be used as an independent risk factor for cardiovascular events in T2DM patients in primary care, for both men and women. This item may be included in future risk assessment for use in clinical practice for cardiovascular risk stratification of T2DM patients.
Trial registration
The study was registered in clinicaltrial.gov (NCT 01049737) in 14 January 2010.
Keywords: cardiovascular diseases, diabetes mellitus, type 2, primary health care, quality of life, risk factors
Novelty statement.
Associations between low quality of life and cardiovascular events in patients with type 2 diabetes mellitus (T2DM) are well known, but screening scales regarding quality of life are seldom incorporated in routine primary care. In this study, we found that one single question regarding vitality from the questionnaire SF36 was associated with cardiovascular events, independent of traditional risk factors and arterial stiffness. We suggest that this question may be included in future risk algorithms in clinical practice for cardiovascular risk stratification in patients with T2DM as an easy way to get additional prognostic information about the risk of cardiovascular disease and death.
1. INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is increasing worldwide and is expected to continue increasing during the next decades. 1 Cardiovascular disease (CVD) is the principal cause of morbidity and mortality in patients with T2DM. 2 Despite a large amount of well‐known risk factors, T2DM patients still have a twofold increased risk for CVD compared to the general population. 2 To optimise care for T2DM patients, there is a need to identify new predictors that may be used to detect individuals with high risk for CVD.
In the general population, the INTERHEART study showed that several psychosocial stressors are associated with increased risk of CVD. 3 Symptoms of depression are also associated with myocardial infarction and death. 4 In patients with diabetes mellitus, the incidence of a major cardiovascular event is associated with decreased quality of life (QoL). 5 Several studies have shown that QoL among diabetes patients is decreased as compared to the general population. 6
Even though strong associations between QoL and CVD have been reported, low QoL is not established as a risk factor for CVD and measurements of QoL are seldom incorporated in clinical practice in primary care. One commonly used measure for QoL is the short form 36 (SF36) questionnaire, which has high validity, reliability and acceptability. 7 , 8 SF36 was designed to assess QoL in a wide variety of patients and settings. 8 , 9 However, in clinical practice, even a short questionnaire may be too time‐consuming, particularly when encountering patients with multiple comorbidities and conditions. 10 Thus, a feasible and easy‐to‐manage marker of QoL might be useful 11 in primary care.
The objective of this study was to investigate if selected single questions in the SF36 were associated with cardiovascular events and mortality in T2DM patients.
2. METHODS
2.1. Patient recruitment and data collection
T2DM patients were included from cardiovascular risk factors in patients with diabetes a prospective study in primary care (CARDIPP), 12 , 13 an observational prospective cohort study where the patients were recruited in 2005–2008 by trained nurses from 22 different primary care centres in the south‐east region of Sweden. 12 , 13 These centres were of different size and located in different areas, but the management and care of T2DM was organised similarly, and all centres adhered to the same national guidelines for diabetes care. Patients were invited to participate in the study when attending a routine annual follow‐up. Inclusion criteria were type 2 diabetes treated in primary care, 55–65 years of age, willingness to participate in the study. The only exclusion criterion was known severe physical or mental disease with a short life expectancy. 13 Standardised medical history was taken, including data on ongoing medication, previous CVD and diabetes duration. The participants filled out a questionnaire regarding demographic and socioeconomic factors, comorbidities, lifestyle factors and QoL. In total, 761 T2DM patients were included in CARDIPP. In five patients, there were no data from any of the questions from SF36, and thus, 756 patients (260 women) remained for further analyses.
2.2. Questionnaire and SF‐36
SF36 measures QoL across eight domains: (1) limitations in physical activities; (2) limitations in social activities due to physical or emotional health; (3) role limitations due to physical problems; (4) physical pain; (5) general mental health and well‐being; (6) role limitations due to emotional problems; (7) vitality (energy/fatigue); and (8) general health. 14 A single item is also included that identifies perceived change in health.
In this study, the patients answered the questions in domains 5 and 7 from SF36. These domains were chosen since no other measurement of mental well‐being was included in the CARDIPP questionnaire and they are both found under the same heading within the SF36 (energy and emotions). In these domains, the answers were graded on a scale from 1 to 6 where 1 meant ‘all the time’ and 6 meant ‘never’. The patients were instructed to give the answer that best represented their feelings during the last 4 weeks. The questions were
‘Domain 5:
Did you feel full of pep?
Did you have a lot of energy?
Did you feel worn out?
Did you feel tired?’
‘Domain 7:
Have you been a very nervous person?
Have you felt so down in the dumps that nothing could cheer you up?
Have you felt calm and peaceful?
Have you felt downhearted and blue?
Have you been a happy person?’
