Abstract
Chemotherapy has a significant positive impact in cancer treatment outcomes, reducing recurrence and mortality. However, many cancer surviving children and adults suffer from aberrant chemotherapy neurotoxic effects on learning, memory, attention, executive functioning, and processing speed. This chemotherapy-induced cognitive impairment (CICI) is referred to as “chemobrain” or “chemofog”. While the underlying mechanisms mediating CICI are still unclear, there is strong evidence that chemotherapy accelerates the biological aging process, manifesting as effects which include telomere shortening, epigenetic dysregulation, oxidative stress, mitochondrial defects, impaired neurogenesis, and neuroinflammation, all of which are known to contribute to increased anxiety and neurocognitive decline. Despite the increased prevalence of CICI, there exists a lack of mechanistic understanding by which chemotherapy detrimentally affects cognition in cancer survivors. Moreover, there are no approved therapeutic interventions for this condition. To address this gap in knowledge, this review attempts to identify how adenosine signaling, particularly through the adenosine A2A receptor, can be an essential tool to attenuate accelerated aging phenotypes. Importantly, the adenosine A2A receptor uniquely stands at the crossroads of cancer treatment and improved cognition, given that it is widely known to control tumor induced immunosuppression in the tumor microenvironment, while also posited to be an essential regulator of cognition in neurodegenerative disease. Consequently, we propose that the adenosine A2A receptor may provide a multi-faceted therapeutic strategy to enhance anticancer activity, while combating chemotherapy induced cognitive deficits, both which are essential to provide novel therapeutic interventions against accelerated aging in cancer survivors.
1. Introduction
The discovery and development of a wide range of chemotherapeutic drugs has a significantly positive impact in cancer treatment. However, while chemotherapy reduced recurrence and mortality and increased survival rate in a variety of cancers, particularly in breast cancer, many cancer survivors also suffer from aberrant neurotoxic effects of chemotherapy on multiple cognitive domains including learning, memory, attention, and executive functioning. This chemotherapy-induced cognitive impairment (CICI) is often referred to as “chemobrain” or “chemofog” (Falleti, Sanfilippo, Maruff, Weih, & Phillips, 2005). While it is possible that cancer can cause cognitive decline due to tumorigenic inflammation (Ahles, Saykin et al., 2008; Wefel, Lenzi et al., 2004), CICI may occur both in the acute period of treatment and chronic period following treatment, and has been reported in up to 75% of patients (Koppelmans, Breteler et al., 2012; Wefel & Schagen, 2012; Weiss, 2008). A subset of survivors (15–20%) experience measurably persistent cognitive decline even after the termination of a chemotherapy regimen (de Ruiter, Reneman et al., 2011; Heflin, Meyerowitz et al., 2005; Lange, Joly et al., 2019; Lange, Licaj et al., 2019). This can affect both adult and pediatric cancer populations, and unfortunately, since there are no current FDA approved clinical treatments, CICI represents a significant public health concern (ACS, 2019). Therefore, understanding the neurobiological underpinnings of CICI will foster development of strategies to alleviate these neurotoxic effects which will ultimately improve the quality of life for cancer survivors.
While the underlying mechanisms mediating CICI are largely unknown, both clinical and preclinical evidence suggests that chemotherapy adversely alters brain function similar to the aging process, manifesting as effects which include increased anxiety, neurocognitive decline, impaired neurogenesis, and increased gliosis. In addition, chemotherapy accelerates biological and molecular aging (Demaria et al., 2017; Sanoff, Deal et al., 2014; Yoo, Tang et al., 2021) and increases the risk for the development of Alzheimer’s disease (Kesler, Rao, Ray, Rao, & I. Alzheimer’s Disease Neuroimaging, 2017), all of which have been observed in breast cancer patients. These observations suggest that CICI and brain aging may share common pathogenic mechanisms mediating cognitive impairment. However, how chemotherapy accelerates brain aging and whether this detrimental effect can be prevented remains unclear. In this regard, adenosine A2A receptor (A2AR) signaling has emerged as a hallmark of age-related diseases which encompass neurodegeneration as well as cancer. Notably, the adenosine A2AR has recently garnered attention as a potential therapeutic target for chemotherapy-induced adverse effects ranging from cognitive impairment, peripheral neuropathy, to nephrotoxicity (Dewaeles, Carvalho et al., 2022; Oliveros, Yoo et al., 2022). Throughout this chapter we will take into consideration how adenosine A2AR receptor function is involved in cognition from a physiological perspective, as well as its involvement in pathological conditions such as accelerated aging and CICI (Fig. 1). To facilitate this discussion, we briefly introduce the phenomenon of chemobrain from a clinical perspective in adult and pediatric cancer survivors, followed by a description of studies associating accelerated aging mechanisms with the chemotherapy treatment related toxicities that are known to bestow neurocellular dysfunctions and cognitive impairments. We then examine adenosine A2AR involvement in cancer-related immunosuppression and posit that the adenosine A2AR is uniquely positioned to be a therapeutic target in neurocellular dysfunctions and cognitive impairments stemming from chemotherapy related accelerated brain aging (Fig. 1).
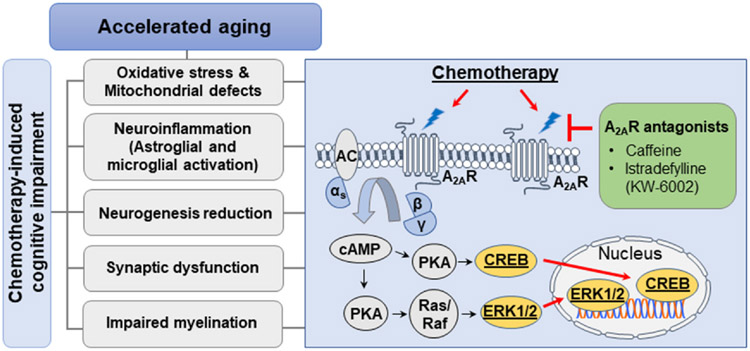
Fig. 1.
Potential cellular and molecular mechanisms mediating the convergence of chemotherapy-induced cognitive impairment and accelerated aging phenotypes. Chemotherapy induced cognitive impairments (CICI) can be experienced by cancer patients with varying genotoxic mechanisms of action, including antifolate anti-metabolites (methotrexate), platinum-based compounds (cisplatin), as well as anthracyclines (doxorubicin), amongst others. Notably, mounting evidence suggests that generally, chemotherapies target multiple cellular and molecular mechanisms that, when disrupted, are widely known to negatively affect hallmarks of cognitive function, such as reductions in the neurogenic potential of the hippocampus, deficient functional neuronal architecture of dendrites yielding stunted dendrite spine densities, and impaired oligodendrocyte progenitor development which hinders myelination capacity. Several lines of evidence suggest that oxidative stress generated by mitochondrial defects, as well as neuroinflammatory sequelae resulting from proinflammatory cytokine penetration of the brain parenchyma (stemming from chemotherapy induced weakening of the blood brain barrier), are candidate hypotheses that may explain how CICI develops. Interestingly, the phenotypes that typify CICI also share a commonality with cellular and molecular detriments observed in cancer-related accelerated aging and neurodegenerative-related accelerated aging. These shared common pathological mechanisms include mitochondrial oxidative stress, and an increased proinflammatory cytokine secretory response engaged by chemotherapy’s apoptotic and necrotic effect on malignancies. Similarly, CICI and neurodegeneration associated accelerated aging share impairments in neurogenesis, synaptic function, and myelination. One pathway that may elucidate the commonality between CICI, cancer-related accelerated aging, and neurodegenerative associated accelerated aging, is through inhibition of adenosine A2AR signaling. Inhibition of A2AR has been demonstrated to sensitize chemotherapy against malignancies as well as prevent tumor induced immune evasion in the tumor microenvironment. Importantly, increased adenosine A2AR is implicated with cognitive deficits in Alzheimer’s disease. A2AR inhibition is associated with in improved cognition in preclinical models of neurodegenerative disease, as well as preservation of neuronal architecture, neurogenesis, and cognitive function in a preclinical model of cisplatin induced CICI. Taken together, we propose that CICI may be classified as a form of accelerated aging, and that the adenosine A2AR could serve as a unique nexus that can provide therapeutic efficacy in cancer survivors and accelerated aging phenotypes.
2. Adenosine A2AR in cognitive function
2.1. Physiological role of A2AR and cognitive improvement
Adenosine is an endogenous neurotransmitter that plays an essential role in synaptic plasticity and maintenance of brain homeostasis through four distinct adenosine receptors: A1R, A2AR, A2BR and A3R (Chen, 2014). Through adenylate cyclase activation, the Gs-protein coupled adenosine A2AR engages cyclic adenosine monophosphate (cAMP) resulting in subsequent protein kinase-A mediated (PKA) phosphorylation of downstream CREB and ERK resulting transcriptional activity (Chen Choi & Cunha, 2023; Nam, Bruner, & Choi, 2013). Known to have high abundance in the striatum, and to a lesser degree, in the hippocampus and cortex (Liu, Chen et al., 2019), the A2AR is increasingly a notable regulator of neuro-glial interactions (Orr, Hsiao et al., 2015; Orr, Orr, Li, Gross, & Traynelis, 2009), neurodevelopment (Rodrigues, Marques, & Cunha, 2019), corticolimbic synaptic plasticity (Reis, Silva et al., 2019), and memory function (Li et al., 2018; Temido-Ferreira, Coelho, Pousinha, & Lopes, 2019). In regards to physiological expression and functionality, the A2AR can be found at presynaptic and postsynaptic terminals of neurons, where through receptor-receptor interactions with the adenosine A1R, or the dopamine D2 receptor, for example, it can fine tune synaptic long-term potentiation (LTP) and long-term depression (LTD), the putative hallmark mechanisms by which memory and emotive behavior are controlled (Chen, Lee, & Chern, 2014; Rebola, Lujan, Cunha, & Mulle, 2008; Rebola, Rodrigues et al., 2005). Importantly, postsynaptic activation/inhibition of the A2AR is effective in the control of aberrant synaptic plasticity, thus making this receptor important for improvement of memory function in normal aging physiology, as well as in pathophysiological conditions that impair learning and memory function (Chen, 2014). The physiological role of A2AR in cognitive improvement is exemplified by the global use of the cognitive enhancer caffeine, which is a partial A2AR antagonist and known to improve cognitive function in normal aging and neurodegenerative conditions (e.g., Alzheimer’s disease), as well as in preclinical rodent models of learning and memory (Canas, Porciuncula et al., 2009; Chen, 2014; Kaster, Machado et al., 2015; Laurent, Burnouf et al., 2016; Merighi, Borea et al., 2022).
2.2. A2AR and cognitive improvement in pathological conditions
Interestingly, a high level of A2AR expression is observed in the aged brain, as well as in brains from Alzheimer’s disease and Parkinson’s disease (PD) patients and mouse models of these conditions (Calon, Dridi et al., 2004; Chen, 2014; Orr, Hsiao et al., 2015; Temido-Ferreira, Ferreira et al., 2020). Importantly, A2AR antagonists are known to improve deficits in synaptic plasticity as well as learning and memory function in mouse models of AD and PD (Da Silva et al., 2016; Ferreira, Temido-Ferreira et al., 2017; Li, Chen et al., 2018; Li, Silva et al., 2015; Orr, Lo et al., 2018; Silva, Lemos et al., 2018; Viana Da Silva Haberl et al., 2016), whereas overactivation of neuronal A2AR impairs memory function (Carvalho, Faivre et al., 2019), suggesting a causal relationship between A2AR in neurodegeneration and therapy. More recently, the A2AR has also been demonstrated to be involved in neurotoxicity and cognitive impairments associated with chemotherapy (Gyau & Deaglio, 2023; Oliveros, Yoo et al., 2022). Given adenosine’s capacity to affect normal and pathological conditions, and in particular those pertaining to brain function through adenosine receptors (Kaur, Weadick et al., 2022), we propose that the adenosine A2A receptor uniquely stands at the crossroads of cancer treatment and cognition, as it may represent a novel therapeutic target for cancer survivors.
