Abstract
Background:
Although primary intraventricular hemorrhage is frequently due to trauma or vascular lesions, the etiology of idiopathic primary intraventricular hemorrhage (IP-IVH) is not defined.
Aims:
Herein, we test the hypothesis that cerebral small vessel diseases (cSVD) including hypertensive cSVD (HTN-cSVD) and cerebral amyloid angiopathy are associated with IP-IVH.
Methods:
Brain magnetic resonance imaging from consecutive patients (January 2011 to September 2019) with non-traumatic intracerebral hemorrhage from a single referral center were reviewed for the presence of HTN-cSVD (defined by strictly deep or mixed-location intracerebral hemorrhage/cerebral microbleeds) and cerebral amyloid angiopathy (applying modified Boston criteria).
Results:
Forty-six (4%) out of 1276 patients were identified as having IP-IVH. Among these, the mean age was 74.4 ± 12.2 years and 18 (39%) were females. Forty (87%) had hypertension, and the mean initial blood pressure was 169.2 ± 40.4/88.8 ± 22.2 mmHg. Of the 35 (76%) patients who received a brain magnetic resonance imaging, two (6%) fulfilled the modified Boston criteria for possible cerebral amyloid angiopathy and 10 (29%) for probable cerebral amyloid angiopathy. Probable cerebral amyloid angiopathy was found at a similar frequency when comparing IP-IVH patients to the remaining patients with primary intraparenchymal hemorrhage (P-IPH) (27%, p = 0.85). Furthermore, imaging evidence for HTN-cSVD was found in 8 (24%) patients with IP-IVH compared to 209 (28%, p = 0.52) patients with P-IPH.
Conclusions:
Among IP-IVH patients, cerebral amyloid angiopathy was found in approximately one-third of patients, whereas HTN-cSVD was detected in 23%—both similar rates to P-IPH patients. Our results suggest that both cSVD subtypes may be associated with IP-IVH.
Keywords: Cerebral hemorrhage, cerebral amyloid angiopathy, cerebral small vessel diseases
Introduction
Intraventricular hemorrhage is often seen as an extension of primary intracerebral hemorrhage (ICH) or subarachnoid hemorrhage. However, isolated intraventricular hemorrhage (termed primary intraventricular hemorrhage1–3) is frequently caused by vascular lesions including aneurysms or arterial venous malformations,4,5 and is seen in 2.7% of patients with spontaneous ICH.6 In the absence of an underlying cause, idiopathic primary intraventricular hemorrhage (IP-IVH) is generally attributed to hypertension, as this risk factor has been frequently observed in these patients.7,8 However, in patients with ICH, hypertension as a comorbidity is found at high frequencies even in patients in whom hypertensive cerebral small vessel disease (HTN-cSVD) is not the primary driver.9,10 Further evidence supporting hypertension as the main substrate for IP-IVH is lacking. The last decade has seen the validation of both hemorrhagic (cerebral microbleeds (CMBs), cortical superficial siderosis (cSS)) and ischemic (white matter hyperintensities (WMHs), lacunes, enlarged perivascular spaces (EPVS)) brain imaging markers, the severity and distribution of which can aid in determining the presence of the two most common types of cSVD, i.e. HTN-cSVD and cerebral amyloid angiopathy (CAA).
Aims/hypothesis
Herein, we sought to characterize the prevalence of cSVD phenotypes in IP-IVH by assessing these established magnetic resonance imaging (MRI) biomarkers of cSVD and comparing them to a cohort of patients with spontaneous primary intraparenchymal hemorrhage (P-IPH). In this preliminary study, our primary hypothesis was that, in addition to severe HTN-cSVD, a diagnosis of CAA can be established in an IP-IVH patient population using the validated modified Boston criteria.11
Methods
Patient selection
Patients with IP-IVH from January 2011 to September 2019 were retrospectively identified from our center’s prospectively entered database of consecutive patients with spontaneous ICH. Patients with any evidence of trauma (recent trauma history, skull fractures, concomitant subdural hemorrhage, or polytrauma) were omitted from this database. Within the IP-IVH cohort, patients who did not receive at least one angiographic study (CT angiogram, MR angiogram, or digital subtraction angiography (DSA)) during their inpatient stay or patients with a causative vascular lesion such as aneurysm or arteriovenous malformation were excluded.
