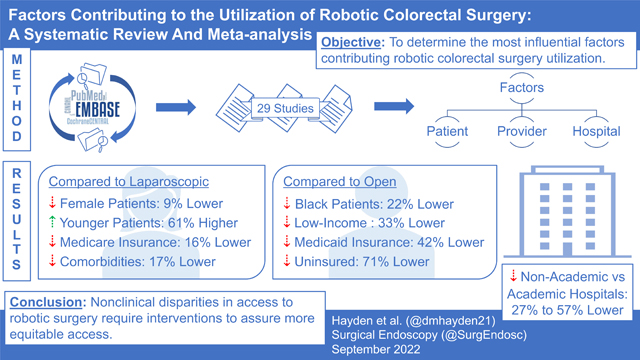
Abstract
Background
Some studies have suggested disparities in access to robotic colorectal surgery, however, it is unclear which factors are most meaningful in the determination of approach relative to laparoscopic or open surgery. This study aimed to identify the most influential factors contributing to robotic colorectal surgery utilization.
Methods
We conducted a systematic review and random-effects meta-analysis of published studies that compared the utilization of robotic colorectal surgery versus laparoscopic or open surgery. Eligible studies were identified through PubMed, EMBASE, CINAHL, Cochrane CENTRAL, PsycINFO, and ProQuest Dissertations in September 2021.
Results
Twenty-nine studies were included in the analysis. Patients were less likely to undergo robotic versus laparoscopic surgery if they were female (OR = 0.91, 0.84–0.98), older (OR = 1.61, 1.38–1.88), had Medicare (OR = 0.84, 0.71–0.99), or had comorbidities (OR = 0.83, 0.77–0.91). Non-academic hospitals had lower odds of conducting robotic versus laparoscopic surgery (OR = 0.73, 0.62–0.86). Additional disparities were observed when comparing robotic with open surgery for patients who were Black (OR = 0.78, 0.71–0.86), had lower income (OR = 0.67, 0.62–0.74), had Medicaid (OR = 0.58, 0.43–0.80), or were uninsured (OR = 0.29, 0.21–0.39).
Conclusion
When determining who undergoes robotic surgery, consideration of factors such as age and comorbid conditions may be clinically justified, while other factors seem less justifiable. Black patients and the underinsured were less likely to undergo robotic surgery. This study identifies nonclinical disparities in access to robotics that should be addressed to provide more equitable access to innovations in colorectal surgery.
Keywords: Colorectal surgery, Robotic surgery, Minimally invasive surgery, Hospital, Characteristics, Patient, characteristics
Graphical Abstract
Since its inception 3 decades ago, minimally invasive surgery has become the gold standard for abdominopelvic surgery, including colorectal cancer (CRC) surgery [1–3]. The use of robotics in colorectal surgery offers numerous benefits to both patients and providers [4, 5].
For patients, robotics use—when compared primarily with open surgery—is associated with decreased postoperative pain, early return of bowel function, decreased blood loss, shorter hospital stays, decreased risk of infection, and reduced postoperative mortality [4–6]. Meta-analyses of randomized controlled trials (RCTs) of robotic compared with laparoscopic colorectal surgery suggest that clinical outcomes for the two approaches are similar [6, 7]. Shorter hospital stays and decreased 30 day readmission rates associated with robotic surgery indicate an accelerated recovery time compared with laparoscopic or open surgery [4, 6, 8–10], suggesting that robotic surgery may allow for a faster return to the workforce, thus offering economic benefits for both the individual and society [11–13]. For providers, robotic colorectal surgery offers advantages such as improved visualization, greater stability because of the surgeon’s control of the camera, and wristed movements that allow for more meticulous operations and improved ergonomics [5, 14]. Overall, robotics in colorectal surgery has numerous advantages over open surgery and for some patients may offer additional benefits when compared with laparoscopic surgery. Thus, all patients should have equitable access to this innovative surgical technique.
Several studies have outlined the influential role of socioeconomic status—one of the strongest predictors of both health and educational outcomes [15, 16]—on the utilization of all forms of minimally invasive colorectal surgery [2, 17, 18]. For example, patients in the highest income quartile are more likely than those in the lowest income groups to undergo minimally invasive procedures such as laparoscopic surgery [17, 18]. Members of socioeconomically disadvantaged groups are also more susceptible to being denied minimally invasive surgery (MIS) due to higher hospital charges for these procedures, particularly for robotic surgery [2]. Additionally, patients with Medicaid or without health insurance are significantly less likely than those with private insurance to undergo MIS [2, 17, 18]. Geographic location has also been associated with surgical approach [2]; Akiny-emiju and colleagues [17] found that individuals residing outside larger metropolitan areas were less likely to undergo laparoscopic surgery.
Despite the increasing use of robotics in colorectal surgery over the past decade [19, 20] and evidence suggesting disparities in access to this innovation, very few studies to date have sought to elucidate the factors contributing to these disparities. To address this knowledge gap, we conducted a systematic review of the literature and a quantitative meta-analysis with two aims: (1) synthesize the evidence from published studies for all factors that contribute to provider recommendation and utilization of the robotic approach in colorectal surgery; and (2) quantitatively assess the evidence from these published studies through meta-analysis to determine the most influential factors contributing to provider recommendation of robotic colorectal surgery.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21] (see Supplementary Material) and was registered on PROSPERO along with the study protocol (CRD42021286022). Further details about the methods can be found in the protocol.
Search strategy
On September 7, 2021, a systematic search was performed in PubMed, EMBASE, CINAHL Complete, Cochrane CENTRAL, PsycINFO, and ProQuest Dissertations and Theses Global. The following search string was adapted to each database with reference to the title and abstract: (robot OR robotic OR robotics OR telerobotic OR laparoscopic OR “minimally invasive”) AND (surgery OR surgical OR technique OR approach OR laparoscopy OR colectomy OR ileocolectomy OR ileocecectomy OR colonic) AND (colorectal OR colon OR rectal OR sigmoid) AND (cancer OR neoplasm OR tumor OR carcinoma OR adenocarcinoma OR “malignant neoplasm” OR “Crohn’s disease” OR “ulcerative colitis” OR “inflammatory bowel disease”) AND (utilization OR “provider recommendation” OR access OR disparity OR disparities OR delivery OR socioeconomic OR race OR racial OR ethnic OR ethnicity). No additional filters were applied. A backward and forward search (via Google Scholar) was also performed based on the studies that met the inclusion criteria.
Selection procedure
Studies identified in the systematic search were uploaded to Rayyan, a web-based application for facilitating the screening of articles for inclusion in systematic reviews [22]. We employed a 2-tier screening procedure: First, two reviewers (EB, KMK) independently screened titles and abstracts for initial inclusion and then met to resolve discrepancies. Second, two reviewers (EB, FT) retrieved and independently examined the full text of each study that met the initial eligibility criteria to confirm eligibility for inclusion. A third reviewer (KMK) resolved discrepancies and made the final inclusion decision.
Eligibility criteria
Published studies were eligible for inclusion regardless of the timing of their publication, year of data collection, or geographic location; however, studies published after September 7, 2021, and in a language other than English were excluded. Studies were included regardless of their design; however, case studies and systematic reviews or meta-analyses of robotic surgical procedures were excluded. The main outcome was utilization of robotic procedures for colorectal surgery. Thus, studies were included if they assessed differences in robotic surgery utilization by hospital (e.g., volume, type); provider/surgeon (e.g., specialization); or patient characteristics (e.g., income, race/ethnicity, health insurance). Studies of robotic procedures for other types of surgery (e.g., prostate) were excluded, along with studies that solely examined disparities in laparoscopic or minimally invasive surgeries overall. Patients diagnosed with CRC or who were required to undergo colon or rectal surgery were eligible for inclusion and were included regardless of age, gender/sex, or race/ethnicity.
Data extraction
Three reviewers (EB, FT, KK) independently extracted the data. For quality assurance purposes, two reviewers coded each study. Each reviewer practiced extracting data on two of the included studies and made revisions as necessary; each then extracted data from the remaining studies, after which they met to resolve discrepancies.
Data items
We assigned each study a unique ID and extracted study identification data, including the first author’s name, year of publication, and publication status. To identify studies from the same parent investigation, we recorded the database used for each study. We coded each study for its design (e.g., cohort, cross-sectional); setting (e.g., geographic region, single hospital versus multiple hospitals, elective versus emergency surgeries); surgical location (e.g., colon versus rectum); and comparison performed (e.g., robotic surgery versus laparoscopic surgery). We extracted data on the characteristics of the study sample, including total sample size, mean age and range, gender/sex, and racial/ethnic composition.
