Abstract
Hepatocyte Nuclear Factor 4 alpha (HNF4α) is a highly conserved member of the nuclear receptor superfamily expressed at high levels in the liver, kidney, pancreas, and gut. In the liver, HNF4α is exclusively expressed in hepatocytes, where it is indispensable for embryonic and postnatal liver development and for normal liver function in adults. It is considered a master regulator of hepatic differentiation because it regulates a significant number of genes involved in hepatocyte specific functions. Loss of HNF4α expression and function is associated with the progression of chronic liver disease. Further, HNF4α is a target of chemical-induced liver injury. In this review, we discuss the role of HNF4α in liver pathophysiology and highlight its potential use as a therapeutic target for liver diseases.
Keywords: Hepatocyte nuclear factor 4alpha, liver development, hepatocyte differentiation, therapeutic target
Graphical Abstract
Introduction to HNF4α
Hepatocyte nuclear factor 4 alpha (HNF4α), also known as NR2A1, is a member of the nuclear receptor superfamily. HNF4α is a transcription factor encoded by the HNF4A gene on chromosome 2 in mice and chromosome 20 in humans. HNF4α was originally identified by Frances M. Sladek and James E. Darnell, Jr using rat liver extracts as a member of steroid hormone receptor superfamily and a protein required for the transcription of genes transthyretin (TTR) and apolipoprotein CIII (APOCIII) [1]. HNF4α is expressed in many tissues, including the intestine, pancreas, and colon, and at high levels in the liver and kidneys.
HNF4α gene is composed of 13 exons and spans over 70 kb. It has multiple alternatively spliced variants encoded by two separate promoters, denoted as P1 and P2. Different transcription patterns from these promoters and alternative splicing result in functionally distinct HNF4α isoforms [2]. The NCBI Reference Sequences (RefSeq) section indicates 10 isoforms of HNF4α but other publications have reported 12 isoforms. Ko et al. have argued that HNF4α4 and HNF4α6 are also functional and distinct homodimers of different HNF4α isoforms regulate different sets of genes [2]. HNF4α1-6 are derived from the P1 promoter, whereas isoform HNF4α7-12 are derived from promoter P2 [3]. It has been proposed that multiple isoforms are thought to have different physiological roles in the transcriptional regulation of target genes [4, 5]. The structural difference between the promoters leads to different capabilities in interacting with transcription factors and cofactors, resulting in the transcriptional regulation of target genes in a specific manner. HNF4α expression is regulated by the P2 promoter during early liver development and controls the expression of fetal liver-specific genes, such as α-fetoprotein and TTR. At birth, regulation of HNF4α expression switches from P2 to P1 promoter and the adult form of HNF4α assumes control of various genes involved in postnatal and adult hepatic differentiation, such as APOCIII, APOB, F12, CLDN1 [6, 7]. Recent work from the Sladek group showed that mice with exclusive expressions of HNF4α-P1 and P2 have physiological role in normal adult liver. HNF4α-P1 regulates gluconeogenesis whereas HNF4α-P2 regulates ketogenesis in an adult liver in a circadian fashion [8].
HNF4α has classic nuclear receptor A-F modular structure domains [9]. It consists of the activation functions (AF)-1 and AF-2, which activate transcription in a cell-type independent manner, and a DNA binding domain, a ligand binding domain, and an activity repressor domain [9]. Despite having a ligand-binding domain, HNF4α is considered an orphan receptor because a ligand that can activate this receptor has yet to be identified. Studies have shown that fatty acids, such as linoleic acid, react with the ligand binding domain of HNF4α and give it structural integrity and stability [10]. HNF4α forms a homodimer and binds to the DNA recognition site of the direct repeat (DR1) element AGGTCA. Upon binding, HNF4α recruits transcriptional coactivators and appropriate accessory proteins to positively regulate the expression of target genes [11].
HNF4α in liver development
The liver development process is divided into three steps including competence, specification and morphogenesis. It involves the interaction of the cardiac mesoderm and foregut endoderm followed by interaction with the mesenchyme of the septum transversum and differentiation. In mice, at embryonic day 8 (ED8), the ventral wall of the foregut endoderm initiates the development toward a hepatic fate in response to growth factor signaling [12, 13]. At ED9, cells within the foregut endoderm start to proliferate and form the liver bud. At this point, hepatoblasts migrate from the foregut endoderm toward the septum transversum [14]. This is followed by the differentiation of cells into hepatocytes, maturation of hepatic vasculature, and biliary tract development[15].
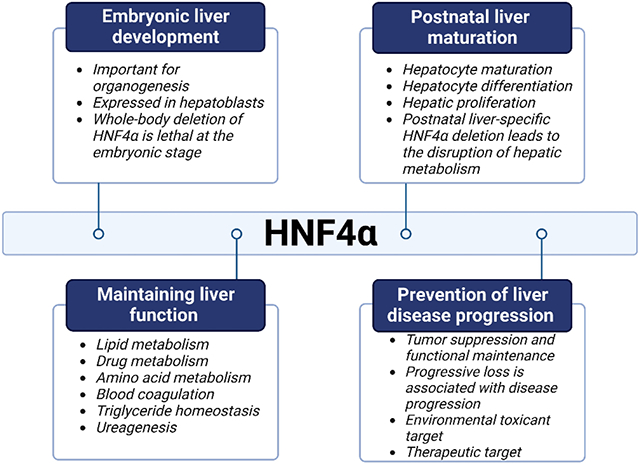
There are many liver-enriched transcription factors, such as HNF1α and β, HNF3α, β, and γ, HNF4α, and HNF6, which act as transcriptional regulators of liver development [16]. Of all these, HNF4α is most critical for regulating genes contributing to hepatocyte maturation and is considered a central regulator of hepatogenesis [17]. HNF4α plays a crucial role during embryonic development, as well as in organogenesis. Homozygous mutants of HNF4α die during gastrulation because of defective visceral endoderm function [18]. HNF4α is expressed first during early development in the primary endoderm and then in the visceral endoderm and is expressed at higher levels in epithelial cells during the differentiation phase [19]. In situ hybridization studies have tracked HNF4α expression in mouse liver development [19]. During the blastocyte stage, HNF4α is found in the primitive endoderm. As the cell differentiates to become the visceral endoderm of the yolk sac, HNF4α levels in the hindgut and midgut decrease. Further, hepatoblasts, the bipotential progenitors in the embryonic liver, primarily express HNF4α along with other factors [19]. Whole-body deletion of HNF4α is lethal at the embryonic stage [18]. Postnatal liver-specific HNF4α deletion under albumin promoter using cre recombinase leads to the disruption of hepatic metabolism, resulting in lethality between 6-8 weeks of age. These data indicate the importance of HNF4α in embryonic liver development and postnatal liver maturation [18, 20].