Clinical and demographic variables
Demographic information and medical history, including vascular risk factors were obtained from patient charts as previously described.12 Pre-hospital antithrombotics, initial blood pressure, pre-morbid modified Rankin Scale (mRS) score,13 admission Glasgow Coma Scale scores, management strategies (including use of extraventricular drain), discharge mRS, discharge Glasgow Outcome Scale scales, and hospital length of stay were also recorded. Within the IP-IVH cohort, the presence of left ventricular hypertrophy (as a surrogate for systemic hypertensive end-organ dysfunction14 and HTN-cSVD15) was determined by reviewing transthoracic echocardiography reports.
CT analysis
The location of the hemorrhage on computed tomography (CT) was classified as either deep, lobar, intraventricular, cerebellar, or mixed by board-certified neurologists (ASD and RWR). Patients were diagnosed with IP-IVH when the hemorrhage was restricted to the ventricles only. The remaining cohort was classified as P-IPH. Hemorrhage volume was calculated with a semiautomated planimetric method using available software (Analyze 11.0, Mayo Clinic) as previously described.16 The modality of angiography and modified Graeb score (Table 1 and Figure 1) were also recorded.17
Table 1.
Modified Graeb scoring system.
| Ventricular blood % | Left lateral ventricle | Right lateral ventricle | Left posterior tip | Right posterior tip | Left temporal tip |
Right temporal tip |
Third ventricle | Fourth ventricle |
|---|---|---|---|---|---|---|---|---|
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ≤25% | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| >25% to ≤50% | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 |
| >50% to ≤75% | 3 | 3 | 2 | 2 | 2 | 2 | 4 | 4 |
| >75% to 100% | 4 | 4 | 2 | 2 | 2 | 2 | 4 | 4 |
| Ventricular expansion | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Table depicting the modified Graeb scoring system. Ventricular regions are giving a score ranging from 0 to 4 based on the percentage of hemorrhage in each area.
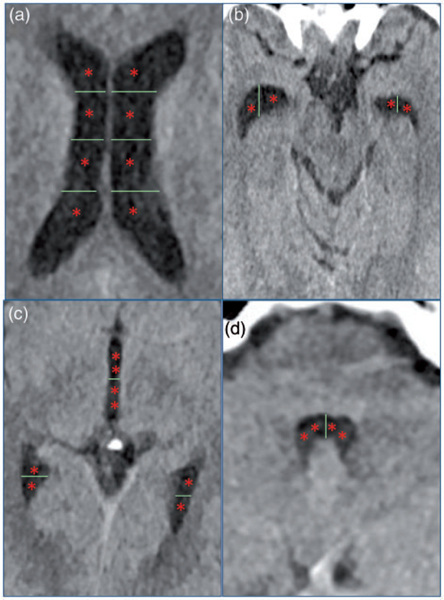
Figure 1.
Visual depiction of modified Graeb score.17 Images depict ventricular regions scored in the modified Graeb score. If a specific ventricular compartment is affected by hemorrhage, a point (one asterisk represents one point) is given. (a) Both right and left lateral ventricles are scored on a 4-point scale. (b) Right and left anterior temporal tips are scored on a 2-point system. (c) Posterior temporal tips are scored on a 2-point system, and the third ventricle is scored on a 4-point system. (d) The fourth ventricle is scored on a 4-point system. If the ventricle is completely filled with hemorrhage to the point where it is expanded, then an additional point is given for each ventricular region. The maximum score possible is 32 in which every region is filled with blood and expanded.
To test the hypothesis that IP-IVH may originate near areas of potentially high vascular amyloid burden, scans in which the IVH was predominantly in the lateral ventricles were identified. Specifically, if the Graeb scores in the lateral ventricles were ≥2, if the temporal tip scores were ≥1, and if the posterior tip scores were ≥1, these patients were identified as having IP-IVH near lobar regions. If patients had Graeb score ≥1 for the third or fourth ventricle, these patients were not considered as having IP-IVH located near lobar regions.
