Abstract
This position statement, updated from the 2015 guidelines for managing Australian and New Zealand children/adolescents and adults with chronic suppurative lung disease (CSLD) and bronchiectasis, resulted from systematic literature searches by a multi‐disciplinary team that included consumers. The main statements are:
Diagnose CSLD and bronchiectasis early; this requires awareness of bronchiectasis symptoms and its co‐existence with other respiratory diseases (e.g., asthma, chronic obstructive pulmonary disease).
Confirm bronchiectasis with a chest computed‐tomography scan, using age‐appropriate protocols and criteria in children.
Undertake a baseline panel of investigations.
Assess baseline severity, and health impact, and develop individualized management plans that include a multi‐disciplinary approach and coordinated care between healthcare providers.
Employ intensive treatment to improve symptom control, reduce exacerbation frequency, preserve lung function, optimize quality‐of‐life and enhance survival. In children, treatment also aims to optimize lung growth and, when possible, reverse bronchiectasis.
Individualize airway clearance techniques (ACTs) taught by respiratory physiotherapists, encourage regular exercise, optimize nutrition, avoid air pollutants and administer vaccines following national schedules.
Treat exacerbations with 14‐day antibiotic courses based upon lower airway culture results, local antibiotic susceptibility patterns, clinical severity and patient tolerance. Patients with severe exacerbations and/or not responding to outpatient therapy are hospitalized for further treatments, including intravenous antibiotics and intensive ACTs.
Eradicate Pseudomonas aeruginosa when newly detected in lower airway cultures.
Individualize therapy for long‐term antibiotics, inhaled corticosteroids, bronchodilators and mucoactive agents.
Ensure ongoing care with 6‐monthly monitoring for complications and co‐morbidities.
Undertake optimal care of under‐served peoples, and despite its challenges, delivering best‐practice treatment remains the overriding aim.
Keywords: adolescents, adults, bronchiectasis, children, evidence base practice, systematic review

INTRODUCTION
Chronic suppurative lung disease (CSLD) and bronchiectasis are characterized by chronic or recurrent wet/productive cough, and lower airway infections. Those with bronchiectasis are distinguished by radiographic evidence of bronchial dilatation secondary to structural airway injury. Other clinical and radiographic features may also exist as bronchiectasis is a heterogenous disease with many underlying aetiologies and co‐morbidities. 1 , 2 , 3
Since our latest guideline, 4 Australia has a diagnostic‐related‐group which recognizes that bronchiectasis is important. Global data have demonstrated increased prevalence, hospitalization and economic burden. 5 , 6 A United Kingdom population‐based study described an increase in point‐prevalence of bronchiectasis between 2004 and 2013 (women = 350.5–566.1/100,000; men = 301.2–485.5/100,000). 7 There are no updated Australian data (prevalence in Central Australian First Nations: children aged ≤15‐years = 1470/100,000; adults = 103/100,00 derived from hospitalization data 4 ), but in New Zealand (NZ) the incidence in children aged ≤14‐years increased from 3.7/100,000 in 2001/2002 to 13.2/100,000 in 2017 and the population‐prevalence was 180/100,000. 8 Recently, a Brisbane‐based paediatric study reported direct health service costs for bronchiectasis exacerbations in 2016 of 30,000 Australian dollars per hospitalization. 9
Although the burden of CSLD/bronchiectasis is now recognized, diagnostic delays remain. 10 , 11 Early diagnosis requires greater clinician awareness, whilst prompt investigations confirm the diagnosis and identify underlying aetiologies, treatable traits and co‐morbidities ensuring prompt and appropriate care.
Currently in Australia and NZ, there are large equity gaps within, 12 , 13 and between, 14 , 15 settings. Australian First Nations with bronchiectasis die >20‐years earlier than other Australians with bronchiectasis. 15 In addition, children/adolescents with bronchiectasis unrelated to cystic fibrosis (CF) have substantially poorer lung function, receive lesser care (as judged by numbers of clinic visits, physiotherapist reviews and lung function tests) than age‐matched children/adolescents with CF. 12 Optimal care for people with bronchiectasis is crucial as poor treatment risks premature and accelerated pulmonary decline. 1 , 2 , 16 Moreover, in children/adolescents, it is acknowledged that mild cylindrical bronchiectasis is potentially reversible, indicating an achievable cure for some patients. 1 , 3 , 16
This Thoracic Society of Australia and NZ (TSANZ) position statement builds upon previous guidelines 4 , 17 , 18 , 19 , 20 for managing CSLD/bronchiectasis in children/adolescents and adults in Australia and NZ, including urban and rural‐remote First Nations people. It seeks to increase awareness of CSLD/bronchiectasis amongst clinicians and health service managers, and to encourage consumers to advocate for best‐practice care. However, it does not substitute for sound clinical judgement when diagnosing and managing these heterogenous disorders. 1 , 2 , 16
The objectives are to:
Increase awareness of the disease burden associated with CSLD/bronchiectasis;
Encourage earlier and improved diagnosis and best‐practice management of CSLD/bronchiectasis, including important co‐morbidities;
Present an updated multi‐disciplinary position statement on managing children/adolescents and adults with CSLD/bronchiectasis living in Australia and NZ.