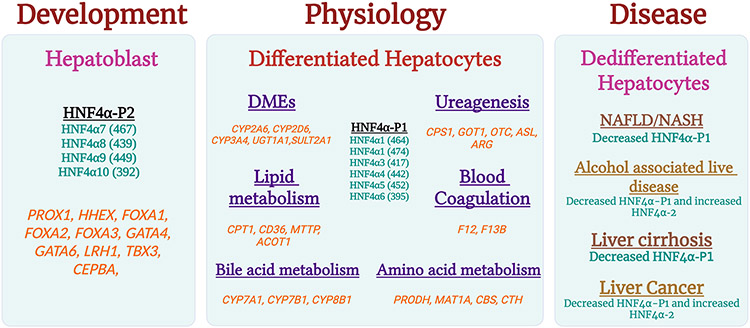
During fetal liver development, the HNF4α-P2 isoform is prevalently present. It activates the promoters of genes in early fetal development. HNF4α-P2 expression decreases at birth and eventually vanishes, whereas, after the differentiation phase, HNF4α-P1 isoform is dominantly present in the adult liver. It induces the promotor of genes expressed postnatally in adult livers [7, 21]. Failure to maintain the HNF4α-P1 isoform in adult livers is a hallmark of multiple liver diseases, including carcinogenesis. Interestingly, downregulation of HNF4α-P1 and elevation in HNF4α-P2 isoform has been reported in alcoholic hepatitis and hepatocellular carcinoma [22, 23] (Fig. 1). [22, 24-37]
Figure 1: HNF4α isoforms in physiological stages with corresponding genes regulated by HNF4α.
Functions and genes regulated by HNF4α isoforms during liver development and physiology, and HNF4α alterations in liver diseases.
HNF4α in liver differentiation
HNF4α is known as a master regulator of hepatic differentiation because it regulates a variety of hepatic functions. HNF4α plays a pivotal role in maintaining hepatocyte function via transcriptional regulation. In a mature adult liver, HNF4α plays a crucial role in maintaining hepatocyte differentiation. It regulates important hepatic metabolic functions (Table 1).
Table 1:
Metabolic processes regulated by HNF4α
| HNF4α manipulation | Organism | Effect | Reference |
|---|---|---|---|
| Liver-specific HNF4α deletion | Mouse | Disrupted bile acid conjugation | [31, 91] |
| Liver-specific HNF4α deletion | Mouse | Disrupted blood coagulation homeostasis | [30] |
| Liver-specific HNF4α deletion | Mouse | Defective ureagenesis | [92] |
| Liver-specific HNF4α deletion | Mouse | Decreased expression of genes involved in amino acid metabolism | [32] |
| INS-1 Cell line with dominant negative mutant HNF4α | Rat | Blunted insulin release induced by glucose | [93] |
| siRNA-mediated knockdown of HNF4α in isolated hepatocytes | Rat | Decreased glucose production | [94] |
| Hepatocyte-specific HNF4α deletion | Mouse | Disrupted lipid and carbohydrate homeostasis | [34] |
| Small hairpin RNA-mediated deletion of HNF4α | Mouse | Disrupted triglyceride and cholesterol homeostasis | [35] |
| Hepatocyte-specific HNF4α deletion | Mouse | Steatosis and depletion of glycogen | [33] |
| Liver-specific deletion | Mouse | Altered hepatic fatty acid metabolism | [95] |
| Antisense RNA or siRNA mediated knockdown of HNF4α in human hepatocytes | Human | Reduced expression of genes involved in drug metabolism | [28, 29] |
Plasma proteins such as albumin, transferrin and TRR are considered markers of hepatocyte differentiation [38, 39]. Targeted knockdown of HNF4α results in decreased expression of albumin and TRR [39, 40]. The absence of HNF4α in the liver results in metabolic disruption and increased mortality. In quiescent hepatocytes, along with regulating differentiation, HNF4α suppresses the expression of the promitogenic genes involved in proliferation [41, 42]. Deletion of HNF4α in hepatocytes results in a significantly increased liver/body weight ratio and a decrease in classic hepatocyte gene expression [33]. Loss of HNF4α has also been shown to result in the accumulation of lipids, increased serum bile acid levels, and reduced serum cholesterol and triglyceride levels [33].
HNF4α in hepatocyte proliferation
One of the first pieces of evidence about HNF4α’s role in maintaining normal liver/body weight ratio and inhibiting hepatocyte proliferation came from Hayhurst et al. They showed that hepatocyte-specific deletion of HNF4α (AlbCre; HNF4αFl/Fl) in mice had increased liver/body weight ratio (4.0 ± 0.3 to 7.3 ± 0.9) [33]. This observation was confirmed by studies from our group in which HNF4α was deleted in adult HNF4α-floxed mice using either a MUP-icre, tamoxifen-inducible albumin cre or AAV8-TBG-cre [41-43]. These studies indicate that in a quiescent hepatocyte, HNF4α positively regulates the expression of genes involved in hepatocyte differentiation and at the same time inhibits the expression of genes involved in hepatocyte proliferation. RNAseq analysis of liver of the hepatocyte specific adult HNF4α-KO mice revealed majority of the genes upregulated following HNF4α deletion were involved in cell proliferation including Ki-67, several cyclins including cyclin A2, B1 and B2, Egr1, Ect2 and cMyc. Microarray analysis of HNF4α-KO mice showed upregulation of many genes involved in cell proliferation and cell cycle progression, ultimately leading to cancer [20]. These studies confirmed that ablation of HNF4α leads to profound changes in hepatocyte-specific gene expression, resulting in dedifferentiation and increased proliferation [44].