For each study, we extracted the type of factor examined in association with the utilization of robotic surgery and the study’s findings, with a focus on results from multivariate analyses. In studies that examined multiple factors, we extracted findings related to each reported factor. These factors fell within three broad categories: hospital, provider/surgeon, and patient characteristics. Hospital factors included the type of hospital (e.g., community or academic) and location (e.g., the respective U.S. census region). Provider/surgeon factors included specialization (e.g., colorectal or general surgeon) and volume (e.g., high or low volume of colorectal-related surgical procedures). Patient factors included race/ethnicity (e.g., Black or White), income (e.g., lower or higher), and insurance status (e.g., private or public).
We also extracted unadjusted odds ratios (ORs) that captured disparities in the utilization of robotic surgery relative to laparoscopic or open surgery, as well as their corresponding 95% confidence intervals (CI). When ORs were not reported, we extracted frequencies (number of patients) for categorical factors to calculate ORs and their standard errors; we also used means and standard deviations to calculate Cohen’s d and transformed to ORs [23].
Methodological quality assessment
To examine risk of bias, we used eight items from the Observational Study Quality Evaluation (OSQE), which was developed from a combination of items across existing riskof-bias measures and reporting guidelines [24]. The OSQE includes separate versions for case–control, cohort, and cross-sectional study designs. Studies received a score of 1 when the criteria for each item were satisfied. A total score was computed by summing across the 8 items, with higher scores indicative of better quality. One reviewer (KMK) evaluated methodological quality using the OSQE; a second reviewer (EB) rated 25% of the studies as a reliability check.
Qualitative synthesis and meta-analysis plan
We synthesized the extracted data by examining consistencies and inconsistencies using a narrative approach. Along with the studies’ methodological quality, we critically evaluated sample or methodological characteristics that contributed to similarities or differences between studies. We quantified the extracted data using frequencies and counts. The entire research team discussed the extracted data for potential patterns of findings across the included studies and reached conclusions regarding the factors and demographics associated with the utilization of robotic approaches to colorectal surgery, as well as areas where additional evidence was needed.
When at least 5 associated ORs could be calculated for a particular factor, we also performed a random-effects meta-analysis [25]. We used a random-effects model because it acknowledges systematic and random error between effect sizes. We used robust variance estimation to adjust standard errors in cases in which effect sizes from the same parent investigation were included [26]. We determined the meta-analytic effect size, its 95% CI, and related variability (i.e., , ). Finally, we used 2 sensitivity tests to examine publication bias for the meta-analytic estimates: Egger’s regression (with robust variance estimation) and the trim-and-fill method [27]. We conducted the meta-analysis using the metafor package [28] in R Studio [29].
Results
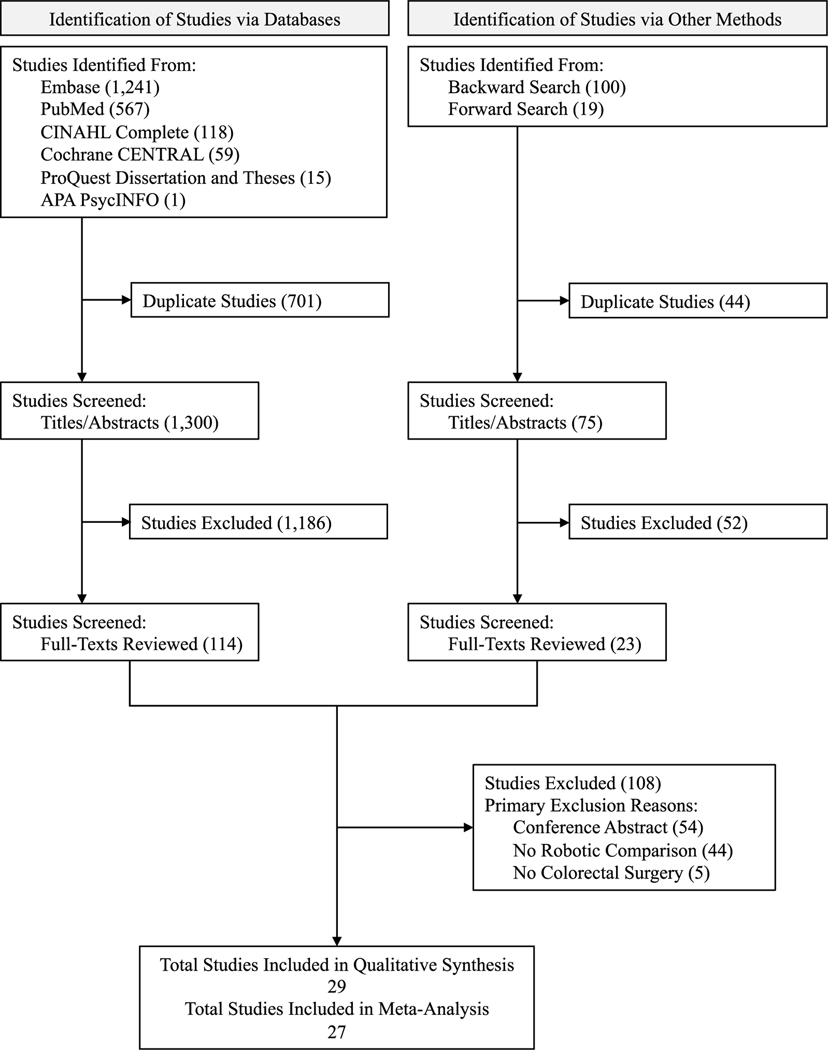
The systematic search identified 1300 studies and the backward and forward search an additional 75. Of these 1375 studies, 137 were retrieved for full-text review; reasons for exclusion are shown in Fig. 1. Of the 137 full-text articles reviewed, 29 met the inclusion criteria for the final sample. A list of all the full-text articles reviewed, along with reasons for exclusion, is available in the Supplementary Material. Several studies that were excluded during the full-text review (41%) investigated differences in surgical approaches but focused on comparing either laparoscopic [30] or minimally invasive surgery (including laparoscopic and robotic) with open surgery without specifically disaggregating differences for robotic surgery [31].
Fig. 1.
Flow diagram of study identification and selection
Study characteristics
A summary of characteristics for the 29 studies [2, 32–59] appears in the Supplementary Material. No studies were RCTs; most (97%) used a retrospective database for their analysis, while 1 study administered a survey to colorectal surgeons [37]. Common databases used were the National Cancer Database (11; 38%), National Inpatient Sample Database (6; 21%), National Quality Improvement Program (3; 10%), University Health System Consortium (2; 7%), and Florida Inpatient Discharge Dataset (2; 7%). Regarding surgical location, 12 studies (41%) examined differences in the utilization of robotic procedures for both colon and rectal surgeries, whereas 9 (31%) focused specifically on rectal surgeries and 8 (28%) specifically on colon surgeries.
The mean or median patient age ranged from 57 to 73 years. Studies differed in their categorization of patients by age. Among studies that reported frequencies, patients were deemed “older” if they were aged over 75, over 80, or over 85 years and “younger” if they were aged either below the “older” cutoff or within a defined range (e.g., 55–64, 65–74 years). The percentage of female participants ranged from 3 to 65%. Seven studies (24%) did not report participants’ race/ethnicity; of those that did, the categories most often used were White (79%), Black/African American (75%), other race (72%), and Hispanic/Latinx (72%). Across studies, more patients were categorized as White (68–87%) followed by Black (5–21%), other race (1–19%), Hispanic/ Latinx (3–15%), Asian/Pacific Islander (1–5%), and American Indian/Native Alaskan (< 1%).
Qualitative synthesis
A summary of findings from the qualitative synthesis appears in the Supplementary Material, including the conclusion for each factor examined, the number of studies for which the direction of the difference was consistent or inconsistent, and studies that reported no evidence of an association between the specific factor and the utilization of robotic surgery. We prioritized multivariate findings, when reported, in informing the qualitative conclusions. Patient-level factors were more commonly examined than hospital-level factors; none of the included studies examined factors related to the provider/surgeon.
Of the patient-level factors, gender/sex, race/ethnicity, age, comorbidities, and insurance status were the most frequently examined factors. These studies were often consistent in suggesting that women; patients who self-identified as Black; patients with comorbidities; and patients with Medicare, Medicaid, or no insurance were less likely to receive robotic surgery compared with men, patients who self-identified as White, patients without comorbidities, and patients with private insurance. In a survey, colorectal surgeons reported that body mass index and gender/sex were important factors to consider when deciding whether to use robotic approaches [37]. Although less examined across the included studies, having lower income or living in low-income areas, living in areas with a greater percentage of adults who did not complete high school, and living in rural areas were also associated with a lower likelihood of receiving robotic surgery. Patients who traveled a greater distance for surgery were more likely to receive a robotic approach.