The accompanying technical report for this position statement is presented in Appendix S1 (in the Supporting Information). We reviewed the latest guideline, 4 and undertook an updated search (Appendix S2 in the Supporting Information); of the 33 statements, one is new, 28 were modified and four unchanged.
AWARENESS AND DEFINITIONS
Statement‐1
1a. Bronchiectasis is a clinical syndrome in children/adolescents or adults with the symptoms and/or signs in Box 1 and presence of characteristic radiographic features on chest computed‐tomography (CT) scans.
1b. CSLD is a clinical syndrome in children/adolescents with the symptoms and/or signs outlined in Box 1, but where radiographic signs of bronchiectasis are lacking.
BOX 1. Definition (related to Statement‐1).
Presentation in children/adolescents is with recurrent wet/productive cough for >4‐weeks, with or without other features (faltering growth, exertional dyspnoea, recurrent chest infections, digital clubbing, hyperinflation and chest wall deformity). While in adults, characteristic clinical features are chronic cough, sputum production and recurrent exacerbations.
In children/adolescents, triggers for referral to a specialist include: >3 episodes of chronic (>4‐weeks) wet/productive cough per year, chronic wet/productive cough not responding to 4‐weeks of antibiotics, recurrent (>2) episodes of pneumonia, severe asthma (with chronic wet/productive cough) and persistent (>6‐weeks) lung parenchymal abnormalities on chest radiographs.
Statement‐2
Patients with symptoms and/or signs suggestive of bronchiectasis require a chest CT‐scan to confirm the diagnosis and to assess severity and extent of bronchiectasis.
In children/adolescents, seek specialist advice before ordering a chest CT‐scan and paediatric‐specific diagnostic radiographic criteria should be used.
In all age groups, multi‐detector CT‐scans with high‐resolution CT reconstruction is the preferred technique to diagnose bronchiectasis.
Statement‐3
Consider a chest CT‐scan in adult patients with other airway diseases (e.g., asthma and chronic obstructive pulmonary disease [COPD]), but who also have the clinical phenotype of bronchiectasis (chronic cough, sputum production and recurrent exacerbations), especially in those with more severe airway disease.
INVESTIGATING A PATIENT WITH CSLD/BRONCHIECTASIS
Statement‐4
Obtaining further history/clinical examination for specific underlying causes may determine subsequent investigation and management.
In children/adolescents, these should include:
CF (faltering growth, chronic diarrhoea, heat prostration with hyponatraemia, positive family history, Pseudomonas aeruginosa in sputum);
Underlying immune deficiency (recurrent severe, protracted infections, infections with unusual pathogens, chronic diarrhoea, severe dermatitis, faltering growth);
Primary ciliary dyskinesia (neonatal respiratory distress in a term infant, recurrent otitis and sinopulmonary infections from the first‐year of life, heterotaxy);
Recurrent aspiration (cough and/or choking with feeds, ‘wet’ sounding voice, cyanotic episodes); and
Inhaled foreign body (history of sudden choking, cough, wheeze/stridor, cyanosis, focal decreased breath sounds or wheezing, refractory pneumonia or persistent atelectasis).
In adults, these should include:
Parameters suggestive of CF (early‐onset symptoms, family history, pancreatitis, chronic gastrointestinal symptoms, co‐existent liver disease, male infertility);
Features of primary ciliary dyskinesia (recurrent upper and lower respiratory tract infections from early life, heterotaxy, infertility);
Evidence of underlying immune deficiency (including recurrent and/or severe sinopulmonary infections, unusual pathogens, severe dermatitis, chronic diarrhoea);
Features of connective tissue diseases (e.g., rheumatoid arthritis);
Features suggestive of COPD (also consider alpha‐1‐antitrypsin deficiency in this group) and asthma;
Features of inflammatory bowel disease;
Recurrent aspiration episodes (these can occur post‐bariatric surgery or with a hiatus hernia, and cause symptoms of cough and/or choking with meals, or may even be occult);
Features suggestive of an inhaled foreign body.
Statement‐5
Perform or refer for baseline investigations (Box 2).
BOX 2. Panel of tests (related to Statement‐5).
The minimum baseline investigations in children/adolescents should include:
Full blood count;
Major immunoglobulin classes G, A, M, E;
Sweat test for chloride testing;
Culturing airway secretions, including specialized cultures for mycobacterial species in sputum‐producing patients (consider referring for induced sputum or bronchoscopy in those unable to expectorate sputum);
Spirometry when age appropriate, usually in those aged >6‐years.
In addition, consider the following after discussion with a specialist
Exhaled fractional nasal nitric oxide, nasal ciliary brushings and/or genetic testing for primary ciliary dyskinesia;
Cystic fibrosis transmembrane conductance regulator gene variant/mutation testing when there is a high index of suspicion for cystic fibrosis, but where sweat testing (for chloride) is equivocal or negative;
Bronchoscopy for foreign body, airway abnormality and specimens for culture of respiratory pathogens, including mycobacteria;
Assessment for aspiration (primary or secondary), for example, modified barium swallow for primary aspiration;
Additional immunological tests (neutrophil function tests and lymphocyte subsets, IgG subclasses, antibody responses to vaccine antigens);
Human immunodeficiency virus testing;
Echocardiogram (when concerned about pulmonary hypertension).
