Abstract
Keeping the global food supply safe necessitates international collaborations between countries. Health and regulatory agencies routinely communicate during foodborne illness outbreaks, allowing partners to share investigational evidence. A 2016–2020 outbreak of Listeria monocytogenes infections linked to imported enoki mushrooms required a multinational collaborative investigation among the United States, Canada, Australia, and France. Ultimately, this outbreak included 48 ill people, 36 in the United States and 12 in Canada, and was linked to enoki mushrooms sourced from one manufacturer located in the Republic of Korea. Epidemiologic, laboratory, and traceback evidence led to multiple regulatory actions, including extensive voluntary recalls by three firms in the United States and one firm in Canada. In the United States and Canada, the Korean manufacturer was placed on import alert while other international partners provided information about their respective investigations and advised the public not to eat the recalled enoki mushrooms. The breadth of the geographic distribution of this outbreak emphasizes the global reach of the food industry. This investigation provides a powerful example of the impact of national and international coordination of efforts to respond to foodborne illness outbreaks and protect consumers. It also demonstrates the importance of fast international data sharing and collaboration in identifying and stopping foodborne outbreaks in the global community. Additionally, it is a meaningful example of the importance of food sampling, testing, and integration of sequencing results into surveillance databases.
Keywords: Enoki mushrooms, Foodborne illness outbreaks, Listeria monocytogenes
Listeria monocytogenes is a foodborne bacterial pathogen that can cause invasive listeriosis, a severe infection that can lead to meningitis, sepsis, encephalitis, or spontaneous abortions and stillbirths. People at higher risk for invasive listeriosis include individuals aged 65 years or older, pregnant people and their newborns, and immunocompromised individuals (Charlier et al., 2017) (Schlech, 2000)). People with invasive listeriosis usually report symptoms starting 1–4 weeks after eating food contaminated with L. monocytogenes; however, some people have reported symptoms starting as late as 70 days after exposure or as early as the same day of exposure (Goulet et al., 2013; U.S. Centers for Disease Control and Prevention, 2022b). The World Health Organization (WHO) estimated that L. monocytogenes caused 23,150 infections and 5,436 deaths worldwide in 2010 (de Noordhout et al., 2014).
Flammulina velutipes, commonly known as enoki or winter mushrooms, are long thin white mushrooms, usually sold in clusters. They are especially popular in Asian cuisines and are also known as enokitake, golden needle, futu, or lily mushrooms (U.S. Food and Drug Administration, 2020e). L. monocytogenes has been isolated from various types of edible mushrooms in both North America and China (Chen et al., 2018), and enoki mushrooms were recalled in Canada in 2019 due to the possible presence of L. monocytogenes, prior to this investigation (Government of Canada, 2019). At the time of this investigation, no known listeriosis outbreaks had been previously linked to the consumption of mushrooms in the United States, Australia, Canada, or France. According to Gould et al. (2017), the number of reported outbreaks in the United States associated with imported foods increased between 1973 and 2014.
The global food supply necessitates international collaborations. Health and regulatory agencies communicate through various channels that allow partners to share genomic and epidemiologic data. The Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC), along with Canadian partners at the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA), routinely communicate about outbreak investigations and contaminated food products in their respective countries (Harvey et al., 2017).
Preliminary findings from this investigation have been previously reported elsewhere (U.S. Food and Drug Administration, 2020e). To the best of our knowledge, this is the first outbreak of listeriosis in the United States, Canada, and Australia, linked to enoki mushrooms. Concurrently to the investigation in the United States, Canada, and France, health officials in Australia investigated six listeriosis illnesses with clinical isolates related to the outbreak strain and illness onset dates between 2017 and 2020 (Food Standards Australia and New Zealand, 2020b). Sample analysis identified Listeria monocytogenes in enoki mushrooms imported from the Republic of Korea (Food Standards Australia and New Zealand, 2020b). As a result, one firm in Australia instituted a recall of enoki mushrooms in 2020 and Australian health officials issued public health communications (Food Standards Australia and New Zealand, 2020a, 2022). The remainder of this paper will focus on investigations in the United States, Canada, and France.
Materials and Methods
Epidemiologic Investigation.
U.S. and Canada.
The Listeria Initiative (LI) is a national surveillance system managed by CDC that collects comprehensive epidemiologic data of ill people with invasive listeriosis and combines that information with laboratory data in PulseNet USA, a national laboratory network that tracks foodborne infections and identifies clusters and outbreaks (U.S. Centers for Disease Control and Prevention, 2016). The standard LI questionnaire is used by state and local health departments to attempt to interview all ill people or their surrogate about a variety of foods, consumed in the 28 days before becoming ill, as well as purchase locations (U.S. Centers for Disease Control and Prevention, 2022c). The questionnaire also collects information on patient demographics including race and ethnicity, clinical signs and symptoms, and illness outcomes. When needed, ill people can be reinterviewed by state or local officials to obtain additional exposure information. Minimal information is collected on the LI questionnaire that would help investigators determine if a suspected food is imported (U.S. Centers for Disease Control and Prevention, 2018). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy*. Similar to the LI, the Enhanced National Listeriosis Surveillance Program (ENLSP), managed by PHAC, collects standardized epidemiologic data of ill people with invasive listeriosis in Canada. Twelve of the 13 provinces and territories in Canada currently participate in the program; the remaining province contributes exposure information during national outbreak investigations. A standardized listeriosis questionnaire is used to collect information on ill persons’ symptom onset dates, clinical signs and symptoms, risk factors, travel histories, demographics, as well as various food exposures in the 28 days prior to illness onset. Information collected through this program is analyzed, in combination with data from PulseNet Canada, to detect and investigate listeriosis outbreaks in Canada.
Epidemiologists at CDC and PHAC routinely exchange information on active outbreak investigations in the United States and Canada. Information shared includes pathogens and serotypes, case counts, illness date ranges, food histories, geographic distribution of cases, and suspected outbreak vehicles. When outbreaks with the potential to cross national borders are identified, partners in Canada and the United States facilitate the exchange of genomic data to identify genetically related clinical isolates and/or nonhuman isolates in each of their respective countries.
Laboratory Investigation.
Genomic data for some clinical, food, and environmental isolates of L. monocytogenes can be uploaded, either on a routine or case-by-case basis, to the public DNA sequence databases at U.S. National Center for Biotechnology Information (NCBI) to facilitate comparisons of isolates. NCBI also mirrors the data uploaded to European Molecular Biology Laboratory European Bioinformatics Institute (EMBL-EBI) and DNA Data Bank of Japan (DDJB) (Benson et al., 2015). CDC also works with PulseNet International to monitor isolates submitted from participating countries and frequently communicates with partners regarding genetic matches to active investigations (Nadon et al., 2017). The Epidemic Intelligence Information System (EPIS), which is part of the European Centre for Disease Prevention and Control (ECDC), includes 52 countries and allows non‐European partners to share information about their clusters within the European system to determine if they may be linked. Finally, International Food Safety Authorities Network (INFOSAN), coordinated by the WHO, facilitates the rapid exchange of information between countries during various food safety events (World Health Organization, 2020).
Cluster Detection.
U.S. L. monocytogenes clinical isolates are submitted for whole-genome sequencing (WGS) and are uploaded to PulseNet USA and NCBI databases. Clusters of illnesses in the United States are identified by PulseNet using a threshold of three or more L. monocytogenes clinical isolates genetically related by ≤25 allele differences, using whole-genome multilocus sequence typing (wgMLST), within a 120-day period (Tolar et al., 2019).
Canada.
Sequencing of clinical and nonclinical isolates is conducted by either the National Microbiology Laboratory (NML) or provincial laboratories. Nonclinical isolates identified through routine sampling or during food safety investigations are also sequenced by the CFIA laboratories. All sequences, both clinical and nonclinical, are uploaded to the PulseNet Canada database. Although Canadian isolates are not yet all uploaded to NCBI in real-time, they may be uploaded by the NML or CFIA laboratory at any time to facilitate international comparisons. Additionally, Canada has been sharing molecular and genomic data routinely with the United States since 2005 under a bilateral agreement. Cluster detection criterion for L. monocytogenes in Canada is two or more isolates genetically related by ≤10 allele differences by wgMLST within 120 days; allele ranges may expand during an investigation based on available laboratory, epidemiologic, and traceback evidence.
Food samples.
U.S. During outbreak investigations, state and local public health agencies will conduct product sampling at retail locations if the epidemiology points to a suspect vehicle or commodity. The Michigan Department of Agriculture and Rural Development (MDARD) and the California Department of Public Health (CDPH) collected and analyzed retail samples of wood ear, maitake, royal oyster, and/or enoki mushrooms in state agricultural and public health laboratories using AOAC Official Method 999.06 and FDA Bacteriological Analytical Manual (BAM): Chapter 10 (Hitchins et al., 2022).
FDA samples of imported products were collected per standard practice (Pereira et al., 2021) and analyzed for L. monocytogenes using the BAM Chapter 10 (Hitchins et al., 2022) in FDA′s Pacific Southwest Food and Feed Laboratory or Northeast Food and Feed Laboratory. Enumeration, as described in the BAM Chapter 10, was performed for a limited number of L. monocytogenes-positive samples (Hitchins et al., 2022).
Canada.
CFIA collects samples of products to determine compliance with Canadian regulations. As part of its food safety investigation, CFIA collected samples of imported enoki mushrooms at various levels of the distribution chain as identified in the traceback investigation. All samples were analyzed in ISO/IEC 17025 accredited CFIA laboratories. Samples consisting of one to ten units of product were analyzed for L. monocytogenes using methods published in Health Canada’s Compendium of Analytical Methods for Microbiological Analysis of Foods (Health Canada, 2021), namely methods MFLP-28 (presence/absence) and MFHPB-30 (cultural confirmation). Enumeration was performed for L. monocytogenes-positive samples from each of up to five of the product units tested individually or in composite using method MFLP-74 (Pagotto et al., 2011).
France.
Food sampling of imported products is conducted by official laboratories of the General Directorate for Competition Policy, Consumer Affairs and Fraud Control (DGCCRF), to identify foods that do not comply with French regulatory standards and European regulations EC 178/2002 (European Commission, 2002, 2005) and EC 2073/2005. One 25 g sample of enoki mushrooms was tested for L. monocytogenes using the ALOA® count method (AES 10/05–09/06, bioMérieux) to ensure compliance with the microbiological criteria of 100 CFU/g of L. monocytogenes applicable for food placed on the market during their shelf-life (International Standards Organization, 2016).
