Abstract
Cascade testing for familial cancer syndromes has historically been difficult to execute. As part of a facilitated cascade testing pathway, we evaluated barriers to completion of cascade testing. Our previously published study evaluated a facilitated cascade testing pathway whereby a genetics team facilitated at-risk relative (ARR) cascade testing through telephone genetic counseling and mailed saliva kit testing. This follow-up study evaluated barriers to completion of cascade genetic testing through six-month follow-up telephone interviews. Probands identified 114 ARRs, of whom 97 were successfully contacted by telephone. Among those contacted, 83 (86%) reported interest in genetic testing and 14 (14%) declined. Among those reporting interest in testing, 71% (69/83) completed testing. Follow-up telephone interviews revealed that 14 ARRs did not complete testing despite reporting interest for the following reasons: concern about genetic discrimination, fear of a positive result and belief that the pathogenic variant was not relevant to his/her health. Five ARRs reported that they remained interested in testing and the telephone call prompted completion of testing. Even when facilitated by a medical team with prioritization of relative convenience, significant barriers to cascade testing ARRs for hereditary breast and ovarian cancer syndrome persist due to concern about genetic discrimination, cost, and fear of positive test results.
Keywords: Cascade genetic testing, Predictive testing, Hereditary breast and ovarian cancer syndrome, Lynch syndrome
Introduction:
In the United States, rates of referral for genetic testing have increased in the past two decades. However, only a modest proportion of individuals with cancer-associated germline pathogenic variants have been identified [1–4]. Cascade testing is a strategy to identify individuals unknowingly carrying germline pathogenic variants. This is accomplished through familial diffusion, or the “cascade” of genetic risk information, whereby, starting with the affected patient (proband), genetic testing is extended to at-risk relatives (ARRs). ARRs found to carry the familial pathogenic variant can take advantage of genetically targeted primary disease prevention through intensive cancer surveillance and risk-reducing surgery, improving morbidity and preventing mortality [2, 3, 5, 6].
Hereditary breast and ovarian cancer (HBOC) syndrome is an inherited genetic condition that causes an increased risk of breast, ovarian, pancreatic, and prostate cancers as well as melanoma [7]. HBOC is most-commonly due to autosomal dominant germline pathogenic variants of BRCA1 and BRCA2; however, other genes have also been associated including ATM, BRIP1, CDH1, CHEK2, NBN, NF1, PALB2, RAD51C, RAD51D [7]. Despite the known benefits of cascade testing for hereditary cancer syndromes like HBOC, it has historically been difficult to execute. While probands disclose genetic results to more than 80% of ARRs, studies demonstrate that only 15–30% actually use recommended genetic services [8, 9]. Because most individuals do not have a cancer diagnosis, the limited existing literature on barriers to cascade testing suggests that testing for cancer-associated syndromes presents unique complexities which contributes to a poor understanding—and limited use—of genetic services. Previously reported barriers include the lack of access to genetic services, socioeconomic factors, lack of health insurance coverage, health care provider-patient communication, patient-family communication, and concerns about misuse of genetic information [10–14]. We now look to investigate barriers to cascade genetic testing when several of these previously demonstrated barriers are controlled for.
In our previously published pilot study, we evaluated a cascade genetic strategy facilitated by a medical team that provided telephone genetic counseling and mailed saliva testing. The facilitated strategy resulted in testing for 58% of ARRs, which was a significantly higher uptake than previously reported (58% vs. 30%, P<0.001, 95% CI 49%−67%) [15]. The most common reasons provided for declining genetic testing from the initial feasibility study were concern about future access to insurance, genetic discrimination, and cost [15]. Even in the initial feasibility trial—which controlled for already known limitations of cascade testing including cost, knowledge about services, and relative ease of obtaining the test—some unknown barriers still existed given the lack of completion among different ARRs. This follow-up prospective study aims to identify these obstacles in order to design an intervention that is even more efficacious.
