Abstract
Reduced renal Na+ reabsorption along with restricted dietary Na+ depletes intravascular plasma volume which can then result in hypotension. Whether hypotension occurs and the magnitude of hypotension depends in part on compensatory angiotensin II-mediated increased vascular resistance. We investigated whether the ability of vascular resistance to mitigate the hypotension was compromised by decreased contractile reactivity. In vitro reactivity was investigated in aorta from mouse models of reduced renal Na+ reabsorption and restricted dietary Na+ associated with considerable hypotension and renin-angiotensin system activation: (1) the Na+-Cl−-Co-transporter (NCC) knockout (KO) with Na+ restricted diet (0.1%, 2 weeks) and (2) the relatively more severe pendrin (apical chloride/bicarbonate exchanger) and NCC double KO. Contractile sensitivity to KCl, phenylephrine, and/or U46619 remained unaltered in aorta from both models. Maximal KCl and phenylephrine contraction expressed as force/aorta length from NCC KO with Na+-restricted diet remained unaltered, while in pendrin/NCC double KO were reduced to 49 and 64%, respectively. Wet weight of aorta from NCC KO with Na+-restricted diet remained unaltered, while pendrin/NCC double KO was reduced to 67%, consistent with decreased medial width determined with Verhoeff-Van Gieson stain. These findings suggest that hypotension associated with severe intravascular volume depletion, as the result of decreased renal Na+ reabsorption, may in part be due to decreased contractile reactivity as a consequence of reduced vascular hypertrophy.
Keywords: Hypotension, Intravascular volume depletion, Knockout, Pendrin, Na-Cl cotransporter, Contraction, Aorta, Na+ reabsorption, Renal
Introduction
A major regulator of arterial pressure is intravascular plasma volume, with increased and decreased volume causing elevated and lowered pressure, respectively. The intravascular plasma volume is regulated by the amount of Na+ absorption by the kidney. Thus, decreased Na+ reabsorption and restricted dietary Na+ represent mechanisms whereby intravascular plasma volume is depleted, thereby lowering arterial pressure. The extent of lowered arterial pressure depends on several factors including the magnitudes of intravascular volume depletion and the compensatory increase in vascular resistance by elevated plasma levels of angiotension II.
Indeed, several mouse models of decreased Na+ reabsorption with and without restricted Na+ diet are associated with hypotension despite substantial activation of the renin-angiotensin system (Schultheis et al. 1998; Wall et al. 2004; Kim et al. 2007; Pech et al. 2010; Soleimani et al. 2012; Lazo-Fernandez et al. 2015; Alshahrani et al. 2016; Sinning et al. 2016). Furthermore, possible altered contractile reactivity was investigated in the aorta from the pendrin knockout (KO; Sutliff et al. 2014). This model is associated with hypotension, albeit at somewhat variable levels (5–17 mmHg; Kim et al. 2007; Pech et al. 2010; Lazo-Fernandez et al. 2015) and plasma renin activity is increased twofold (Kim et al. 2007). In the pendrin KO, maximal force expressed per cross-sectional area of the aorta was non-selectively increased by ~25%, suggesting possible mitigation of the associated hypotension (Sutliff et al. 2014). Although, since aorta wet weight and cross-sectional area decreased by ~20 and ~35%, respectively (Sutliff et al. 2014), one could also consider that force generated per vessel length actually remained unchanged, i.e., despite the decreased hypertrophy.
To further investigate whether vascular reactivity is compromised under conditions of hypotension associated with decreased Na+ reabsorption, we investigated whether aorta contractile reactivity was decreased in the pendrin/NCC double KO and Na+ diet-restricted NCC KO (Schultheis et al. 1998; Soleimani et al. 2012; Alshahrani et al. 2016). These models demonstrate considerable hypotension, with decreases of 20 and 13 mmHg, respectively, and increased renin amounts in the kidney analyzed by Western blot, with seemingly much greater renin in the pendrin/NCC double KO as compared to the NCC KO kidney (Soleimani et al. 2012; S.A. et al., manuscript in preparation). Some of these results have been reported in abstract form (Alshahrani et al. 2015).
Methods and materials
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council USA. NCC KO on normal (1%) and reduced Na+ diet (0.1%, 2 weeks), and pendrin/NCC double KO and wild-type mice (Soleimani et al. 2012) of 4–6 months of either sex were used.