Our group has also investigated the role HNF4α deletion in different liver injury models to analyze the proliferation response. In the first model of chemical-induced HCC, mice were treated with hepatic carcinogen diethylnitrosamine, or DEN, at postnatal day 15. To observe the effect of HNF4α in HCC progression, we deleted HNF4α at 8 months of age and tumor progression was allowed for two additional months. Deletion of HNF4α resulted in significant HCC progression with an increase in tumor size and numbers and a two-fold increase in liver/body weight ratio. HNF4α-KO in combination with DEN showed robust tumor morphology and increased expression of pro-mitogenic genes such as CyclinD1 and cMyc. These data highlighted the role of HNF4α in inhibiting hepatocyte proliferation by down regulation of pro-mitogenic genes [42] .
In the other study, partial hepatectomy (PH) was performed on hepatocyte specific HNF4α-KO mice. Despite showing increased expression of classic proliferation markers such as Cyclin D1 and PCNA, we observed a 100% mortality rate in HNF4α-KO mice by 11 days post PH. Further, to test if test if HNF4α re-expression could rescue HNF4α-KO mice after PH, HNF4α was introduced intravenously (AAV8-CMV-HNF4α) 2 days after PH in these mice. AAV8-mediated re-expression of HNF4α decreased proliferation and improved survival of HNF4α-KO mice post-PH. These results indicated that HNF4α re-expression is critical for the termination phase of liver regeneration after PH [41]. These studies confirm the role HNF4α as a critical player in hepatocyte proliferation.
HNF4α in liver disease
HNF4α function is an important parameter of liver disease progression. HNF4α function declines as liver disease progresses from steatosis to steatohepatitis (NASH) to cirrhosis, ultimately leading to hepatocellular carcinoma (HCC) [36]. In the case of acute liver injury, temporary loss of HNF4α leading to reduced hepatocyte-specific gene expression, can be restored with recovery from injury. However, in the case of chronic liver injury, persistent suppression of HNF4α function contributes to the progression of liver disease [26]. Therefore, it is important to understand the role of HNF4α in liver disease progression.
NAFLD/NASH
HNF4α is a major regulator of genes involved in lipid homeostasis. HNF4α null mice accumulate lipids and show increased bile concentrations and reduced serum cholesterol and triglyceride levels. The absence of HNF4α disrupts VLDL secretion and bile uptake [33]. HNF4α promotes hepatic triglyceride lipolysis, fatty acid oxidation and VLDL secretion and prevents triglyceride accumulation. Overexpression of HNF4α protects against high-fat diet-induced NAFLD to NASH progression [27]. Consistently, deletion of HNF4α in the adult liver results in significant steatosis [20, 42, 43]. Studies have shown that a high-fat diet retains HNF4α in the cytoplasm, causing downregulation of genes involved in VLDL secretion, such as ApoB. Fatty liver-induced oxidative stress activates the protein kinase C-mediated phosphorylation of HNF4α and blocks its nuclear entry [45]. Integrative computational analysis of transcriptomic data in patients with NASH proved HNF4α to be a central gene in the pathogenesis of NASH [46]. Collectively, these studies highlight the importance of HNF4α expression in the pathogenesis of NAFLD and NASH.
Alcoholic liver disease
In humans, studies have indicated that HNF4α expression is significantly reduced in alcoholic liver disease. Alcoholic hepatitis (AH) is characterized by a significant decrease in liver-specific genes regulated by HNF4α. AH is shown to induce HNF4α P2 promoter in hepatocytes, resulting in defective metabolic and synthetic functions [22]. HNF4α and carboxylesterase 1 (CES1), an enzyme involved in triglyceride metabolism, have been shown to decrease in patients with alcoholic steatohepatitis. In this study, alcohol also reduced HNF4α and CES1 expression in primary hepatocyte cell culture [47]. Similarly, chronic alcohol exposure has been proven to repress the HNF4α gene and protein expression, as well as the DNA binding activity of HNF4α in mice [48].
Liver cancer
Many studies have shown that HNF4α expression in reduced in liver cancers [42, 49-51]. Studies have shown a significant decrease in HNF4α expression in human HCC samples, leading to a poor prognosis of the disease [52]. HNF4α plays an important role in HCC metastasis by promoting the process of mesenchymal to epithelial transition [53]. The double negative feedback mechanism of YAP and HNF4α has been shown to regulate cell proliferation. Tissue microarray IHC of HCC indicated increased YAP and decreased HNF4α immunoreactivity compared to adjacent tissue [54]. HNF4α plays a crucial role in liver cancer pathogenesis. One of the reasons behind loss of HNF4α stimulating cancer promotion could be increased proliferation response. Similarly, studies have shown that upregulated genes after HNF4α ablation are involved with cell proliferation, cell cycle progression and cancer [20, 42, 43]. Loss of HNF4α-P1 isoform have is observed in fatty livers [20]. Chronic high fat diet which can cause fatty liver phenotype also results in reduced HNF4-P1 activity [55]. In the study done by Baharan Fekry et al. HNF4α-KO floxed mice were given tamoxifen inducible albumin cre to delete the HNF4α gene and given high fat diet for 38 weeks. Results demonstrated that loss of HNF4α-P1 in combination with high fat diet provide an environment for HCC development in male and female mice. RNA sequencing analysis from the livers of these mice revealed that genes involved in beta-catenin independent WNT signaling were upregulated and genes involved in TNF signaling as well as tumor suppressor genes such as Tp53 (p53) were downregulated in these mice. These data shed light on loss of HNF4α in contributing to NAFLD induced HCC [56].
