Abstract
Purpose:
Preparedness for medical responses to major radiation accidents and the increasing threat of nuclear warfare worldwide necessitates an understanding of the complexity of combined radiation injury (CI) and identifying drugs to treat CI is inevitably critical. The vital sign and survival after CI were presented. The molecular mechanisms, such as microRNA pathways, NF-κB-iNOS-IL-18 pathway, C3 production, the AKT-MAPK cross-talk, and TLR/MMP increases, underlying CI in relation to organ injury and mortality were analyzed. At present, no FDA-approved drug to protect, mitigate, or treat CI is available. The development of CI-specific medical countermeasures was reviewed. Because of the worsened acute radiation syndrome resulting from CI, diagnostic triage can be problematic. Therefore, biodosimetry and CI are bundled together with the need to establish effective triage methods with CI.
Conclusions:
CI mouse model studies at AFRRI are reviewed addressing molecular responses, findings from medical countermeasures, and a proposed plasma proteomic biodosimetry approach based on a panel of radiation-responsive biomarkers (i.e., CD27, Flt-3L, GM-CSF, CD45, IL-12, TPO) negligibly influenced by wounding in an algorithm used for dose predictions is described.
Keywords: Combined radiation injury, wounding, medical countermeasures, mechanisms, acute radiation syndrome, biodosimetry
Introduction
Radiation exposure events on a large scale have always found that victims exposed to radiation are also often suffering from other trauma including hemorrhage, blast, burns or wounds. Combined radiation injuries (CI) were documented at Hiroshima and Nagasaki, Japan, where 60–70% of radiation victims concurrently received thermal burn injury (Iijima 1982; Kishi 2000), while there are reports indicating victims with approximately 60% radiation alone, 35% 2-injuries, and 5% 3-injuries (Table 1). At the Chernobyl reactor meltdown, 10% of 237 victims were exposed to radiation and thermal burns together (Barabanova 2006). Therefore, combined radiation injury is an important area requiring attention, at least with a similar degree as radiation injury alone.
Table 1.
Percentage of victims exposed to atomic bombs with numbers of injury (Joint Commission of USA and Japanese Physicians collected from victims exposed to 15 KT and 25 KT fission Devices 1946).
| Little boy-U235 (N = 5185) | Fat man-Pu239 (N = 4107) | |
|---|---|---|
| Single injury | 60.5% | 57.5% |
| Two injuries | 34.5% | 37.1% |
| Three injuries | 5.0% | 5.2% |
Combined radiation injury
In animal models of combined radiation injury (CI), there are rats (Alpen and Sheline 1954; Valeriote and Baker 1964), guinea pigs (Korlof 1956), dogs (Brooks et al. 1952), and swine (Baxter et al. 1953) with burns and wounds; they usually rise mortality after otherwise non-lethal radiation exposures. In 1970s, Armed Forces Radiobiology Research Institute (AFRRI) began CI investigations. In mice, radiation exposure followed by burns or other wounds further reduced survival compared to burns alone, wounds alone or radiation exposure alone (Ledney et al. 1992; Kiang and Ledney 2013), and radiation delays wound healing times (Ledney et al. 1981). Subsequently, CI resulted in acute suppression of myeloid, inhibition of the immune system, fluid imbalance, macro/microcirculation failure, massive cellular damage and death, and vital organ dysfunctions. Then, multiple organ dysfunction syndrome (MODS) occurs, which is the most frequent cause of death after CI (Koenig et al. 2005; Lausevic et al. 2008; Zou et al. 2008; Kiang and Olabisi 2019).
In addition to penetrating skin wounds (Kiang et al. 2010b) and hemorrhage (Kiang et al. 2015, 2017a), CI with skin burn (Ledney and Elliott 2010; Kiang et al. 2014b) and infection (Brook et al. 2001; Elliott et al. 2002) were established at AFRRI. The radiation dose modified factors (DMF) for radiation combined with skin wound and hemorrhage were 1.08 (Kiang et al. 2010b) and 1.04 (Kiang et al. 2015), respectively, whereas the DMF of radiation in combination with burn was between wound and hemorrhage (Kiang, unpublished data). Radiation combined with infection was toxic but the DMF was not yet determined. Besides worsened survival, body weight loss and delayed wound healing, CI with radiation plus wound (Swift et al. 2015a) or plus hemorrhage (Kiang et al. 2015) appeared to amplify and prolong skeletal tissue loss.
The first study on molecular mechanisms underlying CI (Kiang et al. 2010b) showed that radiation followed by skin wounding immensely increased a) inducible nitric oxide synthase (iNOS), b) cytokine/chemokine concentrations in serum, and c) systemic bacterial infection in liver and heart significantly more than radiation alone (Kiang, et al. 2010b; Kiang, et al. 2020). Their details are described as follows.
The iNOS in these B6D2F1 female mice exposed to CI was largely increased, which was mediated by elevated nuclear factor-keppaB (NF-jB) and nuclear factor-interleukin 6 (NF-IL6) (Kiang et al. 2010b). NF-IL6 was usually stimulated by IL-6 which was increased by radiation. Moreover, the increased iNOS triggered the breakage of the tight junction of the gut barrier as measured by the reduction of Claudin-2, as a well-accepted biomarker for tight junction (Kiang et al. 2020). The magnified increases in systematic bacteria after CI included Enterococcus, Bacillus, Lactobacillus, and Staphylococcus, while systemic bacteria after radiation injury (RI) were Enterococcus and Staphylococcus (Kiang et al. 2010b), which was confirmed by measuring the bacterial DNA and lipopolysaccharide (LPS) concentrations in the liver (Kiang et al. 2020). The onset time of this sepsis was on day 3 after CI and on day 6 after RI (Kiang et al. 2010b). In CD2F1 male mice exposed to radiation followed by 20% hemorrhage, CI mice were detected with Streptococcus sanguinis and Sphingomonas paucimobilis and RI mice were with Proteus mirabilis (Kiang et al. 2017a). The differences in bacterium found after CI could be due to (i) different strains of mice studied, (ii) different sex of mice used, and (iii) different traumas applied, namely, wounding verse hemorrhage.
Using 23 cytokine/chemokine multiplex luminex, we found radiation increased IL-6, IL-10, KC, G-CSF, and MCP-1. In contrast, CI significantly further increased these and many others including IL-1β, IL-6, IL-9, IL-10, IL-13, KC, G-CSF, Eotaxin, IFN-γ, MCP-1, MIP-1α, and MIP-1β (Kiang et al. 2010b). In mice exposed to radiation followed by 20% hemorrhage, RI increased IL-1β, IL-2, IL-12p40, IL-17A, G-CSF, IFN-γ, KC, MCP-1, IL-15, and IL-18, whereas CI increased IL-1α, IL-1β, IL-3, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17A, G-CSF, IFN-γ, KC, MCP-1, MIP-1β, TNF-α, Il-15 and IL-18 (Kiang et al. 2017a).
Radiation with skin wound (CI-wound) significantly increased p21, Bax, Bcl-2, Bax/Bcl-2 ratios, DDB2, Cadd45α, and TERT (Kiang et al. 2010a). Additionally, altered Cdh6, Itga7, Mmp-2, −3, −7, −9, −10, −11, −13, Timp3, Timp4, TLR-1, −2, −4, −6, −7, −8, −9, and myd88 (Kiang and Olabisi, 2019). AKT (pro-survival) and MAPK (anti-survival) were deactivated and activated, respectively, by RI and CI (Kiang et al 2020). It is evident that CI with mixed-field (neutron + gamma) radiation followed by skin wound synergistically increased corticosterone, decreased C-reactive protein (CRP), increased complement component 3 (C3), and prostaglandin E2 (PGE2) and decreased immunoglobulin (IgM) (Kiang and Ledney 2013). CI with Co-60 radiation followed by 20% hemorrhage increased corticosterone, decreased CRP, and increased both C3 and Flt-3 ligands (Kiang et al. 2017a).
It is evident that skin wounds following high-dose radiation exposure amplified and prolonged skeletal tissue loss indicated by biomarkers including transient TRAP 5b increases, decreased osteocalcin, and increased sclerostin (Swift et al. 2015a). Radiation followed by hemorrhage resulted in trabecular bone loss as indicated by increased TRAP 5b, decreased osteocalcin, and increased sclerostin as well (Swift et al. 2015b).
CI with hemorrhage following high-dose radiation exacerbates CI-induced EPO and HIF-1α due to increased NF-κB in the kidney. The inflammation-associated microRNA (miR) expression in the kidney was also altered. Among 29 miRs on day 1 after RCI (Figure 1), 14 were significantly increased and 15 were significantly decreased. Let-7e, miR-29b, miR-30e, miR-27a, miR-32 and miR-135 were associated with NF-κB, HIF-1α, and EPO (Kiang et al. 2017a). However, CI with skin wound following high-dose radiation increased miR-34a to a similar magnitude as RI at early times but not on day 30 (Figure 2). MiR-34a was inhibited by G-CSF. MiR-34a decreased Bcl-2 and increased Bax, whereas G-CSF increased Bcl-2. Therefore, the balance between G-CSF and miR-34a should be critical for cell survival or death (Kiang et al. 2022). It should be kept in mind that FMS-like tyrosine kinase 3 ligand (flt-3L, a bone marrow dysplasia marker) was increased by RI and CI, but the RI-induced increases and CI-induced increases were similar (Jiao et al. 2010; Kiang et al. 2017a). In contrast, citrulline (produced by intestinal epithelial cells) was increased by RI and CI, but the CI-induced decreases were more than RI-induced decreases, confirming the CI-induced GI damage was more than the RI-induced GI damage (Kiang et al. 2020).
Figure 1.
