Abstract
INTRODUCTION
Reproductive health history may contribute to cognitive aging and risk for Alzheimer's disease, but this is understudied among Hispanic/Latina women.
METHODS
Participants included 2126 Hispanic/Latina postmenopausal women (44 to 75 years) from the Study of Latinos‐Investigation of Neurocognitive Aging. Survey linear regressions separately modeled the associations between reproductive health measures (age at menarche, history of oral contraceptive use, number of pregnancies, number of live births, age at menopause, female hormone use at Visit 1, and reproductive span) with cognitive outcomes at Visit 2 (performance, 7‐year change, and mild cognitive impairment [MCI] prevalence).
RESULTS
Younger age at menarche, oral contraceptive use, lower pregnancies, lower live births, and older age at menopause were associated with better cognitive performance. Older age at menarche was protective against cognitive change. Hormone use was linked to lower MCI prevalence.
DISCUSSION
Several aspects of reproductive health appear to impact cognitive aging among Hispanic/Latina women.
Keywords: cognition, Hispanics, Latinas, menopause, mild cognitive impairment, reproductive health, women
1. BACKGROUND
At 45 years of age, estimated lifetime risk for Alzheimer's disease (AD) among men is 10% but is 20% among women. 1 Aging is the strongest risk factor for AD, 2 and the longer average lifespan in women versus men may partially explain their higher lifetime disease risk; however, evidence also suggests that sex‐specific biological factors may contribute to Alzheimer's risk. 3 , 4 , 5
Estrogen confers a wide range of neuroprotective effects, and greater estrogen exposure is associated with decreased risk for AD. 6 Various reproductive health factors, such as birth control use and number of pregnancies, may modify estrogen exposure but are seldom studied in the context of cognitive aging and Alzheimer's risk. For example, exposure to exogenous hormones (eg, estrogen, progesterone) used in some forms of birth control or hormonal therapies may influence brain health and late‐life cognitive performance, though findings are mixed. 7 Additionally, number of pregnancies and live births may be connected to cognitive outcomes through changes to estrogen levels and also improved immunoregulation during pregnancy. 6 , 7 More commonly, age at menarche and menopause are studied in relation to cognitive aging and Alzheimer's risk. For example, a study of women enrolled with Kaiser Permanente linked higher risk of AD and related dementias with several reproductive health factors, including late age at menarche, shorter reproductive span (ie, years between menarche and menopause), earlier menopause age, and history of hysterectomy. 8 Such relationships did not differ by racial or ethnic group, but these sample sizes were relatively small. Hispanic/Latina women made up less than 5% of the sample, and this subgroup may be unique in that they were all insured unlike a significant proportion of the Hispanic/Latino community. 9 , 10 In general, Latinos are at increased risk for Alzheimer's disease compared to non‐Hispanic/Latino Whites, 2 and Hispanic/Latina women have a slightly higher incidence of Alzheimer's disease compared to their male counterparts in late old age. 11 It is unclear whether the latter is related to more severe declines in estrogen in old age or other sex‐specific reproductive experiences among Hispanic/Latina women relative to their male counterparts. Therefore, it is important to examine the role of reproductive health on cognitive aging among Hispanic/Latina women.
Mexican‐heritage women reach menopause earlier than their non‐Hispanic/Latina White counterparts on average. 12 Despite having less access to health care, they often have similar, if not slightly better, pregnancy outcomes (except gestational diabetes prevalence) compared to non‐Hispanic/Latina White individuals. 13 , 14 These factors, combined with longer lifespan among Hispanic/Latina women relative to non‐Hispanic/Latina White women, 15 warrant investigations into the connections between reproductive health and cognitive aging and impairment among diverse Hispanic/Latina women. Notably, Hispanic/Latina women residing in the United States represent various heritage groups (eg, Central American) and immigration histories, which may influence access to resources (eg, educational, health) and reproductive health and pregnancy outcomes. 16 , 17 Therefore, we examined how reproductive health factors relate to cognition, cognitive change, and mild cognitive impairment (MCI) among diverse postmenopausal Hispanic/Latina women using data from the multisite Hispanic Community Health Study/Study of Latinos (HCHS/SOL) and Study of Latinos‐Investigation of Neurocognitive Aging (SOL‐INCA). We hypothesized that later menarche, earlier menopause, and shorter reproductive span would be associated with poorer cognitive performance, greater adverse change in cognition across 7 years, and higher prevalence of MCI. We also explored the relationships between oral contraceptive use, number of pregnancies, number of live births, and female hormone use with these same cognitive outcomes, with no a priori hypotheses.
2. METHODS
2.1. Data
The HCHS/SOL is a multisite prospective cohort study of N = 16,415 self‐identified diverse Hispanic/Latino adults (ages 18 to 74 at recruitment). Participants were from four major US metropolitan areas: Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA. An ancillary study of HCHS/SOL, SOL‐INCA, was conducted during the second HCHS/SOL visit to examine neurocognition among HCHS/SOL participants who were 45 years and older at the time of their initial neurocognitive testing during Visit 1. 18 At Visit 2 of HCHS/SOL, N = 6377 out of N = 7420 eligible individuals completed the SOL‐INCA visit (henceforth Visit 2), approximately 7 years later. The HCHS/SOL Coordinating Center generated complex study designs and sampling procedures to obtain data of diverse Hispanic/Latino adults 50 years and older. The detailed HCHS/SOL study designs and sampling methods have been published. 19 , 20 Our procedures, from the data collection (eg, population‐based sampling, representation from multiple heritage groups [Central Americans, Cubans, Dominicans, Mexicans, Puerto Ricans, South Americans], with testing in their preferred language) to the data analysis (applying sampling weights, including sociodemographic covariates) and interpretation consider several aspects of diversity, equity, and inclusion in order to address health disparities in the Hispanic/Latino population. All participants gave informed consent, and Institutional Review Board approval was obtained at all study sites. Research complied with the Helsinki Declaration and its later amendments.
2.2. Outcomes
2.2.1. Average 7‐year performance and change in cognition
Neurocognitive measures of Brief‐Spanish English Verbal Learning Test Sum of Trials and Delayed Recall (B‐SEVLT Sum and Recall; verbal episodic learning and memory), word fluency (WF; verbal fluency with letters F and A), and digit symbol substitution (DSS; processing speed and executive functioning) were tested at Visits 1 and 2, an average of 7 years later. At Visit 2, two measures on processing speed and executive functioning (Trails A and B) were additionally tested. All tests were Z‐scored [(X − mean)/standard deviation] to facilitate interpretation of results across a common metric. We calculated a global cognitive composite score by averaging the Z‐scores of the repeated tests (from Visits 1 and 2, excluding Trails A and B). To examine cognitive change, we calculated a change index for the repeated cognitive measures and the global measure of cognition. 21 We used survey linear regressions where the cognitive score at Visit 2 was modeled as a function of the Visit 1 cognitive score, adjusting for time between cognitive assessments. Test‐specific standardized measures of change and global cognitive change were calculated using (T2 − T2 pred)/RMSE, where T2 is the respondent's cognitive score at Visit 2, T2 pred is the predicted score, and RMSE is the root mean squared error. Detailed rationales on this technique can be found elsewhere. 22
RESEARCH IN CONTEXT
Systematic review: We reviewed previous peer‐reviewed publications using traditional online search engines (eg, PubMed). The review was largely focused on publications related to female reproductive health (eg, estrogen, number of births, menarche, menopause) and cognitive functioning in middle‐aged and older adults. Relevant articles are cited.
Interpretation: In a sample of over 2000 middle‐aged and older Hispanic/Latina postmenopausal women in the United States, several reproductive health factors (eg, age at menarche, oral contraceptive use, pregnancies, live births, and age at menopause) were associated with cognition and, to a lesser extent, 7‐year cognitive change and mild cognitive impairment prevalence.
