Abstract
OBJECTIVE:
Acute subdural hematoma (aSDH) is a common pathology encountered in neurosurgery. Although most cases are associated with trauma and injuries to draining veins, traumatic aSDH from injury to arteries or spontaneous aSDH because of a ruptured intracranial aneurysm can occur. For some patients without a clear clinical history, it can be difficult to distinguish between these etiologies purely based on radiography. The objective of this research was to describe a case series in which imaging was suggestive of the presence of distal cortical intracranial aneurysm associated with aSDH, but operative management demonstrated no evidence of aneurysm.
METHODS:
We retrospectively reviewed 2 patients known to have aSDH with suspicion for associated aneurysm between May 2019 and September 2019 at our institution. Data collected included demographic, clinical, and operative course, including age, gender, past medical history, presenting symptoms, and pre and postoperative imaging.
RESULTS:
In 2 patients presenting with aSDH with preoperative radiographic imaging suggesting distal middle cerebral artery aneurysms, surgical exploration revealed no aneurysm. In both cases, noniatrogenic active arterial bleeding from an injured cortical middle cerebral artery branch was identified.
CONCLUSIONS:
Although there are prior reports of arterial aSDH, to our knowledge, this is the first to describe the radiographic “ghost aneurysm” sign. It is important for clinicians to be aware of this potential misleading radiographic sign, which indicates active extravasation into a spherical cast of clot.
Keywords: Active extravasation, Intracranial aneurysm, Mycotic aneurysm, Subdural hematoma
INTRODUCTION
Acute subdural hematoma (aSDH) is a common pathology encountered in neurosurgery, often associated with significant morbidity and mortality and occasionally requiring emergent craniotomy or craniectomy for evacuation.1,2 Commonly, patients who present with aSDH have a history of traumatic injury.1 However, a rare subset of patients can present with spontaneous aSDH, without subarachnoid hemorrhage (SAH) or intraparenchymal hematoma (IPH), because of a ruptured aneurysm.3,4 Spontaneous aSDH has been observed with aneurysms located in the distal middle cerebral artery (MCA),5 the posterior communicating artery,6 or distal mycotic aneurysm associated with endocarditis.7 These patients commonly present with delayed diagnosis and represent only a small fraction of total aSDH.4,8,9 Patients who present with aSDH and a suspicion for a ruptured aneurysm typically undergo additional cerebrovascular imaging prior to treatment, including computed tomographic angiography (CTA), magnetic resonance angiography, or digital subtraction angiography (DSA). If imaging is suggestive of the presence of an aneurysm, craniotomy for evacuation of the subdural hematoma, and either simultaneous clipping or postdecompression endovascular treatment of the aneurysm, are performed.4,10,11 However, there are no previous reports of imaging suggestive of aneurysmal aSDH on CTA or DSA with negative operative exploration. In this report, we describe 2 cases in which imaging was highly suggestive of the presence of distal cortical intracranial aneurysm associated with aSDH, but on operative management, no evidence of aneurysm was found.
METHODS
Our institutional review board determined that human subjects review approval and patient consent were not required for this small case series, including deidentified patient information, that is appropriately compliant with institutional requirements. We retrospectively reviewed the electronic medical record at a single institution for 2 known patients in which preoperative imaging suggested distal cortical intracranial aneurysm associated with aSDH, but with no evidence of aneurysm found during operative intervention. For each patient included, demographic, clinical, and operative data were obtained, including age, sex, past medical history, presenting symptoms, and pre- and postoperative imaging.
Surgical Technique
Both patients underwent general anesthesia, pinning in a Mayfield skull clamp (Integra LifeSciences, Plainsboro Township, New Jersey, USA), with a bump under the ipsilateral shoulder, and a standard reverse question mark incision, with a large frontotempero-parietal bone flap craniotomy using a high-speed drill. Stealth neuronavigation (Medtronic, Minneapolis, Minnesota, USA) was registered preoperatively. Mannitol (1 g/kg) and antibiotics were administered. The dura was opened under the microscope in a stellate fashion and carefully peeled back over the suspected location of the aneurysm. Clot was evacuated with gentle suction and irrigation, and active cortical arterial bleeding was coagulated using bipolar cautery. A large piece of DuraGen onlay (Integra LifeSciences) was used as duraplasty, the bone flap was replaced using titanium plates and screws, and the gale and skin were closed after placement of a subgaleal drain. Patients were observed in the intensive care unit overnight, and then in the regular wards.