2.3. Laboratory analyses
As previously described, 12 blood samples were obtained in the morning after 10 h of overnight fast. HbA1c, plasma glucose and serum lipids were analysed directly, according to clinical routine at each primary care centres or hospital. Levels of ApoB and ApoA1 were measured by immunoturbidimetric assays, Bayer Health Care and Siemens Diagnostic Medical Solutions (Tokyo, Japan) and analysed at the Department of Laboratory Medicine, Linköping University Hospital, Sweden.
2.4. Anthropometric measurements
Nurses especially dedicated to the treatment of diabetes at the primary care centres, measured height and weight with the patients wearing light indoor clothing. Sagittal abdominal diameter (SAD) was recorded with the patient in the supine position and with bent knees, with a standardised sliding beam calliper at the highest point of the abdomen. 15 SAD has previously been suggested as a more important risk predictor for arterial stiffness over time, compared with waist circumference (WC) in T2DM patients. 15
2.5. Clinical physiological investigations
All patients were subjected to blood pressure measurements and an echocardiographic evaluation as described before. 16 The determination of pulse wave velocity (PWV) was done at the Department of Physiology, Linköping University Hospital, and at the County Hospital Ryhov, Jönköping. Briefly, the aortic PWV was measured with applanation tonometry (SphygmoCor® system, model MM3, AtCor Medical) over the carotid and femoral arteries. The aortic pulse wave transit times were measured by electrocardiogram guided readings of the femoral arterial pulse waves, using the carotid arterial pulse wave as the reference site. The surface distances were measured from the suprasternal notch to the carotid and femoral measurement sites respectively. PWV was calculated by dividing the surface distance with the pulse wave transit time yielding m/s.
2.6. Outcome
The outcome variable was incidence of major adverse cardiovascular events (MACE). MACE was defined as the occurrence of any cardiovascular mortality, International Classification of Diseases (ICD)‐10 codes: 100–199 or hospitalisation for myocardial infarction; ICD‐10: 121 or stroke; ICD‐10: 160, 161 and 163. The first occurrence of any of these predefined events was classified as an endpoint event. Outcome variable data were retrieved by linkage of the study database with the Swedish Cause of Death Registry (The National Board of Health and Welfare, Stockholm, Sweden) and the Inpatient Register, using the Swedish national personal identification number for each patient. Patients were followed from inclusion until first event occurred, or until 31 December 2018.
2.7. Statistics
SPSS‐27 (IBM SPSS Inc.) was used for statistical analysis. Associations between MACE and the results on the SF36 items were calculated as hazard ratios (HR), with a corresponding 95% confidence interval (CI). Significance was set at 0.05 (two‐sided). Only participants who had data for all the parameters were included. First, crude HR were calculated using univariate Cox regression analyses. Thereafter, adjusted HR were calculated using multivariate Cox regression analyses with the co‐variates: sex, age, HbA1c diabetes duration, SAD, mean systolic blood pressure (SBP) during 24 h, PWV, ApoB/ApoA1 and smoking status. All included items were addressed as risk factors, that is, the positive items from SF36: ‘feeling full of pep’, ‘calm and peaceful’, ‘having a lot of energy’, ‘feeling happy’ were recoded to a lack of that feeling. For associations between MACE and QoL, the ordinal six‐graded scales for each item from SF36 were analysed as continuous variables. When building the final multivariate cox regression models, manual backwards stepwise analysis was used. In additional analyses, the items from SF36 were dichotomised by splitting each in middle, where answer options 1–3 were compared with answer options 4–6. Given the clustered nature of the data, we also explored the degree of clustering by use of frailty models calculated in STATA mp v. 16.1 (StataCorp LLC). Individuals with missing data in any of the included variables were excluded from the analyses.
2.8. Ethics
The study was performed in line with the principles in the Declaration of Helsinki, approved by the Regional Ethical Review Board in Linköping and registered in the clinicaltrial.gov database (NCT 01049737). Informed consent was obtained from all participants.
3. RESULTS
During a follow‐up period of median 11.6 years (range: 38–4998 days), 112 (15%) of the included patients died. No patients were lost to follow‐up. There were 121 (16%) MACE: with ICD‐10 causes as follows: I21‐I509, n = 63; I602‐I620, n = 11; I63, n = 46; I74 n = 1.
All items in the vitality domain in SF36 (‘seldom feeling full of pep’, ‘seldom having a lot of energy’, ‘feeling worn out’, and ‘feeling tired’) were separately associated with MACE in the univariate Cox regression models, as well as two items from the emotional well‐being domain (‘downhearted and blue’ and ‘seldom feeling happy’). Of the other risk factors, male sex, duration of diabetes, HbA1c, mean SBP, SAD and PWV were separately associated with MACE as shown in Table 1.
TABLE 1.