With regard to patient-related clinical factors, tumor site, tumor grade, clinical/pathological stage, and prior treatment were associated with receiving robotic surgery. Patients with surgical sites in the rectum, moderately differentiated tumors, or earlier disease stages, and those who had received radiation or chemotherapy were more likely to undergo robotic surgery than those with surgical sites in the colon, well-differentiated tumors, or later stages of disease, as well as those who had not received prior treatment.
Among hospital-level factors, hospital type was the factor most often examined across studies. Findings consistently suggested that academic, or teaching, hospitals were more likely to use robotic surgery than community or comprehensive community hospitals. Larger hospitals (i.e., those with more beds), those performing a higher volume of colorectal-related surgeries, and those located in metropolitan or urban areas were more likely to use robotic surgery than smaller hospitals, those performing a lower volume of colorectal-related surgeries, and those in rural areas. Three studies reported that hospitals located in the Western region of the USA were less likely to use robotic surgery compared with those in the Northeast [57, 58] and with all other U.S. regions [36].
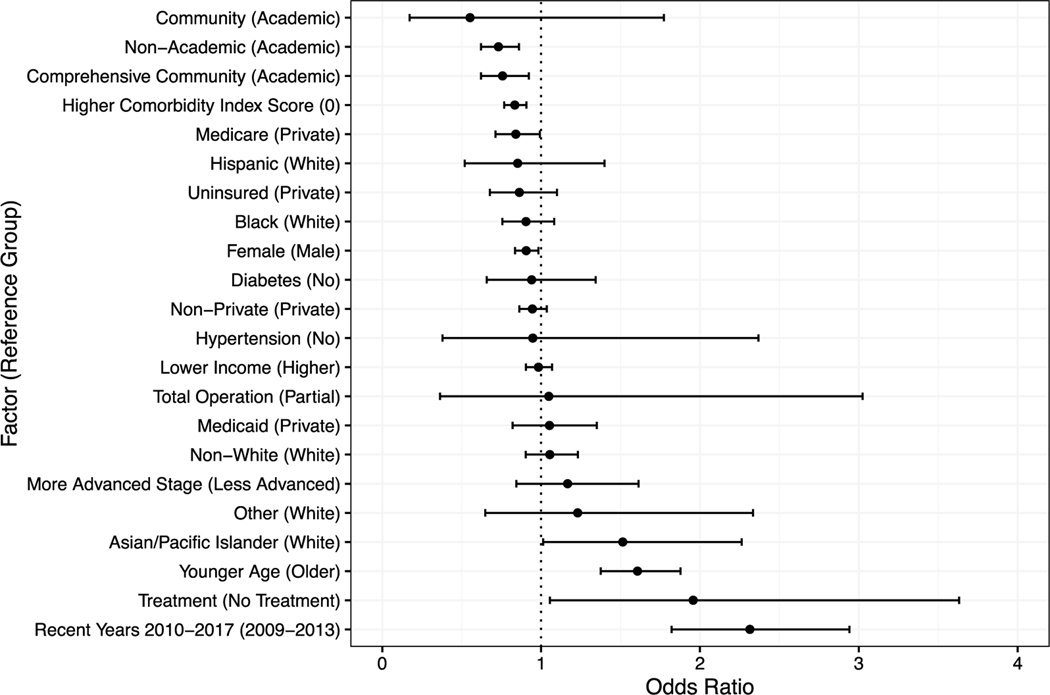
Meta-analysis
The meta-analysis found evidence that sex, race/ethnicity, insurance status, comorbidities, treatment, and year of diagnosis or surgery were associated with the likelihood of receiving robotic versus laparoscopic surgery (Table 1, Fig. 2). Patients who had surgery more recently (up to 2017) and those who had received radiation or chemotherapy had 131% and 96% higher odds, respectively, of undergoing robotic rather than laparoscopic surgery, compared with those who had surgery between 2009 and 2013 and those who had not received radiation or chemotherapy, respectively. Similarly, younger patients and those who identified as Asian/Pacific Islander were 61% more likely than older patients and 51% more likely than White patients, respectively, to undergo robotic than laparoscopic surgery. Patients with Medicare and those with 1 or more comorbidities had 16% and 17% lower odds, respectively, of receiving robotic than laparoscopic surgery.
Table 1.
Meta-analytic results for utilization of robotic vs laparoscopic surgery by patient-level and hospital-level factors
| Factor (reference group) | ORa | 95% CIb | Clustersc | Egger’s | Egger’s -valued | ORTF | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient-level | |||||||||
| Female (male) | 0.91 | [0.84, 0.98] | 18 | 5 | 0.017 | 93.77 | − 0.67 | 0.502 | 0.94 |
| Younger age (older age) | 1.61 | [1.38, 1.88] | 23 | 3 | 0.114 | 98.03 | − 3.39 | 0.453 | 1.61 |
| Non-white (white) | 1.05 | [0.90, 1.23] | 48 | 7 | 0.335 | 98.86 | − 0.99 | 0.110 | 1.29 |
| Black (white) | 0.90 | [0.76, 1.08] | 18 | 7 | 0.047 | 91.55 | − 1.57 | 0.020 | 0.93 |
| Other race (white) | 1.23 | [0.65, 2.33] | 15 | 5 | 0.604 | 99.54 | − 1.80 | 0.140 | 1.57 |
| Hispanic/Latinx (white) | 0.85 | [0.52, 1.40] | 7 | 4 | 0.536 | 99.01 | 0.47 | 0.227 | 0.85 |
| Asian/Pacific islander (white) | 1.51 | [1.01, 2.26] | 6 | 4 | 0.130 | 91.15 | − 1.04 | 0.691 | 1.51 |
| Lower income (higher income) | 0.98 | [0.90, 1.07] | 22 | 3 | 0.002 | 55.92 | − 1.09 | 0.409 | 0.99 |
| Non-private (private insurance) | 0.94 | [0.86, 1.04] | 50 | 5 | 0.108 | 98.45 | 0.32 | 0.727 | 0.94 |
| Medicare (private insurance) | 0.84 | [0.71, 0.99] | 14 | 5 | 0.042 | 98.28 | 4.19 | 0.085 | 0.78 |
| Medicaid (private insurance) | 1.05 | [0.82, 1.35] | 12 | 5 | 0.036 | 90.53 | − 1.43 | 0.290 | 1.05 |
| Uninsured (private insurance) | 0.86 | [0.68, 1.10] | 8 | 3 | 0.292 | 97.09 | 0.32 | 0.833 | 0.86 |
| Higher comorbidity index score (0) | 0.83 | [0.77, 0.91] | 28 | 6 | 0.048 | 94.84 | 0.71 | 0.192 | 0.78 |
| Diabetes (no) | 0.94 | [0.66, 1.34] | 5 | 2 | 0.001 | 16.71 | 1.13 | 0.181 | 0.93 |
| Hypertension (no) | 0.95 | [0.38, 2.37] | 5 | 2 | 0.011 | 82.60 | 0.07 | 0.948 | 0.90 |
| Total operation (partial operation) | 1.05 | [0.36, 3.02] | 5 | 2 | 0.220 | 97.86 | − 2.41 | 0.382 | 1.05 |
| More advanced stage (less advanced) | 1.17 | [0.84, 1.61] | 45 | 6 | 0.347 | 98.17 | 1.37 | 0.208 | 1.17 |
| Treatment (no treatment) | 1.96 | [1.05, 3.63] | 8 | 2 | 0.502 | 99.70 | 0.87 | 0.579 | 1.96 |
| Recent years 2010–2017 (2009–2013)e | 2.31 | [1.82, 2.94] | 21 | 3 | 0.297 | 98.63 | 0.17 | 0.987 | 1.97 |
| Hospital-level | |||||||||
| Non-academic (academic) | 0.73 | [0.62, 0.86] | 30 | 3 | 0.081 | 98.04 | − 1.97 | 0.368 | 0.73 |
| Community (academic) | 0.55 | [0.17, 1.77] | 10 | 2 | 0.107 | 96.90 | − 2.63 | 0.021 | 0.55 |
| Comprehensive community (academic) | 0.76 | [0.62, 0.92] | 9 | 2 | 0.011 | 90.81 | − 0.22 | 0.866 | 0.76 |
OR odds ratio, CI confidence interval, number of extracted odds ratios from individual studies
Bolded factors have confidence intervals that do not overlap with 1
Overall odds ratio from a random-effects meta-analysis
Confidence interval from robust variance estimation
Number of parent investigations used in robust variance estimation
For rows with greater than 2 clusters, the p-value is based on robust variance estimation
Range of years across individual studies
TFEstimated odds ratio adjusted for funnel plot asymmetry using the Trim and Fill method
Fig. 2.