The minimum baseline investigations in adults should include:
Full blood count;
Major immunoglobulin classes (G, A, M, E);
Culturing airway secretions in patients who can expectorate sputum—including for fungi and mycobacterial species—consider referring for induced sputum or bronchoscopy in those unable to expectorate sputum;
Spirometry;
Aspergillus serology (IgG precipitins, specific IgE to aspergillus or skin prick tests to Aspergillus).
In addition, consider the following:
Alpha‐1‐antitrypsin levels if there is evidence of chronic obstructive pulmonary disease/emphysema;
Autoimmune serology if clinically indicated (e.g., anti‐CCP, RF, ANA, ANCA, ENAs);
Vitamin D levels if at risk of deficiency;
Exhaled fractional nasal nitric oxide, nasal ciliary brushings and/or genetic testing for primary ciliary dyskinesia;
Sweat test (for chloride) and/or extended cystic fibrosis transmembrane conductance regulator gene variant/mutation testing (in those with early onset of symptoms, family history, pancreatitis, chronic gastrointestinal symptoms, co‐existent liver disease, male infertility);
Bronchoscopy for investigation of suspected foreign body, airway abnormality or in order to obtain specimens for culture of respiratory pathogens (including mycobacteria) in those who are unable to expectorate sputum;
Assessment for aspiration (oesophageal pH monitoring, multi‐channel intraluminal impedence, endoscopic or radiographic evaluation);
Additional immunological tests (IgG subclasses, functional antibody responses to vaccine antigens (including to 23‐valent pneumococcal polysaccharide vaccine antigens), neutrophil function tests, lymphocyte subsets);
Human immunodeficiency virus or human T‐lymphotrophic virus type 1 testing if clinically suspected;
Echocardiogram, especially in adults (if there is suspicion of secondary pulmonary hypertension);
Detailed lung function testing, including body plethysmography, gas transfers, measures of small airway function and walk test (e.g., 6‐minute walk test, if there are symptoms of dyspnoea).
Statement‐6
Obtain further history/clinical examination to determine markers of severity, impact of illness, co‐morbidities and modifiable risk factors. History should include: frequency and impact of exacerbations and hospitalisations, degree of effort limitation, exposure to tobacco smoke, vaping/e‐cigarettes and other aerotoxicants, immunization and early childhood medical and social history, including housing. Clinicians should be aware that some ethnicities (e.g., Australian First Nations, Māori and Pacific Islanders) have more severe disease and experience worse outcomes than other ethnic groups.
In children/adolescents, growth and developmental assessment should be undertaken.
In adults, for severity and impact assessment, consider using clinical severity (e.g., Bronchiectasis severity index, FACED) and quality‐of‐life scores to guide management at baseline and follow‐up. Assess for modifiable risk factors and treatable co‐morbidities that are more common in those with bronchiectasis (COPD, asthma, atopy, rhinosinusitis, cardiovascular disease, anxiety and depression, stress urinary incontinence and low body‐mass index). In severe cases and those with significant co‐morbidities (e.g., rheumatoid arthritis, other autoimmune conditions, inflammatory bowel disease or those taking corticosteroids, immunosuppressive agents or biologic drugs), referral to specialist bronchiectasis services/clinicians is recommended.
MANAGEMENT AND MONITORING
Box 3 outlines the aims of optimal treatment of people with bronchiectasis.
BOX 3. Main aims of optimal management of children/adolescents and adults with CSLD and bronchiectasis.
Main aims are to: 1
Preserve lung function and halt disease progression.
Optimize well‐being and quality‐of‐life for the patient and family.
Minimize the frequency and severity of respiratory exacerbations.
Prevent complications.
Additional aims in children/adolescents are to:
-
5
Optimize lung growth.
-
6
If possible, reverse structural lung injury.
Statement‐7
Aim to optimize general well‐being, symptom control, lung function, quality‐of‐life and reduce exacerbation frequency and prevent excessive decline in lung function. This may require intensive medical therapy.
Statement‐8
Develop treatment plans for exacerbations for each patient, linking them to primary healthcare and specialist or hospital facilities. When appropriate, this includes individualized and self‐initiated management action plans.
Statement‐9
Base antibiotic selection (Table 1) on lower airway culture results (sputum, bronchoscopy washings [adults and older children/adolescents] or bronchoalveolar lavage [young non‐expectorating children]) when these are available, local antibiotic susceptibility patterns, clinical severity and patient tolerance, including allergy.
TABLE 1.