2.3. Convergence of the adenosine A2AR, aging and AD
The proposed existence of two distinct subtypes of purinergic receptors by Geoffrey Burnstock revealed a distinct difference in affinity for either adenosine (P1 receptors) or conversely, P2 receptors, which have a higher affinity for ATP (Burnstock 1978). Further investigation revealed that P1 receptors could be classified by adenosine’s inherent ability to either inhibit (A1R) or stimulate (A2AR) downstream cAMP signaling in cultured mouse perinatal brain cells (Van Calker, Muller, & Hamprecht, 1979). Similarly, adenosine receptor function was identified by their ability to modulate acetylcholine and glutamatergic neurotransmitter release in the striatum and hippocampus (Ferre, Sarasola, Quiroz, & Ciruela, 2023; Sperlagh & Vizi, 2011; Spignoli, Pedata, & Pepeu, 1984). In terms of AD related neurodegeneration, increased hippocampal expression of astroglial A2AR has been reported in postmortem AD brains, as well as in preclinical AD-related human amyloid precursor protein (hAPP) mouse models (Orr, Hsiao et al., 2015). Dias and colleagues also identified that functional inhibition of astroglial P2 receptors by the specific A2AR antagonist SCH58261 occurred in a PKA dependent manner, whereas the presence of amyloid-ß (1–42) peptides was sufficient to disrupt this interaction (Dias, Madeira et al., 2022). In support of the unique relationship between aging and A2AR expression, A2AR densities and downstream cAMP signaling is increased in 2-year-old rat limbic cortex when compared to 6-week old rats (Lopes, Cunha, & Ribeiro, 1999). Similarly, the frontal cortex of postmortem AD brains have been shown to exhibit increased A2AR expression (Albasanz, Perez, Barrachina, Ferrer, & Martin, 2008), thus emphasizing the adenosine A2AR as a potential regulator of accelerated aging (Dias, Madeira et al., 2022; Lopes, Cunha, & Agostinho, 2021). In support of this hypothesis, a significant body of preclinical studies have demonstrated that A2AR inhibition offers cognitive improvements (Liu, Chen et al., 2019). Of note, activation of the hypothalamic-pituitary-adrenal (HPA) axis can result in excessive cortisol release during advanced aging, and this HPA axis engagement can be further exacerbated by forebrain A2AR activation, thus resulting in synaptic plasticity deficits and memory impairments (Batalha, Ferreira et al., 2016). Conversely, impairments in synaptic plasticity and memory function can be restored by global A2AR deletion, selective deletion of A2AR in forebrain neurons, the non-specific A2AR antagonist caffeine, and the specific A2AR antagonists istradefylline (KW-6002) and SCH-58261 (Batalha, Ferreira et al., 2016; Kaster, Machado et al., 2015). Notably, A2AR elevations have been associated with dysfunctional hippocampal regulation of the HPA axis, resulting in accelerated clinical AD progression, memory impairments, and hippocampal ß-amyloid and Tau accumulation in AD mouse models (Csernansky, Dong et al., 2006; Green, Billings, Roozendaal, McGaugh, & LaFerla, 2006; Temido-Ferreira, Ferreira et al., 2020). Conversely, genetic A2AR deletion or pharmacological inhibition of A2AR that target hippocampal neural cells are reported to improve accelerated aging memory, synaptic plasticity and neurocellular dysfunctions caused by amyloid-ß (1–42) peptide induced neurotoxicity, and Tau accumulation in preclinical mouse models of AD (Canas, Porciuncula et al., 2009; Carvalho, Faivre et al., 2019; Goncalves, Lopes et al., 2019; Kaster, Machado et al., 2015; Laurent, Burnouf et al., 2016; Temido-Ferreira, Coelho et al., 2019). Taken together, these reports suggest that the A2AR plays a significant role in regulation of aging, and neurodegenerative disease. Further investigations are warranted to determine whether the adenosine A2AR inhibition could play a mechanistic role in attenuation of accelerated aging phenotypes.
3. Findings of cognitive dysfunction in chemobrain
3.1. Clinical imaging studies
Due to advanced imaging technology, the impact of chemotherapy on brain functionality has been widely investigated, with magnetic resonance imaging (MRI) playing a pivotal role in identifying which specific brain regions are negatively affected, often typified by reductions in brain volumes. For example, Mc Donald and colleagues demonstrated that breast cancer patients exhibited decreased gray matter in several brain regions, including the frontal and temporal cortices, the cerebellum, and the right thalamus, immediately after chemotherapy (McDonald, Conroy, Ahles, West, & Saykin, 2010). Importantly, frontal gray matter reduction observed in breast cancer patients after chemotherapy was associated with deficits in cortical executive function and memory processes (McDonald, Conroy et al., 2010). Functional MRI (fMRI) studies in chemotherapy-treated women also found reduced activation during cognitive tasks in multiple brain regions including the left caudal lateral prefrontal cortex, the dorsolateral prefrontal cortex, and the parahippocampal gyrus, all of which were significantly correlated with impairments in executive functioning, planning performance and recognition memory (De Ruiter, Reneman et al., 2011; Kesler, Kent, & O’Hara, 2011). In contrast to studies showing hypoactivation, several groups also report increased activation in multiple brain regions. For instance, an fMRI study conducted by Cimprich et al. (2010) investigated verbal working memory in patients with breast cancer both prior and post chemotherapy treatment and found greater levels of activation in the right inferior frontal gyrus and the frontoparietal attentional network (FPN), including bilateral frontal and parietal regions (Cimprich, Reuter-Lorenz et al., 2010). Another study conducted by Scherling and colleagues (2011) examined neurofunctional differences in working memory in breast cancer patients compared to controls prior to chemotherapy treatment (Scherling, Collins, Mackenzie, Bielajew, & Smith, 2011). This investigation revealed that relative to controls, breast cancer patients made fewer commissioned errors and were slower than controls, and these effects were associated with increased activation of the left inferior frontal gyrus, left insula, bilateral thalamus and right midbrain during the working memory tasks. This led the authors to conclude that breast cancer patients found the task more challenging than the control group, thus suggesting that breast cancer patients, to maintain a baseline level of performance, necessitate a greater recruitment of brain regions in order to maintain accuracy in memory tasks (Simo, Rifa-Ros, Rodriguez-Fornells, & Bruna, 2013). Nevertheless, these studies provide evidence that the symptomatology of chemobrain has a solid biological basis, rather than being purely psychological.
4. Chemobrain as an accelerated aging phenotype in adult and pediatric cancer
4.1. Evidence of accelerated aging in adult cancer patients
Aging is the time dependent progression of dynamic cellular, molecular, metabolic, biochemical, and cognitive changes across the lifespan of an organism. Inescapably, aging is typified by pathologies that include accumulation of cellular damage, increased oxidative stress, epigenetic and genomic instability, less-than adequate cellular repair mechanisms, telomere attrition, ineffective signaling cascades, detrimental transcriptional activity, increased peripheral inflammation, blood brain barrier disruption and exacerbated neuroinflammation (Armstrong & Boonekamp, 2023; Chiang, Huo, Kavelaars, & Heijnen, 2019; Knox, Aburto, Clarke, Cryan, & O’Driscoll, 2022; Lopez-Otin, Blasco, Partridge, Serrano, & Kroemer, 2022; Ness, Armstrong et al., 2015; Schmauck-Medina, Moliere et al., 2022; Squassina, Manchia et al., 2020). Despite healthy aging inevitably increasing these pathological processes, and often in the absence of apparent clinical syndromes (Banks, Reed, Logsdon, Rhea, & Erickson, 2021), a biological system’s inherent inability to prevent aging-related processes will eventually manifest into overt clinical disease. The resulting age-related complications include (but are not limited to) cardiovascular and cardiopulmonary pathologies, neurodegenerative related motor and memory dysfunctions, and for the purposes of this review, cancerous malignancies and the accompanying neuropathological and cognitive disturbances associated with chemotherapy (Carroll, Bower, & Ganz, 2022; Lopez-Otin, Blasco et al., 2022).
Not surprisingly, the resulting off-target effects of chemotherapy described from studies involving patients with breast cancer, are hypothesized to resemble pathologies akin to accelerated aging, given that both chemotherapy and accelerated aging are accompanied by dysfunctionality in several cellular hallmarks of cognitive function (Nguyen & Ehrlich, 2020). These include a compromised ability for healthy neural stem cell (NSC) maturation (i.e. adult neurogenesis), a loss of dendritic spines and dendrite arborization complexity, as well as inadequate neurotransmitter release (Onzi et al., 2022). In addition, chemotherapy induced accelerated aging can lead to negative impacts on cellular mechanisms of neuronal support that control adequate cognition, such as inoperative oligodendrocyte maturation leading to myelination deficits (Cardoso et al., 2020; Geraghty, Gibson et al., 2019; Gibson & Monje, 2019; Matsos, Loomes et al., 2017). It has been well established that cancer development, as well as chemotherapy’s apoptotic and necrotic effect on tumor cells, is associated with increased systemic inflammation and an immune response near the tumor site (Stagg & Smyth, 2010). Consequently, chemotherapy’s ability to damage DNA and increase oxidative stress is accompanied by blood brain barrier (BBB) breakdown (Wardill, Mander et al., 2016). Accordingly, chemotherapy induced BBB breakdown increases the propensity for peripheral permeation of the brain parenchyma by proinflammatory cytokines that increase the likelihood of reactive astrogliosis taking precedence over astroglial neuronal support, and in addition, can shift microglia behavior from surveillance to reactivity (Gibson, Nagaraja et al., 2019; Jia, Zhou et al., 2023; Monje, Vogel et al., 2007). Not surprisingly it is posited that the increase in inflammatory cytokines by chemotherapy is an aspect of accelerated aging, termed senescence associated secretory phenotype (SASP), which has also been described to contribute to cognitive dysfunction Alzheimer’s disease (Guerrero, De Strooper, & Arancibia-Carcamo, 2021; Wang, Prizment, Thyagarajan, & Blaes, 2021). Simply stated, cellular senescence is a natural, age-related process by which cells decline in their ability to divide, thus entering into a terminally quiescent, albeit functional state. Cellular senescence can also be engaged by oncogenic signaling, nutrient deprivation, mitochondrial dysfunction, and genotoxic chemotherapeutics, all of which activate accelerated aging sequelae (Gorgoulis, Adams et al., 2019). Consequently, SASP can increase secretion of growth factors, monocyte chemoattractant proteins, receptor proteins, chemokines and proinflammatory cytokines such as IL-6, IL-8, TGF-ß, TNF-α, amongst others (Gorgoulis, Adams et al., 2019; Wang, Prizment et al., 2021). Overlapping with SASP released proinflammatory markers, chemotherapy treated cancer patients also exhibit increased levels of peripheral and brain inflammatory cytokines (e.g. IL-6, IL-8, TNF-α) that are associated with decreased cognitive function (Carroll, Bower et al., 2022; Hoogland, Nelson et al., 2019; Laird, McMillan et al., 2013; Rummel, Chaiswing, Bondada, St Clair, & Butterfield, 2021). Preclinical animal models of methotrexate, doxorubicin and cisplatin CICI also support these findings, as increased BBB breakdown fosters increased entry of peripheral cytokines, such as IL-6, IL-8, IL-1ß and TNF-α which promote both astroglial and microglial reactivity (Alexander, Mahalingam et al., 2022; Chiang, Huo et al., 2019; Gibson, Nagaraja et al., 2019; Savchuk & Monje, 2022), causing further proinflammatory sequelae culminating in neurotoxicity that damages white matter, neurons, and consequently, disrupts cognitive function (Cardoso et al., 2020; George, Semendric, Hutchinson, & Whittaker, 2021; Lacourt & Heijnen, 2017; Ren, Clair, & Butterfield, 2017; Ren, Keeney et al., 2019). Clearly, the complexity of detriments exerted by cancer and cancer chemotherapy that result in cancer related cognitive dysfunction are multifaceted, and can be attributed to several factors, including high stress from cancer diagnosis, peripheral proinflammatory sequelae derived from the innate and/or adaptive immune responses to malignancies, or from chemotherapy and/or radiotherapy treatment devised to eradicate tumors (Argyriou, Assimakopoulos, Iconomou, Giannakopoulou, & Kalofonos, 2011; Wefel, Kesler, Noll, & Schagen, 2015). In regards to the accelerated aging similarities between CICI and neurodegenerative disease, recent studies demonstrate that chemotherapy increases the risk for development of AD. To that effect, the apolipo-protein E4 allele (APOE4), one of the strongest genetic risk factors for AD, links chemotherapy treatment and APOE4 as a potential risk factor in cognitive impairments associated with anthracycline chemotherapy (Fernandez, Varma, Flowers, & Rebeck, 2020). For instance, breast and testicular cancer patients receiving doxorubicin chemotherapy that are genetic carriers of APOE4, are also more likely to exhibit worse attentional processing speed and working memory impairments (Ahles, Li et al., 2014; Ahles, Saykin et al., 2003; Amidi, Agerbaek et al., 2017). Supporting this hypothesis, doxorubicin treatment in preclinical mouse models of familial Alzheimer’s disease mutations (5XFAD), which recapitulate AD cellular pathogenic and behavioral impairments, had increased reactive astrogliosis surrounding cortical amyloid beta deposits (Ng, Biran et al., 2022). CICI is also shown to be associated with accelerated development of tau (a hallmark of AD) clustering in the brain, as a sign of accelerated aging (Chiang, Huo et al., 2019). Furthermore, transgenic APOE4 knock in mice administered doxorubicin displayed reduced cortical and hippocampal volumes alongside impairments in spatial memory (Demby, Rodriguez et al., 2020; Speidell, Demby et al., 2019). Another potentially viable senescence associated target that was recently identified to be increased in cortical and hippocampal tissues of aged rodent models is the cyclin dependent kinase inhibitor and tumor suppressor p16INK4A (Wang Muthu Karuppan et al., 2021; Xie, Zhi, & Meng, 2021). Notably, p16INK4A is also routinely identified as a biomarker of chemotherapy induced senescent tissues in accelerated aging models (Baker, Wijshake et al., 2011; Sanoff, Deal et al., 2014; Uziel, Lahav et al., 2020). Hence, future investigations into the potential functional role that p16INK4A can exert in CICI may provide novel pharmacological or genetic therapeutic strategies to combat accelerated aging in CICI. Collectively, the convergence of these observations suggests that cancer survivors experiencing CICI display various phenotypes that are hallmarks of the accelerated aging process. These include increases in peripheral proinflammatory mediators that penetrate the brain parenchyma due to BBB dysfunction, elevated reactive astrogliosis and microgliosis stemming from increased neuroinflammation, and defective myelination capacity that hinders effective corticolimbic function. Together, these phenotypes foment cognitive deficits that can be exacerbated by genetic risk factors (i.e. APOE4), thus necessitating rapid development of treatment strategies to improve quality of life in cancer survivors.
