Abstract
Background:
There is minimal evidence of relationships between maternal air pollution exposure and spontaneous premature rupture of membranes (SPROM), a critical obstetrical problem that can significantly increase maternal and fetal mortality and morbidity. No prior study has explored the PROM risk related to specific components of particulate matter with aerodynamic diameters of ≤ 2.5 μm (PM2.5). We examined associations between maternal exposure to nitrogen dioxide (NO2), ozone (O3), PM2.5, PM10, and PM2.5 constituents and SPROM.
Methods:
A large retrospective cohort study was conducted and included 427,870 singleton live births from Kaiser Permanente Southern California during 2008–2018. Monthly averages of NO2, O3 (8-h daily maximum), PM2.5, and PM10 were measured using empirical Bayesian kriging based on measurements from monitoring stations. Data on PM2.5 sulfate, nitrate, ammonium, organic matter, and black carbon were obtained from a fine-resolution model. A discrete time approach with pooled logistic regressions was used to estimate associations throughout the pregnancy and based on trimesters and gestational months. The quantile-based g-computation models were fitted to examine the effects of 1) the air pollution mixture of four pollutants of interest and 2) the mixture of PM2.5 components.
Results:
There were 37,857 SPROM cases (8.8%) in our study population. We observed relationships between SPROM and maternal exposure to NO2, O3, and PM2.5. PM2.5 sulfate, nitrate, ammonium, and organic matter were associated with higher SPROM risks in the single-pollutant model. Mixture analyses demonstrated that the overall effects of the air pollution mixture and PM2.5 mixture in this study were mainly driven by O3 and PM2.5 nitrate, respectively. Underweight mothers had a significantly higher risk of SPROM associated with NO2.
Conclusion:
Our findings add to the literature on associations between air pollution exposure and SPROM. This is the first study reporting the impact of PM2.5 constituents on SPROM.
Keywords: Premature rupture of membranes, Air pollution, Ozone, Particulate matter, Maternal exposure, Pregnancy outcome
1. Introduction
Premature rupture of membranes (PROM) is a critical obstetrical event complicating approximately 7–8% of pregnancies (Esteves, 2022) and has been associated with considerable risks of poor maternal and fetal outcomes. Specifically, mothers with PROM have higher risks of intra-amniotic infections, placental abruption, cord prolapse, sepsis, and death (Assefa et al., 2018; Gonzalez-Mesa et al., 2021; Mercer, 2003). For offspring, PROM can result in elevated neonatal mortality and morbidity (Tchirikov et al., 2018; Yagur et al., 2019). Umbilical cord compression or placental abruption following PROM can result in serious fetal complications such as respiratory distress syndrome (Caughey et al., 2008). Of all cases, 30–40% of PROMs can occur before 37 gestational weeks (preterm PROM) and trigger preterm births (Menon and Richardson, 2017). The etiology of PROM remains unclear, but a variety of factors, including apoptosis, oxidative stress, and altered membrane morphology triggered by infection and inflammation, have been suggested (Menon and Richardson, 2017; Tchirikov et al., 2018). Potential risk factors for PROM may include some maternal characteristics, such as cervical incompetence, low body mass index (BMI), low socioeconomic status, and smoking (Caughey et al., 2008; Esteves, 2022; Lyons and McLaughlin, 2020). Environmental factors, such as air pollution, have also been suggested to participate in the pathogenesis of PROM (Dadvand et al., 2014; Yackerson et al., 2008). However, the evidence for different air pollutants remains limited and inconclusive (Dadvand et al., 2014; Pereira et al., 2014; Pereira et al., 2016; Wallace et al., 2016; Wang et al., 2019); these pollutants include nitrogen dioxide (NO2), nitrogen oxides (NOx), ozone (O3), and particulate matter with aerodynamic diameters of ≤ 2.5 μm (PM2.5) and ≤ 10 μm (PM10).
The first investigation regarding air pollution and PROM was conducted in Barcelona using matched case-control analyses and showed that entire-pregnancy exposure to NO2, NOx, and PM2.5 light absorbance (a marker of black carbon aerosol) increased the risk of preterm PROM, while no relationships were found for PM2.5, PM2.5–10, and PM10 (Dadvand et al., 2014). This study was partly in line with another study in the United States where exposure to NOx, PM2.5, and PM10 was not associated with PROM risks (Wallace et al., 2016). Nevertheless, a Chinese cohort study linked PM2.5 exposure to increased risks of PROM regardless of the trimester of exposure (Wang et al., 2019), while a longitudinal study on an Australian cohort only reported PM2.5-related associations in the second trimester (Pereira et al., 2014). Understanding susceptible windows for air pollution can help reveal underlying mechanisms and provide targeted clinical interventions or guidance on behavioral changes to reduce health risks (Warren et al., 2018). Previous epidemiological findings are inconsistent, especially for PM2.5, PM10, and NO2, with some studies observing no association (Dadvand et al., 2014; Pereira et al., 2016; Wallace et al., 2016) and others finding varying critical trimesters (Pereira et al., 2014; Wang et al., 2019). As the biological response to adverse exposure may not be exactly trimester-specific, it is meaningful to capture shorter exposure periods (e.g., months or weeks) that might help identify more specific windows of susceptibility (Sheridan et al., 2019; Sun et al., 2020; Wang et al., 2018).
Ambient PM2.5 pollution has been regarded as the leading environmental risk factor for various health issues globally and contributes to significantly increased premature deaths as well as adverse pregnancy outcomes (Li et al., 2017; Liu et al., 2019). However, research has not reached a clear conclusion on associations between prenatal PM2.5 exposure and PROM (Dadvand et al., 2014; Li et al., 2021; Pereira et al., 2014, 2016; Wallace et al., 2016; Wang et al., 2019; Wu et al., 2023; Zhang et al., 2021). In addition to different study populations and study designs, the difference in study conclusions may be partially explained by the heterogeneity in PM2.5 chemical compositions due to regional differences in types of sources and climatic conditions (Cai et al., 2020; Ebisu and Bell, 2012; Qiao et al., 2014; Sun et al., 2021). Identification of specific impacts of PM2.5 components can assist in effective targeted emission control of particle pollution from a public health viewpoint. However, to our best knowledge, no previous study on PROM looked at the PM2.5 components. Though measuring the PM2.5 elements can be expensive, there have been some models estimating ambient levels of PM2.5 components from high-resolution satellite data and other data sources. Statistical models have also been improved to analyze the effects of air pollution mixtures. Recently, a quantile-based g-computation (QGC) method was developed to evaluate the joint effects of all exposures within a mixture (Keil et al., 2020). This innovative method can estimate the effects of a specific subset of components controlling for possible confounding from other chemicals in the mixture (van den Dries et al., 2021) and would be an appropriate approach to assess the effect of the mixture containing different air pollutants on PROM.
Therefore, we carried out a retrospective cohort study in Southern California to investigate: (1) the associations between maternal exposure to NO2, O3, PM2.5, PM10, and PM2.5 constituents and spontaneous PROM (SPROM) in various exposure windows (i.e., entire pregnancy, trimesters, and gestational months); (2) the joint effects of air pollution mixture on SPROM; and (3) the joint effects of PM2.5 components on SPROM.
2. Methods
2.1. Study population
From 2008 to 2018, over 340,000 mothers who were members of Kaiser Permanente Southern California (KPSC), a large integrated healthcare system, with gestational ages from 20 to 47 weeks were included in this large pregnancy cohort (Fig. S1). More information about the study population can be found elsewhere (Sun et al., 2021). We included mothers who had singleton live births from this cohort for the study on SPROM. Electronic health records (EHRs) provide detailed information including maternal demographic factors, residential histories, medical and obstetrical records, as well as individual health-related behaviors. In total, 429,839 pregnancies were preliminarily identified after excluding multiple births (n = 7,454) and stillbirths (n = 1,961).
2.2. Outcome: SPROM
SPROM is defined as the spontaneous ROM without the onset of labor regardless of gestational age. PROMs due to iatrogenic interventions (non-spontaneous PROM, n = 1,651, 4.1% of all PROM cases) were excluded from our study as the main trigger of those cases would be human factors that may not be attributable to air pollution. More diagnosis information has been provided in our prior study (Jiao et al., 2023). The pregnancy period for our study started from the date of the last menstrual period confirmed by ultrasonography (Sun et al., 2021) and ended at the ROM date (Dadvand et al., 2014; Jiao et al., 2023).
