Abstract
The nucleolus is a common target of viruses and viral proteins, but for many viruses the functional outcomes and significance of this targeting remains unresolved. Recently, the first intranucleolar function of a protein of a cytoplasmically‐replicating negative‐sense RNA virus (NSV) was identified, with the finding that the matrix (M) protein of Hendra virus (HeV) (genus Henipavirus, family Paramyxoviridae) interacts with Treacle protein within nucleolar subcompartments and mimics a cellular mechanism of the nucleolar DNA‐damage response (DDR) to suppress ribosomal RNA (rRNA) synthesis. Whether other viruses utilise this mechanism has not been examined. We report that sub‐nucleolar Treacle targeting and modulation is conserved between M proteins of multiple Henipaviruses, including Nipah virus and other potentially zoonotic viruses. Furthermore, this function is also evident for P3 protein of rabies virus, the prototype virus of a different RNA virus family (Rhabdoviridae), with Treacle depletion in cells also found to impact virus production. These data indicate that unrelated proteins of viruses from different families have independently developed nucleolar/Treacle targeting function, but that modulation of Treacle has distinct effects on infection. Thus, subversion of Treacle may be an important process in infection by diverse NSVs, and so could provide novel targets for antiviral approaches with broad specificity.
Keywords: henipavirus, matrix, nucleus, nucleolus, phosphoprotein, protein trafficking, rabies, ribosome biogenesis, treacle, virus
Synopsis statement: The nucleolus is a common target of RNA viruses, but the roles and significance of this remain unresolved. Previously we found the Hendra virus (a Henipavirus) M protein targets a subnucleolar compartment and interacts with Treacle, impairing ribosome biogenesis. Here we show that targeting of Treacle is conserved among multiple Henipavirus M proteins and also the unrelated P3 protein of rabies virus (a Lyssavirus). These data suggest viral targeting of Treacle/sub‐nucleolar regions may be an important process of diverse viruses.
1. INTRODUCTION
The mammalian nucleolus is composed of three distinct, immiscible compartments (the fibrillar center [FC], dense fibrillar component [DFC], which form a functional unit [FC‐DFC] that are embedded within the granular component [GC]) that mediate successive steps in ribosome biogenesis 1 (Figure 1A). Beyond its long‐established roles in ribosome biogenesis, the nucleolus is now recognised as a highly multifunctional structure involved in cell‐cycle regulation, DNA‐damage sensing and repair, telomere maintenance, gene expression and apoptosis. 1 , 2 It is also becoming increasingly clear that many viruses (including many DNA, RNA and retroviruses) express proteins that localise to nucleoli. 3 , 4 This is particularly notable for RNA viruses that typically have a cytoplasmic replication cycle and limited coding capacity, but often express protein(s) that can target the nucleolus, such as the matrix (M) protein of many paramyxoviruses, P3 protein (an N‐terminally truncated version of full‐length P‐protein) of rabies virus (RABV) and the capsid protein of flaviviruses. 3 , 4 , 5 Despite the apparent conservation of nucleolar targeting across diverse families and classes, functional outcomes are often poorly understood, particularly for RNA viruses with cytoplasmic replication cycles.
FIGURE 1.
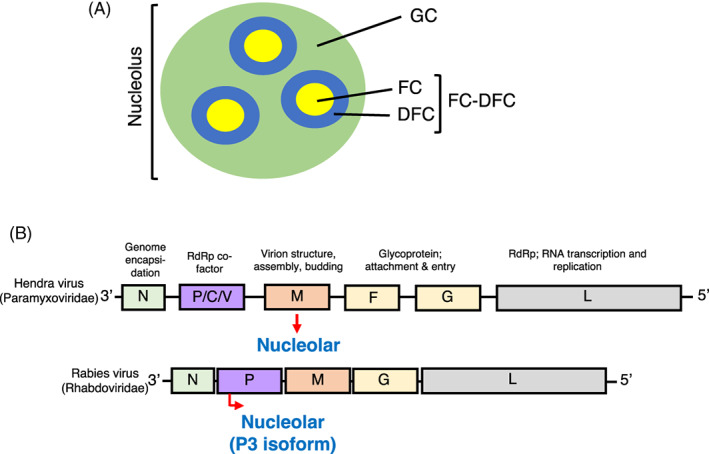
Schematic representations of the structures of the nucleolus and of genomes of viruses of the family Rhabdoviridae and Paramyxoviridae. (A) Schematic of the nucleolus showing the FC, DFC and GC. The FC and DFC form functional units (FC‐DFC) within a single GC. 1 (B) Schematic of genomes of paramyxoviruses and rhabdoviruses, highlighting genes conserved between families of NNSVs (shaded regions) and their function(s) in the infectious cycle. Almost all NNSVs have an equivalent core complement of genes with general structure 3’‐N‐P‐M‐G‐L‐5′. Known nucleolar proteins of rabies virus (P3) and Hendra virus (M) are indicated in blue.
Nonsegmented negative‐sense RNA viruses (NNSVs) include some of the most deadly known pathogens, including Ebola, RABV, Nipah (NiV), and Hendra (HeV) viruses. 6 NNSVs genome structures are generally conserved, encoding a minimal complement of nucleoprotein (N), phosphoprotein (P) (the polymerase co‐factor), M protein (a structural component), glycoprotein (G) and large protein (L) (the RNA‐dependent RNA polymerase), or equivalent proteins with differing nomenclature (Figure 1B). Many NNSV families include members that express at least one viral protein that can localise to nucleoli, including RABV P3 7 and HeV M proteins. 8 , 9 Nucleolar function of NNSV proteins have remained largely unresolved until the recent finding that HeV M protein, which in common with NiV M, traffics between the nucleus/nucleolus and cytoplasm 9 , 10 and targets sub‐nucleolar compartments corresponding to the FC‐DFC, and therein interacts with Treacle protein. 11 Treacle regulates rRNA transcription and processing and is a key player in the nucleolar DNA damage response (DDR) 12 , 13 , 14 whereby, following detection of DNA double‐strand breaks, Treacle recruits NBS1 (a key DDR component) and TOPBP1 into FC‐DFC, causing silencing of rRNA transcription. 15 , 16 , 17 HeV M inhibits rRNA transcription by a Treacle‐dependent mechanism that appears to mimic the NBS1/DDR pathway by binding to Treacle at the same or an overlapping site to that bound by NBS1. 11 The regions of HeV M protein required for nucleolar localisation are not well defined, but mutation of residue K258 to Ala (K258A) prevents accumulation into the FC‐DFC resulting in accumulation in the GC; K258A also prevents interaction with FC‐DFC‐localised Treacle and modulation of rRNA synthesis. 11 A lysine corresponding to K258 is conserved across the M proteins of known henipaviruses (Supplementary Figure S1). Thus, sub‐nucleolar trafficking and Treacle interaction may be conserved, but this has not been examined. During review of this paper, a report was published indicating that mumps virus (MuV) M protein also interacts with Treacle, and that depletion of Treacle can impair MuV production, 18 indicative of conservation of Treacle targeting by M proteins of different genera of the family Paramyxoviridae. However, the functional outcome of MuV M‐Treacle interaction was not defined, so whether MuV M protein, or any other virus/viral protein, impairs rRNA biogenesis through Treacle modulation is unknown. Intriguingly, Treacle depletion reduced MuV production, indicative of potentially different impacts of Treacle targeting in infection by different paramyxoviruses. Importantly, the possibility that nucleolar proteins expressed by viruses of NNSV families other than paramyxoviruses, such as RABV P3, has not been investigated.