MRI analysis
Brain MRIs were reviewed by a board-certified neurologist (ASD) for the presence of cSVD markers (as defined by STRIVE criteria18) including cerebral microinfarcts,19 WMHs (graded using Fazekas score20 and classified according to established patterns10), lacunes,21 EPVS (graded as previously described22), cSS,23 and CMBs. Severe WMHs were defined as Fazekas score ≥2 and severe EPVS were defined as a score >2.22 The presence of CAA was diagnosed based on the ICH location and presence and distribution of strictly lobar CMBs and cSS per modified Boston criteria.24 Patients with strictly deep or mixed-location ICH/CMBs were characterized as having HTN-cSVD.25 The SVD score was determined for each patient.26
Statistical analysis
Baseline demographics, clinical outcomes, and imaging characteristics were compared between patients with IP-IVH and P-IPH. In a subset of patients with P-IPH that were diagnosed with CAA and HTN-cSVD, additional comparisons of baseline demographics and imaging characteristics were made to patients with IP-IVH. Median and interquartile range (IQR) were reported for continuous variables and percent and count were reported for categorical variables. Differences were assessed using either t-test or the nonparametric Wilcoxon rank-sum for continuous variables and Fisher’s Exact test for categorical variables. Two-tailed p values < 0.05 were interpreted as statistically significant. Analyses were performed with SPSS for Windows, version 23.0 (IBM Corp., Armonk, N.Y., USA). Approval for this study was granted by Mass General Brigham Institutional Review Board. Informed consent was waived for this study given its retrospective nature.
Results
A total of 1276 patients were included in our center’s database of consecutive patients with spontaneous ICH between 2011 and 2019. From this cohort, 46 (4%) patients met inclusion criteria and were found to have IP-IVH without trauma or any vascular lesion capable of causing the hemorrhage. Of the patients with IP-IVH, 42 (91%) received a CT angiogram, 17 (37%) received a MR angiogram, 12 (26%) received a DSA, and 20 (43%) received more than one study. Thirty-five (76%) patients with IP-IVH received a brain MRI and 750 (61%) patients with spontaneous P-IPH received a brain MRI (p = 0.04).
The baseline characteristics of the patient population are shown in Table 2. The mean age of patients with IP-IVH was 74.4±12.2 years. Twenty-eight (61%) patients were males and 18 (39%) patients were females, with a male-to-female ratio of 1.55:1 (similar to 1:58:1 reported in another study of IP-IVH7). Hypertension was the most common vascular risk factor and was seen in 87% of patients with IP-IVH compared to 82% with P-IPH (p = 0.56). Within the IP-IVH group, 19 (43%) patients were found to have LVH. Atrial fibrillation, smoking history, dementia, and anticoagulation usage were more common in patients with IP-IVH than patients with P-IPH (p < 0.05 for all comparisons).
Table 2.
Baseline characteristics of patients with intracerebral hemorrhage.
| IP-IVH (n = 46) | P-IPH (n = 1230) | p Value | |
|---|---|---|---|
| Age (years), mean ± SD | 74 (±12) | 73 (±13) | 0.407 |
| Female sex | 18 (39) | 555 (45) | 0.454 |
| Medical history | |||
| Hypertension | 40 (87) | 1008 (82) | 0.555 |
| Hyperlipidemia | 27 (59) | 612 (50) | 0.234 |
| Diabetes | 14 (30) | 291 (24) | 0.293 |
| Coronary artery disease | 15 (33) | 270 (22) | 0.088 |
| Atrial fibrillation | 19 (41) | 289 (24) | 0.008 |
| Prior cerebrovascular events | |||
| Previous ischemic stroke | 12 (26) | 212 (17) | 0.164 |
| Previous hemorrhage | 2 (4) | 125 (10) | 0.311 |
| Substance use | |||
| Smoking history | 31 (67) | 628 (51) | 0.035 |
| Alcohol abuse | 17 (37) | 451 (37) | 0.968 |
| Premorbid disability | |||
| Prior dependence | 15 (33) | 272 (22) | 0.105 |
| Dementia | 9 (20) | 124 (10) | 0.048 |
| Baseline mRS, median (IQR) | 1 (0, 3) | 0 (0, 2) | 0.066 |
| Antithrombotic therapies | |||
| None | 11 (24) | 504 (41) | 0.021 |
| Antiplatelet | 26 (57) | 456 (37) | 0.232 |
| Anticoagulant | 17 (37) | 270 (22) | 0.029 |
Data are counts (n) and percentages (%), means and standard deviations (SD), or medians and interquartile ranges (IQR).
mRS: modified Rankin Scale; IP-IVH: idiopathic primary intraventricular hemorrhage; P-IPH: primary intraparenchymal hemorrhage.