Traceback Investigations.
U.S. and Canada.
The traceback investigation focused on determining the source of product samples that yielded the outbreak strain of L. monocytogenes. Information for the enoki mushroom isolates that yielded L. monocytogenes were analyzed, including bills of lading, import entry documentation, and other relevant product traceability documents from retailers, distributors, and importers along the supply chain. U.S. and Canada investigators used similar investigation methods as described by Irvin et al. (2021).
France.
Traceback information was retrospectively retrieved from the Rapid Alert System for Food and Feed (RASFF) 2017.2213, which is publicly available (European Commission, 2014). Information from enoki mushroom samples yielding L. monocytogenes within one allele difference from the outbreak strain (type cgMLST: L2-SL7-ST7-CT4156 according to Institut Pasteur cgMLST scheme) (European Commission, 2005; European Commission, 2014) were reviewed, including supplier information, receiving dates, dates of product distribution in France, control measures taken, and reported level of contamination of the imported samples.
Results
Epidemiologic Investigation.
U.S.
On October 20, 2017, PulseNet USA detected a cluster of three clinical L. monocytogenes isolates, two from California and one from Hawaii, with isolation dates from September 13, 2017, through September 27, 2017. The isolates were related within 0–1 allele differences by wgMLST, and a multistate investigation was initiated. All three people reported Asian race on the LI questionnaire. No additional illnesses were detected, and the investigation was closed on December 6, 2017, without a suspected vehicle. In early February 2018, three additional illnesses were identified by PulseNet USA, and the investigation was reopened with a total of six illnesses. Four of the six ill people had food exposure histories; nuts and nut butters were the only exposure reported by ill people significantly more often than expected, based on case-case comparison to sporadic listeriosis illnesses from the same states as outbreak cases (U.S. Centers for Disease Control and Prevention, 2022a). A leftover nut butter product was collected from an ill person’s home and tested but did not yield L. monocytogenes. By March 2018, investigators attempted open-ended interviews with available ill people or their surrogates. Five of six ill people reported Asian race, but information on Asian food exposures was limited on the LI questionnaire. At that time, pears were reported by 3/5 (60%) ill people. To collect more information on pear exposures and Asian foods, an Asian-style food supplemental questionnaire was deployed to the states for reinterview of ill people. In May 2018, the investigation was expanded to include another cluster of five illnesses investigated in 2017 and three additional illnesses for a total of 14 cases with isolation dates ranging from November 23, 2016 to April 4, 2018. All isolates were related within 0–14 allele differences (median 5) by wgMLST. Race information was available for 13 ill people and nine (69%) reported Asian race originating from multiple countries. Seven interviews were completed with the Asian-style food supplemental questionnaire, but a suspect vehicle was not identified. The investigation was closed on December 17, 2018, with a total of 26 cases from 13 states.
In February 2020, CFIA uploaded sequence information to NCBI’s pathogen detection database for a historical L. monocytogenes isolate recovered from a Canadian domestic Portobello mushroom sample tested in 2016. CFIA subsequently uploaded a more recent isolate obtained from an imported sample of enoki mushroom tested in 2019; this enoki mushroom sample triggered a recall of enoki mushrooms from a single importer in Canada during that same year (Government of Canada, 2019). Shortly after these isolates were uploaded to NCBI, CDC conducted a wgMLST comparison and determined that the two mushroom isolates were genetically related to the unsolved outbreak of listeriosis in the United States. After being notified of this genetic linkage by CDC, PHAC reviewed their PulseNet Canada database for additional matches and identified six historical clinical isolates and 10 historical food isolates dating from October 2017 to March 2019 that were related to the U.S. cluster within 0–15 allele differences by wgMLST.
A multinational outbreak investigation was initiated in the United States and Canada. At the time, the U.S. outbreak included 36 ill people from 17 states with isolation dates ranging from November 23, 2016, to December 13, 2019. The isolates from ill people were related within 0–38 allele differences by wgMLST. Ages ranged from 0 to 96 years with a median of 67 years, and 21 (58%) ill people were female. Race information was available for 28 ill people and 17 (61%) reported Asian race. Six illnesses were related to pregnancy, and two pregnancies resulted in fetal loss. Hospitalization information was available for 33 ill people; 31 (94%) were hospitalized, and there were four reported deaths (Table 1). A review of available food histories in the United States found that 12 of 22 (55%) ill people with available information reported fresh mushrooms of various types. One person from Michigan, who was ill in 2018, reported consuming various mushrooms including enoki mushrooms purchased at a local Asian market (Point of Sale (POS)-A). Receipts for that purchase were available.
Table 1.
Summary of outbreak-associated illnesses — United States and Canada. (October 4, 2016–December 13, 2019)
| Demographics and Outcomes | United States n (%) | Canada n (%) | Total n (%) |
|---|---|---|---|
| Cases | 36 | 12 | 48 |
| States/Provinces | 17 | 3 | |
| Isolation Dates | 11/23/2016 – 12/13/2019 | 08/04/2016 – 02/19/2019 | 8/04/2016–12/13/2019 |
| Age range (median) | 0–96 (67) | 22–96 (75) | 0–96 |
| Female | 21/36 (58) | 7/12 (58) | 28/48 (58) |
| Asian race | 17/28 (61) | Race data not collected | 17/28 (61) |
| Hospitalized | 31/33 (94) | 6/8 (75) | 37/41 (90) |
| Deaths | 4 | 0 | 4 |
| Pregnancy-associated* | 6 (29) | 1 (14) | 7 (25) |
| Allele range | 8[0–38] | N/A | 8[0–38] |
Two pregnancies resulted in fetal losses.
Canada.
Initially, isolates from six ill people from three Canadian provinces were genetically related to the U.S. illnesses within 0–15 alleles differences by wgMLST. Isolation dates for the ill people were older, ranging from October 5, 2017, to February 19, 2019. Ages ranged from 27 to 96 years with a median of 85 years, and five (83%) ill people were female. Five of six questionnaires were available through the ENLSP. Of these five ill people, three were hospitalized and no deaths were reported. One illness was pregnancy-associated and resulted in one fetal loss. Following the review of available information, no significant commonalities were identified; however, a variety of Asian-style foods were reported. None of the Canadian ill people reported consuming enoki mushrooms upon initial interview; however, the standardized listeriosis questionnaire only includes one question regarding any mushroom consumption and does not ask about enoki mushrooms specifically. The ten additional food isolates related to the U.S. outbreak were detected through provincial sampling at a single sushi restaurant in Canada. Four isolates from sushi samples were detected in June/July 2018, and six isolates from maki (a small segment from a roll of rice and other ingredients wrapped in seaweed) samples were detected in March 2019. Further follow-up at this restaurant in 2020 determined that enoki mushrooms were used as an ingredient/garnish in some of its menu items and cross-contamination could not be ruled out.
Investigators expanded the allele range to 0–48 allele differences by wgMLST because of the results of the U.S. investigation and PHAC completed another check for related isolates. PHAC identified seven additional historical isolates, six clinical isolates from three provinces with isolation dates ranging from August 2016 to January 2019, and one food isolate from ready-to-eat (RTE) salmon dating back to October 2017. The ages of the six additional ill people ranged from 22 to 84 years with a median of 74 years, and two (33%) were female. Questionnaires for three ill people were available through the ENLSP. All three ill people were hospitalized; no pregnancy-associated illnesses or deaths were reported. One ill person specifically reported consuming enoki mushrooms during their exposure period; however, there was no information available on the brand or purchase location of the mushrooms. Due to the older isolation dates of the Canadian ill people, reinterviews by PHAC were not feasible.
This multinational outbreak included 48 ill people (36 in the United States, 12 in Canada) linked to enoki mushrooms sourced from the Republic of Korea. Isolation dates ranged from August 4, 2016, through December 13, 2019 (Fig. 1). Ages ranged from 0 to 96 years, and 28 (58%) of ill people were female. Of 41 ill people with information, 37 (90%) were hospitalized and there were four reported deaths. Seven illnesses were associated with pregnancy and two resulted in fetal loss (Table 1).
Figure 1.
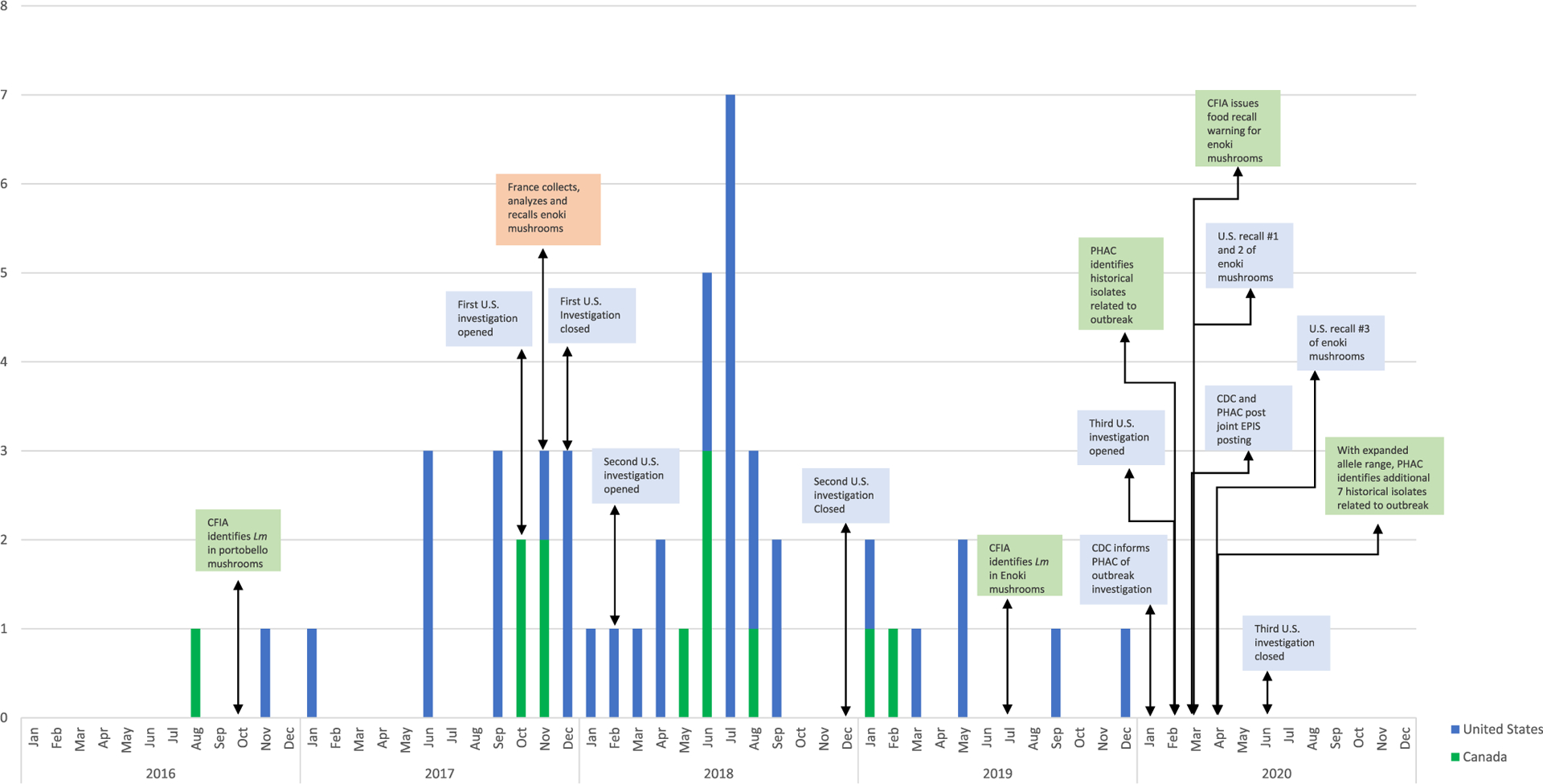
Investigation timeline of multinational outbreak of L. monocytogenes—United States, Canada, and France, 2016–2020.