Here we examined the conservation of targeting of Treacle among M proteins of different henipaviruses including zoonotic and potentially zoonotic viruses, finding that nucleolar localization, Treacle‐binding and suppression of rRNA transcription is consistent with that observed for HeV M or the DDR. Notably, comparable interactions and effects were observed for RABV P3 expression and RABV infection, and Treacle was found to impact on RABV production, indicative of roles in the biology of different viruses.
2. MATERIALS AND METHODS
2.1. Cells, antibodies and other reagents
HeLa (ATCC: CCL‐2), HEK‐293T (ATCC: CRL‐3216), HEK‐293 (ATCC: CRL‐1573) and Neuro2A cells (ATCC: CCL131) were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Calf Serum (FCS) at 37°C, 5% CO2. Plasmids (in pEGFP‐C1 vector) expressing GFP‐tagged henipavirus M proteins, 19 RABV CVS‐11 P3 and mutant (KRm) 20 were used previously. GFP‐Treacle and HA‐Treacle were a kind gift from Prof. Manuel Stucki (University of Zurich). All transfections were performed using Lipofectamine 3000™ (ThermoFisher Scientific) according to the manufacturer instructions. Antibodies used in this study were anti‐TCOF1/treacle (Proteintech; Cat #11003‐1‐AP; immunoblotting (IB), 1:2000; immunofluorescence (IF), 1:100), anti‐FBL (Abcam, Cat #Ab4566; IF, 1:100), anti‐GFP (Sigma‐Aldrich, Cat #11814460001 ROCHE; IB, 1:2000), anti‐RABV N (In‐house, 21 IF, 1:3000), anti‐Phospho‐Histone H2A.X (Ser139) (γH2AX; CST, Cat#2577; IF/IB 1:800) and anti‐actin (Sigma‐Aldrich, Cat#A2228). Secondary antibodies diluted 1:1000 were used for IF (AlexaFluor 568 [ThermoFisher; Cat #A‐11011], and AlexaFluor 647 [Cat #A‐21245]). Secondary antibodies for IB (IgG horse radish peroxidase [HRP]‐conjugated‐antibodies) were diluted 1:10,000 (Merck; Cat #AP307P, Cat #AP308P). DNA damage was induced by treating cells with 50 μM etoposide for 3 h.
2.2. Virus infections
Stocks of RABV Challenge Virus Strandard 11 (CVS) strain was generated in BHK‐21 cells. The viral titre of cell culture supernatants was determined by titration assays on BHK‐21 cells by direct fluorescent antibody test as described previously. 22 For siRNA knockdown experiments, HEK‐293T cells were transfected with siRNA (scrambled (scr) or targeting Treacle (siTCOF1); 100 nM final) using DharmaFECT 1 transfection reagent™ (GE Dharmacon) 48 h prior to infection with RABV at MOI 0.01. At 24 and 48 h p.i. lysates were collected and viral TCID50/ml was determined using Neuro2a cells (mouse neuroblastoma cells) in 96‐well plates as described previously. 22 , 23 Briefly, the plates were fixed with 4% paraformaldehyde for 60 min at room temperature and then stained with FITC conjugated anti‐rabies monoclonal antibody (Fujirebio) at 1:10 dilution in 0.5% BSA/PBSA with 0.005% Evans blue. Plates were read with an Olympus BX51 inverted microscope. For rRNA biogenesis experiments, cells were infected with RABV CVS strain at a MOI of 2. At 23 h post‐infection (p.i.) media was replaced with media with EU reagent, prior to fixation at 24 h p.i. and processing for EU incorporation assay (see below). Antibodies to RABV N protein were used to identify infected cells.
2.3. Confocal laser scanning microscopy and image analysis
Confocal images were acquired by using a Leica SP5 or Nikon C1 with 60× oil immersion objective (NA 1.4) at Monash Micro Imaging (MMI) Facility. Live cell imaging was performed as previously. 11 IF of Henipavirus M expressing cells (Figure 2B), cells were fixed and processed as previously. 11 IF assays for P3 expressing cells and HeV M (Figure 4D) were the same as above except that cells were fixed with 3.7% formaldehyde (37°C for 10 min), prior to 2× PBS wash, permeabilization with 0.5% Triton‐X100 (10 min at RT) and blocking with 2% BSA (30 min at 37°C). Images acquired from live and IF stained cells by CLSM were analysed using ImageJ freeware software as previously. 24 , 25 , 26 , 27 Nucleolar to nuclear fluorescence intensity ratio (Fnu/n) was determined by measuring fluorescence intensity in the nucleolus (Fnu, of the whole nucleolus), nucleus (Fn) and background fluorescence (Fb) and determined by the formula (Fnu‐Fb)/(Fn‐Fb). The mean fluorescence intensity in the GC and FC‐DFC were determined by measuring the fluorescence intensity in the GC region (inside of the nucleolus but outside of the sub‐nucleolar “puncta”) (FGC), and the FC‐DFC (where nucleolar puncta locate) (FFC‐DFC) for individual cells.
FIGURE 2.