Yin Chuan et al. have shown that forced re-expression of HNF4α can re-establish differentiated hepatoma cell morphology and abolish their tumorigenesis in mice. In their study, an adenovirus-mediated gene delivery system, AdCMV-HNF4α, was used to transfer HNF4α in Hep3B and HepG2 cells in vitro. Re-expression of HNF4α significantly reduced cancer stem cell markers in these cells. HNF4α also induced cell cycle arrest in senescence in HepG2 cells and suppressed tumor cell proliferation. Additionally, HNF4α showed an antitumor effect by reducing the carcinogenicity ability of hepatoma cells when introduced in mice. Interestingly, administration of HNF4α gene protected mice from liver metastatic tumor formation [57].
Chemical-induced injury
Drug-induced liver injury (DILI) is a very common cause of acute liver failure in the US [58]. Acetaminophen (APAP) overdose is a classic example of a dose-dependent and acute DILI. APAP is a very commonly used antipyretic and analgesic drug. It is highly effective at therapeutic doses; however, it can cause significant liver injury at higher doses. Maintaining hepatocyte proliferation is critical for regeneration after APAP-induced liver injury. Loss of HNF4α leads to dedifferentiation and increased proliferation of hepatocytes [42]. It has also been shown that deletion of HNF4α induces cMyc mRNA and protein expression [41, 43]. Recent studies from our laboratory show that maintaining HNF4α expression after APAP-induced liver injury is very important for regeneration and recovery. We found that maintaining nuclear HNF4α expression is important for regeneration and recovery after an APAP overdose. Further studies indicated that HNF4α cooperates with Nrf2 to promote GSH replenishment, which contributes to faster recovery after acetaminophen overdose-induced liver injury. In severe APAP overdose, the decrease in HNF4α protein results in activation of cMyc, which inhibits HNF4α-Nrf2 interaction preventing GSH replenishment and promoting injury [59].
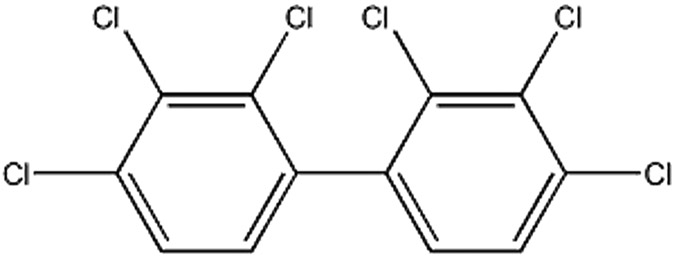
Liver is also a common target of several environmental contaminants. Polychlorinated biphenyls (PCBs) are manmade toxic chemicals that can cause liver injury [60, 61]. Proteomics analysis of mouse liver samples from a PCB chronic exposure study has shown that Aroclor 1260, a mixture of PCB, negatively regulates several nuclear receptors, including HNF4α (Fig.2). It reduces the expression of HNF4α protein and mRNA levels [62, 63].
Figure 2: 2D chemical structure of Aroclor 1260.
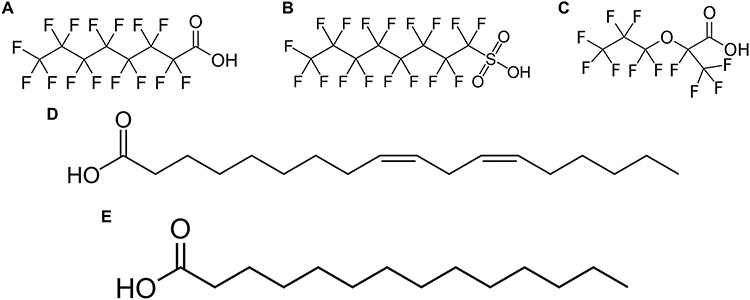
Another class of environmental chemicals that affect HNF4α in hepatocytes are the polyfluoroalkyl substances (PFAS). PFAS are a ubiquitous environmental contaminant with extremely long half-lives that affect several organs including the liver [64-66]. Due to their fluorinated structure, these compounds are extremely stable and resistant to degradation by natural methods [67]. The two most commonly found PFAS in the environment are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) [68]. These two PFAS have a chemical structure similar to that of fatty acids, in which they contain a fluorinated carbon chain and, under deprotonation, a polar head (Fig. 3 A-B). Given this, they are known to bioaccumulate in mammals, with long half-lives in the magnitude of years [69, 70]. In response, the United States government faded out the production of these legacy (PFOA and PFOS) compounds, leading to the production of newer alternative PFAS, such as hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) (Fig. 3C) [71, 72]. Given their ubiquitous presence in human serum, ongoing research is being conducted to study the health impacts of these compounds.
Figure 3. Compounds that bind to HNF4α ligand binding domain.
2D chemical structures of the PFAS: (A) PFOA, (B) PFOS, and (C) GenX. The chemical structure of the proposed HNF4α ligands, (D) linoleic acid and (E) myristic acid.
In rodent livers, these compounds (particularly PFOA and PFOS) are known to induce hepatocyte proliferation. The mechanism is widely thought to be through the induction of the peroxisome proliferator-activated receptor alpha (PPARα), a human-irrelevant mechanism of action [73, 74]. However, recent studies have examined the role in isolated primary human hepatocytes and have found a strong induction of cell proliferation [75, 76]. Studies indicate that increase in cell proliferation by PFAS may involve inhibition of HNF4α [77]. Upon exposure to PFOA, PFOS, and GenX, HNF4α levels significantly decreased with concordant increases in cell proliferation markers [75, 76]. In primary human hepatocytes, PFOA concentrations ranging from 500-10,000 nM reduced HNF4α levels 96 hours after exposure, whereas PFOS HNF4α inhibition ranged from 100-10,000 nM [76]. A proteomic analysis performed on HEPG2 cells treated with 25 μM PFOA showed a loss of HNF4α activity [78]. Further investigation utilizing HNF4α reporters in both HEPG2 and HEK293 cells found that HNF4α is inhibited at concentrations ranging from 1-50 μM, with 50 μM being the highest concentration tested [78]. Lastly, GenX was found to reduce HNF4α levels in primary human hepatocytes at 0.1, 10, and 100 μM concentrations during 96-hour exposure [75]. These data indicate that PFOA, PFOS, and GenX inhibit HNF4α by reducing HNF4α levels.