Future directions: Our findings warrant investigation into whether interventions (medical or public health) to promote specific reproductive health factors might be (1) ethically and culturally appropriate and (2) beneficial to maintaining cognitive functioning in middle age and older adulthood, among Hispanic/Latina women.
2.2.2. MCI prevalence
MCI prevalence was operationalized using National Institute on Aging‐Alzheimer's Association criteria as defined by the SOL‐INCA study and previously published. 23
2.3. Exposures
Exposures included multiple reproductive health factors: self‐reported age at menarche, history of oral contraceptive use (no, yes), number of pregnancies, number of live births, age at menopause (we also considered a categorical operationalization ≤45 years vs >45 years), current female hormone use (no, yes), and reproductive span (years with menses, calculated by subtracting age at menarche from age at menopause). All exposure variables were measured at Visit 1.
2.4. Covariates
All covariates were measured at Visit 1 and included age (continuous), education (less than high school degree/General Educational Development [GED], high school or equivalent, greater than high school degree/GED), language of interview (Spanish, English), Hispanic/Latino heritage (Dominican, Central American, Cuban, Mexican, Puerto Rican, South American), field center (Bronx, Chicago, Miami, San Diego), income (<10,000, 10,001 to 20,000, 20,001 to 40,000, 40,001 to 75,000, >75,000, or not reported), marital status (married/cohabitating, single, separated/divorced/widowed), insurance status (uninsured, insured), nativity (born in U.S. 50 states/DC, born in U.S. territory or foreign country), and body mass index (BMI).
2.5. Statistical analyses
2.5.1. Analytic subpopulation
Out of the unweighted n = 6377 individuals that completed Visit 2, n = 4110 were women. We focused on n = 2197 women who had reached their menopause (either induced: hysterectomy with removal of both ovaries before the natural menopause or natural: no history of hysterectomy or hysterectomy after menopause) at 60 years or younger. More specifically, in the primary analysis, we only included women with a hysterectomy if they had (1) undergone hysterectomy after menopause or (2) reported a bilateral oophorectomy. In the former instance, the women were placed in the natural menopause group. We excluded n = 71 women (less than 5% missing) who had any missing covariates for an analytic sample of unweighted n = 2126. For analyses that examined MCI as an outcome, the unweighted sample included n = 2092 women after also excluding individuals with suspected severe cognitive impairment (n = 34) (Figure S1).
2.5.2. Analytic approach
First, we reported descriptive statistics for the overall analytic sample as well as by menopause type (induced, natural). Distributional differences by type of menopause were tested using survey adjusted chi‐squared tests for categorical variables and t tests for continuous variables. The survey weighted estimates are presented in Table 1. All analyses accounted for the complex survey design and survey weights.
TABLE 1.
Target population characteristics overall and by type of menopause in the Study of Latinos‐Investigation of Neurocognitive Aging.
| Induced menopause n = 282 | Natural menopause n = 1844 | Overall n = 2126 | P | |
|---|---|---|---|---|
| Covariates | ||||
| Age in years (mean [SD]) | 58.6 (8.1) | 59.6 (7.8) | 59.5 (7.8) | .161 |
| Education (% [SE]) | ||||
| <High school | 33.3 (3.7) | 41.2 (1.8) | 40.1 (1.7) | .063 |
| High school | 17.3 (2.8) | 19.3 (1.4) | 19.0 (1.3) | |
| >High school | 49.3 (4.3) | 39.6 (1.7) | 40.9 (1.6) | |
| Language (% [SE]) | ||||
| Spanish | 92.8 (2.6) | 89.5 (1.0) | 90.0 (0.9) | .311 |
| English | 7.2 (2.6) | 10.5 (1.0) | 10.0 (0.9) | |
| Center (% [SE]) | ||||
| Bronx | 21.1 (3.3) | 25.7 (2.0) | 25.1 (1.9) | .368 |
| Chicago | 8.8 (1.7) | 10.8 (0.9) | 10.6 (0.8) | |
| Miami | 45.8 (4.6) | 42.5 (2.8) | 43.0 (2.6) | |
| San Diego | 24.3 (3.8) | 20.9 (1.8) | 21.4 (1.7) | |
| Income (% [SE]) | ||||
| <$10,000 | 17.1 (2.9) | 20.3 (1.5) | 19.8 (1.3) | .647 |
| $10,001 to $20,000 | 31.0 (3.7) | 31.0 (1.5) | 31.0 (1.4) | |
| $20,001 to $40,000 | 30.7 (4.0) | 24.9 (1.5) | 25.7 (1.4) | |
| $40,001 to $75,000 | 8.6 (2.1) | 8.6 (0.9) | 8.6 (0.8) | |
| >$75,000 | 1.2 (0.7) | 1.9 (0.4) | 1.8 (0.3) | |
| Not reported | 11.4 (2.6) | 13.3 (1.2) | 13.0 (1.1) | |
| Marital status (% [SE]) | ||||
| Married/cohabitating | 47.1 (4.2) | 44.5 (2.0) | 44.9 (1.8) | .819 |
| Single | 15.5 (2.6) | 16.1 (1.2) | 16.0 (1.1) | |
| Separated/divorced/widowed | 37.4 (3.9) | 39.4 (1.8) | 39.1 (1.7) | |
| Insurance status (% [SE]) | ||||
| Uninsured | 40.0 (4.2) | 41.6 (1.8) | 41.4 (1.7) | .708 |
| Insured/Medicaid | 60.0 (4.2) | 58.4 (1.8) | 58.6 (1.7) | |
| Nativity (% [SE]) | ||||
| Not born in US | 83.0 (2.8) | 81.6 (1.4) | 81.8 (1.2) | .655 |
| Born in US | 17.0 (2.8) | 18.4 (1.4) | 18.2 (1.2) | |
| BMI (mean [SD]) | 29.8 (6.0) | 30.4 (6.1) | 30.3 (6.1) | .181 |
| Exposures | ||||
| Female hormone use (% [SE]) | ||||
| No | 90.3 (2.3) | 97.2 (0.6) | 96.2 (0.6) | <.001 |
| Yes | 9.7 (2.3) | 2.8 (0.6) | 3.8 (0.6) | |
| History of oral contraceptive use (% [SE]) | ||||
| No | 48.5 (4.3) | 44.2 (1.9) | 44.8 (1.7) | .380 |
| Yes | 51.5 (4.3) | 55.8 (1.9) | 55.2 (1.7) | |
| Age at menarche (mean [SD]) | 12.7 (1.9) | 12.8 (1.9) | 12.7 (1.9) | .520 |
| Number of live births (mean [SD]) | 2.6 (1.8) | 2.9 (2.0) | 2.9 (2.0) | .031 |
| Number of pregnancies (mean [SD]) | 3.4 (2.3) | 3.9 (2.5) | 3.8 (2.5) | .012 |
| Age at menopause (mean [SD]) | 42.1 (7.7) | 48.9 (4.7) | 47.9 (5.8) | <.001 |
| Menopause age (>45 years; % [SE]) | ||||
| ≤45 years | 67.4 (3.7) | 25.5 (1.5) | 31.3 (1.5) | <.001 |
| >45 years | 32.6 (3.7) | 74.5 (1.5) | 68.7 (1.5) | |
| Reproductive span (mean [SD]) | 29.6 (7.8) | 36.1 (4.9) | 35.2 (5.9) | <.001 |
Note: Sample size is unweighted; all other reported values are weighted to represent the target population.
Abbreviations: BMI, body mass index; SD, standard deviation; SE, standard error.
Second, we examined the hypothesized associations between the reproductive health exposures and our cognitive outcomes including cognitive performance at Visit 2 (on average 7 years from Visit 1), cognitive change, and MCI. We fit a series of survey linear and logistic regression models sequentially adjusting for covariates: (1) crude, (2) age and education adjusted, (3) full covariate adjustment (see covariates in the earlier Section 2.4). The estimated beta coefficients and their standard errors (SEs; for cognitive performance at Visit 2 and cognitive change) or odds ratios and their 95% confidence intervals (CIs) (for MCI) are presented in Tables 2 and 3, respectively. In post hoc analyses, we calculated average marginal means and probabilities for significant associations and plotted these with their 95% CIs to facilitate interpretation (Figures 1, 2, 3).