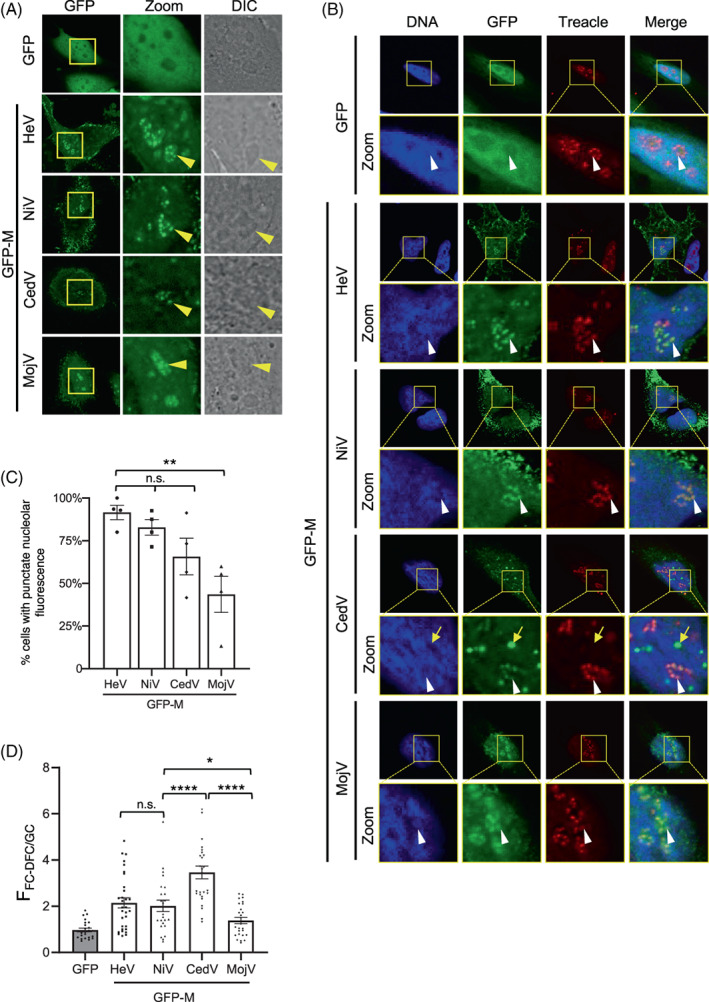
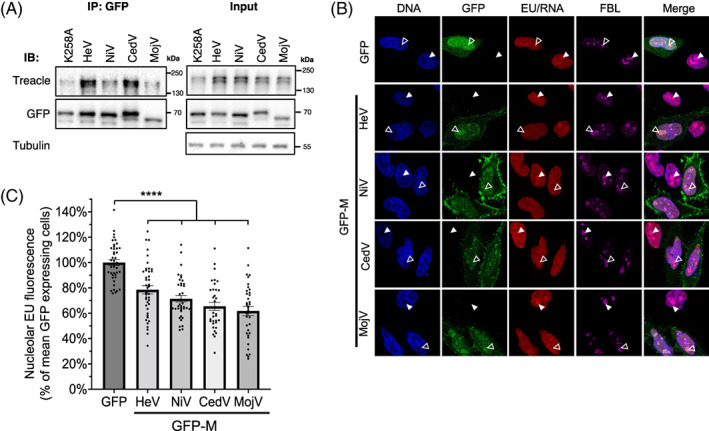
Henipavirus M proteins target the FC‐DFC and colocalise with Treacle. (A) HeLa cells were transfected to express the indicated proteins before analysis of living cells by CLSM. Images are representative of ≥5 independent experiments; ≥10 fields of view were captured for each experiment. Differential interference contrast (DIC) microscopy was used to identify nucleoli. Yellow arrowheads indicate individual nucleoli. (B) HeLa cells transfected to express the indicated proteins were fixed and immunolabelled for Treacle using AlexaFluor‐568‐conjugated secondary antibodies (red fluorescence). Images are representative of ≥7 fields of view. Yellow boxes highlight regions magnified in the Zoom panels. White arrows indicate individual FC‐DFC containing Treacle/M protein; yellow arrow highlights GFP‐CedV M puncta outside of nucleoli. Nuclei/DNA was labelled using Hoechst 33342 dye. (C) Images such as those in A were analysed to determine the percentage of cells expressing the indicated proteins where punctate distribution of nucleolar fluorescence for M protein was evident (mean % ± SEM, n = four independent experiments with analysis of ≥85 cells for each condition). (D) Images such as those shown in A were analysed to determine the FFC‐DFC/GC for GFP alone and the inicated henipavirus GFP‐M proteins. Data shown is from one assay (n ≥ 22 cells for each condition), representative of three independent assays. Statistical analysis used Student's t‐test; *p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; n.s., nonsignificant.
FIGURE 4.
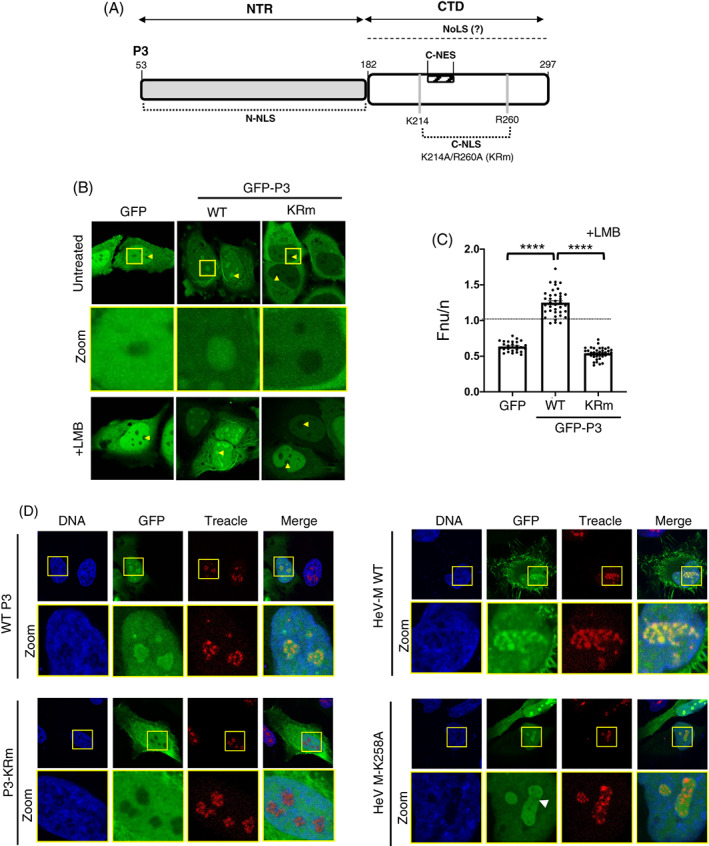
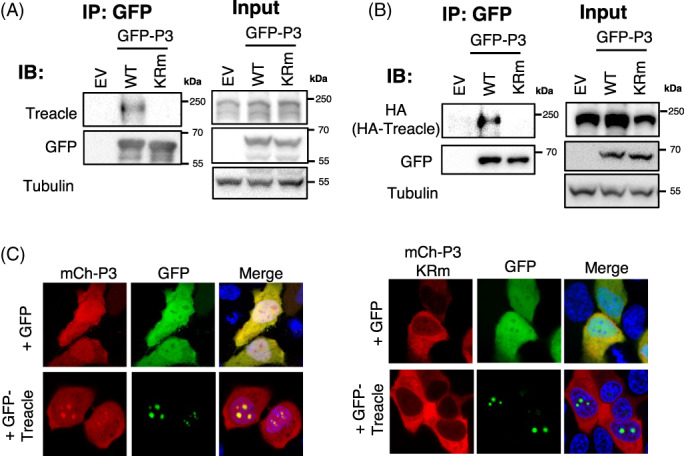
Mutation of residues K214 and R260 to alanine (KRm) abolishes nucleolar targeting of P3. (A) Schematic of the P3 isoform of RABV P protein; P3 is expressed by translation initiation from the codon corresponding to Met‐53 of the full‐length P protein. The N‐terminal region (NTR; residues 53–182) containing the N‐terminal NLS (N‐NLS), and the globular CTD, the C‐terminal NLS and NES (C‐NLS and C‐NES, respectively) are indicated. K214 and R260 form part of the C‐NLS and are substituted for Ala in P3 KRm. Residue numbering corresponds to the full‐length P protein sequence. (B, C) HeLa cells were transfected to express the indicated proteins and treated with or without LMB (3 h) before imaging by live‐cell CLSM at 16–20 h post‐transfection (p.t), as previously. 20 Yellow arrowheads indicate nucleoli. Magnified sections (zoom) of untreated cells are highlighted in yellow boxes. (C) Images such as those B (+ LMB) were used to determine the Fnu/n for individual cells (mean Fnu/n ± SEM, n ≥ 27 cells) as previously. 27 , 46 Statistical analysis used Student's t‐test; ****p < 0.0001. (D) HeLa cells transfected to express the indicated proteins were fixed and immunolabelled for Treacle using AlexaFluor‐647‐conjugated secondary antibodies (red fluorescence). Images are representative of ≥5 fields of view. Yellow boxes highlight regions magnified in the Zoom panels. White arrowhead in HeV M K258A panels highlights regions depleted of GFP fluorescence within FC‐DFC. Nuclei/DNA was labelled using Hoechst 33342 dye.