2.3. Exposure assessment
Monthly averages of NO2, O3–8h (daily maximum from 10 AM to 6 PM), PM2.5, and PM10 were estimated using empirical Bayesian kriging (EBK) based on daily measurements from monitoring stations of the U.S. Environmental Protection Agency during 2007–2018. The EBK method showed a cross-validation R2 ranging from 0.65 to 0.75 for different air pollutants (Wu et al., 2016). Our previous studies have extensively described the used EBK model (Laurent et al., 2016a; Wu et al., 2016). Monthly data on PM2.5 total mass and components at a 1 km resolution, including sulfate, nitrate, ammonium, organic matter, and black carbon, were obtained from a geoscience-derived model from 2007 to 2017 (Meng et al., 2019; van Donkelaar et al., 2019). This model incorporated information from chemical transport modeling, satellites, and ground monitoring stations to provide validated measurements of PM2.5 composition over North America (Meng et al., 2019; van Donkelaar et al., 2019). In the Southwestern United States, the model showed the highest cross-validated agreement for nitrate (R2 = 0.78) and ammonium (R2 = 0.75), followed by sulfate (R2 = 0.59), organic matter (R2 = 0.52), and black carbon (R2 = 0.42). Maternal addresses of residence incorporating detailed information about residential mobility during the entire pregnancy were geocoded. Monthly air pollution data were temporally interpolated to obtain gestational month- and trimester-specific exposure for each participant based on geocoded residential addresses (Sun et al., 2021). Mainly to simplify the monthly analysis, a gestational month was calculated on 30 days, and rough cutoff points for trimesters were applied (i.e., first: gestational months 1–3 [1–90 days]; second: gestational months 4–6 [91–180 days]; third: gestational months 7–9 [181–270 days]). More details for exposure measurement have been provided in our previous work (Sun et al., 2021; Sun et al., 2020).
2.4. Statistical analysis
We summarized the study population characteristics and the exposure to air pollution. The correlation between exposure to each pollutant throughout the pregnancy was measured by Pearson’s correlation. To identify windows of susceptibility, a discrete time approach with the logit function was fitted to estimate associations between air pollution exposure and SPROM during each period, including the entire pregnancy, trimesters, and gestational months. Like the Cox proportional hazard model, the discrete time approach is a method for survival analysis, which is particularly useful for handling large datasets with many ties and time-dependent variables without assuming proportional hazards. As time should be included as a covariate in this approach, in the present study, we included time (i.e., the gestational month) in a flexible manner (i.e. polynomials) as suggested by prior research (Murray et al., 2020). We included the county of residence as a random effect in the model (Sun et al., 2021). Covariates were selected a priori based on existing literature, including maternal age, self-reported race/ethnicity, educational level, neighborhood household income, pre-pregnancy BMI (kg/m2), smoking status, parity, year of infant birth, and season of conception (Dadvand et al., 2014; Jiao et al., 2023; Pereira et al., 2016; Wallace et al., 2016; Wang et al., 2019). To align better with the analysis using monthly exposure data, we included pregnancies with gestational months ≥ 5 (n = 427,870, 99.9%) from the entire pregnancy cohort as the minimum gestational age of mothers in our cohort was 20 weeks.
The trimester-specific associations were jointly examined in a single model by including exposures in three trimesters simultaneously (denoted as the all-trimester model) to provide more accurate estimates (Wilson et al., 2017). We further performed a sensitivity analysis by running single-trimester models to estimate the association for each trimester without controlling for exposures in the other two trimesters. To account for potential correlations between associations across different gestational months, the distributed lag model (DLM) was used to provide less biased results. A natural cubic spline function with 4 degrees of freedom was applied in the DLMs to model the lag structure of associations from gestational month 1 to month 8 (Wang et al., 2018; Wilson et al., 2017). As about 37.2% of mothers giving birth before month 9 did not have exposure data in this month, we did not estimate the association in month 9 to avoid a large decrease in the sample size for the DLM analysis. The results for krigged NO2, O3, PM2.5, and PM10 based on the EBK model and PM2.5 total mass and components from the geoscience-statistical model were reported as odds ratios (ORs) and 95% confidence intervals (CIs) per interquartile range (IQR) increase in average exposure during the pregnancy. To evaluate the impact of physical condition before pregnancy on the susceptibility of pregnant women to SPROM, subgroup analysis stratified by pre-pregnancy BMI was conducted to identify its potential effect modification (Esteves, 2022). We measured the heterogeneity among BMI subgroups using Cochran’s Q test.
The mixture effects were measured by a novel method QGC from the “qgcomp” package in R. This method combines aspects of weighted quantile sum (WQS), a widely used statistical method for mixture analysis, with a causal inference method known as g-computation (Schmidt, 2020). WQS regression measured the combined effects of components in a mixture under two assumptions that associations between each component and the outcome are (1) in the same direction (or null), and (2) linear and additive, while QGC relaxes both assumptions and provides unbiased estimates when its assumptions are violated (Keil et al., 2020; Liu et al., 2021; Sun et al., 2021). We included air pollutants and PM2.5 components associated with SPROM in the single-pollutant model in the mixture analysis.
In sensitivity analyses: (a) We examined the relatively short-term associations in the last one and the last three gestational months (Dadvand et al., 2014). (b) The zip code of residence was used as a random effect for PM2.5 total mass and components to account for smaller spatial clustering. Given that kriging interpolation based on monitoring station data was too coarse to capture within-zip code variability due to relatively sparse stations (Sun et al., 2021), we did not include zip codes in the single-pollutant model for krigged NO2, O3, PM2.5, and PM10. (c) We further included the history of PROM in the analysis as a potential confounder. (d) To check the robustness of our model, we removed the restriction on the time effect in the model by adding a monomial of time as a covariate instead of the polynomials. (e) We examined the associations using the Cox proportional hazards model with the gestational month as the temporal unit. (f) We also conducted a sensitivity analysis using WQS regression as an alternative method to the QGC model. (g) For the PM2.5 mixture, we added PM2.5 total mass in QGC and WQS models to control for any potential effects of other PM2.5 components. (h) We further performed co-pollutant models for entire-pregnancy associations with four krigged air pollutants and five PM2.5 components, respectively, to evaluate the robustness of single-pollutant models. (i) We tested the effect modification by performing models with the interaction term between air pollution exposure and pre-pregnancy BMI. All analyses were conducted with SAS version 9.4 and R 4.1.3.
3. Results
Table 1 summarizes the study population characteristics. A total of 427,870 pregnancies with 37,857 (8.8%) SPROM cases from 2008 to 2018 were included. Approximately 60% of the total population were mothers aged 25–34. Hispanic and non-Hispanic White mothers accounted for approximately 50% and 25% of all subjects, respectively. Among non-SPROM cases, about 60% of them have two or more deliveries, while only about 46% of SPROM cases gave birth two or more times. More SPROM cases had a history of PROM (3.08%) compared with non-SPROM cases (2.13%).
Table 1.
Descriptive statistics of the study population (2008–2018).
| Characteristics | Total pregnancies (n = 427,870) | Non-SPROMs (n = 390,013) | SPROMs (n = 37,857) |
|---|---|---|---|
|
| |||
| Maternal age (n, %) | |||
| < 25 | 83,170 (19.44) | 75,880 (19.46) | 7,290 (19.26) |
| 25–34 | 253,755 (59.31) | 231,148 (59.27) | 22,607 (59.72) |
| ≥ 35 | 90,945 (21.26) | 82,985 (21.28) | 7,960 (21.03) |
| Race/Ethnicity (n, %) | |||
| African American | 32,275 (7.54) | 29,580 (7.58) | 2,695 (7.12) |
| Asian | 53,829 (12.58) | 48,578 (12.46) | 5,251 (13.87) |
| Hispanic | 218,804 (51.14) | 199,269 (51.09) | 19,535 (51.60) |
| Non-Hispanic White | 111,717 (26.11) | 102,338 (26.24) | 9,379 (24.77) |
| Multiple/Others | 11,199 (2.62) | 10,203 (2.62) | 996 (2.63) |
| Missing | 46 (0.01) | 45 (0.01) | 1 (0.00) |
| Education level (n, %) | |||
| Less than college | 133,596 (31.22) | 123,063 (32.55) | 10,533 (27.82) |
| College | 229,984 (53.75) | 208,955 (53.58) | 21,029 (55.55) |
| Higher than college | 55,879 (13.06) | 50,352 (12.91) | 5,527 (14.60) |
| Missing | |||
| BMI, kg/m2 (n, %) | 8,411 (1.97) | 7,643 (1.96) | 768 (2.03) |
| Underweight (<18.5) | 10,453 (2.44) | 9,538 (2.45) | 915 (2.42) |
| Normal weight (18.5–24.9) | 181,905 (42.51) | 165,909 (42.54) | 15,996 (42.25) |
| Overweight (25.0–29.9) | 119,181 (27.85) | 108,336 (27.78) | 10,845 (28.65) |
| Obese (≥30.0) | 112,859 (26.38) | 103,009 (26.41) | 9,850 (26.02) |
| Missing | 3,472 (0.81) | 3,221 (0.83) | 251 (0.66) |
| Smoking status (n, %) | |||
| Never smoker | 357,244 (83.49) | 325,650 (83.50) | 31,594 (83.46) |
| Past smoker | 48,813 (11.41) | 44,488 (11.41) | 4,325 (11.42) |
| Smoker during pregnancy | 21,769 (5.09) | 19,833 (5.09) | 1,936 (5.11) |
| Missing | 44 (0.01) | 42 (0.01) | 2 (0.01) |
| Parity (n, %) | |||
| Primiparous | 176,862 (41.34) | 156,316 (40.08) | 20,546 (54.27) |
| Multiparous | 250,444 (58.53) | 233,172 (59.79) | 17,272 (45.62) |
| Missing | 564 (0.13) | 525 (0.13) | 39 (0.10) |
| Season of conception Cool (November-April) | 216,761 (50.66) | 197,169 (50.55) | 19,592 (51.75) |
| Warm (May-October) | 211,109 (49.34) | 192,844 (49.45) | 18,265 (48.25) |
| History of PROM | |||
| Never having PROM | 418,384 (97.78) | 381,693 (97.87) | 36,691 (96.92) |
| Having PROM before | 9,486 (2.22) | 8,320 (2.13) | 1,166 (3.08) |
| Income level, US Dollars (mean ± SD) | 59,664 (21,801) | 59,652 (21,804) | 59,784 (21,766) |
Note: There were 1,359 (0.32%), 1,279 (0.33%), and 80 (0.21%) pregnancies with missing data on the income level among total pregnancies, non-SPROM cases, and SPROM cases, respectively. Abbreviation: BMI, body mass index; SD, standard deviation; SPROM, spontaneous premature rupture of membranes.