TABLE 2.
Associations of each reproductive history factor with visit 2 (7 years after baseline on average) cognition and 7‐year cognitive change (n = 2126) in Study of Latinos‐Investigation of Neurocognitive Aging.
| 7‐year cognition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B‐SEVLT Sum | B‐SEVLT Recall | WF | DSS | |||||||||
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | |
| B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | |
| Female hormone use | ||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.238 ** (0.083) | 0.065 (0.083) | 0.032 (0.091) | 0.244 * (0.120) | 0.104 (0.105) | 0.086 (0.116) | 0.229 * (0.114) | 0.098 (0.124) | ‐0.020 (0.111) | 0.298 * (0.133) | 0.067 (0.127) | 0.006 (0.104) |
| Contraceptive use | ||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.302 *** (0.065) | 0.189 ** (0.060) | 0.175 ** (0.059) | 0.214 *** (0.062) | 0.120 * (0.061) | 0.092 (0.063) | 0.237 *** (0.071) | 0.172 ** (0.063) | 0.090 (0.065) | 0.384 *** (0.062) | 0.239 *** (0.052) | 0.145 ** (0.049) |
| Age at menarche | 0.002 (0.020) | 0.030 (0.019) | 0.018 (0.018) | 0.001 (0.019) | 0.022 (0.018) | 0.011 (0.018) | ‐0.019 (0.017) | 0.008 (0.016) | ‐0.004 (0.016) | ‐0.090 *** (0.016) | ‐0.050 *** (0.014) | ‐0.042 *** (0.012) |
| Number of live births | ‐0.041 * (0.018) | 0.019 (0.017) | 0.015 (0.016) | ‐0.026 (0.018) | 0.018 (0.018) | 0.006 (0.017) | ‐0.066 *** (0.019) | 0.004 (0.019) | 0.008 (0.020) | ‐0.139 *** (0.014) | ‐0.054 *** (0.014) | ‐0.044 ** (0.014) |
| Number of pregnancies | ‐0.017 (0.014) | 0.014 (0.014) | 0.015 (0.013) | ‐0.005 (0.015) | 0.019 (0.015) | 0.016 (0.014) | ‐0.042 ** (0.014) | ‐0.005 (0.013) | 0.005 (0.013) | ‐0.080 *** (0.014) | ‐0.034 ** (0.012) | ‐0.023 * (0.011) |
| Age at menopause | 0.005 (0.006) | 0.012 * (0.006) | 0.009 (0.005) | 0.008 (0.007) | 0.015 * (0.006) | 0.013 * (0.006) | 0.008 (0.007) | 0.004 (0.006) | 0.000 (0.006) | ‐0.001 (0.006) | 0.004 (0.005) | ‐0.000 (0.004) |
| Menopause age (>45 years) | ||||||||||||
| ≤45 years | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| >45 years | 0.135 (0.075) | 0.133 (0.072) | 0.112 (0.066) | 0.125 (0.078) | 0.139 (0.078) | 0.129 (0.071) | 0.065 (0.081) | ‐0.025 (0.078) | ‐0.063 (0.074) | 0.093 (0.067) | 0.057 (0.059) | 0.024 (0.054) |
| Reproductive span | 0.005 (0.006) | 0.008 (0.006) | 0.007 (0.005) | 0.007 (0.007) | 0.012 (0.007) | 0.011 (0.006) | 0.009 (0.007) | 0.002 (0.006) | 0.000 (0.006) | 0.007 (0.006) | 0.008 (0.004) | 0.003 (0.004) |
| Trail A | Trail B | Global cognition | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | |
| B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | |
| Female hormone use | |||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | ‐0.206 (0.125) | ‐0.023 (0.129) | ‐0.017 (0.118) | ‐0.099 (0.138) | 0.060 (0.139) | 0.106 (0.125) | 0.253 *** (0.073) | 0.084 (0.065) | 0.026 (0.062) |
| Contraceptive use | |||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | ‐0.362 *** (0.085) | ‐0.237 ** (0.075) | ‐0.125 (0.070) | ‐0.261 *** (0.065) | ‐0.169 ** (0.060) | ‐0.082 (0.058) | 0.284 *** (0.049) | 0.179 *** (0.040) | 0.124 ** (0.040) |
| Age at menarche | 0.115 *** (0.024) | 0.083 *** (0.021) | 0.068 *** (0.020) | 0.055 *** (0.015) | 0.033 * (0.014) | 0.028 (0.014) | ‐0.026 (0.013) | 0.003 (0.012) | ‐0.003 (0.011) |
| Number of live births | 0.091 *** (0.020) | 0.015 (0.021) | 0.009 (0.021) | 0.112 *** (0.015) | 0.041 ** (0.015) | 0.029 (0.015) | ‐0.068 *** (0.013) | ‐0.003 (0.012) | ‐0.004 (0.012) |
| Number of pregnancies | 0.040 * (0.017) | ‐0.001 (0.017) | ‐0.006 (0.016) | 0.068 *** (0.013) | 0.029 * (0.011) | 0.015 (0.011) | ‐0.036 *** (0.011) | ‐0.001 (0.010) | 0.003 (0.009) |
| Age at menopause | 0.002 (0.008) | ‐0.001 (0.008) | ‐0.000 (0.006) | 0.002 (0.006) | ‐0.001 (0.005) | 0.001 (0.005) | 0.005 (0.005) | 0.008 * (0.004) | 0.005 (0.004) |
| Menopause age (>45 years) | |||||||||
| ≤45 years | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| >45 years | ‐0.072 (0.082) | ‐0.036 (0.082) | ‐0.022 (0.076) | ‐0.064 (0.069) | ‐0.024 (0.065) | 0.015 (0.061) | 0.103 (0.053) | 0.073 (0.050) | 0.049 (0.043) |
| Years with menses | ‐0.010 (0.008) | ‐0.010 (0.008) | ‐0.007 (0.006) | ‐0.005 (0.005) | ‐0.006 (0.005) | ‐0.002 (0.005) | 0.007 (0.005) | 0.007 (0.004) | 0.005 (0.003) |
| 7‐year cognitive change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ B‐SEVLT Sum | Δ B‐SEVLT Recall | Δ WF | Δ DSS | |||||||||
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | |
| B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | |
| Female hormone use | ||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.144 (0.110) | 0.035 (0.099) | 0.080 (0.100) | 0.217 (0.166) | 0.134 (0.157) | 0.175 (0.161) | 0.075 (0.140) | 0.019 (0.138) | 0.017 (0.138) | 0.270 * (0.112) | 0.188 (0.105) | 0.163 (0.105) |
| Contraceptive use | ||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.167 * (0.072) | 0.084 (0.069) | 0.107 (0.065) | 0.054 (0.062) | ‐0.011 (0.062) | ‐0.009 (0.064) | 0.091 (0.068) | 0.057 (0.068) | 0.053 (0.064) | 0.090 (0.060) | 0.025 (0.060) | 0.049 (0.060) |
| Age at menarche | 0.021 (0.021) | 0.039 (0.020) | 0.040 * (0.019) | 0.021 (0.019) | 0.034 (0.019) | 0.032 (0.019) | ‐0.012 (0.016) | ‐0.000 (0.016) | 0.006 (0.017) | ‐0.001 (0.015) | 0.012 (0.015) | 0.009 (0.015) |
| Number of live births | ‐0.008 (0.017) | 0.029 (0.017) | 0.033 (0.017) | ‐0.011 (0.020) | 0.012 (0.020) | 0.008 (0.021) | ‐0.027 (0.017) | 0.005 (0.018) | 0.022 (0.017) | ‐0.042 * (0.016) | ‐0.022 (0.017) | ‐0.021 (0.018) |