Plot of overall odds ratios for each factor and its association with robotic vs laparoscopic surgery utilization ordered in direction and magnitude
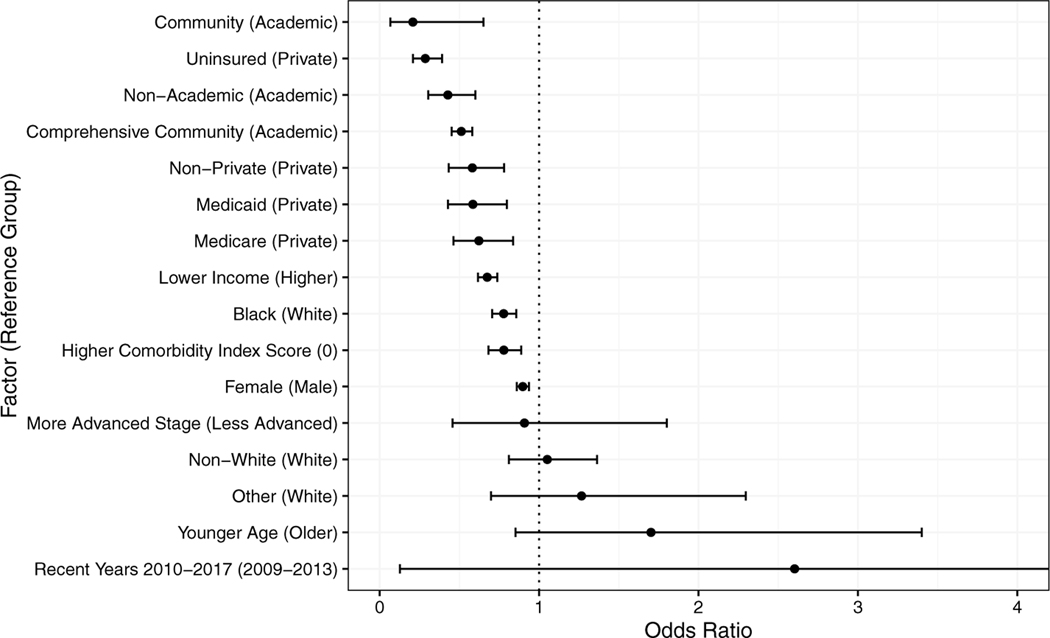
The meta-analysis also revealed that sex, race/ethnicity, income, insurance status, and comorbidities were associated with the odds of receiving robotic versus open surgery (Table 2, Fig. 3). First, when we compared private insurance with all other insurance types, patients with nonprivate insurance had 23% lower odds of receiving robotic than open surgery. These differences were most pronounced for patients with no insurance, Medicare, or Medicaid, who had 71%, 38%, and 42% lower odds of receiving robotic than open surgery, respectively. Similarly, patients with lower income or from lower-income areas (33% lower odds) and those who identified as Black (22% lower odds) were less likely to undergo robotic than open surgery. Patients with 1 or more comorbidities and women also had 22% and 10% lower odds, respectively, of receiving robotic compared with open surgery.
Table 2.
Meta-analytic results for utilization of robotic vs open surgery by patient-level and hospital-level factors
| Factor (reference group) | ORa | 95% CIb | Clustersc | Egger’s | Egger’s -valued | ORTF | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient-level | |||||||||
| Female (male) | 0.90 | 0.86, 0.94 | 11 | 5 | 0.010 | 89.97 | − 0.95 | 0.259 | 0.94 |
| Younger age (older age) | 1.70 | 0.85, 3.40 | 16 | 2 | 0.127 | 98.64 | − 5.00 | 0.000 | 1.70 |
| Non-white (white) | 1.05 | 0.81, 1.36 | 27 | 6 | 0.529 | 99.42 | − 0.69 | 0.119 | 1.41 |
| Black (white) | 0.78 | 0.71, 0.86 | 11 | 6 | 0.004 | 48.77 | − 1.41 | 0.072 | 0.79 |
| Other race (white) | 1.27 | 0.70, 2.30 | 10 | 4 | 1.217 | 99.83 | − 1.05 | 0.079 | 1.97 |
| Lower income (higher income) | 0.67 | 0.62, 0.74 | 15 | 3 | 0.021 | 94.78 | − 0.68 | 0.073 | 0.67 |
| Non-private (private insurance) | 0.58 | 0.43, 0.78 | 35 | 5 | 0.136 | 99.21 | 0.33 | 0.747 | 0.56 |
| Medicare (private insurance) | 0.62 | 0.46, 0.84 | 10 | 5 | 0.113 | 99.49 | 3.45 | 0.397 | 0.55 |
| Medicaid (private insurance) | 0.58 | 0.43, 0.80 | 8 | 5 | 0.106 | 97.40 | − 0.55 | 0.725 | 0.60 |
| Uninsured (private insurance) | 0.29 | 0.21, 0.39 | 5 | 3 | 0.019 | 73.00 | 1.62 | 0.008 | 0.28 |
| Higher comorbidity index score (0) | 0.78 | 0.68, 0.89 | 15 | 4 | 0.029 | 93.98 | 0.10 | 0.906 | 0.78 |
| More advanced stage (less advanced) | 0.91 | 0.46, 1.80 | 26 | 5 | 0.907 | 99.17 | − 0.13 | 0.910 | 0.91 |
| Recent years 2010–2017 (2009–2013)e | 2.60 | 0.13, 53.28 | 8 | 2 | 0.260 | 99.02 | − 11.98 | 0.044 | 2.60 |
| Hospital-level | |||||||||
| Non-academic (academic) | 0.43 | 0.30, 0.60 | 22 | 3 | 0.959 | 99.84 | − 1.80 | 0.598 | 0.43 |
| Community (academic) | 0.21 | 0.07, 0.65 | 7 | 2 | 0.831 | 99.56 | − 0.22 | 0.944 | 0.21 |
| Comprehensive community (academic) | 0.51 | 0.45, 0.58 | 7 | 2 | 0.801 | 99.86 | 3.83 | 0.672 | 0.42 |
OR odds ratio, CI confidence interval, number of extracted odds ratios from individual studies
Bolded factors have confidence intervals that do not overlap with 1
Overall odds ratio from a random-effects meta-analysis
Confidence interval from robust variance estimation
Number of parent investigations used in robust variance estimation
For rows with greater than 2 clusters, the -value is based on robust variance estimation
Range of years across individual studies
TFEstimated odds ratio adjusted for funnel plot asymmetry using the Trim and Fill method
Fig. 3.
Plot of overall odds ratios for each factor and its association with robotic vs open surgery utilization ordered in direction and magnitude
Of the hospital-related factors, hospital type was associated with the use of robotic surgery when compared with laparoscopic or open surgery. Compared with academic centers, nonacademic hospitals (i.e., community, comprehensive, integrated, other) had 17% lower odds of using robotic versus laparoscopic surgery and 57% lower odds of using robotic versus open surgery. When we compared specific types of nonacademic and academic hospitals, only comprehensive community hospitals had lower odds (14%) of using robotic than laparoscopic surgery, whereas community hospitals and comprehensive community hospitals had 79% and 49% lower odds, respectively, of using robotic compared with open surgery.
For some studies, we could extract ORs only for comparisons between robotic and nonrobotic surgery (i.e., laparoscopic and open surgery combined). Neither of the 2 patient-related factors examined across studies (i.e., race/ethnicity, insurance status) indicated an association with the likelihood of undergoing robotic surgery (Table 3).
Table 3.
Meta-analytic results for utilization of robotic vs non-robotic (type unspecified) surgery by patient-level factor
| Factor (reference group) | ORa | 95% CIb | Clustersc | Egger’s | Egger’s -valued | ORTF | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-white (white) | 0.67 | [0.18, 2.59] | 5 | 2 | 2.348 | 99.91 | − 49.87 | 0.002 | 0.67 |
| Non-private (private insurance) | 0.47 | [0.05, 4.66] | 5 | 2 | 2.681 | 99.95 | − 11.25 | 0.658 | 0.47 |
OR odds ratio, CI confidence interval, number of extracted odds ratios from individual studies
Overall odds ratio from a random-effects meta-analysis
Confidence interval from robust variance estimation
Number of parent investigations used in robust variance estimation
For rows with greater than 2 clusters, the -value is based on robust variance estimation
TFEstimated odds ratio adjusted for funnel plot asymmetry using the Trim and Fill method
Publication bias
The Egger’s regression coefficient and associated -value and the bias-adjusted OR based on the trim-and-fill method for each comparison are displayed in Tables 1, 2, 3. For the utilization comparisons between robotic and laparoscopic surgery, neither test suggested that publication or selection bias substantially affected the meta-analytic estimates. For the utilization comparisons between robotic and open surgery, the interpretation was similar except for the OR between patients with private insurance and those with no insurance; for patients without insurance, the odds of receiving robotic compared with open surgery may be lower than our estimate. We found no strong evidence of publication bias for the comparisons between robotic and nonrobotic surgery.
