Abstract
Background:
Increased GR signaling is a proposed compensatory mechanism of resistance to AR inhibition in mCRPC. ORIC-101 is a potent and selective orally-bioavailable GR antagonist.
Methods:
Safety, PK/PD, and antitumor activity of ORIC-101 in combination with enzalutamide were studied in patients with mCRPC progressing on enzalutamide. ORIC-101 doses ranging from 80 to 240 mg QD were tested in combination with enzalutamide 160 mg QD. PK/PD was assessed after a single dose and at steady-state. Disease control rate at 12 weeks (DCR) was evaluated at the RP2D.
Results:
A total of 41 patients were enrolled. There were no dose limiting toxicities and the RP2D was selected as 240 mg of ORIC-101 and 160 mg of enzalutamide daily. At the RP2D, the most common treatment-related AEs were fatigue (38.7%), nausea (29.0%), decreased appetite (19.4%), and constipation (12.9%). PK/PD data confirmed ORIC-101 achieved exposures necessary for GR target engagement. Overall, for 31 patients treated at the RP2D, there was insufficient clinical benefit based on DCR (25.8%; 80% CI: 15.65%, 38.52%) which did not meet the prespecified target rate, leading to termination of the study. Exploratory subgroup analyses based on baseline GR expression, presence of AR resistance variants, and molecular features of aggressive variant PC suggested possible benefit in patients with high GR expression and no other resistance markers, although this would require confirmation.
Conclusions:
Although the combination of ORIC-101 and enzalutamide demonstrated an acceptable tolerability profile, GR target inhibition with ORIC-101 did not produce clinical benefit in men with metastatic prostate cancer resistant to enzalutamide.
INTRODUCTION
For men with de novo or recurrent metastatic prostate cancer, inhibition of the androgen receptor (AR) signaling axis with a next-generation hormonal agent such as abiraterone acetate or enzalutamide is associated with an overall survival benefit and is now a standard of care. However, the emergence of resistance continues to present a clinical challenge. Several mechanisms of resistance have been described, including AR point mutations and amplification, expression of AR splice variants lacking the ligand binding domain, and bypassing of AR signaling through increased expression of the glucocorticoid receptor (GR).
GR is a member of the superfamily of nuclear receptors, is expressed across a wide variety of tissues, and is activated via glucocorticoids that are secreted by the adrenal gland in a circadian and stress-associated manner to regulate metabolism, cell growth, apoptosis, differentiation, inflammation, mood and cognitive function.1 Upon ligand-binding, GR undergoes nuclear translocation and binds to glucocorticoid response elements in DNA to transcriptionally regulate a spectrum of genes that mediate its pleiotropic biological effects.
Specifically for prostate cancer, overexpression of GR has been correlated with poor outcomes in patients with castration-resistant prostate cancer (CRPC) treated with enzalutamide.2 In a LNCaP xenograft model with exogenous AR overexpression (LNCaP AR), acquired resistance to enzalutamide was associated with GR upregulation. Similarly, results from in vitro studies of GR-expressing VCaP cells showed that glucocorticoid-mediated activation of GR was sufficient to confer enzalutamide resistance. Mechanistically, the data suggest that AR and GR drive a partially overlapping transcriptional program.3 Thus, GR activation could circumvent enzalutamide-mediated AR inhibition and sustain prostate cancer cell growth.
Given the evidence that GR acts as a mediator of therapy resistance, the impact of GR antagonists on modulation of therapy response has been explored. Both GR blockade and knockdown in prostate cancer preclinical models effectively reversed GR-mediated pro-survival effects and sensitized them to enzalutamide, apalutamide, and the CYP17A1 inhibitor abiraterone acetate.3,4,5 These preclinical observations provided a rationale for the clinical evaluation of GR antagonists, such as mifepristone and exicorilant, in combination with enzalutamide in patients with prostate cancer progressing on ADT and enzalutamide.6,7
ORIC-1018 is a highly potent and selective small molecule GR antagonist that was developed for the treatment of patients with solid tumors. Mechanistically, ORIC-101 inhibits GR transcriptional activity and blocks the pro-survival signals mediated by the activated nuclear receptor.
MATERIALS AND METHODS
Study Design
This was a Phase 1b open-label, single arm, dose escalation and expansion study designed to evaluate the safety, tolerability, and antitumor activity of ORIC-101 in combination with enzalutamide in patients with metastatic prostate cancer who were progressing on enzalutamide (ClinicalTrials.gov Identifier: NCT04033328). Once patients were deemed eligible for enrollment, they started treatment with ORIC-101 and continued their current enzalutamide therapy without any washout period (i.e., no interruption of enzalutamide administration).
Escalating dose levels of ORIC-101 between 80 mg and 240 mg were administered orally once daily (QD) in combination with enzalutamide 160 mg QD following a modified interval 3+3 dose escalation design (i3+3).9 The Maximal Tolerated Dose (MTD) was defined as the combination dose associated with the probability of dose-limiting toxicity (DLT) occurring between 20 and 30% of patients during the first treatment cycle (28 days).
DLTs were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) version 5.0 and defined as Grade 3 or Grade 4 adverse events (AEs) considered related to study treatment. In order to be considered evaluable for DLT, patients must have received at least 75% of both ORIC-101 and enzalutamide doses. Non-evaluable patients who discontinued treatment prior to completing Cycle 1 for reasons other than toxicity (e.g., disease progression) could be replaced and additional patients could have also been enrolled as backfill patients to supplement missing or insufficient pharmacokinetic (PK) or pharmacodynamic (PD) data as per the i3+3 dose escalation rules.
During dose expansion, patients were treated at the MTD and/or Recommended Phase 2 Dose (RP2D) to assess the preliminary antitumor activity of ORIC-101 in combination with enzalutamide. PSA was collected at the start of every cycle of treatment. Tumor assessments were performed every 8 weeks and included CT/MRI scans of the chest, abdomen, pelvis, plus a bone scan. At screening, a MRI brain scan was performed to rule out the presence of CNS metastases. Radiographic confirmation of objective tumor response or disease progression was based on RECIST 1.1 and the Prostate Cancer Clinical Trials Working Group 3 (PCWG3) criteria10 and assessed locally.
All patients provided written informed consent to participate and were treated until disease progression, unacceptable toxicity, or meeting other criteria for stopping treatment as per the study protocol. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and relevant Institutional Review Board requirements.
Patients
Male patients ≥18 years of age with metastatic prostate cancer with progression on enzalutamide 160 mg QD plus surgical or ongoing chemical castration were eligible for the study; patients must have been on treatment with enzalutamide for at least 3 months prior to documented evidence of PSA progression as per PCWG3. Other key inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 with adequate bone marrow and organ function, and agreement and ability to undergo two tumor biopsies, one pretreatment and one during Cycle 2.
Prior chemotherapy in the mCRPC setting or prior treatment with another second-generation AR pathway inhibitor in the metastatic setting was exclusionary. Other key exclusion criteria included history of or presence of CNS metastases at baseline, seizures, Cushing’s syndrome, adrenal insufficiency, or requirement for chronic use of systemic corticosteroids.
Study Objectives
Dose Escalation: The primary objective was to select the dose of ORIC-101 identified as safe, tolerable, and providing target therapeutic exposure in combination with enzalutamide.
Dose Expansion: The primary objective was to evaluate the preliminary antitumor activity of ORIC-101 in combination with enzalutamide based on Disease Control Rate (DCR), defined as the proportion of patients who did not have PSA or radiographic progression on or before Week 12. Additional endpoints included PSA response rate (≥50% decline from baseline at 12 weeks), objective response rate (ORR), and progression-free survival (PFS), all according to PCWG3.
Pharmacokinetic Assessments
Blood samples were collected for analysis of plasma concentrations of ORIC-101, enzalutamide, and their major metabolites, using a validated bioanalytical method. Pharmacokinetic parameters were determined using noncompartmental analysis (Phoenix WinNonLin software, Certara, NJ, USA) and included maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) from time zero up to 24 hours post-dose (AUC0-t).