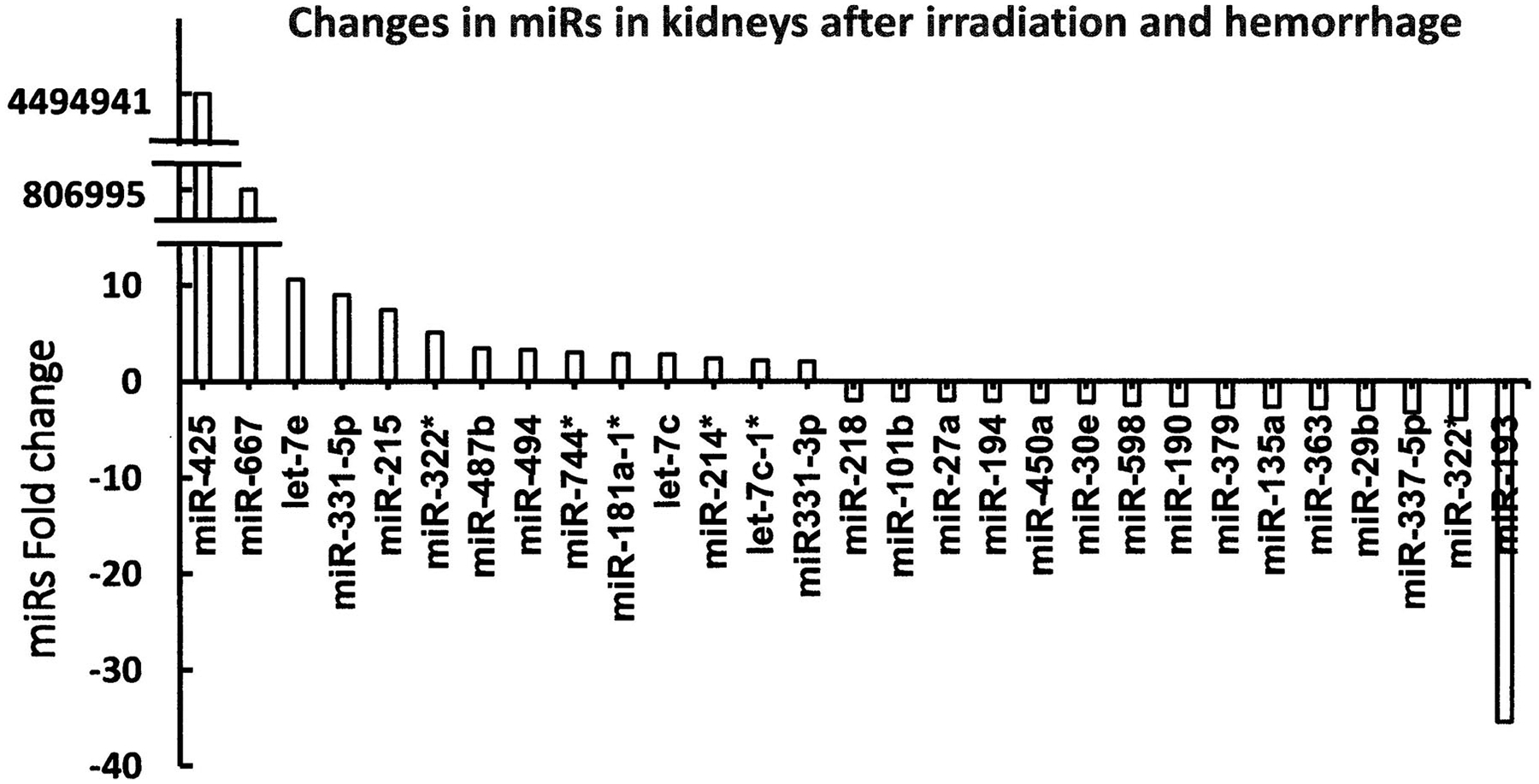
CI increases microRNAs in kidneys of mice exposed to radiation followed by hemorrhage. CD2F1 male mice received 8.75 Gy 60Co followed by 20% bleeding (Kiang et al. 2017a). Kidneys were collected on day 1 after CI (N = 6 per group). miR; microRNA. These results show changes reflecting greater than 2-fold changes (either increased or decreased) fold changes compared to the 0 Gy group.
Figure 2.
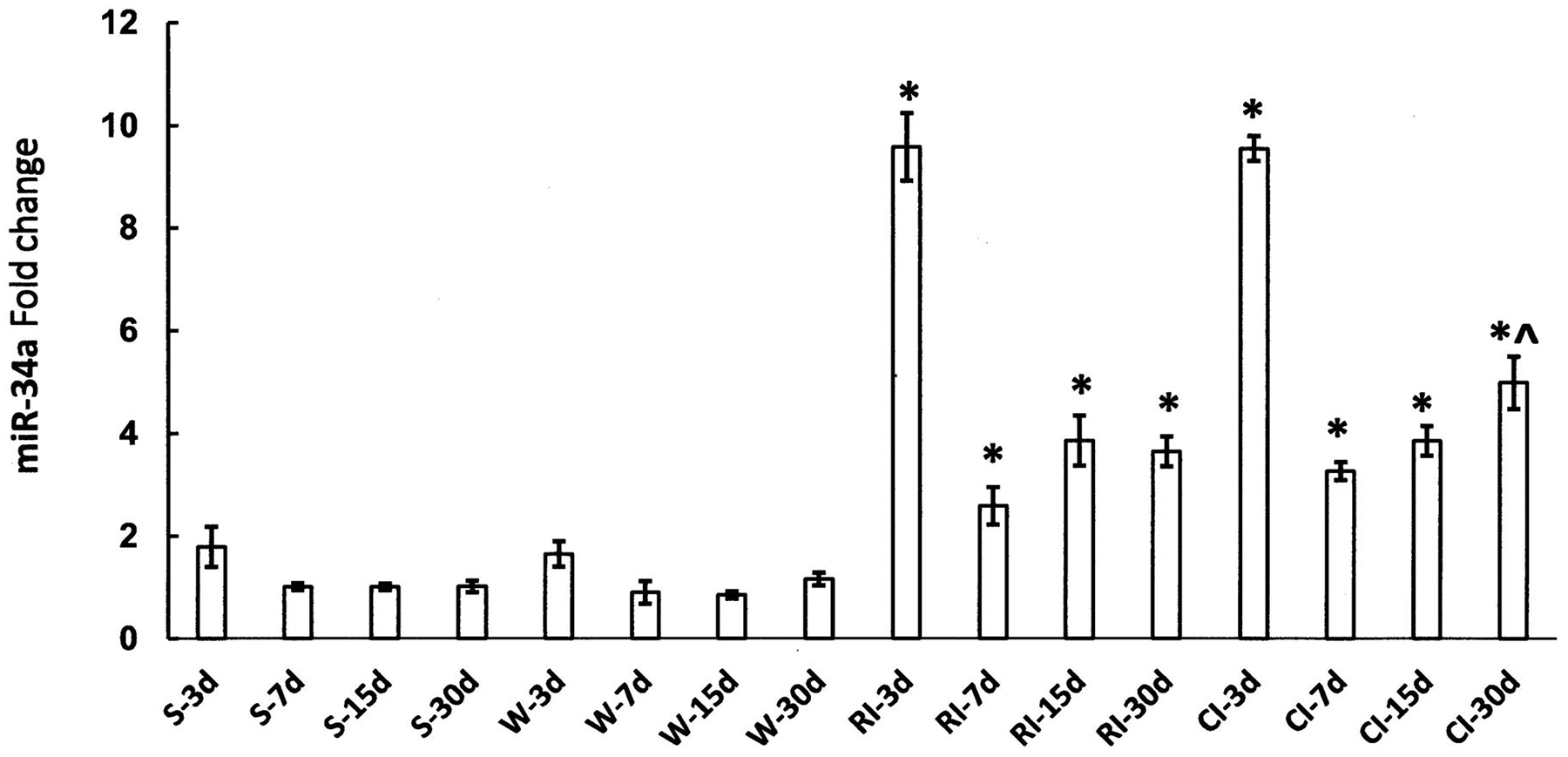
CI-induced miR-34a increases are not different from RI at early time points. B6D2F1 female mice received 9.5 Gy 60Co alone or followed by 15% total body surface area wound trauma (Kiang et al. 2010b). Ileums were collected on days 3, 7, 15 and 30 after RI or CI. No differences between the RI-induced increases in miR-34a and the CI-induced increases in miR-34a were found on days 3, 7, and 15. On day 30, the CI-induced increases were significantly greater than the RI-induced increases (N = 4 per group). *p < .05 vs. respective sham or wound alone; ^p<.05 vs. R-30d using T-Test. miR: microRNA (Kiang et al. 2022 for Materials and Methods). S: Sham; W: 15% total body surface wound; RI: 9.5 Gy; CI: 9.5 Gy + 15% total body surface wound. These results show changes compared to the sham group.
ATP is critical for biomolecular phosphorylation. Our laboratories reported that CI significantly induced ATP depletion in GI, pancreas, spleen, and kidney due to decreases in pyruvate dehydrogenase and increases in pyruvate dehydrogenase kinase 1 (Swift et al. 2015a), decreases in NRF1, NRF2 in cytosol, complexes 1–5 in mitochondria (Kiang et al. 2019) and mitochondrial remodeling (Gorbunov et al. 2015).
Table 2 lists the molecules changed by CI with RI plus wounding, burning, or hemorrhage. CI with RI + wound or RI + hemorrhage was studied extensively and displayed similar changes, whereas studies of CI with RI + burning were limited and required further exploration before a true comparison can be achieved among these 3 CIs. With current comparisons among the three combinations, increases in C3 and corticosterone and decreases in ATP production were commonly found.
Table 2.