Vascular reactivity
Contractile experiments were performed as previously described (Basford et al. 2009). Under a dissecting microscope, thoracic aorta was exposed in situ and cleaned of surrounding tissue. Following placement of the aorta in physiologic salt solution (mmol/L: NaCl 118, KCl 4.73, MgCl2 1.2, EDTA 0.026, KH2PO4 1.2, CaCl2 2.5, 25 NaH2CO3, and glucose 5.5; pH 7.4 with 95% O2/5% CO2 bubbling at 37 °C), each vessel was divided into two, ~5 mm ring segments, with the two segments serving as duplicates. Aorta segments from NCC KO with and without reduced Na+ diet, and segments from pendrin/NCC double KO and wild type, were tested in parallel.
Segments were placed in an isometric contractile apparatus. This procedure involved placement of two wires through the vessel lumen, with one wire stationary and the other movable, such that the distance between the wires could be adjusted and the resultant applied resting tension monitored via a transducer. Optimal 3 g-force resting tension was determined by repeated 50 mM KCl challenge of segments from the four mouse types. After each KCl challenge and wash, as well as following wash of other agonists, resting tension was readjusted to 3 g-force.
After repeated challenge with 50 mM KCl, segments were exposed to cumulative phenylephrine and U46619 concentrations, with the final concentrations at near maximal, i.e., the EC50s should also be considered as approximate. Angiotensin II challenge was performed with a single concentration, 0.1 μM, to avoid desensitization by prior angiotensin II exposure. Wet weight along with length was determined in a number of these vessel segments and also in some additional segments not subjected to tension.
Histology
Aorta was removed, fixed in formaldehyde solution (4 °C, overnight), and transferred to 70% ethanol, fixed in paraffin and 5 μm sections prepared. Hematoxylin-eosin, Verhoeff-Van Gieson, and Masson’s trichrome staining along with immunohistochemistry for smooth muscle actin were then performed. Evaluation included blinded observer.
Statistical analysis
Contraction was calculated as force in millinewton/millimeter aorta length and concentration-contraction curves calculated as percent contraction achieved with the maximally applied agonist concentration. EC50s were determined with GraphPad Prism. Mean ± SE of contractile response and EC50s, expressed as pD2 values, were determined. “n” Represents the number of mice. Statistical significance between means was determined with Student’s unpaired, two-tailed t test and significance accepted at p ≤ .05.
Materials
Phenylephrine and angiotensin II were from Sigma-Aldrich and U46619 from Cayman Chemical.
Results
Contraction
NCC KO with reduced Na+ diet
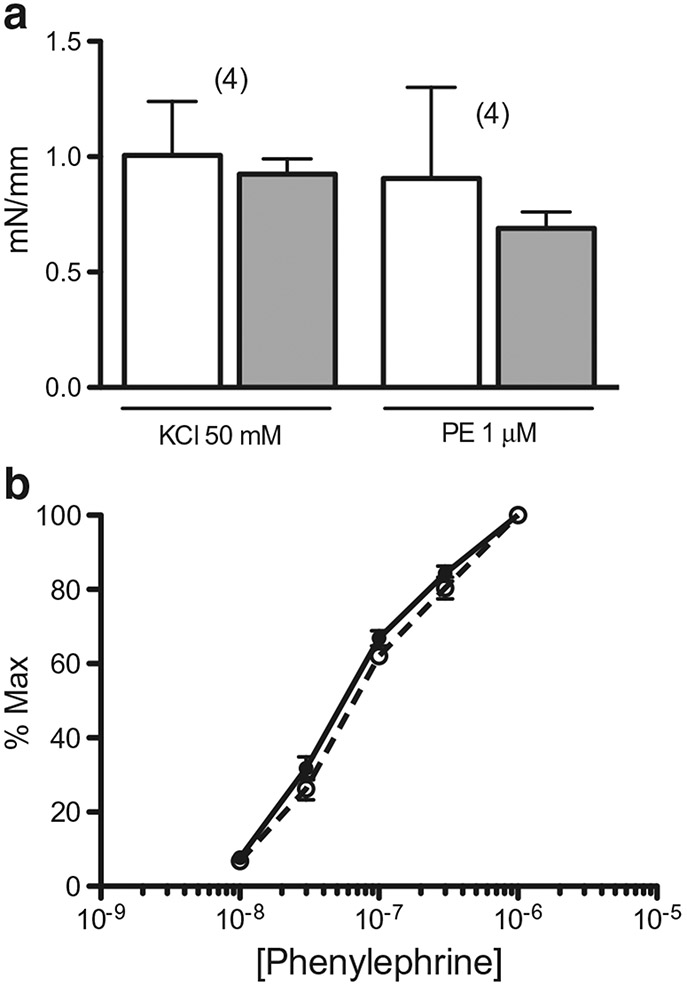
Contractile sensitivity of aorta to phenylephrine was not different in NCC KO with and without reduced Na+ diet (pD2 = 7.30 ± 0.06 and 7.17 ± 0.06, respectively; n = 4 in each case; Fig. 1). Contractions (millinewton/millimeter length) to 1 μM phenylephrine and 50 mM KCl were not different in aorta from NCC KO with and without reduced Na+ diet (Fig. 1).