Pharmacodynamic Assessments
PD modulation in peripheral blood mononuclear cells (PBMCs) collected on multiple days and times in the first two cycles of treatment, before and after dosing, was assessed by RT-qPCR for housekeeping gene RPL27 and GR target genes FKBP5, GILZ and PER1, selected for their consistent stimulation by GR and reversal with ORIC-101 in nonclinical studies and in the first-in-human single and multiple ascending dose studies of ORIC-101 administered to healthy subjects.11, 12, 13 Cortisol levels in blood were simultaneously measured at the time of PBMC collection.
In tumor biopsies obtained at screening, end of Cycle 2, and/or end of treatment, GR, AR and PTEN protein status were evaluated by immunohistochemistry (IHC) in biopsies with >50 tumor cells. A proprietary IHC assay for GR protein status was optimized for staining nuclear GR in the epithelial compartment of major tumor type tissues. AR and GR protein levels were quantified by H-score, ranging from 0 to 300 and defined as (% tumor cells with weak staining) + 2x (% tumor cells with moderate staining) + 3x (% tumor cells with strong staining). Low GR expression was defined as H-score <100. PTEN was reported as % tumor cells with protein loss, with ≥95% of tumor cells lacking PTEN protein considered PTEN-negative.
At those same timepoints, mutations and copy number alterations in cancer genes as well as AR splice variants were captured in plasma and analyzed employing the 152-gene circulating tumor DNA (ctDNA) PredicineCARE™ and the 88-gene cell-free RNA (cfRNA) PredicineRNA™ assays, respectively. Amplification of AR, located on the X chromosome, was defined as cell-free DNA copy number ≥2, corresponding in our study to an estimated ≥8 AR copies in tumor cells by assuming that AR amplification is clonal and using the maximal observed allele frequency of somatic mutations in plasma as an estimate of tumor fraction. AR splice variant AR-v7 positivity was defined as presence of a high amount of AR-v7 in plasma regardless of total AR levels (i.e., number of AR-v7-unique reads >50), or AR-v7 contributing to >5% of total AR expression (i.e., ratio of AR-v7-unique reads to all AR reads extending downstream of exon 3 >5%). Aggressive variant prostate cancer (AVPC) was determined as joint functional loss of two or more of the following markers: (1) RB1, with functional loss defined as homozygous loss or loss of heterozygosity (LOH) detected in ctDNA in plasma, (2) PTEN displaying homozygous loss or LOH, or protein loss in tumor biopsies by IHC, and (3) TP53 displaying a deleterious mutation with LOH or abolished wildtype p53 functionality (dominant negative mutation), or presence of two or more mutations. 14, 15, 16, 17
Statistical Assessments and Sample Size
The sample size for the dose escalation portion of the study was aligned with the objectives of determining a safe and tolerable dose for the ORIC-101 plus enzalutamide combination, based on calibration of the i3+3 design through simulation studies: up to 27 patients in total would be enrolled in groups of 3 to determine the MTD, defined as the highest dose with a DLT rate close to 25% and an equivalence interval between 20% and 30%. These parameters were specified to ensure that the MTD did not induce DLTs with a high rate (no more than 25%) and that the study allowed for an approximate 5% error margin around 25%. The modified i3+3 design has more favorable operating characteristics than the traditional 3+3 design, allowing for backfilling of patients at a level below the current dose under consideration.
For the dose expansion, a minimum of 28 patients were to be enrolled. This sample size was chosen to obtain a preliminary evaluation of antitumor activity as measured by DCR, with an 80% two-sided confidence interval (CI) estimated by the Clopper-Pearson method. If 9 out of 28 patients were to achieve DCR at Week 12, the point estimate of the true DCR was 32% (80% CI: 20.4%, 45.9%) and an additional 20 patients, up to a total of 48 patients, would be enrolled if promising clinical activity was observed (i.e., the lower limit of the two-sided 80% CI for DCR was >20%). The choice of 20% as the threshold of clinical activity in this refractory population was based on review of the literature of the expected proportion of the population without PSA progression at Week 12 on abiraterone acetate plus prednisone following progression on enzalutamide therapy.18
Analysis of DCR as described above was performed for the following two subgroups of patients: (1) those with tumors expressing an AR alteration or AVPC at baseline and (2) those lacking such aberrations at baseline. Exploratory analyses of the association between DCR and baseline GR level of expression were also performed.
Nonclinical Studies
To study the mechanism of action of ORIC-101 + enzalutamide in an AR-mutant context, AR-WT (wildtype) and AR-L702H overexpressing CHO-K1-MMTV-Luciferase (source: DiscoveRx corporation) and VCaP (source: ATCC) stable cell lines were established. Plasmid constructs pWZL AR WT and pWZL AR L702H were obtained from C. Sawyers laboratory (MSKCC, NY, USA). The plasmid constructs pWZL AR WT and pWZL AR L702H were transfected into GP-293T cells and retrovirus containing AR WT or AR L702H was harvested 48 hours post-transfection. VCaP and CHO-K1-MMTV-Luc cells were infected with retrovirus containing AR WT or L702H, followed by the selection of stable cell lines with 10 ug/ml blasticidin (Invivogen, San Diego, CA) for approximately 2 weeks, to establish CHO-K1-MMTV-Luc AR WT or L702H cells and VCaP AR WT or L702H cells.
AR agonism activity of cortisol was assessed in an AR luciferase reporter assay using CHO-K1-MMTV-Luc cells in phenol red free DMEM/10% charcoal stripped FBS. CHO-K1-MMTV-Luc AR WT and AR L702H cells were treated for 24 hours with 10 concentrations of cortisol and analyzed using One-Glo Luciferase reagent (Promega#E6110). The impact of cortisol on cell growth was also evaluated in stable VCaP cells in DMEM/10% charcoal stripped serum (CSS). VCaP cells were treated for 7 days with 8 concentrations of cortisol and analyzed by CellTiter-Glo® Luminescent Cell Viability Assay (Promega#7573). Lastly, stable VCaP AR WT and AR L702H cells in DMEM/CSS media were treated for 14 days with various combinations of ligand stimulation and compound, with media and treatment replenishment every 7 days (Veh: vehicle; R1881: 100 pM R1881; Enza: 2 μM enzalutamide; Cort: 30 nM cortisol; ORIC-101: 0.5 μM ORIC-101). Cell supernatant was collected on Day 16 to assess PSA levels using PSA (human) AlphaLISA Detection Kit (PerkinElmer #AL228C).
Data Availability
The data generated in this study are not publicly available due to patient privacy requirements but can be available upon reasonable request to the corresponding author.