Changes in molecules found in a mouse model of radiation followed by wounding, burning, or hemorrhage trauma.
| Molecule | RI + WND | RI + Burn | RI + Hemo | Molecule | RI + WND | RI + burn | RI + Hemo |
|---|---|---|---|---|---|---|---|
| NF-κB | ↑ | ND | ↑ | MCP-1 | ↑ | ND | ↑ |
| STAT3 | ↑ | ND | ↑ | MIP-1α/β | ↑ | ND | ↑ |
| iNOS | ↑ | ND | ↑ | IFN-γ | — | ND | — |
| Claudin-2 | ↑ | ND | ND | Rantes | — | ND | — |
| IL-1β | ↑ | ND | ↑ | AKT | ↓ | ND | — |
| IL-3 | — | ND | ↑ | MAPK | ↑ | ND | ↑ |
| IL-6 | ↑ | ND | ↑ | CRP | ↑ | ↓ | ↓ |
| KC (i.e., IL-8) | ↑ | ND | ↑ | C3 | ↑ | ↑ | ↑ |
| IL-10 | ↑ | ND | ↑ | Corticosterone | ↑ | ↑ | ↑ |
| IL-12p70 | ↑ | ND | ↑ | IgM | ↓ | ↓ | ↑ |
| IL-13 | ↑ | ND | ↑ | PGE2 | ↑ | ↑ | — |
| IL-18 | ↑ | ND | ↑ | ATP | ↑ | ↑ | ↑ |
| TNF-α | ↑ | ND | ↑ | miR-34 | ↑ | ND | ↑ |
| G-CSF | ↑ | ND | — | Caspase-3 | ↑ | ND | ↑ |
| GM-CSF | ↑ | ND | ↑ | Flt-3L | ↑ | ND | ↑ |
| Exotaxin | ↑ | ND | ↑ | Citrulline | ↓ | ND | ND |
NF-κB: Nuclear factor-keppa B; STAT3: Signal transducer and activator of transcription 3; iNOS: inducible nitric oxide synthase; IL-interlukin; KC: Keratinocyte chemoattractant; TNF: Tumor necrosis factor; G-CSF: Granulocyte-colony stimulating factor; GM-CSF: Granulocyte-Macrophage-colony stimulating factor; MCP-1: Monocyte chemoattractant protein-1; MIP-1: Macrophage inflammatory protein; IFN: Interferon; AKT: Protein kinase B; MAPK: Mitogen-activated protein kinase; CRP: C-reactive protein; C3: Complement component 3; IgM: Immunoglobulin M; PGE2: Prostaglandin E2; ATP: Adrine trisphophate; miR: MicroRNA; Flt-3L: Fms-like tyrosine kinase 3 ligand; RI: Radiation Injury; WND: Wound; Hemo: Hemorrhage; ↑: Increase; ND: Not done; ↓: Decrease;—:Not different from radiation alone.
Countermeasures tested for CI
FDA has approved Neupogen (Amgen Inc 2015a), Neulasta (Amgen Inc2015b), Nplate (Amgen Inc 2021), and Leukine (Safoni-Aventis 2018). They are approved as mitigators to treat hematopoietic syndrome. Despite their efficacy to mitigate radiation-induced hematopoietic acute radiation syndrome, none of them is efficacious for treating CI.
To determine whether a drug is qualified as a medical countermeasure, 30-day survival, body weight loss, and wound healing time are the criteria when the animal is exposed to radiation at LD50/30. Many drugs were tested. Many of them failed to improve survival, mainly due to neither mitigation in body weight loss nor acceleration of wound healing. However, Table 3 lists that treatment with single therapy including gentamicin, Silvadene, WR-151337 (Ledney and Elliott 2010), Alxn4100TPO (Kiang et al. 2017b), bone marrow transplant (Ledney and Elliott 2010), mesenchymal stem cells (Kiang and Gorbunov 2014), Ghrelin (Kiang et al. 2014b; Kiang, Anderson, et al. 2018, 2020), and Ciprofloxacin (Fukumoto et al. 2014; Kiang and Fukumoto 2014). Table 4 lists combined therapy such as 5-S-TDCM plus Gentamicin (Ledney and Elliott 2010), Neulasta plus Alxn4100TPO (Kiang et al. 2017b) and Neulasta plus L-citrulline (Wang et al. 2021) resulted in increases in survival after CI.
Table 3.
Drugs that improve survival after lethal irradiation alone (RI) or combined radiation with wound or burn trauma (CI).
| Single drug | Species | Rad. dose | Admin. route | Admin. doses | Improvement | References | |
|---|---|---|---|---|---|---|---|
| RI | CI | ||||||
| Neupogen (G-CSF) | B6D2F1 female mice | 9.5 Gy 60Co | SC | 10 μg/kg; once daily +1–14d | Yes for survival | No for survival | Kiang et al. Oxid Med Cell Longev 2014a; 2014, 481392. |
| Neulasta (pegylated G-CSF) | B6D2F1 female mice | 9.5 Gy 60Co | SC | 1 mg/kg; once +1d, +8d, +15d | Yes for survival | No for survival | Kiang et al. Oxid Med Cell Longev 2014a; 2014, 481392. |
| Alx4100TPO | B6D2F1 female mice | 9.5 Gy 60Co | SC | 1 mg/kg, once +1d | No for survival | Yes for survival | Kiang et al. Mediators of Inflamm 2017; 2017, 7582079. |
| Mouse MSCs | B6D2F1 female mice | 9.25–9.75 Gy 60Co | IV | 3×106 cells/mouse +1d | ND | Yes for survival | Kiang and Gorbunov, J Cell Sci Ther 2014; 5, 6. |
| Mouse Ghrelin | B6D2F1 female mice | 9.5 Gy 60Co | IV | 113 μg/kg; once +1d, +2d, +3d | NO for survival | Yes for survival | Kiang et al Oxid Med Cell Longe 2014b; 2014, 215858; Cell Biosci 2018; 8, 27 and 2020; 10, 63. |
| Human Ghrelin | Sprague-Dowley male rats | 10 Gy gamma | SC | 20 nmol/rat | Yes for survival | ND for CI | Wang et al. PLoS ONE 2015; 10(2), e0118213. |
| Ciprofloxacin | B6D2F1 female mice | 9.25 Gy 60Co | PO | 90 mg/kg, po once, 2 h and 1d–21d | No for survival | Yes for survival | Fukumoto et al. PLOS ONE 2014; 9(2), e90448 |
| Gentamicin | C3H/HeN-J female mice | Dose–response; 67% neutron + 33% gamma | Topical | 4hr after and +1d–+9d | NA | Yes for survival with DRF = 1.36 (mixed–field) | Ledney and Elliott, Health Phys 2010; 98(2), 145–52 |
| Silvadene | C3H/HeN-J female mice | Dose–response; 67% neutron + 33% gamma | Topical | 4hr after and +1d–+9d | NA | Yes for survival with DRF = 1.16 (mixed–field) | Ledney and Elliott, Health Phys 2010; 98(2), 145–152 |
| WR-151327 | C3H/HeN-J female mice | Dose–response; 67% neutron + 33% gamma or 100% gamma alone | IP | 200 mg/kg, 30min before irradiation | Yes for survival with DRF = 1.53 (gamma) and 1.31 (mixed–field) | Yes for survival with DRF = 1.51 (gamma) and 1.22 (mixed–field) | Ledney and Elliott, Health Phys 2010; 98(2), 145–152 |
| Bone marrow transplantation | B6D2F1 female mice | 10 Gy 60Co | IV | Given after RI or CI. Time was not known. For CI, needed 10X more than RI | Yes for survival | Yes for survival | Ledney and Elliott, Health Phys 2010; 98(2), 145–152 |
Rad.: radiation; Admin.: administration; SC: subcutaneous; PO: per os (i.e., oral feed); IV: intravenous; IP: intrapreural; RI: radiation injury; CI: combined radiation injury; ND: not done; MSC: mesenchymal stem cells; TPO: thrombopoietin; DRF: dose reduction factor.
Table 4.
Combined drugs that improve survival after lethal irradiation alone (RI) or combined radiation with wound or burn trauma (CI).
| 2 drugs | Species | Rad. dose | Admin. route | Admin. doses | Improvement | References | |
|---|---|---|---|---|---|---|---|
| RI | CI | ||||||
| Neulasta + Alxn4100TPO | B6D2F1 female mice | 9.5 Gy 60Co | SC; SC | 1mg/kg sc + 1d, +8d, +15d; 1 mg/kg sc, +1d | Yes for survival | Yes for survival | Kiang et al. Radiat Res 2017; 188, 476–490. |
| Neulasta + Ghrelin | B6D2F1 female mice | 9.5 Gy 60Co | SC; SC | 1mg/kg sc + 1d, +8d, +15d; 113 μg/kg; once +1d, +2d, +3dμ | Yes for survival | No for survival | Kiang et al. Front Pharmacol 2021; 2021:628018 |
| Neulasta + Ciprofloxacin | B6D2F1 female mice | 9.5 Gy Co-60 | SC; PO | 1mg/kg sc + 1d, +8d, +15d; 90 mg/kg, po once, +2 hr, +1d–+21dμ | Yes for survival | No for survival | Kiang et al. in preparation |
| Neulasta + L-Citrulline | B6D2F1 female mice | 9.5 Gy 60Co | SC; PO | 1mg/kg sc +1d, +8d, +15d; 1 g/kg; po once +1d-21d | Yes for survival | almost for survival | Wang et al. Radiat Res 2021; 196, 113–127 |
| S-TDCM + Gentamicin | C3H/HeN-J female mice | 8Gy 60Co | IP | 200 mg/kg, immediately after irradiation | No for survival | Yes for survival | Ledny and Elliott, Health Phys 2010; 98(2), 145–152 |
Rad.: radiation; Admin.: administration; SC: subcutaneous; PO: per os (i.e., oral feed); IP: intrapreural; RI: radiation injury; CI: combined radiation injury.