Fig. 1.
Effect of NCC KO with reduced Na+ diet on agonist-induced aorta contraction. a Contractile responses of ring segments from NCC KO with (closed bar) and without reduced Na+ diet (open bar) to 50 mM KCl and 1 μM phenylephrine (PE) are expressed as millinewton/millimeter (mN/mm) vessel length. Number in parenthesis represents number of animals in each case. b Concentration-contraction curve of aorta to phenylephrine (n = 4 in each case) from NCC KO with reduced Na+ (open circle plus dashed line) and normal diet (closed circle plus uninterrupted line) was determined from the experiments in a and expressed as percent maximal contraction
Pendrin/NCC double KO
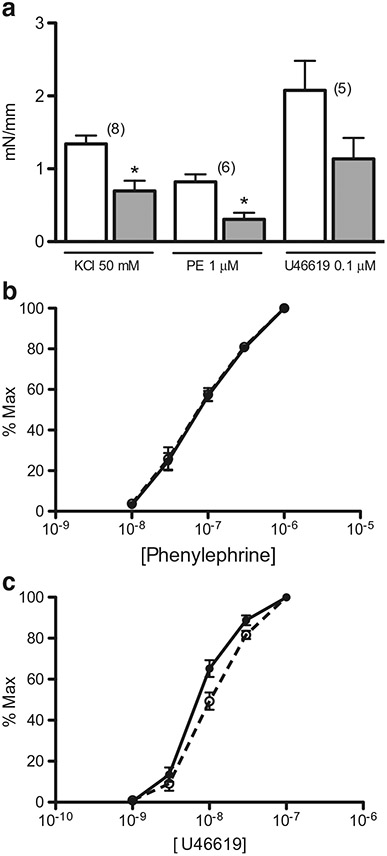
Contractile sensitivity to phenylephrine and U46619 (thromboxane A2 receptor antagonist) was not different in aorta from pendrin/NCC double KO compared to wild type (phenylephrine pD2 = 7.13 ± 0.08 and 7.11 ± 0.06, respectively; n = 6 each case; U46619 pD2 = 8.03 ± 0.07 and 8.21 ± 0.08, respectively; n = 5 in each case; Fig. 2).
Fig. 2.
Effect of pendrin/NCC double KO on agonist-induced aorta contraction. a Contractile responses of ring segments from pendrin/NCC double KO (closed bar) and wild type (open bar) to 50 mM KCl, 1 μM phenylephrine (PE), and 0.1 μM U46619 were expressed as millinewton/millimete r (mN/mm) vessel length. Number in parenthesis represents number of mice in each case. b, c Concentration-contraction curves of aorta to phenylephrine (n = 6 in each case) and U46619 (n = 5 in each case), respectively, from pendrin/NCC double KO (open circle plus dashed line) and wild type (closed circle plus uninterrupted line) were determined from the experiments in a and expressed as percent maximal contraction. *p < .05
Contractions (millinewton/millimeter length) to 1 μM phenylephrine and 50 mM KCl in aorta from pendrin/NCC double KO were 64 and 49%, respectively, of wild type (Fig. 2). Contraction to 0.1 μM U46619 tended to be reduced (46% of wild type), but the reduction was not statistically significant (Fig. 2).
Contraction to 0.1 μM angiotensin II of aorta from pendrin/NCC double KO and wild type was only 0.04 ± 0.04 and 0.03 ± 0.02 millinewton/millimeter length, respectively (n = 3 in each case).