4.2. Evidence of accelerated aging phenotypes in pediatric cancer patients
Similar as what was observed in adult cancer patients, approximately 500,000 survivors from childhood cancer live in the United States and up to 35% will experience persistent neurocognitive impairment months or years after treatment (Williams & Cole, 2021). Given that chemotherapies target proliferating cells, it is not surprising that neural stem cells and oligodendrocyte progenitors undergoing neurogenic development are particularly vulnerable (Mogavero, Bruni, DelRosso, & Ferri, 2020; Sekeres, Bradley-Garcia, Martinez-Canabal, & Winocur, 2021). Therefore, CICI in pediatric and adolescent cancer survivors is particularly egregious and damaging to brain development in this population. Studies utilizing fMRI in ALL pediatric patients have identified reduced volumes in multiple brain structures, including the cortex, hippocampus, white matter structures (e.g. corpus callosum), amygdala, thalamus and striatum (van der Plas, Schachar et al., 2017; Stefancin, Cahaney et al., 2020). More importantly, these brain volume deficits are significantly associated with deficiencies in working memory, attentional processing, sleep disruptions, behavioral response inhibition, and poor planning, which are confirmed by studies showing that 67% of pediatric cancer survivors report attention deficits, and up to 35% of survivors report memory dysfunction (Brace, Lee, Cole, & Sussman, 2019; Cole, Finkelstein et al., 2015; Mogavero, Bruni et al., 2020). Confirming these findings, preclinical adolescent rodent studies that employ methotrexate, a frontline chemotherapy utilized to treat pediatric cancer patients, similarly show disruptions in cognition, synaptic plasticity, myelination and neurogenesis (Geraghty, Gibson et al., 2019; Gibson, Nagaraja et al., 2019; Wen, Maxwell et al., 2018; Wen, Patel et al., 2022). Several neurobiological processes are disrupted in chemotherapy induced accelerated aging which are hypothesized to contribute to the frail physicality and cognitive impairments in young cancer survivors, which strikingly resemble similar patterns observed in frail advanced aged older populations. These include increased oxidative stress, telomere shortening, and inflammatory sequelae which can explain the aggravated physical frailty, and increased morbidity from secondary cardiovascular and cardiopulmonary pathologies, as well as attention, memory, and learning dysfunction, in addition to psychological distress experienced by pediatric cancer survivors (Dewar, Ahn, Eraj, Mahal, & Sanford, 2021; Williams & Cole, 2021; Williams, Krull et al., 2021).
4.3. Accelerated aging mitochondrial defects associated with cancer and chemobrain
Mitochondria are essential cellular organelles that provide energy for biochemical processes and metabolic function and dictate successful longitudinal survivorship across the lifespan. However, despite the essentiality of this organelle for survival, mitochondrial function deteriorates with normal aging (Tang, Oliveros, & Jang, 2019). Aging-related impairments in mitochondria include decreased function of respiratory chain protein complexes, reduced oxidative phosphorylation capacity, deficits in adenosine triphosphate (ATP) generation, and enhanced cytochrome-C release which engage the mitochondrial apoptotic cascade (Tang, Oliveros et al., 2019). Moreover, mitochondrial impairments elevate generation of reactive oxygen species (ROS) which promote downstream inflammasome activation of caspases, and eventually cell death (Lopez-Otin, Blasco et al., 2022). Not surprisingly, exacerbated deterioration of mitochondria is exhibited by diseases that typify accelerated aging, including neurodegenerative conditions and cancer treatment (Amorim, Coppotelli et al., 2022). Consequently, many cancer chemotherapies, including doxorubicin, cyclophosphamide, cisplatin, fluorouracil (5-FU), methotrexate, paclitaxel and others, in their efforts to induce death of cellular malignancies, can generate mitochondrial ROS that negligently affect non-cancerous cells near the tumor site, and permeate the BBB to negatively affect the function of distal corticolimbic brain structures (Rummel, Chaiswing et al., 2021). Chemotherapy treatment depletes the abundance of free-radical scavenging antioxidant biomolecules (e.g., glutathione, catalase, superoxide dismutase, and others), resulting in heightened oxidative stress that is posited to be a main contributing mechanism in central nervous system (CNS) toxicity in CICI (Joshi, Aluise et al., 2010; Sahu, Langeh, Singh, & Singh, 2021; Welbat, Naewla et al., 2020). In terms of aging related diseases, metformin, an antihyperglycemic compound that lowers hepatic glucose production to treat type-2 diabetes (Bailey, 2017), has recently garnered attention for its promising ability to reduce hippocampal-related oxidative stress and cognitive deficits through its effects on the mitochondrial fission/fusion protein Drp1 in a mouse model of diabetes (Hu, Zhou et al., 2022). As an antiaging compound, metformin is also purported to be effective in attenuating cognitive decline in diabetic patients (Samaras, Makkar et al., 2020), whereas metformin’s action on cortical mitochondrial complex I protein NDUFA2 was recently linked to reductions in Alzheimer’s disease (Zheng, Xu et al., 2022). Metformin has also been demonstrated to be effective in reducing cancer related oxidative stress (Kulkarni, Gubbi, & Barzilai, 2020) and more importantly, ameliorating cisplatin-induced cognitive dysfunctions, dendrite spine density impairments and neuronal myelination deficits in a mouse model of CICI (Zhou, Kavelaars, & Heijnen, 2016). Notably, it is hypothesized that cisplatin-induced CICI fosters an accelerated aging phenotype, as cisplatin stimulates the accumulation of Tau aggregates which are accompanied by decreased expression of the postsynaptic marker PSD-95 and adjacent astrogliosis (Chiang, Huo et al., 2019). Further supporting the role of mitochondria in chemotherapy induced accelerated aging, a significant body of work by Cobi Heijen’s group posits that accumulation of the tumor suppressor protein p53 compromises synaptic mitochondrial integrity, which culminates in cisplatin-induced neurotoxicity, cortical myelin impairments, and hippocampal memory dysfunction (Chiu, Boukelmoune et al., 2018; Chiu, Maj et al., 2017). Naturally, implementation of compounds that promote metabolic stabilization of mitochondrial dynamics and reduction of oxidative stress can be indispensable strategies to restore mitochondrial function in CICI. For example, cisplatin administration to cortical neurons derived from human induced pluripotent stem cells (iPSCs), as well as studies from rat hippocampus, exhibited significant swollen vacuolizations in mitochondria, increased ROS levels, and ƔH2AX DNA damage, coupled with disruptions in membrane potential and deficits in ATP generation (Lomeli, Di, Czerniawski, Guzowski, & Bota, 2017; Rashid, Oliveros, Kim, & Jang, 2022). This is reminiscent of the oxidative stress and neurotoxicity observed in patients treated with chemotherapy (Torre, Dey, Woods, & Feany, 2021). However, interventions with compounds that increase antioxidant activity and improve metabolic function in mitochondria, including nicotinamide mononucleotide (NMN) and N-acetylcisteine (NAC), have been effective in restoring mitochondrial respiration, and increasing ATP production, while preventing mitochondrial vacuolization, DNA damage and cognitive dysfunction (Lomeli, Di et al., 2017; Rashid, Oliveros et al., 2022). In support, restoration of healthy mitochondria via non-invasive nasal administration shows significant promise in attenuation of cisplatin-induced CICI (Alexander, Mahalingam et al., 2022; Alexander, Seua et al., 2021). Given that nasal administration of mitochondria is a simple, non-invasive route of administration that can be applied in the clinic (Lofts, Abu-Hijleh, Rigg, Mishra, & Hoare, 2022), further investigations are needed to ascertain whether mitochondrial replacement therapy in CICI is a general mechanism that can be implemented only with cisplatin or whether this strategy can also be applied to chemotherapies with different mechanisms of action (e.g. methotrexate, cyclophosphamide, 5-FU, etc.) to ameliorate the neurocellular and cognitive deficits typified by this condition.
4.4. Accelerated aging, telomeres, and chemobrain
Across the lifespan of organisms, including humans, the ribonucleo-protein enzyme telomerase provides chromosomal endpoint stabilization and telomere lengthening to protect genomic DNA integrity upon repeated cell divisions, thereby avoiding telomere attrition (Blackburn, Epel, & Lin, 2015). Interestingly, telomere attrition has been described as a risk factor in neuropsychiatric conditions (e.g. depression, psychosis, post-traumatic stress disorder), and recently, it was revealed as a mechanism in preclinical Alzheimer’s disease models (Lindqvist, Epel et al., 2015; Shim, Horner et al., 2021). Exemplifying this, a recent study examining telomere reverse transcriptase (TERT) haploinsufficiency in Alzheimer’s disease mouse models found accumulation of amyloid precursor protein and amyloid-ß, resulting in decreased dendrite spine densities and cognitive deficits, whereas overexpression of TERT effectively reversed these aging pathologies (Shim, Horner et al., 2021). Interestingly, there is some evidence for the involvement of adenosine and its receptors, in regulation of telomere function. For example, a study conducted by Fishman and colleagues showed that exposure of adenosine suppressed telomere signals in Nb2-11C rat lymphoma cells (Fishman, Bar-Yehuda et al., 2000), whereas the adenosine A2AR antagonist SCH-442416 was demonstrated to stabilize the telomeric G-quadruplex DNA complex, suggesting that the A2AR may play a notable function in telomerase dynamics (Salem, Haty, & Ghattas, 2022). In spite of the necessity for possessing active telomerase for healthy aging, the telomerase-telomere complex decreases in expression and function with age, consequently making telomere shortening a key hallmark in aging-related disease (Lopez-Otin, Blasco et al., 2022). Paradoxically, cancer cells undergoing unchecked cell division beyond the Hayflick limit (Romaniuk, Paszel-Jaworska et al., 2019), avoid senescence or apoptosis by overactivation of telomerase, thus necessitating genotoxic chemotherapy treatment to treat malignancies (Blackburn, Epel et al., 2015). Over the last several decades, tactful utilization of chemotherapy has garnered positive outcomes in terminating malignancies at the cost of inducing telomere attrition, which is posited to be a potential mechanism that effectuates CICI and is akin to accelerated aging (Wang, Prizment et al., 2021). Recent studies in breast cancer survivors treated with chemotherapy and longitudinally assessed up to 6 years following treatment, exhibited higher levels of DNA damage and lower peripheral blood mononuclear cells (PBMCs) telomerase activity, which was associated with impairments in executive function and attention (Carroll, Van Dyk et al., 2019; Scuric, Carroll et al., 2017). Supporting this hypothesis, breast cancer patients treated with combinations of either docetaxel, doxorubicin, cyclophosphamide, carboplatin and trastuzumab showed decreased telomere lengths associated with older age, while significant associations were also found between shorter chromosomal telomere lengths and disruptions in cognitive domains spanning visual memory, psychomotor processing speed, reaction time, and executive functioning (Alhareeri, Archer et al., 2020). Further evidence implicating adenosine regulation of telomeres was described in a study where increased adenosine levels via the loss of adenosine deaminase (ADA), which converts adenosine to inosine, reduced telomerase activity and accelerated senescence in CD8+ T-lymphocytes (Parish, Kim et al., 2010). Conversely, decreased production of adenosine by mesenchymal stromal cells (MSC) immortalized via retroviral transfection of the telomerase reverse transcriptase (TERT), were less immunosuppressive towards lymphocytes, hence denoting the bi-directional relationship between telomerase function and adenosine (Beckenkamp, da Fontoura et al., 2020). Given that chemotherapies can detrimentally reduce telomerase activity and prevent accelerated aging mediated telomere lengthening (Cupit-Link, Kirkland et al., 2017), future studies are required to determine whether adenosine A2AR antagonists can modify telomerase activity or telomere lengthening in the context of chemobrain. Investigation of this concept can engender novel mechanisms for attenuation of CICI in cancer-related accelerated aging. Addressing these critical issues may help devise effective therapeutic strategies that eradicate tumors, while preserving cognitive function in cancer survivors.