Risk factors for MACE in T2DM patients
| Total no. of respondents | MACE (mean ± SD or percentage) | No MACE (mean ± SD or percentage) | Univariate Cox regression | |||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p‐value | ||||
| Male sex | 756 | n = 92/121 (75%) | n = 404/635 (64%) | 1.70 | 1.13–2.57 | 0.01* |
| Age (years) | 756 | 61.0 ± 3.0 | 61.0 ± 3.2 | 1.04 | 0.98–1.11 | 0.17 |
| Duration of diabetes (years) | 711 | 9.7 ± 7.7 | 6.8 ± 5.8 | 1.05 | 1.03–1.07 | <0.001** |
| HbA1c IFCC (mmol/mol) (HbA1c DCCT, %) |
746 746 |
58 ± 11 6.5 ± 1.1 |
52 ± 12 6.0 ± 1.1 |
1.03 1.35 |
1.02–1.04 1.19–1.53 |
<0.001** <0.001** |
| Current smoker | 747 | n = 26/117 (22%) | n = 115/630 (18%) | 1.34 | 0.86–2.06 | 0.19 |
| SAD (cm) | 712 | 26.8 ± 3.6 | 25.3 ± 3.8 | 1.09 | 1.04–1.14 | <0.001** |
| ApoB/ApoA1 | 702 | 0.76 ± 0.17 | 0.73 ± 0.17 | 2.49 | 0.88–7.71 | 0.09 |
| Mean SBP (mm Hg) | 718 | 133 ± 15 | 129 ± 13 | 1.01 | 1.00–1.03 | 0.04* |
| PWV (m/s) | 697 | 11.2 ± 2.6 | 10.2 ± 2.0 | 1.18 | 1.10–1.27 | <0.001** |
| Short Form 36 | ||||||
| Domain: Vitality | ||||||
| Seldom feeling full of pep | 745 | 3.30 ± 1.53 | 2.80 ± 1.33 | 1.25 | 1.11–1.42 | <0.001** |
| Seldom having a lot of energy | 749 | 3.61 ± 1.45 | 3.08 ± 1.35 | 1.29 | 1.13–1.46 | <0.001** |
| Worn out | 748 | 2.66 ± 1.47 | 2.23 ± 1.30 | 1.23 | 1.09–1.39 | 0.001** |
| Tired | 751 | 3.25 ± 1.49 | 2.88 ± 1.28 | 1.21 | 1.07–1.38 | 0.003** |
| Domain: Emotional well‐being | ||||||
| Very nervous | 748 | 1.74 ± 1.15 | 1.56 ± 0.89 | 1.16 | 0.98–1.38 | 0.09 |
| Impossible to cheer up | 739 | 1.53 ± 1.00 | 1.43 ± 0.86 | 1.10 | 0.91–1.32 | 0.34 |
| Seldom calm and peaceful | 751 | 2.66 ± 1.49 | 2.46 ± 1.37 | 1.10 | 0.97–1.24 | 0.14 |
| Downhearted and blue | 749 | 1.86 ± 1.08 | 1.65 ± 0.97 | 1.19 | 1.01–1.39 | 0.03* |
| Seldom happy | 748 | 3.00 ± 1.46 | 2.63 ± 1.23 | 1.21 | 1.06–1.39 | 0.004** |
Note: Baseline characteristics of the study cohort and Cox regressions analyses of MACE in relation to all included vascular risk factors as well as all items from the two domains vitality and emotional well‐being in SF36. Regarding the domains from SF36, all items were analysed as continuous variables. All analyses are made by univariate Cox regression analyses.
Abbreviations: CI, confidence interval; SAD, sagittal abdominal diameter; SBP, systolic blood pressure; PWV, pulse wave velocity; SF36, short form 36.
p < 0.05
p < 0.005.
The included items from SF36 were analysed separately in multivariate Cox regression models adjusted for all included risk factors. Three items from the vitality domain remained independently associated with MACE: ‘seldom feeling full of pep’, ‘seldom having a lot of energy’, and ‘worn out’, as well as one item from the emotional well‐being domain: ‘seldom happy’, see supplemental digital content (SDC) T1. Figure 1 illustrates the Cox regression analyses of event‐free survival in relation to the items ‘full of pep’ (A) and ‘seldom having a lot of energy’ (B). The association between MACE and the items from SF 36 was also analysed as dichotomised data. In this multivariate model, ‘seldom feeling full of pep’, ‘seldom having a lot of energy’ and ‘seldom happy’ remained independently associated with MACE, see Table S2.
FIGURE 1.