Wet weight and protein
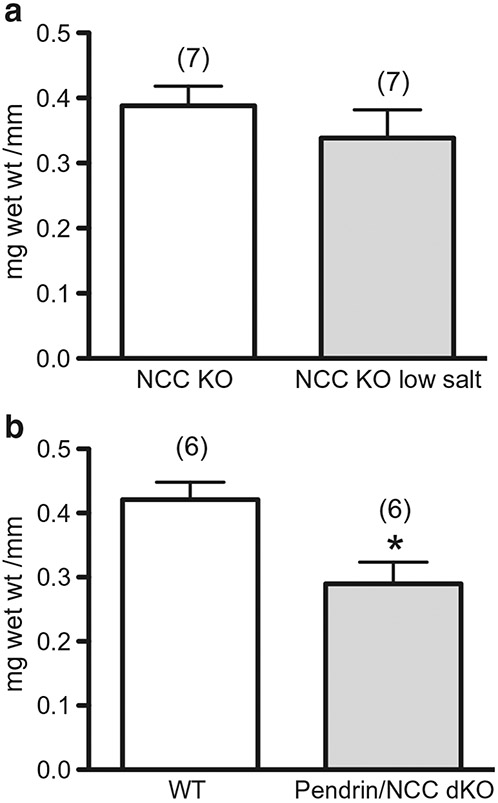
Wet weight/aorta length of segments from NCC KO with Na+ restricted diet and pendrin/NCC double KO remained unchanged and was 67% of wild type, respectively (Fig. 3). In an additional experiment in which milligram protein was determined in aorta segments (segments combined to increase protein measurement detection), milligram protein/millimeter vessel length was 20% less in aorta from pendrin/NCC double KO as compared to aorta from wild type.
Fig. 3.
Wet weight of aorta from NCC KO with reduced Na+ diet and pendrin/NCC double KO. Shown are milligram wet weight per millimeter (mg wet wt )/mm) ring segment length from a NCC KO with (closed bar) and without reduced Na+ diet (open bar) and b pendrin/NCC double KO (dKO; closed bar) and wild type (open bar). Number in parenthesis represents number of animals. *p < .05
Histology
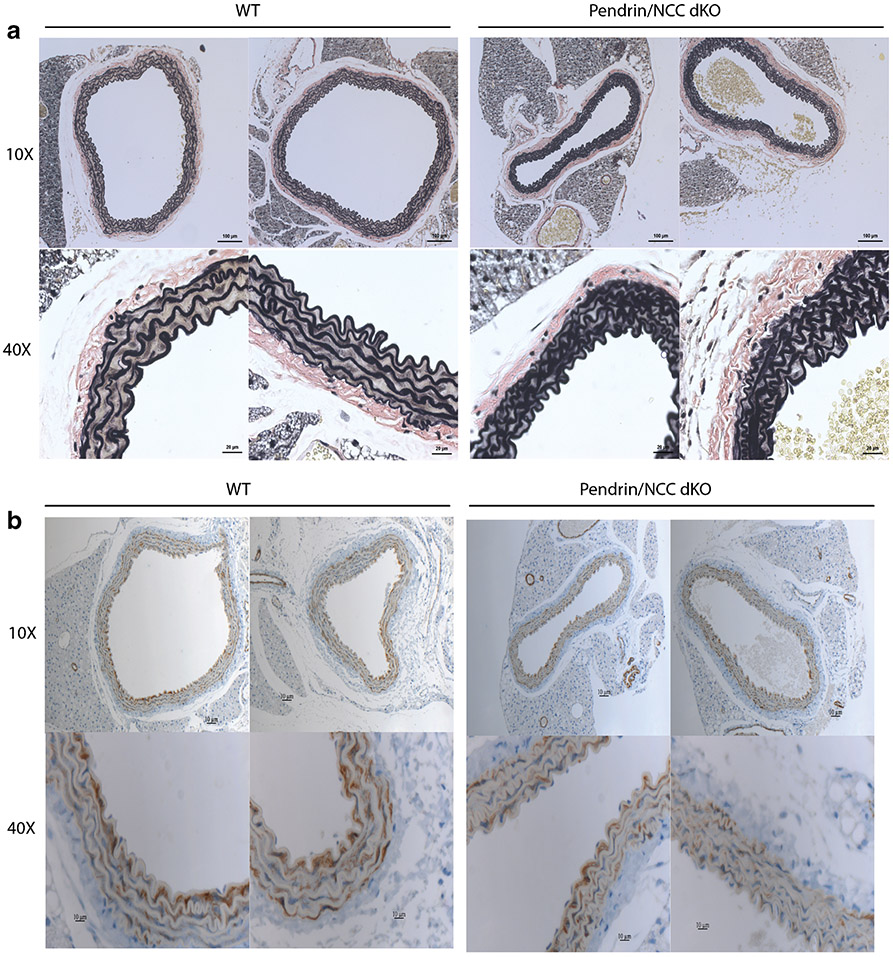
Verhoeff-Van Gieson staining revealed reduced medial extracellular matrix and greater convolution of laminae in aorta from pendrin/NCC double KO as compared to wild type (Fig. 4). Although these differences were difficult to discern in hematoxylin-eosin and Masson’s trichrome stained sections (not shown). Reduction of smooth muscle actin assessed with immunohistochemistry was also difficult to discern in pendrin/NCC double KO (Fig. 4). Differences were not observed in extracellular matrix and laminae in aorta from NCC KO with and without reduced Na+ diet as assessed with Verhoeff-Van Gieson staining (data not shown).