4.5. Epigenetic related alterations in chemobrain and accelerated aging
Dysfunctional histone acetylation or methylation of the chromatin structures encapsulating DNA in the epigenome can promote aberrant transcriptional modifications that contribute to the aging process (Lopez-Otin, Blasco et al., 2022). Epigenetically, decreases in the function of the sirtuin (SIRT) family of protein deacetylases is associated with impaired nicotinamide adenine dinucleotide (NAD+) metabolic regulation, resulting in accelerated aging (SIRT7 deletion) and degenerative (SIRT6 deletion) phenotypes in mouse models similar to BubR1 progeroid mice, a well-known genotype that exhibits accelerated aging defects (Cho, Yoo et al., 2019; Corujo-Ramirez, Dua, Yoo, Oliveros, & Jang, 2020; Lopez-Otin, Blasco et al., 2022). Conversely, a mouse model of SIRT6 overexpression that increases de novo NAD+ synthesis, has been shown to delay age-related frailty and preserve healthy aging (Roichman, Elhanati et al., 2021). Interestingly, inhibition of the histone deacetylase HDAC6 has been associated with pathogenic Tau accumulation in preclinical studies of Alzheimer’s disease (Trzeciakiewicz, Ajit et al., 2020; Tseng, Xie et al., 2017), whereas selective HDAC6 inhibition with Ricolinostat (ACY-1215) showed significant antitumor activity in multiple myeloma clinical studies (Vogl, Raje et al., 2017). Given the importance of histone modification in conveying antitumor efficacy cancer, while preserving healthy aging related phenotypes as discussed above, it is conceivable that control of histone modification may confer some neuroprotective effects in chemobrain. Accordingly, administration of the selective HDAC6 inhibitor Ricolinostat in young mice (8–10 weeks of age) showed effective attenuation of cisplatin-induced cognitive deficits, in conjunction with reversal of hippocampal Tau phosphorylation, restoration of mitochondrial function, and synaptic integrity (Ma, Huo, Jarpe, Kavelaars, & Heijnen, 2018). In aged mice (7–8 months of age), cisplatin chemotherapy decreased PSD-95 expression and significantly accumulated Tau cluster formations in the hippocampus, suggesting that cisplatin accelerates aging akin to what is observed in rodent models of Alzheimer’s disease (Chiang, Huo et al., 2019; Tseng, Xie et al., 2017). Indeed, our own recent study demonstrated how cisplatin chemotherapy decreased functional activity of nicotinamide phosphorybosyltransferase (Nampt) and SIRT2, thus disrupting NAD+ metabolic activity, which contributes to hippocampal neural stem cell impairments, neuronal maturation deficits, as well as spatial and recall memory dysfunction (Yoo, Tang et al., 2021), akin to neurodegenerative conditions and accelerated aging. Intriguingly, North and colleagues (2014) performed an investigation using the BubR1 accelerated aging mouse model which revealed that administration of the NAD+ precursor nicotine mononucleotide (NMN), as well as SIRT2 overexpression, causally increased BubR1 protein levels to promote life extension (North, Rosenberg et al., 2014). Importantly, the work of North and colleagues (2014) is uniquely in line with our findings where we demonstrate that cisplatin chemotherapy foments accelerated aging, given that NMN supplementation or NAMPT overexpression, can efficaciously attenuate cisplatin-induced chemobrain by regulating expression levels of SIRT2 (Yoo, Tang et al., 2021). Overall, targeting the NAD+ metabolic pathway may provide future effective treatment strategies that can provide clinical benefits to cancer survivors experiencing accelerated aging sequelae related to chemotherapy.
5. The adenosine A2A receptor as a potential mechanism of accelerated aging in chemobrain and cancer
5.1. Anticancer effects and immune cell modulation of the adenosine A2AR in malignancies
Adenosine can exert tight control of adenosine receptor function to control cellular proliferation and exert immunosuppression during cancer and chemotherapy treatment, through (1) the extracellular enzymatic conversion of existing ATP, ADP or AMP by the ectonucleotidases CD39 and CD73, or (2) through the release of ATP from mitochondrial intracellular stores, which again, utilize CD39 and CD73 to generate adenosine (Boison & Yegutkin, 2019; Congreve, Brown, Borodovsky, & Lamb, 2018). Indeed, in the CNS, astrocyte mediated ATP release and subsequent Ca2+ influx in neural cells triggers mitochondrial oxidative stress culminating in neuronal damage and cell death, which are neurotoxic biological consequences associated with accelerated aging and neurodegeneration (Rodrigues, Tome, & Cunha, 2015). However, the above mechanisms are by no means the only pathways that generate adenosine, and a detailed description of these processes have been extensively reviewed (Boison & Yegutkin, 2019; Ohta, 2016). The net effect of CD39 and CD73 enzymatic activity is to elevate extracellular levels of adenosine in the tumor microenvironment, thereby naturally activating adenosine A2AR on lymphocytes in an immunosuppressive capacity, thus protecting tumors from destruction (Ohta, Gorelik et al., 2006; Sitkovsky, Kjaergaard, Lukashev, & Ohta, 2008).
In chronic hypoxic microenvironments typified by extensive cellular proliferation, such as B-lymphocyte immune memory development in germinal centers, or the tumor microenvironment, cellular stress can induce persistent adenosine release and subsequent activation of A2AR, which plays an important role in conducting immune cell development, and tumor-controlled immunosuppression of T-lymphocytes (Abbott, Silva et al., 2017; Blay, White, & Hoskin, 1997; Ohta, 2016; Ohta, Gorelik et al., 2006; Young, Mittal et al., 2014). In the tumor microenvironment, adenosine achieves this by accumulating in the extracellular space surrounding solid tumors (Blay, White et al., 1997). Extracellular adenosine accumulation, in addition to the degradation of ATP by the ectonucleotidases CD39 and CD73 (released by apoptotic or necrotic cells exposed to chemotherapy), can also be released by activated monocytes, neutrophils, and dendritic cells, all of which are chemotactically summoned in response to ATP “danger signals" emanating from the tumor microenvironment (Stagg & Smyth, 2010). ATP mediated “danger signal” adenosine accumulation can result from mechanical injury to epithelium (Mikolajewicz, Mohammed, Morris, & Komarova, 2018; Yin, Xu, Zhang, Kumar, & Yu, 2007), or immunogenic cell death started by cytotoxic chemotherapy and subsequent responses by tumor targeting lymphocytes (Martins, Tesniere et al., 2009; Martins, Wang et al., 2014). Mechanistically, overexpression of cell surface ectonucleotidase CD39 promotes ATP conversion towards adenosine, thus attenuating ATP mediated “danger signaling” (Imai, Goepfert, Kaczmarek, & Robson, 2000). Indeed, Ghiringhelli and collaborators further demonstrated that oxaliplatin and doxorubicin chemotherapy causes release of ATP derived from dying tumor cells that subsequently activate dendritic cell NLRP1 inflammasome to release proinflammatory IL-1ß (Ghiringhelli, Apetoh et al., 2009). Therefore, adenosine acting through the A2AR can tightly regulate proinflammatory mediators to eradicate malignancies, and anti-inflammatory mediators to engage in immunosuppression, to protect against general tissue destruction by injury, genotoxicity or immune cell responses (Stagg & Smyth, 2010).
Interestingly, A2AR activation can have significant regulatory effects on natural killer (NK) and lymphokine activated killer (LAK) cell function. A2AR activation can potentiate immunosuppression by limiting proinflammatory cytokine release (e.g., INF-Ɣ, TNF-α) and effective recognition and elimination of malignancies by NK (Raskovalova, Huang et al., 2005) and LAK (Lokshin, Raskovalova et al., 2006) cells in the tumor microenvironment. Conversely, Young and colleagues found that A2AR deficiency in mice allows full maturation and proliferation of NK cells, resulting in decreased tumor initiation (Young, Ngiow et al., 2018). Not surprisingly, during LPS induced inflammation, adenosine levels can also have an anti-inflammatory effect by acting on A2AR function in antigen presenting dendritic cells in a cAMP-PKA dependent manner, so that these cells preferentially release immunosuppressive IL-10 (Kayhan, Koyas, Akdemir, Savas, & Cekic, 2019), and limit proinflammatory IL-2 release, thus extending immune tolerance (Challier, Bruniquel, Sewell, & Laugel, 2013).
Immunogenic cell death notwithstanding, tumor cells have cleverly circumvented ATP signaled immune cell destruction through upregulation of cell surface expression of CD39 and CD73 ectonucleotidases, both of which respectively convert ATP/ADP and AMP into immunosuppressive adenosine in the tumor microenvironment (Kaur, Weadick et al., 2022). In the tumor microenvironment, a mechanism involving adenosine receptors on dendritic cells can foster release of immunosuppressive cytokines to prevent tumor destruction by NK cells or lymphocytes as has been reported to occur through the ectonucleotidases CD39 and CD73 on tumors, tumor associated macrophages (TAMs), and T-lymphocytes to promote immunosuppressive adenosine circulation and generation (Jacoberger-Foissac et al., 2023; Montalban de Barrio et al., 2016). Nonetheless, enhancing chemotherapy effectiveness can be achieved by several strategies that reduce adenosine generation, including monoclonal antibody mediated blockade of CD39 and CD73 (Perrot, Michaud et al., 2019), by the antidiabetic medication metformin inhibiting CD39/CD73 function to increase CD8+ T-lymphocytes antitumor activity which prevens adenosine elevation-mediated immunosuppression in the ovarian cancer tumor microenvironment (Li, Wang et al., 2018). Similarly, the CD73 inhibitor AB680 (Jacoberger-Foissac et al., 2023; Lawson, Kalisiak et al., 2020), the CD39 inhibitor POM-1, as well as adenosine deaminase (d’Almeida et al., 2016) are also known to enhance chemotherapy effectiveness.
Despite the fact that anti-inflammatory mechanisms via adenosine at the A2AR may be at play, inhibition of this receptor has shown promise in preventing immunosuppression and increasing effective anticancer therapy in immuno-oncology (Congreve, Brown et al., 2018; Leone, Lo, & Powell, 2015). Indeed, preclinical studies have demonstrated that A2AR antagonists markedly enhance antitumor immunity, tumor vaccines, checkpoint blockade and adoptive T cell therapy (Mediavilla-Varela, Luddy et al., 2013; Ohta, Gorelik et al., 2006; Willingham, Ho et al., 2018). For example, global A2AR deletion (A2AR−/− mice) fostered 60% tumor rejection, and increased survival, effects that were not detected in control mice (Ohta, Gorelik et al., 2006). Accordingly, specific A2AR antagonism with ZM-241385, SCH-58261 or the non-specific antagonist caffeine enhanced the antitumor effects of CD8+ T-lymphocytes in tumor xenograft models (Mediavilla-Varela, Luddy et al., 2013; Ohta, Gorelik et al., 2006). Similarly, potent A2AR blockade with CPI-444 increased IL-2 and INF-Ɣ production in vitro, while blunting tumor growth and extending survival, either alone or in combination with anti-PD-L1 or anti-CTLA-4 antibodies in syngeneic mouse tumor models (Willingham, Ho et al., 2018). Moreover, adoptive immunotherapy transplantation of A2AR−/− T-lymphocytes in tumor bearing mice yielded increased INF-Ɣ secretion, while specific antagonism with the FDA-approved drug Istradefylline (KW-6002) improved adoptive T-lymphocyte immunotherapy, likely in a CREB mediated fashion (Bai, Zhang et al., 2022; Kjaergaard, Hatfield, Jones, Ohta, & Sitkovsky, 2018). Collectively, A2AR antagonists potentially represent the next generation of immune checkpoint inhibition in cancer immunotherapy, which may have far-reaching therapeutic effects on both cancer and CICI (Ohta, 2016). This is important, given that istradefylline was recently demonstrated to prevent cisplatin induced cognitive dysfunctions without affecting antitumor efficacy in a mouse model of chemobrain (Oliveros, Yoo et al., 2022).
5.2. Interaction between chemotherapy, adenosine A2AR and chemobrain
The exact mechanism by which chemotherapy acts on adenosine receptors to potentiate chemobrain is not well understood. To this effect, Bednarska-Szczepaniak and colleagues (2019) provide a clue for how A2AR inhibition may prevent aberrant cisplatin uptake by mature and/or developing neurons in the CNS. In their study, the adenosine A1 receptor antagonist PSB-36 was recently shown to sensitize cisplatin’s cytotoxic effects in an ovarian cancer cell line known to be resistant to the platinum-based compound, while conversely, application of the specific adenosine A2AR antagonist ZM241385 to this cisplatin-resistant ovarian cancer cell line reduced cellular uptake of cisplatin and attenuated cisplatin-induced cell cycle arrest (Bednarska-Szczepaniak, Krzyzanowski, Klink, & Nowak, 2019). Echoing these findings, increased uptake of adenosine via the equilibrative nucleoside transporter-1 (ENT1) in an ovarian cancer cell line was reported to enhance chemosensitivity to cisplatin in an AMPK mediated fashion, whereas this effect was abrogated by the phosphodiesterase inhibitor dipyridamole (Sureechatchaiyan, Hamacher, Brockmann, Stork, & Kassack, 2018). These studies suggest that increased adenosine presence enhances cisplatin’s apoptotic effects, which is good for malignancy eradication outside the CNS, but detrimental to neural stem cells in the brain, hence fostering induction of chemobrain. Further support highlighting the regulatory control that A2AR exerts in preventing chemotherapy induced mitochondrial dysfunction was described by Silva and colleagues, where selective A2AR antagonism by SCH58261 and ZM241385 prevented mitochondrial cytochrome-C release and caspase-3 activation from hippocampal neurons exposed to the antitumor compound Staurosporine (Silva, Porciuncula, Canas, Oliveira, & Cunha, 2007). Other adenosine receptors may also be involved in chemobrain, as one study demonstrated that the selective adenosine A3 receptor (A3R) agonist MRS5980 ameliorates cisplatin induced neurotoxicities, including increases in oxidative stress, mitochondrial dysfunction, cognitive deficits, and neuropathy (Singh, Mahalingam et al., 2022). Therefore, targeting adenosine receptors (A2AR, A3R), and nucleoside transporters (ENT1) can similarly provide novel therapeutic strategies to protect neuronal function during chemotherapy.