| Number of pregnancies | 0.009 (0.015) | 0.028 (0.015) | 0.026 (0.014) | 0.003 (0.017) | 0.016 (0.017) | 0.013 (0.016) | ‐0.013 (0.012) | 0.004 (0.012) | 0.012 (0.012) | ‐0.017 (0.014) | ‐0.005 (0.014) | ‐0.003 (0.014) |
| Age at menopause | 0.000 (0.006) | 0.007 (0.006) | 0.004 (0.006) | 0.005 (0.006) | 0.011 (0.006) | 0.008 (0.006) | 0.009 (0.006) | 0.009 (0.006) | 0.007 (0.006) | 0.003 (0.006) | 0.009 (0.005) | 0.010 (0.005) |
| Menopause age (>45 years) | ||||||||||||
| ≤45 years | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| >45 years | 0.117 (0.080) | 0.133 (0.081) | 0.109 (0.077) | 0.095 (0.073) | 0.117 (0.075) | 0.100 (0.069) | 0.050 (0.073) | 0.015 (0.074) | ‐0.015 (0.072) | 0.069 (0.064) | 0.091 (0.063) | 0.098 (0.064) |
| Reproductive span | ‐0.001 (0.006) | 0.003 (0.006) | 0.001 (0.005) | 0.002 (0.006) | 0.007 (0.006) | 0.004 (0.005) | 0.009 (0.005) | 0.007 (0.006) | 0.004 (0.006) | 0.002 (0.005) | 0.007 (0.005) | 0.008 (0.005) |
| Δ Global cognition | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| B (SE) | B (SE) | B (SE) | |
| Female hormone use | |||
| No | Ref | Ref | Ref |
| Yes | 0.204 (0.158) | 0.110 (0.144) | 0.167 (0.141) |
| Contraceptive use | |||
| No | Ref | Ref | Ref |
| Yes | 0.073 (0.064) | ‐0.003 (0.062) | 0.027 (0.061) |
| Age at menarche | 0.012 (0.018) | 0.029 (0.017) | 0.036 * (0.017) |
| Number of live births | ‐0.028 (0.020) | 0.004 (0.019) | 0.014 (0.019) |
| Number of pregnancies | ‐0.002 (0.016) | 0.016 (0.015) | 0.017 (0.015) |
| Age at menopause | 0.004 (0.006) | 0.009 (0.005) | 0.007 (0.005) |
| Menopause age (>45 years) | |||
| ≤45 years | Ref | Ref | Ref |
| >45 years | 0.098 (0.071) | 0.109 (0.073) | 0.082 (0.069) |
| Reproductive span | 0.002 (0.005) | 0.005 (0.005) | 0.002 (0.005) |
Note: Sample size is unweighted; all other reported values are weighted to represent the target population.
Each reproductive history factor was entered separately into model with each cognitive outcome.
Model 1 was crude, Model 2 was adjusted for age and education, Model 3 was adjusted for Model 2 + language, Hispanic/Latino heritage, field center, income, marital status, insurance status, nativity, and body mass index.
In cognitive change models, time in years between cognitive function assessments were included in the calculation of the cognitive function outcome.
Abbreviations: B‐SEVLT, Brief‐Spanish English Verbal Learning Test; DSS, digit symbol substitution; M#, model; SE, standard error; WF, word fluency; Δ, change; B, beta.
p < .05.
p < .01.
p < .001.
TABLE 3.
Associations between each reproductive history factor with MCI (n = 2092) in Study of Latinos‐Investigation of Neurocognitive Aging.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| OR [95% CI] | OR [95% CI] | OR [95% CI] | |
| Female hormone use | |||
| No | Ref | Ref | Ref |
| Yes | 0.29 * [0.11;0.76] | 0.34 * [0.13;0.91] | 0.35 * [0.13;0.94] |
| Contraceptive use | |||
| No | Ref | Ref | Ref |
| Yes | 0.77 [0.52;1.12] | 0.85 [0.57;1.25] | 0.81 [0.53;1.23] |
| Age at menarche | 1.01 [0.91;1.11] | 0.98 [0.89;1.08] | 0.99 [0.90;1.09] |
| Number of live births | 1.08 [0.99;1.19] | 1.02 [0.92;1.12] | 1.00 [0.91;1.11] |
| Number of pregnancies | 1.04 [0.97;1.12] | 1.01 [0.94;1.08] | 0.99 [0.92;1.07] |
| Age at menopause | 1.00 [0.96;1.03] | 0.99 [0.96;1.03] | 1.00 [0.97;1.03] |
| Menopause age (>45 years) | |||
| ≤45 years | Ref | Ref | Ref |
| >45 years | 1.19 [0.81;1.75] | 1.24 [0.85;1.83] | 1.29 [0.87;1.91] |
| Reproductive span | 0.99 [0.96;1.03] | 0.99 [0.96;1.03] | 1.00 [0.96;1.03] |
Note: Sample size is unweighted; all other reported values are weighted to represent the target population.
Each reproductive history factor was entered separately into model. Model 1 was crude, Model 2 was adjusted for age and education, Model 3 was adjusted for Model 2 + language, Hispanic/Latino heritage, field center, income, marital status, insurance status, nativity, and body mass index.
Abbreviations: CI, confidence interval; M#, model; MCI, mild cognitive impairment; OR, odds ratio.
p < .05.
FIGURE 1.

Associations between reproductive history factors with cognitive performance (Z‐scored) at Visit 2 in Study of Latinos‐Investigation of Neurocognitive Aging. Model 1 is crude; Model 2 is adjusted for age and education; Model 3 is additionally adjusted for language preference, Hispanic/Latino heritage, field center, income, marital status, insurance status, place of birth, and body mass index. B‐SEVLT = Brief‐Spanish English Verbal Learning Test; DSS = Digit Symbol Substitution.
FIGURE 2.
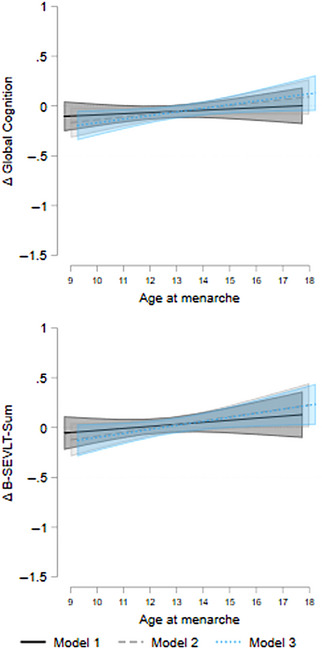
Associations between age at menarche with 7‐year cognitive change in Study of Latinos‐Investigation of Neurocognitive Aging. Model 1 is crude; Model 2 is adjusted for age and education; Model 3 is additionally adjusted for language preference, Hispanic/Latino heritage, field center, income, marital status, insurance status, place of birth, and body mass index. Δ = change; B‐SEVLT = Brief‐Spanish English Verbal Learning Test.
FIGURE 3.

Association between female hormone use at Visit 1 with prevalent MCI at Visit 2. Model 1 is crude; Model 2 is adjusted for age and education; Model 3 is additionally adjusted for language preference, Hispanic/Latino heritage, field center, income, marital status, insurance status, place of birth, and body mass index. MCI = mild cognitive impairment.