It is well established that phosphorylation of HNF4α leads to the targeting of HNF4α to the proteome. Protein chemical docking has been utilized to determine the extent of binding of PFOA and PFOS to HNF4α [76]. Linoleic acid and myristic acid are two known ligands for HNF4α (Fig.3 D-E) [79, 80]. Given PFOA chemical structure similarities to linoleic acid and myristic acid, it is not surprising that the two PFAS bind to the HNF4α [76]. PFOA binds in the ligand-binding domain pocket and PFOS binds outside of this domain, eliciting the same response [76]. This difference in HNF4α binding location is likely due to the sulfonic acid in PFOS rather than the carboxylic acid in PFOA. In the case of GenX, it is unknown whether it directly interacts with HNF4α. However, given its similarities to PFOA, it is reasonable to assume it is binding, to HNF4α, in the same location as PFOA. These PFAS binding events are then speculated to cause HNF4α destabilization, targeting HNF4α to the proteome and causing a reduction in HNF4α protein levels [76]. Altogether, HNF4α is a susceptible target for environmental pollutants that affect hepatocyte function.
HNF4α as a therapy
For patients with end-stage liver disease and terminal liver failure, liver transplantation is the only treatment. The role of HNF4α in the development and differentiation of hepatocytes has made it a potential therapeutic target and an alternative to transplantation [51, 81]. Increased expression of HNF4α has been shown as a potential option for hepatic fibrosis therapy by suppressing the epithelial-to-mesenchymal transition [82]. Bin Shi et al. provided evidence that enforced HNF4α downregulates profibrogenic markers and can resolve early-stage cirrhosis via suppressing ERK signaling pathways and increasing collagenolytic activity [83]. HNF4α functions as a tumor suppressor in HCC. Upregulation of HNF4α can block HCC development by inhibiting β- catenin signaling pathways [49].
Nishikawa Taichiro et al. developed a carbon tetrachloride (CCl4) induced progressive liver failure model in rats and found that along with HNF4α, other hepatocyte network transcription factors were downregulated in end-stage hepatocytes isolated from the rats. Further, forceful re-expression of HNF4α through AAV vectors restored functionality in diseased hepatocytes from CCl4 treated rats and induced expression of other transcription factors. It also restored diseased hepatocytes and reversed fatal liver failure [84]. These studies suggest the role of HNF4α as a potential therapeutic target for the prevention of liver disease progression.
The mRNA-based approach has been used for the development of cancer immunotherapy and vaccines for infectious diseases [85, 86]. A recent study showed that mRNA-mediated reinforcement of HNF4α can restore the expression of hepatic genes and transcription factors to some degree in hepatocytes isolated from the cirrhotic liver [81]. Yang et al. identified that mRNA-mediated delivery of HNF4α via lipid nanoparticles can attenuate liver fibrosis and cirrhosis in mice [87]. This suggests that restoring HNF4α expression in damaged hepatocytes during the disease condition can be therapeutic.
Recently, our lab developed a HNF4α target gene signature. We the performed in silico analysis of 30 independent datasets containing around 3500 individual samples and found that changes in HNF4α were associated with chronic liver disease progression [36].Our studies demonstrated a novel HNF4α target gene signature that is used to predict outcomes in cirrhosis and HCC. This can also be used as a prognostic tool with the use of advanced technology such as Nanostring to measure mRNA expression of HNF4α target genes in liver tissue samples.
Regardless of its potential as a therapeutic target, the search for a pharmacological activator for HNF4α has been fruitless. Finding a natural ligand or a small molecule or biological activator of HNF4α could transform the therapeutic for many liver diseases. Earlier, small molecule regulators that can modulate HNF4α were investigated. Few compounds containing a nitro group and naphthofuran backbone were found to enhance HNF4α transcriptional activity by binding to HNF4α ligand binding domain [88]. Meijer et al., recently screened 480 drug fragments for HNF4α modulation in cell culture. They found three validated compounds that modulated mRNA expression of HNF4α genes in human hepatocytes by directly binding to the recombinant HNF4α ligand binding domain with micromolar potencies [89]. For phenotypic studies these HNF4α modulators can serve as a preliminary chemogenic tool. However, further investigation is required to develop high potential ligands for HNF4α.
In the recent study from our laboratory, we treated mice with mouse specific activators of Aryl hydrocarbon Receptor (AhR), Constitutive Androstane Receptor (CAR), Pregnane X Receptor (PXR), and Peroxisome Proliferator-Activated Receptor-alpha (PPARα) for their activation. We found that genes positively regulated by HNF4α were significantly downregulated after activation of CAR, PXR and PPARα. Thus, we speculate that maybe HNF4α does not require ligand for the activation and its interaction with other nuclear receptors is what changes the expression of HNF4α (Unpublished work) [90].
Summary
HNF4α is a unique nuclear receptor in multiple ways. It is expressed in multiple tissues of endodermal origin, but it is most known for its central role in liver homeostasis and function. There is currently no known ligand for HNF4α but it is known to work with several cofactors. In the liver, HNF4α is required for hepatic differentiation and inhibits hepatocyte proliferation. HNF4α expression is reduced in chronic liver diseases, and its decline in HFN4α function is associated with progression of liver diseases from more manageable early stages to a severe end stage liver disease. Because of this, and because of its pro-differentiation and anti-proliferative roles, it has become an attractive therapeutic target in several liver diseases. The next major challenge in developing HNF4α based therapies is to identify novel mechanisms by which sustained HFN4α reactivation can be achieved.