5.3. Adenosine A2AR and chemobrain
Cisplatin is an efficacious platinum-based compound widely used as a frontline therapy to treat cancers of the prostate, ovaries and breast (Balmana, Tung et al., 2014; Dasari & Tchounwou, 2014). Recent evidence shows that this chemotherapy affects cognitive function and neurogenic development in preclinical models of chemobrain (Matsos & Johnston, 2019; Zhou, Kavelaars et al., 2016). In agreement, our group recently revealed that one mechanism by which cisplatin detrimentally affects functional neuronal morphology and cognition is through disruption of nicotinamide adenine dinucleotide (NAD+) metabolic pathways (Yoo, Tang et al., 2021), which importantly, can be ameliorated by nicotinamide mononucleotide (NMN) supplementation. While cisplatin can accumulate in the cortex to exert cognitive impairments (Alexander, Seua et al., 2021; Huo, Reyes, Heijnen, & Kavelaars, 2018; Yoo, Tang et al., 2021), we and others have found particular vulnerability to neuronal cells in the hippocampus, a brain structure known for its control of learning and memory. In chemobrain, the molecular pathways by which cognitive dysfunction occurs is largely unexplored. Therefore, given the prominence for the hippocampus to replenish neuronal populations in its function as a neurogenic niche (Ming & Song, 2011), and in light of the fact that impairments in neurogenesis and neuronal dendrite architecture are pathogenic hallmarks of accelerated aging and cognitive disorders (Hering & Sheng, 2001), shedding light on novel mechanisms by which chemotherapy can impair cognition is of paramount importance. Hence, we posit that activation of cellular stress response mediators, such as adenosine, frequently occurs at the convergence of cancer and chemotherapy.
In the brain, the functional expression of A2AR in the striatum and hippocampus uniquely places this receptor as an important investigative target in the study of how chemobrain detrimentally affects synaptic plasticity, neural stem cell development, memory, and emotive behavior (Horgusluoglu-Moloch, Nho et al., 2017; Peyton, Oliveros, Choi, & Jang, 2021; Ribeiro, Glaser, Oliveira-Giacomelli, & Ulrich, 2019). Interestingly, preclinical animal models and clinical studies examining the effects of chemotherapy on brain function have reported a stark similarity in expression of molecular markers that underlie accelerated brain aging and chemobrain (Carroll, Van Dyk et al., 2019; Chiang, Huo et al., 2019). Cisplatin targets several corticolimbic structures involved in cognitive function, including the cortex (Alexander, Seua et al., 2021; Huo, Reyes et al., 2018). Our recent study identified disruptions in the adenosine A2AR and its downstream signaling effectors, cAMP, and CREB in the hippocampus, as key contributors in cisplatin-induced chemobrain (Oliveros, Yoo et al., 2022). Notably, we found A2AR in hippocampal excitatory neurons to be uniquely vulnerable to cisplatin insult since we detected specific elevations in hippocampal A2AR concomitant with impaired neurogenesis, increased anxiety-like behavior, as well as dysfunctions in recall memory and spatial memory. Furthermore, specific A2AR inhibition with istradefylline attenuated these detrimental effects (Oliveros, Yoo et al., 2022). Our findings thus prompts the question: can robust elevations in hippocampal A2AR by cisplatin, which are associated with cognitive dysfunctions, be akin to accelerated aging? Indeed, the evidence proposed in this review certainly agrees with this notion, given how increased A2AR is closely associated with accelerated aging in neurodegenerative conditions, such as Alzheimer’s disease, and cancer (Carvalho, Faivre et al., 2019; Orr, Hsiao et al., 2015; Temido-Ferreira, Ferreira et al., 2020). In support, selective A2AR inhibition through other antagonists such as SCH58261 or through the nonselective A2AR antagonist caffeine, also enhances synaptic plasticity and spatial working memory in aging and Alzheimer’s disease conditions in mice (Kaster, Machado et al., 2015; Reis, Silva et al., 2019; Viana Da Silva Haberl et al., 2016).
Currently, there is a dearth of therapeutics available to treat chemobrain. Given adenosine’s increasing prominence in emerging cancer therapies (Boison & Yegutkin, 2019), in conjunction with chemotherapy’s inherent ability to potentiate neuronal impairments and cognitive dysfunction resembling accelerated brain aging (Yoo, Tang et al., 2021), it stands to reason that A2AR inhibition may ameliorate cisplatin induced CICI, thus offering a novel therapeutic intervention against this chemobrain-associated condition. Our group’s recent findings demonstrate that istradefylline, an FDA approved drug for the treatment of Parkinson’s disease (Chen & Cunha, 2020), can be efficacious in cisplatin chemobrain (Oliveros, Yoo et al., 2022). Istradefylline has been shown to rectify synaptic plasticity, dendritic spine densities and improve cognitive function in a fragile-x-syndrome mouse model (Ferrante, Boussadia et al., 2021). However, other studies also show that A2AR activation can increase neurogenic potential (Ribeiro, Ferreira et al., 2021) while loss of A2AR function impairs cognition (Moscoso-Castro, Lopez-Cano, Gracia-Rubio, Ciruela, & Valverde, 2017), suggesting that pharmacological or genetic neuromodulation of the A2AR may be dependent on downstream transcriptional activities which can alter A2AR abundance to regain homeostatic functionality. Indeed, our results show the neuromodulatory nature of A2AR, as istradefylline attenuated cisplatin-induced increases in cAMP, CREB and ERK (unpublished observations) in conjunction with hippocampal cellular and neurogenic improvements (Oliveros, Yoo et al., 2022). This is interesting since increases in CREB and ERK are generally associated with increased neurogenesis, whereas our results show an inverse relationship. However, in support of our findings, pathogenic conditions that generate injury elevate Ras/Raf/ERK with impaired neurogenesis and dendrite spine development, while conversely, inhibition of Ras/Raf/ERK restores these impairments (Chen, Rusnak, Lombroso, & Sidhu, 2009; Xu, Cao, Sun, Liu, & Feng, 2016; Yang, Cao et al., 2013). Nonetheless, it is possible that cisplatin-mediated activation of A2AR and cAMP, promotes a B-RAF and Rap1 mediated compensatory increase in CREB and ERK (Stork & Schmitt, 2002; Takahashi, Li, Dillon, & Stork, 2017). Further studies are required to ascertain the relationship of diminished neurogenesis and increased neuronal CREB or ERK activity that may occur due to cisplatin induced neurotoxic injury. Finally, A2AR antagonism via istradefylline may have broad neuroprotective effects against chemotherapies with non-platinum mechanisms of action (Chang, Chung et al., 2020; Gibson, Nagaraja et al., 2019). However, further studies are warranted to test this hypothesis.
6. Concluding remarks
In conclusion, this review attempts to bridge the current gap in knowledge between how adenosine, through A2AR activation, may be a novel constituent of accelerated aging caused by cancer and chemotherapy, thus resulting in cognitive dysfunction. More importantly, we also provide information on the potential therapeutic potential that A2AR inhibition may confer, not only in chemobrain, but also in cancer treatment. Given that A2AR antagonists are proven to enhance antitumor activity (Congreve, Brown et al., 2018), as well as safely provide neuroprotection in neurodegenerative conditions (Chen & Cunha, 2020), inhibiting A2AR may have far-reaching synergistic effects on cancer treatment and prevention of the development of chemobrain, as well as providing a novel therapeutic strategy in accelerated aging phenotypes.
Acknowledgments
This work was supported by the NIH (R01CA242158, R01AG058560), Regenerative Medicine Minnesota (RMM091718DS005), and Rutgers Cancer Institute of New Jersey (CINJ) survivorship award to M.H.J., and the NIH (NS103740, NS065957, NS127846) and the US Department of the Army through contract W81XWH2210638 to D.B. Support to AO was provided by the American Association for Cancer Research-Bosarge Family Foundation-Waun Ki Hong Scholar Regenerative Cancer Medicine Award (19-40-60-OLIV) and the Rutgers CINJ Pediatric Cancer and Blood Disorders Research Center.
Footnotes
Conflict of interest statement
Dr. Detlev Boison is cofounder and CDO of PrevEp Inc. Other authors report no conflicts of interest.
References
- Abbott RK, Silva M, Labuda J, Thayer M, Cain DW, Philbrook P, … Sitkovsky M (2017). The GS protein-coupled A2a adenosine receptor controls T cell help in the germinal center. The Journal of Biological Chemistry, 292(4), 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACS. (2019). Cancer treatment and survivorship: Facts and figures 2019-2021. Atlanta: American Cancer Society, 2019, 1–48. [Google Scholar]
- Ahles TA, Li Y, McDonald BC, Schwartz GN, Kaufman PA, Tsongalis GJ, … Saykin AJ (2014). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psycho-oncology, 23(12), 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, … Kaufman PA (2008). Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Research and Treatment, 110(1), 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, & Mott LA (2003). The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-oncology, 12(6), 612–619. [DOI] [PubMed] [Google Scholar]
- Albasanz JL, Perez S, Barrachina M, Ferrer I, & Martin M (2008). Up-regulation of adenosine receptors in the frontal cortex in Alzheimer's disease. Brain Pathology (Zurich, Switzerland), 18(2), 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JF, Mahalingam R, Seua AV, Wu S, Arroyo LD, Horbelt T, … Heijnen CJ (2022). Targeting the meningeal compartment to resolve chemobrain and neuropathy via nasal delivery of functionalized mitochondria. Advanced Healthcare Materials, 11(8) e2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JF, Seua AV, Arroyo LD, Ray PR, Wangzhou A, Heibeta-Luckemann L, … Heijnen CJ (2021). Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics, 11(7), 3109–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhareeri AA, Archer KJ, Fu H, Lyon DE, Elswick RK, Kelly JRDL, … Jackson-Cook CK (2020). Telomere lengths in women treated for breast cancer show associations with chemotherapy, pain symptoms, and cognitive domain measures: A longitudinal study. Breast Cancer Research: BCR, 22(1), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amidi A, Agerbaek M, Wu LM, Pedersen AD, Mehlsen M, Clausen CR, … Zachariae R (2017). Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging and Behavior, 11(3), 769–783. [DOI] [PubMed] [Google Scholar]
- Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, & Sinclair DA (2022). Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nature Reviews Endocrinology, 18(4), 243–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou AA, Assimakopoulos K, Iconomou G, Giannakopoulou F, & Kalofonos HP (2011). Either called “chemobrain” or “chemofog,” the long-term chemotherapy-induced cognitive decline in cancer survivors is real. Journal of Pain and Symptom Management, 41(1), 126–139. [DOI] [PubMed] [Google Scholar]
- Armstrong E, & Boonekamp J (2023). Does oxidative stress shorten telomeres in vivo? A meta-analysis. Ageing Research Reviews, 85, 101854. [DOI] [PubMed] [Google Scholar]
- Bai Y, Zhang X, Zheng J, Liu Z, Yang Z, & Zhang X (2022). Overcoming high level adenosine-mediated immunosuppression by DZD2269, a potent and selective A2aR antagonist. Journal of Experimental & Clinical Cancer Research: CR, 41(1), 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ (2017). Metformin: Historical overview. Diabetologia, 60(9), 1566–1576. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, Van de Sluis B, … Van Deursen JM (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 479(7372), 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmana J, Tung NM, Isakoff SJ, Grana B, Ryan PD, Saura C, … Garber JE (2014). Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO, 25(8), 1656–1663. [DOI] [PubMed] [Google Scholar]
- Banks WA, Reed MJ, Logsdon AF, Rhea EM, & Erickson MA (2021). Healthy aging and the blood-brain barrier. Nature Aging, 1(3), 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalha VL, Ferreira DG, Coelho JE, Valadas JS, Gomes R, Temido-Ferreira M, … Lopes LV (2016). The caffeine-binding adenosine A2a receptor induces age-like HPA-axis dysfunction by targeting glucocorticoid receptor function. Scientific Reports, 6, 31493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckenkamp LR, Da Fontoura DMS, Korb VG, De Campos RP, Onzi GR, Iser IC, … Wink MR (2020). Immortalization of mesenchymal stromal cells by TERT affects adenosine metabolism and impairs their immunosuppressive capacity. Stem Cell Reviews and Reports, 16(4), 776–791. [DOI] [PubMed] [Google Scholar]
- Bednarska-Szczepaniak K, Krzyzanowski D, Klink M, & Nowak M (2019). Adenosine analogues as opposite modulators of the cisplatin resistance of ovarian cancer cells. Anti-cancer Agents in Medicinal Chemistry, 19(4), 473–486. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, & Lin J (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science (New York, N. Y.), 350(6265), 1193–1198. [DOI] [PubMed] [Google Scholar]
- Blay J, White TD, & Hoskin DW (1997). The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Research, 57(13), 2602–2605. [PubMed] [Google Scholar]
- Boison D, & Yegutkin GG (2019). Adenosine metabolism: Emerging concepts for cancer therapy. Cancer Cell, 36(6), 582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace KM, Lee WW, Cole PD, & Sussman ES (2019). Childhood leukemia survivors exhibit deficiencies in sensory and cognitive processes, as reflected by event-related brain potentials after completion of curative chemotherapy: A preliminary investigation. Journal of Clinical and Experimental Neuropsychology, 41(8), 814–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon F, Dridi M, Hornykiewicz O, Bedard PJ, Rajput AH, & Di Paolo T (2004). Increased adenosine A2A receptors in the brain of Parkinson's disease patients with dyskinesias. Brain, 127 (Pt 5), 1075–1084. [DOI] [PubMed] [Google Scholar]