RESULTS
Case 1
An 87-year-old man on warfarin for atrial fibrillation presented with headache after multiple episodes of light-headedness and falls. His past medical history was also notable for congestive heart failure, diabetes, and hypertension. On arrival, the patient was afebrile with a normal neurologic examination. The patient showed no signs of infection with a normal white blood cell count and no history of intravenous drug abuse.
Head computed tomography (CT) revealed a right-sided convexity aSDH with maximum thickness of 12.5 and 3 mm of right-to-left midline shift (Figure 1A). CTA revealed a 5 × 6 × 7 mm saccular aneurysm in the right frontal convexity, immediately adjacent to the aSDH (Figure 1B). The patient underwent DSA, which confirmed an aneurysm with opacification in the late arterial phase and persistence into the venous phase in the distal M4 MCA (posterior parietal branch; Figure 1C–D).
Figure 1.
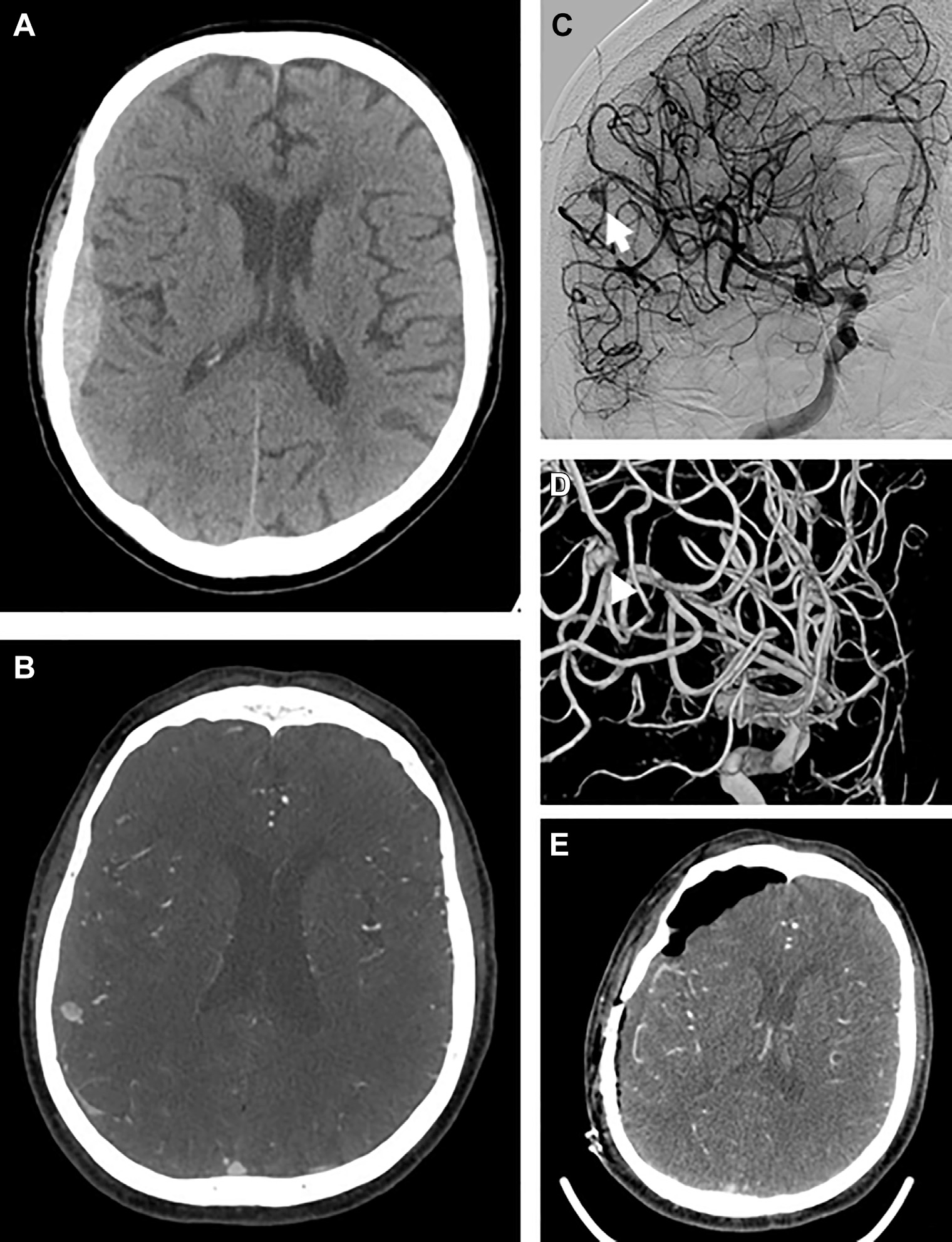
Case 1. Initial axial noncontrast computed tomography scan demonstrating subdural hematoma with local mass effect suggestive of acute hemorrhage (A). Computed tomographic angiography (CTA) with contrast demonstrating focal dilation contiguous with cortical branch of middle cerebral artery (MCA) in axial cut (B). Digital subtraction angiography, left internal carotid injection, transorbital oblique view demonstrating aneurysmal dilation (arrow) of the distal M4 segment of the MCA with contrast extravasation suggestive of M4 aneurysm rupture (C), with 3D reconstruction of the angiogram (D). Postoperative CTA axial cut demonstrating interval removal of subdural hematoma and no residual aneurysm (E).