We conducted three separate sets of supplemental analysis. First, we estimated an additional model that expanded the primary analysis to include adjustments for cardiovascular risk as measured by Framingham Cardiovascular Risk score 24 and depressive symptoms measured using the Center for Epidemiologic Studies Depression Scale (CES‐D‐10). 25 The results yielded by these models are included in Tables S1 and S2. Second, we examined the associations between age at hysterectomy with our outcomes of interest for the subsample of women who had undergone hysterectomy before or after menopause (unweighted n = 612; Figure S2). We followed the same sequence of model adjustments as described previously. The estimated coefficients and their SEs for our continuous cognitive outcomes (Visit 2 performance and change) are presented in Table S3, and odds ratios and their 95% CIs for our categorical outcome (MCI) are presented in Table S4. In post hoc analyses, we calculated average marginal estimates and probabilities for significant associations and plotted these with their 95% CIs to facilitate interpretation (Figures S3 and S4). Third, given potential chronological age differences in the associations between our reproductive health exposures and cognitive outcomes, we tested for modification by age split by the mean (<60 vs 60+ years) by adding an interaction effect between age and the reproductive health exposures, independently, to fully adjusted models in the primary analysis (Model 3 as specified earlier). See Tables S5 and S6. Note, we did not have duration for female hormone use and so did not test age interactions with this exposure. When the interaction effects were consistently significant, we estimated stratified models whereby we refit Model 3 within each age group (<60 vs 60+ years) (Table S7 and Figures S5 and S6).
3. RESULTS
3.1. Descriptive statistics
The descriptive characteristics for the overall target female population, as described above as well as by the type of menopause (induced vs natural), are shown in Table 1. The average age at Visit 1 was 59.5 years, more than 40% had an education level of high school or above, and 90% conducted their interviews in Spanish. Nearly 20% had income of less than $10,000, 45% either were married or cohabited, roughly 60% had health insurance coverage, and 82% were born outside the United States. The average BMI was 30.3. There was no significant difference between induced and natural menopause groups in the distributions of these characteristics.
For the reproductive health measures, 4% reported current female hormone use at Visit 1. In the full sample, 55% indicated a history of oral contraceptive use with 62% of those under 60 years reporting use versus 48% of those 60 years and older. The average age at menarche was 12.7 years, and the number of pregnancies and live births were 3.8 and 2.9, respectively. The average age at menopause was 47.9 years, and 69% underwent menopause after 45 years of age. The average reproductive span was 35.2 years. Individuals with induced menopause were more likely to use female hormone and had lower numbers of pregnancies and live births. The induced menopause group had a lower average age at menopause, higher proportion of women with early menopause (at or before 45‐years old), and shorter reproductive span.
In the full sample, 10.8% met criteria for MCI. When split by age, 9.9% of those under 60 years and 11.8% of those 60 years and older met the criteria.
3.2. Primary analysis
The history of oral contraceptive use was associated with better cognitive performance across all outcomes in crude and age‐ and education‐adjusted models. In fully adjusted models, oral contraceptive use maintained associations with global cognitive performance (βGlobal = 0.124 [SE = 0.040], p < .01), learning (βB‐SEVLT‐Sum = 0.175 [SE = 0.059], p < .01), and processing speed (βDSS = 0.145 [SE = 0.049], p < .01) at Visit 2. In fully adjusted models, older age at menarche was associated with worse Visit 2 performance in processing speed (βDSS = −0.042 [SE = 0.012], p < .001 and βTrails A = 0.068 [SE = 0.020], p < .001). Higher number of pregnancies and live births were each associated with slower processing speed on the DSS (pregnancies: βDSS = −0.023 [SE = 0.011], p < .05 and live births: βDSS = −0.044 [SE = 0.014], p < .01) in fully adjusted models. Older age at menopause (continuous) was associated with better memory (βB‐SEVLT‐Recall = 0.013 [SE = 0.006], p < .05), whereas categorical age at menopause was not associated with cognitive outcomes at Visit 2. Reproductive span and female hormone use were not associated with Visit 2 performance on any cognitive outcomes.
In fully adjusted models, older age at menarche was protective against 7‐year average adverse change in global cognition (βGlobal = 0.036 [SE = 0.017], p < .05) and learning (βB‐SEVLT‐Sum = 0.040 [SE = 0.019], p < .05). No other reproductive health factor was linked to change in cognitive outcomes. Only female hormone use was associated with lower odds of MCI prevalence (OR = 0.35 [95% CI = 0.13;0.94], p < .05) (Tables 1, 2, 3).
3.3. Supplemental analysis
Additional adjustments for cardiovascular disease risk and depressive symptoms to the primary analysis did not have any quantitative or qualitative effects on the main results reported above (Tables S1 and S2).
Older age at hysterectomy was associated with better (average) 7‐year cognitive performance in executive functioning (βTrails B = −0.011 [SE = 0.005], p < .05). In terms of cognitive change, older age at hysterectomy was linked to adverse change in learning (βB‐SEVLT‐Sum = −0.011 [SE = 0.005], p < .05) and memory (βB‐SEVLT‐Recall = −0.012 [SE = 0.006], p < .05), yet it was associated with maintenance/improvement in verbal fluency (βWF = 0.015 [SE = 0.006], p < .01). Age at hysterectomy was not associated with MCI prevalence (Tables S3 and S4 and Figures S3 and S4).
Our tests of age interactions indicated that the associations between binary age at menopause (≤45, >45 years) and reproductive span were significant with respect to global cognitive function, learning, and delayed recall. Specifically, women over the age of 60 years with later menopause (>45 years) had better learning and memory performance compared to their counterparts who underwent menopause earlier in life, whereas age at menopause did not result in cognitive differences for women under 60 years of age. Similarly, later age of menopause was also protective against adverse change in learning and global cognition among older women but was unrelated to cognitive change in younger women. Additionally, longer reproductive span was associated with better memory only among women over 60 years of age (Tables S5 and S7 and Figures S5 and S6).
4. DISCUSSION
In a population‐based cohort of 2126 Hispanic/Latina postmenopausal women, aspects of reproductive health (ie, oral contraceptive use, number of pregnancies, number of live births, age at menarche, and age at menopause) were associated with cognitive performance and/or change in cognition over an average 7‐year period. Additionally, hormone use at Visit 1 was linked to lower prevalence of MCI, suggesting it may be a protective factor against cognitive impairment. Our results inform our understanding of cognitive aging among Hispanic/Latina women, a population with a wide range of educational backgrounds, immigration histories, ancestral backgrounds, and lower rates of access to insurance.
Generally, existing literature suggests that higher levels of estrogen exposure are protective to cognition, 26 and we found mixed evidence to support this across reproductive health measures. Reproductive health factors had relatively distinct associations with cognitive outcomes. For example, consistent with existing studies, history of oral contraceptive use was associated with better cognitive outcomes (ie, learning, processing speed/executive functioning [DSS], and global cognition). 27 , 28 , 29 , 30 Null findings have been reported elsewhere and may be due to small sample size, low prevalence of oral contraceptive use, and/or variability in the specific cognitive domains assessed. 6 , 31 , 32 , 33 Importantly, the prevalence of history of oral contraceptive in this Hispanic/Latina sample (57%) lags behind the US average (82%), 34 suggesting many more Hispanic/Latina women may stand to benefit from oral contraception, but thorough investigation is needed to confirm. Several factors impact oral contraception use in this population, including but not limited to medical mistrust (particularly in light of forced sterilizations of Hispanic/Latina women in the United States), 35 , 36 cultural and religious beliefs surrounding conception, 37 and limited access to health care. 9 , 10
Higher numbers of pregnancies and live births were each associated with slower processing speed. Fox and colleagues 38 found that the number of first trimesters rather than third trimesters in pregnancy was protective against AD. This might suggest that the improved immunoregulation that occurs early in pregnancy is driving the protective effect more than the increase in estrogen levels in later stages of pregnancy, which then decreases drastically following the birth with the delivery of the placenta. 39 , 40 , 41 Complicating this further, estrogen levels tend to stay lower in the postpartum period for individuals who breastfeed, 42 nulliparous women may have higher estrogen levels than parous individuals, 43 and those with four or more births may have particularly low estrogen levels after menopause. 44 Alternatively, births may capture the influence of parenting on cognition. 45 , 46 Birthing has been associated with declines in frontal and temporal volumes for up to 2 years among new mothers but not among fathers, 47 yet at older ages mothers and fathers seem to show reduced brain aging relative to non‐parents, 46 suggesting a long‐term benefit of parenting. Although, we did not detect associations between number of pregnancies or births with cognitive change or MCI, number of full‐term births has been connected to risk for cognitive impairment/AD. 7 However, the specific number of births that confer risk varies widely, with some investigations finding that zero births increases risk, 48 others stating that zero births decreases risk, 31 and still others finding that several births increases risk. 29 , 30 , 49 This variation may be indicative of cohort differences in the psychosocial changes that accompany pregnancy and raising children.