France.
On March 5, 2020, CDC and PHAC posted a joint EPIS posting with epidemiologic information, genomic data, and food isolates in Canada. On March 6, 2020, France responded with five related enoki mushroom isolates matching the outbreak strain within one allele difference by cgMLST. These five isolates received at the National Reference Center for Listeria (NRCL) on November 28, 2017 had been integrated in the weekly French genomic surveillance of Listeria by the NRCL on December 11, 2017 without any related clinical or nonclinical French isolates since the beginning of genomic surveillance of Listeria in 2015 (Moura et al., 2017). No illnesses linked to the outbreak were reported in France.
Republic of Korea.
On March 12, 2020, PulseNet International sent out an inquiry to worldwide regional coordinators for clinical and nonclinical matches to the outbreak. The Republic of Korea PulseNet Coordinator responded to the inquiry with no related matches. The Korean Ministry of Food and Drug Safety compared the U.S. isolates to their databases and had no related matches by cgMLST.
Laboratory Investigation.
U.S. food samples.
MDARD collected 13 samples of multiple mushroom varieties after wgMLST linked the historical Canadian mushroom isolates and the clinical isolate from a 2018 Michigan ill person who consumed enoki mushrooms. Samples included wood ear, maitake, royal oyster, and enoki mushrooms samples from POS-A where the 2018 Michigan ill person reported purchasing their enoki mushrooms. Two enoki mushroom samples taken at retail were positive for L. monocytogenes, with counts by FDA of 8.0 × 105 CFU/g and 9.0 × 103 CFU/g, respectively. Based on the findings of the MDARD sampling, CDPH collected a total of 27 enoki mushroom samples from two grocery store locations (POS-B, POS-E). One sample collected from POS-B yielded L. monocytogenes.
On March 11, 2020, FDA began increased sampling of enoki mushrooms imported into the United States from three foreign firms located in the Republic of Korea identified in the product traceback: Foreign Manufacturer A, Broker A, and Foreign Manufacturer B. FDA collected samples at import or at domestic import of enoki, king oyster, or beech mushrooms. FDA collected 16 mushroom samples; 15 samples were collected as part of increased screening and included enoki (10), beech (1) and king oyster (4) mushrooms, and one domestic import sample of enoki mushrooms was collected during the Foreign Supplier Verification Program (FSVP) inspection at Importer A. Five samples of enoki mushrooms yielded L. monocytogenes. Other Listeria species were also found among the samples: L. innocua (eight samples), L. grayi (one sample) and L. welshimeri (one sample). Enumeration was performed on the domestic import and one increased surveillance sample. Both samples yielded L. monocytogenes populations of >1.1 × 103 CFU/g.
In total, 56 enoki mushroom samples were collected by FDA and state partners in response to the outbreak investigation. Eight of the 56 samples yielded 15 L. monocytogenes isolates which were related to the clinical isolates within 0–53 alleles by wgMLST, and the enoki samples were products of Foreign Manufacturer B, a manufacturer in the Republic of Korea. L. monocytogenes was not identified in any of the other mushroom varieties analyzed.
Canadian food samples.
The enoki mushroom isolate from the 2019 sample collected at a Canadian port of entry was related to the clinical isolates in the outbreak within 4–26 allele differences by wgMLST. Additionally, L. monocytogenes was identified from two samples of enoki mushrooms collected by CFIA from a retail location (POS-C) in 2020, and the isolates were related to the U.S. and Canadian clinical isolates within 2–33 allele differences by wgMLST. Enumeration results indicated levels of L. monocytogenes up to 3.0 × 103 CFU/g and >6.0 × 103 CFU/g for these two samples. In response to the positive retail samples, CFIA collected seven enoki mushroom samples from Importer D, the distributor to POS-C. Three of the samples yielded L. monocytogenes, and these isolates were related to the outbreak strain by wgMLST. For these samples, counts of L. monocytogenes up to 1.1 × 102, 2.9 × 102, and <10 CFU/g respectively were identified. Based on the packaging of the Canadian products and additional traceback conducted by CFIA, the three samples were determined to be products of Foreign Manufacturer B.
French food samples.
In November 2017, one official laboratory of the DGCCRF enumerated by ALOA® count method (bioMérieux) an enoki mushroom sample collected from Distributor D on November 6, 2017. The sample was contaminated with L. monocytogenes at a level of 9.0 × 102 CFU/g. This enoki sample yielded five L. monocytogenes isolates that were related to the clinical isolates within one allele by cgMLST (Table 2) (Moura et al., 2017). This sample of enoki mushrooms was collected as part of official control sampling at a food distributor. French DGCCRF’s investigation determined that the enoki mushrooms were imported from the Republic of Korea via the Netherlands (Exporter A) and manufactured by Foreign Manufacturer B.
Table 2.
Sample and enumeration results of L. monocytogenes enoki mushroom samples collected by United States, Canada, and France from retail, distributors or import and sourced from Foreign Manufacturer B
| Sample # | Collection Country | Collection Agency | Collection Year | Collection Site | WGS ID | Enumeration (CFU/g) |
|---|---|---|---|---|---|---|
| 1 | USA | MDARD* | 2020 | POS-A | PNUSAL007024 | 9.0 × 103 |
| PNUSAL007025 | ||||||
| PNUSAL007026 | ||||||
| PNUSAL007027 | ||||||
| PNUSAL007028 | ||||||
| 2 | USA | MDARD* | 2020 | POS-A | PNUSAL007029 | 8.0 × 105 |
| PNUSAL007030 | ||||||
| PNUSAL007031 | ||||||
| 3 | USA | CDPH^ | 2020 | POS-B | PNUSAL007188 | – |
| PNUSAL007189 | ||||||
| 4 | USA | FDA~ | 2020 | Import | FDA1142048-001 | >1.1 × 103 |
| FDA1142048-002 | ||||||
| 5 | USA | FDA~ | 2020 | Domestic Import | FDA942342-001 | >1.1 × 103 |
| FDA942342-002 | ||||||
| 6 | USA | FDA~ | 2020 | Import | FDA1142655- C001 | – |
| FDA1142655- C002 | ||||||
| 7 | USA | FDA~ | 2020 | Import | FDA1142657-C001 | – |
| FDA1142657-C002 | ||||||
| 8 | USA | FDA~ | 2020 | Import | FDA1142659-C001 | – |
| FDA1142659-C002 | ||||||
| 9 | Canada | CFIA# | 2019 | Import | CFIAFB20190030 | – |
| 10 | Canada | CFIA# | 2020 | POS-C | CFIAFB20200081 | 1: <10 |
| 2: >6.0 × 103 | ||||||
| 3: <10 | ||||||
| 4: 1.6 × 102 | ||||||
| 5: >6.0 × 103 | ||||||
| 11 | Canada | CFIA# | 2020 | POS-C | CFIAFB20200080 | 1: 4.1 × 10 |
| 2: <10 | ||||||
| 3: <10 | ||||||
| 4: 3.0 × 103 | ||||||
| 5: 1.8 × 102 | ||||||
| 12 | Canada | CFIA# | 2020 | Importer D | CFIAFB20200090 | 1: <10 |
| 2: <10 | ||||||
| 3: <10 | ||||||
| 4: <10 | ||||||
| 5: <10 | ||||||
| 13 | Canada | CFIA# | 2020 | Importer D | CFIAFB20200089 | 1: <10 |
| 2: <10 | ||||||
| 3: <10 | ||||||
| 4: <10 | ||||||
| 5: <10 | ||||||
| 14 | Canada | CFIA# | 2020 | Importer D | CFIAFB20200088 | 1: <10 |
| 2: <10 | ||||||
| 3: <10 | ||||||
| 4: 2.9 × 102 | ||||||
| 5: <10 | ||||||
| 15 | France | DGCCRF+ | 2017 | Distributor D | ERR6355681 | 9.0 × 102 |
| ERR6355682 | ||||||
| ERR6355683 | ||||||
| ERR6355684 | ||||||
| ERR6355685 |
Michigan Department of Agriculture and Rural Development.
California Department of Public Health.
Food & Drug Administration.
Canadian Food Inspection Agency.
General Directorate for Competition Policy, Consumer Affairs and Fraud Control.
Whole-Genome Sequencing.
Using a reference-based variant detection method, sixty-seven isolates associated with this multinational outbreak were found to be within 23 SNPs of one another and likely from the same source based on analysis of WGS data produced with the CFSAN SNP Pipeline and analyzed by FDA (Fig. 2) (Davis et al., 2015). The allele range of these isolates was 0–53 by wgMLST.
Figure 2.