RESULTS
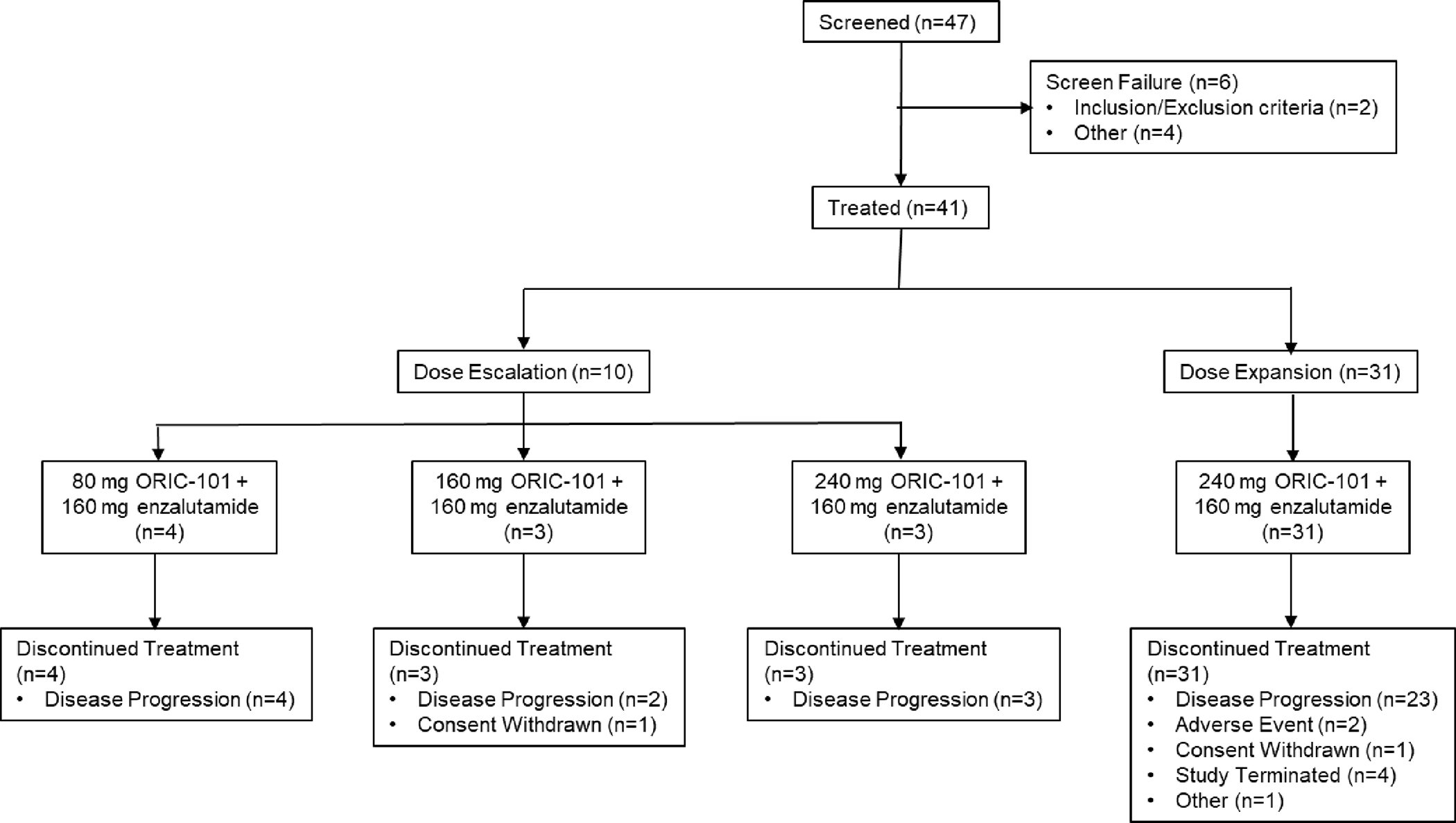
Between December 2019 and November 2022, a total of 41 patients were enrolled: 10 during dose escalation and 31 in dose expansion at the RP2D. Demographics were consistent with a metastatic prostate cancer population following frontline treatment with enzalutamide and are shown in Table 1 (and Supplementary Table 1). During dose escalation, 3 dose levels of ORIC-101 (80 mg [n=4], 160 mg [n=3], 240 mg [n=3]) were evaluated in combination with enzalutamide 160 mg, both administered on a once daily continuous daily dosing regimen. There were no DLTs and the RP2D was selected as 240 mg ORIC-101 plus 160 mg enzalutamide daily, based on overall tolerability, PK, and PD.
Table 1.
Baseline Characteristics and Demographics
| Dose Escalation | Dose Expansion | All Patients | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 80 mg ORIC-101 + 160 mg enzalutamide (N=4) | 160 mg ORIC-101 + 160 mg enzalutamide (N=3) | 240 mg ORIC-101 + 160 mg enzalutamide (N=3) | Total (N=10) | 240 mg ORIC-101 + 160 mg enzalutamide (N=31) | Total (N=41) | |
|
| ||||||
| Age, years | ||||||
| Median (range) | 71 (70, 83) | 68 (60, 68) | 72 (70, 79) | 70 (60, 83) | 70 (53, 82) | 70 (53, 83) |
|
| ||||||
| Race, n (%) | ||||||
| White | 4 (100) | 3 (100) | 3 (100) | 10 (100) | 24 (77.4) | 34 (82.9) |
| African American | 0 | 0 | 0 | 0 | 5 (16.1) | 5 (12.2) |
| Asian | 0 | 0 | 0 | 0 | 1 (3.2) | 1 (2.4) |
| Other | 0 | 0 | 0 | 0 | 1 (3.2) | 1 (2.4) |
|
| ||||||
| ECOG, n (%) | ||||||
| 0 | 4 (100) | 3 (100) | 3 (100) | 10 (100) | 17 (54.8) | 27 (65.9) |
| 1 | 0 | 0 | 0 | 0 | 14 (45.2) | 14 (34.1) |
|
| ||||||
| Total Gleason Score, n (%) | ||||||
| <7 | 2 (50) | 0 | 2 (66.7) | 4 (40) | 6 (19.4) | 10 (24.4) |
| 8–10 | 1 (25) | 2 (66.7) | 1 (33.3) | 4 (40) | 18 (58.0) | 22 (53.6) |
| Unknown | 1 (25) | 1 (33.3) | 0 | 2 (20) | 7 (22.6) | 9 (22.0) |
|
| ||||||
| Site of Metastasis, n (%) | ||||||
| Bone | 4 (100) | 3 (100) | 3 (100) | 10 (100) | 23 (74.2) | 33 (80.5) |
| Lung | 0 | 0 | 0 | 0 | 2 (6.5) | 2 (4.9) |
| Lymph | 0 | 0 | 0 | 0 | 3 (9.7) | 3 (7.3) |
| Other | 0 | 0 | 0 | 0 | 2 (6.5) | 2 (4.9) |
|
| ||||||
| PSA (ng/mL) | ||||||
| Median (range) | 20.5 (1.4, 69.7) | 13.1 (2.6, 24.9) | 7.4 (2.4, 16.1) | 10.3 (1.4, 69.7) | 13.2 (0.8, 583.5) | 13.1 (0.8, 583.5) |
|
| ||||||
| Prior therapies in mCRPC, n (%) | ||||||
| Hormonal | 4 (100) | 3 (100) | 3 (100) | 9 (90) | 31 (100) | 41 (100) |
| Chemotherapy | 0 | 0 | 0 | 0 | 7 (22.6) | 7 (17.1) |
| Other | 1 (25) | 0 | 1 (33.3) | 2 (20) | 8 (25.8) | 10 (24.4) |
|
| ||||||
| Prior Anticancer Surgery, n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Prior Radiation Therapy, n (%) | 2 (50) | 0 | 0 | 2 (20) | 4 (12.9) | 6 (14.6) |
ECOG: Eastern Oncology Cooperative Group; PSA: prostate-specific antigen; ADT: androgen-deprivation therapy (pharmacological)
Pharmacokinetic data demonstrated increasing ORIC-101 exposures with dose increase, with a steady-state mean Cmax (%CV) of 427 ng/mL (48.7), Cmin (%CV) of 54.1 ng/mL (48.1), AUC (%CV) of 4090 h*ng/mL (48.1), and half-life (%CV) of 10.7 hours (34.7) at 240 mg daily. These parameters were in line with the predicted human PK based on nonclinical studies and confirmed ORIC-101 achieved exposures necessary for GR target coverage without affecting enzalutamide exposure.
PD modulation did not show dose dependency during dose escalation, with GR target suppression achieved starting at the first dose level tested in dose escalation, 80 mg ORIC-101.
Overall, disease progression (radiological, clinical, and/or PSA progression) was the most common primary reason for treatment discontinuation: 9 (90%) patients in dose escalation and 23 (74%) patients during dose expansion. Two (6.5%) patients discontinued due to AEs during dose expansion, both events considered related to both study drugs: Grade 2 fatigue and Grade 2 AST elevation. Study patient disposition is presented in Figure 1.
Figure 1. Study Disposition.