The patient underwent craniotomy for evacuation of aSDH and aneurysm clipping. After opening tense dura and evacuating the hematoma carefully under the operative microscope, the dura was reflected and a pinhole site of active arterial extravasation from a cortical M4 branch was encountered, which corresponded to the site of the aneurysm on CTA-guided neuronavigation (Video 1; Figure 2). This cortical bleeding was not provoked as it was apparent immediately on evacuation of the local clot without any cortical manipulation. The artery was coagulated and extensive subarachnoid and sulcal dissection was performed without any evidence of aneurysm. This was confirmed with intraoperative ultrasonography and intraoperative CTA (Figure 1E). The patient’s postoperative course was uneventful, and he was discharged home 2 days later.
Video 1.
Figure 2.
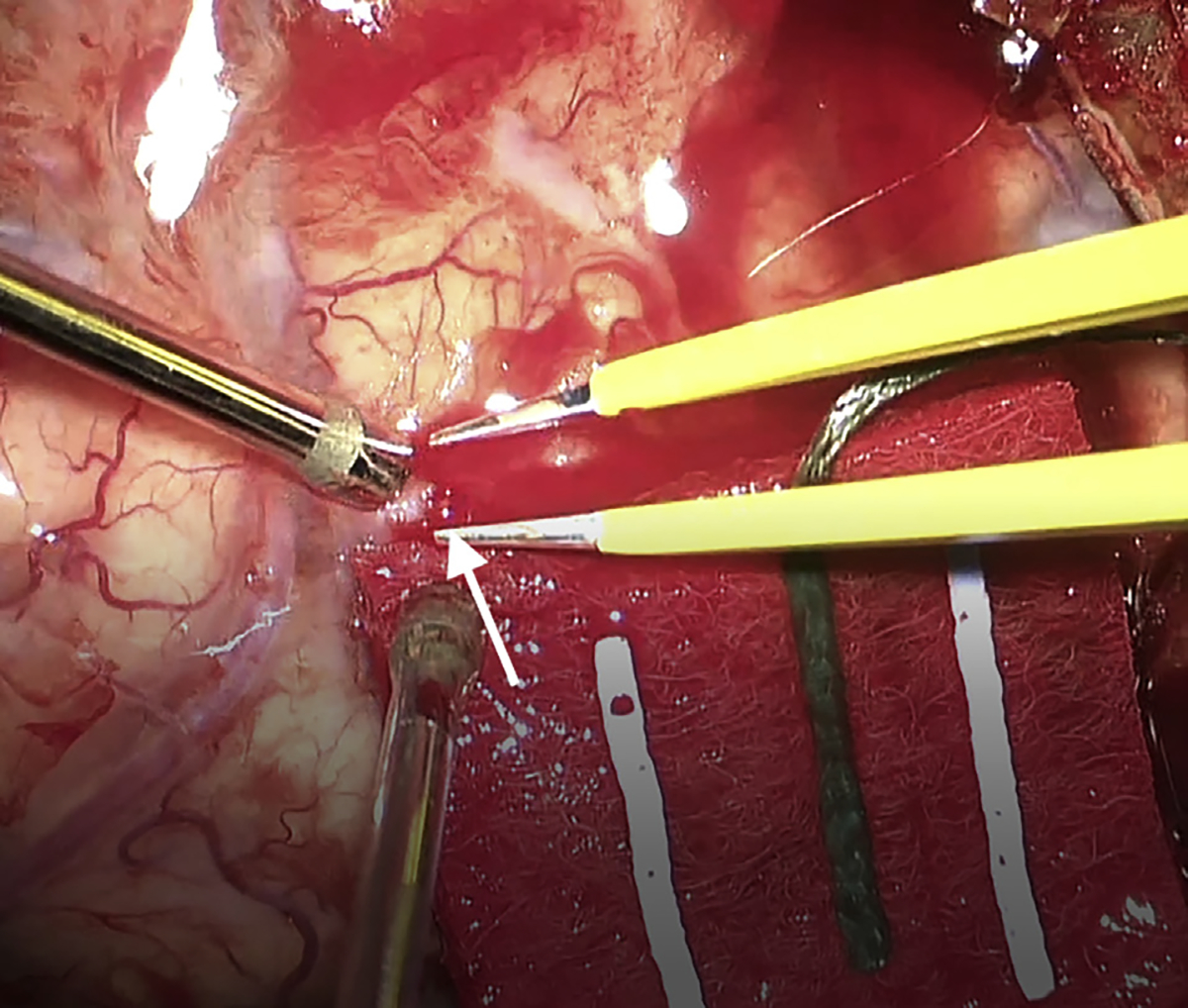
Case 1. Intraoperative image showing the pinhole site of arterial bleeding just prior to coagulation. White arrow indicates site of arterial rent.