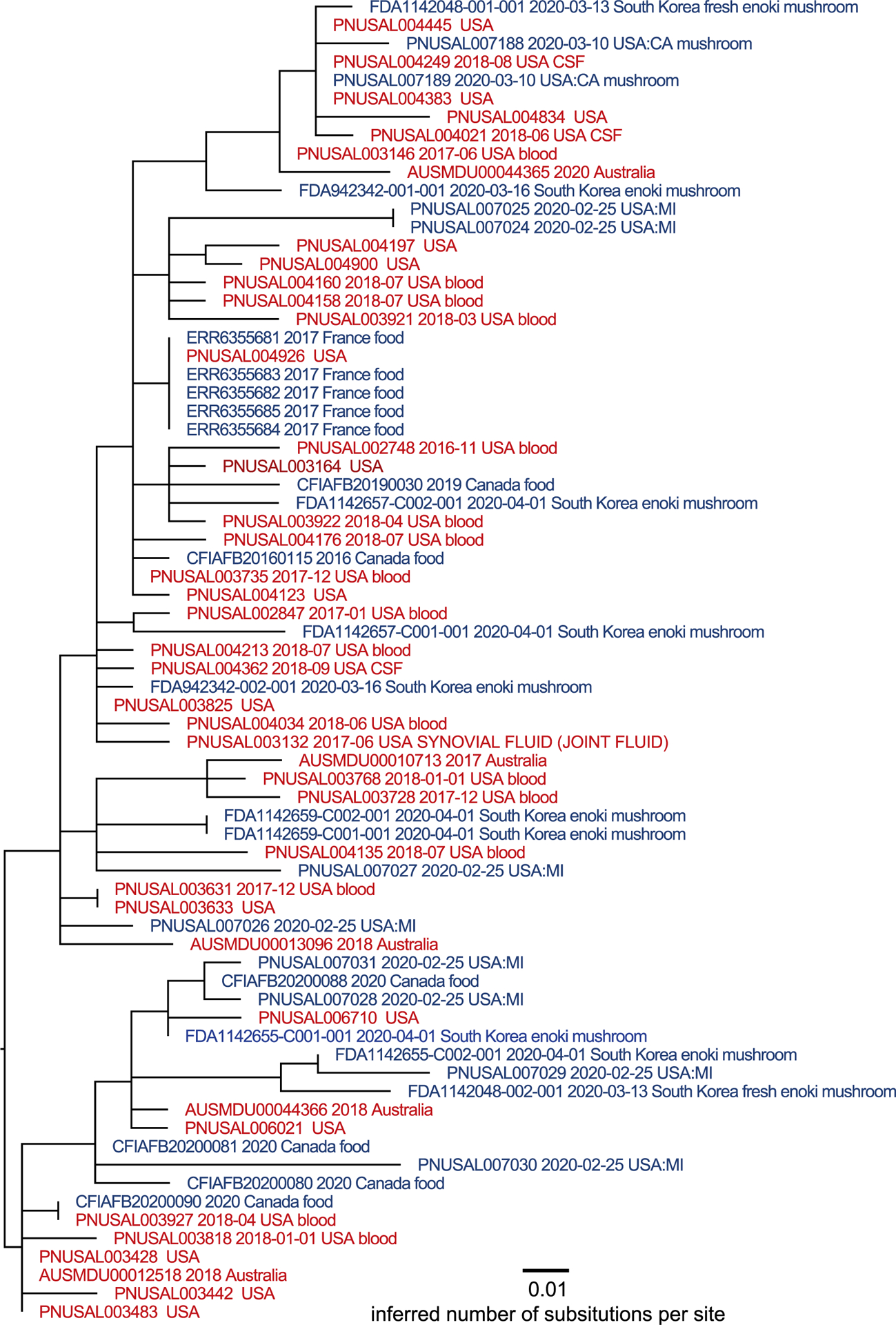
Maximum-likelihood phylogenetic tree inferred via GARLI and a SNP matrix constructed by the CFSAN SNP Pipeline. Tips are color coded as to whether the isolate was collected from a clinical specimen (red) or product sample (blue). Metadata includes isolation date and isolation source in the NCBI Pathogen Database where available for the isolate*. *Single isolate collected by CFIA unrelated to the larger cluster of isolates by WGS. This isolate can be found at https://www.ncbi.nlm.nih.gov/pathogens/tree#Listeria/PDG000000001.2941/PDS000024856.160?key=4qlNyg9jYqcV9ViU95REyULDp6vK-41h91iOKeRJthP_ULcf9H67&labels=epi_type,collection_date,target_creation_date,geo_loc_name,isolation_source,strain,accession.
Traceback Investigation.
The U.S. investigation traced back 15 enoki mushroom samples that yielded L. monocytogenes isolates that were genetically related to the outbreak strain. There were seven traceback legs, four legs representing the eight U.S. samples, two legs representing the six Canadian samples, and one leg representing the one French sample (Fig. 3). FDA identified January 2020 to March 2020 as the timeframe of interest for record collection for the U.S. samples. The French traceback investigation in 2017, as well as the record review and package labeling on the enoki mushroom of the positive samples from Canada and France, identified Foreign Manufacturer B as the product manufacturer. The seven traceback legs converged on Foreign Manufacturer B which confirmed that the 15 positive samples, collected in three different countries between 2017 and 2020, were the product of one manufacturer in the Republic of Korea.
Figure 3.
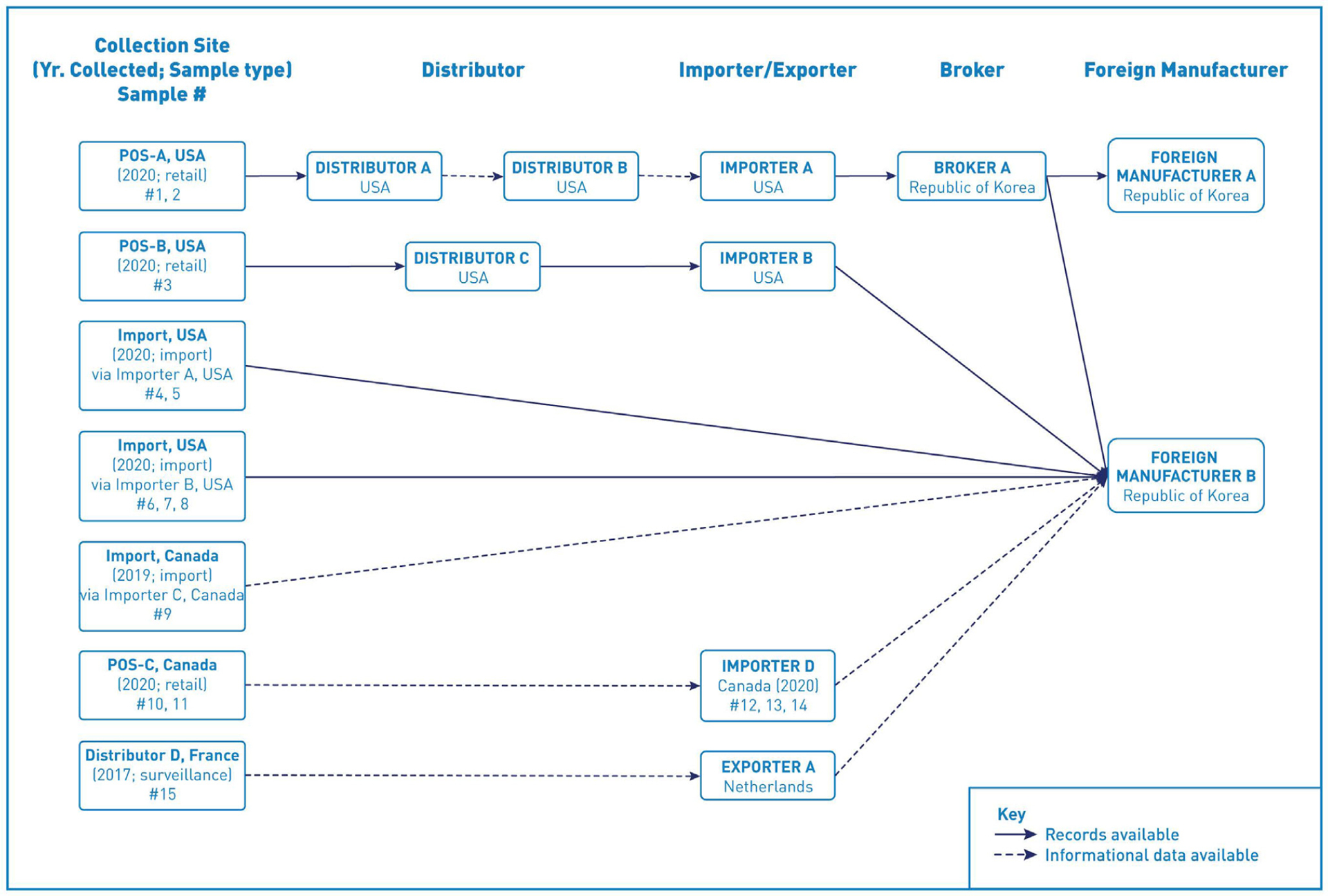
Traceback diagram for multistate outbreak of listeriosis illnesses associated with consumption of enoki mushrooms from the Republic of Korea. L. monocytogenes-positive enoki mushroom samples identified in the United States, Canada, and France were traced from the sample collection site, through the distribution chain, to the foreign manufacturer, denoted on the right side of the diagram.
Foreign Manufacturer B produced and packaged fresh enoki mushrooms, king oyster mushrooms, and beech mushrooms. The enoki mushrooms were sourced from five indoor farms operated by Foreign Manufacturer B. The firm had nine packaging facilities in Gyoungbuk, Republic of Korea and confirmed that they conduct business directly with one U.S. firm (Distributor C) and indirectly conducts business with two Korean trading companies. These Korean trading companies, Broker A and Broker B, supplied two U.S. importers, Importer A and Importer F, respectively. Foreign Manufacturer B exported enoki mushrooms to the United States, Canada, Europe, Australia, and Southeast Asian countries.
Facility Inspections.
U.S.
FDA conducted FSVP inspections at three firms (Importer A, B, and F) that imported enoki mushrooms from Foreign Manufacturer B. FSVP requires that importers verify that their suppliers are producing food using processes and procedures that offer the same level of public health protection as the preventive controls requirements in the preventive controls and current good manufacturing practices rules for human food and animal food and produce safety FDA Food Safety Modernization Act (FSMA) rules, and that the food is not adulterated and properly labeled with respect to allergens (U.S. Food and Drug Administration, 2018c). The firms received FDA Form 483a for failure to develop a FSVP for enoki mushrooms. Each firm received an FDA Warning Letter (U.S. Food and Drug Administration, 2020g, 2020h, 2020i).