References:
- 1.Sladek FM, et al. , Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev, 1990. 4(12B): p. 2353–65. [DOI] [PubMed] [Google Scholar]
- 2.Ko HL, Zhuo Z, and Ren EC, HNF4alpha Combinatorial Isoform Heterodimers Activate Distinct Gene Targets that Differ from Their Corresponding Homodimers. Cell Rep, 2019. 26(10): p. 2549–2557 e3. [DOI] [PubMed] [Google Scholar]
- 3.Lau HH, et al. , The molecular functions of hepatocyte nuclear factors - In and beyond the liver. J Hepatol, 2018. 68(5): p. 1033–1048. [DOI] [PubMed] [Google Scholar]
- 4.Jiang S., et al. , Expression and localization of P1 promoter-driven hepatocyte nuclear factor-4alpha (HNF4alpha) isoforms in human and rats. Nucl Recept, 2003. 1: p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briancon N and Weiss MC, In vivo role of the HNF4alpha AF-1 activation domain revealed by exon swapping. EMBO J, 2006. 25(6): p. 1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drewes T., et al. , Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol Cell Biol, 1996. 16(3): p. 925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Padilla ME, Fougere-Deschatrette C, and Weiss MC, Expression of HNF4alpha isoforms in mouse liver development is regulated by sequential promoter usage and constitutive 3' end splicing. Mech Dev, 2001. 109(2): p. 183–93. [DOI] [PubMed] [Google Scholar]
- 8.Deans JR, et al. , HNF4α isoforms regulate the circadian balance between carbohydrate and lipid metabolism in the liver. bioRxiv, 2021: p. 2021.02.28.433261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadzopoulou-Cladaras M., et al. , Functional domains of the nuclear receptor hepatocyte nuclear factor 4. J Biol Chem, 1997. 272(1): p. 539–50. [DOI] [PubMed] [Google Scholar]
- 10.Yuan X., et al. , Identification of an endogenous ligand bound to a native orphan nuclear receptor. PLoS One, 2009. 4(5): p. e5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez FJ, Regulation of hepatocyte nuclear factor 4 alpha-mediated transcription. Drug Metab Pharmacokinet, 2008. 23(1): p. 2–7. [DOI] [PubMed] [Google Scholar]
- 12.Duncan SA, Mechanisms controlling early development of the liver. Mech Dev, 2003. 120(1): p. 19–33. [DOI] [PubMed] [Google Scholar]
- 13.Jung J., et al. , Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science, 1999. 284(5422): p. 1998–2003. [DOI] [PubMed] [Google Scholar]
- 14.Zhao R and Duncan SA, Embryonic development of the liver. Hepatology, 2005. 41(5): p. 956–67. [DOI] [PubMed] [Google Scholar]
- 15.Lemaigre F and Zaret KS, Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev, 2004. 14(5): p. 582–90. [DOI] [PubMed] [Google Scholar]
- 16.Duncan SA, Transcriptional regulation of liver development. Dev Dyn, 2000. 219(2): p. 131–42. [DOI] [PubMed] [Google Scholar]
- 17.Watt AJ, Garrison WD, and Duncan SA, HNF4: a central regulator of hepatocyte differentiation and function. Hepatology, 2003. 37(6): p. 1249–53. [DOI] [PubMed] [Google Scholar]
- 18.Chen WS, et al. , Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev, 1994. 8(20): p. 2466–77. [DOI] [PubMed] [Google Scholar]
- 19.Duncan SA, et al. , Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci U S A, 1994. 91(16): p. 7598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonzo JA, et al. , Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4alpha in adult mice. J Biol Chem, 2012. 287(10): p. 7345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briancon N., et al. , Expression of the alpha7 isoform of hepatocyte nuclear factor (HNF) 4 is activated by HNF6/OC-2 and HNF1 and repressed by HNF4alpha1 in the liver. J Biol Chem, 2004. 279(32): p. 33398–408. [DOI] [PubMed] [Google Scholar]
- 22.Argemi J., et al. , Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis. Nat Commun, 2019. 10(1): p. 3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka T., et al. , Dysregulated expression of P1 and P2 promoter-driven hepatocyte nuclear factor-4alpha in the pathogenesis of human cancer. J Pathol, 2006. 208(5): p. 662–72. [DOI] [PubMed] [Google Scholar]
- 24.NCBI.
- 25.DeLaForest A., et al. , HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development, 2011. 138(19): p. 4143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois V., et al. , Control of Cell Identity by the Nuclear Receptor HNF4 in Organ Pathophysiology. Cells, 2020. 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y., et al. , Hepatocyte Nuclear Factor 4alpha Prevents the Steatosis-to-NASH Progression by Regulating p53 and Bile Acid Signaling (in mice). Hepatology, 2021. 73(6): p. 2251–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jover R., et al. , Cytochrome P450 regulation by hepatocyte nuclear factor 4 in human hepatocytes: a study using adenovirus-mediated antisense targeting. Hepatology, 2001. 33(3): p. 668–75. [DOI] [PubMed] [Google Scholar]
- 29.Kamiyama Y., et al. , Role of human hepatocyte nuclear factor 4alpha in the expression of drug-metabolizing enzymes and transporters in human hepatocytes assessed by use of small interfering RNA. Drug Metab Pharmacokinet, 2007. 22(4): p. 287–98. [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y., et al. , Role of hepatocyte nuclear factor 4alpha in control of blood coagulation factor gene expression. J Mol Med (Berl), 2006. 84(4): p. 334–44. [DOI] [PubMed] [Google Scholar]
- 31.Inoue Y., et al. , Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J Lipid Res, 2006. 47(1): p. 215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamiya A., et al. , Hepatocyte nuclear factors 1alpha and 4alpha control expression of proline oxidase in adult liver. FEBS Lett, 2004. 578(1-2): p. 63–8. [DOI] [PubMed] [Google Scholar]