Safety Profile
During dose escalation (n=10), 7 (70%) patients experienced treatment-emergent AEs that were considered related to study treatment (TRAEs) (Table 2). There were no discontinuations due to AEs and there were no DLTs reported. The most common TRAEs occurring in at least 2 patients were fatigue (50%), nausea (30%), and decreased appetite, constipation, headache, aspartate aminotransferase (AST) increased, and blood alkaline phosphatase increased, each with 20% incidence. There was only one Grade 3 TRAE of syncope reported at the 240 mg ORIC-101 dose level, which resolved with study drug interruption.
Table 2.
Treatment-Related Adverse Events Occurring in at Least 5% of Patients or Grade 3 in Severity
| Dose Escalation | Dose Expansion | All Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 80 mg ORIC-101 + 160 mg enzalutamide (N=4) | 160 mg ORIC-101 + 160 mg enzalutamide (N=3) | 240 mg ORIC-101 + 160 mg enzalutamide (N=3) | Total (N=10) | 240 mg ORIC-101 + 160 mg enzalutamide (N=31) | Total (N=41) | |||||||
| Patients with at least one TRAE n (%) | All Grades | Grade 3 | All Grades | Grade 3 | All Grades | Grade 3 | All Grades | Grade 3 | All Grades | Grade 3 | All Grades | Grade 3 |
| Fatigue | - | - | 2 (66.7) | - | 3 (100) | - | 5 (50) | - | 12 (38.7) | - | 17 (41.5) | |
| Nausea | - | - | 1 (33.3) | - | 2 (66.7) | - | 3 (30) | - | 9 (29.0) | 1 (3.2) | 12 (29.3) | 1 (2.4) |
| Decreased appetite | 1 (25) | - | - | - | 1 (33.3) | - | 2 (20) | - | 6 (19.4) | - | 8 (19.5) | |
| Constipation | 1 (25) | - | - | - | 1 (33.3) | - | 2 (20) | - | 4 (12.9) | - | 6 (14.6) | |
| Headache | - | - | 1 (33.3) | - | 1 (33.3) | - | 2 (20) | - | 3 (9.7) | - | 5 (12.2) | |
| AST increased | - | - | 2 (66.7) | - | - | - | 2 (20) | - | 2 (6.5) | - | 4 (9.8) | |
| ALT increased | - | - | 1 (33.3) | - | - | - | 1 (10) | - | 2 (6.5) | 1 (3.2) | 3 (7.3) | 1 (2.4) |
| Alk Phos increased | - | - | 2 (66.7) | - | - | - | 2 (20) | - | 1 (3.2) | - | 3 (7.3) | |
| TSH increased | - | - | - | - | - | - | - | - | 3 (9.7) | - | 3 (7.3) | |
| Dyspepsia | - | - | - | - | - | - | - | - | 3 (9.7) | - | 3 (7.3) | |
| Hot flush | - | - | - | - | 1 (33.3) | - | 1 (10) | - | 2 (6.5) | - | 3 (7.3) | |
| Vomiting | - | - | - | - | - | - | - | - | 3 (9.7) | - | 3 (7.3) | |
| Syncope | - | - | - | - | 1 (33.3) | - | 1 (10) | - | 1 (3.2) | 2 (4.9) | 2 (4.9) | |
| Hypokalemia | 1 (25) | - | - | - | - | - | 1 (1) | - | - | 1 (3.2) | 2 (4.9) | 1 (2.4) |
TRAE: treatment-related adverse events (related to ORIC-101 or enzalutamide); AEs coded using MedDRA v22.0; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Alk Phos: blood alkaline phosphatase; TSH: thyroid stimulating hormone
Despite not meeting protocol criteria for dose limiting toxicities, the events of fatigue, nausea, and syncope experienced by some patients treated with 240 mg ORIC-101 led to the joint investigators and sponsor’s decision to select that dose as the ORIC-101 RP2D for combination with 160 mg enzalutamide and not proceed with further ORIC-101 dose escalation to determine the MTD.
During dose expansion at the RP2D (n=31), 25 (80.6%) patients experienced TRAEs, with the most commonly reported in at least 10% of patients being fatigue (38.7%), nausea (29.0%), decreased appetite (19.4%), and constipation (12.9%). Grade 3 TRAEs of alanine aminotransferase (ALT) increased, nausea, hypokalemia, and syncope were reported, all recovered with study drug interruptions. One patient experienced an SAE of osteonecrosis, deemed possibly related to enzalutamide and prior treatment with Ra-223 and radiation therapy, and one patient discontinued the study due to fatigue.
There were no Grade 4 or Grade 5 adverse events reported in the overall study.
Dose Expansion Results
Antitumor Activity
At the planned interim analysis of futility (n=28), the DCR was 25.0% (80% CI: 10.7%, 44.9%) which did not meet the target rate defined in the statistical analysis plan of a lower limit of the two-sided 80% CI >20% to warrant continuation of the study.
PSA response rate was 0% (95% CI: 0.0, 11.2), ORR was 3.2% (95% CI: 0.08, 16.70), and the median PFS was 4.1 months (95% CI: 1.87, NE).
Pharmacodynamics
ORIC-101 reduced GR target gene expression in PBMCs in 23/28 (82%) patients, as early as after one dose of ORIC-101 and independent of drug exposure, including patients where cortisol levels increased post-dose due to physiologic negative feedback between cortisol and GR (Figure 2A).19
Figure 2. ORIC-101 Pharmacodynamics.
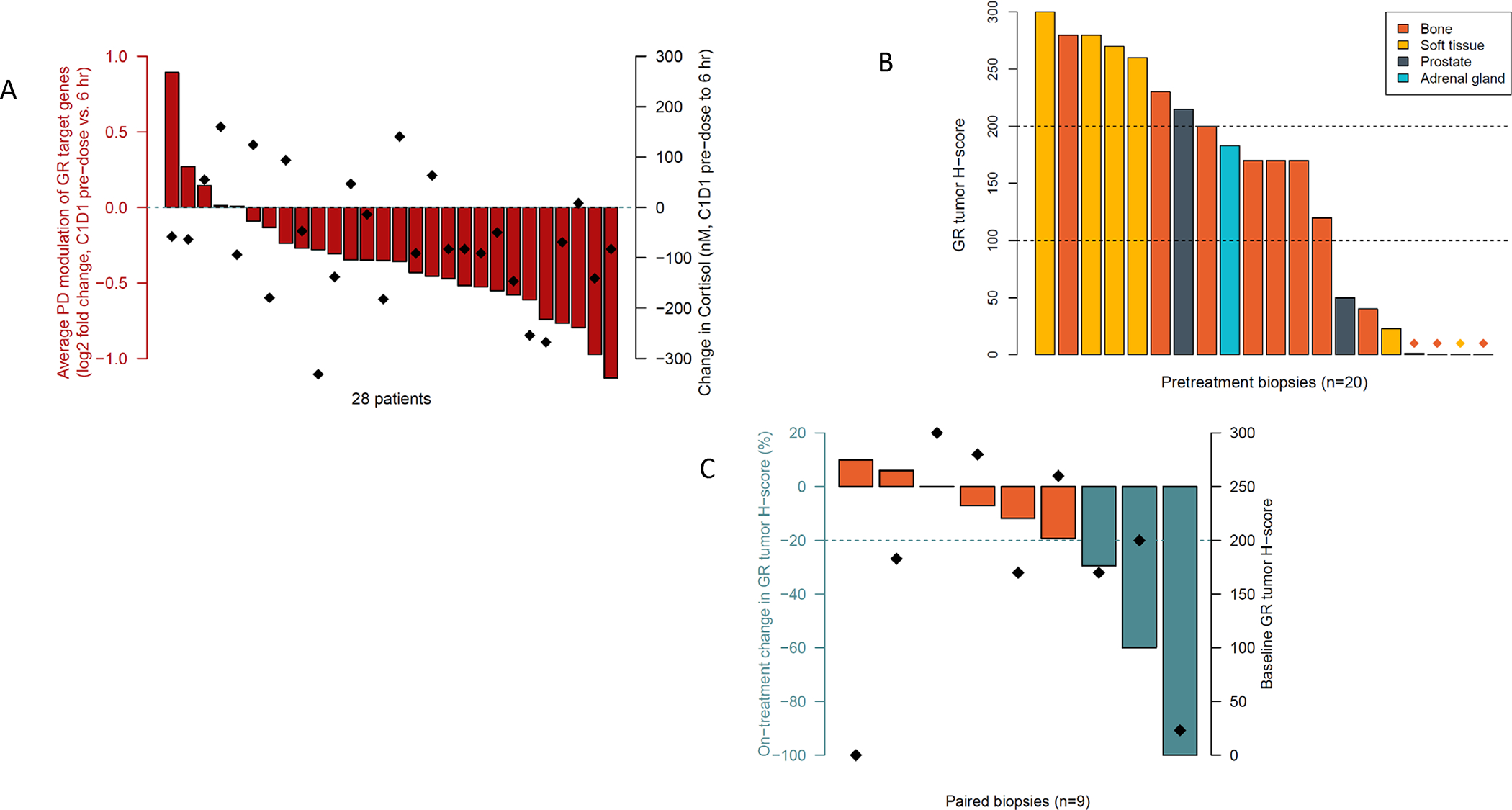
(A) PD modulation after one dose of ORIC-101 in dose expansion patients treated at the RP2D. Results are displayed as the average fold change in the expression of GR target genes FKBP5, GILZ and PER1 from pre-dose to 6 hours post-dose (in red). Diamonds represent change in cortisol pre-dose to 6 hours post-dose. (B) Barplot with GR IHC H-scores in tumor cells in pretreatment biopsies, colored by biopsy site, from dose expansion patients treated at the RP2D. Low GR expression was defined as H-score <100, and high GR expression as H-score ≥200. (C) Barplot with proportional on-treatment change in GR tumor H-score, defined as (on-treatment GR H-score – pretreatment GR H-score) / pretreatment GR H-score, for 9 dose expansion patients with matched tumor biopsies from the same biopsy site. Diamonds represent GR tumor H-score at pretreatment.