As shown in Table 3, the mean systolic blood pressure on presentation was similar between groups (169.2 ± 40.4mmHg in the IP-IVH group and 175.4 ± 34.7mmHg in the P-IPH group, p = 0.30). 89% of patients in each group had poor discharge functional outcomes (mRS > 2). However, the length of stay was greater in the IP-IVH group than in the P-IPH group (p = 0.01). Furthermore, 52% of patients in the IP-IVH group were still hospitalized after one week compared to 35% in the P-IPH group (p = 0.03).
Table 3.
Presenting characteristics and clinical outcomes of patients with intracerebral hemorrhage.
| IP-IVH (n = 46) | P-IPH (n = 1230) | p Value | |
|---|---|---|---|
| Glasgow coma scale score, median (IQR) | 14 (8, 15) | 14 (7, 15) | 0.370 |
| Systolic blood pressure, mean ± SD | 169.2 (±40.4) | 175.4 (±34.7) | 0.303 |
| Diastolic blood pressure, mean ± SD | 88.8 (±22.2) | 92.3 (±21.8) | 0.295 |
| Laboratory values, mean ± SD | |||
| White blood cell count (103/μL) | 11.4 (±4.6) | 9.7 (±4.3) | 0.015 |
| Hemoglobin (g/dL) | 13.9 (±1.9) | 13.6 (±1.8) | 0.223 |
| Platelets (×103/μL) | 227.0 (±78.0) | 222.8 (±74.5) | 0.727 |
| Creatinine (mg/dL) | 1.1 (±0.8) | 1.0 (±0.5) | 0.197 |
| International normalized ratio | 1.5 (±0.9) | 1.4 (±0.9) | 0.529 |
| Clinical outcomes, median (IQR) | |||
| Discharge modified Rankin scale score | 4 (4, 6) | 4 (4, 6) | 0.494 |
| Length of stay | 10 (5, 18) | 6 (3, 12) | 0.005 |
| Poor outcomes | |||
| Discharge modified Rankin scale score > 2 | 41 (89) | 1099 (89) | 0.962 |
| Discharge mortality | 12 (26) | 379 (31) | 0.625 |
Data are counts (n) and percentages (%), means and standard deviations (SD), or medians and interquartile ranges (IQR).
IP-IVH: idiopathic primary intraventricular hemorrhage; P-IPH: primary intraparenchymal hemorrhage.
To determine whether cSVD is prominent in patients with IP-IVH, we compared the presence and burden of MRI-based cSVD biomarkers in IP-IVH patients to those with P-IPH (summarized in Table 4). There were no significant differences in the presence of cSVD markers between the IP-IVH cohort and the P-IPH cohort, although there was a trend toward an increased frequency of multiple subcortical spots pattern in patients with IPH-IVH compared to those with P-IPH (22% vs. 11%, p = 0.06).
Table 4.
Imaging characteristics of intracerebral hemorrhage.