- 33.Hayhurst GP, et al. , Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol, 2001. 21(4): p. 1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huck I., et al. , Hepatocyte-Specific Hepatocyte Nuclear Factor 4 Alpha (HNF4) Deletion Decreases Resting Energy Expenditure by Disrupting Lipid and Carbohydrate Homeostasis. Gene Expr, 2021. 20(3): p. 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin L., et al. , Hepatic hepatocyte nuclear factor 4alpha is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol, 2011. 31(2): p. 328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunewardena S., et al. , Progressive loss of hepatocyte nuclear factor 4 alpha activity in chronic liver diseases in humans. Hepatology, 2022. 76(2): p. 372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai SH, et al. , Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Therap Adv Gastroenterol, 2017. 10(10): p. 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal S, Holton KL, and Lanza R, Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells, 2008. 26(5): p. 1117–27. [DOI] [PubMed] [Google Scholar]
- 39.Nagaki M., et al. , Regulation of hepatic genes and liver transcription factors in rat hepatocytes by extracellular matrix. Biochem Biophys Res Commun, 1995. 210(1): p. 38–43. [DOI] [PubMed] [Google Scholar]
- 40.Kimata T., et al. , Hepatocyte nuclear factor-4alpha and −1 small interfering RNA inhibits hepatocyte differentiation induced by extracellular matrix. Hepatol Res, 2006. 35(1): p. 3–9. [DOI] [PubMed] [Google Scholar]
- 41.Huck I., et al. , Hepatocyte Nuclear Factor 4 Alpha Activation Is Essential for Termination of Liver Regeneration in Mice. Hepatology, 2019. 70(2): p. 666–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walesky C., et al. , Hepatocyte nuclear factor 4 alpha deletion promotes diethylnitrosamine-induced hepatocellular carcinoma in rodents. Hepatology, 2013. 57(6): p. 2480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walesky C., et al. , Hepatocyte-specific deletion of hepatocyte nuclear factor-4alpha in adult mice results in increased hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol, 2013. 304(1): p. G26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walesky C and Apte U, Role of hepatocyte nuclear factor 4alpha (HNF4alpha) in cell proliferation and cancer. Gene Expr, 2015. 16(3): p. 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu D., et al. , High fat diet-induced oxidative stress blocks hepatocyte nuclear factor 4alpha and leads to hepatic steatosis in mice. J Cell Physiol, 2018. 233(6): p. 4770–4782. [DOI] [PubMed] [Google Scholar]
- 46.Baciu C., et al. , Systematic integrative analysis of gene expression identifies HNF4A as the central gene in pathogenesis of non-alcoholic steatohepatitis. PLoS One, 2017. 12(12): p. e0189223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J., et al. , Carboxylesterase 1 Is Regulated by Hepatocyte Nuclear Factor 4alpha and Protects Against Alcohol- and MCD diet-induced Liver Injury. Sci Rep, 2016. 6: p. 24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong W, et al. , Inactivation of hepatocyte nuclear factor-4alpha mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol, 2010. 299(3): p. G643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ning BF, et al. , Hepatocyte nuclear factor 4 alpha suppresses the development of hepatocellular carcinoma. Cancer Res, 2010. 70(19): p. 7640–51. [DOI] [PubMed] [Google Scholar]
- 50.Ning BF, et al. , Hepatocyte nuclear factor 4alpha-nuclear factor-kappaB feedback circuit modulates liver cancer progression. Hepatology, 2014. 60(5): p. 1607–19. [DOI] [PubMed] [Google Scholar]
- 51.Zeng X., et al. , Recombinant adenovirus carrying the hepatocyte nuclear factor-1alpha gene inhibits hepatocellular carcinoma xenograft growth in mice. Hepatology, 2011. 54(6): p. 2036–47. [DOI] [PubMed] [Google Scholar]
- 52.Lazarevich NL, et al. , Deregulation of hepatocyte nuclear factor 4 (HNF4)as a marker of epithelial tumors progression. Exp Oncol, 2010. 32(3): p. 167–71. [PubMed] [Google Scholar]
- 53.Yao D, Peng S, and Dai C, The role of hepatocyte nuclear factor 4alpha in metastatic tumor formation of hepatocellular carcinoma and its close relationship with the mesenchymal-epithelial transition markers. BMC Cancer, 2013. 13: p. 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai WY, et al. , Yes-associated protein/TEA domain family member and hepatocyte nuclear factor 4-alpha (HNF4alpha) repress reciprocally to regulate hepatocarcinogenesis in rats and mice. Hepatology, 2017. 65(4): p. 1206–1221. [DOI] [PubMed] [Google Scholar]
- 55.Fekry B., et al. , Incompatibility of the circadian protein BMAL1 and HNF4alpha in hepatocellular carcinoma. Nat Commun, 2018. 9(1): p. 4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fekry B., et al. , HNF4alpha-Deficient Fatty Liver Provides a Permissive Environment for Sex-Independent Hepatocellular Carcinoma. Cancer Res, 2019. 79(22): p. 5860–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin C., et al. , Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology, 2008. 48(5): p. 1528–39. [DOI] [PubMed] [Google Scholar]
- 58.Ostapowicz G., et al. , Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med, 2002. 137(12): p. 947–54. [DOI] [PubMed] [Google Scholar]
- 59.Kotulkar M., et al. , Role of HNF4alpha-cMyc interaction in liver regeneration and recovery after acetaminophen-induced acute liver injury. Hepatology, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drinker CK WM, and Bennett GA THE PROBLEM OF POSSIBLE SYSTEMIC EFFECTS FROM CERTAIN CHLORINATED HYDROCARBONS. Journal of Industrial Hygiene and Toxicology, 1937(19(7): p. ): p. 283–311. [Google Scholar]
- 61.Maroni M., et al. , Occupational exposure to polychlorinated biphenyls in electrical workers. II. Health effects. Br J Ind Med, 1981. 38(1): p. 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardesty JE, et al. , Proteomic Analysis Reveals Novel Mechanisms by Which Polychlorinated Biphenyls Compromise the Liver Promoting Diet-Induced Steatohepatitis. J Proteome Res, 2019. 18(4): p. 1582–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahlang B., et al. , Identifying sex differences arising from polychlorinated biphenyl exposures in toxicant-associated liver disease. Food Chem Toxicol, 2019. 129: p. 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David S and Hamilton JP, Drug-induced Liver Injury. US Gastroenterol Hepatol Rev, 2010. 6: p. 73–80. [PMC free article] [PubMed] [Google Scholar]