Methods and Materials
Our study was reviewed by an institutional review board/human investigations committee/ethics committee (Weill Cornell Institutional Review Board) and approved as human subjects research.
Probands were included if they met the following criteria, 1) age greater than or equal to 18 years, and 2) presence of a cancer-associated germline pathogenic variant identified in the preceding 12 months. ARRs were included if they met the following criteria: 1) age greater than or equal to 18 years, 2) relative of a proband enrolled in the study, 3) proband consented to contact of ARRs. ARRs were eligible for repeat telephone contact in this follow-up study if they initially expressed interest in genetic testing but did not return the mailed saliva kit for completion of genetic testing.
This prospective study was approved by the Institutional Review Board. All experiments were performed in accordance with relevant guidelines and regulations. Complete study details are available in the original pilot publication [15]. The facilitated cascade testing pathway bundled multiple components: 1) the genetics team (genetics physician and genetics team navigator) facilitated identification of ARRs through creation of a pedigree, educating the proband on which family members were at-risk for carrying the pathogenic variant, 2) the genetics team contacted ARRs to review the familial pathogenic variant, provide telephone genetic counseling, and offer genetic testing, 3) ARRs interested in genetic testing were mailed saliva kits to complete at home, 4) the genetics team contacted ARRs to disclose results, 5) ARRs underwent telephone genetic counseling to review results.
For this follow-up study, ARRs who reported interest in genetic testing and received a saliva kit but did not return the saliva kit for genetic testing were re-contacted by telephone at six months. The genetics team, using a script, performed a standardized telephone survey inquiring about the reasons for not completing the mailed saliva kit. ARRs were then asked whether they were still considering completing genetic testing. Those ARRs interested in completing testing were informed that they still had the opportunity to return the mailed saliva kit and would be contacted by the study team for disclosure of results and post-test genetic counseling. Cost of testing was covered by the study.
The primary objective was to evaluate the reasons ARRs did not complete oncologic cascade testing. This discussion regarding barriers occurred during a follow-up telephone call taking place 6–12 months following the initial genetic counseling. Secondary objectives included evaluating demographic and clinical predictors of genetic testing completion as well as future completion of testing following the telephone survey. ARR characteristics evaluated included type of familial pathogenic variant, age, gender, highest level of education, parental status, personal history of cancer, family history of cancer, number of relatives affected by cancer and whether or not the proband had a cancer diagnosis.
Univariate tests were applied based on whether the variable of interest was normally distributed (i.e., t-test, analysis of variance) or not (i.e., Mann-Whitney U test). Associations between categorical variables were evaluated by the chi-square test or Fisher’s exact tests, as appropriate for category size. All tests were two-sided with statistical significance set at P<0.05. All analyses were performed in SPSS Version 24 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
Results
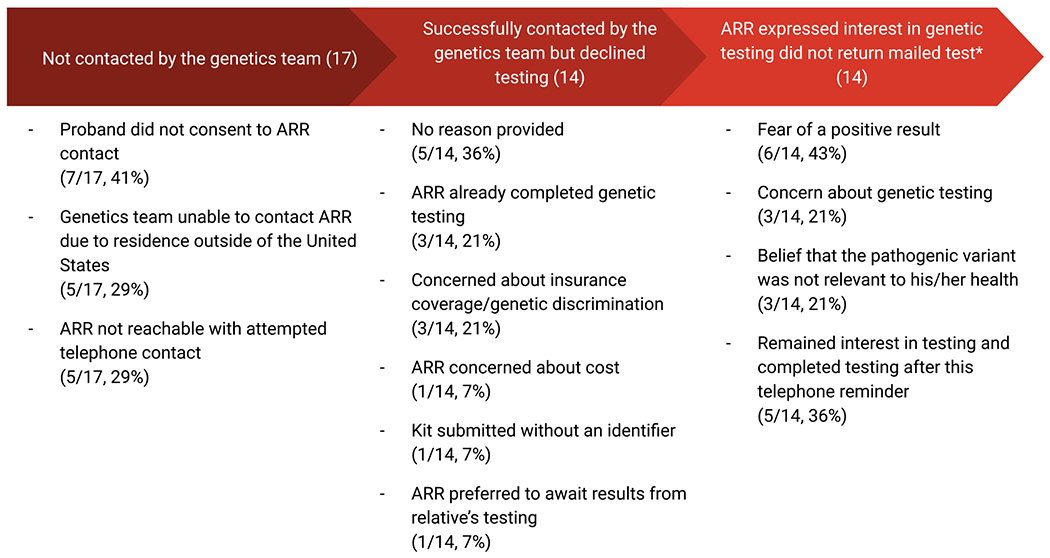
The initial pilot study reported on 30 probands and 114 identified ARRs [15]. Five ARRs were ineligible due to residence outside of the United States. Seven ARRs were ineligible because, despite the genetics team recommending genetic counseling and testing, the proband did not consent to contact for the following reasons: decision not to disclose results to children younger than 25 years (2), concern that ARR was too ill from other medical issues (1), strained family relationship (1), and no reason provided (3). The genetics team attempted to contact the remaining 102 ARRs by telephone. Three telephone attempts were made for each ARR; if these attempts were not successful the ARR was deemed to be uncontacted. Among ARRs where race/ethnicity information was provided, 57/72 (79%) identified as white as 15/72 (21%) identified as non-white.