2.4. Co‐immunoprecipitation
HEK‐293T cells expressing the indicated GFP or GFP‐fused proteins were lysed at 24 h post‐transfection (p.t.) in lysis buffer (10 mM Tris/Cl pH 7.5; 150 mM NaCl; 0.5 mM EDTA; 0.5% NP‐40, 1 mM PMSF, 1 × Protease Inhibitor Cocktail [PIC; Sigma‐Aldrich Cat#11697498001]) for 30 min at 4°C. Supernatants were cleared by centrifugation at 20000× g for 15 min at 4°C, and 10% of the supernatant was collected for analysis of “input”. The remaining lysate was subjected to IP by incubation with 10 μl of GFP‐Trap® beads (Chromotek) for 45 min at 4°C on a rotary mixer. Beads were then washed three times with dilution buffer (10 mM Tris/Cl pH 7.5; 150 mM NaCl; 0.5 mM EDTA; 1 mM PMSF; 1× PIC), prior to resuspension in 2× SDS‐PAGE sample buffer to solubilise the proteins. Note, all wash steps were completed within 15 min at 4°C to minimise degradation of Treacle that is often observed. 15 , 16 , 17 Proteins were then separated using SDS‐PAGE and transferred to nitrocellulose membranes before probing with antibodies (see above), developed using Western Lightning Plus reagent (Perkin Elmer Cat# NEL103001EA) and imaged on a Gel Doc™ XR + Gel Documentation System (BioRad).
2.5. 5‐ethnyl uridine incorporation assays
Levels of rRNA synthesis were determined using an image based technique (Click iT® RNA Alexa Fluor 594 Imaging kit [Thermo‐Fisher, Cat# C10330]) as previously. 11 , 16 , 17 Briefly, cells were incubated for 1 h in the presence of EU before fixation in 4% paraformaldehyde at RT for 12 min, and permeabilization in 0.25% Triton X‐100 for 5 min. Samples were then processed according to the manufacturer's recommendations to label incorporated EU with Alexa Fluor 594. Cells were imaged by CLSM to detect labelling of nascent rRNA by measuring the fluorescence intensity of Alexa Fluor 594 within nucleoli. Quantitative analysis to determine EU fluorescence was performed using ImageJ software, with nucleoli identified using a IF nucleolar stain (anti‐FBL or anti‐Treacle) and/or Hoescht 33342 DNA stain.
3. RESULTS AND DISCUSSION
3.1. Subnucleolar targeting is conserved among M proteins of diverse henipaviruses
NiV and HeV are prototypes of the genus Henipavirus (family Paramyxoviridae) and are zoonotic viruses that are highly pathogenic in humans (40%–75% case‐fatality rate in outbreaks 28 , 29 ) and other nonreservoir species, but are nonpathogenic in the natural reservoir hosts, Pteropus bats. 30 , 31 , 32 Cedar virus (CedV), Mojiang paramyxovirus (MojV, the M protein of which is the most distantly related among henipaviruses to HeV M) and Kumasi virus (KV) are also maintained in bats; CedV appears to be nonpathogenic in model animals, 33 but KV and MojV are suggested to cause disease in humans, although neither has been isolated from human cases. 34 , 35 Ongoing outbreaks of HeV and NiV highlight bat‐borne henipaviruses as a source of concern for human health with henipaviruses listed as priority diseases with epidemic potential by the World Health Organisation (WHO). 28
To test whether targeting of nucleoli is conserved between M proteins of different henipaviruses, we expressed GFP‐fused M proteins of HeV, NiV, CedV and MojV, in HeLa cells before imaging of living cells by confocal laser scanning microscopy (CLSM), as previously used to demonstrate sub‐nucleolar localization of HeV M protein. 11 All of the proteins showed strong nucleolar accumulation and could accumulate into sub‐nucleolar compartments, indicated by a punctate appearance of nucleolar fluorescence (Figure 2A); as expected, GFP alone did not accumulate in any part of the nucleolus, consistent with the known lack of nucleolar targeting signals. 36 We previously found that the punctate structures in HeV M protein‐expressing cells are FC‐DFC11 based on colocalization with fibrillarin (FBL) and Treacle (FC‐DFC‐resident proteins), and we confirmed here that the punctate accumulation of all of the Henipavirus M proteins tested corresponds to FC‐DFC (based on colocalization with Treacle following immunofluorescent (IF) staining) (Figure 2B). The diffuse region is thus consistent with the GC.
Interestingly, the extent of sub‐nucleolar localization of the M proteins differed (Figure 2C), with 80%–90% of HeV and NiV M protein‐expressing cells displaying punctate nucleolar fluorescence compared with 65% for CedV M protein (a nonsignificant reduction compared to HeV M protein), and 40% for MojV M protein (significantly reduced compared to HeV M protein; p < 0.01). The images of cells with evident punctate localization also indicated differing distribution within the nucleolus, whereby HeV and NiV M proteins accumulated in puncta (shown previously to be the FC‐DFC11) but also clearly localised to the diffuse region (the GC), whereas GC localization of CedV M was less evident. Image analysis to calculate the ratio of fluorescence intensity (F) in the FC‐DFC relative to that in the GC (FFC‐DFC/GC; Figure 2D) to quantify the relative accumulation of M protein in the FC‐DFC confirmed that CedV M had the greatest FFC‐DFC/GC (~3.5), consistent with the low signal in the GC, followed by HeV and NiV M (~2), and MojV M protein (~1.5). Intriguingly, CedV M also accumulated in nuclear bodies distinct from nucleoli and Treacle (e.g., Figure 2B, yellow arrow); similar accumulation of other M proteins was rarely observed or not observed. The significance of this localisation is not known.
While sub‐nucleolar FC‐DFC localisation of MojV M was lower than that of other henipavirus M proteins, we cannot dismiss the possibility that this results from lower expression of MojV M rather than specific differences in trafficking/interactions (see immunoblot in Figure 3A). Nonetheless, it is apparent that targeting of FC‐DFC is conserved among the henipavirus M proteins tested, including the most distantly related, MojV. The extent of targeting to different compartments is similar between M proteins of closely related HeV and NiV, but appears to diverge for the more distantly related CedV and MojV.
FIGURE 3.
Henipavirus M proteins bind to Treacle and inhibit rRNA synthesis. (A) IPs of the indicated proteins from transfected HEK‐293T cells were analysed by IB with the indicated antibodies; results are representative of >3 independent experiments. (B) HeLa cells were transfected to express the indicated GFP‐fused proteins for 20 h before labelling of nascent RNA and fixation using the ClickiT™ RNA synthesis imaging kit, as previously described. 11 Cells were labelled using antibodies to the nucleolar protein, fibrillarin (FBL; marker for the FC‐DFC), and Hoechst 33342 (DNA), before imaging using CLSM. White unfilled arrowheads indicate individual nucleoli of cells expressing GFP/GFP‐M, and filled arrowheads indicate nucleoli of cells not expressing GFP. (C) Images such as those in B were analysed to quantify nascent rRNA (histogram shows mean nucleolar EU fluorescence ± SEM as a percentage of the mean intensity of GFP alone expressing cells (control); n ≥ 34 cells analysed for each condition; data from a single assay; comparable inhibition of rRNA synthesis by the M proteins was observed in ≥3 independent assays. Statistical analysis used Student's t‐test; ****p < 0.0001.