- 65.Melaram R., Environmental Risk Factors Implicated in Liver Disease: A Mini-Review. Front Public Health, 2021. 9: p. 683719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costello E., et al. , Exposure to per- and Polyfluoroalkyl Substances and Markers of Liver Injury: A Systematic Review and Meta-Analysis. Environ Health Perspect, 2022. 130(4): p. 46001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saez M, de Voogt P, and Parsons JR, Persistence of perfluoroalkylated substances in closed bottle tests with municipal sewage sludge. Environ Sci Pollut Res Int, 2008. 15(6): p. 472–7. [DOI] [PubMed] [Google Scholar]
- 68.Thompson J, Eaglesham G, and Mueller J, Concentrations of PFOS, PFOA and other perfluorinated alkyl acids in Australian drinking water. Chemosphere, 2011. 83(10): p. 1320–5. [DOI] [PubMed] [Google Scholar]
- 69.Dunder L., et al. , Changes in plasma levels of per- and polyfluoroalkyl substances (PFAS) are associated with changes in plasma lipids - A longitudinal study over 10 years. Environ Res, 2022. 211: p. 112903. [DOI] [PubMed] [Google Scholar]
- 70.Li Y., et al. , Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med, 2018. 75(1): p. 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brennan NM, et al. , Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int J Environ Res Public Health, 2021. 18(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sunderland EM, et al. , A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol, 2019. 29(2): p. 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corton JC, Peters JM, and Klaunig JE, The PPARalpha-dependent rodent liver tumor response is not relevant to humans: addressing misconceptions. Arch Toxicol, 2018. 92(1): p. 83–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klaunig JE, et al. , PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol, 2003. 33(6): p. 655–780. [DOI] [PubMed] [Google Scholar]
- 75.Robarts DR, et al. , GenX induces fibroinflammatory gene expression in primary human hepatocytes. Toxicology, 2022. 477: p. 153259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beggs KM, et al. , The role of hepatocyte nuclear factor 4-alpha in perfluorooctanoic acid- and perfluorooctanesulfonic acid-induced hepatocellular dysfunction. Toxicol Appl Pharmacol, 2016. 304: p. 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robarts DR, et al. , Regulation of Liver Regeneration by Hepatocyte O-GlcNAcylation in Mice. Cell Mol Gastroenterol Hepatol, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scharmach E., et al. , Perfluorooctanoic acid affects the activity of the hepatocyte nuclear factor 4 alpha (HNF4alpha). Toxicol Lett, 2012. 212(2): p. 106–12. [DOI] [PubMed] [Google Scholar]
- 79.Kiselyuk A., et al. , HNF4alpha antagonists discovered by a high-throughput screen for modulators of the human insulin promoter. Chem Biol, 2012. 19(7): p. 806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhe-Paganon S., et al. , Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. J Biol Chem, 2002. 277(41): p. 37973–6. [DOI] [PubMed] [Google Scholar]
- 81.Tafaleng EN, et al. , Hepatocyte Nuclear Factor 4 alpha 2 Messenger RNA Reprograms Liver-Enriched Transcription Factors and Functional Proteins in End-Stage Cirrhotic Human Hepatocytes. Hepatol Commun, 2021. 5(11): p. 1911–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yue HY, et al. , Hepatocyte nuclear factor 4alpha attenuates hepatic fibrosis in rats. Gut, 2010. 59(2): p. 236–46. [DOI] [PubMed] [Google Scholar]
- 83.Fan TT, et al. , Regression effect of hepatocyte nuclear factor 4alpha on liver cirrhosis in rats. J Dig Dis, 2013. 14(6): p. 318–27. [DOI] [PubMed] [Google Scholar]
- 84.Nishikawa T., et al. , Resetting the transcription factor network reverses terminal chronic hepatic failure. J Clin Invest, 2015. 125(4): p. 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laczko D., et al. , A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity, 2020. 53(4): p. 724–732 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verbeke R., et al. , Co-delivery of nucleoside-modified mRNA and TLR agonists for cancer immunotherapy: Restoring the immunogenicity of immunosilent mRNA. J Control Release, 2017. 266: p. 287–300. [DOI] [PubMed] [Google Scholar]
- 87.Yang T., et al. , Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J Hepatol, 2021. 75(6): p. 1420–1433. [DOI] [PubMed] [Google Scholar]
- 88.Le Guevel R., et al. , Identification of small molecule regulators of the nuclear receptor HNF4alpha based on naphthofuran scaffolds. Bioorg Med Chem, 2009. 17(19): p. 7021–30. [DOI] [PubMed] [Google Scholar]
- 89.Meijer I., et al. , Chemical Starting Matter for HNF4alpha Ligand Discovery and Chemogenomics. Int J Mol Sci, 2020. 21(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kotulkar M, P.-C. D, Robarts DR, and Apte U, Regulation of Hepatic Xenosensor Function by HNF4alpha. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inoue Y, et al. , Hepatocyte nuclear factor 4alpha is a central regulator of bile acid conjugation. J Biol Chem, 2004. 279(4): p. 2480–9. [DOI] [PubMed] [Google Scholar]
- 92.Inoue Y., et al. , Defective ureagenesis in mice carrying a liver-specific disruption of hepatocyte nuclear factor 4alpha (HNF4alpha ). HNF4alpha regulates ornithine transcarbamylase in vivo. J Biol Chem, 2002. 277(28): p. 25257–65. [DOI] [PubMed] [Google Scholar]
- 93.Wang H., et al. , Hepatocyte nuclear factor 4alpha regulates the expression of pancreatic beta -cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J Biol Chem, 2000. 275(46): p. 35953–9. [DOI] [PubMed] [Google Scholar]
- 94.Park EY, et al. , HNF4alpha contributes to glucose formation in aged rat hepatocytes. Exp Gerontol, 2013. 48(12): p. 1518–25. [DOI] [PubMed] [Google Scholar]
- 95.Martinez-Jimenez CP, et al. , Hepatocyte nuclear factor 4alpha coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol Cell Biol, 2010. 30(3): p. 565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]