| IP-IVH | P-IPH | p Value | |
|---|---|---|---|
| CT-based imaging characteristics | (n = 46) | (n = 1230) | |
| Hemorrhage volume, mean ± SD (mm3) | 54.6 (±51.8) | 44.2 (±51.3) | 0.482 |
| Modified Graeb score, median (IQR) | 5 (3, 8) | ||
| IVH near lobar regions | 28 (61) | ||
| MRI-based imaging characteristics | (n = 35) | (n = 750) | |
| Presence of cerebral microinfarcts | 6 (16) | 97 (13) | 0.614 |
| Severe white matter hyperintensities (Fazekas ≥ 2) | 32 (87) | 584 (77) | 0.228 |
| White matter hyperintensity patterns | |||
| Multiple subcortical spots | 8 (22) | 81 (11) | 0.057 |
| Anterior subcortical patches | 10 (27) | 114 (15) | 0.062 |
| Large posterior subcortical patches | 13 (35) | 198 (26) | 0.254 |
| Peri-basal ganglia pattern | 2 (5) | 57 (8) | 0.628 |
| Presence of lacunes | 18 (49) | 253 (34) | 0.075 |
| Presence of deep lacunes | 15 (41) | 220 (29) | 0.143 |
| Presence of lobar lacunes | 5 (14) | 73 (10) | 0.399 |
| Presence of severe perivascular spaces (BG) | 7 (19) | 82 (11) | 0.175 |
| Presence of severe perivascular spaces (CSO) | 5 (14) | 69 (9) | 0.380 |
| Presence of cortical superficial siderosis | 7 (20) | 121 (16) | 0.489 |
| Presence of cerebral microbleeds | 18 (51) | 424 (57) | 0.603 |
| Presence of cerebral microbleeds (deep) | 8 (23) | 193 (26) | 0.844 |
| Presence of cerebral microbleeds (lobar) | 14 (40) | 344 (46) | 0.603 |
| Strictly deep ICH/microbleeds | 4 (11) | 58 (7) | 0.349 |
| Strictly lobar ICH/microbleeds | 10 (29) | 209 (28) | 0.928 |
| Mixed-location ICH/microbleeds | 4 (11) | 135 (18) | 0.495 |
| No microbleeds | 17 (49) | 348 (46) | 0.863 |
| Presence of any cSVD biomarker | 33 (94) | 667 (89) | 0.415 |
| SVD score, median (IQR) | 2 (1, 3) | 2 (1, 3) | 0.066 |
| Etiologic classification | |||
| Undetermined | 15 (43) | 188 (25) | 0.028 |
| Hypertensive cerebral small vessel disease | 8 (23) | 209 (28) | 0.517 |
| Possible cerebral amyloid angiopathy | 2 (6) | 148 (20) | 0.045 |
| Probable cerebral amyloid angiopathy | 10 (29) | 205 (27) | 0.848 |
| Cerebral amyloid angiopathy (total) | 12 (34) | 353 (47) | 0.166 |
Data are counts (n) and percentages (%), means and standard deviations (SD), or medians and interquartile ranges (IQR).
CT: computed tomography; MRI: magnetic resonance imaging; IVH: idiopathic primary intraventricular hemorrhage; P-IPH: intraparenchymal hemorrhage; BG: basal ganglia; CSO: centrum semiovale; ICH: intracerebral hemorrhage; cSVD: cerebral small vessel disease.
Utilizing the presence and distribution of CMBs and cSS, the frequency of HTN-cSVD and CAA was compared between groups. In the IP-IVH group, possible CAA was diagnosed in 2 (6%) patients and probable CAA was diagnosed in 10 (29%) patients using the modified Boston criteria. While the presence of possible CAA was lower in patients with IP-IVH compared to those with P-IPH (6% vs. 20%, p = 0.05), notably, probable CAA was found at a similar frequency compared to the P-IPH group (29% vs. 27%, p = 0.85). Moreover, a diagnosis of possible/probable CAA was found at similar frequencies in each group (34% in the IP-IVH group and 47% in the P-IPH group, p = 0.17). In addition to CAA, the frequency of HTN-cSVD was also similar between groups (23% in IP-IVH and 28% in P-IPH, p = 0.52).
In the subset of patients with P-IPH diagnosed with CAA or HTN-cSVD, the mean age of patients with IP-IVH was similar to those with CAA-related IPH (CAA-IPH) (74 ± 12 years vs. 75 ± 11 years, p = 0.90) as compared to patients with HTN-cSVD-related IPH (HTN-IPH) (70 ± 14 years, p = 0.01) (Supplementary Table 1). Similar to the results of Table 1, smoking history was more common in patients with IP-IVH (67%) than in patients with HTN-IPH (52%) and CAA-IPH (50%) (p < 0.05 for both comparisons). Patients with IP-IVH had higher rates of baseline dependence, disability, and dementia than patients with either HTN-IPH or CAA-IPH (p < 0.05 for all comparisons). Lastly, patients with IP-IVH had a higher burden of atrial fibrillation and anticoagulant usage than patients with either subtype of P-IPH (p < 0.05 for all comparisons).