Table 2 shows the distribution of exposure levels during pregnancy for the study population. The average levels of entire-pregnancy exposure to NO2, O3, PM2.5, and PM10 were 15.62 ppb, 44.12 ppb, 11.63 μg/m3, and 28.65 μg/m3, respectively. Table S1 describes Pearson correlation coefficients between air pollution exposure throughout pregnancy. NO2 was negatively correlated with O3 (correlation coefficient r = − 0.39) and moderately correlated with PM2.5 and its components (0.55 ≤ r ≤ 0.66), except for PM2.5 sulfate (r = 0.08). O3 was moderately correlated with PM2.5 sulfate (r = 0.38), while other correlations were relatively weaker. PM2.5 exposure measurements based on the EBK model and the geoscience-derived model were highly correlated with each other (r = 0.83) and both were moderately to highly correlated with PM2.5 components (0.43 ≤ r ≤ 0.91). Those correlations were similar to our previous findings for the same population (Sun et al., 2021).
Table 2.
Summary statistics (Mean ± SD) of the average exposure to air pollutants and PM2.5 total mass and components throughout pregnancy for the study population.
| Air pollutants | Total pregnancies | Non-SPROMs | SPROMs |
|---|---|---|---|
|
| |||
| Krigged NO2, ppb | 15.62 ± 4.21 | 15.64 ± 4.23 | 15.46 ± 4.06 |
| Krigged O3, ppb | 44.12 ± 6.80 | 44.04 ± 6.79 | 44.94 ± 6.88 |
| Krigged PM2.5, μg/m3 | 11.63 ± 2.37 | 11.64 ± 2.38 | 11.48 ± 2.21 |
| Krigged PM10, μg/m3 | 28.65 ± 5.64 | 28.69 ± 5.69 | 28.31 ± 5.13 |
| PM2.5 total mass, μg/m3 | 12.88 ± 2.64 | 12.88 ± 2.65 | 12.81 ± 2.53 |
| PM2.5 sulfate, μg/m3 | 1.28 ± 0.30 | 1.28 ± 0.30 | 1.27 ± 0.32 |
| PM2.5 nitrate, μg/m3 | 2.41 ± 0.66 | 2.41 ± 0.66 | 2.44 ± 0.63 |
| PM2.5 ammonium, μg/m3 | 0.95 ± 0.32 | 0.95 ± 0.32 | 0.93 ± 0.30 |
| PM2.5 organic matter, μg/m3 | 5.39 ± 1.34 | 5.40 ± 1.34 | 5.30 ± 1.29 |
| PM2.5 black carbon, μg/m3 | 1.49 ± 0.62 | 1.49 ± 0.62 | 1.42 ± 0.64 |
Note: The exposure to four krigged air pollutants was measured based on empirical Bayesian kriging. The exposure to PM2.5 total mass and components was measured based on a fine-resolution geoscience-derived model. Abbreviation: ppb, parts per billion; SD, standard deviation; SPROM, spontaneous premature rupture of membranes.
Adjusted pooled ORs and corresponding 95% CIs of SPROM associated with air pollution exposure in trimesters and entire pregnancy based on the single-pollutant model are shown in Table 3. Throughout pregnancy, 2–7% higher risks of SPROM were related to per IQR increase in exposure to krigged NO2, O3, PM2.5, and PM10. The strongest associations were found for O3 and PM2.5, followed by NO2 and PM10. In terms of trimester-specific results, associations with NO2 and O3 were observed in the first and third trimesters, while associations with PM2.5 were observed in the second and third trimesters. The trimester-specific estimates for PM10 exposure were not statistically precise. Results for PM2.5 components showed similar trends with PM2.5 exposure (Table 3). Every IQR increase in sulfate, nitrate, ammonium, and organic matter throughout the pregnancy was associated with 6%−8% higher risks of SPROM in the single-constituent model, except black carbon (1%, 95% CI: − 2%, 4%). Trimester-specific results showed relatively stronger associations in the second trimester for all PM2.5 components.
Table 3.
Adjusted pooled odds ratios (95% confidence intervals) of spontaneous premature rupture of membranes associated with air pollution exposure during trimesters and throughout the pregnancy in the single-pollutant model.
| Air pollutants | Exposure windows |
|||
|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | Entire pregnancy | |
|
| ||||
| Krigged NO2 | 1.05 (1.03, 1.08) | 0.98 (0.95, 1.01) | 1.07 (1.05, 1.09) | 1.06 (1.03, 1.09) |
| Krigged O3 | 1.04 (1.02, 1.05) | 1.00 (0.99, 1.02) | 1.02 (1.01, 1.04) | 1.07 (1.05, 1.10) |
| Krigged PM2.5 | 1.01 (0.99, 1.03) | 1.04 (1.02, 1.06) | 1.02 (1.01, 1.04) | 1.07 (1.05, 1.09) |
| Krigged PM10 | 1.01 (1.00, 1.02) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.02) | 1.02 (1.00, 1.04) |
| PM2.5 total mass | 0.99 (0.97, 1.02) | 1.07 (1.04, 1.09) | 1.02 (1.00, 1.05) | 1.09 (1.06, 1.11) |
| PM2.5 sulfate | 1.03 (1.01, 1.05) | 1.04 (1.01, 1.06) | 1.04 (1.02, 1.06) | 1.08 (1.05, 1.12) |
| PM2.5 nitrate | 1.00 (0.99, 1.02) | 1.04 (1.03, 1.06) | 1.02 (1.00, 1.03) | 1.06 (1.04, 1.08) |
| PM2.5 ammonium | 1.01 (1.00, 1.03) | 1.04 (1.03, 1.06) | 1.01 (1.00, 1.03) | 1.07 (1.05, 1.09) |
| PM2.5 organic matter | 0.98 (0.96, 1.00) | 1.07 (1.04, 1.10) | 1.01 (0.99, 1.03) | 1.06 (1.04, 1.09) |
| PM2.5 black carbon | 1.00 (0.98, 1.03) | 1.01 (0.98, 1.05) | 1.01 (0.98, 1.04) | 1.01 (0.98, 1.04) |
Note: The exposure to four krigged air pollutants was measured based on empirical Bayesian kriging. The exposure to PM2.5 total mass and components was measured based on a fine-resolution geoscience-derived model. IQR increments are 6.69 ppb for NO2, 9.89 ppb for O3, 3.26 μg/m3 for PM2.5, 6.82 μg/m3 for PM10, 3.91 μg/m3 for PM2.5 total mass, 0.627 μg/m3 for PM2.5 sulfate, 0.946 μg/m3 for PM2.5 nitrate, 0.421 μg/m3 for PM2.5 ammonium, 1.99 μg/m3 for PM2.5 organic matter, and 1.05 μg/m3 for PM2.5 black carbon. All models were adjusted for maternal age, race/ethnicity, education level, income level, pre-pregnancy body mass index, smoking status, parity, year of infant birth, and season of conception.