Twenty-nine pretreatment or archival tumor biopsies were collected, with 20 (69%) having sufficient tumor cell content for protein quantification by IHC. Nuclear GR protein was present (H-score ≥100) pretreatment in 65% (13/20) of patients, with a median H-score of 170 (Figure 2B). GR protein levels were not associated with biopsy site, equally present in bone lesions, lymph node tissue and prostate tumors. A >20% on-treatment reduction in GR protein levels in tumor cells was observed in 3/9 patients with matched tumor biopsies (Figure 2C), suggestive of on-target therapeutic effect of ORIC-101 on GR-positive tumor cells, as demonstrated by disease control of approximately 7, 8, and 11 months, respectively.
Exploratory Genomic Analyses
Based on genomic profiles and amount of tumor shedding (Supplementary Table 2), patients enrolled in the dose expansion portion of the study were representative of an mCRPC disease setting, with pretreatment AR protein levels consistently high (median IHC H-score 290, IQR 237–300). AR alterations observed in ctDNA and cfRNA pretreatment included amplification, AR-v7, and AR L702H in 8 patients (Supplementary Table 3). We specifically showed that the AR mutation L702H altered the desired mechanism of action of ORIC-101 in combination with enzalutamide in nonclinical studies. Cortisol drove the growth of AR L702H-mutant prostate cells, as previously reported, 20, 21 but this growth could not be reversed with the combination of enzalutamide and ORIC-101 (Supplementary Figure 1A–C). Other prevalent genomic alterations observed in the dose expansion cohort included functional loss of tumor suppressor genes TP53, RB1 and PTEN, gain or activation of MYC, MYCN, PIK3CA, CDK6, FGFR1/3, and the TMPRSS2-ERG fusion (Figure 3).
Figure 3. Genomic Landscape of Dose Expansion Patients.
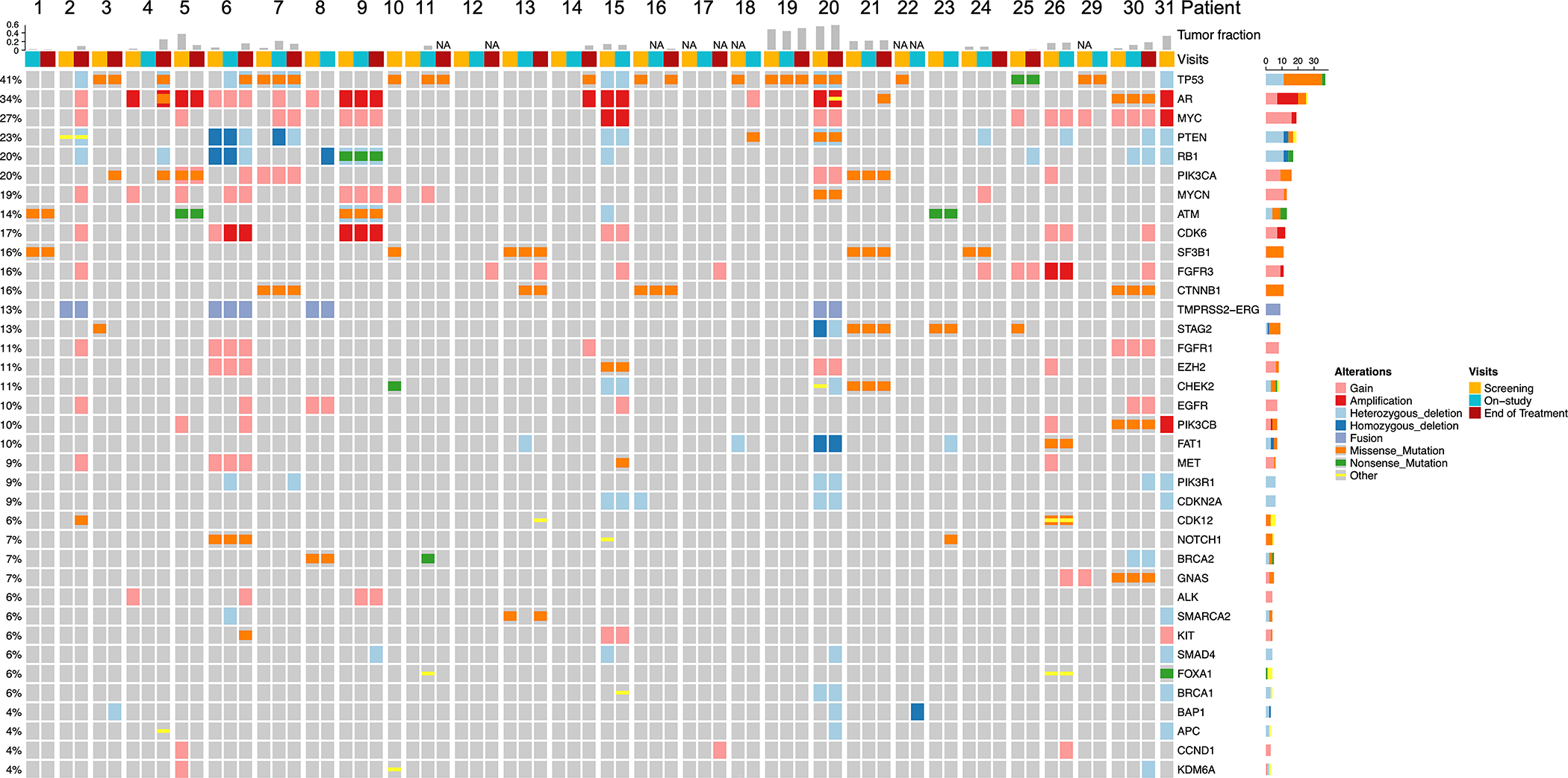
OncoPrint overview of the most prevalent genomic alterations in plasma samples from dose expansion patients with plasma collected before treatment, at the end of Cycle 2, and/or at the end of treatment. Plasma could not be collected in 2 patients. Displayed are genes from the targeted 152-gene panel that were altered at any timepoint in ≥3 (3/29, 10%) of the study patients. Type of alteration is indicated by color, with “Other” including frame-shift insertions and deletions, in-frame insertions and deletions, and splice site mutations. Screening, on-study and end of treatment samples are shown as columns, grouped per patient. Tumor fraction is shown on top; NA indicates tumor fraction could not be estimated.