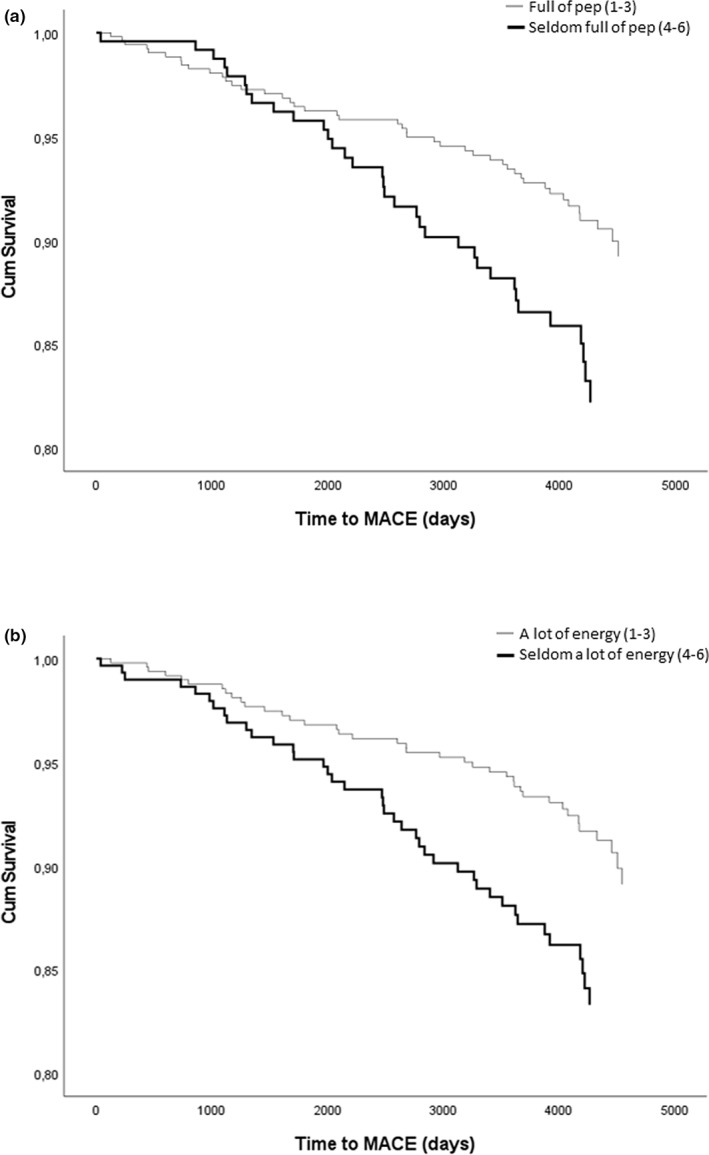
Cox regression analyses of event‐free survival in relation to the items ‘seldom feeling full of pep’ (a) hazard ratio 1.8, 95% confidence interval from 1.1 to 2.9, p = 0.013 and ‘seldom having a lot of energy’ (b) hazard ratio 1.8, 95% confidence interval from 1.2 to 2.9, p = 0.009. The grey line represents answer options 1–3 (all of the time–most of the time–a good bit of the time) and the black line represents answer options 4–6 (some of the time–a little bit of the time–none of the time). Results are adjusted for sex, age, duration of diabetes, HbA1c, smoking status, SAD, ApoB/ApoA1, blood pressure and PWV.
Finally, multivariate Cox regression models only including independent vascular risk factors were constructed for each SF36 item associated with MACE. In summary, ‘seldom feeling full of pep’ (HR 1.2, 95%CI: 1.1–1.4, p = 0.006), ‘seldom having a lot of energy’ (HR 1.3, 95%CI: 1.1–1.5, p < 0.001), ‘feeling worn out’ (HR 1.2, 95%CI: 1.0–1.4, p = 0.020) and ‘seldom feeling happy’ (HR 1.2, 95%CI: 1.0–1.4, p = 0.012) remained independent risk factors for MACE, as well as male sex, duration of diabetes, HbA1c, PWV and SAD (only independent for variables ‘feeling worn out’ and ‘seldom feeling happy’). For details, see Tables 2,b. The assumption for proportional hazards was tested using Schoenfeld residuals in Stata. The lowest p‐value was for ‘seldom full of pep’ (p = 0.31). Thus, the assumption of proportional hazard was fulfilled. The results from the frailty models were only marginally different from the original models, with no effect on the significances. Finally, a model with all items from SF36 associated with MACE as well as all the other risk factors was tried out. Using manual backwards stepwise analysis, only the item ‘seldom having a lot of energy’ from SF36 remained in this additional model (supplementary content T3).
TABLE 2.