Case 2
A 73-year-old man on aspirin and clopidogrel for a recent ischemic cerebrovascular accident requiring thrombectomy (without residual deficit), coronary artery disease, and prior cardiac stent placement was found unresponsive by family. Head CT revealed a right-sided convexity aSDH measuring 9 mm in maximum thickness with 4 mm of right-to-left midline shift (Figure 3A). The patient was afebrile, and the neurologic examination was notable for baseline proximal right lower extremity weakness and mild confusion. The patient had an elevated white blood cell count (16,000), but all blood cultures were negative. There was no history of intravenous drug abuse.
Figure 3.
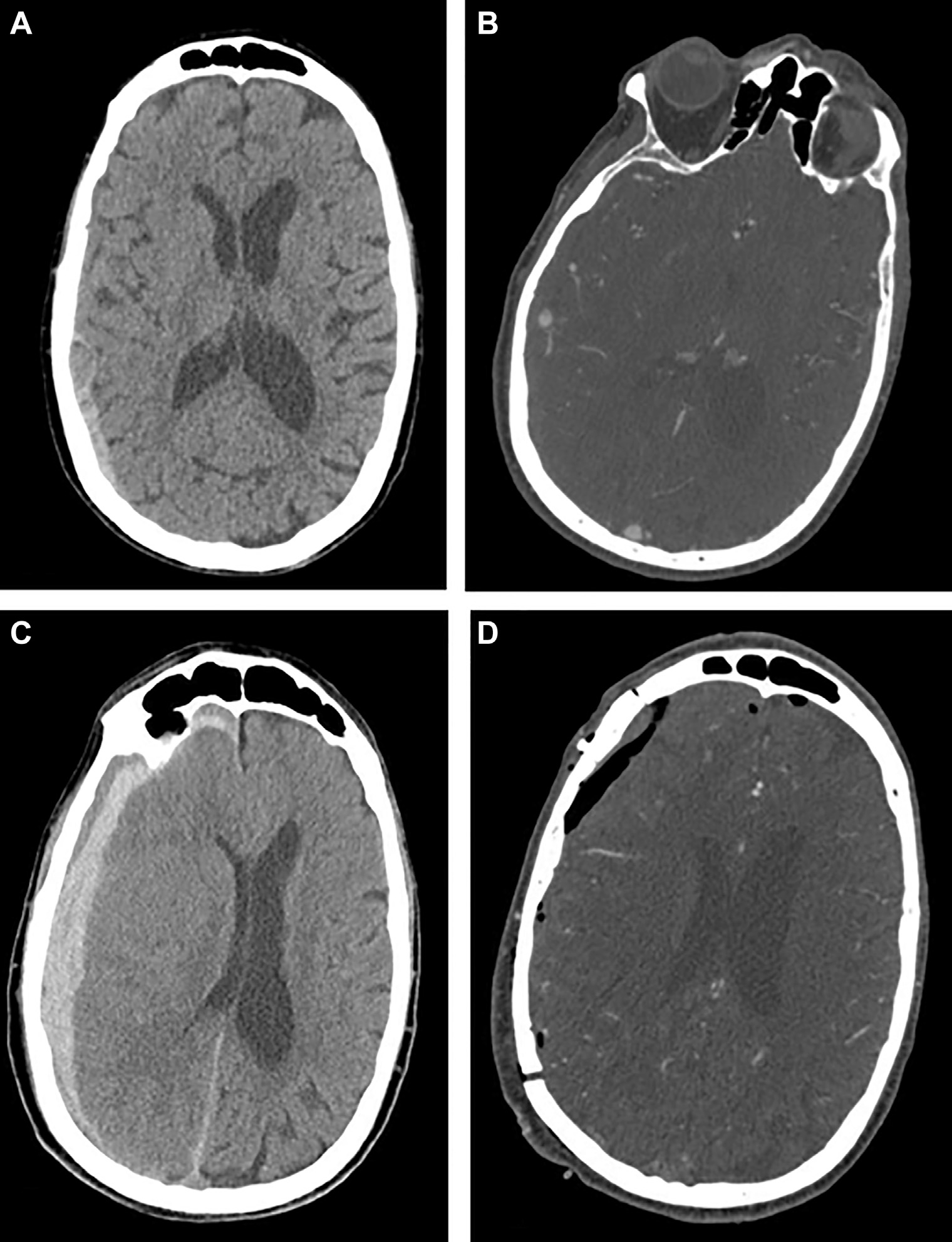
Case 2. Initial axial noncontrast computed tomography (CT) scan demonstrating subdural hematoma (A). Computed tomographic angiography (CTA) with contrast demonstrating concern for aneurysmal dilation immediately adjacent to nearby vessel in axial cut (B). Follow-up repeat head CT 4 hours later showing progression and enlarging hyperacute subdural hematoma and significant midline shift (C). Postoperative CTA confirming no residual aneurysm (D).