- Canas PM, Porciuncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, … Cunha RA (2009). Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. The Journal of Neuroscience, 29(47), 14741–14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso CV, De Barros MP, Bachi ALL, Bernardi MM, Kirsten TB, De Fatima Monteiro Martins M, … Bondan EF (2020). Chemobrain in rats: Behavioral, morphological, oxidative and inflammatory effects of doxorubicin administration. Behavioural Brain Research, 378, 112233. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Bower JE, & Ganz PA (2022). Cancer-related accelerated ageing and biobehavioural modifiers: A framework for research and clinical care. Nature Reviews Clinical Oncology, 19(3), 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Van Dyk K, Bower JE, Scuric Z, Petersen L, Schiestl R, … Ganz PA (2019). Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer, 125(2), 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho K, Faivre E, Pietrowski MJ, Marques X, Gomez-Murcia V, Deleau A, … Blum D (2019). Exacerbation of C1q dysregulation, synaptic loss and memory deficits in tau pathology linked to neuronal adenosine A2A receptor. Brain, 142(11), 3636–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier J, Bruniquel D, Sewell AK, & Laugel B (2013). Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity. Immunology, 138(4), 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Chung NC, Lawther AJ, Ziegler AI, Shackleford DM, Sloan EK, & Walker AK (2020). The anti-inflammatory drug aspirin does not protect against chemotherapy-induced memory impairment by paclitaxel in mice. Frontiers in Oncology, 10, 564965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rusnak M, Lombroso PJ, & Sidhu A (2009). Dopamine promotes striatal neuronal apoptotic death via ERK signaling cascades. The European Journal of Neuroscience, 29(2), 287–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF (2014). Adenosine receptor control of cognition in normal and disease. International Review of Neurobiology, 119, 257–307. [DOI] [PubMed] [Google Scholar]
- Chen JF, Choi DS, & Cunha RA (2023). Striatopallidal adenosine A(2A) receptor modulation of goal-directed behavior: Homeostatic control with cognitive flexibility. Neuropharmacology, 226, 109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, & Cunha RA (2020). The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signalling, 16(2), 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Lee CF, & Chern Y (2014). Adenosine receptor neurobiology: Overview. International Review of Neurobiology, 119, 1–49. [DOI] [PubMed] [Google Scholar]
- Chiang ACA, Huo X, Kavelaars A, & Heijnen CJ (2019). Chemotherapy accelerates age-related development of tauopathy and results in loss of synaptic integrity and cognitive impairment. Brain, Behavior, and Immunity, 79, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu GS, Boukelmoune N, Chiang ACA, Peng B, Rao V, Kingsley C, … Heijnen CJ (2018). Nasal administration of mesenchymal stem cells restores cisplatin-induced cognitive impairment and brain damage in mice. Oncotarget, 9(85), 35581–35597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu GS, Maj MA, Rizvi S, Dantzer R, Vichaya EG, Laumet G, … Heijnen CJ (2017). Pifithrin-mu prevents cisplatin-induced chemobrain by preserving neuronal mitochondrial function. Cancer Research, 77(3), 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH, Yoo KH, Oliveros A, Paulson S, Hussaini SMQ, Van Deursen JM, & Jang MH (2019). sFRP3 inhibition improves age-related cellular changes in BubR1 progeroid mice. Aging Cell, 18(2) e12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, … Welsh RC (2010). Prechemotherapy alterations in brain function in women with breast cancer. Journal of Clinical and Experimental Neuropsychology, 32(3), 324–331. [DOI] [PubMed] [Google Scholar]
- Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, Silverman LB, … Waber DP (2015). Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 33(19), 2205–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congreve M, Brown GA, Borodovsky A, & Lamb ML (2018). Targeting adenosine A2A receptor antagonism for treatment of cancer. Expert Opinion on Drug Discovery, 13(11), 997–1003. [DOI] [PubMed] [Google Scholar]
- Corujo-Ramirez AM, Dua M, Yoo KH, Oliveros A, & Jang MH (2020). Genetic inhibition of sFRP3 prevents glial reactivity in a mouse model of accelerated aging. International Neurourology Journal, 24(Suppl 2), 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, & Morris JC (2006). Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. The American Journal of Psychiatry, 163(12), 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupit-Link MC, Kirkland JL, Ness KK, Armstrong GT, Tchkonia T, LeBrasseur NK, … Hashmi SK (2017). Biology of premature ageing in survivors of cancer. ESMO Open, 2(5), e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Almeida SM, Kauffenstein G, Roy C, Bassett L, Papargyris L, Henrion D, … Tabiasco J (2016). The ecto-ATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancer tumor-associated macrophages: Regulatory role of IL-27. Oncoimmunology, 5(7), e1178025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva SV, Haberl MG, Zhang P, Bethge P, Lemos C, Goncalves N, … Mulle C (2016). Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A(2A) receptors. Nature Communications, 7, 11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, & Tchounwou PB (2014). Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology, 740, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, … Schagen SB (2011). Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human Brain Mapping, 32(8), 1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, … Campisi J (2017). Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discovery, 7(2), 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demby TC, Rodriguez O, McCarthy CW, Lee YC, Albanese C, Mandelblatt J, & Rebeck GW (2020). A mouse model of chemotherapy-related cognitive impairments integrating the risk factors of aging and APOE4 genotype. Behavioural Brain Research, 384, 112534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewaeles E, Carvalho K, Fellah S, Sim J, Boukrout N, Caillierez R, … Cauffiez C (2022). Istradefylline protects from cisplatin-induced nephrotoxicity and peripheral neuropathy while preserving cisplatin antitumor effects. The Journal of Clinical Investigation, 132(22), e152924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar EO, Ahn C, Eraj S, Mahal BA, & Sanford NN (2021). Psychological distress and cognition among long-term survivors of adolescent and young adult cancer in the USA. Journal of Cancer Survivorship, 15(5), 776–784. [DOI] [PubMed] [Google Scholar]
- Dias L, Madeira D, Dias R, Tome AR, Cunha RA, & Agostinho P (2022). Abeta (1-42) peptides blunt the adenosine A(2A) receptor-mediated control of the interplay between P(2)X(7) and P(2)Y(1) receptors mediated calcium responses in astrocytes. Cellular and Molecular Life Sciences: CMLS, 79(8), 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falleti MG, Sanfilippo A, Maruff P, Weih L, & Phillips KA (2005). The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain and Cognition, 59(1), 60–70. [DOI] [PubMed] [Google Scholar]
- Fernandez HR, Varma A, Flowers SA, & Rebeck GW (2020). Cancer chemotherapy related cognitive impairment and the impact of the Alzheimer’s disease risk factor APOE. Cancers (Basel), 12(12), 3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Boussadia Z, Borreca A, Mallozzi C, Pedini G, Pacini L, … Martire A (2021). Adenosine A2A receptor inhibition reduces synaptic and cognitive hippocampal alterations in Fmr1 KO mice. Translational Psychiatry, 11(1), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Sarasola LI, Quiroz C, & Ciruela F (2023). Presynaptic adenosine receptor heteromers as key modulators of glutamatergic and dopaminergic neurotransmission in the striatum. Neuropharmacology, 223, 109329. [DOI] [PubMed] [Google Scholar]
- Ferreira DG, Temido-Ferreira M, Vicente Miranda H, Batalha VL, Coelho JE, Szego EM, … Outeiro TF (2017). Alpha-synuclein interacts with PrP(C) to induce cognitive impairment through mGluR5 and NMDAR2B. Nature Neuroscience, 20(11), 1569–1579. [DOI] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Ohana G, Pathak S, Wasserman L, Barer F, & Multani AS (2000). Adenosine acts as an inhibitor of lymphoma cell growth: A major role for the A3 adenosine receptor. European Journal of Cancer, 36(11), 1452–1458. [DOI] [PubMed] [Google Scholar]
- George RP, Semendric I, Hutchinson MR, & Whittaker AL (2021). Neuroimmune reactivity marker expression in rodent models of chemotherapy-induced cognitive impairment: A systematic scoping review. Brain, Behavior, and Immunity, 94, 392–409. [DOI] [PubMed] [Google Scholar]
- Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, … Monje M (2019). Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron, 103(2), 250–265.e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, … Zitvogel L (2009). Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature Medicine, 15(10), 1170–1178. [DOI] [PubMed] [Google Scholar]
- Gibson EM, & Monje M (2019). Emerging mechanistic underpinnings and therapeutic targets for chemotherapy-related cognitive impairment. Current Opinion in Oncology, 31(6), 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, … Monje M (2019). Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell, 176(1–2), 43–55.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves FQ, Lopes JP, Silva HB, Lemos C, Silva AC, Goncalves N, … Cunha RA (2019). Synaptic and memory dysfunction in a beta-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiology of Disease, 132, 104570. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, … Demaria M (2019). Cellular senescence: Defining a path forward. Cell, 179(4), 813–827. [DOI] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, & LaFerla FM (2006). Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. The Journal of Neuroscience, 26(35), 9047–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A, De Strooper B, & Arancibia-Carcamo IL (2021). Cellular senescence at the crossroads of inflammation and Alzheimer's disease. Trends in Neurosciences, 44(9), 714–727. [DOI] [PubMed] [Google Scholar]
- Gyau BB, & Deaglio S (2023). A(2A) receptor signaling drives cisplatin-mediated hippocampal neurotoxicity and cognitive defects in mice. Purinergic Signalling. 10.1007/s11302-023-09919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin LH, Meyerowitz BE, Hall P, Lichtenstein P, Johansson B, Pedersen NL, & Gatz M (2005). Cancer as a risk factor for long-term cognitive deficits and dementia. Journal of the National Cancer Institute, 97(11), 854–856. [DOI] [PubMed] [Google Scholar]
- Hering H, & Sheng M (2001). Dendritic spines: Structure, dynamics and regulation. Nature Reviews. Neuroscience, 2(12), 880–888. [DOI] [PubMed] [Google Scholar]
- Hoogland AI, Nelson AM, Gonzalez BD, Small BJ, Breen EC, Sutton SK, … Jim HSL (2019). Worsening cognitive performance is associated with increases in systemic inflammation following hematopoietic cell transplantation. Brain, Behavior, and Immunity, 80, 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgusluoglu-Moloch E, Nho K, Risacher SL, Kim S, Foroud T, Shaw LM, … Saykin AJ & I. Alzheimer's Disease Neuroimaging. (2017). Targeted neurogenesis pathway-based gene analysis identifies ADORA2A associated with hippocampal volume in mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging, 60, 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhou Y, Yang Y, Tang H, Si Y, Chen Z, … Fang H (2022). Metformin protects against diabetes-induced cognitive dysfunction by inhibiting mitochondrial fission protein DRP1. Frontiers in Pharmacology, 13, 832707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X, Reyes TM, Heijnen CJ, & Kavelaars A (2018). Cisplatin treatment induces attention deficits and impairs synaptic integrity in the prefrontal cortex in mice. Scientific Reports, 8(1), 17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Goepfert C, Kaczmarek E, & Robson SC (2000). CD39 modulates IL-1 release from activated endothelial cells. Biochemical and Biophysical Research Communications, 270(1), 272–278. [DOI] [PubMed] [Google Scholar]
- Jacoberger-Foissac C, Cousineau I, Bareche Y, Allard D, Chrobak P, Allard B, … Stagg J (2023). CD73 inhibits cGAS-STING and cooperates with CD39 to promote pancreatic cancer. Cancer Immunology Research, 11(1), 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Zhou Y, Ma L, Li W, Chan C, Zhang S, & Zhao Y (2023). Inhibition of NLRP3 alleviated chemotherapy-induced cognitive impairment in rats. Neuroscience Letters, 793, 136975. [DOI] [PubMed] [Google Scholar]
- Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, … Butterfield DA (2010). Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: Implications for oxidative stress-mediated chemobrain. Neuroscience, 166(3), 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, … Cunha RA (2015). Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proceedings of the National Academy of Sciences of the United States of America, 112(25), 7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Weadick B, Mace TA, Desai K, Odom H, & Govindarajan R (2022). Nucleoside transporters and immunosuppressive adenosine signaling in the tumor microenvironment: Potential therapeutic opportunities. Pharmacology & Therapeutics, 240, 108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayhan M, Koyas A, Akdemir I, Savas AC, & Cekic C (2019). Adenosine receptor signaling targets both PKA and Epac pathways to polarize dendritic cells to a suppressive phenotype. Journal of Immunology, 203(12), 3247–3255. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, & O’Hara R (2011). Prefrontal cortex and executive function impairments in primary breast cancer. Archives of Neurology, 68(11), 1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Rao V, Ray WJ, & Rao AI Alzheimer’s Disease Neuroimaging. (2017). Probability of Alzheimer’s disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimer’s & Dementia (Amst), 9, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaergaard J, Hatfield S, Jones G, Ohta A, & Sitkovsky M (2018). A2A adenosine receptor gene deletion or synthetic A2A antagonist liberate tumor-reactive CD8(+) T cells from tumor-induced immunosuppression. Journal of Immunology, 201(2), 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox EG, Aburto MR, Clarke G, Cryan JF, & O’Driscoll CM (2022). The blood-brain barrier in aging and neurodegeneration. Molecular Psychiatry, 27(6), 2659–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, & Schagen SB (2012). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 30(10), 1080–1086. [DOI] [PubMed] [Google Scholar]
- Kulkarni AS, Gubbi S, & Barzilai N (2020). Benefits of metformin in attenuating the hallmarks of aging. Cell Metabolism, 32(1), 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourt TE, & Heijnen CJ (2017). Mechanisms of neurotoxic symptoms as a result of breast cancer and its treatment: Considerations on the contribution of stress, inflammation, and cellular bioenergetics. Current Breast Cancer Reports, 9(2), 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird BJ, McMillan DC, Fayers P, Fearon K, Kaasa S, Fallon MT, & Klepstad P (2013). The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. The Oncologist, 18(9), 1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, … Castel H (2019). Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO, 30(12), 1925–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Licaj I, Clarisse B, Humbert X, Grellard JM, Tron L, & Joly F (2019). Cognitive complaints in cancer survivors and expectations for support: Results from a web-based survey. Cancer Medicine, 8(5), 2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent C, Burnouf S, Ferry B, Batalha VL, Coelho JE, Baqi Y, … Blum D (2016). A2A adenosine receptor deletion is protective in a mouse model of tauopathy. Molecular Psychiatry, 21(1), 97–107. [DOI] [PubMed] [Google Scholar]
- Lawson KV, Kalisiak J, Lindsey EA, Newcomb ET, Leleti MR, Debien L, … Powers JP (2020). Discovery of AB680: A potent and selective inhibitor of CD73. Journal of Medicinal Chemistry, 63(20), 11448–11468. [DOI] [PubMed] [Google Scholar]
- Leone RD, Lo YC, & Powell JD (2015). A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Computational and Structural Biotechnology Journal, 13, 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, … Zhang Y (2018). Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Research, 78(7), 1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Silva HB, Real J, Wang YM, Rial D, Li P, … Chen JF (2015). Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington’s disease models. Neurobiology of Disease, 79, 70–80. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen X, Wang T, Gao Y, Li F, Chen L, … Chen JF (2018). The corti-costriatal adenosine A2A receptor controls maintenance and retrieval of spatial working memory. Biological Psychiatry, 83(6), 530–541. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, … Wolkowitz OM (2015). Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neuroscience and Biobehavioral Reviews, 55, 333–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Chen J, Li X, Zhou X, Hu YM, Chu SF, … Chen NH (2019). Research progress on adenosine in central nervous system diseases. CNS Neuroscience & Therapeutics, 25(9), 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofts A, Abu-Hijleh F, Rigg N, Mishra RK, & Hoare T (2022). Using the intranasal route to administer drugs to treat neurological and psychiatric illnesses: Rationale, successes, and future needs. CNS Drugs, 36(7), 739–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, & Gorelik E (2006). Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Research, 66(15), 7758–7765. [DOI] [PubMed] [Google Scholar]
- Lomeli N, Di K, Czerniawski J, Guzowski JF, & Bota DA (2017). Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radical Biology & Medicine, 102, 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CR, Cunha RA, & Agostinho P (2021). Astrocytes and adenosine A(2A) receptors: Active players in Alzheimer’s disease. Frontiers in Neuroscience, 15, 666710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, & Ribeiro JA (1999). Increase in the number, G protein coupling, and efficiency of facilitatory adenosine A2A receptors in the limbic cortex, but not striatum, of aged rats. Journal of Neurochemistry, 73(4), 1733–1738. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, & Kroemer G (2022). Hallmarks of aging: An expanding universe. Cell, 186, 243–278. [DOI] [PubMed] [Google Scholar]
- Ma J, Huo X, Jarpe MB, Kavelaars A, & Heijnen CJ (2018). Pharmacological inhibition of HDAC6 reverses cognitive impairment and tau pathology as a result of cisplatin treatment. Acta Neuropathologica Communications, 6(1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, … Kroemer G (2009). Chemotherapy induces ATP release from tumor cells. Cell Cycle (Georgetown, Tex.), 8(22), 3723–3728. [DOI] [PubMed] [Google Scholar]
- Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, … Kroemer G (2014). Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death and Differentiation, 21(1), 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsos A, & Johnston IN (2019). Chemotherapy-induced cognitive impairments: A systematic review of the animal literature. Neuroscience and Biobehavioral Reviews, 102, 382–399. [DOI] [PubMed] [Google Scholar]
- Matsos A, Loomes M, Zhou I, Macmillan E, Sabel I, Rotziokos E, … Johnston IN (2017). Chemotherapy-induced cognitive impairments: White matter pathologies. Cancer Treatment Reviews, 61, 6–14. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, & Saykin AJ (2010). Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Research and Treatment, 123(3), 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediavilla-Varela M, Luddy K, Noyes D, Khalil FK, Neuger AM, Soliman H, & Antonia SJ (2013). Antagonism of adenosine A2A receptor expressed by lung adenocarcinoma tumor cells and cancer associated fibroblasts inhibits their growth. Cancer Biology & Therapy, 14(9), 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Borea PA, Varani K, Vincenzi F, Jacobson KA, & Gessi S (2022). A(2A) adenosine receptor antagonists in neurodegenerative diseases. Current Medicinal Chemistry, 29(24), 4138–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajewicz N, Mohammed A, Morris M, & Komarova SV (2018). Mechanically stimulated ATP release from mammalian cells: Systematic review and meta-analysis. Journal of Cell Science, 131(22), jcs223354. [DOI] [PubMed] [Google Scholar]
- Ming GL, & Song H (2011). Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron, 70(4), 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogavero MP, Bruni O, DelRosso LM, & Ferri R (2020). Neurodevelopmental consequences of pediatric cancer and its treatment: The role of sleep. Brain Sciences, 10(7), 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, & Palmer TD (2007). Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Annals of Neurology, 62(5), 515–520. [DOI] [PubMed] [Google Scholar]
- Montalban de Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wöchel A, … Häusler SFM (2016). Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages — a self amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. Journal for ImmunoTherapy of Cancer, 4(49). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso-Castro M, Lopez-Cano M, Gracia-Rubio I, Ciruela F, & Valverde O (2017). Cognitive impairments associated with alterations in synaptic proteins induced by the genetic loss of adenosine A2A receptors in mice. Neuropharmacology, 126, 48–57. [DOI] [PubMed] [Google Scholar]
- Nam HW, Bruner RC, & Choi DS (2013). Adenosine signaling in striatal circuits and alcohol use disorders. Molecules and Cells, 36(3), 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness KK, Armstrong GT, Kundu M, Wilson CL, Tchkonia T, & Kirkland JL (2015). Frailty in childhood cancer survivors. Cancer, 121(10), 1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CAS, Biran LP, Galvano E, Mandelblatt J, Vicini S, & Rebeck GW (2022). Chemotherapy promotes astrocytic response to Abeta deposition, but not Abeta levels, in a mouse model of amyloid and APOE. Neurobiology of Disease, 175, 105915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LD, & Ehrlich BE (2020). Cellular mechanisms and treatments for chemobrain: Insight from aging and neurodegenerative diseases. EMBO Molecular Medicine, 12(6), e12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Rosenberg MA, Jeganathan KB, Hafner AV, Michan S, Dai J, … Sinclair DA (2014). SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. The EMBO Journal, 33(13), 1438–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A. (2016). A metabolic immune checkpoint: Adenosine in tumor microenvironment. Frontiers in Immunology, 7, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, … Sitkovsky M (2006). A2A adenosine receptor protects tumors from antitumor T cells. Proceedings of the National Academy of Sciences of the United States of America, 103(35), 13132–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros A, Yoo KH, Rashid MA, Corujo-Ramirez A, Hur B, Sung J, … Jang MH (2022). Adenosine A2A receptor blockade prevents cisplatin-induced impairments in neurogenesis and cognitive function. Proceedings of the National Academy of Sciences of the United States of America, 119(28) e2206415119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onzi GR, D’Agustini N, Garcia SC, Guterres SS, Pohlmann PR, Rosa DD, & Pohlmann AR (2022). Chemobrain in breast cancer: Mechanisms, clinical manifestations, and potential interventions. Drug Safety: An International Journal of Medical Toxicology and Drug Experience, 45(6), 601–621. [DOI] [PubMed] [Google Scholar]
- Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, … Mucke L (2015). Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nature Neuroscience, 18(3), 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Lo I, Schumacher H, Ho K, Gill M, Guo W, … Mucke L (2018). Istradefylline reduces memory deficits in aging mice with amyloid pathology. Neurobiology of Disease, 110, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li XJ, Gross RE, & Traynelis SF (2009). Adenosine A(2A) receptor mediates microglial process retraction. Nature Neuroscience, 12(7), 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish ST, Kim S, Sekhon RK, Wu JE, Kawakatsu Y, & Effros RB (2010). Adenosine deaminase modulation of telomerase activity and replicative senescence in human CD8 T lymphocytes. Journal of Immunology, 184(6), 2847–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, … Bonnefoy N (2019). Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Reports, 27(8), 2411–2425 e2419. [DOI] [PubMed] [Google Scholar]
- Peyton L, Oliveros A, Choi DS, & Jang MH (2021). Hippocampal regenerative medicine: Neurogenic implications for addiction and mental disorders. Experimental & Molecular Medicine, 53(3), 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MA, Oliveros A, Kim YS, & Jang MH (2022). Nicotinamide mononucleotide prevents cisplatin-induced mitochondrial defects in cortical neurons derived from human induced pluripotent stem cells. Brain Plasticity, 8(2), 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, & Gorelik E (2005). Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. Journal of Immunology, 175(7), 4383–4391. [DOI] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, & Mulle C (2008). Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron, 57(1), 121–134. [DOI] [PubMed] [Google Scholar]
- Rebola N, Rodrigues RJ, Lopes LV, Richardson PJ, Oliveira CR, & Cunha RA (2005). Adenosine A1 and A2A receptors are co-expressed in pyramidal neurons and co-localized in glutamatergic nerve terminals of the rat hippocampus. Neuroscience, 133(1), 79–83. [DOI] [PubMed] [Google Scholar]
- Reis SL, Silva HB, Almeida M, Cunha RA, Simoes AP, & Canas PM (2019). Adenosine A1 and A2A receptors differently control synaptic plasticity in the mouse dorsal and ventral hippocampus. Journal of Neurochemistry, 151(2), 227–237. [DOI] [PubMed] [Google Scholar]
- Ren X, Keeney JTR, Miriyala S, Noel T, Powell DK, Chaiswing L, … Butterfield DA (2019). The triangle of death of neurons: Oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-alpha. Free Radical Biology & Medicine, 134, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Clair DKST, & Butterfield DA (2017). Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacological Research: The Official Journal of the Italian Pharmacological Society, 117, 267–273. [DOI] [PubMed] [Google Scholar]
- Ribeiro DE, Glaser T, Oliveira-Giacomelli A, & Ulrich H (2019). Purinergic receptors in neurogenic processes. Brain Research Bulletin, 151, 3–11. [DOI] [PubMed] [Google Scholar]
- Ribeiro FF, Ferreira F, Rodrigues RS, Soares R, Pedro DM, Duarte-Samartinho M, … Xapelli S (2021). Regulation of hippocampal postnatal and adult neurogenesis by adenosine A2A receptor: Interaction with brain-derived neurotrophic factor. Stem Cells, 39, 1362–1381. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Marques JM, & Cunha RA (2019). Purinergic signalling and brain development. Seminars in Cell & Developmental Biology, 95, 34–41. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Tome AR, & Cunha RA (2015). ATP as a multi-target danger signal in the brain. Frontiers in Neuroscience, 9, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roichman A, Elhanati S, Aon MA, Abramovich I, Di Francesco A, Shahar Y, … Cohen HY (2021). Restoration of energy homeostasis by SIRT6 extends healthy lifespan. Nature Communications, 12(1), 3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk A, Paszel-Jaworska A, Toton E, Lisiak N, Holysz H, Krolak A, … Rubis B (2019). The non-canonical functions of telomerase: To turn off or not to turn off. Molecular Biology Reports, 46(1), 1401–1411. [DOI] [PubMed] [Google Scholar]