(A–B) Cox regression models including independent predictors of MACE
| Model 1 ‘Seldom feeling full of pep’ (n = 555) | Model 2 ‘Seldom having a lot of energy’ (n = 558) | Model 3 ‘Feeling worn out’ (n = 558) | Model 4 ‘Seldom feeling happy’ (n = 558) | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p‐value | Hazard ratio (95% CI) | p‐value | Hazard ratio (95% CI) | p‐value | Hazard ratio (95% CI) | p‐value | |
| A | ||||||||
| Item in Short Form 36 | 1.20 (1.03–1.40) | 0.02 | 1.31 (1.12–1.54) | 0.001 | 1.17 (1.01–1.37) | 0.04 | 1.23 (1.04–1.46) | 0.02 |
| Male sex | 2.06 (1.16–3.66) | 0.01 | 2.26 (1.27–4.03) | 0.005 | 1.97 (1.11–3.49) | 0.02 | 2.09 (1.18–3.72) | 0.01 |
| Duration of diabetes | 1.04 (1.01–1.06) | 0.01 | 1.03 (1.01–1.06) | 0.01 | 1.04 (1.01–1.06) | 0.005 | 1.04 (1.01–1.06) | 0.009 |
| HbA1c IFCC | 1.03 (1.02–1.05) | <0.001 | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 | 1.04 (1.02–1.05) | <0.001 |
| PWV | 1.13 (1.02–1.25) | 0.02 | 1.13 (1.03–1.26) | 0.02 | 1.13 (1.02–1.25) | 0.02 | 1.14 (1.03–1.26) | 0.02 |
| SAD | 1.05 (0.98–1.12) | 0.14 | 1.05 (0.99–1.12) | 0.13 | 1.06 (0.99–1.13) | 0.08 | 1.06 (0.99–1.12) | 0.09 |
| Age | 0.99 (0.92–1.07) | 0.77 | 1.00 (0.93–1.08) | 1.00 | 1.00 (0.92–1.08) | 0.98 | 1.00 (0.92–1.08) | 0.91 |
| Current smoker | 1.13 (0.61–2.08) | 0.70 | 1.14 (0.62–2.10) | 0.67 | 1.09 (0.59–2.00) | 0.79 | 1.08 (0.58–2.00) | 0.81 |
| ApoB/ApoA1 | 1.42 (0.34–5.85) | 0.63 | 1.57 (0.38–6.49) | 0.53 | 1.37 (0.34–5.60) | 0.66 | 1.45 (0.35–5.90) | 0.61 |
| Mean SBP | 1.00 (0.98–1.02) | 0.90 | 1.00 (0.99–1.02) | 0.87 | 1.00 (0.98–1.02) | 0.98 | 1.00 (0.99–1.02) | 0.83 |
| B | ||||||||
| Item in Short Form 36 | 1.21 (1.05–1.38) | 0.01 | 1.28 (1.12–1.48) | <0.001 | 1.18 (1.03–1.36) | 0.02 | 1.23 (1.05–1.44) | 0.01 |
| Male sex | 2.01 (1.23–3.27) | 0.01 | 2.13 (1.31–3.47) | 0.002 | 2.23 (1.31–3.79) | 0.003 | 2.39 (1.40–4.07) | 0.001 |
| Duration of diabetes | 1.03 (1.01–1.05) | 0.02 | 1.03 (1.00–1.05) | 0.03 | 1.03 (1.01–1.06) | 0.01 | 1.03 (1.01–1.06) | 0.02 |
| HbA1c IFCC | 1.03 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 |
| PWV | 1.15 (1.06–1.24) | 0.001 | 1.16 (1.07–1.25) | <0.001 | 1.12 (1.03–1.23) | 0.01 | 1.14 (1.04–1.24) | 0.01 |
| SAD | — | — | — | — | 1.06 (1.00–1.13) | 0.04 | 1.06 (1.00–1.12) | 0.04 |
| Age | — | — | — | — | — | — | — | — |
| Current smoker | — | — | — | — | — | — | — | — |
| ApoB/ApoA1 | — | — | — | — | — | — | — | — |
| Mean SBP | — | — | — | — | — | — | — | — |
Note: Models for each item in SF36 that were associated with MACE in the multivariate Cox regression analyses adjusted for all included risk factors. All items from SF36 were analyzed as continuous variables. Model 1 comprised the item ‘Seldom feeling full of pep’, model 2 comprised the item ‘seldom having a lot of energy’, model 3 comprised the item ‘Feeling worn out’ and model 4 comprised the item ‘seldom feeling happy’. In table A, Hazard ratios, 95% CI and p‐values for each included variable are reported before further analyses were conducted. In table B, all variables not independently associated with MACE have been removed using manual backwards stepwise analysis until only the variables that remained independent predictors of MACE remained.
Abbreviations: CI, confidence interval; PWV, pulse wave velocity; SAD, sagittal abdominal diameter; SF36, short form 36.