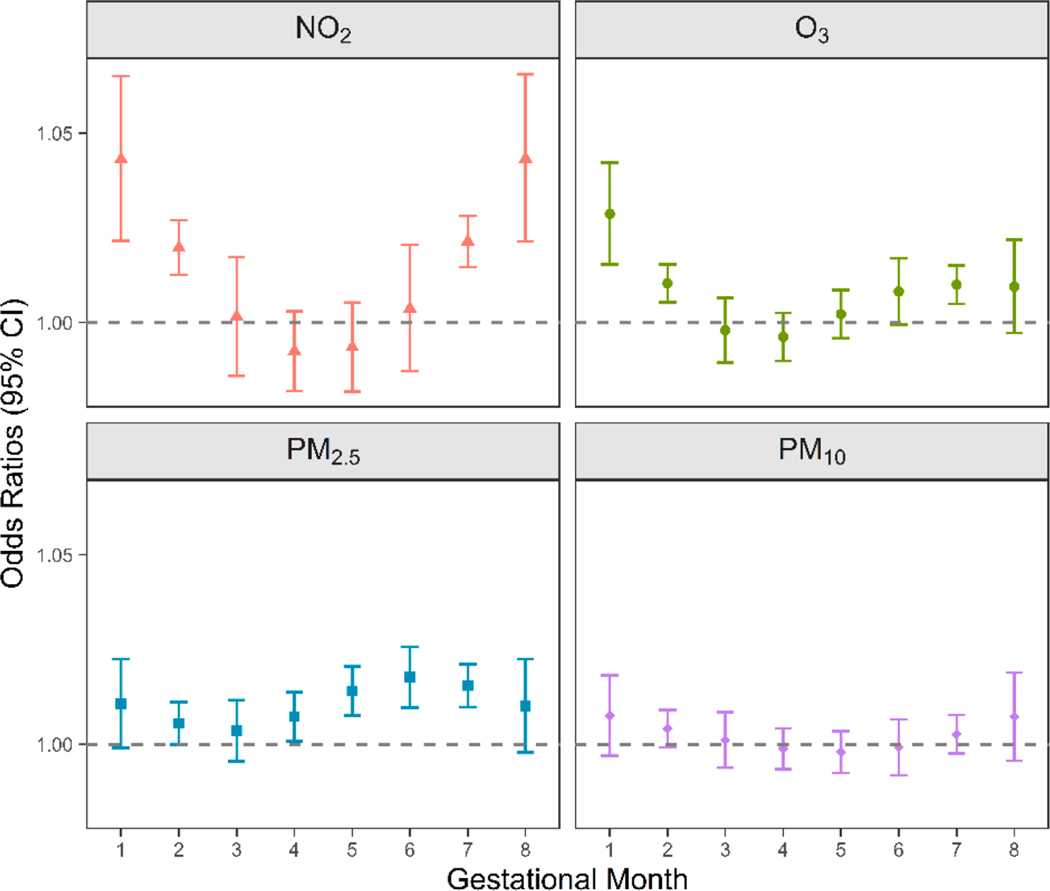
Fig. 1 shows the monthly associations between air pollution estimated by the EBK model and SPROM based on the DLMs. In general, the monthly associations and the trimester-specific results showed consistent critical exposure windows. Windows of increased risks associated with NO2 occurred at the beginning and the end of pregnancy (gestational months 1–2 and 7–8). The highest risk of SPROM was associated with O3 exposure in gestational month 1. For PM2.5, the period of increased risks was mainly identified from month 4 to month 7. For PM10, being consistent with the trimester-specific results, we did not observe any associations. Details on the effect sizes are provided in Table S2.
Fig. 1.
Monthly associations between maternal exposure to NO2, O3, PM2.5, and PM10 and spontaneous premature rupture of membranes in the distributed lag models (odds ratios [ORs] with 95% confidence intervals [CIs]). Exposure data were obtained from the empirical Bayesian kriging model. Models were adjusted for maternal age, race/ethnicity, education level, income level, pre-pregnancy body mass index, smoking status, parity, year of infant birth, and season of conception.
The results of mixture analyses based on the QGC model are shown in Table 4. Krigged NO2, O3, PM2.5, and PM10 as well as PM2.5 sulfate, nitrate, ammonium, and organic matter were included in the mixture analysis. Positive associations of SPROM were observed for both the krigged air pollution mixture and the PM2.5 mixture during the pregnancy. Each quartile increase in the mixture containing four krigged air pollutants was associated with 15% (95% CI: 12%, 18%) higher odds of SPROM. Increasing all four air pollutants at once by a quartile, the positive weights of associations with SPROM were mainly driven by O3 (49.6%), followed by PM2.5 (25.4%) and NO2 (25.0%), while PM10 was the only pollutant negatively and weakly associated with SPROM. Each quartile increase in the mixture containing four PM2.5 components was associated with 7% (95% CI: 5%, 9%) higher odds of SPROM. The positive weights of contributions for sulfate, nitrate, and organic matter were similar to each other, while ammonium was negatively associated with SPROM in this model.
Table 4.
Mixture effects on spontaneous premature rupture of membranes for one quartile increase in the air pollution mixture and PM2.5 mixture throughout the pregnancy based on quantile-based g computation.
| Mode | Contribution to positive/negative effect (%) | Positive/Negative coefficient (β)a | Overall mixture coefficient (β [95% CI])b | Overall mixture effect (OR [95% CI]) |
|---|---|---|---|---|
|
| ||||
| Model 1: Krigged air pollutants | ||||
| Positive mixturec | 0.14 (0.12, 0.17) | 1.15 (1.12, 1.18) | ||
| NO2 | 25.0 | 0.16 | (p <.001) | (p <.001) |
| O3 | 49.6 | |||
| PM2.5 | 25.4 | |||
| Negative mixtured | ||||
| PM10 | 100.0e | −0.02 | ||
| Model 2: PM2.5 components | ||||
| Positive mixturec | 0.07 (0.05, 0.09) | 1.07 (1.05, 1.09) | ||
| PM2.5 sulfate | 34.1 | 0.10 | (p <.001) | (p <.001) |
| PM2.5 nitrate | 43.3 | |||
| PM2.5 organic matter | 22.6 | |||
| Negative mixtured | ||||
| PM2.5 ammonium | 100.0e | −0.03 | ||
Note: The exposure to four krigged air pollutants was measured based on empirical Bayesian kriging. The exposure to PM2.5 components was measured based on a fine-resolution geoscience-derived model. Models were adjusted for maternal age, race/ethnicity, education level, income level, pre-pregnancy body mass index, smoking status, parity, year of infant birth, and season of conception. Abbreviation: CI, confidence interval; OR, odds ratio.
The coefficient for the positive or negative mixture.
The overall mixture coefficient is the sum of coefficients of the positive mixture and negative mixture.
The positive mixture includes pollutants positively associated with the outcome in the model.
The negative mixture includes pollutants negatively associated with the outcome in the model.
The contribution of the pollutant is 100.0% as it is the only pollutant negatively associated with the outcome in the model.
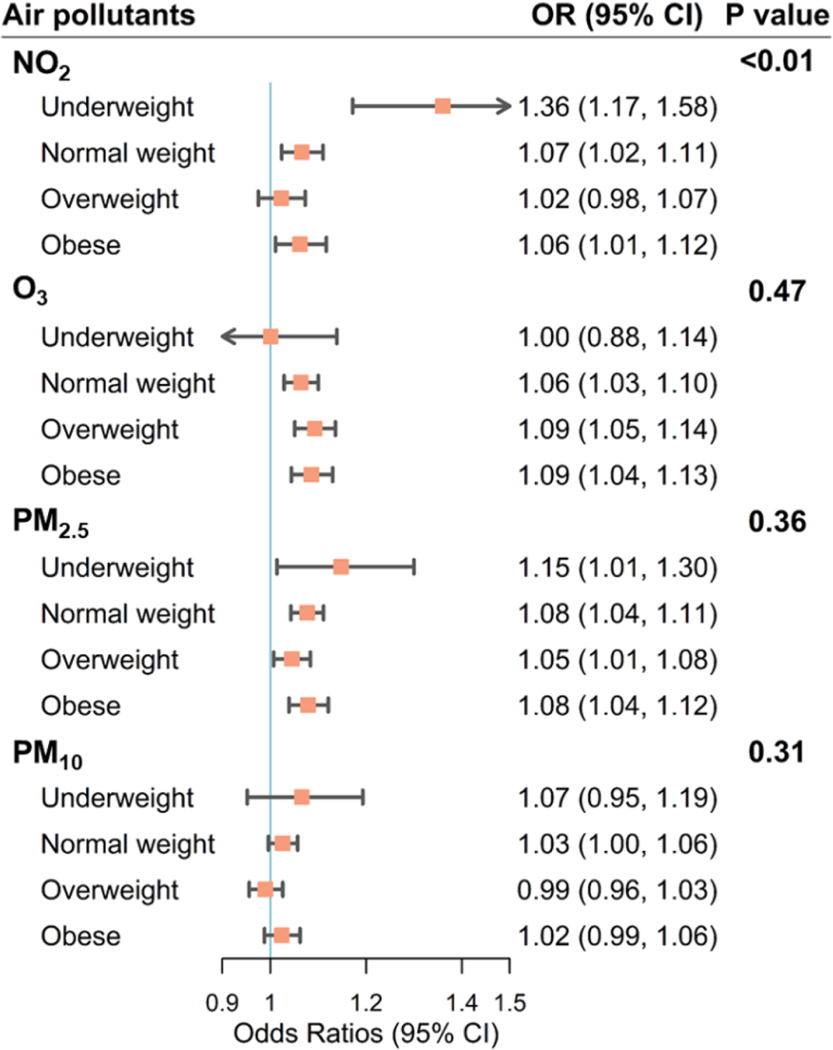
We conducted subgroup analyses stratified by pre-pregnancy BMI (Fig. 2 and Table S3). Significant associations were observed in most of the subgroups for different air pollutants, except PM10. The mothers with the lowest pre-pregnancy BMI (< 18.5 kg/m2) tended to have higher risks of SPROM associated with NO2 and PM2.5, compared with higher pre-pregnancy BMI subgroups. Each IQR increase in exposure to NO2 and PM2.5 in underweight mothers was associated with 36% (95% CI: 17%, 58%) and 15% (95% CI: 1%, 30%) higher odds of SPROM during the pregnancy, respectively. Significant heterogeneity in associations with NO2 exposure was detected across different BMI subgroups (p-value = 0.005).