3.2. Interaction with Treacle and inhibition of rRNA synthesis is conserved between M proteins of different henipaviruses
To assess whether the co‐localisation of M proteins with Treacle in FC‐DFC (Figure 2) correlates with physical interaction, as previously identified for HeV M protein, 11 we immunoprecipitated (IP) henipavirus M proteins before immunoblot (IB) analysis. HeV M protein containing the K258A mutation was included as a negative control. 11 As expected, 11 Treacle was detected in IPs of HeV M, but reduced for HeV M‐K258A protein, and also co‐precipitated with NiV, CedV and MojV M proteins, albeit to differing levels, indicating that co‐localization in FC‐DFC enables or results from Treacle interaction, which is conserved across diverse henipaviruses (Figure 3A). However, the differing level of co‐precipitation of Treacle by the different henipavirus M proteins suggests there may be differences in the mechanisms of regulation of Treacle and/or rRNA synthesis by the different henipavirus M proteins.
Treacle interaction of HeV M protein is associated with Treacle‐dependent suppression of rRNA synthesis. 11 To determine whether this is conserved for other M proteins we measured rRNA synthesis in cells using an imaging‐based approach, as previously 11 , 16 , 17 (Figure 3B). CLSM images indicated a reduction in newly synthesised rRNA in the nucleoli of cells with detectable expression of each of the M proteins tested, compared with cells expressing a GFP control, or nonexpressing cells (Figure 3B). A significant reduction of rRNA synthesis was confirmed for all M proteins by quantitative image analysis (Figure 3C). Thus, the capacity to suppress rRNA production is conserved among M proteins of diverse henipaviruses. The observed reduction (c. 20%–40% reduction compared to control) is comparable to that observed previously for Treacle‐dependent silencing by HeV M protein, or silencing of rRNA transcription by Treacle depletion or induction of a DDR. 11 , 16 These data are consistent with the conservation of inhibition of the Treacle‐dependent component of rRNA synthesis among M proteins. Thus, it appears that Treacle targeting and suppression of rRNA synthesis has roles at the henipavirus‐host interface; notably, the potent targeting by CedV, which appears to be nonpathogenic in nonreservoir species, indicates that the process is distinct from disease mechanisms.
3.3. Mutation of residues K214‐A and R260‐A (KRm) in RABV P3 abolishes nucleolar targeting
We next examined whether Treacle targeting is conserved beyond paramyxoviruses and M proteins. RABV belongs to a different family (family Rhabdoviridae), and the P3 protein of RABV can localise to nucleoli and bind to nucleolin (NCL); depletion of NCL inhibits replication, 7 but no direct function of intranucleolar localization or interactions have been defined. Importantly, M protein of rhabdoviruses and of paramyxoviruses are expressed from the M gene, while P3 protein of RABV is a multifunctional accessory protein expressed as an N‐terminally truncated isoform of the polymerase co‐factor P protein by translation from internal in frame start codons 37 ; thus, P3 and M protein are unrelated in sequence and function. Similar to henipaviruses, the replication of RABV is cytoplasmic, but P3 shuttles between the nucleus and cytoplasm via several nuclear localisation sequences (NLSs) and nuclear export sequences (NESs), enabling nucleolar interaction. 7 While M protein K258 is implicated in sub‐nucleolar localization, the nucleolar targeting sequence of P3 is unknown beyond localization to the P3 C‐terminal domain (CTD) 7 (Figure 4A). Nucleolar localization signals (NoLSs) are highly variable and typically poorly defined, but they often overlap with NLSs, 38 so we initially examined whether residues K214/R260, which form part of a previously defined NLS in the CTD (C‐NLS), affect nucleolar targeting. 39 , 40 , 41 , 42 Analysis of GFP‐fused P3 in HeLa cells by live‐cell CLSM confirmed previous observations 20 that wild‐type (wt) P3 localises in the nucleus and cytoplasm, and can accumulate in nucleoli (Figure 4B). In contrast to henipavirus M proteins, no strong accumulation into FC‐DFC was observed, with P3 having a more diffuse appearance throughout the nucleolus 11 (Figure 4D). However, P3 could clearly localise within FC‐DFC (Figure 4B, zoom), including in cells co‐immunostained for Treacle (Figure 4D), where K258A, but not P3 was depleted from Treacle‐labelled FC‐DFC (consistent with previous data for HeV M‐K258A 11 ). Thus, it appears that P3 uses targeting mechanisms to access these structures.
Mutation of K214 and R260 to alanine (K214‐A/R260‐A, KRm; Figure 4A) inhibits nuclear localisation, 20 , 43 and consistent with this, P3‐KRm was impaired for nuclear localization compared with wt P3, but was not excluded from the nucleus (Figure 4B). However, P3‐KRm was excluded from nucleoli, similar to GFP alone, indicating a lack of functional nucleolar localization sequences. To confirm that the absence of nucleolar targeting of P3‐KRm does not derive from defective nuclear localization, we analysed cells following treatment with leptomycin B (LMB, which inhibits NES‐mediated nuclear export of P3 by inhibiting the exportin CRM1 43 , 44 ). LMB treatment increased P3‐KRm nuclear accumulation, as expected, 20 but the protein remained excluded from nucleoli (Figure 4B) and absent from Treacle‐specific sub‐nucleolar FC‐DFC regions (Figure 4D). Quantitative image analysis of LMB‐treated cells to determine the nucleolar to nuclear fluorescence ratio (Fnu/n) confirmed that P3‐KRm is largely absent from nucleoli, similar to GFP alone (Figure 4C). Thus, the basic C‐NLS residues K214/R260 appear to be important to accumulation entry to the nucleus, and critical to accumulation within nucleoli. This finding provided a control that can be used to examine functions/interactions associated with nucleolar targeting of P3.
3.4. RABV P3 interacts with Treacle
To examine whether RABV P3 interacts with Treacle, we performed co‐immunoprecipitation (co‐IP) assays, which indicated that P3, but not P3‐KRm, binds to endogenous Treacle (Figure 5A) and to HA‐fused Treacle expressed in co‐transfected cells (Figure 5B). To confirm the interaction of P3 with Treacle in living cells, we co‐expressed GFP‐Treacle or GFP‐alone with mCherry (mCh)‐fused P3 or P3‐KRm for live cell CLSM (Figure 5C). Expression of GFP‐Treacle caused strong accumulation of mCh‐P3, but not mCh‐P3‐KRm into nucleoli, resulting in clear colocalization. Thus, P3, but not P3‐KRm targets nucleoli and interacts with Treacle. Notably, RABV P3 clearly co‐IPs less Treacle than HeV M (Figure S2), consistent with fundamental differences in the sequences/structure and observed localisation, and potentially indicative of differing mechanisms to regulate rRNA synthesis (Figure 4). These data suggest that, while M protein and P3 protein are unrelated proteins expressed from distinct genes, they have independently developed the capacity to enter the FC‐DFC and bind to Treacle, suggestive of a general importance of this interface between different NNSV families.