Republic of Korea.
On March 18, 2020, the Korean Ministry of Food and Drug Safety published its investigation findings and steps it will take to prevent future illnesses (Korean Ministry of Agriculture, 2020). They found L. monocytogenes in enoki mushrooms produced by two firms in the Republic of Korea, but it is unknown whether those isolates were related to the outbreak.
Public Health Actions.
U.S.
Enoki mushrooms manufactured by Foreign Manufacturer B were recalled in the United States by three domestic firms: Distributor B (March 10, 2020) (U.S. Food and Drug Administration, 2020f), Distributor C (March 23, 2020) (U.S. Food and Drug Administration, 2020c), and Importer F (April 7, 2020) (U.S. Food and Drug Administration, 2020d). These recalls subsequently removed all enoki mushrooms manufactured by Foreign Manufacturer B from the U.S. market.
Foreign Manufacturer B was placed on two separate import alerts. In 2020, FDA-placed Foreign Manufacturer B on Import Alert 99–35 (U.S. Food and Drug Administration, 2021e) (Detention Without Physical Examination of Fresh Produce that Appears to Have Been Prepared, Packed, or Held Under Insanitary Conditions) based on the laboratory, traceback, and epidemiological evidence found during the outbreak investigation. Foreign Manufacturer B was also placed on Import Alert 99–23 (U.S. Food and Drug Administration, 2021d) (Detention Without Physical Examination of Produce Due to Contamination with Human Pathogens) based on the analytic results of an FDA-collected import sample. This import alert applies to produce that appears to bear or contain a poisonous or deleterious substance that may render it injurious to health. The addition of Foreign Manufacturer B to these import alerts subjected future shipments of their enoki mushrooms to detention without physical examination.
Canada.
Based on the positive retail sample of enoki mushrooms collected by CFIA, enoki mushrooms imported from Foreign Manufacturer B, which were distributed to several provinces within Canada, were recalled on March 24, 2020 (Government of Canada, 2020). Following the recall, CFIA confirmed that no additional product from Foreign Manufacturer B was on the market in Canada. CFIA also initiated a border lookout to prevent any additional enoki mushrooms from Foreign Manufacturer B from entering Canada.
France.
The presence of L. monocytogenes at levels of 9.0 × 102 CFU/g signified noncompliance with the European microbiological criterion which resulted in a national alert (FNA 2017/0141). Identified contaminated batches of enoki mushrooms distributed in France between October 25, 2017, and November 17, 2017 were recalled from the French market on November 23, 2017. The enoki mushrooms were imported from the Republic of Korea via The Netherlands, leading France to issue a European alert (RASFF 2017.2213) on December 26, 2017, to inform Dutch authorities.
Public Communication.
U.S..
CDC and FDA both published an outbreak notice on their websites and updated their notices five times (U.S. Centers for Disease Control and Prevention, 2020; U.S. Food and Drug Administration, 2020e). FDA and CDC relayed information on enoki mushroom recalls from Distributer B, Distributer C, and Importer F produced by Foreign Manufacturer B; subsequent Import Alerts were also communicated. On June 9, 2020, CDC and FDA declared the outbreak over.
In addition to online communications, CDC and FDA took several steps to ensure outbreak and recall information reached consumers in the Asian-American population. This was particularly important as enoki mushrooms are typically consumed in Asian cuisine and almost two-thirds of ill people in this outbreak reported Asian race. CDC and FDA translated communications to Korean and simplified Chinese. CDC’s media affairs specialist also provided outbreak information to Asian-American media outlets, which published numerous articles about the outbreak.
Discussion
General Overview.
We report on a multiyear, multinational outbreak of listeriosis linked to fresh enoki mushrooms. These findings suggest that uncooked enoki mushrooms should be considered as a potential vehicle that may lead to listeriosis. The geographic distribution of this outbreak emphasizes the global reach of the food industry. Based on available laboratory data, epidemiologic evidence, traceback analysis of records, and information supplied by firms along the distribution chain, enoki mushrooms manufactured by Foreign Manufacturer B were confirmed as the source of illnesses in this outbreak. This outbreak highlights the importance of international collaboration and public genomic repositories, such as NCBI and EBI (European Bioinformatics Institute). Prompt identification of potential multinational outbreaks and subsequent implementation of public health prevention and control measures benefit greatly from real-time sharing of WGS data (Pettengill et al., 2020). The use of WGS, combined with judicious international data sharing, are essential for identifying and stopping future outbreaks of infectious diseases. This outbreak illustrates that there is still work to be done to ensure actionable data are made public (Pettengill et al., 2021; Pettengill et al., 2020).
In the U.S., the FSMA, and its implementing regulations, has enabled FDA to better protect public health by strengthening the food safety system (U.S. Food and Drug Administration, 2018a). One component of FSMA is the Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, also known as the Produce Safety Rule (PSR) (U.S. Food and Drug Administration, 2020b). The PSR established science-based, minimum standards for the safe production and harvesting of fruits and vegetables, including mushrooms. This includes produce raw agricultural commodities (RACs) that are grown domestically and produce RACs that will be imported or offered for import in any State or territory of the United States, the District of Columbia, or the Commonwealth of Puerto Rico. The FSVP rule for importers of food for humans and animals requires that importers perform certain risk-based activities to verify that food imported into the United States has been produced in a manner that meets applicable U. S. safety standards (U.S. Food and Drug Administration, 2020a). Each of the three firms (Importer A, B, and F) that imported enoki mushrooms from Foreign Manufacturer B to the United States received an FDA Warning Letter for significant FSVP violations.
Potential Contamination Pathway.
Growing mushrooms requires certain temperature and humidity conditions (Carrasco & Preston, 2020; Ikeda et al., 2021; Ostrom Muchroom Farms, 2022). While specific practices may vary, the general principles include having the initial mushroom growing environment comparatively humid with a fresh air exchange, and the temperature is maintained at about 72–77°F. The second stage of growing mushrooms requires lowering the temperature to 45–55°F, still in a comparatively humid environment, and a fresh air exchange. This rapid change in environment shocks the mycelium into forming tiny fruiting bodies that will eventually grow into mature mushrooms. Enoki mushrooms require a much cooler environment (e.g., 45°F), compared to temperatures which other mushroom varieties require (e.g., 60°F). After about 90 days, enoki mushrooms are harvested and are usually packaged into shrink-wrap bags. L. monocytogenes are intracellular bacteria that can thrive in diverse environments and thus, are ubiquitous in nature (Freitag & Port, 2009; Pennone, Lehardy, Coffey, & McAuliffe, 2018). Unlike many other foodborne pathogens, L. monocytogenes can multiply at cooler temperatures, are found more frequently in moist environments (Chapin et al., 2014; Strawn et al., 2013), and can grow under both aerobic and anaerobic conditions. A study by Dygico et al. (2020) showed that isolates of L. monocytogenes can form high levels of biofilm on surfaces of materials commonly found in the mushroom industry. L. monocytogenes may be entering mushroom production operations through incoming raw materials, such as improperly handled spawn or inadequately pasteurized growing substrate, and survive and grow in production and packing house environments when there are insufficient hygiene practices (Pennone et al., 2020; Yuk et al., 2007) and possible cross-contamination occurring due to insanitary practices (Dygico et al., 2020)). L. monocytogenes could also survive on the surface of mushrooms during storage and at retail (Viswanath et al., 2013). The level of contamination for the enoki mushrooms ranged from <10 to 8.0 × 105 CFU/g; higher than levels found in outbreak-associated ice cream (median 4.52 MPN/g) (Chen, Burall, Macarisin, et al., 2016), stone fruit (median of 5 CFU/fruit) (Chen, Burall, Luo, et al., 2016), and Ricotta Salata cheese samples (median, 4.8 × 104 CFU/g) (Heiman et al., 2016), indicating that the level of contamination was relatively high (Heiman et al., 2016).
From June 2020, when the outbreak was declared over, until August 2022, twelve U.S. firms issued 18 recalls and five Canadian firms issued 12 recalls of enoki mushrooms due to potential L. monocytogenes contamination (Food Safety News, 2021; U.S. Food and Drug Administration, 2021a, 2021b, 2021c, 2021g, 2021h, 2021i). In response to the outbreak findings, FDA implemented the Imported Enoki Mushroom and Imported Wood Ear Mushroom Prevention Strategy in May 2021 to ensure and enhance the safety of imported specialty mushrooms (U.S. Food and Drug Administration, 2022a, 2022c). Additionally, FDA’s continued sampling of imported enoki mushrooms at ports of entry yielded L. monocytogenes from multiple firms in the Republic of Korea, which were subsequently added to FDA’s Import Alert 99–23 and Import Alert 99–35. On July 1, 2022, FDA implemented a country-wide Import Alert #25–21, ‘Detention Without Physical Examination of Enoki Mushrooms from Korea (the Republic of) due to Listeria monocytogenes, to assist in preventing potential outbreaks and illnesses (U.S. Food and Drug Administration, 2022b). These actions underscore the ongoing and widespread food safety concern of L. monocytogenes contamination in enoki mushrooms and the need for education and outreach regarding the minimum, science-based food safety provisions promulgated by several FSMA regulations.
International Engagement and Cultural Considerations.
The volume of food imports into the U.S. has been increasing in the past 15 years. As of 2019, 32% of fresh vegetables and 55% of the fresh fruit were supplied by other countries (U.S. Food and Drug Administration, 2019). The U.S. imports food from more than 200 countries or territories and approximately 125,000 exporting food facilities and farms (U.S. Food and Drug Administration, 2019). The sheer volume of food imported into the U.S. contributes to the diversity of the food supply but is also a prominent reason why FDA’s food safety regulations extend beyond U.S. borders.
During outbreaks, sharing information with international partners and foreign firms is paramount in preventing additional illnesses and deaths. This outbreak likely would have continued if not for routine communication between the U.S. and Canada, the EPIS forum, PulseNet International, INFOSAN, and NCBI to share outbreak information. Gathering information from importers through the FSVP inspection and engaging with the Korean Ministry of Food and Drug Safety helped FDA determine the production and distribution of the implicated mushrooms in the Republic of Korea. This information better informed the U.S. investigation, which allowed FDA to target resources and inform international and domestic partners about the potential scope of contamination and risk to consumers.