Fig. 2.
Subgroup analyses of associations (odds ratios [ORs] with 95% confidence intervals [CIs]) between air pollution exposure throughout the pregnancy and spontaneous premature rupture of membranes stratified by pre-pregnancy body mass index (BMI), kg/m2, where underweight refers to pre-pregnancy BMI < 18.5, normal weight refers to 18.5 ≤ pre-pregnancy BMI ≤ 24.9, overweight refers to 25.0 ≤ pre-pregnancy BMI ≤ 29.9, and obese refers to pre-pregnancy BMI ≥ 30.0. Exposure data were obtained from the empirical Bayesian kriging model. Models are adjusted for maternal age, race/ethnicity, education level, income level, smoking status, parity, year of infant birth, and season of conception. The p-value refers to the comparison among BMI subgroups and is obtained from Cochran’s Q test.
Sensitivity analyses are shown in Tables S4–S10. Trimester-specific associations based on the single-trimester models are shown in Table S4. Associations with exposure to each air pollutant and PM2.5 components in the last one and three gestational months (Table S5) were similar to estimates for the third trimester shown in Table 3. Associations with NO2 and PM2.5 in later pregnancy were slightly higher compared to associations with O3. The associations for entire pregnancy exposure were robust among different models in the sensitivity analyses (Table S5). The WQS and QGC analyses showed similar results (Table S6). A quartile increment in the air pollution mixture of NO2, O3, PM2.5, and PM10 resulted in 13% (95% CI: 10%, 17%) higher risk of SPROM in the WQS model and the highest weighted pollutant was O3 (weight: 51%). For PM2.5 components, the OR for each quartile increase in the mixture of nitrate, sulfate, ammonium, and organic matter was 1.08 (95% CI: 1.05, 1.11) and sulfate contributed most to the association (weight: 46%). Adding the PM2.5 total mass in the model for the mixture analyses of PM2.5 components did not change our conclusions (Table S7). The results of entire-pregnancy associations changed minimally by performing co-pollutant models (Table S8-S9), while the associations with NO2 and with PM2.5 nitrate were attenuated to be insignificant after adjusting for PM2.5 and PM2.5 ammonium, respectively. This was likely due to moderate to high correlations between PM2.5 and NO2 (r = 0.61) and between PM2.5 nitrate and PM2.5 ammonium (r = 0.80). For effect modification by pre-pregnancy BMI evaluated in the interaction model, underweight mothers were at higher risks of NO2- and PM2.5-related SPROM (Table S10).
4. Discussion
In a large retrospective cohort of 427,870 births with detailed individual-level EHRs in the KPSC healthcare system from 2008 to 2018, our results suggested that maternal exposure to NO2, O3, and PM2.5 was consistently associated with increased risks of SPROM, and the critical exposure windows varied for each air pollutant: the first and third trimesters for NO2 and O3 and the latter two trimesters for PM2.5. The mixture analysis showed that, among air pollutants examined in this study, the overall adverse effect on SPROM was mostly driven by O3, followed by PM2.5 and NO2. In terms of PM2.5 components, sulfate, nitrate, and organic matter contributed to the most SPROM risk. Mothers with lower pre-pregnancy BMI were more susceptible to SPROM related to air pollution, especially to NO2.
PROM is a common pregnancy complication arising from complicated pathophysiological factors and it has attracted growing interest for its association with elevated levels of ambient air pollution (Esteves, 2022; Goldenberg et al., 2008). To date, evidence of air pollution effects is still very limited and remains inconclusive to a large extent, with five studies conducted in China (Li et al., 2021; Song et al., 2019; Wang et al., 2019; Wu et al., 2023; Zhang et al., 2021), two in the United States (Pereira et al., 2016; Wallace et al., 2016), one in Spain (Dadvand et al., 2014), and another one in Australia (Pereira et al., 2014). Dadvand et al. first linked air pollution exposure and preterm PROM risks (Dadvand et al., 2014). They performed a matched case-control study on a hospital-based cohort with 5,555 singleton births in Spain and observed adverse effects of NO2 and NOx during the entire pregnancy. NO2 often serves as a widely used marker of complex traffic pollution (Durant et al., 2014). Similarly, our study identified significant associations for NO2, especially at the beginning and the end of pregnancy, based on a larger cohort in Southern California. There is only one other study examining long-term exposure to NOx, but that retrospective cohort study did not replicate findings regarding NOx exposure during the whole pregnancy (Wallace et al., 2016). The authors suggested that different levels of NOx pollution might be a possible explanation for the inconsistency, as the median NOx concentration was 28.9 μg/m3 in their study, while the median level was much higher in the study from Spain (i.e., 102.6 μg/m3) (Dadvand et al., 2014; Wallace et al., 2016). However, our study offered evidence in the context of lower pollution. The different characteristics of study populations, such as the population composition and susceptibility, might lead to possible discrepancies. As few studies analyzing these relationships have been reported, further investigations are necessary to confirm our findings.
Prior studies mainly examined the short-term effects of O3 on PROM. Song et al. carried out a time series study in Xinxiang, China, and included 3,255 PROM admissions from 2015 to 2017 (Song et al., 2019). They found that higher exposure to O3 at lag 1, 2, 0–1, 0–2, 0–3, and 0–4 significantly increased PROM admissions, and the strongest effect was observed at lag 0–2 (5.42% [95% CI: 1.45%, 9.39%] changes in hospitalization per 10 μg/m3 increase in daily O3–8h concentration). Following this study, Zhang et al. reported a similar but slightly smaller effect of O3 in their time series research conducted in Hefei, China (Zhang et al., 2021). In addition, a retrospective cohort study in the United States observed very acute effects of O3 exposure several hours (1–5) before the delivery, while no association related to entire-pregnancy exposure was observed (Wallace et al., 2016). However, they used the delivery admission time as a proxy for the time that PROM occurred due to data unavailability. Even though most of the women would deliver their babies soon after the PROM, some mothers will experience a prolonged PROM (PROM greater than 24 h) (about 14% in our study population) and deliver their babies even after several weeks from PROM occurrence (Caughey et al., 2008). Neglecting the time interval between the PROM event and delivery may lead to bias, especially for the study on acute effects focusing on hourly or daily associations. Based on the rich clinical database from the KPSC healthcare system, we were able to obtain the accurate dates of PROM diagnosis and exclude non-spontaneous PROMs in our study and first observed long-term associations for O3 during the whole pregnancy, especially in the first trimester. According to the results from the QGC model, O3 was the highest positively weighted air pollutant associated with SPROM in the present study, indicating that O3 might be the most harmful component when the concentration level of four air pollutants increased simultaneously. Some previous studies also reported long- or short-term relationships between maternal exposure to carbon monoxide and sulfur dioxide and PROM (Li et al., 2021; Wallace et al., 2016; Wu et al., 2023; Zhang et al., 2021). The contribution to positive associations with PROM may vary accordingly for their study populations due to different exposure profiles. Thus, more studies from different regions or countries are warranted to identify the most important air pollutants for corresponding public interventions.
PM2.5 is the criteria pollutant of the greatest public concern in California due to its high emission level and great health impacts (Anderson et al., 2018). There have been several papers examining the long-(Dadvand et al., 2014; Pereira et al., 2014, 2016; Wallace et al., 2016; Wang et al., 2019; Wu et al., 2023) or short-term (Li et al., 2021; Zhang et al., 2021) relationships between PM2.5 and PROM, and conclusions remain mixed possibly due to differential study designs, populations, or PM2.5 chemical compositions. Pereira et al. conducted a longitudinal study in an Australian cohort and reported a 3% (95% CI: 0%, 6%) higher risk of PROM per 1 μg/m3 increase in the second-trimester exposure (Pereira et al., 2014), while they did not replicate the results in another study in New York (Pereira et al., 2016). Dadvand et al. and Wallace et al. also did not find associations for PM2.5 or PM10 (Dadvand et al., 2014; Wallace et al., 2016). By contrast, a study in Wuhan, China, demonstrated more conclusive associations across trimesters in the context of more severe PM2.5 pollution and reported a 9%−35% higher risk of PROM per 10 μg/m3 increase in PM2.5 exposure (Wang et al., 2019). However, their study may suffer from a relatively smaller sample size and underlying measurement error, since it included only about 5,000 singleton births from a single hospital and used air pollution measurement from a single ground-based monitoring station as a proxy for personal exposure without considering maternal residential changes in the study period. In our study, we included mothers from a large pregnancy cohort spanning 10 counties that covers 146,350 km2 area of California. Based on air pollution data with a higher spatial resolution, higher SPROM risks were observed with increased PM2.5 exposure in the second and third trimesters and throughout the pregnancy, further strengthening the existing evidence of associations for PM2.5.