Single therapy
• Effects of Gentamicin on CI
Gentamicin (0.1% in Garamycin cream) is one of antibiotics. C3H/HeN female mice received gentamicin topically covering the entire wounded area 4 hr after CI and thereafter once daily for 9 days. The mixed-field (67% neutron + 33% gamma) irradiation increased LD50/30 from 3.13 Gy to 4.26 Gy with a dose reduction factor (DRF) of 1.36. No body weight loss and wound healing measurements were conducted (Ledney and Elliott 2010).
• Effects of silvadene on CI
Silvadene (1% silver sulfadiazine) is one of the antibiotics. C3H/HeN female mice received silvadene topically covering the entire wounded area 4 hr after CI and thereafter once daily for 9 days. The mixed-field (67% neutron + 33% gamma) irradiation increased L50/30 from 3.13 Gy to 3.74 Gy with DRF of 1.16. No body weight loss and wound healing measurements were conducted (Ledney and Elliott 2010).
• Effects of WR-151327 on CI
WR-151327 is S-3-(3-methylaminopropylamino) propylthiophosphorothioic acid. C3H/HeN female mice received this drug (200 mg/kg, i.p.) 30 min before RCI. The mixed-field (67% neutron + 33% gamma) irradiation increased CI LD50/30 from 3.75 Gy to 4.55 Gy with DRF of 1.22. The pure gamma irradiation increased CI LD50/30 from 7.60 to 11.50 with DRF of 1.51. No body weight loss and wound healing measurements were conducted (Ledney and Elliott 2010).
• Effects of Alxn4100TPO on CI
Alxn4100TPO is a thrombopoietin (TPO) receptor agonist. It was subcutaneously administered with 1 mg/kg s.c. 24 hr to B6D2F1 female mice after CI. It significantly attenuated body weight loss and increased 30-day survival by 20%, but failed to accelerate wound healing. It significantly increased platelet counts (Kiang et al. 2017c). The results suggest that failure in wound healing acceleration limits its efficacy in improving survival after CI.
• Effects of bone marrow transplant on CI
Whole bone marrow collected from femurs (1 × 104 to 20 × 106 cells per mouse in 0.5 ml RPMI-1640 media) was intravenously delivered to C3H3 female mice within 2 hr after CI. 100% survival was observed. Similar observations were also obtained in mice exposed to RI, but the bone marrow cells required were 10 times lower than CI (Ledney and Elliott 2010). Neither body weight loss nor wound healing rates were reported.
• Effects of mesenchymal stem cells on CI
B6D2F1 female mice were administered with bone marrow-derived mesenchymal stem cells extracted from femurs at 3×106 cells in 0.4 ml DMEM through the tail vein 24 hr after LD70/30 CI. The survival was increased by 30% above the vehicle-treated group. The body weight loss was mitigated. The wound was healed 7 days earlier than the vehicle-treated group. The histopathology with H&E staining appeared to increase cellularity (Kiang and Gorbunov 2014).
• Effects of Ghrelin on CI
Ghrelin, 28 amino acids, is released from the stomach during hunger and triggers appetite through the thalamus. B6D2F1 female mice were administered with ghrelin at 113 mg/kg i.v. 24 hr, 48 hr, 72 hr after CI. This hunger-stimulating peptide therapy increased survival, mitigated body-weight loss, accelerated wound healing, and numbers of neutrophils, lymphocytes, and platelets and ameliorated bone-marrow cell depletion (Kiang et al. 2014b). Additionally, this therapy appeared to effectively inhibit RCI-induced brain surface hemorrhage and intracranial hemorrhage (Gorbunov and Kiang 2017; Kiang et al. 2019).
This laboratory reported that Ghrelin enabled to mitigate the of CI-induced bone marrow injury and splenocytopenia by sustaining G-CSF and KC increases in circulation (Kiang et al. 2018). Additionally, Ghrelin therapy mitigated CI-induced increases in IL-1β, IL-6, IL-17A, IL-18, KC and TNF-α in serum but sustained G-CSF, KC and MIP-1αincreases in GI. Moreover, Ghrelin increased AKT activation and ERK activation and suppressed JNK activation, caspase-3 activation, NF-κB activation, iNOS, and BAX. In turn, the tight junction in GI was repaired so to mitigate bacterial translocation from GI lumen into the tissue (Kiang et al. 2020), suggesting Ghrelin improves survival mediated by organ repairs such as bone marrow, GI, and brain repair, or mitigation of hematopoietic ARS, GI ARS, and Neurovascular ARS.
• Effects of Ciprofloxacin on CI
Ciprofloxacin, Ciprofloxacin is an FDA-approved fluoroquinolone (FQ), which is widely used as an antimicrobial. Ciprofloxacin has been included in the Strategic National Stockpile, which is maintained by the U.S. Department of Health and Human Services, to control bacterial infection during a national emergency such as a nuclear detonation or other radiological incidents. Besides the antimicrobial activity, several groups reported immunomodulatory effects that Ciprofloxacin exerts in rodent models and human clinical trials (Dalhoff and Shalit 2003; Dalhoff 2005), improving a wide spectrum of conditions including thrombocytopenia (Shoenfeld et al. 1998; Savion et al. 2000; Blank et al. 1998), Crohn’s disease (Stein and Hanauer 1999; Rath et al. 2001), rheumatoid arthritis (Breban et al. 1992; Lewis and Keft 1995) and chemotherapy-induced neutropenia (Freifeld et al. 1999). Most importantly, oral administration with Ciprofloxacin at 90 mg/kg, 2 hr after CI and once daily thereafter for 21 days, significantly increased survival, mitigated body weight loss and accelerated skin-wound healing (Fukumoto et al. 2014; Kiang and Fukumoto 2014). Ciprofloxacin improved recovery of bone marrow cellularity (Fukumoto et al. 2014), enhanced stress erythropoiesis in the spleen that was stimulated by circulating IL-3 increases (Fukumoto et al. 2014; Kiang and Fukumoto 2014), and mitigated ATP loss in GI, pancreas, spleen, and kidney via preservation of pyruvate dehydrogenase and inhibition of pyruvate dehydrogenase kinase 1 (Swift et al. 2015a). Furthermore, Ciprofloxacin acted as a radio-sensitizer to tumor cells and a radio-protectant for normal cells via differential effects on γ-H2AX formation, p53 phosphorylation and Bcl-2 production (Kiang et al. 2014c). Repurposing Ciprofloxacin in this regard will be beneficial to treat CI in the near future due to the increasing threat of using nuclear weapons worldwide.
Combined therapy
Combined therapies of 5-S-TDCM plus Gentamicin (Ledney and Elliott 2010), Neulasta plus Alxn4100TPO (Kiang et al. 2017b), or Neulasta plus L-Citrulline (Wang et al. 2021) are effective in increasing the survival after RCI, likely by enhancing the survival of the hematopoietic stem/progenitor cells, GI repair, or accelerating recovery of cutaneous wounds. Moreover, co-therapy of Neulasta with Ghrelin mitigated the radiation-induced brain hemorrhage (Kiang et al. 2019), even though treatment with Ghrelin alone showed to significantly diminish the brain hemorrhage induced by radiation combined with burn trauma (Gorbunov and Kiang 2017).
• Effects of S-TDCM plus Gentamicin on CI
Synthetic trehalose dicorynomycolate (S-TDCM), a nonspecific immuno- and hematopoietic modulator. C3H3 female mice were exposed to 8 Gy RCI and immediately received S-TDCM (200 mg/kg, i.p.) with topical gentamicin. This combined therapy increased 30-day animal survival. There were no reports on body weight loss and wound healing impacted by this combined therapy (Ledney and Elliott 2010).
• Effects of Neulasta plus Alxn4100TPO on CI
Alxn4100TPO is a TPO receptor agonist and known to increase platelets and survival after RCI (Kiang et al. 2017b). B6D2F1 female mice were injected with Neulasta™ at 1 mg/kg, subcutaneously on days 1, 8 and 15 after RCI and Alxn4100TPO (1 g/kg), once 4 hr after RCI. The results suggest that combined treatment with Neulasta and Alxn4100TPO is effective for mitigating the effects of both radiation alone and in combination with wound injury. Each individual drug alone or the combined treatment resulted in the mitigation of body weight loss, WBC depletion, and platelet depletion, but did not accelerate wound healing (Kiang et al. 2017b).
• Effects of Neulasta plus L-Citrulline on CI
L-citrulline is a neutral alpha-amino acid shown to improve vascular endothelial function in cardiovascular diseases (Romero et al. 2006; Matsuo et al. 2017). B6D2F1 female mice were injected with Neulasta at 1 mg/kg, subcutaneously on days 1, 8 and 15 after RCI and L-citrulline (1 g/kg), once daily from day 1 to day 21 after CI. The combined therapy significantly improved the 30-day survival by 27% above the vehicle controls. This co-therapy significantly mitigated body weight loss increased bone marrow stem and progenitor cell clonogenicity, and accelerated recovery from intestinal injury. Although treatment with L-citrulline alone accelerated skin wound healing after CI, the co-therapy did not (Wang et al. 2021).
Data from our laboratory and others suggest that sex disparity to radiation sensitivity is present. Females were found to be more resistant to radiation than males (Kiang et al. 2022; Krukowski et al. 2018). In addition, other confounding factors including age, hypertension, diabetes, and high cholesterol should be addressed. However, it is imminent and imperative to explore whether the sex disparity is also present in the efficacy of radiation medical countermeasures for RI or CI.