Antibiotic selection guide (related to Statement‐9).
| Mild‐to‐moderate exacerbations | Moderate‐to‐severe exacerbations | |
|---|---|---|
| (Oral therapy) | (Intravenous therapy) | |
| Initial empiric therapy a |
Children: amoxycillin‐clavulanate Adults/adolescents: amoxycillin‐clavulanate or doxycycline b |
All ages: amoxycillin‐clavulanate, cefotaxime or ceftriaxone (amoxycillin, amoxycillin‐clavulanate or cefuroxime in New Zealand) |
|
All ages: ciprofloxacin if P. aeruginosa in recent cultures |
All ages: piperacillin‐tazobactam or ceftazidime ± tobramycin c if severe or P. aeruginosa in recent cultures | |
| Specific pathogens | ||
| H. influenzae d | ||
| β‐lactamase −ve | Amoxycillin | Ampicillin (amoxycillin in New Zealand) |
| β‐lactamase +ve | Amoxycillin‐clavulanate or doxycycline b | Amoxycillin‐clavulanate, cefotaxime or ceftriaxone (amoxycillin‐clavulanate or cefuroxime in New Zealand) |
| S. pneumoniae | Amoxycillin | Benzylpenicillin G, ampicillin (amoxycillin in New Zealand) |
| M. catarrhalis | Amoxycillin‐clavulanate | Amoxycillin‐clavulanate, cefotaxime or ceftriaxone (amoxycillin‐clavulanate or cefuroxime in New Zealand) |
| S. aureus MRSA | Di‐/flucloxacillin seek specialist advice e | Flucloxacillin seek specialist advice e |
| P. aeruginosa | Ciprofloxacin (max 14‐days) | Piperacillin‐tazobactam or ceftazidime ± tobramycin c |
| Aspergillus or NTM | Seek specialist advice | Seek specialist advice |
Abbreviations: MRSA, methicillin‐resistant S. aureus; NTM, non‐tuberculous mycobacteria species.
In addition to clinical severity, initial empiric therapy is also guided by previous lower airway culture results (sputum, bronchoscopy washings or bronchoalveolar lavage), local antibiotic susceptibility patterns, patient tolerance and hypersensitivity to antibiotics and prior responses to antibiotic treatments. In children too young to expectorate sputum, and in older patients when no previous lower airway culture results are available, prescribed empiric antibiotic therapy should be active against Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis. 16
Doxycycline is used only in adults and adolescents.
Although treating serious Pseudomonas aeruginosa infections in adults with combined beta‐lactam and aminoglycoside antibiotic therapy provides no additional clinical benefit than when using a single beta‐lactam agent, 21 the role of single beta‐lactam antibiotics compared with combination antibiotic therapy for P. aeruginosa associated exacerbations in bronchiectasis remains unproven. 22 Thus, in the absence of evidence, the current standard of care of combination parenteral antibiotic therapy for moderate to‐severe exacerbations in children/adolescents should continue, as it should in adults when multi‐resistant P. aeruginosa strains are suspected. Otherwise, in elderly patients and those with significant medical co‐morbidities, beta‐lactam monotherapy is recommended. Whenever systemic aminoglycoside antibiotics are prescribed, careful monitoring for toxicity and measurement of serum drug levels are required. Seek specialist advice when treating multi‐resistant organisms, including P. aeruginosa.
Routine beta‐lactam susceptibility testing for H. influenzae is unreliable and no longer performed by many microbiology laboratories for respiratory isolates. Amoxycillin/ampicillin remains standard therapy for beta‐lactamase negative strains, while amoxycillin‐clavulanate, cefotaxime/ceftriaxone or cefuroxime (New Zealand) are appropriate for beta‐lactamase positive strains. Seek specialist advice if further susceptibility testing is required.
Specialist advice should be sought for treating MRSA strains in accordance with local susceptibility patterns and infection control policies.
Statement‐10
When P. aeruginosa is newly detected, eradication therapy should be offered (Figure 1).
FIGURE 1.
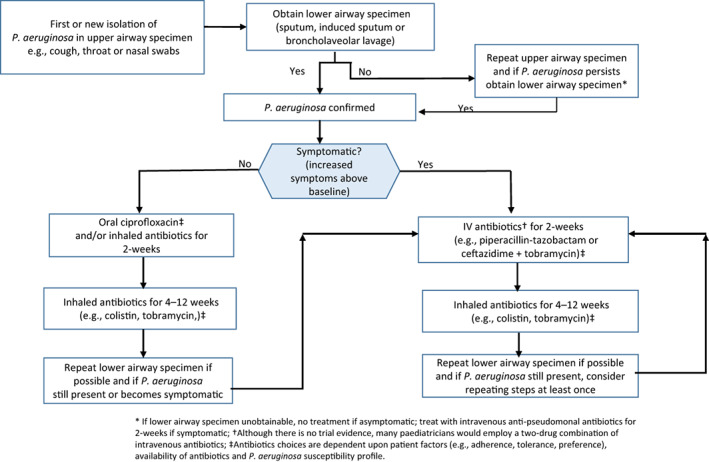
Suggested pathway for treatment when Pseudomonas aeruginosa is newly detected. Figure adapted with permission from the European Respiratory Society paediatric guideline for managing children/adolescents with bronchiectasis. 16
Statement‐11
In patients not requiring parenteral antibiotics for an acute exacerbation, oral antibiotics are prescribed for at least 14‐days based upon available airway microbiology results. Close follow‐up to assess treatment response is necessary.
Statement‐12
Inadequate response should prompt repeating lower airway cultures, including for potential pathogens not detected on routine culture (e.g., non‐tuberculous mycobacteria; NTM) and assessment of whether a change in antibiotic therapy, including parenteral antibiotics, and hospitalization are required.