Telephone contact was established with 97 ARRs (95% of all attempted ARRs). Among the 97 contacted ARRs, 69 (71%) agreed to and completed genetic testing (an additional three patients returned saliva kits following the publication of the pilot study). Among contacted relatives, 14 declined genetic testing for the following reasons: already completed genetic testing (3), concern about genetic discrimination (3), no reason provided (5), concern about cost associated with genetic testing (1), genetic testing saliva kit was submitted without an identifier (1), and preferred to await his mother’s genetic testing results (1).
Fourteen ARRs stated that they were interested in genetic testing and requested that a saliva kit be mailed to their home however did not return the kit for evaluation (Table 1). We contacted these 14 ARRs six months after the initial telephone contact. These ARRs reported the following reasons for not completing the saliva kit genetic testing: fear of a positive result (6), concern about genetic discrimination (3) and belief that the pathogenic variant was not relevant to his/her health (3). Of note, some ARRs provided more than one reason. Five ARRs reported that they remained interested in testing but had forgotten to return the saliva kit. Following this telephone call, all five of these ARRs completed genetic testing via the mailed saliva kit. Three were found to carry the familial pathogenic variant and two tested negative. The relatives that stated the pathogenic variant was not relevant to his/her health included: 1) a 56-year-old male with a CHEK2 familial pathogenic variant, 2) a 51-year-old male with a BRIP1 familial pathogenic variant, 3) a 20-year-old male with a BRCA1 familial pathogenic variant. In our pilot study, when including ARRs who completed testing following a six-month telephone call prompt, 74 (76%) of the 97 ARRs with whom contact was established completed cascade testing and 40% (30/74) were found to have pathogenic variants.
Table 1.
At-risk relatives (ARRs) that did not complete cascade genetic testing among 114 proband-designated relatives. (ARR: At-Risk Relative)
|
ARR with more than one response
At the conclusion of the follow-up telephone interview for ARRs who initially agreed to genetic testing, however did not complete the mailed saliva kit, participants were invited to provide comments addressing why they did not complete the requested saliva kit. A selection of some of the responses are shown below:
“I have multiple other medical co-morbidities including aortic valve dysfunction for which I see a physician every three months, I did not want an additional diagnosis looming over me.” [45-year-old female with a family history of a MSH2 pathogenic variant]
“I am worried about results affecting my children negatively with insurance. Also, I did not feel that BRIP1 applied to me as a male.” [51-year-old male with a family history of a BRIP1 pathogenic variant]
“I am too stressed and busy with studying for school. I am not interested in knowing at this time.” [22-year-old male with as family history of a MSH2 pathogenic variant]
We compared characteristics for ARRs who completed versus ARRs who declined genetic testing. There was no significant difference in uptake of genetic testing when comparing gender, highest level of education, parental status, personal history of cancer, family history of cancer, number of relatives affected by cancer, and whether or not the proband had a cancer diagnosis. However, ARRs completing genetic testing were significantly older than those who did not complete testing, 54 (range 24-85) vs. 43 (20-78) years respectively (P = 0.002). ARRs completing genetic testing were also significantly older than those who initially agreed to testing but did not complete testing, 54 (range 24-85) vs. 41 (29-73) (P = 0.003) (Table 2).