Among all FDA-approved drugs and non-FDA-approved candidates, combined therapy with mesenchymal stem cells (Kiang and Gorbunov 2014) and Neulasta (Kiang et al. 2014a) should stand a great chance to significantly increase survival after CI, because whole bone marrow (containing pregenital stem cells and mesenchymal stem cells) transplantation displayed 100% survival after lethal CI (Ledney and Elliott. 2010), treatment with mesenchymal stem cells showed survival improvement by 30% after lethal CI (Kiang and Gorbunov 2014), and Neulasta demonstrated to mobilize lymphocytes from bone marrow to peripheral blood (Kiang et al. 2014a). In this case, if Neulasta can enhance mesenchymal stem cell capability to further save more lives after CI, then fewer numbers of mesenchymal stem cells are needed, thereby, leading to fewer chances to develop lung fibrosis later. However, there is a drawback to using mesenchymal stem cells, because medical assistance is required that is not feasible under a mass casualty scenario. In addition, the quality control and/or safety use of mesenchymal stem cells are not officially regulated yet.
CI and biodosimetry
Studies were performed to evaluate the effects of CI (radiation plus wounding) on the use of plasma proteomic biomarkers to assess radiation dose (Ossetrova and Blakely 2009; Sigal et al. 2013; Blakely et al. 2014; Ossetrova et al. 2014). A mouse study using female B6D2F1/J mice (N = 8 per dose and time point) was performed comparing a panel of candidate proteomic plasma radiation-responsive biomarkers after exposure to radiation compared to radiation and wounding involving a dorsal puncture over 15% body surface (CI) within 1–2 hr after radiation exposure. Blood was biosampled at various times after exposure to 60Co gamma rays (0.6 Gy/min) to a dose spanning from 0 to 10 Gy. Several radiation-response biomarkers (i.e. serum amyloid A or SAA, G-CSF, CD27) showed large wounding injury effects compared with the radiation-only response. For example, Figure 3 illustrates the response when measuring SAA, which illustrates significant (p < .05) elevations of SAA levels by wounding at equivalent radiation exposures. In contrast, several blood plasma proteomic biomarkers (i.e., Fms-like tyrosine kinase 3 ligand or Flt-3L, GM-CSF, TPO, EPO, IL-5, and CD27) were negligibly affected by wounding. For example Figures 4 and 5 illustrate the response when measuring Flt-3L (Figure 4) and CD27 (Figure 5), where RI and wounding (CI) showed either minor changes (i.e., fold differences less than ~2) or no significant differences (p < .05); see Supplementary Table 1 for data and statistical analysis. CI did not worsen or diminish radiation-induced increases in Flt-3 ligand (Jiao et al. 2009; Kiang et al. 2017b) or miR-34a at early time points before day 7 after CI (Figure 2).
Figure 3.
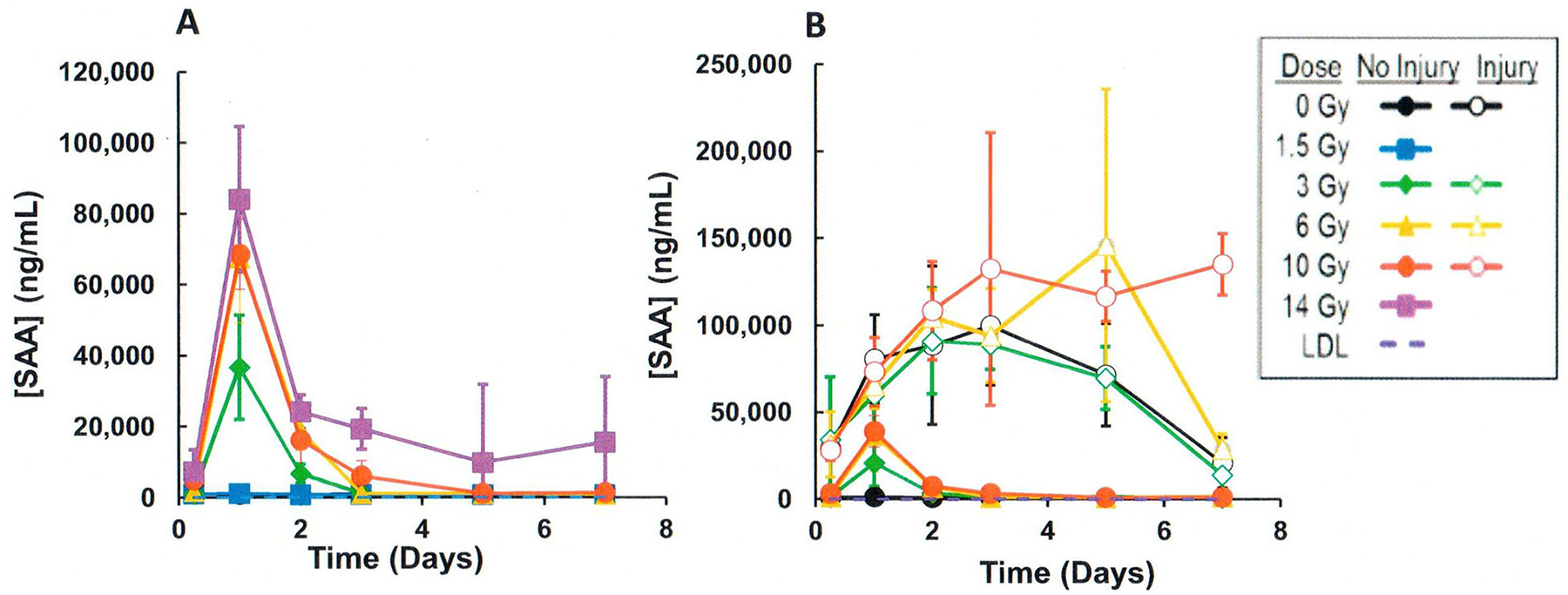
Comparison of effects of radiation vs CI (radiation plus wounding) on plasma serum amyloid A (SAA) in mouse radiation model. (A) Radiation exposure, (B) CI (radiation plus wounding) exposure. Symbols represent the mean and bar the standard error of the mean. Comparison of radiation vs radiation plus wounding samples showed significant differences using the T-test (p < .05) (Supplementary Table 1).
Figure 4.
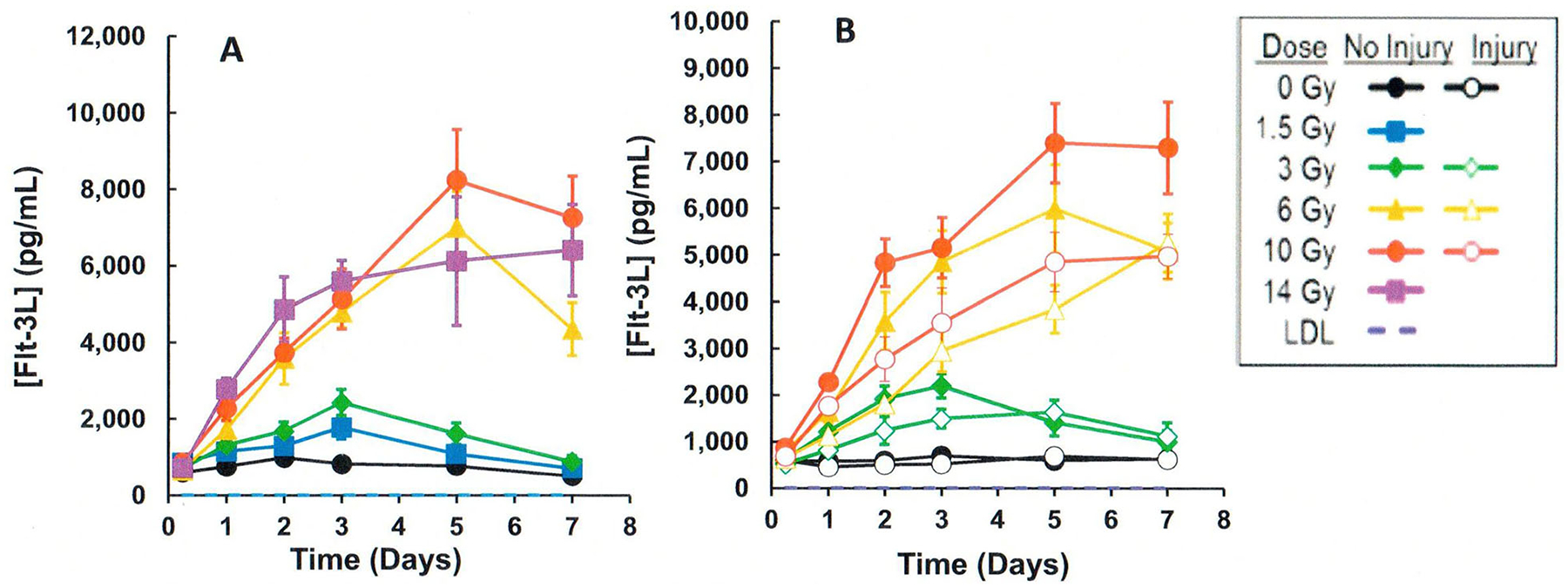
Comparison of effects of radiation vs. CI (radiation plus wounding) on plasma Fms-like tyrosine kinase 3 ligands (Flt-3L) in mouse radiation model. (A) Radiation exposure, (B) CI (radiation plus wounding) exposure. Symbols represent the mean and bar the standard error of the mean. Comparison of radiation vs radiation plus wounding samples showed either significant differences using the T-test (p < .05) or were less than ~2-fold different (Supplementary Table 1).
Figure 5.