In the imaging analysis of patients with HTN-IPH and CAA-IPH (Supplementary Table 2), a similar frequency of multiple subcortical spots pattern was found among patients with IP-IVH and CAA-IPH (22% vs. 11%, p = 0.11) compared to those with HTN-IPH (22% vs. 10%, p = 0.04). However, the presence of deep lacunes and deep EPVS was lower in patients with CAA-IPH than those with IP-IVH (p < 0.05 for both comparisons). Notably, deep CMBs were more common in patients with HTN-IPH compared to those with IP-IVH (48% vs. 23%, p < 0.01), and cSS was less common in patients with HTN-IPH compared to those with IP-IVH (8% vs. 20%, p = 0.02).
Within the IP-IVH group, of the 15 patients that did not have any CMBs or cSS, 13 (87%) had at least one marker of severe cSVD including moderate-to-severe WMHs, lacunes, or severe EPVS. No patients diagnosed with CAA received an autopsy or had tissue sent for pathological evaluation. Only one patient in the study received an autopsy which did not show any causative vascular lesion or CAA but did reveal an incidental capillary telangiectasia. The same patient received a brain MRI during the time of their hemorrhage which did not reveal any CMBs or cSS.
In the IP-IVH cohort, 28 (61%) patients were identified as having IP-IVH near lobar regions. A greater proportion of patients with CAA had IVH located near lobar areas compared to non-lobar areas (or in both locations) (83% vs. 50%, p = 0.18); however, this was not statistically significant. To further confirm the validity of the HTN-cSVD diagnosis within IP-IVH patients, the presence of left ventricular hypertrophy was compared between patients with CAA and those with HTN-cSVD. Notably, 6 of the 8 (75%) patients with HTN-cSVD had evidence of left ventricular hypertrophy on transthoracic echocardiography compared to 4 of the 10 (40%) patients with CAA (p = 0.07). Interestingly, the presence of hypertension was not significantly different between the two groups (p = 0.24).
Discussion
Herein, we demonstrate that the frequencies of the cSVD subtypes (CAA and HTN-cSVD) and cSVD biomarkers were similar between the IP-IVH cohort and a cohort of spontaneous P-IPH, suggesting that cSVD may be a likely etiology of IP-IVH. Contrasting the purported notion that IP-IVH is mediated by HTN-cSVD (of which hypertension is a risk factor),7 approximately one-third of patients with IP-IVH had underlying possible/probable CAA based on the presence and distribution of validated hemorrhagic markers on brain MRI. Furthermore, the frequency of LVH found in our entire IP-IVH cohort (43%) was similar to the frequency of LVH that has been reported in P-IPH patients (32%–60%),25,27 suggesting that HTN-cSVD is not overrepresented in patients with IP-IVH. When IP-IVH was compared to the patients with HTN-IPH and CAA-IPH, we found a similar frequency of CAA-related markers such as multiple subcortical spots pattern and cSS in patients with IP-IVH and CAA-IPH. Furthermore, patients with HTN-IPH had a much higher burden of deep CMBs than did patients with IP-IVH. Although we found a similar burden of deep lacunes and severe basal ganglia perivascular spaces in patients with IP-IVH and HTN-IPH, we believe these findings confirm the notion that HTN-cSVD may also contribute to the development of IP-IVH.
The vast majority (83%) of the patients with CAA had probable CAA, which carries a specificity of 95.5% for the diagnosis of CAA based on the validation study of modified Boston criteria.11 Interestingly, only 4 (11%) patients had strictly deep CMBs despite the high prevalence of hypertension in our study. A similar amount however, had mixed-location microbleeds, which have been suggested to be caused by HTN-cSVD.25,28 Collating the patients with strictly deep or mixed-location CMBs, 8 (23%) patients with IP-IVH likely had HTN-cSVD as the primary etiology of their hemorrhage. In this cohort, there was a trend toward an increased presence of left ventricular hypertrophy in patients with HTN-cSVD compared to those with CAA, further confirming the validity of the underlying HTN-cSVD diagnosis. Therefore, it appears that both CAA and HTN-cSVD are both plausible explanations for the development of IP-IVH. Even in patients without any CMBs or cSS, an overwhelming majority (87%) had imaging evidence of cSVD, suggesting that cSVD is indeed an etiology of IP-IVH.