In addition, separate multivariate Cox regression models for each SF36 item were constructed separately for sexes. In the models where each SF36 item was analysed along with all other accessible risk factors ‘seldom full of pep’ and ‘seldom having a lot of energy’ remained significant for men, while no SF36 item was significant for women, see SDC T4. However, when conducting separate manual backwards analyses for each SF36 variable, for women, ‘seldom feeling full of pep’ (HR: 1.4, 95% CI: 1.0–1.9, p = 0.030) and ‘seldom having a lot of energy’ (HR: 1.5, 95% CI: 1.1–2.0, p = 0.011) were associated with MACE, where the other remaining significant variables were HbA1c, smoking status and PWV. ‘Feeling worn out’ (HR: 1.4, 95% CI: 1.0–1.9, p = 0.029) was also independently associated with MACE in women. For men, the SF36 items ‘seldom feeling full of pep’ (HR: 1.2, 95% CI: 1.0–1.4, p = 0.015) and ‘seldom having a lot of energy’ (HR: 1.3, 95% CI: 1.1–1.5, p = 0.003) remained associated with MACE when conducting separate manual backwards analyses for each SF36 variable, where the other significant variables were duration of diabetes, HbA1c and PWV. When all items from SF36 associated with MACE as well as all other included risk factors were included in the same model, using manual backwards stepwise analysis only the item ‘seldom having a lot of energy’ from SF36 remained significant for both men (p = 0.003) and women (p = 0.046).
4. DISCUSSION
We found that self‐reported ‘low feeling of pep’ and ‘low energy levels’ were associated with MACE in T2DM patients, independent of both established cardiovascular risk factors, arterial stiffness 17 and abdominal obesity. 15 Only the item ‘low energy levels’ remained when all items were comprised in the same model, suggesting this variable is most reflective of future risk for MACE.
It is previously known that T2DM patients have a lower QoL than the general population and often present with a wide variety of comorbidities possibly influencing QoL. 6 , 18 Low QoL has also been reported as a risk factor for CVD in T2DM. 10 , 11 , 19 , 20 , 21 What does then this study add to previous knowledge? First, our results suggested that self‐reported sense of vitality may be an equally strong risk factor for MACE as several previously well‐known risk factors such as duration of diabetes and HbA1c. When using the multivariate models that only included variables independently associated with MACE, the separate items ‘seldom feeling full of pep’ and ‘seldom having a lot of energy’ were among the risk factors most strongly associated with MACE.
Second, the long‐time follow‐up of median 11 years extends most previous studies with follow‐up times for 2–4 years. 11 , 19 , 21 , 22 Third, in the present study, questions from the two domains vitality and emotional well‐being from SF36 were analysed as separate items. It has been claimed before that a single measure of self‐rated health using the EuroQol group's visual analogue scale (EQ‐VAS) in T2DM provides valuable information on patient risk for subsequent outcomes. 19 The use of selected single questions from SF36 as risk factors for cardiovascular disease is to our knowledge a novel approach. The identification of specific questions that can be used as independent markers for CVD may be an advantage in clinical practice because of the simplicity of the process compared with utilisation of more extensive questionnaires.
Screening scales regarding QoL are seldom used in routine primary care. The limited time for each patient in combination with the stressful clinical reality 10 is probably the most important cause, possibly in combination with the challenge to choose between multiple QoL measurements. 23 Based on the results in our study, we suggest that the question: ‘During the last 4 weeks, did you have a lot of energy?’ may be included in future risk algorithms for use in clinical practice for cardiovascular risk stratification of T2DM patients. This may be an easy way to get additional prognostic information about risk for cardiovascular disease and death and should imply further investigation of other potential treatable causes for low QoL, for example, depression. 3 , 4 In addition, a heightened risk for MACE may facilitate early and individually adjusted interventions with, for example, adjustment of antihypertensive medication or intensified diabetes treatment.
When conducting separate analyses for men and women, the items ‘seldom feeling full of pep’ and ‘seldom having a lot of energy’ were risk factors for MACE for both sexes. The item ‘feeling worn out’ was the second most important risk factor for MACE in women, only exceeded by PWV. Sex differences related to QoL have been reported before. 24 , 25 Women with diabetes or CVD report lower QoL than men, 26 , 27 , 28 and in a general population, it has been shown that men report higher physical and psychosocial health and less pain than women. 29 Regarding predicting factors for QoL, social support appears to be more important for emotional QoL among women with CVD than men. 30 In our study, the separate questions from SF36 were independently associated with MACE in both men and women, in contrast to one previous study where the association between low QoL and cardiovascular death and all‐cause mortality was only observed in men. 22 Further research is needed to identify how different aspects of QoL may affect men and women differently and how it can be managed in clinical practice.