Head CTA was obtained due to history of recent stroke, which revealed a 6 × 8 mm cortical aneurysm (Figure 3B). A follow-up head CT was obtained 4 hours later, which showed significant progression of the aSDH (Figure 3C).
The patient underwent urgent craniotomy after administering platelets. As with case 1, tense dura was opened, and the clot was evacuated carefully under the microscope. On reflecting the dura, we immediately encountered arterial bleeding from a pinhole in an M4 branch of MCA (posterior temporal branch), corresponding to the location of the aneurysm on neuronavigation. As with case 1, this defect was not iatrogenic and was encountered without any cortical manipulation or dissection. The artery was coagulated, and no aneurysm or vascular lesion was identified on extensive subarachnoid dissection or intraoperative ultrasound. No residual lesion was seen on postoperative CTA (Figure 3D). The patient’s postoperative course was uneventful, and he was eventually discharged to a skilled nursing facility for further rehabilitation.
DISCUSSION
aSDH is one of the most common neurologic pathologies encountered by the neurosurgeon, and can be because of a number of causative factors, including trauma, intracranial hypotension, cerebral atrophy, or a ruptured cerebrovascular abnormality (aneurysm, arteriovenous malformation, or arteriovenous fistula).7,12–15 In the majority of cases, patients present with either a clear spontaneous ictus or traumatic event that either leads to arterial injury or the shearing of bridging veins within the subdural space, given their variability in wall thickness.16 In rare occasions, subdural hematomas can be caused by arterial bleeding due to spontaneous rupture of a cortical artery,17 neoplasm,18 or hematological abnormality.19,20 Patients with arterial causes of subdural hematoma commonly present similarly to those with venous ruptures, reporting headache, nausea, vomiting, and changes in mental status.17 In these cases, the presence of any aneurysmal dilation of cortical vessels is typically negative on both CTA and DSA. In some cases of ruptured intracranial aneurysm, there is aSDH associated with SAH.8,21 However, there are several reports of ruptured aneurysms presenting with isolated aSDH in the absence of SAH or IPH.5–7,22 Kondziolka et al.22 report 2 cases in which operative management revealed the presence of an aneurysm, which was amenable to clip ligation.