Statement‐13
Patients failing oral antibiotic therapy for an acute exacerbation should receive intensive airway clearance strategies and parenteral antibiotics based upon the latest lower airway culture results. Close follow‐up is required.
In children/adolescents, this requires supervised treatment for at least 14‐days;
In adults, intravenous antibiotics should be for at least 5‐days and only transitioned to an oral regimen following clear evidence of clinical improvement. A total of at least 14‐days antibiotic therapy should be prescribed. The transition from intravenous to oral antibiotics should depend upon appropriate oral alternatives (based upon sputum culture results) and, if effective, adjunct therapies, such as airway clearance strategies, can be maintained in an ambulatory care setting and where there is ongoing outpatient review.
Statement‐14
Long‐term oral macrolides should be considered for patients with the frequent exacerbator phenotype (≥3 exacerbations requiring antibiotics in the preceding 12‐months) and without contraindications to these agents. Such courses of macrolides should be for at least 6‐months with regular assessments to determine if there is a clinical benefit. Those receiving longer courses should be reviewed regularly for adherence, development of adverse events (especially gastrointestinal and auditory) and emergent macrolide‐resistant bacteria, and to determine risk versus benefit.
Before commencing long‐term macrolides; (a) seek specialist advice, (b) ensure NTM infection is excluded in patients capable of providing a sputum specimen, (c) perform an electrocardiogram in adults to determine their QTc interval (an electrocardiogram should also be ordered in children/adolescents when there is a history of acute cardiac events, if there is a family history of prolonged QT syndrome and/or arrhythmias, or if already receiving QT prolonging medication) and (d) warn adults that macrolides may adversely affect their hearing, and if confirmed the antibiotic will be discontinued.
Statement‐15
Long‐term (>3‐months) inhaled antibiotics (e.g., aminoglycosides, colistin) should not be prescribed routinely for patients with clinically stable bronchiectasis. They should be considered in adults with bronchiectasis who have P. aeruginosa infection and frequent exacerbations (≥3 episodes/year). Similarly, long‐term inhaled antibiotics should be considered in adult patients if oral macrolides are either contraindicated or poorly tolerated or if they have not reduced the frequency of pulmonary exacerbations (irrespective of P. aeruginosa status). Seek specialist advice for children/adolescents.
Those receiving long‐term inhaled antibiotics should be reviewed regularly to monitor their clinical progress, and to assess therapy adherence. Also, discuss (with the patient) if the antibiotic is still being tolerated, and determine the overall risk versus benefit of this treatment.
Statement‐16
Inhaled and oral corticosteroids should not be prescribed routinely in either the short or long‐term, unless there is an established diagnosis of co‐existing asthma and/or eosinophilic airway inflammation. In adults with bronchiectasis, inhaled corticosteroids may be beneficial in those with peripheral blood eosinophilia (>3%).
Statement‐17
Inhaled bronchodilators should not be prescribed routinely and instead used only on an individual basis (e.g., before using inhaled mucoactive agents or inhaled antibiotics or before undertaking ACTs).
Statement‐18
Recombinant human deoxyribonuclease is contraindicated in CSLD/bronchiectasis.
Statement‐19
Mucoactive agents, including inhaled isotonic saline, hypertonic saline and mannitol, are currently not recommended routinely. Consider a therapeutic trial in children/adolescents and adults with continuing frequent exacerbations despite other interventions (e.g., long‐term antibiotics). If bronchial hyper‐responsiveness is present, a rapid‐onset bronchodilator should be given pre‐administration of hypertonic saline or mannitol.
Statement‐20
ACTs are recommended. These should be individualized, and their methods and frequency reviewed, preferably biannually by a respiratory physiotherapist.
Statement‐21
Offer pulmonary rehabilitation (including exercise training) to adults with bronchiectasis, especially those who have reduced exercise tolerance and/or frequent exacerbations.
Statement‐22
Regular physical activity is recommended for children/adolescents and adults with CSLD/bronchiectasis.
Statement‐23
Assess and optimize nutritional status.
Statement‐24
Promote elimination of smoking, including vaping/e‐cigarettes, cannabis and second‐hand smoke exposure.
Statement‐25
Promote avoidance of airborne pollutants, including traffic emissions, biomass smoke and other settings with poor air quality.
Statement‐26
Regularly monitor and manage complications and co‐morbidities (described below). When present, these are managed following standard guidelines. Review at least annually in adults and 6‐monthly in children/adolescents, ideally using a multi‐disciplinary team approach. The review includes assessment of:
Severity, which includes oximetry and spirometry;
Sputum culture (when available) for routine bacterial and annual mycobacterial culture;
Management of treatable traits and possible complications and/or co‐morbidities, particularly for nutritional deficiencies, gastro‐oesophageal reflux disease/aspiration, asthma, COPD (in adults), allergic broncho‐pulmonary aspergillosis and specific pathogens (e.g., P. aeruginosa or NTM), odontogenic and otorhinolaryngeal disorders, urinary incontinence, anxiety and mental health disorders. Less commonly, patients require assessments for sleep‐disordered breathing and cardiac complications;
General well‐being and bronchiectasis‐specific health education, including that related to exacerbations.