Older age at menarche was associated with cognitive advantages and disadvantages. Similar to a study of French women, 32 we found that older age at menarche was associated with slower processing speed on both the DSS and Trails A, yet it was associated with better 7‐year maintenance of learning and global cognition. Differences in outcomes may reflect age at menarche serving as an indicator of various early‐life exposures that are independently associated with cognitive outcomes. Certain early‐life exposures may lower menarche age (eg, high childhood body mass 50 and cardiovascular disease risk, 51 childhood sexual abuse 52 ), whereas others may raise menarche age (eg, early‐life nutritional deprivation, financial hardships). Adverse early life exposures may be more prevalent among the Hispanic/Latino community relative to the non‐Latino White population. 53
The associations between age at menarche with change in learning suggest that younger age at menarche could be a risk factor for AD. Later age at menarche was associated with lower levels of AD biomarkers (amyloid beta and tau) among postmenopausal Swedish women. 54 However, we did not detect associations between age at menarche with MCI, possibly due to our small proportion of participants with MCI. The existing literature primarily focuses on dementia status, and results range from null findings 6 , 49 , 55 , 56 to older age at menarche being associated with increased risk for dementia. 8 , 57 Of note, Prince and colleagues’ 49 population‐based study, which included over 7000 women in Latin America, did not find differential risk for dementia by age at menarche, whereas Gilsanz and colleagues 8 conducted a smaller study that included 274 US Hispanic/Latina women (<5% of the sample) and found that older age at menarche (16 to 17 years) was associated with increased risk for dementia. Our largely foreign‐born cohort may be more similar to individuals still living in Latin America, or discrepancies with Gilsanz et al. 8 may be related to differences in study design (ie, medical record review of individuals enrolled at Kaiser Permanente in Northern California vs multistate population‐based cohort), which may sample groups that differ in socioeconomic background on the whole.
The use of female hormones at Visit 1 was the only reproductive health factor associated with MCI, specifically lower prevalence. Notably, only 4% of the overall sample indicated female hormone use at Visit 1. There may be a critical period to capture the benefits of hormone therapy on cognition (eg, closer to menopause or before 65 years), but findings are not definitive. 26 , 58 , 59 , 60 While we found no associations between female hormone use with cognitive performance or cognitive change, female hormone use may nonetheless impact subjective cognitive decline – another component of our MCI measure. 23
Longer reproductive span was associated with better memory performance only among women over 60 years old, suggesting a potential delayed protection in older adulthood. Nonetheless, we did not find evidence of reproductive span‐related protection in 7‐year cognitive change or MCI for older women. In a recent study, Najar and colleagues 56 observed that longer reproductive period was associated with increased incidence of AD, especially among individuals 75 years and older, but not dementia with cerebrovascular disease. Given this distinction between dementia etiologies, relationships between reproductive span and cognitive status may be more difficult to detect in populations with higher risk for cerebrovascular disease pathology, such as Hispanic/Latino adults. 61 Importantly, in the present study, results remained significant when controlling for a variety of sociodemographic factors, depressive symptoms, and cardiovascular disease risk, suggesting robust influences of reproductive health factors on cognitive aging among Hispanic/Latina women.
Among Hispanic/Latina women who had undergone hysterectomy, age at hysterectomy had cognitive domain‐specific associations. The majority of existing studies link younger age of hysterectomy with poorer cognitive outcomes, 62 yet we observed better maintenance of learning and memory performance over time. Despite this, younger age at hysterectomy appeared to be disadvantageous to executive functioning, specifically worse cross‐sectional performance on Trails B and greater negative change in phonemic fluency over an average 7‐year period. Younger age at hysterectomy has been associated with increased presence of neuritic plaques 63 and increased risk for dementia, 62 but we did not detect associations with MCI. Women who undergo hysterectomies often have medical comorbidities that contribute to the decision to undergo the procedure. 64 Disentangling the role of these contributing factors on brain and cognitive health from that of the hysterectomy alone should be further examined among Hispanic/Latina women, particularly because of the difficulties accessing health care that are unique to this population. 9
Our findings should be interpreted within the context of limitations. First, we used retrospective, self‐report data to ascertain reproductive health information, though this has been shown to be reliable. 65 Second, regarding oral contraceptives, we did not examine other forms of contraception (eg, injectable contraceptives), specific hormonal components (exogenous estrogen and progestin may provide the most neuroprotection), 66 , 67 or duration of use. Some have found that use for ≤5 years is associated with lower risk of cognitive impairment, 30 whereas others have found better cognitive performance with longer use. 27 , 28 Third, we lacked information on onset of hormone use and previous use. Initiating hormone use more than 5 years after menopause may be associated with greater cognitive declines and higher tau deposition. 68 , 69 Fourth, we had a small number of women who reached menopause before 40 years of age (n = 81), indicating premature menopause. Future studies should examine differences in cognitive decline between premature menopause, early menopause (ages 40 to 45 years), and menopause after 45 years. Fifth, we do not have information on transgender/non‐binary experiences. Individuals who are transgender/non‐binary often have complicated reproductive health histories and report more subjective cognitive decline than cis‐gender individuals. 70 , 71 Finally, other reproductive factors (eg, age at first child), 72 potential confounds (eg, parenthood), 45 and sociocultural factors 73 may be critical in the context of reproductive health and cognitive aging.
5. CONCLUSION
Hispanic/Latina women's reproductive health throughout the life course was linked to various aspects of later life cognition, 7‐year average change in cognition, and MCI. Our findings underscore the need to investigate sex‐/gender‐specific factors in relation to cognitive aging among underserved populations.
CONFLICT OF INTEREST STATEMENT
Nothing to disclose. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants gave informed consent, and Institutional Review Board approval was obtained at all study sites. Research complied with the Helsinki Declaration and its later amendments.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank our study staff and participants for their contributions to advancing scientific knowledge. This work is supported by R01AG048642, R56AG048642 RF1AG054548, RF1AG061022, and R01 AG075758 (National Institute on Aging). Additional support includes K08AG075351, L30AG074401, and U54CA267789 to Dr. Stickel, R01AG062711 to Dr. Lamar; P30AG062429 to Dr. González; and R01AG066088 to Dr. Banks. The Hispanic Community Health Study/Study of Latinos is carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01HC65233), University of Miami (N01HC65234), Albert Einstein College of Medicine (N01HC65235), Northwestern University (N01HC65236), and San Diego State University (N01HC65237). The following institutes/centers/offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution‐Office of Dietary Supplements.
Stickel AM, Tarraf W, Kuwayama S, et al. Connections between reproductive health and cognitive aging among women enrolled in the HCHS/SOL and SOL‐INCA. Alzheimer's Dement. 2024;20:1944–1957. 10.1002/alz.13575
Hector M. Gonzalez and Sarah J. Banks are co‐senior authors.
Contributor Information
Ariana M. Stickel, Email: astickel@sdsu.edu.
Sarah J. Banks, Email: sbanks@health.ucsd.edu.