In this series, we present 2 cases of patients who presented with aSDH without SAH, but with radiographic evidence of cortical aneurysm. In both cases, operative exploration revealed no evidence of aneurysm, despite preoperative radiographic evidence. Similar to the “swirl sign,” seen on noncontrast head CT with IPH or the “spot sign” seen on CTA with IPH,23,24 the “ghost aneurysm” of contrast enhancement on CTA (Figures 1B) represents a radiographic correlate to active bleeding into aSDH. Romero et al.25 initially describe this phenomenon in a series of 157 patients presenting with aSDH. Thirty of 199 aSDHs (15.1%) had evidence of active contrast extravasation, but this included one or more foci of contrast pooling, foci of contrast that were discontinuous of neighboring vascular structures (i.e., cortical arteries or veins), and foci of any size or morphology. In this report, we specifically describe a single focus of contrast extravasation in a saccular shape, immediately continuous with a nearby suspected cortical vascular structure. We contend that this represents active extravasation from a distal cortical artery into a partially formed cast of thrombus in the shape of a sphere, thus giving the allusion of a ghost aneurysm. Ruptured cortical arteries without an underlying aneurysm or cerebrovascular abnormality in the setting of an aSDH are rare but have been described.26,27 Interestingly, most of these cases are reported in the absence of any definite trauma (i.e., are spontaneous) and in the setting of hypertension. The underlying anatomy and mechanisms potentially responsible for this phenomenon have also been described; early autopsies of patients with aSDH revealed perpendicular branches from cortical arteries directly connecting to the dura mater.26,28 These “twigs” are susceptible to being torn during trauma, and thus might explain the pinhole defect we observed in the operating room for both cases. Additionally, distal cortical arteries can have focal protrusions through the arachnoid with firm adhesions to the dura mater; Drake29 described this in 1961 and postulated that even minor traumatic brain injuries in these cases have the capacity for tearing an artery, leaving a resultant “tiny rent” in the vessel. Regardless of ultimate pathology, the surgeon must be prepared for aneurysmal clip ligation prior to opening the dura. CTA-guided neuronavigation is also essential to ensure accurate surgical planning. At the time of treatment, the arterial rent associated with the ghost aneurysm—if large enough, which was not the case in either patient presented earlier—can either be repaired primarily with a single 10–0 suture or coagulated completely to prevent any further bleeding. Postoperative management for these patients should be similar to any patient that requires craniotomy for a subdural hematoma, with a head CT obtained at a delayed time interval (i.e., 6 weeks) to ensure no reaccumulation or rebleeding. Because the culprit in ghost aneurysms is a simple rent in a distal cortical artery and the solution usually requires complete electrocautery of the defect and associated arterial segment, delayed cerebrovascular imaging is not necessary as long as immediate postoperative CTA does not reveal any new or persistent intracranial cerebrovascular abnormalities (Table 1).30–34 If a primary repair is pursued, a delayed cerebrovascular follow-up study (i.e., CTA at 6 weeks) should be obtained to ensure no development of pseudoaneurysm.
Table 1.
Comparison of Etiology, Pathophysiology, Radiographic Findings, and Treatment for Ghost, Pseudo, and Distal Cortical Aneurysms
| Ghost Aneurysm | Pseudo Aneurysm | Distal Cortical Aneurysm | |
|---|---|---|---|
| Etiology and pathophysiology | Noniatrogenic distal cortical injury, usually a pinhole defect, caused by either avulsion of a perpendicular branch to dura or torsion across a dural adhesion. Active extravasation into spherical case of clot within aSDH. No layers of artery surrounding ghost aneurysm. | Partial arterial wall injury (at least of tunica intima) due to infection, trauma, surgery, and others.30 As a result, aneurysmal dilation occurs either 1) within the tunica media and tunica adventitia, 2) within the tunica adventitia, 3) without any layer of artery (i.e., within soft tissue), or 4) any combination of the 3. | Etiology and pathophysiology not well understood. Usually includes mycotic, iatrogenic, and cryptogenic aneurysms.31,32 All 3 layers of artery comprise the aneurysmal dilatation. |
| Radiographic findings | Saccular appearing aneurysmal dilatation in the presence of aSDH. Diagnostic imaging includes CTA and DSA. | Can be saccular or fusiform appearing on imaging. Diagnostic imaging includes CTA, MRA, and DSA.33 Can be associated with parent vessel dissection or stenosis. Usually extracranial or along the skull base, but can be intracranial. | Commonly small, saccular aneurysms located at unusually distal locations (i.e., distal MCA or ACA), associated with ICH or SAH.32 |
| Treatment | Open: Craniotomy for evacuation of aSDH and coagulation or primary repair of arterial rent. | Open: Trapping, excision, or bypass. Rarely amenable to microsurgical clipping. Endovascular: Flow diversion, coil/stent- assisted coil embolization, or parent vessel sacrifice. Rarely glue or particle embolization.30,33,34 |
Open: Microsurgical clipping. Endovascular: Coil embolization or parent vessel sacrifice.31,32 |
aSDH, acute subdural hematoma; CTA, computed tomographic angiography; DSA, digital subtraction angiography; MRA, magnetic resonance angiography; MCA, middle cerebral artery; ACA, anterior cerebral artery; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
CONCLUSIONS
We describe 2 cases of aSDH with ghost aneurysms on preoperative imaging, found to have nonaneurysmal distal cortical arterial bleeding during surgical exploration. Surgeons should be aware of the possibility of nonaneurysmal pathology in cases of aSDH evacuation. Future investigations might aim to 1) quantify the actual rate of this radiographic finding across a larger series of aSDH patients, and 2) utilize blood vessel wall imaging to better delineate true aneurysms from ghost aneurysms.35
ACKNOWLEDGMENTS
The authors thank Sharon Durfy, Ph.D. for her assistance with manuscript preparation.