Fig. 4.
Verhoeff-Van Gieson and smooth muscle actin staining in aorta from pendrin/NCC double KO. a Verhoeff-Van Gieson and b smooth muscle actin immunohistochemical stained aorta sections from two pendrin/NCC double KO and wild type (WT) mice at ×10 and ×40 magnification. See text for description
Discussion
The present findings suggest that decreased vascular contractile reactivity may limit the ability of vasoconstrictors to reverse the hypotension associated with intravascular volume depletion caused by severely reduced Na+ reabsorption. This suggestion is supported by the decreased force per length of aorta from pendrin/NCC double KO. Whether this suggestion is in conflict with the essentially lack of decreased force of aorta from pendrin/NCC double KO when accounting for the accompanying reduced aorta wet weight should also be considered.
An alternative explanation for the decreased contraction of aorta from pendrin/NCC double KO is the depressed overall growth in this model, i.e., body weight is decreased to 60% (Soleimani et al. 2012). However, 7% Na+ diet for 2 weeks reversed the hypotension, as well as reduced the elevated renin mRNA levels in the kidney, but only slightly increased body weight (Soleimani et al. 2012; S.A., unpublished observation). Thus, it is likely that the decreased contractile reactivity was due to depleted intravascular volume rather than depressed overall growth.
The reduced contraction in aorta from pendrin/NCC double KO appears to result from decreased amounts of media, based on histological and protein determinations. These findings are consistent with the observed decreased contractile efficacy and lack of effect on sensitivity.
Interestingly, the decreased hypertrophy of aorta from pendrin/NCC double KO may be unexpected based on the considerable activation of the renin-angiotension II system in this model (Soleimani et al. 2012; S.A. et al., manuscript in preparation) and the well-known ability of angiotensin II to increase hypertrophy (Berk 2001). A speculation regarding the mechanism underlying the decreased hypertrophy in aorta from the pendrin/NCC double KO is that angiotensin II under hypotensive conditions interacts with additional factors to decrease hypertrophy. Although the effects of hypotension on hypertrophy have not been studied (to our knowledge), also of possible relevance is that elevated pressure elicits hypertrophy (Anwar et al. 2012).
In apparent conflict with the suggestion that angiotensin II under hypotensive conditions interacts with additional factors to decrease hypertrophy is the finding that pretreatment (7 days) with an angiotensin1 receptor antagonist normalized the reduction in cross-sectional area of the aorta from the pendrin KO (Sutliff et al. 2014). However, arterial pressure was not determined in this study (Sutliff et al. 2014) and variable levels of hypotension have been reported in this model (5–17 mmHg; Kim et al. 2007; Pech et al. 2010; Lazo-Fernandez et al. 2015). In any case, reduced hypertrophy was not detected in aorta from the Na+ diet restricted NCC KO (present results), in which arterial pressure is decreased although not to the level observed in pendrin/NCC double KO (Schultheis et al. 1998; Soleimani et al. 2012;Alshahrani et al. 2016) and in which there is relatively less activation of the renin-angiotensin system (Soleimani et al. 2012; S.A. et al., manuscript in preparation). Thus, possible interaction between angiotensin II and factors associated with hypotension to reduce hypertrophy may require a certain balance between angiotensin II and these factors.