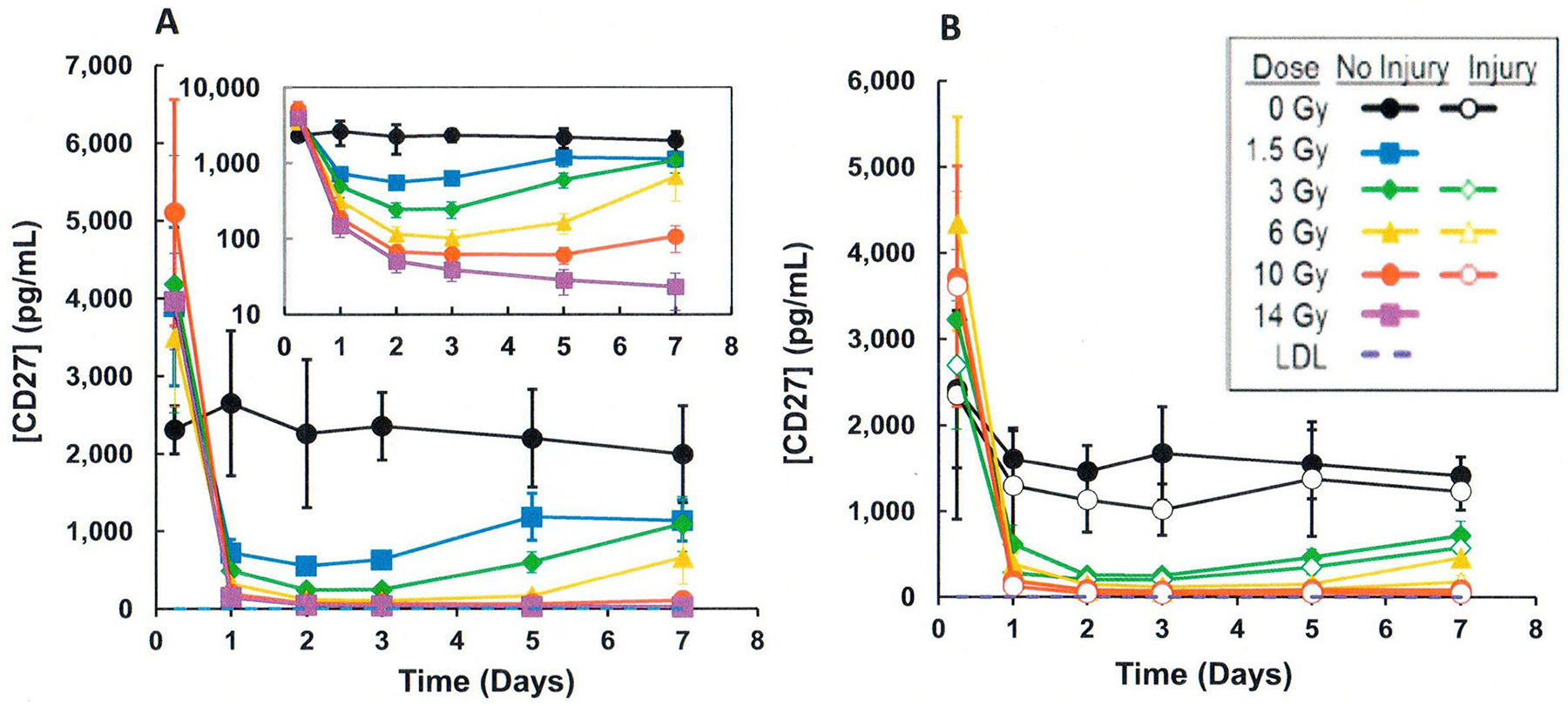
Comparison of effects of radiation vs. CI (radiation plus wounding) on plasma cluster differentiation 27 (CD27; lymphocyte surface biomarker) in mouse radiation model. Materials and methods as described in Figure 3 legend. (A) Radiation exposure, (B) CI (radiation plus wounding) exposure. Symbols represent the mean and bar the standard error of the mean. Comparison of radiation vs radiation plus wounding samples showed either significant differences using the T-test (p < .05) or were less than ~2-fold different (Supplementary Table 1).
A multivariate algorithm was developed using the panel of radiation-responsive blood plasma proteomic biomarkers that were negligibly affected by wounding (data not shown). The robustness of this algorithm to accurately predicted the dose for combined injury samples was evaluated. Mice received 0 or 6 Gy 60Co gamma rays with (labeled I) or without (labeled N) skin wounding. Figure 6 shows the results from this study and illustrates individual animal dose predictions. Points within the dashed lines that showed acceptable accuracy criteria are found within the dashed lines shown in Figure 6. These findings illustrate that the selection of appropriate biomarkers, which are negligibly responsive to wounding, can minimize potential confounding effects of combined wound injury when using plasma proteomic biomarkers for dose assessment using a mouse radiation combined injury (wounding) model.
Figure 6.
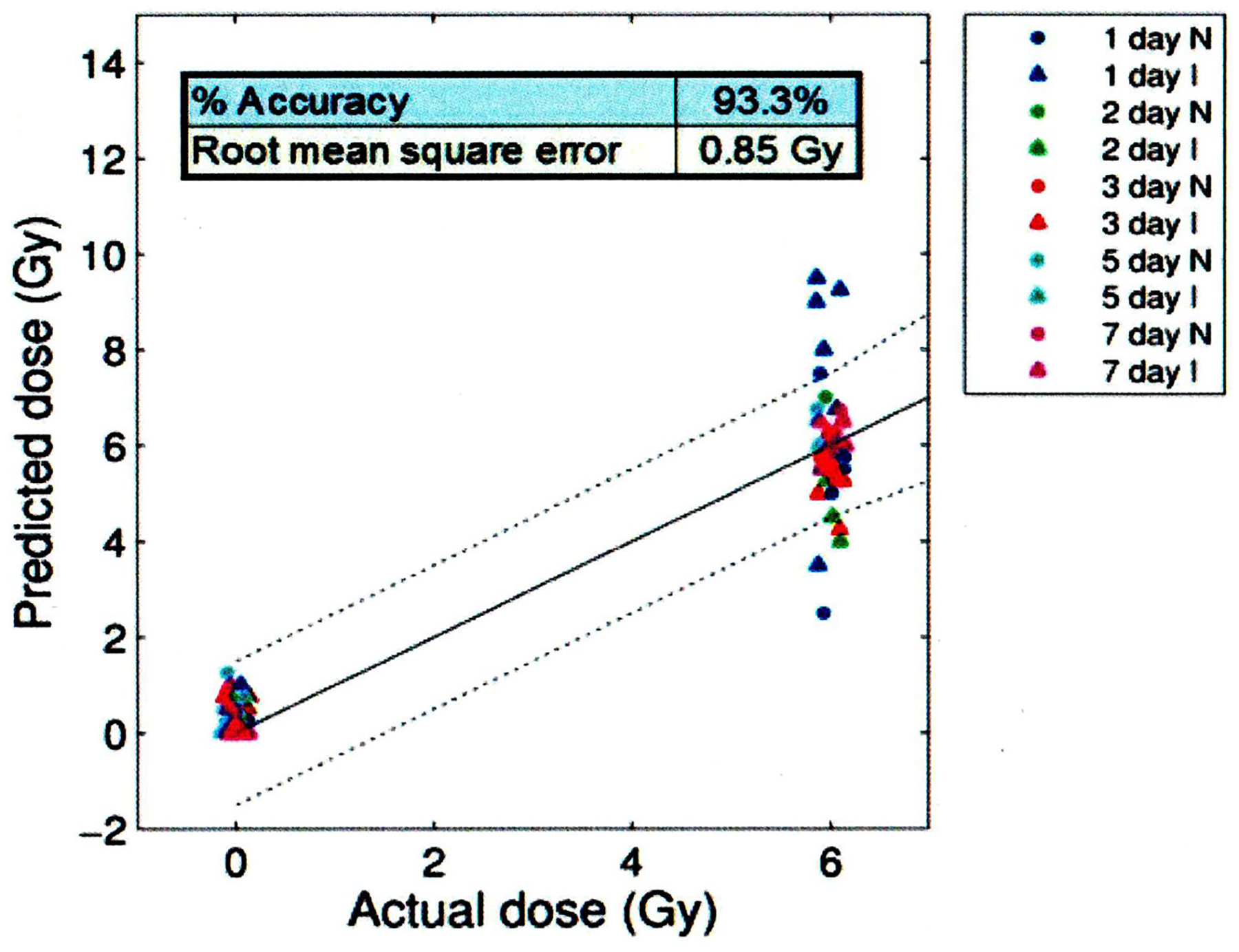
Effect of CI (radiation plus wounding) on radiation dose prediction accuracy based on an algorithm using a panel (i.e., CD27, Flt-3L, GM-CSF, CD45, IL-12, TPO) of plasma biomarkers negligibly affect by combined radiation and wounding. Mice received 0 and 6 Gy with or without skin wounding. Symbols represent the results from individual animals at the designated time points after irradiation. Time points within the dashed lines meet the established dose prediction accuracy criteria. CD27: cluster differentiation 27; Flt-3L: Fms-like tyrosine kinase 3 ligand; GM-CSF: granulocyte-macrophage-colony stimulating factor; CD45: cluster differentiation 45; IL-12: interleukin 12; TPO: thrombopoietin; N: not injured; I: injured.
The time window between radiation and wounding may affect the proteomic biomarker response. Further studies including an investigation of the possibility of a time-windows confounder need to be performed to evaluate the utility of a panel of proteomic biomarkers for dose assessment involving combined injury.