Abbreviations and Acronyms
- aSDH
Acute subdural hematoma
- CT
Computed tomography
- CTA
Computed tomographic angiography
- DSA
Digital subtraction angiography
- IPH
Intraparenchymal hematoma
- MCA
Middle cerebral artery
- SAH
Subarachnoid hemorrhage
Footnotes
Conflict of interest statement: Dr. Levitt has equity interest in eLoupes, Inc., Cerebrotech, and Synchron; has received unrestricted educational grants from Stryker, Medtronic, and Philips Volcano; and has received grants from the National Institutes of Health (R01NS105692, U24NS100654, R01NS088072), American Heart Association (18CDA34110295), and Aneurysm and AVM Research Foundation.
CRediT AUTHORSHIP CONTRIBUTION STATEMENT
Zachary A. Abecassis: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Dominic A. Nistal: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Isaac Josh Abecassis: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Rajeev D. Sen: Data curation, Formal analysis, Writing - review & editing. Michael R. Levitt: Conceptualization, Formal analysis, Project administration, Resources, Supervision, Writing - review & editing.
REFERENCES
- 1.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 suppl):S16–S24 [discussion: Si-iv]. [PubMed] [Google Scholar]
- 2.Wilberger JE Jr, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74:212–218. [DOI] [PubMed] [Google Scholar]
- 3.Park SM, Han YM, Park YS, Park IS, Baik MW, Yang JH. Acute aneurysmal subdural hematoma: clinical and radiological characteristics. J Korean Neurosurg Soc. 2005;37:329–335. [Google Scholar]
- 4.De Blasi R, Salvati A, Renna M, Chiumarulo L. Pure subdural hematoma due to cerebral aneurysmal rupture: an often delayed diagnosis. Cardiovasc Intervent Radiol. 2010;33:870–873. [DOI] [PubMed] [Google Scholar]
- 5.Awaji K, Inokuchi R, Ikeda R, Haisa T. Non-traumatic pure acute subdural hematoma caused by a ruptured cortical middle cerebral artery aneurysm: case report and literature review. NMC Case Rep J. 2016;3:63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mrfka M, Pistracher K, Augustin M, Kurschel-Lackner S, Mokry M. Acute subdural hematoma without subarachnoid hemorrhage or intraparenchymal hematoma caused by rupture of a posterior communicating artery aneurysm: case report and review of the literature. J Emerg Med. 2013;44:e369–e373. [DOI] [PubMed] [Google Scholar]
- 7.Boukobza M, Duval X, Laissy JP. Mycotic intracranial aneurysms rupture presenting as pure acute subdural hematoma in infectious endocarditis. Report of 2 cases and review of the literature. J Clin Neurosci. 2019;62:222–225. [DOI] [PubMed] [Google Scholar]
- 8.Kamiya K, Inagawa T, Yamamoto M, Monden S. Subdural hematoma due to ruptured intracranial aneurysm. Neurol Med Chir (Tokyo). 1991;31:82–86. [DOI] [PubMed] [Google Scholar]
- 9.Barton E, Tudor J. Subdural haematoma in association with intracranial aneurysm. Neuroradiology. 1982;23:157–160. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa E, Sugimoto K, Yanaka K, et al. Interhemispheric subdural hematoma caused by a ruptured internal carotid artery aneurysm: case report. Surg Neurol. 2000;54:82–86. [DOI] [PubMed] [Google Scholar]
- 11.Inamasu J, Saito R, Nakamura Y, et al. Acute subdural hematoma caused by ruptured cerebral aneurysms: diagnostic and therapeutic pitfalls. Resuscitation. 2002;52:71–76. [DOI] [PubMed] [Google Scholar]
- 12.Al-Mufti F, Mayer SA. Neurocritical care of acute subdural hemorrhage. Neurosurg Clin N Am. 2017;28:267–278. [DOI] [PubMed] [Google Scholar]
- 13.Sciubba DM, Kretzer RM, Wang PP. Acute intracranial subdural hematoma following a lumbar CSF leak caused by spine surgery. Spine (Phila Pa 1976). 2005;30:E730–E732. [DOI] [PubMed] [Google Scholar]
- 14.Choi HJ, Lee JI, Nam KH, Ko JK. Acute spontaneous subdural hematoma due to rupture of a tiny cortical arteriovenous malformation. J Korean Neurosurg Soc. 2015;58:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa K, Oishi M, Mizutani T, Maejima S, Mori T. Dural arteriovenous fistula on the convexity presenting with pure acute subdural hematoma. Acta Neurol Belg. 2010;110:190. [PubMed] [Google Scholar]