The pathophysiological mechanisms for the relationship between self‐reported sense of vitality and MACE in T2DM patients are unknown. In agreement with previous studies, we consider that the measurements of QoL may provide additional insight to health problems or other processes that influence mortality and cardiovascular events but may not be captured by medical history and established risk factors. 19 , 20 , 21
5. STRENGTHS AND LIMITATIONS
The major strengths of this study were the large number of included participants, the longitudinal long‐term follow‐up, the large number of collected variables and the reliable follow‐up data from two national registers with high validity: the Swedish National Inpatient Register and the Swedish Cause of Death Register. In addition, the generalisability of the results to primary care T2DM patients should be high, since study participants were recruited from primary care adjacent to a routine annual follow‐up. One of the main limitations was that the presence of depression or antidepressant medication was not screened for. Depression is both a risk factor for cardiovascular events and low QoL 3 , 4 and as such may be a confounder for our result. Other limitations include that the risk factors low education, physical inactivity and living alone were not investigated, and the potential bias affecting sex differences since cardiovascular disease develops later in women than in men. Finally, only two domains of the SF‐36 were analysed. However, the objective for this study was to investigate if separate questions from SF‐36 could be used as independent risk factors for MACE, in order to find practical, easy‐to‐manage clinical tools to facilitate a measurement of QoL in daily praxis.
6. CONCLUSION
Short questions regarding sense of vitality are associated with cardiovascular events and death in T2DM patients in primary care, independent of traditional vascular risk factors as well as arterial stiffness. We suggest that the question from SF36: ‘During the last 4 weeks, did you have a lot of energy?’ may be included in future risk algorithms for use in clinical practice for cardiovascular risk stratification of patients with T2DM.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Marta Vergara, Carl Johan Östgren, Fredrik H Nyström, Hanna Israelsson. The first draft of the manuscript was written by Marta Vergara. All authors were involved in drafting the manuscript, which was completed by Hanna Israelsson. All authors revised the manuscript for important intellectual content. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
FUNDING INFORMATION
The study was supported by grants from the Medical Research Council of Southeast Sweden, Futurum, King Gustaf V and Queen Victoria Freemason Foundation, GE Healthcare and the Swedish Heart‐Lung Foundation and the Swedish Research Council Grant 12661. The County Council of Östergötland and Linköping University, Department of Medical and Health Sciences, also supported the trial with grants and other resources.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this article.
ETHICAL APPROVAL
The study was approved by the Regional Ethical Review Board in Linköping and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Supporting information
Table S1
Vergara M, Östgren CJ, Nyström FH, Israelsson H. Sense of vitality is associated with cardiovascular events in type 2 diabetes independently of traditional risk factors and arterial stiffness. Diabet Med. 2023;40:e14938. doi: 10.1111/dme.14938
DATA AVAILABILITY STATEMENT
Data and material from the CARDIPP study are not deposited in a public repository, due to the large amount of personal data that could easily identify single patients (e.g. date of death and comorbidities) and still ongoing follow‐up of patients. However, upon request during the peer‐review process, data may be shared to reviewers to perform their own calculations. For potential future collaboration agreements and data accessibility, please contact Carl Johan Östgren at Linköping University: carl.johan.ostgren@liu.se.
REFERENCES
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4‐14. [DOI] [PubMed] [Google Scholar]
- 2. Abdul‐Ghani M, DeFronzo RA, Del Prato S, Chilton R, Singh R, Ryder REJ. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived? Diabetes Care. 2017;40(7):813‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364(9438):953‐962. [DOI] [PubMed] [Google Scholar]
- 4. Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93(11):1976‐1980. [DOI] [PubMed] [Google Scholar]
- 5. Briggs AH, Bhatt DL, Scirica BM, et al. Health‐related quality‐of‐life implications of cardiovascular events in individuals with type 2 diabetes mellitus: a subanalysis from the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus (SAVOR)‐TIMI 53 trial. Diabetes Res Clin Pract. 2017;130:24‐33. [DOI] [PubMed] [Google Scholar]
- 6. Schram MT, Baan CA, Pouwer F. Depression and quality of life in patients with diabetes: a systematic review from the European depression in diabetes (EDID) research consortium. Curr Diabetes Rev. 2009;5(2):112‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coons SJ, Rao S, Keininger DL, Hays RD. A comparative review of generic quality‐of‐life instruments. Pharmacoeconomics. 2000;17(1):13‐35. [DOI] [PubMed] [Google Scholar]