Compared to patients with HTN-IPH, patients with IP-IVH were older and were of a similar age to those with CAA-IPH. Such age disparity between patients with HTN-cSVD and CAA is well-described in the literature and suggests that CAA may be an important contributor to IP-IVH.10,21,25 Interestingly, smoking was found at a higher frequency in patients with IP-IVH than those with P-IPH (or HTN-IPH and CAA-IPH). While tobacco is both a risk factor for ICH and CMB development, smoking has not been specifically linked to IP-IVH in previous studies; however, we speculate that smoking may increase the propensity for ependymal vessels to rupture leading to IP-IVH.29,30 Consistent with previous studies showing increased intraventricular extension in anticoagulation users,31–34 anticoagulation usage (and consequently atrial fibrillation) was shown to be more common among patients with IP-IVH and suggests that oral anticoagulation-related hemorrhages are more likely to dissect into the ventricular system.
Another noteworthy result of our study was the finding that patients with IP-IVH had higher levels of pre-morbid disability and dementia. Such findings are consistent with the trend toward higher SVD scores found in patients with IP-IVH compared to those with P-IPH. Furthermore, it is likely that patients with higher disability have a higher likelihood of having atrial fibrillation and using oral anticoagulants, which in turn, are related to the development of IP-IVH. Given the high frequency of premorbid disability, it is not surprising that patients with IP-IVH had equally devastating functional outcomes and longer lengths of stay as those with P-IPH. However, these associations warrant further study in larger data sets to provide further mechanistic insight.
In several instances of IP-IVH, the hemorrhage is often seen arising from the lateral ventricles, near either the occipital or temporal cortex. These posterior lobar regions are potential sites of high amyloid-β (Aβ)35 deposition leading to CMBs in CAA.36 Conceivably, the ependymal vessels that supply the lateral ventricles may become fragile with Aβ deposition and subsequently rupture, leading to IP-IVH. In our study, although a greater proportion of patients with CAA had IVH located near lobar regions, this did not meet the threshold for statistical significance likely due to the small sample size.
Some limitations to this study include its retrospective nature and relatively small sample size; however, due to the rarity of this disease these challenges are not easily overcome. Not all patients underwent a brain MRI (76%), so subtle intraparenchymal hemorrhage may have been missed. Furthermore, not all patients underwent cerebral DSA so a small vascular lesion may have been undetected. However, the entire cohort underwent CTA or MRA, and 20 (43%) patients received more than one angiographic study thereby mitigating this concern. Another limitation of the study is that the Boston criteria have not been validated in patients with primary IVH, so our findings should be interpreted with some caution. Lastly, because our database included only patients without any culprit vascular lesions, an analysis of all patients with primary IVH (both IP-IVH and primary IVH caused by vascular abnormalities) was not possible.
In summary, in a relatively large cohort of patients with IP-IVH, we confirm the notion that HTN-cSVD and CAA may represent underlying etiologies for IP-IVH. The similar frequency of both major cSVD subtypes in the IP-IVH cohort compared to the P-IPH cohort suggests that cSVD is associated with the development of IP-IVH. This novel association warrants further study using advanced imaging and/or confirmatory pathological correlations.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Heitman Young Investigator Fund as well as the National Institutes of Neurological Disorders and Stroke NS083711, R01NS114526, 5R01NS096730–04, and 5R01AG026484.
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Gurol received research grants to his hospital from AVID, Pfizer and Boston Scientific Corporation. The other authors have no relevant financial or non-financial interests to disclose.
Supplemental material
Supplemental material for this article is available online.
Data availability
Unpublished data are available upon reasonable request by a qualifying investigator.