- Rummel NG, Chaiswing L, Bondada S, St Clair DK, & Butterfield DA (2021). Chemotherapy-induced cognitive impairment: Focus on the intersection of oxidative stress and TNFalpha. Cellular and Molecular Life Sciences: CMLS, 78(19–20), 6533–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu K, Langeh U, Singh C, & Singh A (2021). Crosstalk between anticancer drugs and mitochondrial functions. Current Research in Pharmacology and Drug Discovery, 2, 100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem AA, Haty IAEL, & Ghattas MA (2022). GW-2974 and SCH-442416 modulators of tyrosine kinase and adenosine receptors can also stabilize human telomeric G-quadruplex DNA. PLoS One, 17(12) e0277963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras K, Makkar S, Crawford JD, Kochan NA, Wen W, Draper B, … Sachdev PS (2020). Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: The Sydney memory and ageing study. Diabetes Care, 43(11), 2691–2701. [DOI] [PubMed] [Google Scholar]
- Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, … Muss HB (2014). Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. Journal of the National Cancer Institute, 106(4) dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchuk S, & Monje M (2022). Mini-review: Aplastic myelin following chemotherapy. Neuroscience Letters, 790, 136861. [DOI] [PubMed] [Google Scholar]
- Scherling C, Collins B, Mackenzie J, Bielajew C, & Smith A (2011). Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: An FMRI study. Frontiers in Human Neuroscience, 5, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmauck-Medina T, Moliere A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, … Fang EF (2022). New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging (Albany NY), 14(16), 6829–6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuric Z, Carroll JE, Bower JE, Ramos-Perlberg S, Petersen L, Esquivel S, … Schiestl R (2017). Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer, 3, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Bradley-Garcia M, Martinez-Canabal A, & Winocur G (2021). Chemotherapy-induced cognitive impairment and hippocampal neurogenesis: A review of physiological mechanisms and interventions. International Journal of Molecular Sciences, 22(23), 12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim HS, Horner JW, Wu CJ, Li J, Lan ZD, Jiang S, … DePinho RA (2021). Telomerase reverse transcriptase preserves neuron survival and cognition in Alzheimer’s disease models. Nature Aging, 1(12), 1162–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lemos C, Goncalves FQ, Pliassova AV, Machado NJ, Silva HB, … Agostinho P (2018). Blockade of adenosine A2A receptors recovers early deficits of memory and plasticity in the triple transgenic mouse model of Alzheimer’s disease. Neurobiology of Disease, 117, 72–81. [DOI] [PubMed] [Google Scholar]
- Silva CG, Porciuncula LO, Canas PM, Oliveira CR, & Cunha RA (2007). Blockade of adenosine A(2A) receptors prevents staurosporine-induced apoptosis of rat hippocampal neurons. Neurobiology of Disease, 27(2), 182–189. [DOI] [PubMed] [Google Scholar]
- Simo M, Rifa-Ros X, Rodriguez-Fornells A, & Bruna J (2013). Chemobrain: A systematic review of structural and functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37(8), 1311–1321. [DOI] [PubMed] [Google Scholar]
- Singh AK, Mahalingam R, Squillace S, Jacobson KA, Tosh DK, Dharmaraj S, … Heijnen CJ (2022). Targeting the A(3) adenosine receptor to prevent and reverse chemotherapy-induced neurotoxicities in mice. Acta Neuropathologica Communications, 10(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitkovsky MV, Kjaergaard J, Lukashev D, & Ohta A (2008). Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 14(19), 5947–5952. [DOI] [PubMed] [Google Scholar]
- Speidell AP, Demby T, Lee Y, Rodriguez O, Albanese C, Mandelblatt J, & Rebeck GW (2019). Development of a human APOE knock-in mouse model for study of cognitive function after cancer chemotherapy. Neurotoxicity Research, 35(2), 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlagh B, & Vizi ES (2011). The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: Pharmacological and clinical aspects. Current Topics in Medicinal Chemistry, 11(8), 1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spignoli G, Pedata F, & Pepeu G (1984). A1 and A2 adenosine receptors modulate acetylcholine release from brain slices. European Journal of Pharmacology, 97(3–4), 341–342. [DOI] [PubMed] [Google Scholar]
- Squassina A, Manchia M, Pisanu C, Ardau R, Arzedi C, Bocchetta A, … Carpiniello B (2020). Telomere attrition and inflammatory load in severe psychiatric disorders and in response to psychotropic medications. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 45(13), 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, & Smyth MJ (2010). Extracellular adenosine triphosphate and adenosine in cancer. Oncogene, 29(39), 5346–5358. [DOI] [PubMed] [Google Scholar]
- Stefancin P, Cahaney C, Parker RI, Preston T, Coulehan K, Hogan L, & Duong TQ (2020). Neural correlates of working memory function in pediatric cancer survivors treated with chemotherapy: An fMRI study. NMR in Biomedicine, 33(6) e4296. [DOI] [PubMed] [Google Scholar]
- Stork PJ, & Schmitt JM (2002). Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends in Cell Biology, 12(6), 258–266. [DOI] [PubMed] [Google Scholar]
- Sureechatchaiyan P, Hamacher A, Brockmann N, Stork B, & Kassack MU (2018). Adenosine enhances cisplatin sensitivity in human ovarian cancer cells. Purinergic Signalling, 14(4), 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Li Y, Dillon TJ, & Stork PJ (2017). Phosphorylation of Rap1 by cAMP-dependent protein kinase (PKA) creates a binding site for KSR to sustain ERK activation by cAMP. The Journal of Biological Chemistry, 292(4), 1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Oliveros A, & Jang MH (2019). Dysfunctional mitochondrial bioenergetics and synaptic degeneration in Alzheimer disease. International Neurourology Journal, 23(Suppl 1), S5–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temido-Ferreira M, Coelho JE, Pousinha PA, & Lopes LV (2019). Novel players in the aging synapse: Impact on cognition. Journal of Caffeine and Adenosine Research, 9(3), 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temido-Ferreira M, Ferreira DG, Batalha VL, Marques-Morgado I, Coelho JE, Pereira P, … Lopes, L. V. (2020). Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Molecular Psychiatry, 25(8), 1876–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre M, Dey A, Woods JK, & Feany MB (2021). Elevated oxidative stress and DNA damage in cortical neurons of chemotherapy patients. Journal of Neuropathology and Experimental Neurology, 80(7), 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciakiewicz H, Ajit D, Tseng JH, Chen Y, Ajit A, Tabassum Z, … Cohen TJ (2020). An HDAC6-dependent surveillance mechanism suppresses tau-mediated neurodegeneration and cognitive decline. Nature Communications, 11(1), 5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng JH, Xie L, Song S, Xie Y, Allen L, Ajit D, … Cohen TJ (2017). The deacetylase HDAC6 mediates endogenous neuritic tau pathology. Cell Reports, 20(9), 2169–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel O, Lahav M, Shargian L, Beery E, Pasvolsky O, Rozovski U, … Yeshurun M (2020). Premature ageing following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation, 55(7), 1438–1446. [DOI] [PubMed] [Google Scholar]
- Van Calker D, Muller M, & Hamprecht B (1979). Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. Journal of Neurochemistry, 33(5), 999–1005. [DOI] [PubMed] [Google Scholar]
- Van Der Plas E, Schachar RJ, Hitzler J, Crosbie J, Guger SL, Spiegler BJ, … Nieman BJ (2017). Brain structure, working memory and response inhibition in childhood leukemia survivors. Brain and Behavior, 7(2), e00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana Da Silva S, Haberl MG, Zhang P, Bethge P, Lemos C, Goncalves N, … Mulle C (2016). Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nature Communications, 7, 11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl DT, Raje N, Jagannath S, Richardson P, Hari P, Orlowski R, … Lonial S (2017). Ricolinostat, the first selective histone deacetylase 6 inhibitor, in combination with bortezomib and dexamethasone for relapsed or refractory multiple myeloma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 23(13), 3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Muthu Karuppan MK, Devadoss D, Nair M, Chand HS, & Lakshmana MK (2021). TFEB protein expression is reduced in aged brains and its overexpression mitigates senescence-associated biomarkers and memory deficits in mice. Neurobiology of Aging, 106, 26–36. [DOI] [PubMed] [Google Scholar]
- Wang S, Prizment A, Thyagarajan B, & Blaes A (2021). Cancer treatment-induced accelerated aging in cancer survivors: Biology and assessment. Cancers (Basel), 13(3), 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardill HR, Mander KA, Van Sebille YZ, Gibson RJ, Logan RM, Bowen JM, & Sonis ST (2016). Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. International Journal of Cancer. Journal International du Cancer, 139(12), 2635–2645. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Kesler SR, Noll KR, & Schagen SB (2015). Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: A Cancer Journal for Clinicians, 65(2), 123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, & Meyers CA (2004). ‘Chemobrain’ in breast carcinoma?: A prologue. Cancer, 101(3), 466–475. [DOI] [PubMed] [Google Scholar]
- Wefel JS, & Schagen SB (2012). Chemotherapy-related cognitive dysfunction. Current Neurology and Neuroscience Reports, 12(3), 267–275. [DOI] [PubMed] [Google Scholar]
- Weiss B. (2008). Chemobrain: A translational challenge for neurotoxicology. Neurotoxicology, 29(5), 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbat JU, Naewla S, Pannangrong W, Sirichoat A, Aranarochana A, & Wigmore P (2020). Neuroprotective effects of hesperidin against methotrexate-induced changes in neurogenesis and oxidative stress in the adult rat. Biochemical Pharmacology, 178, 114083. [DOI] [PubMed] [Google Scholar]
- Wen J, Maxwell RR, Wolf AJ, Spira M, Gulinello ME, & Cole PD (2018). Methotrexate causes persistent deficits in memory and executive function in a juvenile animal model. Neuropharmacology, 139, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Patel C, Diglio F, Baker K, Marshall G, Li S, & Cole PD (2022). Cognitive impairment persists at least 1 year after juvenile rats are treated with methotrexate. Neuropharmacology, 206, 108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, & Cole PD (2021). Biomarkers of cognitive impairment in pediatric cancer survivors. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 39(16), 1766–1774. [DOI] [PubMed] [Google Scholar]
- Williams AM, Krull KR, Howell CR, Banerjee P, Brinkman TM, Kaste SC, … Ness KK (2021). Physiologic frailty and neurocognitive decline among young-adult childhood cancer survivors: A prospective study from the St Jude lifetime cohort. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 39(31), 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Ho PY, Hotson A, Hill C, Piccione EC, Hsieh J, … Miller RA (2018). A2AR antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-(L)1 and anti-CTLA-4 in preclinical models. Cancer Immunology Research, 6(10), 1136–1149. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhi K, & Meng X (2021). Effects and mechanisms of synaptotagmin-7 in the hippocampus on cognitive impairment in aging mice. Molecular Neurobiology, 58(11), 5756–5771. [DOI] [PubMed] [Google Scholar]
- Xu D, Cao F, Sun S, Liu T, & Feng S (2016). Inhibition of the Ras/Raf/ERK1/2 signaling pathway restores cultured spinal cord-injured neuronal migration, adhesion, and dendritic spine development. Neurochemical Research, 41(8), 2086–2096. [DOI] [PubMed] [Google Scholar]
- Yang K, Cao F, Sheikh AM, Malik M, Wen G, Wei H, … Li X (2013). Up-regulation of Ras/Raf/ERK1/2 signaling impairs cultured neuronal cell migration, neurogenesis, synapse formation, and dendritic spine development. Brain Structure & Function, 218(3), 669–682. [DOI] [PubMed] [Google Scholar]
- Yin J, Xu K, Zhang J, Kumar A, & Yu FS (2007). Wound-induced ATP release and EGF receptor activation in epithelial cells. Journal of Cell Science, 120(Pt 5), 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KH, Tang JJ, Rashid MA, Cho CH, Corujo-Ramirez A, Choi J, … Jang MH (2021). Nicotinamide mononucleotide prevents cisplatin-induced cognitive impairments. Cancer Research, 81(13), 3727–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Mittal D, Stannard K, Yong M, Teng MW, Allard B, … Smyth MJ (2014). Co-blockade of immune checkpoints and adenosine A2A receptor suppresses metastasis. Oncoimmunology, 3(10) e958952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, … Smyth MJ (2018). A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Research, 78(4), 1003–1016. [DOI] [PubMed] [Google Scholar]
- Zheng J, Xu M, Walker V, Yuan J, Korologou-Linden R, Robinson J, … Bi Y (2022). Evaluating the efficacy and mechanism of metformin targets on reducing Alzheimer’s disease risk in the general population: A Mendelian randomisation study. Diabetologia, 65(10), 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kavelaars A, & Heijnen CJ (2016). Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS One, 11(3), e0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]