Table 2.
Characteristics of at-risk relative (ARR) cohorts that completed genetic testing versus those that did not complete genetic testing. First degree family members include parent, full sibling, child.
| Completed Testing (69) | Declined testing (14) | Agreed to testing did not complete (14) | |
|---|---|---|---|
| Mean Age, year (Range) | 54 (24-85) | 47 (20-67) | 41 (29-73) |
| Gender | |||
| Male | 32 | 9 | 8 |
| Female | 37 | 5 | 6 |
| Familial Mutation | |||
| APC | 5 | 0 | 0 |
| BRCA1 | 27 | 4 | 4 |
| BRCA2 | 17 | 3 | 3 |
| BRIP1 | 7 | 3 | 1 |
| CHEK2 | 3 | 1 | 1 |
| MSH2 | 0 | 0 | 0 |
| MSH6 | 1 | 2 | 1 |
| MUTYH | 2 | 1 | 0 |
| PTEN | 3 | 0 | 3 |
| RAD51C | 4 | 0 | 0 |
| Proband with cancer | |||
| Yes | 20 | 6 | 8 |
| No | 49 | 8 | 6 |
| Highest level of education* | |||
| Elementary School | 2 | 0 | 0 |
| High School | 14 | 2 | 1 |
| College | 25 | 5 | 9 |
| Graduate School | 26 | 3 | 4 |
| Mean Number of Children | 2 (0-10) | 1 (0-3) | 2 (0-9) |
| Mean Number of Living Relatives | 4 (1-16) | 5 (2-9) | 6 (3-12) |
| Personal Cancer Diagnosis* | |||
| Yes | 13 | 1 | 1 |
| No | 56 | 11 | 13 |
| Family Cancer History* | |||
| Yes | 68 | 12 | 14 |
| No | 0 | 0 |
Among patients with reported responses
Discussion
Our study demonstrates that even when facilitated by a medical team with prioritization of testing convenience, significant barriers to cascade genetic testing for HBOC persist. We reported on 30 probands and 114 identified ARRs; telephone contact was then established with 97 ARRs (95% of all attempted ARRs). Among the 97 contacted ARRs, 28 (29%) did not complete genetic testing. Fourteen of whom declined testing and 14 expressed interest in genetic testing but did not return mailed saliva kit test. After 6-months of follow-up from the initial feasibility trial, we found that the most significant barriers to completing genetic testing for ARRs were fear of a positive result, concern about genetic discrimination and the belief that the familial pathogenic variant was not relevant to his/her personal health.
Cascade testing has been designated by the Centers for Disease Control and Prevention (CDC) as a Tier 1 genetic application for hereditary breast and ovarian cancer and Lynch syndrome [16, 17]. The Society of Gynecologic Oncology (SGO), National Comprehensive Cancer Network (NCCN), American College of Obstetricians and Gynecologists (ACOG) and American Society of Clinical Oncology (ASCO) all emphasize the importance of cascade testing [3, 7, 18–20]. However, the literature reveals rates of cascade testing success are low, with rates as low as 15-30% [8, 9, 21–24]. The most commonly cited barrier to cascade testing is the lack of family members being informed about the pathogenic variant and recommendation for testing [24, 25]. Our study facilitated the process of informing family members and, therefore, highlights other barriers to successful oncologic cascade testing. Despite utilizing a medical genetics team to contact ARRs, disclose and explain the familial pathogenic variant and importance of genetic testing and facilitate and subsidize testing performed at home, 29% of contacted ARRs did not complete genetic testing.