To have a further understanding of the PM2.5 effect, we estimated the associations for some PM2.5 components. In the single-component model, sulfate, nitrate, ammonium, and organic matter showed comparable estimates with the result of PM2.5 total mass. Considering any potential interaction of the intake of PM2.5 components (Li et al., 2022), we applied the QGC model for multiple components and noticed that the effect of the PM2.5 mixture was mainly dominated by nitrate, sulfate, and organic matter. Although ammonium was positively associated with SPROM in the single-pollutant model, it did not show a similar effect in the mixture analysis. As the levels of nitrate and ammonium were highly correlated (r = 0.80), we were unable to determine the independent impact for each of them by only conducting the single-pollutant analysis. The QGC approach can leverage the correlation among exposures (Keil et al., 2020). The positive association of SPROM with nitrate but a negative association with ammonium in the QGC model indicates that nitrate may play a more crucial role in the SPROM risk, while ammonium is likely a surrogate of nitrate in the single-pollutant model (Dadvand et al., 2014). Interestingly, we did not find a relationship between black carbon and SPROM during any exposure windows in our study, which is inconsistent with the study conducted in Barcelona, Spain, that found an association between PM2.5 light absorbance and preterm PROM (Dadvand et al., 2014). Sulfate, nitrate, and ammonium are soluble ions and constitute the secondary inorganic aerosols formed by the photochemical transformation of precursor pollutants mainly produced by coal burning and vehicle exhaust (Cai et al., 2020; Cyrys et al., 2003; Weagle et al., 2018; Zhang et al., 2015). Black carbon is a typical primary pollutant and usually serves as an important indicator of traffic-related particles, as its emission has been strongly associated with diesel engines (Andrew Gray and Cass, 1998; Cyrys et al., 2003; Zhang et al., 2017). Those ingredients of PM2.5 have been linked to systematic inflammation, oxidative stress, and coagulation in previous studies (Chuang et al., 2007; Lei et al., 2019; Liu et al., 2017; Wu et al., 2012). A recent experimental study found evidence of particle translocation and reported black carbon accumulating on the fetal side of the placenta (Bove et al., 2019). Black carbon exposure has also been linked to preterm births. (Basu et al., 2017; Cai et al., 2020; Laurent et al., 2016b; Wilhelm et al., 2011). As PROM is an important contributor to preterm births (Esteves, 2022; Goldenberg et al., 2008), understanding the relationships between PM2.5 components and PROM to identify the most hazardous constituents may help to further clarify their effects on preterm births. Our findings may be informative for future research to identify the critical sources of ambient PM2.5 pollution and thus to provide some references for emission controls and mitigation strategies.
Trimester- and gestational month-specific results demonstrated the susceptible windows depending on exposure to different air pollutants. We observed a more important role for O3 in early pregnancy, for PM2.5 in mid to late pregnancy, and for NO2 in early and late pregnancy. Researchers have suggested that amniotic membranes grow rapidly with the production of collagen during the first half of pregnancy and stretch in the rest of the pregnancy period to provide more space for fetal growth. The pathogenesis of membrane ruptures has been reported to be associated with the reduction, degradation, damage, or deficiency of chorioamniotic collagen (MacDermott and Landon, 2000; Stuart et al., 2005). Previous studies have linked reactive oxygen species (ROS) to the biological changes in the chorionic and amniotic membranes (Wall et al., 2002; Woods, 2001). Subsequent oxidative stress induced by excessive ROS can cause tearing in membranes by impairing the integrity of collagen (i.e., strength and elasticity) and lead to PROM as a result of collagen damage (Aponte and Agarwal, 2013; Huang et al., 2018; Longini et al., 2007). As a reactive form of oxygen gas, O3 can cause serious oxidative stress damage, induce inflammation responses and cell apoptosis, then disturb the production of collagen or affect the fluidity of membranes, thereby leading to PROM (Gervasi et al., 2002; Huang et al., 2018; Kataoka et al., 2002; Parry and Strauss, 1998; Song et al., 2019; Wallace et al., 2016). Exposure to PM2.5 or NO2 can also promote oxidative stress or inflammation to weaken the structure of membranes or impair placental functions (Li et al., 2017; Li et al., 2019; Shah and Balkhair, 2011).
Our stratified analysis indicates that mothers with the lowest pre-pregnancy BMI can be more vulnerable to the effect of air pollution, especially for NO2, even though the results for those underweight mothers might be less precise (e.g., with wider CIs) compared to results of other subgroups due to a smaller sample size (10,453 underweight mothers, 2.44% of the entire study population). With the interaction term, the heterogeneity of SPROM risk among different BMI groups for NO2 exposure was attenuated to be marginally significant (p-value = 0.108). Pre-pregnancy BMI is a potential risk factor for SPROM, and lower BMI may be associated with a lack of micro and macronutrients that may amplify the vulnerability to air pollution exposure during pregnancy (Caughey et al., 2008; Esteves, 2022). Women who are planning for a pregnancy should pay more attention to their living environment (e.g., taking measures to prevent adverse air pollution) as well as pre-conception conditions (e.g., keeping BMI ≥ 18.5 kg/m2) to avoid higher risks of adverse pregnancy outcomes.
Our study had some unique strengths. First, to the best of our knowledge, this is the first study examining associations between PM2.5 chemical components and SPROM, which may have important intervention implications in source-specific emission control of particulate matter pollution. Second, we collected important characteristics and high-quality medical and obstetrical information on mothers from the large and diverse pregnancy cohort of the KPSC healthcare system, including exact dates of PROM occurrence with its types (spontaneous/non-spontaneous) and maternal residential changes with the accurate address history. More than 40% of participants moved during their pregnancies and exposure assessment irrespective of such changes may result in more bias (Sun et al., 2021). Third, we investigated the effect modification of pre-pregnancy BMI to provide a reference for pre-pregnancy care.
Limitations in this study must be acknowledged. First, although residential mobility was considered in this study, using ambient pollutant levels as a proxy for personal exposure may lead to exposure misclassification. We were unable to take indoor air pollution and maternal time-activity patterns into account due to data unavailability. Besides, residual confounding resulting from other factors that were not controlled may exist in this study. Second, we used rough definitions of gestational months and trimesters and did not examine narrower exposure windows or any acute effects of air pollution exposure, including weekly or daily associations, as we obtained only monthly air pollution data. Our trimester-specific results may not be accurate enough based on monthly exposure data. Given the acute effects of air pollution detected in earlier research, both chronic and acute associations merit further examination with the air pollution data in a finer temporal resolution (e.g., daily air pollutant concentrations). Moreover, as only five PM2.5 components were included in this study, potential associations between other components (i.e., heavy metals in PM2.5) and SPROM merit further investigations.
5. Conclusions
The findings of this retrospective cohort study in Southern California add to the literature on associations between maternal exposure to NO2, O3, PM2.5, and PM10 and spontaneous PROM and corresponding critical susceptible windows. O3 appeared to be more harmful among the four air pollutants of interest and the effects of PM2.5 were mainly driven by nitrate, sulfate, and organic matter in this study. As air pollution mixture varies regionally, more studies are warranted in different locations to replicate previous findings and explore more air pollutants or PM2.5 components.
Supplementary Material
Acknowledgment
This study was supported by the National Institute of Environmental Health Sciences (NIEHS; R01ES030353).
Footnotes
CRediT authorship contribution statement
Anqi Jiao: Methodology, Software, Data curation, Formal analysis, Writing – original draft. Yi Sun: Methodology, Software, Data curation, Writing – review & editing. Chantal Avila: Data curation, Writing – review & editing. Vicki Chiu: Software, Data curation. John Molitor: Writing – review & editing. Jeff Slezak: Writing – review & editing. David A. Sacks: Writing – review & editing. Jiu-Chiuan Chen: Writing – review & editing. Tarik Benmarhnia: Methodology, Software, Writing – review & editing. Darios Getahun: Conceptualization, Supervision, Project administration, Funding acquisition, Methodology, Data curation, Writing – review & editing. Jun Wu: Conceptualization, Supervision, Project administration, Funding acquisition, Methodology, Data curation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2023.108030.
Data availability
The data that has been used is confidential.