References
- 1.Sanders E. A study of primary, immediate, or direct hemorrhage into the ventricles of the brain. Am J Med Sci 1881; 82: 85–128. [Google Scholar]
- 2.Gates PC, Barnett HJM, Vinters HV, Simonsen RL and Siu K. Primary intraventricular hemorrhage in adults. Stroke 1986; 17: 872–877. [DOI] [PubMed] [Google Scholar]
- 3.Darby DG, Donnan GA, Saling MA, Walsh KW and Bladin PF. Primary intraventricular hemorrhage: clinical and neuropsychological findings in a prospective stroke series. Neurology 1988; 38: 68–75. [DOI] [PubMed] [Google Scholar]
- 4.Gaberel T, Magheru C and Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev 2012; 35: 485–495. [DOI] [PubMed] [Google Scholar]
- 5.Hilkens N, van Asch C, Rinkel G and Klijn C. Yield of angiographic examinations in isolated intraventricular hemorrhage: a case series and systematic review of the literature. Eur stroke J 2016; 1: 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flint AC, Roebken A and Singh V. Primary intraventricular hemorrhage: yield of diagnostic angiography and clinical outcome. Neurocrit Care 2008; 8: 330–336. [DOI] [PubMed] [Google Scholar]
- 7.Guo R, Ma L, Shrestha BK, Yu Z, Li H and You C. A retrospective clinical study of 98 adult idiopathic primary intraventricular hemorrhage cases. Medicine 2016; 95: e5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein R, Ess K, Sirdar B, Song S and Cutting S. Primary intraventricular hemorrhage: clinical characteristics and outcomes. J Stroke Cerebrovasc Dis 2017; 26: 995–999. [DOI] [PubMed] [Google Scholar]
- 9.Vonsattel JPG, Myers RH, Tessa Hedley-Whyte E, Ropper AH, Bird ED and Richardson EP. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 1991; 30: 637–649. [DOI] [PubMed] [Google Scholar]
- 10.Charidimou A, Boulouis G, Haley K, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2016; 86: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg SM and Charidimou A. Diagnosis of cerebral amyloid angiopathy. Stroke 2018; 49: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006; 66: 23–29. [DOI] [PubMed] [Google Scholar]
- 13.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ and van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 14.Sagie A, Benjamin EJ, Galderisi M, et al. Echocardiographic assessment of left ventricular structure and diastolic filling in elderly subjects with borderline isolated systolic hypertension (the Framingham Heart Study). Am J Cardiol 1993; 72: 662–665. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos A, Palaiopanos K, Protogerou AP, Paraskevas GP, Tsivgoulis G and Georgakis MK. Left ventricular hypertrophy and cerebral small vessel disease: a systematic review and meta-analysis. J Stroke 2020; 22: 206–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulouis G, Van Etten ES, Charidimou A, et al. Association of key magnetic resonance imaging markers of cerebral small vessel disease with hematoma volume and expansion in patients with lobar and deep intracerebral hemorrhage. JAMA Neurol 2016; 73: 1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan TC, Dawson J, Spengler D, et al. The modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke 2013; 44: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Veluw SJ, Shih AY, Smith EE, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol 2017; 16: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993; 43: 1683–1689. [DOI] [PubMed] [Google Scholar]
- 21.Pasi M, Boulouis G, Fotiadis P, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology 2017; 88: 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017; 88: 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charidimou A, Boulouis G, Roongpiboonsopit D, et al. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: a prospective study. Neurology 2017; 89: 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010; 74: 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasi M, Charidimou A, Boulouis G, et al. Mixed-location cerebral hemorrhage/microbleeds. Neurology 2018; 90: e119–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staals J, Makin SDJ, Doubal FN, Dennis MS and Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014; 83: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallesen L-P, Wagner J, Lambrou D, et al. Association of hypertensive intracerebral hemorrhage with left ventricular hypertrophy on transthoracic echocardiography. J Clin Med 2020; 9: 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai H-H, Pasi M, Tsai L-K, et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds. Neurology 2019; 92: e774–e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S, Rehni AK and Dave KR. Tobacco use: a major risk factor of intracerebral hemorrhage. J Stroke 2021; 23: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das AS, Regenhardt RW, Vernooij MW, Blacker D, Charidimou A and Viswanathan A. Asymptomatic cerebral small vessel disease: insights from population-based studies. J Stroke 2019; 21: 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biffi A, Battey TWK, Ayres AM, et al. Warfarin-related intraventricular hemorrhage: Imaging and outcome. Neurology 2011; 77: 1840–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson D, Seiffge DJ, Traenka C, et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology 2017; 88: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner T, Weitz JI and Veltkamp R. Anticoagulant-associated intracranial hemorrhage in the era of reversal agents. Stroke 2017; 48: 1432–1437. [DOI] [PubMed] [Google Scholar]
- 34.Rosand J, Eckman MH, Knudsen KA, Singer DE and Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004; 164: 880. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert JJ and Vinters HV. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke 1983; 14: 915–923. [DOI] [PubMed] [Google Scholar]
- 36.Rosand J, Muzikansky A, Kumar A, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol 2005; 58: 459–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Unpublished data are available upon reasonable request by a qualifying investigator.