Our study identified three ARRs who declined cascade testing because they believed that the familial pathogenic variant was not clinically relevant to their health. This included a 56-year-old male with a family history of a CHEK2 pathogenic variant, a 20-year-old male with a family history of a BRCA1 pathogenic variant and a 51-year-old male with a family history of a BRIP1 pathogenic variant. For males with CHEK2 pathogenic variants, the NCCN guidelines recommend colonoscopy screening starting at age 40, every 5 years (or 10 years prior to age of first-degree relative’s age at colorectal cancer diagnosis), and therefore identifying a CHEK2 pathogenic variant in this individual would be clinically actionable.26 For males with BRCA1 pathogenic variants, the NCCN guidelines recommend: for breast cancer risk, breast self-exam training and education, as well as clinical breast exams every 12 months starting at age 35; for prostate cancer risk, consideration of shared decision-making about prostate-specific antigen (PSA) screening at age 40 and to consider screening at annual intervals.7 While currently there are no guidelines for males with BRIP1 pathogenic variants, this patient has one daughter; for women with BRIP1 pathogenic variants, the NCCN guidelines recommend consideration of risk-reducing salpingo-oophorectomy at 45-50 years of age, given an increased risk of ovarian cancer [7, 26].
In one of the largest studies to date evaluating barriers to oncologic cascade testing, Donenberg et al. report on a cohort of 268 women with breast cancer in Trinidad and Tobago [27]. This study employed a similar strategy of using board-certified genetic counselors to conduct family counseling sessions which included a discussion about genetic test results, gene-associated cancer risks, risks for cancer recurrence and options for cancer screening and risk reduction. They identified the following barriers to cascade testing: inability to contact relatives, fear of knowing results, loss to follow-up after agreeing to participate, and strained family relations, similar to findings from our study [27]. This study, along with our data, suggests that a formal approach to building a genetic risk assessment program that incorporates promotion of awareness and empowerment amongst probands and family members is an effective method to improve uptake of cascade testing [27, 28].
This study has important limitations. As ARRs were contacted via telephone for surveys six months following initial genetic counseling, we are unable to account for recall bias. This study could not accurately address cost as a barrier to cascade testing, as testing was funded as part of study participation. Although many genetic testing laboratories offer free testing for relatives of an affected individual, to demonstrate precisely the significance of cost as a barrier, future work, where coverage is not guaranteed through a research mechanism, is needed. We did not include data on whether the proband discussed results with the ARRs during the genetic counseling visit. In addition, all probands in this study sought care at a tertiary care center, and the results are not easily generalizable to more vulnerable communities and different patient populations.
It is important to note that our follow-up telephone contact had the unexpected consequence of prompting five relatives who had not completed their saliva tests to complete testing. Furthermore, 60% (3/5) were found to carry the familial pathogenic variant. While this finding suggests that reminders from the medical team may improve testing uptake, we have not identified the ideal timing and manner in which to re-contact ARRs who have not completed testing.
With our facilitated cascade genetic testing strategy, we were able to eliminate many of the barriers to genetic testing previously cited in the literature, including cost, lack of awareness, and inaccessible services [8, 9, 27]. Yet, nearly one third of ARRs contacted still did not complete cascade testing for HBOC. This allowed us to identify additional important barriers to cascade testing including fear of positive results, concern of genetic discrimination and lack of awareness of pertinent evidence-based screening and management guidelines.