Methodological quality
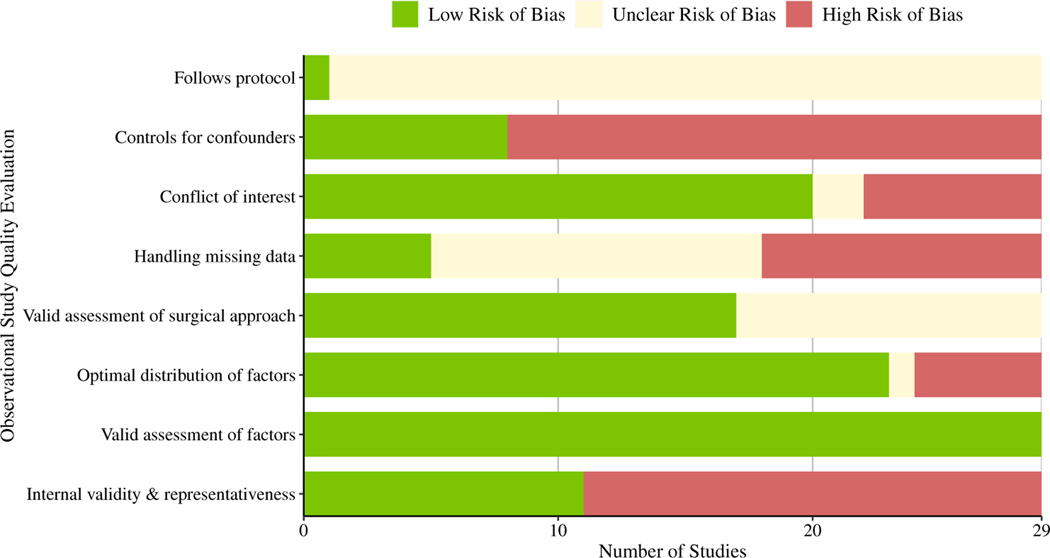
The overall methodological quality score across the 29 studies ranged from 2 to 6 (out of 8, the highest possible quality score; Fig. 4). Ten studies (34%) received a quality score between 5 and 6, ten (34%) received a 4, five (17%) received a 3, and four (14%) received a 2. There was low risk of bias for the assessment of patient and hospital-level factors across all studies and for variability in these factors across most studies (79%). All but 1 study did not refer to a protocol; thus, it was unclear whether these studies reported patient-level or hospital-level factors that were decided prior to data analysis. The amount of missing data across factors was unclear in 13 studies (45%); only 5 studies (38%) properly dealt with missing data. Twelve studies (41%) did not report surgical codes, which made it difficult to evaluate the validity of the surgical procedures. Eighteen studies (62%) showed evidence of bias regarding internal validity and representativeness, mostly attributed to a lack of transparent reporting of their sample-selection process. Additionally, only 8 studies (28%) reported using a multivariate model when testing differences by patient-level and hospital-level factors; the remaining studies did not control for relevant confounders. Finally, 7 studies (24%) reported a conflict of interest, while 2 studies (7%) did not include a conflicts disclosure statement.
Fig. 4.
Distribution of studies that met each methodological quality assessment item
Discussion
We conducted a systematic review and random-effects meta-analysis of all published studies comparing robotic colorectal surgery utilization with that of laparoscopic or open surgery to identify the most influential factors contributing to provider recommendation and use of robotic colorectal surgery. Our findings reveal several patient- and institution-related factors associated with the utilization of robotic surgery as well as nonclinical disparities in access to this innovative modality.
Women were less likely than men to receive robotic surgery. This held true for the comparisons of robotic surgery with both laparoscopic and open surgery. One possible explanation for this finding is the perceived greater difficulty of abdominopelvic surgery in men given anatomical differences. Studies have shown that the male and female pelvis differ significantly in dimension. Men have a shorter, narrower pelvic inlet and a deeper pelvis, leading to a more technically difficult operation, especially in the case of a low rectal tumor. Therefore, while all approaches may be available for women, men may be more likely to be offered robotic surgery due to the visualization benefits in the more difficult male pelvis. Veenhoff and colleagues [60] reviewed 50 patients who underwent proctectomy for rectal cancer and found that even though male anatomy was perceived to be more difficult, there were no differences in surgical outcomes between men and women [60, 61]. On the other hand, in cases of total mesorectal excision for rectal cancer in male patients, robotic surgery offers a demonstrated benefit of reduced urinary and sexual impairment compared with laparoscopic approaches [62, 63]. This finding is likely related to improved pelvic autonomic nerve preservation secondary to better visualization and dexterity in difficult anatomical locations [64]. While there is a demonstrated benefit in using robotic surgery for low rectal cancers and total mesorectal excision in men, there seems to be no disadvantage to the use of robotic surgery in women.
It is noteworthy that Black patients were less likely than White patients to receive robotic surgery than open surgery, whereas Asian American/Pacific Islanders were more likely than White patients to receive robotic than laparoscopic surgery. Results from studies of racial and ethnic disparities in MIS have been equivocal [2, 33]. For example, a 2016 analysis of data from the Nationwide Inpatient Sample found that, compared with White patients, Black patients were less likely to undergo surgery for CRC and more likely to have worse outcomes; however, when surgery was performed, no racial disparities associated with surgical approach (i.e., robotic, laparoscopic, or open) were seen [17]. A review using data from the American College of Surgeons National Surgical Quality Improvement Program reported that Black patients were less likely than White patients to receive the most routinely performed laparoscopic surgeries [65]. Lower income level and nonprivate insurance remain risk factors consistently associated with a lower likelihood of receiving MIS [33, 66]. Because some of these studies report that a higher proportion of the patients in these groups are Black, there may be mediation between these factors that warrants further investigation.
In this study, rates of robotic surgery utilization were highest for patients with private insurance compared with those with Medicare or Medicaid or those who were uninsured, a difference that was most noticeable when robotic surgery was compared with open surgery. This disparity is presumed to be related to the operating-room costs associated with robotic surgery compared with laparoscopic and open colorectal procedures. In our review, 18 studies considered insurance status as a factor in the decision to perform a robotic procedure. As noted in Table 2, 14 of these studies were concordant in demonstrating that patients with private insurance more often received robotic surgery. Ramji et al. [67] compared the operating-room costs of robotic, laparoscopic, and open surgery for rectal cancer as well as the overall cost of the episode of care. While there were no major differences in procedural costs between laparoscopic and open procedures, the robotic approach was found to add approximately $6000 CAD (Canadian dollars; approximately $4600 USD) to the cost of each procedure. However, no significant additional costs were associated with the overall episode of care for patients receiving a robotic procedure. By comparison, the overall hospital costs for open, laparoscopic, and robotic rectal cancer resections were $12,558.56, $11,493.46, and $18,273.35 USD, respectively [67]. It is important to note that these figures do not reflect the overall economic cost associated with surgical care for rectal cancer, which ideally would consider not only hospital costs but also loss of productivity for the patient and caregiver. Loss of productivity due to surgery-related work absenteeism is estimated to be around $14,000 USD, indicating the importance of promoting operations that decrease recovery time in mitigating economic consequences for patients, employers, and healthcare systems [68].
Several studies comparing MIS (robotic or laparoscopic) with open surgery have shown decreases in length of stay and decreased rates of wound infections and hernias [3–5, 69]. Of note, when complications are present, open surgery may be more expensive than robotic surgery. Few studies show decreased length of stay for robotic versus laparoscopic surgery [10, 70]. Even though cost is consistently higher for robotic versus laparoscopic surgery, the difference in cost may reduce over time as operative duration decreases and small differences in length of stay become lower in certain patients [69]. For some surgeons the learning curve for laparoscopy is much steeper than for robotics, making transition to robotic surgery much more realistic, resulting in improved outcomes and lower cost for those patients who would have otherwise underwent an open approach.
Difficulty in comparing the costs associated with robotic versus laparoscopic surgery is partly attributed to differences in how procedures are performed (e.g., fully robotic or laparoscopic procedures compared with hand-assisted procedures, conversion rates), variability of surgical skill, level of complexity of the case, as well as short-term versus long-term costs. Therefore, outcome evaluation should extend beyond economics and morbidity/mortality to include measurement of oncologic success in terms such as lymphnode harvest and conversion rate and long-term costs [10].