Information sharing between regulatory agencies in the U.S. and Canada was facilitated by FDA’s Systems Recognition. The Systems Recognition arrangements complement existing Confidentiality Commitments which prioritize information exchange, especially during recalls/outbreaks and food-related emergencies, to maximize consumer protection (U.S. Food and Drug Administration, 2018b). The information flow among FDA and Canada was swift and equivalent to how FDA shares information with domestic partners. This allowed each country to make important, better-informed decisions regarding sampling and regulatory actions.
In contrast, given the language barrier, lack of confidentiality commitment, and diplomatic considerations, FDA could only share limited information with Republic of Korea officials. In instances where direct agreements are not in place, international collaboration through networks such as INFOSAN can be invaluable. During this outbreak, INFOSAN aided in the exchange of information between the Republic of Korea food safety officials and international partners.
Likewise, FDA could not reach out directly to Foreign Manufacturer B to address traceability and outbreak-related information in real time due to the language barrier. To help accelerate information sharing and public health actions in future outbreaks, a foreign firm should select a U.S. agent that is bilingual and informed of food safety regulations to serve as a liaison to FDA. Foreign manufacturers who export food to the U.S. for human consumption are required to register with FDA (21 CFR 1, subpart H) and identify an individual or corporation in the U.S. as their representative. However, farms are exempt from registration per subpart H, so if a farm was also the exporter, then they would not need to register but would still need to identify an individual or corporation in the U.S. as their representative. Similar observations were made during a 2019 outbreak of scombrotoxin fish poisoning linked to tuna imported from Vietnam (Pereira et al., 2021). The World Health Organization has published a toolkit with strategies promoting effective communication in settings of international foodborne disease outbreaks (World Health Organization, 2012).
One outcome from engagement with Republic of Korea officials was the development of a mitigation strategy by Republic of Korea officials. Enoki mushrooms are typically consumed cooked in the Republic of Korea. In the U.S. and Canada, enoki mushrooms may be consumed uncooked in salads, sandwiches, and as a garnish on a variety of dishes. There is a need to continue communication with other countries that mushrooms, whether grown in the U.S. or offered for entry into the U.S. as a raw agricultural commodity, are considered by FDA to be covered produce subject to the requirements of the PSR and, when imported, FSVP.
Epidemiologic Challenges.
This outbreak spanned multiple years and was investigated in the United States several times. Each investigation yielded limited food history and multiple attempts to reinterview cases did not identify mushrooms as a suspect vehicle. If CFIA had not uploaded their historical mushroom isolates to NCBI in February 2020, illnesses would likely have continued, and the outbreak would have remained unsolved. Routinely sharing both epidemiologic and sequencing data in real time can lead to swift public health actions and prevent further illness.
The use of enoki mushrooms in dishes served at restaurants as a small ingredient of a larger dish, or as a garnish, may have led ill people to not recall eating enoki mushrooms. There is also the possibility of ill people not eating enoki mushrooms but being exposed to L. monocytogenes via cross-contamination in a restaurant setting. Given that most ill people from Canada and the United States had older isolation dates, the investigation relied on information collected during initial interview. For all countries, the length of this outbreak made it difficult to reinterview ill people. Ultimately, only two ill people, one each in the United States and Canada reported consuming enoki mushrooms. Remarkably, one U.S. ill person from 2018 kept receipts from their enoki mushroom purchase allowing investigators to pursue sampling at the POS-A and ultimately leading to the identification of L. monocytogenes in enoki mushrooms on the market in 2020. Since specific questions on enoki mushroom consumption are not included in the U.S. or Canada’s standardized listeriosis questionnaire, it was difficult to gather information on this exposure.
There may be some benefits to making routine questionnaires more inclusive of the increased variety of food products available to consumers. However, the LI standard questionnaire is already long, and it would be impossible to include every food that could cause listeriosis. These questionnaires often focus on specific, high-risk food items or regionally established food/hazard combinations, such as deli meat or cheeses. As illustrated by this outbreak, it is important to consider the addition of new food items to the Listeria questionnaire as new laboratory or epidemiologic evidence becomes available through outbreak investigations.
Traceback Challenges.
Several traceback challenges were faced by all partners involved. For example, illnesses dated back to 2016 which resulted in limited epidemiologic information. As a result, the traceback investigation was limited to the traceback of positive samples that matched the outbreak strain. Traceability was also limited by the lack of record keeping along the supply chain. Specifically, for the positive sample collected from POS-A, Importer A could not decipher the traceability system used by the foreign firm to determine which Republic of Korea manufacturer supplied the positive sample. Additionally, Foreign Manufacturer B maintained a traceability system for their shipments; however, given the language barriers, lack of a confidentiality commitment agreement, and diplomatic considerations, FDA could not reach out directly to Foreign Manufacturer B to address traceability questions in real time. Unlike the product shipped to the United States, the labeling of the positive enoki mushroom samples distributed to Canada and France identified Foreign Manufacturer B as the product manufacturer. The informative labels allowed investigators to quickly identify the foreign firm of interest and implement public health actions without depleting traceback resources.
This outbreak highlights the successes and challenges of international collaboration during outbreak detection, investigations, and response. Data sharing through multiple channels allowed the United States and Canada to recognize that ill people in their respective countries were part of the same outbreak with a common source of imported enoki mushrooms. This also represents the first known outbreak of listeriosis linked to mushrooms in the United States or Canada, a novel pathogen/food vehicle pair for consideration during future outbreaks. Continual surveillance for L. monocytogenes is needed along with the persistence of investigators to continually monitor for new clinical and nonclinical isolates, review epidemiologic information, pursue records at retail establishments, and collect product for testing. This investigation provides a powerful example of the impact of national and international collaborative efforts to respond to food-borne illness outbreaks and protect consumers and public health (Pettengill et al., 2020). It also demonstrates the importance of routine, international data sharing and food testing in identifying and stopping foodborne outbreaks.
*45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241 (d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
Acknowledgments
The response efforts for this outbreak included numerous public health officials at local and state health departments and public health laboratories in the United States, who serve as the backbone of any multistate foodborne illness outbreak investigation. The efforts of Michigan Department of Health and Human Services, Bureau of Laboratories staff in the bacteriology unit, especially Stephen Dietrich and Elizabeth Burgess were essential during the outbreak investigation. The contributions of the California Department of Public Health Food and Drug Branch Emergency Response Unit aided public health actions. We also appreciate the assistance provided by FDA Emergency Response Coordinators who coordinated the response between FDA and state partners.
CDC would like to acknowledge our partners at the Arizona Department of Health Services (Mackenzie Tewell), California Department of Public Health (Akiko Kimura), Florida Department of Health (Jenny Crain, Kimberly Stockdale, Teresa Gorden), Hawaii State Department of Health (Myra Ching-Lee, Diana Connor), Indiana Department of Health (Hailey Vest), Kentucky Department for Public Health (Stacy Davidson), Massachusetts Department of Public Health (Emily Harvey, Johanna Vostock, Scott Troppy, Elizabeth Traphagen) Maryland Department of Health (Jordan Cahoon, Michelle Boyle), Michigan Department of Health & Human Services (Katie Arends, Justin Henderson, Danielle Donovan), Missouri Department of Health and Senior Services (John Bos, Ashley Wigginton), Nevada Department of Health and Human Services (Brian Parrish), North Carolina Department of Health and Human Services (Tammra Morrison, Nicole Lee), New Jersey Department of Health (Deepam Thomas, Erica Rauch), New York State Department of Health (Amy Robbins, Katherine Purcell, Madhu Anand, David Nicholas), New York City Department of Health and Mental Hygiene (HaeNa Waechter, Vasudha Reddy), Rhode Island Department of Health (Seth Peters, Michael Gosciminski), Tennessee Department of Health (Brenda Rue), Virginia Department of Health, (Kelsey Holloman, Katherine McCombs, and Washington State Department of Health (Beth Melius, Krisandra Allen, Laurie Stewart) for their continued support and efforts during this long and challenging investigation. We appreciate our coinvestigators willingness to change directions throughout this multiyear investigation.
PHAC and CFIA would like to acknowledge the contributions of local and provincial public health authorities, the Enhanced National Listeriosis Surveillance Program, the PulseNet Canada Steering Committee, provincial public health laboratories, the CFIA laboratory Network and Austin Markell from CFIA Science Branch.
French partners would like to acknowledge Mrs. Yasmine Abdallah, former Deputy Head of the Alert Unit, General Directorate for Competition Policy, Consumer Affairs and Fraud Control, French Ministry for the Economy and Finance, Paris, France; Dr. Jean-Philippe Rosec, Head of Joint Laboratory service, French Ministry for the Economy and Finance, Bordeaux, France, and Dr. Alexandra Moura, French National Reference Centre and WHO Collaborating Centre Listeria, Institut Pasteur, Paris, France.