Conclusion
Our initial pilot study and follow-up telephone interviews add to our understanding of barriers to successful cascade testing for cancer-associated pathogenic variants. ARRs are concerned about genetic discrimination and implications for insurance coverage as well as cost associated with testing (even those enrolled on a study where the cost of testing was covered as part of study participation). Furthermore, many ARRs who initially reported interest in testing did not follow through with testing due similar concerns in addition to fear of positive results and an erroneous belief that the pathogenic variant was not clinically relevant. Finally, a telephone call at six-months prompted some ARRs initially interested in testing to complete their saliva kits, emphasizing the need for built in mechanisms for patient follow-up. Efforts to improve uptake of cascade genetic testing should address concerns about genetic discrimination, cost of testing, fear of positive results, evidence-based cancer prevention strategies based on the familial pathogenic variant and should embed follow-up systems into the cascade testing workflow. Our results demonstrate that improving effective communication strategies surrounding medical genetic testing has the potential to improve the identification of HBOC, which may improve care and ultimately prevent cancer in high-risk families.
Acknowledgement of research support:
This project was supported by the Weill Cornell Medicine Clinical & Translational Science Center (CTSC) and Invitae Genetics.
Paul Christos was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000457. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Statements and Declarations
Kevin Holcomb serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics.
None of the remaining authors have a conflict of interest to disclose.
Funding:
There are no sources of funding for the research reported
Footnotes
Ethics approval: Approved by Weill Cornell Medicine Institutional Review Board
Compliance with Ethical Standards
Our study was reviewed by an institutional review board/human investigations committee/ethics committee (Weill Cornell Institutional Review Board) and approved as human subjects research.
Human studies and informed consent
All appropriate steps were taken in obtaining informed consent of all human subjects participating in the research comprising the manuscript submitted for publication. There is no identifying information. All study participants have given informed consent to be included in the study.
Consent for publication: Not applicable
Conflict of Interest / Competing Interests
Ryan M. Kahn declares that he has no conflict of interest
Muhammad Danyal Ahsan declares that he has no conflict of interest
Eloise Chapman-Davis declares that she has no conflict of interest
Kevin Holcomb declares that he has no conflict of interest
Roni Nitecki declares that she has no conflict of interest
Jose Alejandro Rauh-Hain declares that he has no conflict of interest
Rana Khan Fowlkes declares that she has no conflict of interest
Francesca Tubito declares that she has no conflict of interest
Maira Pires declares that she has no conflict of interest
Paul J Christos declares that he has no conflict of interest
Kaitlyn Tkachuk declares that she has no conflict of interest
Hannah Krinsky declares that she has no conflict of interest
Ravi N. Sharaf declares that he has no conflict of interest
Kenneth Offit declares that he has no conflict of interest
Steven Lipkin declares that he has no conflict of interest
Melissa K. Frey declares that she has no conflict of interest
Availability of data and material:
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study
References
- 1.Drohan B, Roche CA, Cusack JC, Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. Jun 2012;19(6):1732–7. doi: 10.1245/s10434-012-2257-y [DOI] [PubMed] [Google Scholar]
- 2.Practice CoG. ACOG Committee Opinion No. 727: Cascade Testing: Testing Women for Known Hereditary Genetic Mutations Associated With Cancer. Obstet Gynecol. January 2018;131(1):e31–e34. doi: 10.1097/AOG.0000000000002457 [DOI] [PubMed] [Google Scholar]