Material and methods
Radiation model and proteomic analysis
Female B6D2F1/J mice radiation vs CI model and radiation dosimetry was performed as described in Kiang et al. (2010b) except mice were double-loaded in mouse irradiation Plexiglass boxes and exposed to 60Co gamma rays doses of 0, 1.5, 3, 6, 10, 12, and 14 Gy at 0.6 Gy min−1. Wound trauma involving a dorsal puncture over 15% total body surface area in the mouse CI model was performed 1–2 hr after irradiation. Blood biosampling was performed at 0, 1, 2, 3, 5, and 7 d after irradiation. The number of mice per dose and time point was 8. A panel of candidate radiation-responsive plasma protein biomarkers were measured using the Meso Scale Diagnostic (MSD) MULTI-ARRAY electro-chemiluminescence-detection technology, which exhibited high sensitivity and dynamic range capabilities, as previously described (Debad et al. 2004). Assays were developed as multiplex panels in 16-spot MULTIARRAY 96-well plates and analyzed on an MSD PR2 Model 1800 plate reader as previously described (Sigal et al. 2013).
Dose prediction algorithm
Dose prediction algorithms were developed as previously described (Sigal et al. 2013). Briefly, plot average response surface in dose and time for each biomarker was developed to predict the dose for an individual sample. The target-specific algorithms selected the dose that best matches the response surface for all of the selected biomarkers of a panel in the model. All panels were evaluated and the best panel that predicted doses for ~94% of samples were selected using a root mean square error analysis.
Statistical analysis
Data analysis and graphs were done using MS excel (Microsoft Inc.). Comparisons of results for radiation vs radiation and wounding were evaluated using a two-sample T-test using an online tool (Statistics Kingdom) accessible at the website https://www.statskingdom.com/140MeanT2eq.html.
Summary
The effects of combined radiation with wounding, burns, hemorrhage, or infection represent significant confounders in understanding the resultant radio-response (in particular, organ impairment and survival as well as delayed outcomes) and the development of preparation for medical response both in the assessment and medical management of injuries. Efforts to investigate the CI murine model established at AFRRI have been reviewed with a focus on characterizing the early-phase molecular responses, promising results on the use of single and combined medical countermeasures and preliminary findings on a proposed use of plasma proteomic biomarker panel, negligibly influenced by the combined insults of radiation and wounding, for radiation dose assessment. Follow-on studies are needed to further characterize the effects of CI on molecular mechanisms underlying RI, and validate both uses of medical countermeasures and radiation diagnosis following CI.
Supplementary Material
Acknowledgements
G. Sigal (MSD Technologies) contributed to the experimental design, analysis of proteomic biomarkers, and dose-prediction algorithm development. Author (JGK) thanks all scientists, technicians, and research assistants for their contributions to the combined radiation injury field over all these years. Author (WFB) wishes to credit the efforts of AFRRI contributors Dr. V Nagy, Dr. NI Ossetrova, K Krasnopolsky, KP Hieber, DP Condiffe, and DJ Sandgren, who contributed to support radiation dosimetry and the proteomic biodosimetry study involving comparison of radiation with combined radiation injury (wounding).
Funding
The studies were supported by NIAID-AFRRI Inter-Agency Agreement YI-AI-5045-04, NIAID R21/R33 AI080553, JPC-7 VP000276-01, SAPO: G17070 (a subaward of 5U19AI067773-18) and AFRRI intramural RAB32164, 33336, 33529, 310934, AFR-B2-12950 and AFR-B2-12812 (to JGK). Project funded by Biomedical Advanced Research and Development Authority BARDA (BAA-BARDA-09-36), which is part of the Office of the Assistant Secretary for Preparedness and Response within the US Department of Health and Services. AFRRI’s contributions were part of a sub-award to Drs. NI Ossetrova and WF Blakely. This study was also funded by National Institute of Allergy and Infectious Diseases, National Institute of Allergy and Infectious Diseases, and U.S. Department of Defense.
Footnotes
Dedication
Professor John Edward Moulder (1945–2022) scientific contributions spanned a wide-range of interests in radiobiology research and clinical radiotherapy. His contributions also extended into the area of medical readiness to provide radiation diagnosis to triage and guide medical management of potential radiation-exposed individuals with a focus on assessment and medical management of radiation injury to the kidney. We dedicate our manuscript to honor the rich contributions and guidance Professor Moulder provided in this area. He will be truly missed.
Disclosure statement
The authors report no financial conflicts of interest. The manuscript was cleared for publication by the Armed Forces Radiobiology Research Institute, The Uniformed Services of the Health Sciences. The views, opinions, and findings contained in this report are those of the authors and do not reflect official policy or positions of the Armed Forces Radiobiology Research Institute, the Uniformed Services University of the Health Sciences, the Department of Defense, or the United States government.
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09553002.2023.2188933
Data availability statement
Data presented in this review are available in it.
References
- Alpen EL, Sheline GE. 1954. The combined effects of thermal burns and whole body X irradiation on survival time and mortality. Ann Surg. 140(1):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amgen Inc. 2015a. NEULASTA® (pegfilgrastim) injection, for subcutaneous use.
- Amgen Inc. 2015b. Neupogen (filgrastim) injection for subcutaneous or intravenous use.
- Amgen Inc. 2021. NPLATE® (romiplostim) for injection, for subcutaneous use.
- Barabanova AV. 2006. Significance of beta-radiation skin burns in Chernobyl patients for the theory and practice of radiopathology. Vojnosanit Pregl. 63(5):477–480. [DOI] [PubMed] [Google Scholar]
- Baxter H, Drummond JA, Stephens-Newsham LG, Randall RG. 1953. Reduction of mortality in swine from combined total body radiation and thermal burns by streptomycin. Ann Surg. 37:450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely WF, Sandgren DJ, Nagy V, Kim SY, Sigal GB, Ossetrova NI. 2014. Further biodosimetry investigations using murine partial-body irradiation model. Radiat Prot Dosimetry. 159(1–4):46–51. [DOI] [PubMed] [Google Scholar]
- Blank M, George J, Fishman P, Levy Y, Toder V, Savion S, Barak V, Koike T, Shoenfeld Y. 1998. Ciprofloxacin immunomodulation of experimental antiphospholipid syndrome associated with elevation of interleukin-3 and granulocyte-macrophage colony-stimulating factor expression. Arthritis Rheum. 41:224–232. [DOI] [PubMed] [Google Scholar]
- Breban M, Fournier C, Gougerot-Pocidalo MA, Muffat-Joly M, Pocidalo JJ. 1992. Protective effects of ciprofloxacin against type II collagen induced arthritis in rats. J Rheumatol. 19:216–222. [PubMed] [Google Scholar]
- Brook I, Elliott TB, Harding RA, Bouhaouala SS, Peacock SJ, Ledney GD, Knudson GB. 2001. Susceptibility of irradiated mice to Bacillus anthracis sterne by the intratracheal route of infection. J Med Microbiol. 50(8):702–711. [DOI] [PubMed] [Google Scholar]
- Brooks JW, Evans EI, Ham WT Jr., Reid JD. 1952. The influence of external body radiation on mortality from thermal burns. Ann Surg. 136:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff A, Shalit I. 2003. Immunomodulatory effects of quinolones. Lancet Infect Dis. 3:359–371. [DOI] [PubMed] [Google Scholar]
- Dalhoff A 2005. Immunomodulatory activities of fluoroquinolones. Infection. 33(Suppl 2):5–70. [DOI] [PubMed] [Google Scholar]
- Debad JD, Glezer EN, Wohlstadter JN, Sigal GB. 2004. Clinical and biological applications of ECL. In: Bard AJ (Ed.) Electrogenerated Chemiluminescence. New York (NY): Marcel Dekker. [Google Scholar]
- Elliott TB, Brook I, Harding RA, Bouhaouala SS, Shoemaker MO, Knudson GB. 2002. Antimicrobial therapy for bacillus anthracis-induced polymicrobial infection in (60)Co gamma-irradiated mice. Antimicrob Agents Chemother. 46(11):3463–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifeld A, Marchigiani D, Walsh T, Chanock S, Lewis L, Hiemenz J, Hiemenz S, Hicks JE, Gill V, Steinberg SM, et al. 1999. A double blind comparison of empirical oral and intravenous antibiotic therapy for low risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 341(5):305–311. [DOI] [PubMed] [Google Scholar]
- Fukumoto R, Burns TM, Kiang JG. 2014. Ciprofloxacin enhances stress erythropoiesis in spleen and increases survival after whole-body irradiation combined with skin-wound trauma. PLoS One. 9(2): e90448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunov NV, Kiang JG. 2017. Ghrelin therapy decreases incidents of intracranial hemorrhage in mice after whole-body ionizing irradiation combined with burn trauma. Int J Mol Sci. 18:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunov NV, McDaniel DP, Zhai M, Liao PJ, Garrison BR, Kiang JG. 2015. Autophagy and mitochondrial remodelling in mouse mesenchymal stromal cells challenged with Staphylococcus epidermidis. J Cell Mol Med. 19(5):1133–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima S 1982. Pathology of atomic bomb casualties. Acta Pathol Jpn. 32(Suppl 2):237–270. [PubMed] [Google Scholar]
- Jiao W, Kiang JG, Cary L, Elliott TB, Pellmar TC, Ledney GD. 2009. COX-2 inhibitors are contraindicated for therapy of combined injury. Radiat Res. 172(6):686–697. [DOI] [PubMed] [Google Scholar]
- Joint Commission of USA and Japanese Physicians collected from victims exposed to 20 KT fission Devices, 1946.