FIGURE 5.
Rabies P3 interacts with Treacle dependent on K214/R260. (A, B) HEK‐293T cells transfected to express the indicated GFP proteins without (A) or with (B) co‐expression of HA‐Treacle were subjected to IP using GFP‐Trap, before analysis of by immunoblotting (IB) with the indicated antibodies as previously. 11 (C) HeLa cells transfected to co‐express GFP or GFP‐Treacle with mCh‐P3 or mCh‐P3‐KRm were analysed by live cell CLSM (16–18 h p.t.). Images are representative of ≥7 fields of view; co‐localization of P3 with Treacle is indicated by yellow colouration in merged images.
3.5. Depletion of Treacle impairs RABV production
To assess the importance of Treacle in RABV infection, we transfected cells with scrambled (scr) or Treacle‐targeting (siTCOF1) siRNA 48 h prior to infection of cells with RABV. Viral titres were then determined at 24 and 48 h p.i. (Figure 6A). Depletion of Treacle (Figure S3) resulted in a significant decrease in virus production at 48 h p.i., suggesting that Treacle is important for RABV production. Intriguingly, this contrasts with observations for HeV, in which depletion of Treacle resulted in increased virus production, 11 but is similar to MuV virus (a paramyxovirus), in which Treacle depletion decreased virus production. 18 Thus, these data suggest Treacle is a common target of viruses in different genera and families, but has distinct effects on the replication of different viruses, indicative of specific requirements in the lifecycles of these viruses. One possibility is that regulation of rRNA biogenesis by Treacle impacts on replication and on cellular antiviral protein production, thus having potentially pro‐viral or anti‐viral effects.
FIGURE 6.
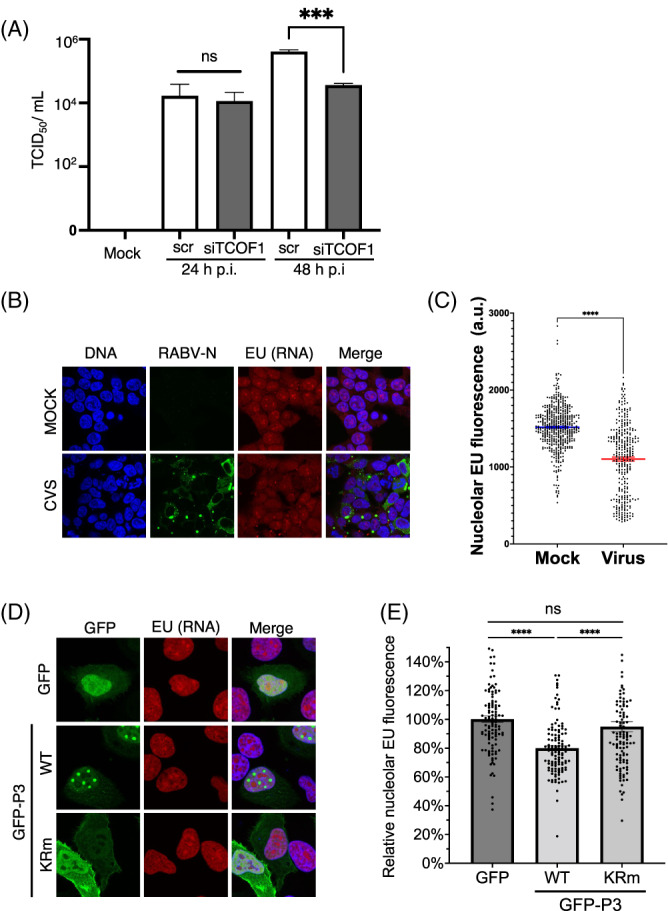
Depletion of Treacle reduces RABV production and RABV infection or P3 expression impairs rRNA synthesis. (A) HEK‐293T cells were transfected with scrambled (scr) or Treacle‐targeting (siTCOF1) siRNA 2 days prior to infection with RABV (MOI 0.01). RABV titers were measured at 24 and 48 h p.i. (mean TCID50/ml ± SEM, n = 3). Statistical analysis used Student's t‐test, ***, p = 0.0002. (B, C) HEK‐293 cells infected with RABV (MOI of 2) or (D, E) HeLa cells transfected to express RABV P3 or RABV P3‐KRm, were analysed to determine nucleolar EU incorporation (as described in the legend to Figure 3). EU reagent was added 1 h prior to fixation at 24 h p.t. or p.i. Infected cells (B, C) were detected by IF staining for RABV N protein, and transfected cells (D, E) by expression of GFP. Hoechst 33342 was used to detect nuclei and assist with identification of nucleoli. (C) Mean nucleolar EU fluorescence (arbitrary units [a.u.]) of mock‐ and virus‐infected samples (n ≥ 309 nucleoli for each condition from three independent samples). Microscope settings were identical for all samples. (E) Histogram of nucleolar EU fluorescence represented as relative to cells expressing GFP alone (control) (n ≥ 110 nucleoli for each condition, from three independent experiments); Statistical analysis used Student's t‐test; ***p < 0.001; ****p < 0.0001; ns, nonsignificant (p > 0.05).
3.6. RABV infection and expression of P3 alone inhibit rRNA biogenesis
To examine whether the interaction of P3 with Treacle is associated with inhibition of rRNA biogenesis in a similar fashion to M protein of HeV and other henipaviruses 11 (above), we analysed rRNA synthesis in cells infected by RABV (Figures 6B,C) or transfected to express P3 alone (Figures 6D,E), using the Click‐iT™ RNA Imaging kit as above. 11 , 16 , 17 CLSM and quantitative image analysis indicated high levels of rRNA synthesis in nucleoli of mock‐infected cells and cells expressing a GFP alone control. rRNA synthesis was significantly (p < 0.0001) reduced in cells infected with RABV compared with mock‐infected cells (Figure 6C) and in cells expressing wt P3 compared with cells expressing GFP alone or P3‐KRm (Figure 6E). We noted in these assays that nucleolar localisation of P3 was greatly reduced, resulting in the protein becoming more nuclear, and suggesting that the fixation conditions may cause mislocalisation. The observed effects (c. 20%–25% reduction by infection or expression of wt P3) are comparable with those observed for HeV infection, expression of HeV M protein, or Treacle depletion. 11 , 16 Importantly, similar to HeV M11, the effect of P3 on rRNA synthesis was not the result of induction of a DDR, as we found no significant increase in phosphorylation of the histone variant, 𝛾H2AX, at S189 (𝛾H2AX‐p, a standard indicator of DNA damage) using both IF (Figure 7A) and IB in HEK‐293T (Figure 7B) or HeLa (Figure S4) cells. Thus, RABV infection induces rRNA transcriptional silencing, with P3 sufficient to replicate this effect. The dependence on nucleolar localization/Treacle interaction is consistent with modification of Treacle‐dependent rRNA synthesis.
FIGURE 7.