REFERENCES
- 1. Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid‐adult life. Alzheimers Dement. 2015;11(3):310‐320. doi: 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alzheimer's Association. 2021 Alzheimer's disease facts and figures . Alzheimers Dement. 2021;17(3):327‐406. [DOI] [PubMed] [Google Scholar]
- 3. Espeland MA, Brinton RD, Manson JE, et al. Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology. 2015;85(13):1131‐1138. doi: 10.1212/WNL.0000000000001816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klosinski LP, Yao J, Yin F, et al. White matter lipids as a ketogenic fuel supply in aging female brain: implications for Alzheimer's disease. EBioMedicine. 2015;2(12):1888‐1904. doi: 10.1016/j.ebiom.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snyder HM, Asthana S, Bain L, et al. Sex biology contributions to vulnerability to Alzheimer's disease: a think tank convened by the women's Alzheimer's research initiative. Alzheimers Dement. 2016;12(11):1186‐1196. doi: 10.1016/j.jalz.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer's risk in a cohort of British women. Psychoneuroendocrinology. 2013;38(12):2973‐2982. doi: 10.1016/j.psyneuen.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 7. Peterson A, Tom SE. A lifecourse perspective on female sex‐specific risk factors for later life cognition. Curr Neurol Neurosci Rep. 2021;21(9):46. doi: 10.1007/s11910-021-01133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilsanz P, Lee C, Corrada MM, Kawas CH, Quesenberry CP Jr, Whitmer RA. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology. 2019;92(17):e2005‐e2014. doi: 10.1212/WNL.0000000000007326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doty MM, Blumenthal D, Collins SR. The Affordable Care Act and health insurance for Latinos. JAMA. 2014;312(17):1735‐1736. doi: 10.1001/jama.2014.13841 [DOI] [PubMed] [Google Scholar]
- 10. Schneiderman N, Chirinos DA, Aviles‐Santa ML, Heiss G. Challenges in preventing heart disease in hispanics: early lessons learned from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog Cardiovasc Dis. 2014;57(3):253‐261. doi: 10.1016/j.pcad.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216‐224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the multiethnic cohort study. Am J Epidemiol. 2008;167(11):1287‐1294. doi: 10.1093/aje/kwn046 [DOI] [PubMed] [Google Scholar]
- 13. McDonald JA, Suellentrop K, Paulozzi LJ, Morrow B. Reproductive health of the rapidly growing Hispanic population: data from the pregnancy risk assessment monitoring system. Matern Child Health J. 2008;12(3):342‐356. doi: 10.1007/s10995-007-0244-x 2002 [DOI] [PubMed] [Google Scholar]
- 14. Brown HL, Chireau MV, Jallah Y, Howard D. The “Hispanic paradox”: an investigation of racial disparity in pregnancy outcomes at a tertiary care medical center. Am J Obstet Gynecol. 2007;197(2):197 e1‐197 doi: 10.1016/j.ajog.2007.04.036 [DOI] [PubMed] [Google Scholar]
- 15. Arias E, Xu JQ. United States life tables, 2017. National Vital Statistics Reports; 2019. [PubMed]
- 16. Acevedo‐Garcia D, Soobader MJ, Berkman LF. Low birthweight among US Hispanic/Latino subgroups: the effect of maternal foreign‐born status and education. Soc Sci Med. 2007;65(12):2503‐2516. doi: 10.1016/j.socscimed.2007.06.033 [DOI] [PubMed] [Google Scholar]
- 17. Tapales A, Douglas‐Hall A, Whitehead H. The sexual and reproductive health of foreign‐born women in the United States. Contraception. 2018;98(1):47‐51. doi: 10.1016/j.contraception.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González HM, Tarraf W, Fornage M, et al. A research framework for cognitive aging and Alzheimer's disease among diverse US Latinos: design and implementation of the Hispanic Community Health Study/Study of Latinos—Investigation of Neurocognitive Aging (SOL‐INCA). Alzheimer's & Dementia. 2019;15(12):1624‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LaVange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sorlie PD, Avilés‐Santa LM, Wassertheil‐Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629‐641. doi: 10.1016/j.annepidem.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramos AR, Tarraf W, Daviglus M, et al. Sleep duration and neurocognitive function in the Hispanic Community Health Study/Study of Latinos. Sleep. 2016;39(10):1843‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duff K. Evidence‐based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27(3):248‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. González HM, Tarraf W, Schneiderman N, et al. Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: study of Latinos‐investigation of Neurocognitive aging results. Alzheimer's & Dementia. 2019;15(12):1507‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743‐753. [DOI] [PubMed] [Google Scholar]
- 25. Wassertheil‐Smoller S, Arredondo EM, Cai J, et al. Depression, anxiety, antidepressant use, and cardiovascular disease among Hispanic men and women of different national backgrounds: results from the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2014;24(11):822‐830. doi: 10.1016/j.annepidem.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matyi JM, Rattinger GB, Schwartz S, Buhusi M, Tschanz JT. Lifetime estrogen exposure and cognition in late life: the Cache County Study. Menopause. 2019;26(12):1366‐1374. doi: 10.1097/GME.0000000000001405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egan KR, Gleason CE. Longer duration of hormonal contraceptive use predicts better cognitive outcomes later in life. J Womens Health (Larchmt). 2012;21(12):1259‐1266. doi: 10.1089/jwh.2012.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karim R, Dang H, Henderson VW, et al. Effect of reproductive history and exogenous hormone use on cognitive function in mid‐ and late life. J Am Geriatr Soc. 2016;64(12):2448‐2456. doi: 10.1111/jgs.14658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li FD, He F, Chen TR, et al. Reproductive history and risk of cognitive impairment in elderly women: a cross‐sectional study in eastern China. J Alzheimers Dis. 2016;49(1):139‐147. doi: 10.3233/JAD-150444 [DOI] [PubMed] [Google Scholar]
- 30. Song X, Wu J, Zhou Y, et al. Reproductive and hormonal factors and risk of cognitive impairment among Singapore Chinese women. Am J Obstet Gynecol. 2020;223(3):410 e1‐23 doi: 10.1016/j.ajog.2020.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci. 2003;15(2):161‐167. doi: 10.1176/jnp.15.2.161. Spring. [DOI] [PubMed] [Google Scholar]
- 32. Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML. Life‐time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34(2):287‐298. doi: 10.1016/j.psyneuen.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 33. Tierney MC, Ryan J, Ancelin ML, et al. Lifelong estrogen exposure and memory in older postmenopausal women. J Alzheimers Dis. 2013;34(3):601‐608. doi: 10.3233/JAD-122062 [DOI] [PubMed] [Google Scholar]
- 34. Mosher WD, Jones J. Use of contraception in the United States: 1982‐2008. Vital and Health statistics. Series 23, Data from the National Survey of Family Growth. 2010; 1‐44 [PubMed] [Google Scholar]
- 35. Gutiérrez E, Fuentes L. Population control by sterilization: the cases of Puerto Rican and Mexican‐origin women in the United States. Latino(a) Res Rev. 2009;7(3):85‐100 [Google Scholar]
- 36. Khan E. The forced sterilization of Spanish‐speaking Women in Los Angeles: a denial of the Latina immigrant identity from 1965 to 1978. History matters: An undergraduate journal of historical research. 2019;16:35‐43 [Google Scholar]
- 37. Gonzalez EU, Sable MR, Campbell JD, Dannerbeck A. The influence of patriarchal behavior on birth control access and use among recent Hispanic immigrants. J Immigr Minor Health. 2010;12(4):551‐558. doi: 10.1007/s10903-009-9272-5 [DOI] [PubMed] [Google Scholar]
- 38. Fox M, Berzuini C, Knapp LA, Glynn LM. Women's pregnancy life history and Alzheimer's risk: can immunoregulation explain the link? Am J Alzheimers Dis Other Demen. 2018;33(8):516‐526. doi: 10.1177/1533317518786447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nott PN, Franklin M, Armitage C, Gelder MG. Hormonal changes and mood in the puerperium. Br J Psychiatry. 1976;128:379‐383. doi: 10.1192/bjp.128.4.379 [DOI] [PubMed] [Google Scholar]