- 8. Brazier JE, Harper R, Jones NM, et al. Validating the SF‐36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40‐66. [DOI] [PubMed] [Google Scholar]
- 10. Pinheiro LC, Reshetnyak E, Sterling MR, Richman JS, Kern LM, Safford MM. Using health‐related quality of life to predict cardiovascular disease events. Qual Life Res. 2019;28(6):1465‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamarul Imran M, Ismail AA, Naing L, Wan Mohamad WB. Type 2 diabetes mellitus patients with poor glycaemic control have lower quality of life scores as measured by the Short Form‐36. Singapore Med J. 2010;51(2):157‐162. [PubMed] [Google Scholar]
- 12. Vavruch C, Lanne T, Fredrikson M, Lindstrom T, Ostgren CJ, Nystrom FH. Serum leptin levels are independently related to the incidence of ischemic heart disease in a prospective study of patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wijkman M, Lanne T, Engvall J, Lindstrom T, Ostgren CJ, Nystrom FH. Masked nocturnal hypertension—a novel marker of risk in type 2 diabetes. Diabetologia. 2009;52(7):1258‐1264. [DOI] [PubMed] [Google Scholar]
- 14. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473‐483. [PubMed] [Google Scholar]
- 15. Dahlen EM, Bjarnegard N, Lanne T, Nystrom FH, Ostgren CJ. Sagittal abdominal diameter is a more independent measure compared with waist circumference to predict arterial stiffness in subjects with type 2 diabetes‐‐a prospective observational cohort study. Cardiovasc Diabetol. 2013;12:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wijkman M, Lanne T, Grodzinsky E, Ostgren CJ, Engvall J, Nystrom FH. Ambulatory systolic blood pressure predicts left ventricular mass in type 2 diabetes, independent of central systolic blood pressure. Blood Press Monit. 2012;17(4):139‐144. [DOI] [PubMed] [Google Scholar]
- 17. Wijkman M, Lanne T, Ostgren CJ, Nystrom FH. Aortic pulse wave velocity predicts incident cardiovascular events in patients with type 2 diabetes treated in primary care. J Diabetes Complications. 2016;30(7):1223‐1228. [DOI] [PubMed] [Google Scholar]
- 18. Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self‐rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795‐797. [DOI] [PubMed] [Google Scholar]
- 20. Williams ED, Rawal L, Oldenburg BF, Renwick C, Shaw JE, Tapp RJ. Risk of cardiovascular and all‐cause mortality: impact of impaired health‐related functioning and diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2012;35(5):1067‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McEwen LN, Kim C, Haan MN, et al. Are health‐related quality‐of‐life and self‐rated health associated with mortality? Insights from Translating Research Into Action for Diabetes (TRIAD). Prim Care Diabetes. 2009;3(1):37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venskutonyte L, Brismar K, Ohrvik J, Ryden L, Kjellstrom B. Self‐rated health predicts outcome in patients with type 2 diabetes and myocardial infarction: a DIGAMI 2 quality of life sub‐study. Diab Vasc Dis Res. 2013;10(4):361‐367. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs JE. Quality of life: what does it mean for general practice? Br J Gen Pract. 2009;59(568):807‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schunk M, Reitmeir P, Schipf S, et al. Health‐related quality of life in women and men with type 2 diabetes: a comparison across treatment groups. J Diabetes Complications. 2015;29(2):203‐211. [DOI] [PubMed] [Google Scholar]
- 25. Jonsson PM, Sterky G, Gafvels C, Ostman J. Gender equity in health care: the case of Swedish diabetes care. Health Care Women Int. 2000;21(5):413‐431. [DOI] [PubMed] [Google Scholar]
- 26. Unden AL, Elofsson S, Andreasson A, Hillered E, Eriksson I, Brismar K. Gender differences in self‐rated health, quality of life, quality of care, and metabolic control in patients with diabetes. Gend Med. 2008;5(2):162‐180. [DOI] [PubMed] [Google Scholar]
- 27. Sparring V, Nystrom L, Wahlstrom R, Jonsson PM, Ostman J, Burstrom K. Diabetes duration and health‐related quality of life in individuals with onset of diabetes in the age group 15‐34 years – a Swedish population‐based study using EQ‐5D. BMC Public Health. 2013;13:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riedinger MS, Dracup KA, Brecht ML, Padilla G, Sarna L, Ganz PA. Quality of life in patients with heart failure: do gender differences exist? Heart Lung. 2001;30(2):105‐116. [DOI] [PubMed] [Google Scholar]
- 29. Cherepanov D, Palta M, Fryback DG, Robert SA, Hays RD, Kaplan RM. Gender differences in multiple underlying dimensions of health‐related quality of life are associated with sociodemographic and socioeconomic status. Med Care. 2011;49(11):1021‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emery CF, Frid DJ, Engebretson TO, et al. Gender differences in quality of life among cardiac patients. Psychosom Med. 2004;66(2):190‐197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data and material from the CARDIPP study are not deposited in a public repository, due to the large amount of personal data that could easily identify single patients (e.g. date of death and comorbidities) and still ongoing follow‐up of patients. However, upon request during the peer‐review process, data may be shared to reviewers to perform their own calculations. For potential future collaboration agreements and data accessibility, please contact Carl Johan Östgren at Linköping University: carl.johan.ostgren@liu.se.