Across 13 studies, when robotic surgery was compared with both laparoscopic and open surgery, a comorbidity score greater than 0 on the Elixhauser or Charlson-Deyo comorbidity index was consistently associated with a lower likelihood of receiving robotic surgery (although results were less consistent when specific comorbidities were examined). Surgeons may be less likely to pursue robotic approaches with more medically complex patients, instead relying on more conventional techniques. In a study of 884 patients who underwent robotic procedures at the University of Illinois at Chicago, univariate analysis showed that cardiovascular and renal disease, hypertension, and cancer were associated with higher morbidity and mortality risk [71].
The literature also suggests that operative time is longer for robotic surgery relative to other approaches, which may increase risk for peri- and postoperative complications [14, 72]. Surgeons may be more inclined to elect a faster surgical approach for patients who are already at high risk for surgical complications due to comorbidities. Furthermore, patients with more medical problems are more likely to have an increased post-surgical length of stay for monitoring secondary to their comorbid conditions [73], which would negate the benefit of a shorter hospital stay (and corresponding decreased costs and increased reported quality of life) associated with a robotic approach [4, 6, 8, 9].
In this study, American Society of Anesthesiologists Physical Status Classification System class was not associated with receiving robotic surgery. However, robotic surgery may be beneficial for patients with certain comorbidities, such as obesity. A study examining outcomes in rectal cancer surgery showed decreased length of stay and reduced morbidity in patients with obesity who underwent robotic versus laparoscopic surgery [9]. Additional studies are warranted to examine how and why specific comorbidities contribute to a patient’s likelihood of undergoing robotic surgery.
Lastly, academic, or teaching, hospitals had the highest rates of utilization of robotic surgery compared with community and comprehensive community hospitals, a difference that was more noticeable when robotic surgery was compared with open surgery. The use of more technically advanced procedures tends to start at academic centers and then expand to community hospitals over time. The use of laparoscopic and robotic approaches still seems to be associated with higher hospital volume and with urban, teaching centers [35]. As these more-innovative approaches, including robotics, are more widely employed, it is important to ensure that they are offered equitably in the communities into which they are expanding.
Limitations
Our study is not without limitations. First, none of the primary studies included in our systematic review and meta-analysis were RCTs; the included studies primarily used retrospective datasets. Second, our qualitative and meta-analysis findings were primarily informed by unadjusted models because only 28% of the studies included in the analysis reported a multivariate examination of differences between factors. Our focus on unadjusted ORs in the meta-analysis increased the comparability of ORs across studies and allowed us to estimate ORs, which also increased the number of studies in the meta-analysis, as only two of the included studies reported effect sizes. Third, we did not aim to expound on the moderate to large heterogeneity between ORs for each comparison we examined (see in Tables 1, 2, 3). Nevertheless, the factors we investigated are sample characteristics that are often included in tests of moderation in meta-analyses. Therefore, our study emphasizes the importance of including these factors to account for heterogeneity in meta-analyses comparing robotic surgery with other procedures, as well as in primary studies. Fourth, we analyzed only the peer-reviewed literature published before September 7, 2021, excluding works such as conference abstracts and works in progress. Thus, some relevant studies may have been excluded from our review. However, given the frequent use of common parent investigations (e.g., National Cancer Database) and a paucity of evidence of publication/selection bias, we deem that our estimated ORs and CIs, using robust meta-analytic methods to handle the clustered structure of the data, represent a plausible range of effect sizes. Despite these limitations, this study is to our knowledge the first to synthesize and quantitatively assess the literature on disparities in the utilization of robotic colorectal surgery.
Conclusion
Our study provides a strong reference point to guide future research on disparities in the utilization of robotic approaches to colorectal surgery, while emphasizing several patient- and institution-related factors. Some factors associated with lower utilization of robotic surgery (e.g., comorbidities, age) seemed clinically justified, while other factors (e.g., nonteaching, nonacademic hospital type) were also likely to be associated with lower robotic surgery utilization. These institution-related influences are likely to become less important as utilization of robotic surgery increases over time and expands beyond academic centers. The nonclinical disparities in access to robotic surgery that we highlight require intervention to assure more equitable access to innovative technology in colorectal surgery, specifically among Black and underinsured patients who are less likely to undergo surgery using this innovative approach. Ultimately, increasing access to robotic surgery may also increase overall access to minimally invasive surgical approaches.
Supplementary Material
Acknowledgements
The authors extend gratitude to Eleanor Mayfield, ELS, for editorial support.
Funding
This research was supported by 5 For The Fight, Huntsman Cancer Institute, the Medical College of Wisconsin, and the V Foundation for Cancer Research; by the National Cancer Institute (NCI)—an entity of the National Institutes of Health (NIH)—under Grant K01CA234319; and by the Research Foundation of the American Society of Colon and Rectal Surgeons. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00464-022-09793-8.
Declarations
Disclosures Although unrelated to this study, Charles Rogers offers scientific input to research studies through an investigator services agreement with Exact Sciences, and Erin King-Mullins offers input as an Educational Consultant for THD America. Dana Hayden, Kevin Korous, Erin Brooks, Fa Tuuhetaufa, Abigail Martin, and Chassidy Grimes, have no conflicts of interest or financial ties to disclose.
References
- 1.Abu Gazala M, Wexner SD (2017) Re-appraisal and consideration of minimally invasive surgery in colorectal cancer. Gastroenterol Rep 5:1–10. 10.1093/gastro/gox001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osagiede O, Spaulding AC, Cochuyt JJ, Naessens J, Merchea A, Colibaseanu DT (2019) Disparities in minimally invasive surgery for colorectal cancer in Florida. J Laparoendosc Adv Surg Tech 29:926–933. 10.1089/lap.2019.0016 [DOI] [PubMed] [Google Scholar]
- 3.Keller DS, Delaney CP, Hashemi L, Haas EM (2016) A national evaluation of clinical and economic outcomes in open versus laparoscopic colorectal surgery. Surg Endosc 30:4220–4228. 10.1007/s00464-015-4732-6 [DOI] [PubMed] [Google Scholar]
- 4.Hollis RH, Cannon JA, Singletary BA, Korb ML, Hawn MT, Heslin MJ (2016) Understanding the value of both laparoscopic and robotic approaches compared to the open approach in colorectal surgery. J Laparoendosc Adv Surg Tech 26:850–856. 10.1089/lap.2015.0620 [DOI] [PubMed] [Google Scholar]
- 5.Papanikolaou IG (2014) Robotic surgery for colorectal cancer: systematic review of the literature. Surg Laparosc Endosc Percutan Tech 24:478–483. 10.1097/SLE.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 6.Sheng S, Zhao T, Wang X (2018) Comparison of robot-assisted surgery, laparoscopic-assisted surgery, and open surgery for the treatment of colorectal cancer: a network meta-analysis. Med (Baltimore) 97:e11817. 10.1097/MD.0000000000011817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2018) Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 267:1034–1046. 10.1097/SLA.0000000000002523 [DOI] [PubMed] [Google Scholar]
- 8.Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16:1480–1487. 10.1245/s10434-009-0435-3 [DOI] [PubMed] [Google Scholar]