Overall, 12 (39%) patients’ tumors harbored potential AR resistance alterations or were characterized by tumor suppressor gene loss, with AR amplification the most prevalent genomic finding (n=6), followed by AVPC molecular features (n=5), AR-v7 (n=3), and AR L702H (n=1), with some overlap (Supplementary Table 3; Figure 4). Among the remaining 19 patients with normal or unknown AR/AVPC status, 7 (23%) patients had moderate to high GR levels, 4 (13%) patients had low levels of GR protein, and in 8 (26%) patients, GR was not evaluable, or a tumor biopsy could not be collected. We assessed the association of these molecular characteristics with clinical outcome for patients enrolled at the RP2D (Figure 4). In patients lacking AR resistance variants and AVPC molecular features (n=19), DCR was 42.1% (80% CI: 26.3%, 59.2%). DCR was 53.3% (80% CI: 34.2%, 71.8%) in the subset with moderate to high, or unknown, GR protein level at baseline (n=15). Conversely, in the subgroup of patients whose tumors harbored AR resistance variants, AVPC molecular features, and/or expressed low GR protein levels (n=16), DCR was 0% (80% CI: 0.0%, 13.4%). PFS results followed the same trend.
Figure 4.
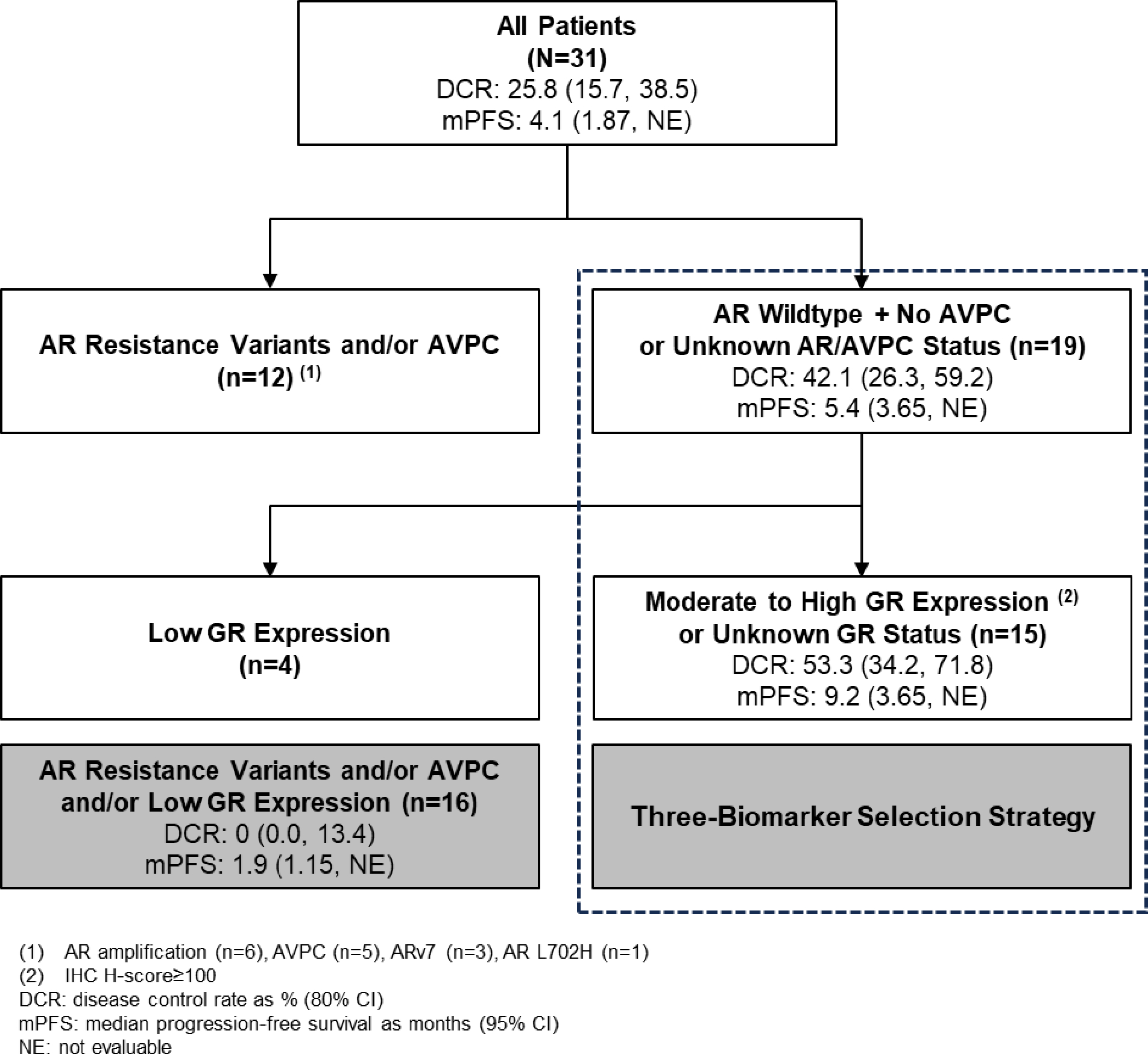
Biomarker Patient Selection Strategy Consort, with Antitumor Activity at the RP2D Across Patients Defined by AR, AVPC, and GR Status
Lastly, the genomic profiles at baseline revealed intra- and inter-tumor heterogeneity, with the co-existence of markers of AR-dependent or -independent resistance mechanisms. Serial tumor sampling from individual patients demonstrated further acquisition of potential AR-dependent resistance markers such as AR-v7 and AR L702H as well as AR-independent resistance markers such as PI3K-AKT-mTOR and Wnt pathway alterations and markers of neuroendocrine transformation including RB1 or TP53 loss and MYC gain (Figure 5).
Figure 5.