- Kiang JG, Anderson MN, Smith JT. 2018. Ghrelin therapy mitigates bone marrow injury and splenocytopenia by sustaining circulating G-CSF and KC increases after irradiation combined with wound. Cell Biosci. 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Cannon G, Olson MG, Smith JT, Anderson MN, Zhai M, Umali MV, Ho K, Ho C, Cui W, et al. 2022. Female mice are more resistant to the mixed-field (67% neutron + 33% gamma) radiation-induced injury in bone marrow and small intestine than male mice due to sustained increases in G-CSF and the bcl-2/bax ratio and lower miT-34a and MAPK activation. Radiat Res. 198:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Fukumoto R. 2014. Ciprofloxacin increases survival after ionizing irradiation combined injury: gamma-H2AX formation, cytokine/chemokine, and red blood cells. Health Phys. 106(6):720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Garrison BR, Smith JT, Fukumoto R. 2014c. Ciprofloxacin as a potential radio-sensitizer to tumor cells and a radioprotectant for normal cells: Differential effects on α-H2AX formation, p53 phosphorylation, Bcl-2 production, and cell death. Mol Cell Biochem. 393(1–2):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Garrison BR, Gorbunov NV. 2010a. Radiation combined injury: DNA damage, apoptosis, and autophagy. Adpt Med. 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Gorbunov NV. 2014. Bone marrow mesenchymal stem cells increases survival after ionizing irradiation combined with wound trauma: Characterization and therapy. J Cell Sci Ther. 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC, Ledney GD. 2010b. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 173(3):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Ledney GD. 2013. Skin injuries reduce survival and modulate corticosterone, C-reactive protein, complement component 3, IgM and prostaglandin E2 after whole-body reactor-produced mixed field (n + gamma-photons) irradiation. Oxid Med Cell Longev. 2013:821541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Olabisi AO. 2019. Radiation: a poly-traumatic hit leading to multi-organ injury. Cell Biosci. 9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Smith JT, Anderson MN, Swift JM, Christensen CL, Gupta P, Balakathiresan N, Maheshwari RK. 2015. Hemorrhage exacerbates radiation effects on survival, leukocytopenia, thrombopenia, erythropenia, bone marrow cell depletion and hematopoiesis, and inflammation-associated microRNAs expression in kidney. PLoS ONE. 10(9):e0139271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Smith J, Anderson M, Umali M, Ho C, Zhai M, Lin B, Jiang S. 2019. A novel therapy, using ghrelin with pegylated G-CSF, inhibits brain hemorrhage from ionizing radiation or combined radiation injury. Pharm Pharmacol Int J. 7(3):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Smith JT, Anderson MN, Elliott TB, Gupta P, Balakathiresan NS, Maheshwari RK, Knollmann-Ritschel B. 2017a. Hemorrhage enhances cytokine, complement component 3, and caspase-3, and regulates microRNAs associated with intestinal damage after whole-body gamma-irradiation in combined injury. PLoS One. 12: e0184393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Smith JT, Cannon G, Anderson MN, Ho C, Zhai M, Cui W, Xiao M. 2020. Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma. Cell Biosci. 10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Zhai M, Bolduc DL, Smith JT, Anderson MN, Ho C, Lin B, Jiang S. 2017b. Combined therapy of pegylated g-csf and alxn4100tpo improves survival and mitigates acute radiation syndrome after whole-body ionizing irradiation alone and followed by wound trauma. Radiat Res. 188:476–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Zhai M, Liao PJ, Bolduc DL, Elliott TB, Gorbunov NV. 2014a. Pegylated G-CSF inhibits blood cell depletion, increases platelets, blocks splenomegaly, and improves survival after whole-body ionizing irradiation but not after irradiation combined with burn. Oxid Med Cell Longev. 2014:481392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Zhai M, Liao PJ, Elliott TB, Gorbunov NV. 2014b. Ghrelin therapy improves survival after whole-body ionizing irradiation or combined with burn or wound: amelioration of leukocytopenia, thrombocytopenia, splenomegaly, and bone marrow injury. Oxid Med Cell Longev. 2014:315858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Zhai M, Liao PJ, Ho C, Gorbunov NV, Elliott TB. 2017c. Thrombopoietin receptor agonist mitigates hematopoietic radiation syndrome and improves survival after whole-body ionizing irradiation followed by wound trauma. Mediators Inflamm. 2017:7582079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Zhai M, Lin B, Smith JT, Anderson MN, Jiang S. 2015. Co-therapy of pegylated G-CSF and ghrelin for enhancing survival after exposure to lethal radiation. Front Pharmacol. 12:628018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi HS. 2000. Effects of the “special bomb”: recollections of a neuro-surgeon in Hiroshima, August 8–15, 1945. Neurosurgery. 47:441–445. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- Koenig KL, Goans RE, Hatchett RJ, Mettler FA Jr., Schumacher TA, Noji EK, Jarrett DG. 2005. Medical treatment of radiological casualties: current concepts. Ann Emerg Med. 45(6):643–652. [DOI] [PubMed] [Google Scholar]
- Korlof B 1956. Infection of burns. I. A bacteriological and clinical study of 99 cases. II. Animal experiments; burns and total body x-irradiation. Acta Chir Scand Suppl. 209:1–144. [PubMed] [Google Scholar]
- Krukowski K, Grue K, Frias ES, Pietrykowski J, Jones T, Nelson G. 2018. Female mice are protected from space radiation-induced mal-adaptive responses. Brain Behav Immun. 74:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausevic Z, Lausevic M, Trbojevic-Stankovic J, Krstic S, Stojimirovic B. 2008. Predicting multiple organ failure in patients with severe trauma. Can J Surg. 51:97–102. [PMC free article] [PubMed] [Google Scholar]
- Ledney GD, Elliott TB, Moore MM. 1992. Modulations of mortality by tissue trauma and sepsis in mice after radiation injury. In: Mossman KL, Mills WA, editors. The Biological Basis of Radiation Protection Practice. Philadelphia (PA): Lippincott Williams and Wilkins; p. 202–217. [Google Scholar]
- Ledney GD, Elliott TB. 2010. Combined injury: factors with potential to impact radiation dose assessments. Health Phys. 98(2):145–152. [DOI] [PubMed] [Google Scholar]
- Ledney GD, Stewart DA, Exum ED, Sheehy PA. 1981. Skin wound-enhanced survival and myelocytopoiesis in mice after whole-body irradiation. Acta Radiol Oncol. 20(1):29–38. [DOI] [PubMed] [Google Scholar]
- Lewis AJ, Keft AF. 1995. A review on the strategies for the development and application of new anti-arthritic agents. Immunopharmacol Immunotoxicol. 17(4):607–663. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Yabuki Y, Fukunaga K. 2017. Combined l-citrulline and glutathione administration prevents neuronal cell death following transient brain ischemia. Brain Res. 1663:123–131. [DOI] [PubMed] [Google Scholar]
- Ossetrova NI, Blakely WF. 2009. Multiple blood-proteins approach for early-response exposure assessment using an in vivo murine radiation model. Int J Radiat Biol. 85:837–850. [PubMed] [Google Scholar]
- Ossetrova NI, Condliffe DP, Ney PH, Krasnopolsky K, Hieber KP, Rahman A, Sandgren DJ. 2014. Early-response biomarkers for assessment of radiation exposure in a mouse total-body irradiation model. Health Phys. 106(6):772–786. [DOI] [PubMed] [Google Scholar]
- Rath HC, Schultz M, Freitag R, Dieleman LA, Li F, Linde HJ, Schölmerich J, Sartor RB. 2001. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 69(4):2277–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MJ, Platt DH, Caldwell RB, Caldwell RW. 2006. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 24(3–4):275–290. [DOI] [PubMed] [Google Scholar]
- Sanofi-Aventis U.S. LLC. 2018. LEUKINE® (sargramostim) for injection, for subcutaneous or intravenous use.
- Savion S, Blank M, Shepshelovich J, Fishman P, Shoenfeld Y, Toder V. 2000. Ciprofloxacin affects pregnancy loss in CBA/JxDBA/2J mice possibly via elevation of interleukin-3 and granulocyte macrophage-colony stimulating factor production. Am J Reprod Immunol. 44(5): 293–298. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Sherer Y, Fishman P. 1998. Interleukin-3 and pregnancy loss in antiphospholipid syndrome. Scand J Rheumatol Suppl. 107: 19–22. [DOI] [PubMed] [Google Scholar]
- Sigal GB, Glezer E, Kenten J, Kumar S, Ossetrova NI, Blakely WF. 2013. Biomarker-based radiation-dosimetry diagnostics. In Christensen DM, Sugarman SL and O’Hara FM, editors. The medical basis for radiation-accident preparedness. Oak Ridge (TN): Oak Ridge Associated Universities; p. 375–378. [Google Scholar]
- Stein RB, Hanauer SB. 1999. Medical therapy for inflammatory bowel disease. Gastroenterol Clin North Am. 28(2):297–321. [DOI] [PubMed] [Google Scholar]
- Swift JM, Swift SN, Smith JT, Kiang JG, Allen MR. 2015a. Skin wound trauma, following high-dose radiation exposure, amplifies and prolongs skeletal tissue loss. Bone. 81:487–494. [DOI] [PubMed] [Google Scholar]
- Swift JM, Smith JT, Kiang JG. 2015b. Hemorrhage trauma increases radiation-induced trabecular bone loss and marrow cell depletion in mice. Radiat Res. 183(5):578–583. [DOI] [PubMed] [Google Scholar]
- Valeriote FA, Baker DG. 1964. The combined effects of thermal trauma and X-irradiation on early mortality. Radiat Res. 22:693–702. [PubMed] [Google Scholar]
- Wang L, Zhai M, Lin B, Cui W, Hull L, Li X, Kiang JG, Xiao M. 2021. Peg-G-CSF and L-citrulline combinational therapy for mitigating skin wound combined radiation injury in a mouse model. Radiat Res. 196(1):113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Sun H, Su Y, Cheng T, Luo C. 2008. Progress in research on radiation combined injury in China. Radiat Res. 169(6):722–729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this review are available in it.