Statement‐27
Although surgery is not indicated normally, there may be circumstances requiring assessment by a multi‐disciplinary team expert in bronchiectasis. Factors to address are firstly ensuring it is focal disease, then optimizing medical management, and next considering the patient's age, symptoms, underlying aetiology (likelihood of recurrence), and the facility where surgery can be undertaken (expertise and availability of pre‐ and post‐surgical care).
Statement‐28
Vaccinate according to National Immunization Program Schedules, which include pneumococcal and influenza vaccine recommendations for high‐risk patients with chronic lung disorders. Ensure timely annual seasonal influenza vaccination. COVID‐19 vaccination should follow national public health policies. Finally, consider vaccinating household contacts annually with the seasonal influenza vaccine.
Statement‐29
A multidisciplinary approach to healthcare is recommended. Key considerations should focus upon tailoring care to the individual, reducing barriers to care, improving adherence to treatment and providing culturally responsive healthcare. Evaluation by clinicians with expertise in CSLD/bronchiectasis is recommended to confirm the diagnosis, investigate the aetiology, assess severity, provide education and develop individualized treatment and self‐management plans. Clinical deterioration should prompt early referral to specialist CSLD/bronchiectasis services.
Statement‐30
All people with CSLD/bronchiectasis should receive care by a specialist respiratory service with expertise in bronchiectasis. More frequent review for those with bronchiectasis should be undertaken in patients with any of the following: moderate disability, deteriorating lung disease, haemoptysis, pulmonary hypertension, frequent exacerbations (≥3/year), lower airway P. aeruginosa or NTM infection, severe asthma symptoms, presence of underlying cause (e.g., primary ciliary dyskinesia, immunodeficiency, autoimmune diseases), advanced disease and if they are being considered for lung transplantation.
Statement‐31
Providing healthcare for Australian First Nations, Māori and Pacific Islanders and under‐serviced communities in rural‐remote regions requires flexible and adaptive arrangements. However, it should not alter the objective of delivering best‐practice treatment to these populations.
Statement‐32
Given the high prevalence of CSLD/bronchiectasis in Australian First Nations, Māori and Pacific Islanders, a high index of suspicion with early diagnostic investigation, and institution of best‐practice treatment should be established. Interpreters and local health‐workers should be available for education regarding the disease and its management.
Statement‐33
Health services should provide transitional care that supports the requirements of adolescents with bronchiectasis. A purposeful, planned and developmentally appropriate transition, which includes engagement of the paediatric and adult multi‐disciplinary teams, utilization of transition guidelines, and clear documentation of transfer processes are key goals as they transition from child to adult health services.
CONCLUSION
Although CSLD/bronchiectasis are described as amongst the most neglected pulmonary disorders, 23 since the latest TSANZ guideline in 2015 4 our knowledge and evidence base 1 , 3 , 24 , 25 for managing these conditions has expanded considerably. An important advance was recognizing that with optimal care, early cylindrical bronchiectasis in children/adolescents is potentially reversible. 1 , 16 Another was identifying the importance of ‘treatable traits’, which seek to address the heterogeneity of CSLD/bronchiectasis by individualizing the pulmonary, aetiological, extra‐pulmonary and environmental/lifestyle impacts of each disorder. 1 , 3 , 26 Accordingly, and with the assistance of consumers, this TSANZ position statement is designed to help clinicians, health service managers, patients and families achieve the patient‐oriented goals of optimizing lung growth in children, preserving lung function, minimizing exacerbations, optimizing quality‐of‐life, preventing complications and disease progression and if possible, reversing early bronchiectasis. Finally, the inequity of resources available for people with bronchiectasis living in Australia and NZ 12 , 13 , 14 , 15 also needs addressing.
AUTHOR CONTRIBUTION
Anne B Chang: Conceptualization (lead); data curation (lead); methodology (lead); project administration (lead); writing – original draft (lead). Scott Cameron Bell: Data curation (supporting); writing – review and editing (supporting). Catherine A Byrnes: Data curation (supporting); writing – review and editing (supporting). Anne Holland: Data curation (supporting); writing – review and editing (supporting). Emma Kennedy: Data curation (supporting); writing – review and editing (supporting). Paul King: Data curation (supporting); writing – review and editing (supporting). Pamela Laird: Data curation (supporting); writing – review and editing (supporting). Sarah Mooney: Data curation (supporting); writing – review and editing (supporting). Lucy Morgan: Data curation (supporting); writing – review and editing (supporting). Marianne Parsons: Data curation (supporting); writing – review and editing (supporting). Betty Poot: Data curation (supporting); writing – review and editing (supporting). Maree Toombs: Data curation (supporting); writing – review and editing (supporting). Paul J Torzillo: Data curation (supporting); writing – review and editing (supporting). Keith Grimwood: Data curation (supporting); writing – review and editing (equal).