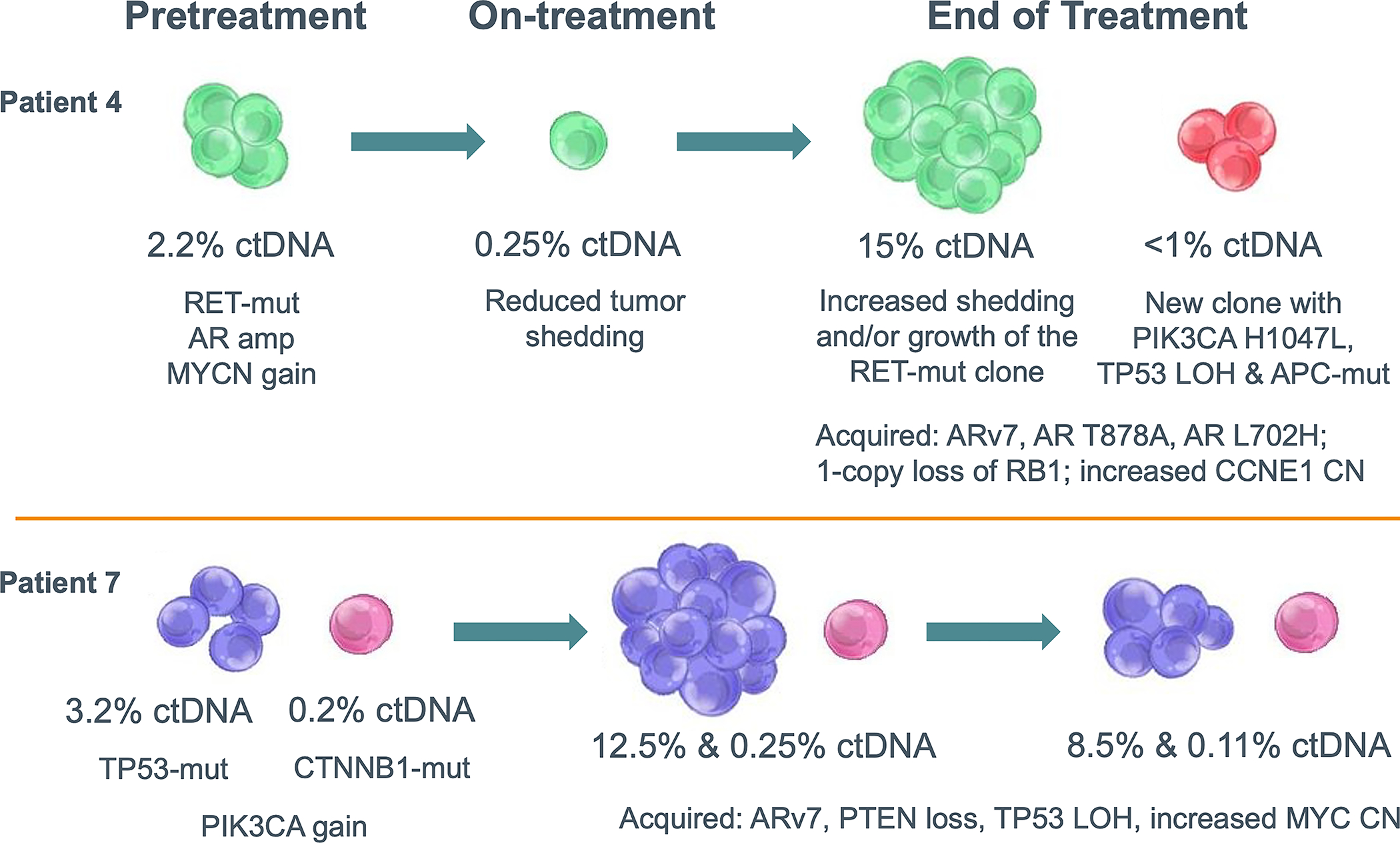
Representative Examples of Tumor Heterogeneity and Resistance Mechanism Redundancy
DISCUSSION
In this Phase 1/1b study of ORIC-101 in combination with enzalutamide in patients with mCRPC progressing on enzalutamide, we tested the hypothesis that GR antagonism would counter mechanisms of resistance to AR signaling mediated through GR signaling. As the data demonstrated, effective target engagement was achieved without toxicity, but no meaningful antitumor effect (PSA or radiographic) was observed. These observations suggest that in enzalutamide-resistant cancer, GR expression is not a primary driver of clinical outcomes. AR-dependent and -independent resistance mechanisms may co-exist, and our findings indicate additional work is needed to further elucidate which disease setting and patient population would benefit from dual AR and GR inhibition, and potentially suppression of other biological pathways.
Despite the negative outcome, this study has an important strength in the comprehensive molecular characterization that allowed for exploring the outcome of GR inhibition in molecular subsets of mCRPC, including tumors with high GR expression. No clinical benefit was observed despite effective target engagement. In 6/9 patients with matched tumor biopsies, the ORIC-101 plus enzalutamide combination did not reduce GR-expressing tumor cells despite pharmacologic inhibition. This suggests that suppressing GR signaling with ORIC-101 did not suffice to re-sensitize cells to enzalutamide. Alternate pathways of resistance to enzalutamide include the development of AR resistance mutations, constitutively active AR splice variants (AR-v7), or loss of dependence on the AR pathway altogether via lineage plasticity, which sometimes manifests clinically as AVPC. In all of these contexts, dual AR/GR inhibition may not be beneficial.
Similar outcomes were observed in other studies of GR and AR antagonism. In heavily pretreated mCRPC patients with prior enzalutamide exposure, only one PSA response and no radiographic responses were observed with the combination of enzalutamide and GR antagonist exicorilant.6 The combination of enzalutamide with GR antagonist mifepristone in mCRPC patients similarly did not delay time to PSA, radiographic or clinical PFS, resulting in early trial termination due to futility, though these results may have further been hampered by the partial AR agonism behavior of mifepristone.7 In these studies, GR was found to be expressed in tumors and on circulating tumor cells, but the target level did not associate with clinical outcome. Systemic GR modulation was observed by reduction in GR target genes. It was postulated in these studies that the CRPC setting may be too late for a dual AR/GR inhibition approach, thereby inadvertently enriching for intra-patient pleiotropic AR antagonist resistant mechanisms beyond GR. Furthermore, the mifepristone trial may have had an imbalance in AR alterations that have been implicated in castration resistance between the combination and enzalutamide single-agent arms, yet such alterations were not tracked.7
In exploratory analyses in our study, a higher disease control rate was observed in patients whose tumors did not harbor potential AR resistance variants or AVPC molecular features, and additionally lacked GR expression. It is possible, however, that the better outcome observed in these patients could be due to inherently more indolent disease biology rather than an effect of GR inhibition. Nonetheless, a future selection strategy for AR/GR inhibitor combination therapy in prostate cancer could focus on an earlier disease setting, when the likelihood of presenting with alternate, non-GR resistance pathways is lower, or exclude patients with AR resistance variants, AVPC molecular features, and low GR expression.
The strength of our trial is the comprehensive biomarker data collection, which led us to establish intra-patient tumor heterogeneity and the existence of possible pleiotropic AR-dependent and -independent resistance mechanisms. Limitations of our study are the small sample size in context of the observed tumor heterogeneity, the impact that patients with AVPC molecular features and poorer outcomes may have had on the clinical endpoints, and lastly the unconventional choice of DCR, defined as the proportion of patients who did not have PSA or radiographic progression on or before Week 12, as primary endpoint. The main premise in this study was the potential for ORIC-101 to reverse resistance to enzalutamide and prolong clinical benefit from the therapeutic agent, delaying the need to transition to more toxic therapies. In that context, DCR was selected as an endpoint thought to better capture the duration of clinical benefit in keeping with the expected mechanism of action of ORIC-101.
In conclusion, based on a planned interim analysis, although the combination of ORIC-101 and enzalutamide was safe and tolerable, antitumor efficacy was not observed in the context of effective target engagement. The clinical and translational observations of our study led us to conclude that GR was not the principal driver of resistance to AR antagonists in most men with mCRPC. Further development of GR inhibition in prostate cancer will require an understanding of the mechanism(s) that drives resistance to AR antagonists and upregulates GR expression. A more homogeneous patient population in an earlier prostate cancer disease setting with the right biomarker-based selection may still lead to therapeutic benefit.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Upregulation of the glucocorticoid receptor (GR) is a potential mechanism of resistance to androgen receptor (AR) inhibition, leading to poor outcomes in patients with mCRPC treated with enzalutamide. ORIC-101 inhibits GR transcriptional activity and blocks pro-survival signals mediated by the activated nuclear hormone receptor. In this Phase 1b study, a comprehensive characterization of patients’ tumors at multiple timepoints during treatment identified heterogeneity in AR alterations and variant expression, molecular features of aggressive variant prostate cancer, and low GR expression as potential factors explaining poor response to treatment with ORIC-101 and enzalutamide. These observations suggest that future efforts to target GR should explore the optimal point in prostate cancer evolution for effective interruption of the GR axis to account for heterogeneity that usurps GR dependency.
Funding:
This study was funded by ORIC Pharmaceuticals. Supported in part by the National Cancer Institute (NCI) Cancer Center Support grant number P30-CA008748 and the Prostate Cancer Foundation (to Wassim Abida).
Footnotes
Conflicts of Interest:
WA: Speaking honoraria from Roche, Pfizer, Medscape, Aptitude Health, Clinical Education Alliance, touchIME and Onclive/MJH Life Sciences, consulting fees from Clovis Oncology, Janssen, ORIC Pharmaceuticals (prior to opening of the study in this publication), Daiichi Sankyo and AstraZeneca, and Research Funding (to his institution) from AstraZeneca, Zenith Epigenetics, Clovis Oncology, ORIC Pharmaceuticals, Epizyme, and Nuvation Bio.