- 9.Panteleimonitis S, Pickering O, Abbas H, Harper M, Kandala N, Figueiredo N, Qureshi T, Parvaiz A (2018) Robotic rectal cancer surgery in obese patients may lead to better short-term outcomes when compared to laparoscopy: a comparative propensity scored match study. Int J Colorectal Dis 33:1079–1086. 10.1007/s00384-018-3030-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters PS, Cheung FP, Peacock O, Heriot AG, Warrier SK, O’Riordain DS, Pillinger S, Lynch AC, Stevenson ARL (2020) Successful patient-oriented surgical outcomes in robotic vs laparoscopic right hemicolectomy for cancer—a systematic review. Colorectal Dis 22:488–499. 10.1111/codi.14822 [DOI] [PubMed] [Google Scholar]
- 11.Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ (2011) Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech 24:479–484. 10.1097/BSD.0b013e3182055cac [DOI] [PubMed] [Google Scholar]
- 12.Starkweather AR, Witek-Janusek L, Nockels RP, Peterson J, Mathews HL (2008) The multiple benefits of minimally invasive spinal surgery: results comparing transformational lumbar interbody fusion and posterior lumbar fusion. J Neurosci Nurs 40:32–39 [PMC free article] [PubMed] [Google Scholar]
- 13.Whitecloud TS, Roesch WW, Ricciardi JE (2001) Transforaminal interbody fusion versus anterior–posterior interbody fusion of the lumbar spine: a financial analysis. J Spinal Disord 14:100–103. 10.1097/00002517-200104000-00002 [DOI] [PubMed] [Google Scholar]
- 14.Kim CW, Kim CH, Baik SH (2014) Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg 18:816–830. 10.1007/s11605-014-2469-5 [DOI] [PubMed] [Google Scholar]
- 15.Kivimäki M, Batty GD, Pentti J, Shipley MJ, Sipilä PN, Nyberg ST, Suominen SB, Oksanen T, Stenholm S, Virtanen M, Marmot MG, Singh-Manoux A, Brunner EJ, Lindbohm JV, Ferrie JE, Vahtera J (2020) Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 5:e140–e149. 10.1016/S2468-2667(19)30248-8 [DOI] [PubMed] [Google Scholar]
- 16.Vukojević M (2017) Parental socioeconomic status as a predictor of physical and mental health outcomes in children—literature review. Acta Clin Croat. 10.20471/acc.2017.56.04.23 [DOI] [PubMed] [Google Scholar]
- 17.Akinyemiju T, Meng Q, Vin-Raviv N (2016) Race/ethnicity and socio-economic differences in colorectal cancer surgery outcomes: analysis of the nationwide inpatient sample. BMC Cancer 16:715. 10.1186/s12885-016-2738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson CN, Balentine CJ, Sansgiry S, Berger DH (2012) Disparities in the use of minimally invasive surgery for colorectal disease. J Gastrointest Surg 16:897–904. 10.1007/s11605-012-1844-3 [DOI] [PubMed] [Google Scholar]
- 19.Koerner C, Rosen SA (2019) How robotics is changing and will change the field of colorectal surgery. World J Gastrointest Surg 11:381–387. 10.4240/wjgs.v11.i10.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei D, Johnston S, Goldstein L, Nagle D (2020) Minimally invasive colectomy is associated with reduced risk of anastomotic leak and other major perioperative complications and reduced hospital resource utilization as compared with open surgery: a retrospective population-based study of comparative effectiveness and trends of surgical approach. Surg Endosc 34:610–621. 10.1007/s00464-019-06805-y [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10:89. 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borenstein M, Hedges LV, Higgins J, Rothstein HR (2021) Introduction to meta-analysis, 2nd edn. Wiley, Hoboken [Google Scholar]
- 24.Drukker M, Weltens I, van Hooijdonk CFM, Vandenberk E, Bak M (2021) Development of a methodological quality criteria list for observational studies: the observational study quality evaluation. Front Res Metr Anal 6:675071. 10.3389/frma.2021.675071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson D, Turner R (2017) Power analysis for random-effects meta-analysis: power analysis for meta-analysis. Res Synth Methods 8:290–302. 10.1002/jrsm.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanner-Smith EE, Tipton E, Polanin JR (2016) Handling complex meta-analytic data structures using robust variance estimates: a tutorial in R. J Dev Life-Course Criminol 2:85–112. 10.1007/s40865-016-0026-5 [DOI] [Google Scholar]
- 27.Vevea JL, Coburn K, Sutton A (2019) Publication bias. In: Cooper H, Hedges LV, Valentine JC (eds) The handbook of research synthesis and meta-analysis. Russell: Sage, pp 383–429 [Google Scholar]
- 28.Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 29.RStudio Team (2020) RStudio: integrated development environment for R [Google Scholar]
- 30.Cairns AL, Schlottmann F, Strassle PD, Di Corpo M, Patti MG (2019) Racial and socioeconomic disparities in the surgical management and outcomes of patients with colorectal carcinoma. World J Surg 43:1342–1350. 10.1007/s00268-018-04898-5 [DOI] [PubMed] [Google Scholar]
- 31.Hawkins AT, Ford MM, Benjamin Hopkins M, Muldoon RL, Wanderer JP, Parikh AA, Geiger TM (2018) Barriers to laparoscopic colon resection for cancer: a national analysis. Surg Endosc 32:1035–1042. 10.1007/s00464-017-5782-8 [DOI] [PubMed] [Google Scholar]
- 32.Damle RN, Flahive JM, Davids JS, Maykel JA, Sturrock PR, Alavi K (2016) Examination of racial disparities in the receipt of minimally invasive surgery among a national cohort of adult patients undergoing colorectal surgery. Dis Colon Rectum 59:1055–1062. 10.1097/DCR.0000000000000692 [DOI] [PubMed] [Google Scholar]
- 33.Gabriel E, Thirunavukarasu P, Al-Sukhni E, Attwood K, Nurkin SJ (2016) National disparities in minimally invasive surgery for rectal cancer. Surg Endosc 30:1060–1067. 10.1007/s00464-015-4296-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abd El Aziz MA, Grass F, Perry W, Behm KT, Shawki SF, Larson DW, Mathis KL (2020) Colectomy for patients with super obesity: current practice and surgical morbidity in the United States. Surg Obes Relat Dis 16:1764–1769. 10.1016/j.soard.2020.06.033 [DOI] [PubMed] [Google Scholar]
- 35.Addae JK, Gani F, Fang SY, Wick EC, Althumairi AA, Efron JE, Canner JK, Euhus DM, Schneider EB (2017) A comparison of trends in operative approach and postoperative outcomes for colorectal cancer surgery. J Surg Res 208:111–120. 10.1016/j.jss.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 36.Alharthi S, Reilly M, Arishi A, Ahmed AM, Chulkov M, Qu W, Ortiz J, Nazzal M, Pannell S (2020) Robotic versus laparoscopic sigmoid colectomy: analysis of healthcare cost and utilization project database. Am Surg 86:256–260. 10.1177/000313482008600337 [DOI] [PubMed] [Google Scholar]
- 37.Bell SW, Heriot AG, Warrier SK, Farmer CK, Stevenson ARL, Bissett I, Kong JC, Solomon M (2019) Surgical techniques in the management of rectal cancer: a modified Delphi method by colorectal surgeons in Australia and New Zealand. Tech Coloproctology 23:743–749. 10.1007/s10151-019-02052-4 [DOI] [PubMed] [Google Scholar]
- 38.Chung G, Hinoul P, Coplan P, Yoo A (2021) Trends in the diffusion of robotic surgery in prostate, uterus, and colorectal procedures: a retrospective population-based study. J Robot Surg 15:275–291. 10.1007/s11701-020-01102-6 [DOI] [PubMed] [Google Scholar]
- 39.Concors SJ, Murken DR, Hernandez PT, Mahmoud NN, Paulson EC (2020) The volume–outcome relationship in robotic protectectomy: does center volume matter? results of a national cohort study. Surg Endosc 34:4472–4480. 10.1007/s00464-019-07227-6 [DOI] [PubMed] [Google Scholar]
- 40.Fantus RJ, Cohen A, Riedinger CB, Kuchta K, Wang CH, Yao K, Park S (2019) Facility-level analysis of robot utilization across disciplines in the National Cancer Database. J Robot Surg 13:293–299. 10.1007/s11701-018-0855-9 [DOI] [PubMed] [Google Scholar]
- 41.Fernandez R, Anaya DA, Li LT, Orcutt ST, Balentine CJ, Awad SA, Berger DH, Albo DA, Artinyan A (2013) Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg 206:509–517. 10.1016/j.amjsurg.2013.01.036 [DOI] [PubMed] [Google Scholar]
- 42.Halabi WJ, Kang CY, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Stamos MJ, Pigazzi A (2013) Robotic-assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg 37:2782–2790. 10.1007/s00268-013-2024-7 [DOI] [PubMed] [Google Scholar]
- 43.Konstantinidis IT, Ituarte P, Woo Y, Warner SG, Melstrom K, Kim J, Singh G, Lee B, Fong Y, Melstrom LG (2020) Trends and outcomes of robotic surgery for gastrointestinal (GI) cancers in the USA: maintaining perioperative and oncologic safety. Surg Endosc 34:4932–4942. 10.1007/s00464-019-07284-x [DOI] [PubMed] [Google Scholar]