- 16.Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:399–406. [PubMed] [Google Scholar]
- 17.McDermott M, Fleming JF, Vanderlinden RG, Tucker WS. Spontaneous arterial subdural hematoma. Neurosurgery. 1984;14:13–18. [DOI] [PubMed] [Google Scholar]
- 18.Nery B, Costa RAF, Pereira LCT, et al. Spontaneous subdural hematoma associated with microcystic meningioma: first case report in the literature. Br J Neurosurg. 2019;33:428–431. [DOI] [PubMed] [Google Scholar]
- 19.Entezami P, Raval MP, Sanders LN, Adepoju A, Yamamoto J. Spontaneous subdural hematoma in patient with polycythemia vera. World Neurosurg. 2019;125:354–356. [DOI] [PubMed] [Google Scholar]
- 20.Moledina S, Shanmuganathan M, Pathak S, Simon A. Spontaneous subdural haematoma in a patient with a total artificial heart on warfarin. BMJ Case Rep. 2019;12:e230519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shekarchizadeh A, Masih S, Reza P, Seif B. Acute subdural hematoma and subarachnoid hemorrhage caused by ruptured cortical artery aneurysm: case report and review of literature. Adv Biomed Res. 2017;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondziolka D, Bernstein M, ter Brugge K, Schutz H. Acute subdural hematoma from ruptured posterior communicating artery aneurysm. Neurosurgery. 1988;22:151–154. [DOI] [PubMed] [Google Scholar]
- 23.Morotti A, Jessel MJ, Brouwers HB, et al. CT angiography spot sign, hematoma expansion, and outcome in primary pontine intracerebral hemorrhage. Neurocrit Care. 2016;25:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng D, Churilov L, Mitchell P, Dowling R, Yan B. The CT swirl sign is associated with hematoma expansion in intracerebral hemorrhage. AJNR Am J Neuroradiol. 2018;39:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero J, Kelly H, Almandoz JD, et al. Contrast extravasation on CT angiography predicts hematoma expansion and mortality in acute traumatic subdural hemorrhage. AJNR Am J Neuroradiol. 2013;34:1528–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhiber SS, Singh JP. Acute spontaneous subdural hematoma of arterial origin: a report of four cases and review of literature. Neurol India. 2010;58:654–658. [DOI] [PubMed] [Google Scholar]
- 27.Koc RK, Pasaoglu A, Kurtsoy A, Oktem IS, Kavuncu I. Acute spontaneous subdural hematoma of arterial origin: a report of five cases. Surg Neurol. 1997;47:9–11. [DOI] [PubMed] [Google Scholar]
- 28.Vance BM. Ruptures of surface blood vessels on cerebral hemispheres as a cause of subdural hemorrhage. AMA Arch Surg. 1950;61:992–1006. [DOI] [PubMed] [Google Scholar]
- 29.Drake CG. Subdural haematoma from arterial rupture. J Neurosurg. 1961;18:597–601. [DOI] [PubMed] [Google Scholar]
- 30.Medel R, Crowley RW, Hamilton DK, Dumont AS. Endovascular obliteration of an intracranial pseudoaneurysm: the utility of Onyx. J Neurosurg Pediatr. 2009;4:445–448. [DOI] [PubMed] [Google Scholar]
- 31.Lewis SB, Chang DJ, Peace DA, Lafrentz PJ, Day AL. Distal posterior inferior cerebellar artery aneurysms: clinical features and management. J Neurosurg. 2002;97:756–766. [DOI] [PubMed] [Google Scholar]
- 32.Lehecka M, Dashti R, Hernesniemi J, et al. Microneurosurgical management of aneurysms at A4 and A5 segments and distal cortical branches of anterior cerebral artery. Surg Neurol. 2008;70:352–367. [DOI] [PubMed] [Google Scholar]
- 33.Ciceri E, Regna-Gladin C, Erbetta A, et al. Iatrogenic intracranial pseudoaneurysms: neuroradiological and therapeutical considerations, including endovascular options. Neurol Sci. 2006;27:317–322. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Yang M, Cai M, Qin J, Pan L. Treatment of pediatric traumatic intracranial pseudoaneurysm using endovascular covered stent: three case reports. World Neurosurg. 2016;88:693.e1–693.e6. [DOI] [PubMed] [Google Scholar]
- 35.Mossa-Basha M, Alexander M, Gaddikeri S, Yuan C, Gandhi D. Vessel wall imaging for intracranial vascular disease evaluation. J Neurointerv Surg. 2016;8:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]