AWH: Advisory board consulting: Janssen, Intellisphere; Honoraria: Medscape; Travel support: Dava Oncology.
NS: Consulting: Abbvie, Accord, Alessa Therapeutics, Amgen, Antev, Arquer, Asieris, Astellas, Astra Zeneca, Aura Biosciences, Bayer, Bioprotect, Bristol Myers Squibb, Boston Scientific, CG Oncology, Clarity, Cold Genesys, Dendreon, Exact Imaging, Genentech Roche, Ferring, Fize Medical, Foundation Medicine, Genesis Care, Immunity Bio, Incyte, Invitae, Janssen, Lantheus, Lilly, MDXHEALTH, Merck, Minomic, Myovant, Myriad, Nonagen, Novartis, Nymox, Palette Life, PlatformQ, Pacific Edge, Pfizer, Preview, Profound Medical, Promaxo, Protara, Photocure, Propella, Sanofi, Genzyme, Specialty Networks, Telix, Tolmar, Urogen.
NA: Consultancy to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics. Research funding (to his institution): Arnivas, Astellas, Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Crispr, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Lava, Medivation, Merck, Nektar, Neoleukin, New Link Genetics, Novartis, Oric, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon.
PS: Speaker: Bayer, Astellas, Myovant, Dendreon, Pfizer, Merck, Janssen, Accord; compensated consultant: NovoNordisk; research trials: AstraZeneca, Astellas, Bayer, Merck, CG Therapeutics, Pfizer, Janssen, Dendreon, ORIC
MRS: Consulting fees from Ambrx, Astellas, Bayer, Pfizer, Janssen, Lilly; speaking honoraria from Astellas, Bayer, Pfizer, Janssen, Lilly.
TBD: Consulting fees from AstraZeneca, Bayer, Janssen, Sanofi.
PM: Research: Bioxcel, Genentech, Dendreon, Regeneron; consulting: Pfizer, Myovant, Sanofi, Dendreon.
MR: Speaker: Bayer, Janssen; Compensated Consultant: Amgen, Janssen, Bayer, Inmune Bio, Ambryx, Astra-Zeneca, Myovant; Research Support: Merck, Novartis, Lantheus, Janssen.
CJL: Honoraria: Bayer, Amgen, Novartis, Boehringer Ingelheim, Merck, Sharp & Dohme, Exelixis; Clinical Grants: Janssen, ORIC Pharmaceuticals, Novartis.
MJM: Uncompensated advisor to Bayer, and Novartis. Compensated advisor to AstraZeneca, Lantheus, Daiichi, Convergent Therapeutics, Pfizer, ITM Isotope Technologies, Clarity Pharmaceuticals, Blue Earth Diagnostics, POINT Biopharma, Telix, and Z-Alpha. His institution receives research funding from Bayer, Corcept, Roche, Janssen, Celgene, Novartis, and Astellas.
ORIC Authors: Stock and employment at ORIC Pharmaceuticals.
Ethics approval: The study was approved by the relevant ethics committees, and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent to participate: Informed consent was obtained from the patients, parents or legal guardians.
Consent for publication: No sensitive identifying patient information is included in this manuscript.
All authors were involved in revising the manuscript critically for important intellectual content, approved the final version, and agreed to be accountable for the work.
REFERENCES
- 1.Azher S, Azami O, Amato C, McCullough M, Celentano A, Cirillo N. The non-conventional effects of glucocorticoids in cancer. J Cell Physiol. 2016; 231:2368–2373 [DOI] [PubMed] [Google Scholar]
- 2.Arora VK, Schenkein E, Murali R, Subudhi S, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N, Wang P, Wongvipat J, et al. Regulation of the glucocorticoid receptor via a BET-dependent enhancer drives antiandrogen resistance in prostate cancer. eLife 2017;6:e27861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5:72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puhr M, Hoefer J, Eigentler A, Ploner C, Handle F, Schaefer G, et al. The glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved antiandrogen therapy. Clin Cancer Res. 2018;24:927–938. [DOI] [PubMed] [Google Scholar]
- 6.Morris MJ, Linch MD, Crabb SJ, Beer TM, Heath EI, Gordon MS, et al. : Phase 1 efficacy and pharmacodynamic results of exicorilant + enzalutamide in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2023; 41.6 suppl.145.36112962 [Google Scholar]
- 7.Serritella AV, Shevrin D, Heath EI, Wade JL, Martinez E, Anderson A, et al. Phase I/II trial of enzalutamide and mifepristone, a glucocorticoid receptor antagonist, for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2022;28:1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rew Y, Du X, Eksterovicz J, Zhou H, Jahchan N, Zhu L, et al. Discovery of a potent and selective steroidal glucocorticoid receptor antagonist (ORIC-101). J Med Chem. 2018;61:7767–7784. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Wang SJ, Ji Y. The i3+3 design for phase I clinical trials. J Biopharm Stat. 2020; 30:294–304 [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pankov A, Zhou H, Barkund S, Hegde G, Narayanan P, Kabbarah O, et al. Abstract 4120: ORIC-101 comprehensively inhibits glucocorticoid pathways to overcome therapeutic resistance in pan-cancer models. Cancer Res. 2020; 80(16_Supplement):4120. [Google Scholar]
- 12.Zhou H, Ye Q, Pankov A, Kong W, Barkund S, Friedman LS, et al. Abstract P6–03-24: ORIC-101 robustly inhibits the glucocorticoid pathway and overcomes chemoresistance in triple-negative breast cancer. Cancer Res. 2020; 80(4_Supplement):P6–03-24. [Google Scholar]
- 13.Daemen A, Pankov A, Barkund S, Zhou H, Duff M, Johnson A, et al. Biomarker results supporting selection of RP2D from a phase 1b study of ORIC-101, a glucocorticoid receptor antagonist, in combination with nab-paclitaxel in patients with advanced solid tumors. J Clin Oncol. 2021; 39(15_suppl):3110. [Google Scholar]
- 14.Aparicio AA, Shen L, Tapia ELN, et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin Cancer Res. 2016; 22:1520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotler E, Shani O, Goldfeld G, Lotan-Pompan M, Tarcic O, Gershoni A, et al. A systematic p53 mutation library links differential functional impact to cancer mutation pattern and evolutionary conservation. Mol Cell. 2018;71:178–190. [DOI] [PubMed] [Google Scholar]
- 16.Giacomelli AO, Yang X, Lintner RE, McFarland JM, Duby M, Kim J, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet. 2018;50:1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donehower LA, Soussi T, Korkut A, Liu X, Schultz A, Cardenas M, et al. Integrated analysis of TP53 gene and pathway alterations in The Cancer Genome Atlas. Cell Rep. 2019;28:1370–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–1739. [DOI] [PubMed] [Google Scholar]
- 19.Daemen A, Barkund S, Johnson A, Pankov A, Wang AW, Zhou H, et al. Abstract P015: Biomarker results supporting selection of RP2D from a phase 1b study of ORIC-101, a glucocorticoid receptor antagonist, in combination with enzalutamide in patients with metastatic prostate cancer progressing on enzalutamide. Mol Cancer Ther. 2021; 20(12_Supplement):P015. [Google Scholar]
- 20.Torquato S, Pallavajjala A, Goldstein A, Valda Toro P, Silbertstein JL, Lee J, et al. Genetic alterations detected in cell-free DNA are associated with enzalutamide and abiraterone resistance in castration-resistant prostate cancer. JCO Precis Oncol. 2019;3:PO.18.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanel A, Gasi Tandefelt D, Conteduca V, Jayaram A, Casiragui N, Wettersskog D, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are not publicly available due to patient privacy requirements but can be available upon reasonable request to the corresponding author.