CONFLICTS OF INTEREST STATEMENT
Catherine A. Byrnes: Trustee, Bronchiectasis Foundation; Editor, New Zealand Formulary for Children at the Starship Children's Hospital. Has received funds from Health Research Council of New Zealand research, CureKids research and FluLab International research on the subject matter. Anne B. Chang: European Respiratory Society (ERS) Guidelines Committee Member, ERS Long Strategy Member, APSR Strategic Planning Member and Australian Bronchiectasis Consortium and Registry. Has written for UpToDate and has received multiple NHMRC and MRFF grants on the subject matter. Sarah Mooney has received funds from Asthma NZ to present at GP and annual conference. Lucy Morgan: Past Chair of Australian Bronchiectasis Consortium and Registry. Has received personal funds from Astra Zeneca, Zambon, Insmed and Glaxo; and administrative support from the Lung Foundation of Australia. Received payment as a PI in commercial clinical trials. Betty Poot: Currently involved in recruiting patients to the NZ bronchiectasis registry where data has been used for conference presentations and for writing up manuscript for publication. Scott C. Bell, Paul Dawkins, Keith Grimwood, Anne E. Holland, Emma Kennedy, Paul T. King, Pamela Laird, Marianne Parsons and Paul J. Torzillo have nothing to disclose.
Supporting information
APPENDIX S1: Full technical report for the Thoracic Society of Australia and New Zealand (TSANZ) position statement.
APPENDIX S2: Search strategy used for statements.
Visual Abstract Chronic suppurative lung disease and bronchiectasis in children, adolescents and adults in australia and new zealand Thoracic Society of Australia and New Zealand (TSANZ) Position Statement
ACKNOWLEDGEMENTS
Health professionals who contributed to the Delphi statement: Nurses and Physiotherapists: Kathleen Hall, Christine Wilson, Annemarie Lee, Donna Mason, Gabrielle McCallum, Anna Middleton, Lesley Versteegh, Kathy Lawton. Adult‐based Medical Practitioners: Lucy Burr, Bart Currie, John Kolbe, Lata Jayaram, Chen Li Holmes‐Liew, Peter Middleton, Jonathan Rutland, Frank Thien, Grant Waterer, Conroy Wong, Tim Baird, Daniel Smith, James Geake, Rachel Thompson. Paediatric‐based Medical Practitioners: Vikas Goyal, Peter Morris, Anna Mulholland, Brent Masters, Andre Schultz, Gurmeet Singh, Dhanusya Sivananthan, Rahul Thomas, Danielle Wurzel, Julie Marchant, Bernadette Prentice, Hiran Selvadurai. General Practice: Kylie Vuong.
Research funding: Open access fees were provided by the TSANZ. Supported by the National Health and Medical Research Council (NHMRC) Centre for Research Excellence in bronchiectasis for children (grant 1170958). Anne B. Chang is funded by a NHMRC practitioner fellowship (grant 1154302) and the Queensland Children's Hospital Foundation. Open access publishing facilitated by Charles Darwin University, as part of the Wiley ‐ Charles Darwin University agreement via the Council of Australian University Librarians.
Chang AB, Bell SC, Byrnes CA, Dawkins P, Holland AE, Kennedy E, et al. Thoracic Society of Australia and New Zealand (TSANZ) position statement on chronic suppurative lung disease and bronchiectasis in children, adolescents and adults in Australia and New Zealand . Respirology. 2023;28(4):339–349. 10.1111/resp.14479
This document has been endorsed by the Thoracic Society of Australia and New Zealand on 15 February 2023.
Handling Editor: Paul Reynolds
Funding information National Health and Medical Research Council, Grant/Award Numbers: 1170958, 1154302; Children's Hospital Foundation
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Chang AB, Bush A, Grimwood K. Bronchiectasis in children: diagnosis and treatment. Lancet. 2018;392:866–79. [DOI] [PubMed] [Google Scholar]
- 2. Hill AT, Sullivan L, Chalmers JD, De SA, Elborn JS, Floto AR, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74:1–69. [DOI] [PubMed] [Google Scholar]
- 3. Chalmers JD, Chang AB, Chotirmall SH, Dhar R, McShane PJ. Bronchiectasis. Nature Rev Dis Primers. 2018;4:45. [DOI] [PubMed] [Google Scholar]
- 4. Chang AB, Bell SC, Torzillo PJ, King PT, Byrnes CA, Maguire GP, et al. Bronchiectasis and chronic suppurative lung disease (CSLD) in children and adults in Australia and New Zealand: Thoracic Society of Australia and New Zealand Guideline: an update. Med J Aust. 2015;202:21–3. [DOI] [PubMed] [Google Scholar]
- 5. Quint JK, Smith MP. Paediatric and adult bronchiectasis: diagnosis, disease burden and prognosis. Respirology. 2019;24:413–22. [DOI] [PubMed] [Google Scholar]
- 6. Guan WJ, Han XR, Rosa‐Carrillo D, Martinez‐Garcia MA. The significant global economic burden of bronchiectasis: a pending matter. Eur Respir J. 2019;53:1802392. [DOI] [PubMed] [Google Scholar]