References
- Anderson CM, Kissel KA, Field CB, Mach KJ, 2018. Climate change mitigation, air pollution, and environmental justice in California. Environ. Sci. Tech. 52, 10829–10838. [DOI] [PubMed] [Google Scholar]
- Andrew Gray H, Cass GR, 1998. Source contributions to atmospheric fine carbon particle concentrations. Atmos. Environ. 32, 3805–3825. [Google Scholar]
- Aponte A, Agarwal A, 2013. Premature Rupture of Membranes and Oxidative Stress. Studies on Women’s Health. Springer. [Google Scholar]
- Assefa NE, Berhe H, Girma F, Berhe K, Berhe YZ, Gebreheat G, Werid WM, Berhe A, Rufae HB, Welu G, 2018. Risk factors of premature rupture of membranes in public hospitals at Mekele city, Tigray, a case control study. BMC Pregn. Childbirth 18, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Pearson D, Ebisu K, Malig B, 2017. Association between PM2.5 and PM2.5 constituents and preterm delivery in California, 2000–2006. Paediatr. Perinat. Epidemiol. 31, 424–434. [DOI] [PubMed] [Google Scholar]
- Bove H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, Van Eyken P, Plusquin M, Roeffaers MBJ, Ameloot M, Nawrot TS, 2019. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 10, 3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Kan J, Chen R, Martin R, van Donkelaar A, Ao J, Zhang J, Kan H, Hua J, 2020. Prenatal exposure to specific PM2.5 chemical constituents and preterm birth in China: a nationwide cohort study. Environ. Sci. Tech. 54, 14494–14501. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Robinson JN, Norwitz ER, 2008. Contemporary diagnosis and management of preterm premature rupture of membranes. Rev. Obstet. Gynecol. 1, 11–22. [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS, 2007. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am. J. Respir. Crit. Care Med. 176, 370–376. [DOI] [PubMed] [Google Scholar]
- Cyrys J, Heinrich J, Hoek G, Meliefste K, Lewne M, Gehring U, Bellander T, Fischer P, van Vliet P, Brauer M, Wichmann HE, Brunekreef B, 2003. Comparison between different traffic-related particle indicators: elemental carbon (EC), PM2.5 mass, and absorbance. J. Expo. Anal. Environ. Epidemiol. 13, 134–143. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Basagaña X, Figueras F, Martinez D, Beelen R, Cirach M, de Nazelle A, Hoek G, Ostro B, Nieuwenhuijsen MJ, 2014. Air pollution and preterm premature rupture of membranes: a spatiotemporal analysis. Am. J. Epidemiol. 179, 200–207. [DOI] [PubMed] [Google Scholar]
- Durant JL, Beelen R, Eeftens M, Meliefste K, Cyrys J, Heinrich J, Bellander T, Lewne M, Brunekreef B, Hoek G, 2014. Comparison of ambient airborne PM(2). (5), PM(2).(5) absorbance and nitrogen dioxide ratios measured in 1999 and 2009 in three areas in Europe. Sci. Total Environ. 487, 290–298. [DOI] [PubMed] [Google Scholar]
- Ebisu K, Bell ML, 2012. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environ. Health Perspect. 120, 1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves JS, 2022. Premature rupture of membranes. In: Moreira de Sá RA, Fonseca E. B.d. (Eds.), Perinatology: Evidence-Based Best Practices in Perinatal Medicine. Springer International Publishing, Cham. [Google Scholar]
- Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, Kim JC, Kim YM, Yoshimatsu J, Espinoza J, Romero R, 2002. Maternal intravascular inflammation in preterm premature rupture of membranes. J. Matern. Fetal Neonatal Med. 11, 171–175. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R, 2008. Epidemiology and causes of preterm birth. Lancet 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mesa E, Blasco-Alonso M, Benitez MJ, Gomez-Munoz C, Sabonet-Morente L, Gomez-Castellanos M, Ulloa O, Gonzalez-Cazorla E, Puertas-Prieto A, Mozas-Moreno J, Jimenez-Lopez J, Lubian-Lopez D, 2021. Obstetric and Perinatal Outcomes after Very Early Preterm Premature Rupture of Membranes (PPROM)-A Retrospective Analysis over the Period 2000–2020. Medicina (Kaunas). 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Xia W, Sheng X, Qiu L, Zhang B, Chen T, Xu S, Li Y, 2018. Maternal lead exposure and premature rupture of membranes: a birth cohort study in China. BMJ Open 8, e021565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao A, Sun Y, Sacks DA, Avila C, Chiu V, Molitor J, Chen J-C, Sanders KT, Abatzoglou JT, Slezak J, Benmarhnia T, Getahun D, Wu J, 2023. The role of extreme heat exposure on premature rupture of membranes in southern California: a study from a large pregnancy cohort. Environ. Int, 107824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Furuta I, Yamada H, Kato EH, Ebina Y, Kishida T, Kobayashi N, Fujimoto S, 2002. Increased apoptosis of human fetal membranes in rupture of the membranes and chorioamnionitis. Placenta 23, 224–231. [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ, 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 128, 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, Escobedo L, Wu J, 2016a. Low birth weight and air pollution in California: Which sources and components drive the risk? Environ. Int. 92–93, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, Escobedo L, Wu J, 2016b. A statewide nested case-control study of preterm birth and air pollution by source and composition: California, 2001–2008. Environ. Health Perspect. 124, 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Chen R, Wang C, Shi J, Zhao Z, Li W, Yan B, Chillrud S, Cai J, Kan H, 2019. Personal fine particulate matter constituents, increased systemic inflammation, and the role of DNA hypomethylation. Environ. Sci. Tech. 53, 9837–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu JJ, He YC, Chen L, Dennis CL, Huang HF, Wu YT, 2021. Effects of acute ambient pollution exposure on preterm prelabor rupture of membranes: a time-series analysis in Shanghai, China. Environ. Pollut. 276, 116756. [DOI] [PubMed] [Google Scholar]
- Li G, Su W, Zhong Q, Hu M, He J, Lu H, Hu W, Liu J, Li X, Hao J, Huang F, 2022. Individual PM2.5 component exposure model, elevated blood pressure and hypertension in middle-aged and older adults: a nationwide cohort study from 125 cities in China. Environ. Res. 215, 114360. [DOI] [PubMed] [Google Scholar]
- Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, Xue X, Chu Y, Liu F, Liu Y, Ren M, Chen X, Li N, Lu Y, Mao Z, Tian L, Xiang H, 2017. Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ. Pollut. 227, 596–605. [DOI] [PubMed] [Google Scholar]
- Li Z, Tang Y, Song X, Lazar L, Li Z, Zhao J, 2019. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 169, 248–254. [DOI] [PubMed] [Google Scholar]
- Liu C, Cai J, Qiao L, Wang H, Xu W, Li H, Zhao Z, Chen R, Kan H, 2017. The acute effects of fine particulate matter constituents on blood inflammation and coagulation. Environ. Sci. Tech. 51, 8128–8137. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, Coelho MSZS, Saldiva PHN, Lavigne E, Matus P, Valdes Ortega N, Osorio Garcia S, Pascal M, Stafoggia M, Scortichini M, Hashizume M, Honda Y, Hurtado-Díaz M, Cruz J, Nunes B, Teixeira JP, Kim H, Tobias A, Íñiguez C, Forsberg B, Åström C, Ragettli MS, Guo Y-L, Chen B-Y, Bell ML, Wright CY, Scovronick N, Garland RM, Milojevic A, Kyselý J, Urban A, Orru H, Indermitte E, Jaakkola JJK, Ryti NRI, Katsouyanni K, Analitis A, Zanobetti A, Schwartz J, Chen J, Wu T, Cohen A, Gasparrini A, Kan H, 2019. Ambient particulate air pollution and daily mortality in 652 cities. New Engl. J. Med. 381, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu M, Song L, Bi J, Wang L, Chen K, Liu Q, Xiong C, Cao Z, Li Y, Xia W, Xu S, Wang Y, 2021. Association between prenatal rare earth elements exposure and premature rupture of membranes: Results from a birth cohort study. Environ. Res. 193, 110534. [DOI] [PubMed] [Google Scholar]
- Longini M, Perrone S, Vezzosi P, Marzocchi B, Kenanidis A, Centini G, Rosignoli L, Buonocore G, 2007. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin. Biochem. 40, 793–797. [DOI] [PubMed] [Google Scholar]
- Lyons P, McLaughlin N, 2020. Premature rupture of membranes. In: Lyons P, McLaughlin N (Eds.), Obstetrics in Family Medicine: A Practical Guide. Springer International Publishing, Cham. [Google Scholar]
- MacDermott RI, Landon CR, 2000. The hydroxyproline content of amnion and prelabour rupture of the membranes. Eur. J. Obstet. Gynecol. Reprod. Biol. 92, 217–221. [DOI] [PubMed] [Google Scholar]