Footnotes
Disclaimer
The findings and conclusions in this article are those of the author (s) and do not necessarily represent the views or opinions of the California Department of Public Health or the California Health and Human Services Agency. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control (CDC) or the Food and Drug Administration (FDA).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, & Sayers EW (2015). GenBank. Nucleic Acids Research, 43(Database issue), D30–D35. 10.1093/nar/gku1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco J, & Preston GM (2020). Growing Edible Mushrooms: A Conversation Between Bacteria and Fungi. Environmental Microbiology, 22(3), 858–872. 10.1111/1462-2920.14765. [DOI] [PubMed] [Google Scholar]
- Chapin TK, Nightingale KK, Worobo RW, Wiedmann M, & Strawn LK (2014). Geographical and meteorological factors associated with isolation of Listeria species in New York State produce production and natural environments. Journal of Food Protection, 77(11), 1919–1928. 10.4315/0362-028x.Jfp-14-132. [DOI] [PubMed] [Google Scholar]
- Charlier C, Perrodeau É, Leclercq A, Cazenave B, Pilmis B, Henry B, Lopes A, Maury MM, Moura A, Goffinet F, Dieye HB, Thouvenot P, Ungeheuer MN, Tourdjman M, Goulet V, de Valk H, Lortholary O, Ravaud P, & Lecuit M (2017). Clinical Features and Prognostic Factors of Listeriosis: The MONALISA National Prospective Cohort Study. The Lancet Infectious Diseases, 17(5), 510–519. 10.1016/s1473-3099(16)30521-7. [DOI] [PubMed] [Google Scholar]
- Chen M, Cheng J, Wu Q, Zhang J, Chen Y, … Zeng H (2018). Prevalence, Potential Virulence, and Genetic Diversity of Listeria monocytogenes Isolates From Edible Mushrooms in Chinese Markets. Frontiers in Microbiology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Burall LS, Luo Y, Timme R, Melka D, Muruvanda T, Payne J, Wang C, Kastanis G, Maounounen-Laasri A, De Jesus AJ, Curry PE, Stones R, K’Aluoch O, Liu E, Salter M, Hammack TS, Evans PS, Parish M, … Brown EW (2016). Listeria monocytogenes in Stone Fruits Linked to a Multistate Outbreak: Enumeration of Cells and Whole-Genome Sequencing. Applied and Environmental Microbiology, 82(24), 7030–7040. 10.1128/aem.01486-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Burall LS, Macarisin D, Pouillot R, Strain E, AJ DEJ, Laasri A, Wang H, Ali L, Tatavarthy A, Zhang G, Hu L, Day J, Kang J, Sahu S, Srinivasan D, Klontz K, Parish M, Evans PS, … Datta AR (2016). Prevalence and Level of Listeria monocytogenes in Ice Cream Linked to a Listeriosis Outbreak in the United States. Journal of Food Protection, 79(11), 1828–1832. 10.4315/0362-028x.Jfp-16-208. [DOI] [PubMed] [Google Scholar]
- Davis S, P JB, Luo Y, Payne J, Shpuntoff A, Rand H, & Strain E (2015). CFSAN SNP Pipeline: An Automated Method for Constructing SNP Matrices From Next-Generation Sequence Data. Peer Journal of Computer Science, 1. 10.7717/peerj-cs.20. [DOI] [Google Scholar]
- de Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, Havelaar A, & Speybroeck N (2014). The Global Burden of Listeriosis: A Systematic Review and Meta-Analysis. The Lancet Infectious Diseases, 14(11), 1073–1082. 10.1016/S1473-3099(14)70870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dygico LK, Gahan CGM, Grogan H, & Burgess CM (2020). The Ability of Listeria monocytogenes to Form Biofilm on Surfaces Relevant to the Mushroom Production Environment. International Journal of Food Microbiology, 317. 10.1016/j.ijfoodmicro.2019.108385 108385. [DOI] [PubMed] [Google Scholar]
- European Commission (2002). Commission Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Official Journal of the European Communities, 31, 1–24. [Google Scholar]
- European Commission (2005). Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Official Journal of the European Communities, 50, 1–26. [Google Scholar]
- European Commission (2014). Rapid Alert System for Food and Feed. Retrieved March 5 from https://ec.europa.eu/food/safety/rasff_en
- Food Safety News. (2021). CFIA Testing Leads to Enoki Mushroom Recall Because of Listeria Concerns. Retrieved September 20 from https://www.foodsafetynews.com/2021/09/cfia-testing-leads-to-enoki-mushroom-recall-because-of-listeria-concerns/?utm_source=Food+Safety+News&utm_campaign=be1a73cc4a-RSS_EMAIL_CAMPAIGN&utm_medium=email&utm_term=0_f46cc10150-be1a73cc4a-40337703
- Food Standards Australia and New Zealand. (2020a). Green Co. Enoki Mushrooms 200g and 300g Retrieved August 15 from https://www.foodstandards.gov.au/industry/foodrecalls/recalls/Pages/Green-Co.-Enoki-Mushrooms-200g%20and%20300g.aspx
- Food Standards Australia and New Zealand. (2020b). Listeria monocytogenes Linked to Fresh Enoki Mushrooms Imported From South Korea Retrieved August 15 from https://www.foodstandards.gov.au/consumer/generalissues/Pages/Listeria-Monocytogenes-linked-to-fresh-enoki-mushrooms-imported-from-South-Korea.aspx
- Food Standards Australia and New Zealand. (2022). Listeria monocytogenes Linked to Fresh Enoki Mushrooms Imported From South Korea Retrieved September 29 from https://www.foodstandards.gov.au/consumer/generalissues/Pages/Listeria-Monocytogenes-linked-to-fresh-enoki-mushrooms-imported-from-South-Korea.aspx
- Freitag NE, & Port GC (2009). Listeria monocytogenes - From Saprophyte to Intracellular Pathogen. Nat Rev Microbiol, 7, 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould LH, Kline J, Monahan C, & Vierk K (2017). Outbreaks of Disease Associated with Food Imported into the United States, 1996–2014(1). Emerging Infectious Diseases, 23(3), 525–528. 10.3201/eid2303.161462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet V, King LA, Vaillant V, & de Valk H (2013). What Is the Incubation Period For Listeriosis? BMC Infectious Diseases, 13, 11. 10.1186/1471-2334-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Canada. (2019). Notification - Jongilpoom brand Enoki Mushrooms Recalled due to Listeria monocytogenes. Retrieved March 5 from https://www.inspection.gc.ca/food-recall-warnings-and-allergy-alerts/2019-07-05-r13072/eng/1563312477630/1563312479417
- Government of Canada. (2020). Food Recall Warning - Golden Mushroom Brand Enoki Mushroom Recalled Due to Listeria monocytogenes. Retrieved January 6 from https://inspection.canada.ca/food-recall-warnings-and-allergy-alerts/2020-03-24/eng/1585081415872/1585081416327
- Harvey RR, Heiman Marshall KE, Burnworth L, Hamel M, Tataryn J, Cutler J, Meghnath K, Wellman A, Irvin K, Isaac L, Chau K, Locas A, Kohl J, Huth PA, Nicholas D, Traphagen E, Soto K, Mank L, Holmes-Talbot K, … Gieraltowski L (2017). International Outbreak of Multiple Salmonella Serotype Infections Linked to Sprouted Chia Seed Powder - USA and Canada, 2013–2014. Epidemiology and Infection, 145(8), 1535–1544. 10.1017/s0950268817000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. (2021). The compendium of analytical methods. Retrieved April 9 from http://www.hc-sc.gc.ca/fn-an/res-rech/analy-meth/microbio/index-eng.php
- Heiman KE, Garalde VB, Gronostaj M, Jackson KA, Beam S, Joseph L, Saupe A, Ricotta E, Waechter H, Wellman A, Adams-Cameron M, Ray G, Fields A, Chen Y, Datta A, Burall L, Sabol A, Kucerova Z, Trees E, … Silk BJ (2016). Multistate Outbreak of Listeriosis Caused by Imported Cheese and Evidence of Cross-Contamination of other Cheeses, USA, 2012. Epidemiology and Infection, 144 (13), 2698–2708. 10.1017/s095026881500117x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins AD, Jinneman K, & Chen Y (2022). BAM Chapter 10: Detection of Listeria monocytogenes in Foods and Environmental Samples, and Enumeration of Listeria monocytogenes in Foods.
- Ikeda S, Yamauchi M, Watari T, Hatamoto M, Yamada M, Maki S, Hara H, & Yamaguchi T (2021). Development of Enokitake (Flammulina velutipes) mushroom cultivation technology using spent mushroom substrate anaerobic digestion residue. Environmental Technology & Innovation, 24 102046. [Google Scholar]
- International Standards Organization (2016). 16140–2: Microbiology of the Food Chain Method Validation-Part 2: Protocol For the Validation of Alternative (Proprietary) Methods Against a Reference Method. Geneva: I J International Standards Organization. [Google Scholar]
- Irvin K, Viazis S, Fields A, Seelman S, Blickenstaff K, Gee E, Wise M, Marshall K, Gieraltowski L, & Harris S (2021). An Overview of Traceback Investigations and Three Case Studies of Recent Outbreaks of Escherichia coli O157:H7 Infections Linked to Romaine Lettuce. Journal of Food Protection, 84(8), 1340–1356. 10.4315/jfp-21-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korean Ministry of Agriculture. (2020). Strengthening hygiene management for enoki mushroom producers. Retrieved September 28 from https://www.mafra.go.kr/mafra/293/subview.do?enc=Zm5jdDF8QEB8JTJGYmJzJTJGbWFmcmElMkY2OCUyRjMyMzMyMiUyRmFydGNsVmlldy5kbyUzRmJic0NsU2VxJTNEJTI2cmdzRW5kZGVTdHIlM0QlMjZiYnNPcGVuV3JkU2VxJTNEJTI2cGFzc3dvcmQlM0QlMjZzcmNoQ29sdW1uJTNEJTI2cGFnZSUzRDglMjZyZ3NCZ25kZVN0ciUzRCUyNnJvdyUzRDEwJTI2aXNWaWV3TWluZSUzRGZhbHNlJTI2c3JjaFdyZCUzRCUyNg%3D%3D
- Moura A, Tourdjman M, Leclercq A, Hamelin E, Laurent E, Fredriksen N, Van Cauteren D, Bracq-Dieye H, Thouvenot P, Vales G, Tessaud-Rita N, Maury MM, Alexandru A, Criscuolo A, Quevillon E, Donguy MP, Enouf V, de Valk H, Brisse S, & Lecuit M (2017). Real-Time Whole-Genome Sequencing for Surveillance of Listeria monocytogenes, France. Emerging Infectious Diseases, 23(9), 1462–1470. 10.3201/eid2309.170336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon C, Van Walle I, Gerner-Smidt P, Campos J, Chinen I, Concepcion-Acevedo J, Gilpin B, Smith AM, Kam KM, Perez E, Trees E, Kubota K, Takkinen J, Nielsen EM, Carleton H, & Panel F-NE (2017). PulseNet International: Vision for the Implementation of Whole Genome Sequencing (WGS) for Global Food-Borne Disease Surveillance. Eurosurveillance, 22(23), 30544. 10.2807/1560-7917.ES.2017.22.23.30544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom Muchroom Farms. (2022). How Enoki Mushrooms Grow. Retrieved December 29 from https://www.ostrommushrooms.com/varieties/enoki/how-they-grow#:~:text=Enokis%20are%20usually%20cultivated%20on,takes%20from%2012%2D30%20days
- Pagotto F, Trottier Y-L,Upham J, & Iugovaz I (2011). Laboratory Procedure MFLP-74 February 2011 Health Products and Food Branch. Health.