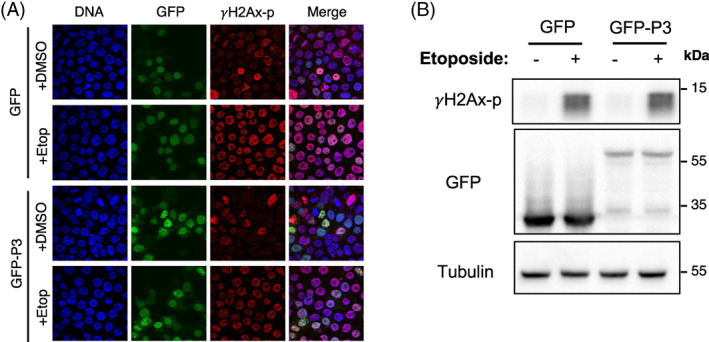
RABV P3 does not induce DNA damage. HEK‐293T cells transfected to express GFP or GFP‐RABV‐P3 protein were treated with DMSO or etoposide (which induces DNA double‐stranded breaks) 11 at 21 h p.t. for 3 h prior to (A) fixation and immunostaining for γH2AX phosphorylated at S139 (γH2AX‐p), and imaging by CLSM (images representative of 10 fields of view taken from two independent experiments) or (B) lysis for IB analysis using the indicated antibodies (data representative of three independent experiments).
Taken together, our data indicate that viral targeting of intranucleolar Treacle and silencing rRNA synthesis, previously described only for HeV M protein, is conserved among M proteins of diverse henipaviruses, and is mediated by the unrelated P3 protein of RABV, representing the first intranucleolar function for RABV P3 protein. Thus, Treacle targeting appears to have developed independently in two different proteins of NNSVs of different families, indicating it plays important roles during viral infection, including by pathogenic and nonpathogenic viruses. Since the effects of Treacle depletion on virus production differed between HeV 11 and RABV or MuV (this study and Reference 18) it appears that modulation of Treacle has different functional outcomes for the different viruses. Delineating the mechanistic basis for these differences will form the basis of future research in our program.
As viral infection is associated with activation of various stress responses and HeV infection induces DNA damage, one possibility is that modulation of the DDR by viral proteins may serve to protect infected cells from deleterious effects. 11 , 45 Notably, DNA‐damage and the DDR appears to be specialised in bats, due to adaptation to flight, and so may present specific requirements during infection. 11 An intriguing alternative possibility, both for the cellular DDR and viral subversion of nucleolar pathways, comes from reports that Treacle and its intranuclear partners not only affect rRNA transcription, but also rRNA processing, thus having both quantitative and qualitative effects on ribosomes. 14 Viral modulation of Treacle and associated pathways might thereby alter the translational landscape (or “translatome”) of cells by affecting the cellular complement of specialised ribosomes, as a mechanism to modify host cell biology. Many diverse viruses target proteins to nucleoli, so our finding that products of genes with different conserved functions in replication/assembly (M and P) suggest that modulation of Treacle function may form part of the nucleolar subversion program of multiple viruses.
AUTHOR CONTRIBUTIONS
Conceptualization and methodology: Stephen M. Rawlinson, Vinod Sundaramoorthy and Gregory W. Moseley; Performed experiments: Stephen M. Rawlinson, Tianyue Zhao, Katie Ardipradja, Yilin Zhang, Patrick F. Veugelers, Jennifer A. Harper and Cassandra T. David; Writing manuscript: Stephen M. Rawlinson, Tianyue Zhao and Gregory W. Moseley; Editing and reviewing manuscript: Stephen M. Rawlinson, Vinod Sundaramoorthy and Gregory W. Moseley; Funding acquisition: Vinod Sundaramoorthy and Gregory W. Moseley.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
We acknowledge the facilities and technical assistance of Monash Micro Imaging (Monash University) and the CSIRO‐ACDP tissue culture team for maintenance and provision of cell lines. This study was supported by National Health and Medical Research Council grants 1125704, 1160838 and 1079211 to Gregory W. Moseley; Australian Research Council (ARC) grants DP210100998, DP150102569 to Gregory W. Moseley, ARC Discovery Early Career Researcher Award grant DE210101145 to Vinod Sundaramoorthy, and the Miegunyah Trust Grimwade Fellowship to Gregory W. Moseley. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Rawlinson SM, Zhao T, Ardipradja K, et al. Henipaviruses and lyssaviruses target nucleolar treacle protein and regulate ribosomal RNA synthesis. Traffic. 2023;24(3):146‐157. doi: 10.1111/tra.12877
Stephen M. Rawlinson and Gregory W. Moseley contributed equally to this study.
Funding information National Health and Medical Research Council (NHRMC), Grant/Award Numbers: 1125704, 1160838, 1079211; Australian Research Council (ARC), Grant/Award Numbers: DP210100998, DP150102569; ARC Discovery Early Career Researcher Award, Grant/Award Number: DE210101145
Contributor Information
Stephen M. Rawlinson, Email: stephen.rawlinson@monash.edu.
Gregory W. Moseley, Email: greg.moseley@monash.edu.
REFERENCES
- 1. Lafontaine DLJ, Riback JA, Bascetin R, Brangwynne CP. The nucleolus as a multiphase liquid condensate. Nat Rev Mol Cell Biol. 2021;22(3):165‐182. [DOI] [PubMed] [Google Scholar]
- 2. Iarovaia OV, Minina EP, Sheval EV, et al. Nucleolus: a central hub for nuclear functions. Trends Cell Biol. 2019;29(8):647‐659. [DOI] [PubMed] [Google Scholar]
- 3. Rawlinson SM, Moseley GW. The nucleolar interface of RNA viruses. Cell Microbiol. 2015;17(8):1108‐1120. [DOI] [PubMed] [Google Scholar]
- 4. Salvetti A, Greco A. Viruses and the nucleolus: the fatal attraction. Biochim Biophys Acta. 2014;1842(6):840‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iarovaia OV, Ioudinkova ES, Velichko AK, Razin SV. Manipulation of cellular processes via nucleolus Hijaking in the course of viral infection in mammals. Cell. 2021;10(7):1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb RA. Mononegavirales. In: Knipe DM, Howley PM, eds. Fields virology. 6th ed. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA; 2013. [Google Scholar]
- 7. Oksayan S, Nikolic J, David CT, Blondel D, Jans DA, Moseley GW. Identification of a role for nucleolin in rabies virus infection. J Virol. 2015;89(3):1939‐1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deffrasnes C, Marsh GA, Foo CH, et al. Genome‐wide siRNA screening at biosafety level 4 reveals a crucial role for Fibrillarin in Henipavirus infection. PLoS Pathog. 2016;12(3):e1005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pentecost M, Vashisht AA, Lester T, et al. Evidence for ubiquitin‐regulated nuclear and subnuclear trafficking among Paramyxovirinae matrix proteins. PLoS Pathog. 2015;11(3):e1004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang YE, Park A, Lake M, et al. Ubiquitin‐regulated nuclear‐cytoplasmic trafficking of the Nipah virus matrix protein is important for viral budding. PLoS Pathog. 2010;6(11):e1001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rawlinson SM, Zhao T, Rozario AM, et al. Viral regulation of host cell biology by hijacking of the nucleolar DNA‐damage response. Nat Commun. 2018;9(1):3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzales B, Henning D, So RB, Dixon J, Dixon MJ, Valdez BC. The Treacher Collins syndrome (TCOF1) gene product is involved in pre‐rRNA methylation. Hum Mol Genet. 2005;14(14):2035‐2043. [DOI] [PubMed] [Google Scholar]