- 40. Hendrick V, Altshuler LL, Suri R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 1998;39(2):93‐101. doi: 10.1016/S0033-3182(98)71355-6 [DOI] [PubMed] [Google Scholar]
- 41. Deems NP, Leuner B. Pregnancy, postpartum and parity: resilience and vulnerability in brain health and disease. Front Neuroendocrinol. 2020;57:100820. doi: 10.1016/j.yfrne.2020.100820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeppsson S, Rannevik G, Thorell JI, Wide L. Influence of LH/FSH releasing hormone (LRH) on the basal secretion of gonadotrophins in relation to plasma levels of oestradiol, progesterone and prolactin during the post‐partum period in lactating and in non‐lactating women. Acta Endocrinol (Copenh). 1977;84(4):713‐728. doi: 10.1530/acta.0.0840713 [DOI] [PubMed] [Google Scholar]
- 43. Bernstein L, Pike MC, Ross RK, Judd HL, Brown JB, Henderson BE. Estrogen and sex hormone‐binding globulin levels in nulliparous and parous women. J Natl Cancer Inst. 1985;74(4):741‐745. doi: 10.1093/jnci/74.4.741 [DOI] [PubMed] [Google Scholar]
- 44. Chubak J, Tworoger SS, Yasui Y, Ulrich CM, Stanczyk FZ, McTiernan A. Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1296‐1301. doi: 10.1158/1055-9965.1296.13.8 [DOI] [PubMed] [Google Scholar]
- 45. Bordone V, Weber D. Number of children and cognitive abilities in later life. Vienna Year book of Popul Res. 2012;10:95‐126. http://www.jstor.org/stable/41940999 [Google Scholar]
- 46. Ning K, Zhao L, Franklin M, et al. Parity is associated with cognitive function and brain age in both females and males. Sci Rep. 2020;10(1):6100. doi: 10.1038/s41598-020-63014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoekzema E, Barba‐Muller E, Pozzobon C, et al. Pregnancy leads to long‐lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287‐296. doi: 10.1038/nn.4458 [DOI] [PubMed] [Google Scholar]
- 48. Bae JB, Lipnicki DM, Han JW, et al. Does parity matter in women's risk of dementia? A COSMIC collaboration cohort study. BMC Med. 2020;18(1):210. doi: 10.1186/s12916-020-01671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prince MJ, Acosta D, Guerra M, et al. Reproductive period, endogenous estrogen exposure and dementia incidence among women in Latin America and China; A 10/66 population‐based cohort study. PLoS One. 2018;13(2):e0192889. doi: 10.1371/journal.pone.0192889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Terry MB, Ferris JS, Tehranifar P, Wei Y, Flom JD. Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72‐79. doi: 10.1093/aje/kwp095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yermachenko A, Dvornyk V. Nongenetic determinants of age at menarche: a systematic review. Biomed Res Int. 2014;2014:371583. doi: 10.1155/2014/371583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boynton‐Jarrett R, Harville EW. A prospective study of childhood social hardships and age at menarche. Ann Epidemiol. 2012;22(10):731‐737. doi: 10.1016/j.annepidem.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suglia SF, Appleton AA, Bleil ME, et al. Timing, duration, and differential susceptibility to early life adversities and cardiovascular disease risk across the lifespan: implications for future research. Prev Med. 2021;153:106736. doi: 10.1016/j.ypmed.2021.106736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Najar J, Hallstrom T, Zettergren A, et al. Reproductive period and preclinical cerebrospinal fluid markers for Alzheimer disease: a 25‐year study. Menopause. 2021;28(10):1099‐1107. doi: 10.1097/GME.0000000000001816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Geerlings MI, Ruitenberg A, Witteman JC, et al. Reproductive period and risk of dementia in postmenopausal women. JAMA. 2001;285(11):1475‐1481. doi: 10.1001/jama.285.11.1475 [DOI] [PubMed] [Google Scholar]
- 56. Najar J, Ostling S, Waern M, et al. Reproductive period and dementia: a 44‐year longitudinal population study of Swedish women. Alzheimers Dement. 2020;16(8):1153‐1163. doi: 10.1002/alz.12118 [DOI] [PubMed] [Google Scholar]
- 57. Yoo JE, Shin DW, Han K, et al. Female reproductive factors and the risk of dementia: a nationwide cohort study. Eur J Neurol. 2020;27(8):1448‐1458. doi: 10.1111/ene.14315 [DOI] [PubMed] [Google Scholar]
- 58. LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta‐analysis. JAMA. 2001;285(11):1489‐1499. doi: 10.1001/jama.285.11.1489 [DOI] [PubMed] [Google Scholar]
- 59. Lobo RA. Hormone‐replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13(4):220‐231. doi: 10.1038/nrendo.2016.164 [DOI] [PubMed] [Google Scholar]
- 60. Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20(6):695‐709. doi: 10.1097/GME.0b013e3182960cf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Filshtein TJ, Dugger BN, Jin LW, et al. Neuropathological diagnoses of demented Hispanic, black, and non‐Hispanic white decedents seen at an Alzheimer's Disease Center. J Alzheimers Dis. 2019;68(1):145‐158. doi: 10.3233/Jad-180992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Georgakis MK, Beskou‐Kontou T, Theodoridis I, Skalkidou A, Petridou ET. Surgical menopause in association with cognitive function and risk of dementia: a systematic review and meta‐analysis. Psychoneuroendocrinology. 2019;106:9‐19. doi: 10.1016/j.psyneuen.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 63. Bove R, Secor E, Chibnik LB, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82(3):222‐229. doi: 10.1212/WNL.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brett KM, Higgins JA. Hysterectomy prevalence by Hispanic ethnicity: evidence from a national survey. Am J Public Health. 2003;93(2):307‐312. doi: 10.2105/ajph.93.2.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lucas R, Azevedo A, Barros H. Self‐reported data on reproductive variables were reliable among postmenopausal women. J Clin Epidemiol. 2008;61(9):945‐950. doi: 10.1016/j.jclinepi.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 66. Kaur P, Jodhka PK, Underwood WA, et al. Progesterone increases brain‐derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen‐activated protein kinase‐ and phosphoinositide‐3 kinase‐dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85(11):2441‐2449. doi: 10.1002/jnr.21370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17(5):367‐388. doi: 10.1089/neu.2000.17.367 [DOI] [PubMed] [Google Scholar]
- 68. Lee JK, Frank RD, Christenson LR, Fields JA, Rocca WA, Mielke MM. Associations of reproductive factors and exogenous estrogens with global and domain‐specific cognition in later life. Alzheimers Dement. 2023. doi: 10.1002/alz.13394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coughlan GT, Betthauser TJ, Boyle R, et al. Association of age at menopause and hormone therapy use with tau and beta‐amyloid positron emission tomography. JAMA Neurol. 2023;80(5):462‐473. doi: 10.1001/jamaneurol.2023.0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cicero E, Flatt JD, Wharton W. Transgender adults report greater cognitive and related functional challenges: findings from the 2015‐2019 behavioral risk factor surveillance system. Alzheimer's & Dementia. 2021;17(S10). doi: 10.1002/alz.053902 [DOI] [Google Scholar]
- 71. Lambrou NH, Norton DL, Gleason CE, Flatt JD. Prevalence of modifiable risk factors for Alzheimer's disease and related dementias, and association with cognitive disability among transgender and gender non‐binary adults in the U.S.: BRFSS 2019. Alzheimer's & Dementia. 2021;17(S10). doi: 10.1002/alz.055822 [DOI] [Google Scholar]
- 72. Read SL, Grundy EMD. Fertility history and cognition in later life. J Gerontol B Psychol Sci Soc Sci. 2017;72(6):1021‐1031. doi: 10.1093/geronb/gbw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ruiz JM, Hamann HA, Mehl MR, O'Connor MF. The Hispanic health paradox: from epidemiological phenomenon to contribution opportunities for psychological science. Group Process Intergr Relat. 2016;19(4):462‐476. doi: 10.1177/1368430216638540 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