- Meng J, Li C, Martin RV, van Donkelaar A, Hystad P, Brauer M, 2019. Estimated long-term (1981–2016) concentrations of ambient fine particulate matter across north america from chemical transport modeling, satellite remote sensing, and ground-based measurements. Environ. Sci. Tech. 53, 5071–5079. [DOI] [PubMed] [Google Scholar]
- Menon R, Richardson LS, 2017. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin. Perinatol. 41, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer BM, 2003. Preterm premature rupture of the membranes. Obstet. Gynecol. 101, 178–193. [DOI] [PubMed] [Google Scholar]
- Murray EJ, Caniglia EC, Petito LC, 2020. Causal survival analysis: a guide to estimating intention-to-treat and per-protocol effects from randomized clinical trials with non-adherence. Res. Methods Med. Health Sci. 2, 39–49. [Google Scholar]
- Parry S, Strauss JF, 1998. Premature rupture of the fetal membranes. New Engl. J. Med. 338, 663–670. [DOI] [PubMed] [Google Scholar]
- Pereira G, Bell ML, Belanger K, de Klerk N, 2014. Fine particulate matter and risk of preterm birth and pre-labor rupture of membranes in Perth, Western Australia 1997–2007: a longitudinal study. Environ. Int. 73, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G, Evans KA, Rich DQ, Bracken MB, Bell ML, 2016. Fine particulates, preterm birth, and membrane rupture in Rochester, NY. Epidemiology 27, 66–73. [DOI] [PubMed] [Google Scholar]
- Qiao L, Cai J, Wang H, Wang W, Zhou M, Lou S, Chen R, Dai H, Chen C, Kan H, 2014. PM2.5 constituents and hospital emergency-room visits in Shanghai, China. Environ. Sci. Technol. 48, 10406–10414. [DOI] [PubMed] [Google Scholar]
- Schmidt S, 2020. Quantile g-computation: a new method for analyzing mixtures of environmental exposures. Environ. Health Perspect. 128, 104004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, Balkhair T, 2011. Knowledge synthesis group on determinants of preterm, L. B.W.b. Air pollution and birth outcomes: a systematic review. Environ. Int. 37, 498–516. [DOI] [PubMed] [Google Scholar]
- Sheridan P, Ilango S, Bruckner TA, Wang Q, Basu R, Benmarhnia T, 2019. Ambient fine particulate matter and preterm birth in California: identification of critical exposure windows. Am. J. Epidemiol. 188, 1608–1615. [DOI] [PubMed] [Google Scholar]
- Song J, Lu M, An Z, Liu Y, Zheng L, Li Y, Chao L, Xu D, Yao S, Wu W, 2019. Estimating the acute effects of ambient ozone pollution on the premature rupture of membranes in Xinxiang, China. Chemosphere 227, 191–197. [DOI] [PubMed] [Google Scholar]
- Stuart EL, Evans GS, Lin YS, Powers HJ, 2005. Reduced collagen and ascorbic acid concentrations and increased proteolytic susceptibility with prelabor fetal membrane rupture in women. Biol. Reprod. 72, 230–235. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li X, Benmarhnia T, Chen JC, Avila C, Sacks DA, Chiu V, Slezak J, Molitor J, Getahun D, Wu J, 2021. Exposure to air pollutant mixture and gestational diabetes mellitus in Southern California: Results from electronic health record data of a large pregnancy cohort. Environ. Int. 158, 106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sheridan P, Laurent O, Li J, Sacks DA, Fischer H, Qiu Y, Jiang Y, Yim IS, Jiang LH, Molitor J, Chen JC, Benmarhnia T, Lawrence JM, Wu J, 2020. Associations between green space and preterm birth: Windows of susceptibility and interaction with air pollution. Environ. Int. 142, 105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchirikov M, Schlabritz-Loutsevitch N, Maher J, Buchmann J, Naberezhnev Y, Winarno AS, Seliger G, 2018. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J. Perinat. Med. 46, 465–488. [DOI] [PubMed] [Google Scholar]
- van den Dries MA, Keil AP, Tiemeier H, Pronk A, Spaan S, Santos S, Asimakopoulos AG, Kannan K, Gaillard R, Guxens M, Trasande L, Jaddoe VWV, Ferguson KK, 2021. Prenatal exposure to nonpersistent chemical mixtures and fetal growth: a population-based study. Environ. Health Perspect. 129, 117008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Li C, Burnett RT, 2019. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ. Sci. Tech. 53, 2595–2611. [DOI] [PubMed] [Google Scholar]
- Wall PD, Pressman EK, Woods JR Jr., 2002. Preterm premature rupture of the membranes and antioxidants: the free radical connection. J. Perinat. Med. 30, 447–457. [DOI] [PubMed] [Google Scholar]
- Wallace ME, Grantz KL, Liu D, Zhu Y, Kim SS, Mendola P, 2016. Exposure to ambient air pollution and premature rupture of membranes. Am. J. Epidemiol. 183, 1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Tian Y, Zheng H, Shan S, Zhao X, Liu C, 2019. Maternal exposure to ambient fine particulate matter and risk of premature rupture of membranes in Wuhan, Central China: a cohort study. Environ. Health 18, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Benmarhnia T, Zhang H, Knibbs LD, Sheridan P, Li C, Bao J, Ren M, Wang S, He Y, Zhang Y, Zhao Q, Huang C, 2018. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ. Int. 121, 317–324. [DOI] [PubMed] [Google Scholar]
- Warren JL, Son JY, Pereira G, Leaderer BP, Bell ML, 2018. Investigating the impact of maternal residential mobility on identifying critical windows of susceptibility to ambient air pollution during pregnancy. Am. J. Epidemiol. 187, 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weagle CL, Snider G, Li C, van Donkelaar A, Philip S, Bissonnette P, Burke J, Jackson J, Latimer R, Stone E, Abboud I, Akoshile C, Anh NX, Brook JR, Cohen A, Dong J, Gibson MD, Griffith D, He KB, Holben BN, Kahn R, Keller CA, Kim JS, Lagrosas N, Lestari P, Khian YL, Liu Y, Marais EA, Martins JV, Misra A, Muliane U, Pratiwi R, Quel EJ, Salam A, Segev L, Tripathi SN, Wang C, Zhang Q, Brauer M, Rudich Y, Martin RV, 2018. Global sources of fine particulate matter: interpretation of PM2.5 chemical composition observed by SPARTAN using a global chemical transport model. Environ. Sci. Tech. 52, 11670–11681. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B, 2011. Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles County, California. Environ Health 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA, 2017. Potential for bias when estimating critical windows for air pollution in children’s health. Am. J. Epidemiol. 186, 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JR Jr., 2001. Reactive oxygen species and preterm premature rupture of membranes-a review. Placenta 22 Suppl A, S38–S44. [DOI] [PubMed] [Google Scholar]
- Wu J, Laurent O, Li L, Hu J, Kleeman M, 2016. Adverse reproductive health outcomes and exposure to gaseous and particulate-matter air pollution in pregnant women. Research Reports. Health Effects Institute, p. 2016. [PMC free article] [PubMed] [Google Scholar]
- Wu S, Deng F, Wei H, Huang J, Wang H, Shima M, Wang X, Qin Y, Zheng C, Hao Y, Guo X, 2012. Chemical constituents of ambient particulate air pollution and biomarkers of inflammation, coagulation and homocysteine in healthy adults: a prospective panel study. Part. Fibre Toxicol. 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Yin WJ, Yu LJ, Wang YH, Jiang XM, Zhang Y, Tao FB, Tao RX, Zhu P, 2023. Prenatal Exposure to Air Pollution and Pre-Labor Rupture of Membranes in a Prospective Cohort Study: The Role of Maternal Hemoglobin and Iron Supplementation. Environ Health Perspect 131, 47013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yackerson N, Piura B, Sheiner E, 2008. The influence of meteorological factors on the emergence of preterm delivery and preterm premature rupture of membrane. J. Perinatol. 28, 707–711. [DOI] [PubMed] [Google Scholar]
- Yagur Y, Weitzner O, Ravid E, Biron-Shental T, 2019. Can we predict preterm delivery in patients with premature rupture of membranes? Archiv. Gynecol. Obstet. 300, 615–621. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li S, Guo GL, Hao JW, Cheng P, Xiong LL, Chen ST, Cao JY, Guo YW, Hao JH, 2021. Acute associations between air pollution on premature rupture of membranes in Hefei, China. Environ. Geochem. Health 43, 3393–3406. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang G, Guo S, Zamora ML, Ying Q, Lin Y, Wang W, Hu M, Wang Y, 2015. Formation of urban fine particulate matter. Chem. Rev. 115, 3803–3855. [DOI] [PubMed] [Google Scholar]
- Zhang Z-H, Khlystov A, Norford LK, Tan Z-K, Balasubramanian R, 2017. Characterization of traffic-related ambient fine particulate matter (PM 2.5) in an Asian city: environmental and health implications. Atmos. Environ. 161, 132–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.