- 3.Randall LM, Pothuri B, Swisher EM, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. August 2017;146(2):217–224. doi: 10.1016/j.ygyno.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Offit K, Tkachuk KA, Stadler ZK, et al. Cascading After Peridiagnostic Cancer Genetic Testing: An Alternative to Population-Based Screening. J Clin Oncol. Jan 2020:JCO1902010. doi: 10.1200/JCO.19.02010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offit K The future of clinical cancer genomics. Semin Oncol. Oct 2016;43(5):615–622. doi: 10.1053/j.seminoncol.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. May 2014;32(15):1547–53. doi: 10.1200/JCO.2013.53.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly MB, et al. , NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment:Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Blandy C, Chabal F, Stoppa-Lyonnet D, Julian-Reynier C. Testing participation in BRCA1/2-positive families: initiator role of index cases. Genetic testing. Fall 2003;7(3):225–33. doi: 10.1089/109065703322537241 [DOI] [PubMed] [Google Scholar]
- 9.Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns. Oct 2013;22(5):603–12. doi: 10.1007/s10897-013-9592-4 [DOI] [PubMed] [Google Scholar]
- 10.Hafertepen L, Pastorino A, Morman N, et al. Barriers to genetic testing in newly diagnosed breast cancer patients: Do surgeons limit testing?. Am J Surg. 2017;214(1):105–110. [DOI] [PubMed] [Google Scholar]
- 11.Hinchcliff EM, Bednar EM, Lu KH, Rauh-hain JA. Disparities in gynecologic cancer genetics evaluation. Gynecol Oncol. 2019;153(1):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard VB, Mays D, LaVeist T, Tercyak KP. Medical mistrust influences black women’s level of engagement in BRCA 1/2 genetic counseling and testing. J Natl Med Assoc . 2013;105(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J. 2009;15 Suppl 1:S56–62. [DOI] [PubMed] [Google Scholar]
- 14.Ford ME, Alford SH, Britton D, Mcclary B, Gordon HS. Factors influencing perceptions of breast cancer genetic counseling among women in an urban health care system. J Genet Couns. 2007;16(6):735–53. [DOI] [PubMed] [Google Scholar]
- 15.Frey MK, Kahn RM, Chapman-davis E, et al. Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling. J Clin Oncol. 2020;:JCO1902005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George R, Kovak K, Cox SL. Aligning policy to promote cascade genetic screening for prevention and early diagnosis of heritable diseases. J Genet Couns. Jun 2015;24(3):388–99. doi: 10.1007/s10897-014-9805-5 [DOI] [PubMed] [Google Scholar]
- 17.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. Nov 2011;108(44):18032–7. doi: 10.1073/pnas.1115052108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu KH, Wood ME, Daniels M, et al. American Society of Clinical Oncology Expert Statement: collection and use of a cancer family history for oncology providers. J Clin Oncol. Mar 2014;32(8):833–40. doi: 10.1200/JCO.2013.50.9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Committee on Practice Bulletins–Gynecology CoG, S.ciety of Gynecologic Oncology. Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. September 2017;130(3):e110–e126. doi: 10.1097/AOG.0000000000002296 [DOI] [PubMed] [Google Scholar]
- 20.Konstantinopoulos PA, Norquist B, Lacchetti C, et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J Clin Oncol. 2020;:JCO1902960. [DOI] [PubMed] [Google Scholar]
- 21.Katapodi MC, Viassolo V, Caiata-Zufferey M, et al. Cancer Predisposition Cascade Screening for Hereditary Breast/Ovarian Cancer and Lynch Syndromes in Switzerland: Study Protocol. JMIR Res Protoc. Sep 2017;6(9):e184. doi: 10.2196/resprot.8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. Aug 2006;43(8):665–70. doi: 10.1136/jmg.2005.039172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. Sep 2013;11(9):1093–100. doi: 10.1016/j.cgh.2013.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieberman S, Lahad A, Tomer A, et al. Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med. 2018;20(11):1446–1454. [DOI] [PubMed] [Google Scholar]
- 25.Narod SA, Butler R, Bobrowski D, et al. Short report: Follow-up of Bahamian women with a BRCA1 or BRCA2 mutation. Mol Genet Genomic Med. 2018;6(2):301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provenzale D, et al. , Genetic/Familial High-Risk Assessment: Colorectal Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 2019. [Google Scholar]
- 27.Donenberg T, George S, Ali J, et al. A clinically structured and partnered approach to genetic testing in Trinidadian women with breast cancer and their families. Breast Cancer Res Treat. 2019;174(2):469–477. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald DJ, Blazer KR, Weitzel JN (2010) Extending com- prehensive cancer center expertise in clinical cancer genetics and genomics to diverse communities: the power of partnership. J Natl Compr Cancer Netw 8(5):615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study