- 7. Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. Eur Respir J. 2016;47:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Telfar‐Barnard L, Zhang J. The impact of respiratory disease in New Zealand: 2018 update. University of Otago. 2018 [cited 2022 Jan 12]. Available from: https://www.asthmafoundation.org.nz/research/the‐impact‐of‐respiratory‐disease‐in‐new‐zealand‐2018‐update; 2018
- 9. Goyal V, McPhail SM, Hurley F, Grimwood K, Marchant JM, Masters IB, et al. Cost of hospitalisation for bronchiectasis exacerbations in children. Respirology. 2020;25:1250–6. [DOI] [PubMed] [Google Scholar]
- 10. Giron RM, de Gracia RJ, Olveira C, Vendrell M, Martinez‐Garcia MA, de la Rosa D, et al. Sex bias in diagnostic delay in bronchiectasis: an analysis of the Spanish historical registry of bronchiectasis. Chron Respir Dis. 2017;14:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goyal V, Grimwood K, Masters IB, Marchant JM, Chang AB. State of the art: pediatric bronchiectasis. Pediatr Pulmonol. 2016;51:450–69. [DOI] [PubMed] [Google Scholar]
- 12. Prentice BJ, Wales S, Doumit M, Owens L, Widger J. Children with bronchiectasis have poorer lung function than those with cystic fibrosis and do not receive the same standard of care. Pediatr Pulmonol. 2019;54:1921–6. [DOI] [PubMed] [Google Scholar]
- 13. Moss R, Farrant B, Byrnes CA. Transitioning from paediatric to adult services with cystic fibrosis or bronchiectasis: what is the impact on engagement and health outcomes? J Paediatr Child Health. 2021;57:548–53. [DOI] [PubMed] [Google Scholar]
- 14. Blackall SR, Hong JB, King P, Wong C, Einsiedel L, Remond MGW, et al. Bronchiectasis in Indigenous and non‐Indigenous residents of Australia and New Zealand. Respirology. 2018;23:743–9. [DOI] [PubMed] [Google Scholar]
- 15. McCallum GB, Chang AB. 'Good enough' is 'not enough' when managing Indigenous adults with bronchiectasis in Australia and New Zealand. Respirology. 2018;23:725–6. [DOI] [PubMed] [Google Scholar]
- 16. Chang AB, Fortescue R, Grimwood K, Alexopoulou E, Bell L, Boyd J, et al. Task Force report: European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur Respir J. 2021;58:2002990. [DOI] [PubMed] [Google Scholar]
- 17. Chang AB, Bell SC, Torzillo PJ, King PT, Byrnes CA, Maguire GP, et al. Thoracic Society of Australia and New Zealand. Chronic suppurative lung disease and bronchiectasis in children and adults in Australian and New Zealand. Available from: http://www.thoracic.org.au/journal‐publishing/command/download_file/id/36/filename/TSANZ‐ChronicSuppurativeLungDisease‐Guidelines‐2016‐web.pdf.2014 [DOI] [PubMed]
- 18. Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes PW, King PT, et al. Bronchiectasis and chronic suppurative lung disease (CSLD) in children and adults in Australian and New Zealand: Thoracic Society of Australia and New Zealand and Australian Lung Foundation Position Statement. Med J Aust. 2010;193:356–65. [DOI] [PubMed] [Google Scholar]
- 19. Chang AB, Grimwood K, Macguire G, King PT, Morris PS, Torzillo PJ. Management of bronchiectasis and chronic suppurative lung disease (CSLD) in Indigenous children and adults from rural and remote Australian communities. Med J Aust. 2008;189:386–93. [DOI] [PubMed] [Google Scholar]
- 20. Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in Indigenous children in remote Australian communities: a position statement. Med J Aust. 2002;177:200–4. [DOI] [PubMed] [Google Scholar]
- 21. Heffernan AJ, Sime FB, Sun J, Lipman J, Kumar A, Andrews K, et al. B‐lactam antibiotic versus combined B‐lactam antibiotics and single daily dosing regimens of aminoglycosides for treating serious infections: a meta‐analysis. Int J Antimicrob Agents. 2020;55:105839. [DOI] [PubMed] [Google Scholar]
- 22. Felix LM, Grundy S, Milan SJ, Armstrong R, Harrison H, Lynes D, et al. Dual antibiotics for bronchiectasis. Cochrane Database Syst Rev. 2018;6:CD012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Respiratory Society . Bronchiectasis. The European lung white book. Volume 15. Sheffield: European Respiratory Society; 2014. p. 176–83. [Google Scholar]
- 24. Laska IF, Crichton ML, Shoemark A, Chalmers JD. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta‐analysis. Lancet Respir Med. 2019;7:855–69. [DOI] [PubMed] [Google Scholar]
- 25. Chalmers JD, Boersma W, Lonergan M, Jayaram L, Crichton ML, Karalus N, et al. Long‐term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta‐analysis. Lancet Respir Med. 2019;7:845–54. [DOI] [PubMed] [Google Scholar]
- 26. Boaventura R, Sibila O, Agusti A, Chalmers JD. Treatable traits in bronchiectasis. Eur Respir J. 2018;52:1801269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1: Full technical report for the Thoracic Society of Australia and New Zealand (TSANZ) position statement.
APPENDIX S2: Search strategy used for statements.
Visual Abstract Chronic suppurative lung disease and bronchiectasis in children, adolescents and adults in australia and new zealand Thoracic Society of Australia and New Zealand (TSANZ) Position Statement
Data Availability Statement
Not applicable.