Also in contrast to aorta from pendrin/NCC double KO, force per aorta length was not decreased in the Na+ diet restricted NCC KO. The unchanged force is consistent with the unaltered medial layer. Thus, the reduced ability of vasoconstrictors to mitigate the hypotension is limited to higher levels of severity of intravascular volume depletion, i.e., the pendrin/NCC double KO.
Limitations of the in vitro contractile studies include that (1) the aorta is a conduit vessel, negligibly contributing to vascular resistance (although mouse conduit vessels are commonly used in vitro to determine isometric vascular contractility due to their relatively greater mass and, thus, increased force generation) and (2) the effects of angiotensin II, a major constrictor associated with intravascular volume depletion, minimally constricted the aorta. Weak contractile efficacy of the mouse aorta to angiotensin II was previously reported (Russell and Watts 2000), although in another study 0.1 μM angiotensin II elicited 35% of maximal phenylephrine contraction (Sutliff et al. 2014). Likely explanations for the variable angiotensin II constrictor efficacy include the mouse strain. Experiments utilizing resistance-type vessels and, further, which respond with significant contractile efficacy to angiotensin II are warranted.
Acknowledgements
This study was supported by a Merit Review award from the Department of Veterans Affairs, funds from the Center on Genetics of Transport and Epithelial Biology at the University of Cincinnati, and grants from the Dialysis Clinic, Inc. (DCI) and US Renal Care (MS) and a predoctoral scholarship from Jazan University, Saudi Arabia (SA). We thank Dr. David Witte (Department Pathology, Cincinnati Children’s Hospital Medical Center) for his suggestions.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards All procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council USA.
References
- Alshahrani S, Rubinstein J, Jiang M, Barone S, Xu J, Zahedi K, Soleimani M (2015) Hydrochlorothiazide lowers systemic blood pressure predominantly through vasodilation in conditions associated with vasoconstriction. Hypertension 66:A115 [Google Scholar]
- Alshahrani S, Rapoport RM, Soleimani M (2016) Differential role of vascular contractile reactivity in salt wasting- and salt restriction-induced hypotension. J Am Soc Nephrol 27:A339 [Google Scholar]
- Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH (2012) The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res 49:463–478 [DOI] [PubMed] [Google Scholar]
- Basford JE, Moore ZW, Zhou L, Herz J, Hui DY (2009) Smooth muscle LDL receptor-related protein-1 inactivation reduces vascular reactivity and promotes injury-induced neointima formation. Arterioscler Thromb Vasc Biol 29:1772–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk BC (2001) Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev 81:999–1030 [DOI] [PubMed] [Google Scholar]
- Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, Wall SM (2007) Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Phys 293:F1314–F1324 [DOI] [PubMed] [Google Scholar]
- Lazo-Fernandez Y, Aguilera G, Pham TD, Park AY, Beierwaltes WH, Sutliff RL, Verlander JW, Pacak K, Osunkoya AO, Ellis CL, Kim YH, Shipley GL, Wynne BM, Hoover RS, Sen SK, Plotsky PM, Wall SM (2015) Pendrin localizes to the adrenal medulla and modulates catecholamine release. Am J Phys 309:E534–E545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM (2010) Pendrin modulates ENaC function by changing luminal HCO3. J Am Soc Nephrol 21:1928–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A, Watts S (2000) Vascular reactivity of isolated thoracic aorta of the C57BL/6J mouse. J Pharmacol Exp 294:598–604 [PubMed] [Google Scholar]
- Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE (1998) Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl− cotransporter of the distal convoluted tubule. J Biol Chem 273:29150–29155 [DOI] [PubMed] [Google Scholar]
- Sinning A, Radionov N, Trepiccione F, López-Cayuqueo KI, Jayat M, Baron S, Cornière N, Alexander RT, Hadchouel J, Eladari D, Hübner CA, Chambrey R (2016) Double knockout of the Na+-Driven Cl−/HCO3− exchanger and Na+/Cl− cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H (2012) Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A 109:13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutliff RL, Walp ER, Kim YH, Walker LA, El-Ali AM, Ma J, Bonsall R, Ramosevac S, Eaton DC, Verlander JW, Hansen L, Gleason RL Jr, Pham TD, Hong S, Pech V, Wall SM (2014) Contractile force is enhanced in aortas from pendrin null mice due to stimulation of angiotensin II-dependent signaling. PLoS One 22:e105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW (2004) NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44:982–987 [DOI] [PubMed] [Google Scholar]