- 13. Valdez BC, Henning D, So RB, Dixon J, Dixon MJ. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci U S A. 2004;101(29):10709‐10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werner A, Iwasaki S, McGourty CA, et al. Cell‐fate determination by ubiquitin‐dependent regulation of translation. Nature. 2015;525(7570):523‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ciccia A, Huang JW, Izhar L, Sowa ME, Harper JW, Elledge SJ. Treacher Collins syndrome TCOF1 protein cooperates with NBS1 in the DNA damage response. Proc Natl Acad Sci U S A. 2014;111(52):18631‐18636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen DH, Hari F, Clapperton JA, et al. The NBS1‐treacle complex controls ribosomal RNA transcription in response to DNA damage. Nat Cell Biol. 2014;16(8):792‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mooser C, Symeonidou IE, Leimbacher PA, et al. Treacle controls the nucleolar response to rDNA breaks via TOPBP1 recruitment and ATR activation. Nat Commun. 2020;11(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wakata A, Katoh H, Kato F, Takeda M. Nucleolar protein treacle is important for the efficient growth of mumps virus. J Virol. 2022;96(19):e0072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McLinton EC, Wagstaff KM, Lee A, et al. Nuclear localization and secretion competence are conserved among henipavirus matrix proteins. J Gen Virol. 2017;98(4):563‐576. [DOI] [PubMed] [Google Scholar]
- 20. Rowe CL, Wagstaff KM, Oksayan S, Glover DJ, Jans DA, Moseley GW. Nuclear trafficking of the rabies virus interferon antagonist P‐protein is regulated by an importin‐binding nuclear localization sequence in the C‐terminal domain. PLoS One. 2016;11(3):e0150477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahmadane I, Certoma AF, Peck GR, et al. Development and validation of an immunoperoxidase antigen detection test for improved diagnosis of rabies in Indonesia. PLoS Negl Trop Dis. 2017;11(11):e0006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sundaramoorthy V, Godde N, Farr RJ, et al. Modelling lyssavirus infections in human stem cell‐derived neural cultures. Viruses. 2020;12(4):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27(3):493‐497. [Google Scholar]
- 24. Brice AM, Rozario AM, Rawlinson SM, et al. Lyssavirus P protein isoforms diverge significantly in subcellular interactions underlying mechanisms of interferon antagonism. J Virol. 2022;96(20):e0139622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brice AM, Watts E, Hirst B, Jans DA, Ito N, Moseley GW. Implication of the nuclear trafficking of rabies virus P3 protein in viral pathogenicity. Traffic. 2021;22:482‐489. [DOI] [PubMed] [Google Scholar]
- 26. Harrison AR, David CT, Rawlinson SM, Moseley GW. The Ebola virus interferon antagonist VP24 undergoes active nucleocytoplasmic trafficking. Viruses. 2021;13(8):1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison AR, Todd S, Dearnley M, et al. Antagonism of STAT3 signalling by Ebola virus. PLoS Pathog. 2021;17(6):e1009636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization (WHO) . Nipah virus. 2022; Available online at: https://www.who.int/news-room/fact-sheets/detail/nipah-virus (accessed December, 2022).
- 29. Wang LF, Mackenzie JS, Broder CC. Henipaviruses. In: Knipe DMH, ed. Fields Virology. Vol 2. 6th ed. Lippincott Williams & Wilkins; 2013:286‐313. [Google Scholar]
- 30. Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81(Pt 8):1927‐1932. [DOI] [PubMed] [Google Scholar]
- 31. Weatherman S, Feldmann H, de Wit E. Transmission of henipaviruses. Curr Opin Virol. 2018;28:7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yob JM, Field H, Rashdi AM, et al. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg Infect Dis. 2001;7(3):439‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marsh GA, de Jong C, Barr JA, et al. Cedar virus: a novel Henipavirus isolated from Australian bats. PLoS Pathog. 2012;8(8):e1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pernet O, Schneider BS, Beaty SM, et al. Evidence for henipavirus spillover into human populations in Africa. Nat Commun. 2014;5:5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Z, Yang L, Yang F, et al. Novel Henipa‐like virus, Mojiang paramyxovirus, in rats, China, 2012. Emerg Infect Dis. 2014;20(6):1064‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin RM, Ter‐Avetisyan G, Herce HD, Ludwig AK, Lattig‐Tunnemann G, Cardoso MC. Principles of protein targeting to the nucleolus. Nucleus. 2015;6(4):314‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chenik M, Chebli K, Blondel D. Translation initiation at alternate in‐frame AUG codons in the rabies virus phosphoprotein mRNA is mediated by a ribosomal leaky scanning mechanism. J Virol. 1995;69(2):707‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emmott E, Hiscox JA. Nucleolar targeting: the hub of the matter. EMBO Rep. 2009;10(3):231‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hossain MA, Larrous F, Rawlinson SM, et al. Structural elucidation of viral antagonism of innate immunity at the STAT1 Interface. Cell Rep. 2019;29(7):1934‐1945. [DOI] [PubMed] [Google Scholar]
- 40. Mavrakis M, McCarthy AA, Roche S, Blondel D, Ruigrok RW. Structure and function of the C‐terminal domain of the polymerase cofactor of rabies virus. J Mol Biol. 2004;343(4):819‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sugiyama A, Nomai T, Jiang X, et al. Structural comparison of the C‐terminal domain of functionally divergent lyssavirus P proteins. Biochem Biophys Res Commun. 2020;529(2):507‐512. [DOI] [PubMed] [Google Scholar]
- 42. Zhan J, Hossain MA, Sethi A, Ose T, Moseley GW, Gooley PR. (1)H, (15)N and (13)C resonance assignments of the C‐terminal domain of the P protein of the Nishigahara strain of rabies virus. Biomol NMR Assign. 2019;13(1):5‐8. [DOI] [PubMed] [Google Scholar]
- 43. Pasdeloup D, Poisson N, Raux H, Gaudin Y, Ruigrok RW, Blondel D. Nucleocytoplasmic shuttling of the rabies virus P protein requires a nuclear localization signal and a CRM1‐dependent nuclear export signal. Virology. 2005;334(2):284‐293. [DOI] [PubMed] [Google Scholar]
- 44. Moseley GW, Filmer RP, DeJesus MA, Jans DA. Nucleocytoplasmic distribution of rabies virus P‐protein is regulated by phosphorylation adjacent to C‐terminal nuclear import and export signals. Biochemistry. 2007;46(43):12053‐12061. [DOI] [PubMed] [Google Scholar]
- 45. Ryan EL, Hollingworth R, Grand RJ. Activation of the DNA damage response by RNA viruses. Biomolecules. 2016;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Audsley MD, Jans DA, Moseley GW. Nucleocytoplasmic trafficking of Nipah virus W protein involves multiple discrete interactions with the nuclear import and export machinery. Biochem Biophys Res Commun. 2016;479(3):429‐433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