- 44.Lee MTG, Chiu CC, Wang CC, Chang CN, Lee SH, Lee M, Hsu TC, Lee CC (2017) Trends and outcomes of surgical treatment for colorectal cancer between 2004 and 2012- an analysis using National Inpatient Database. Sci Rep 7:1–8. 10.1038/s41598-017-02224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo BD, Zhang GQ, Stem M, Sahyoun R, Efron JE, Safar B, Atallah C (2021) Do specific operative approaches and insurance status impact timely access to colorectal cancer care? Surg Endosc 35:3774–3786. 10.1007/s00464-020-07870-4 [DOI] [PubMed] [Google Scholar]
- 46.Miller PE, Dao H, Paluvoi N, Bailey M, Margolin D, Shah N, Vargas HD (2016) Comparison of 30-day postoperative outcomes after laparoscopic vs robotic colectomy. J Am Coll Surg 223:369–373. 10.1016/j.jamcollsurg.2016.03.041 [DOI] [PubMed] [Google Scholar]
- 47.Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E (2018) Robotic versus laparoscopic colectomy for stage I-III colon cancer: oncologic and long-term survival outcomes. Surg Endosc 32:2894–2901. 10.1007/s00464-017-5999-6 [DOI] [PubMed] [Google Scholar]
- 48.Moghadamyeghaneh Z, Phelan M, Smith BR, Stamos MJ (2015) Outcomes of open, laparoscopic, and robotic abdominoperineal resections in patients with rectal cancer. Dis Colon Rectum 58:1123–1129. 10.1097/DCR.0000000000000475 [DOI] [PubMed] [Google Scholar]
- 49.Ofshteyn A, Bingmer K, Towe CW, Steinhagen E, Stein SL (2020) Robotic proctectomy for rectal cancer in the US: a skewed population. Surg Endosc 34:2651–2656. 10.1007/s00464-019-07041-0 [DOI] [PubMed] [Google Scholar]
- 50.Panteleimonitis S, Popeskou S, Harper M, Kandala N, Figueiredo N, Qureshi T, Parvaiz A (2018) Minimally invasive colorectal surgery in the morbid obese: does size really matter? Surg Endosc 32:3486–3494. 10.1007/s00464-018-6068-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parascandola SA, Hota S, Sparks AD, Boulos S, Cavallo K, Kim G, Obias V (2021) Trends in utilization, conversion rates, and outcomes for minimally invasive approaches to non-metastatic rectal cancer: a national cancer database analysis. Surg Endosc 35:3154–3165. 10.1007/s00464-020-07756-5 [DOI] [PubMed] [Google Scholar]
- 52.Sastow DL, White RS, Mauer E, Chen Y, Gaber-Baylis LK, Turnbull ZA (2019) The disparity of care and outcomes for medicaid patients undergoing colectomy. J Surg Res 235:190–201. 10.1016/j.jss.2018.09.056 [DOI] [PubMed] [Google Scholar]
- 53.Schootman M, Hendren S, Loux T, Ratnapradipa K, Eberth JM, Davidson NO (2017) Differences in effectiveness and use of robotic surgery in patients undergoing minimally invasive colectomy. J Gastrointest Surg 21:1296–1303. 10.1007/s11605-017-3460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon HL, Reif de Paula T, Spigel ZA, Keller DS (2021) National disparities in use of minimally invasive surgery for rectal cancer in older adults. J Am Geriatr Soc. 10.1111/jgs.17467 [DOI] [PubMed] [Google Scholar]
- 55.Spaulding AC, Hamadi H, Osagiede O, Lemini R, Cochuyt JJ, Watson J, Naessens JM, Colibaseanu DT (2021) Hospital robotic use for colorectal cancer care. J Robot Surg 15:561–569. 10.1007/s11701-020-01142-y [DOI] [PubMed] [Google Scholar]
- 56.Sujatha-Bhaskar S, Jafari MD, Gahagan JV, Inaba CS, Koh CY, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2017) Defining the role of minimally invasive proctectomy for locally advanced rectal adenocarcinoma. Ann Surg 266:574–581. 10.1097/SLA.0000000000002357 [DOI] [PubMed] [Google Scholar]
- 57.Villano AM, Zeymo A, Houlihan BK, Bayasi M, Al-Refaie WB, Chan KS (2020) Minimally invasive surgery for colorectal cancer: hospital type drives utilization and outcomes. J Surg Res 247:180–189. 10.1016/j.jss.2019.07.102 [DOI] [PubMed] [Google Scholar]
- 58.Yeo HL, Isaacs AJ, Abelson JS, Milsom JW, Sedrakyan A (2016) Comparison of open, laparoscopic, and robotic colectomies using a large national database: outcomes and trends related to surgery center volume. Dis Colon Rectum 59:535–542. 10.1097/DCR.0000000000000580 [DOI] [PubMed] [Google Scholar]
- 59.Buonpane C, Efiong E, Hunsinger M, Fluck M, Shabahang M, Wild J, Halm K, Long K, Buzas C, Blansfield J (2017) Predictors of utilization and quality assessment in robotic rectal cancer resection: a review of the National Cancer Database. Am Surg 83:918–924. 10.1177/000313481708300847 [DOI] [PubMed] [Google Scholar]
- 60.Veenhof AAFA, Engel AF, van der Peet DL, Sietses C, Meijerink WJHJ, de Lange-de Klerk ESM, Cuesta MA(2008) Technical difficulty grade score for the laparoscopic approach of rectal cancer: a single institution pilot study. Int J Colorectal Dis 23:469–475. 10.1007/s00384-007-0433-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogiso S, Yamaguchi T, Hata H, Fukuda M, Ikai I, Yamato T, Sakai Y (2011) Evaluation of factors affecting the difficulty of laparoscopic anterior resection for rectal cancer: “narrow pelvis” is not a contraindication. Surg Endosc 25:1907–1912. 10.1007/s00464-010-1485-0 [DOI] [PubMed] [Google Scholar]
- 62.Fleming CA, Cullinane C, Lynch N, Killeen S, Coffey JC, Peirce CB (2021) Urogenital function following robotic and laparoscopic rectal cancer surgery: meta-analysis. Br J Surg 108:128–137. 10.1093/bjs/znaa067 [DOI] [PubMed] [Google Scholar]
- 63.Kim HJ, Choi GS, Park JS, Park SY, Yang CS, Lee HJ (2018) The impact of robotic surgery on quality of life, urinary and sexual function following total mesorectal excision for rectal cancer: a propensity score-matched analysis with laparoscopic surgery. Colorectal Dis 20:O103–O113. 10.1111/codi.14051 [DOI] [PubMed] [Google Scholar]
- 64.Kim NK, Kim YW, Cho MS (2015) Total mesorectal excision for rectal cancer with emphasis on pelvic autonomic nerve preservation: expert technical tips for robotic surgery. Surg Oncol 24:172–180. 10.1016/j.suronc.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 65.Wood KL, Haider SF, Bui A, Leitman IM (2020) Access to common laparoscopic general surgical procedures: do racial disparities exist? Surg Endosc 34:1376–1386. 10.1007/s00464-019-06912-w [DOI] [PubMed] [Google Scholar]
- 66.Schneider MA, Gero D, Müller M, Horisberger K, Rickenbacher A, Turina M (2021) Inequalities in access to minimally invasive general surgery: a comprehensive nationwide analysis across 20 years. Surg Endosc 35:6227–6243. 10.1007/s00464-020-08123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramji KM, Cleghorn MC, Josse JM, MacNeill A, O’Brien C, Urbach D, Quereshy FA (2016) Comparison of clinical and economic outcomes between robotic, laparoscopic, and open rectal cancer surgery: early experience at a tertiary care center. Surg Endosc 30:1337–1343. 10.1007/s00464-015-4390-8 [DOI] [PubMed] [Google Scholar]
- 68.Hah JM, Lee E, Shrestha R, Pirrotta L, Huddleston J, Goodman S, Amanatullah DF, Dirbas FM, Carroll IR, Schofield D (2021) Return to work and productivity loss after surgery: a health economic evaluation. Int J Surg 95:106100. 10.1016/j.ijsu.2021.106100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright JP, Albert MR (2020) A current review of robotic colorectal surgery. Ann Laparosc Endosc Surg 5:9. 10.21037/ales.2019.12.01 [DOI] [Google Scholar]
- 70.Hopkins MB, Geiger TM, Bethurum AJ, Ford MM, Muldoon RL, Beck DE, Stewart TG, Hawkins AT (2020) Comparing pathologic outcomes for robotic versus laparoscopic surgery in rectal cancer resection: a propensity adjusted analysis of 7616 patients. Surg Endosc 34:2613–2622. 10.1007/s00464-019-07032-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchs NC, Addeo P, Bianco FM, Gorodner V, Ayloo SM, Elli EF, Oberholzer J, Benedetti E, Giulianotti PC (2012) Perioperative risk assessment in robotic general surgery: lessons learned from 884 cases at a single institution. Arch Surg 147:701–708. 10.1001/archsurg.2012.496 [DOI] [PubMed] [Google Scholar]
- 72.Kirchhoff P, Clavien P-A, Hahnloser D (2010) Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 4:5. 10.1186/1754-9493-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider EB, Hyder O, Brooke BS, Efron J, Cameron JL, Edil BH, Schulick RD, Choti MA, Wolfgang CL, Pawlik TM (2012) Patient readmission and mortality after colorectal surgery for colon cancer: impact of length of stay relative to other clinical factors. J Am Coll Surg 214:390–398. 10.1016/j.jamcollsurg.2011.12.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.