- Pennone V, Dygico KL, Coffey A, Gahan CGM, Grogan H, McAuliffe O, Burgess CM, & Jordan K (2020). Effectiveness of current hygiene practices on minimization of Listeria monocytogenes in different mushroom production-related environments. Food Science & Nutrition, 8(7), 3456–3468. 10.1002/fsn3.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennone V, Lehardy A, Coffey A, & McAuliffe O (2018). Diversity of Listeria monocytogenes Strains Isolated from Agaricus bisporus Mushroom Production. J Appl Microbiol, 125, 586–595. [DOI] [PubMed] [Google Scholar]
- Pereira E, Elliot EL, Singleton LS, Otto M, Tesfai A, Doyle M, Hawk H, Bloodgood S, Benner RA, Ross MP, Scott A, Kristof MC, Fox T, Bridgman B, Long N, Livsey K, Rubenstein A, Garner K, Nicholas D, … Viazis S (2021). An Outbreak Investigation of Scombrotoxin Fish Poisoning Illnesses in the United States Linked to Yellowfin Tuna Imported from Vietnam-2019. Journal of Food Protection, 84(6), 962–972. 10.4315/jfp-20-456. [DOI] [PubMed] [Google Scholar]
- Pettengill JB, Beal J, Balkey M, Allard M, Rand H, & Timme R (2021). Interpretative Labor and the Bane of Nonstandardized Metadata in Public Health Surveillance and Food Safety. Clinical Infectious Diseases, 73(8), 1537–1539. 10.1093/cid/ciab615. [DOI] [PubMed] [Google Scholar]
- Pettengill JB, Markell A, Conrad A, Carleton HA, Beal J, Rand H, Musser S, Brown EW, Allard MW, Huffman J, Harris S, Wise M, & Locas A (2020). A Multinational Listeriosis Outbreak And The Importance of Sharing Genomic Data. The Lancet Microbe, 1(6), e233–e234. 10.1016/S2666-5247(20)30122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech WF 3rd. (2000). Foodborne listeriosis. Clinical Infectious Diseases, 31(3), 770–775. 10.1086/314008. [DOI] [PubMed] [Google Scholar]
- Strawn LK, Fortes ED, Bihn EA, Nightingale KK, Gröhn YT, Worobo RW, Wiedmann M, & Bergholz PW (2013). Landscape and meteorological factors affecting prevalence of three food-borne pathogens in fruit and vegetable farms. Applied and Environmental Microbiology, 79(2), 588–600. 10.1128/aem.02491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar B, Joseph LA, Schroeder MN, Stroika S, Ribot EM, Hise KB, & Gerner-Smidt P (2019). An Overview of PulseNet USA Databases. Foodborne Pathogens and Disease, 16(7), 457–462. 10.1089/fpd.2019.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2016). PulseNet Methods and Protocols. Retrieved July 30, 2021 from https://www.cdc.gov/pulsenet/pathogens/protocols.html
- U.S. Centers for Disease Control and Prevention. (2018). National Listeria Surveillance: Listeria Initiative. Retrieved May 5 from https://www.cdc.gov/nationalsurveillance/listeria-surveillance.html
- U.S. Centers for Disease Control and Prevention. (2020). Outbreak of Listeria Infections Linked to Enoki Mushrooms. Retrieved October 26 from https://www.cdc.gov/listeria/outbreaks/enoki-mushrooms-03-20/index.html
- U.S. Centers for Disease Control and Prevention. (2022a). Foodborne Diseases Active Surveillance Network (FoodNet) Population Survey. Retrieved November 18 from https://www.cdc.gov/foodnet/surveys/population.html
- U.S. Centers for Disease Control and Prevention. (2022b). Listeria (Listeriosis). Retrieved December 29 from https://www.cdc.gov/listeria/faq.html
- U.S. Centers for Disease Control and Prevention. (2022c). National Listeria Surveillance. Retrieved April 11 from https://www.cdc.gov/nationalsurveillance/listeriasurveillance.html
- U.S. Food and Drug Administration. (2018a). Background on the FDA Food Safety Modernization Act (FSMA). Retrieved May 22 from https://www.fda.gov/food/food-safety-modernization-act-fsma/background-fda-food-safety-modernization-act-fsma
- U.S. Food and Drug Administration. (2018b). Frequently Asked Questions on Systems Recognition for Foreign Governments. Retrieved November 12 from https://www.fda.gov/food/international-interagency-coordination/frequently-asked-questions-systems-recognition-foreign-governments
- U.S. Food and Drug Administration. (2018c). What Do Importers Need to Know About FSVP. Retrieved January 3 from https://www.fda.gov/food/conversations-experts-food-topics/what-do-importers-need-know-about-fsvp
- U.S. Food and Drug Administration. (2019). FDA Strategy for the Safety of Imported Food. Retrieved November 12 from https://www.fda.gov/media/120585/download
- U.S. Food and Drug Administration. (2020a). FSMA Final Rule on Foreign Supplier Verification Programs (FSVP) for Importers of Food for Humans and Animals. Retrieved May 22 from https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-foreign-supplier-verification-programs-fsvp-importers-food-humans-and-animals
- U.S. Food and Drug Administration. (2020b). FSMA Final Rule on Produce Safety: Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption. Retrieved May 22 from https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-produce-safety
- U.S. Food and Drug Administration. (2020c). Guan’s Mushroom Co Recalls Enoki Because of Possible Health Risk. Retrieved May 12 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/guans-mushroom-co-recalls-enoki-because-possible-health-risk
- U.S. Food and Drug Administration. (2020d). H&C Food Inc. Recalls Enoki Mushroom Because of Possible Health Risk. Retrieved May 12 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/hc-food-inc-recalls-enoki-mushroom-because-possible-health-risk
- U.S. Food and Drug Administration. (2020e). Outbreak Investigation of Listeria monocytogenes: Enoki Mushrooms (March 2020). Retrieved May 6 from https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-enoki-mushrooms-march-2020
- U.S. Food and Drug Administration. (2020f). Sun Hong Foods, Inc. Recalls Enoki Mushroom Because of Possible Health Risk. Retrieved May 12 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/sun-hong-foods-inc-recalls-enoki-mushroom-because-possible-health-risk
- U.S. Food and Drug Administration. (2020g). Warning Letter: H & C Food Inc. Retrieved April 12 from https://wayback.archive-it.org/7993/20201219144916/ https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/h-c-food-inc-607742-06222020
- U.S. Food and Drug Administration. (2020h). Warning Letter: Royal International Trading LLC. Retrieved May 12 from https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/royal-international-trading-llc-606915-05272020
- U.S. Food and Drug Administration. (2020i). Warning Letter: Ventura Garden Inc. Retrieved April 12 from https://wayback.archive-it.org/7993/20201219151645/ https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/ventura-terra-garden-inc-608649-07292020
- U.S. Food and Drug Administration. (2021a). Concord Farms Recalls Enoki Mushrooms Due to Possible Health Risk. Retrieved June 21 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/concord-farms-recalls-enoki-mushrooms-due-possible-health-risk
- U.S. Food and Drug Administration. (2021b). Golden Medal Mushroom Inc. Recalls Enoki Mushrooms Because of Possible Health Risk. Retrieved June 21 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/golden-medal-mushroom-inc-recalls-enoki-mushrooms-because-possible-health-risk
- U.S. Food and Drug Administration. (2021c). Guan’s Mushroom Co Recalls Enoki Because of Possible Health Risk. Retrieved June 21 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/guans-mushroom-co-recalls-enoki-because-possible-health-risk-0
- U.S. Food and Drug Administration. (2021d). Import Alert 99–23. https://www.accessdata.fda.gov/cms_ia/importalert_266.html
- U.S. Food and Drug Administration. (2021e). Import Alert 99–35. Retrieved May 12 from https://www.accessdata.fda.gov/cms_ia/importalert_1128.html
- U.S. Food and Drug Administration. (2021g). Marquis Worldwide Specialty Inc. Recalls Organic Enoki Mushroom 200g Because of Possible Health Risk. Retrieved June 21 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/marquis-worldwide-specialty-inc-recalls-organic-enoki-mushroom-200g-because-possible-health-risk
- U.S. Food and Drug Administration. (2021h). Rainfield Marketing Group, Inc. of Vernon, CA is Recalling Enoki Mushrooms (Product of Korea) Because it has the Potential to be Contaminated with Listeria monocytogenes. Retrieved June 21 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/rainfield-marketing-group-inc-vernon-ca-recalling-enoki-mushrooms-product-korea-because-it-has
- U.S. Food and Drug Administration. (2021i). Revised Guan’s Mushroom Co Recalls Enoki Because of Possible Health Risk. Retrieved June 21 from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/revised-guans-mushroom-co-recalls-enoki-because-possible-health-risk
- U.S. Food and Drug Administration. (2022a). FDA Issues Country-Wide Import Alert for Enoki Mushrooms from the Republic of Korea. Retrieved Septemer 23 from https://www.fda.gov/food/cfsan-constituent-updates/fda-issues-country-wide-import-alert-enoki-mushrooms-republic-korea
- U.S. Food and Drug Administration. (2022b). Import Alert 25–21. Retrieved August 15 from https://cms.fda.gov/vts/imports_publish/private/importalert_1177.html
- U.S. Food and Drug Administration. (2022c). Summary of FDA’s Strategy to Help Prevent Listeriosis and Salmonellosis Outbreaks Associated with Imported Enoki and Imported Wood Ear Mushrooms. Retrieved September 29 from https://www.fda.gov/food/new-era-smarter-food-safety/summary-fdas-strategy-help-prevent-listeriosis-and-salmonellosis-outbreaks-associated-imported-enoki?utm_medium=email&utm_source=govdelivery
- World Health Organization. (2012). Communication for Behavioural Impact (COMBI): A Toolkit for Behavioural and Social Communication in Outbreak Response. World Health Organization. Retrieved August 18 from https://www.who.int/ihr/publications/combi_toolkit_outbreaks/en/ [Google Scholar]
- World Health Organization. (2020). Responding to Food Safety Emergencies (INFOSAN). Retrieved November 12 from https://www.who.int/activities/responding-to-food-safety-emergencies-infosan
- Viswanath P, Murugesan L, Knabel SJ, Verghese B, & Chikthimmah N (2013). Incidence of Listeria monocytogenes and Listeria spp. in a Small-Scale Mushroom Production Facility. J Food Prot, 76, 608–615. [DOI] [PubMed] [Google Scholar]
- Yuk H-G, Yoo M-Y, Yoon J-W, Marshall DL, & Oh D-H (2007). Effect of combined ozone and organic acid treatment for control of Escherichia coli O157:H7 and Listeria monocytogenes on enoki mushroom. Food Control, 18(5), 548–553. 10.1016/j.foodcont.2006.01.004. [DOI] [Google Scholar]
Further reading
- U.S. Food and Drug Administration. (2021f). Investigations Operations Manual. Retrieved September 20 from https://www.fda.gov/media/113432/download


