Abstract
Background:
Early endpoints in clinical trials of high-risk localized prostate cancer (HRLPC) that resemble those monitored in real-world practice could expedite clinical development.
Objective:
To assess the association of prostate-specific antigen (PSA) recurrence (PSA-R)-based early endpoints with metastasis-free survival (MFS), overall survival (OS), and prostate cancer (PC)-specific survival (PCSS), and to identify clinically undetectable disease.
Design, setting, and participants:
A post hoc analysis of patients with HRLPC from Radiation Therapy Oncology Group studies 9202, 9902, and 0521 was performed.
Intervention:
Long-term adjuvant androgen-deprivation therapy (ADT) and post-primary definitive radiotherapy.
Outcome measurements and statistical analysis:
Event-free survival (EFS; PSA-R, locoregional recurrence [LRR], distant metastasis [DM], or death), biochemical failure (PSA-R), general clinical failure (PSA-R, LRR, DM, ADT initiation, or death), and no evidence of disease (NED; alive patients without PSA-R, LRR, DM, and subsequent PC therapy, and with testosterone recovery) were assessed for association with MFS, OS, and PCSS using correlation and landmark analyses, Kaplan-Meier method, and Cox proportional-hazard model. PSA-R was defined as PSA nadir + 2 ng/ml; PSA nadir + 2 ng/ml and rising; PSA >5, 10, and 25 ng/ml; or PSA doubling time (PSADT) <6 mo.
Results and limitations:
Among assessed early endpoints, EFS with PSA nadir + 2 ng/ml and rising, or with PSA >5 ng/ml was associated with MFS, OS, and PCSS. No development of EFS with PSADT <6 mo or ADT initiation event or achievement of NED at 3 yr was associated with prolonged OS, MFS, and PCSS (hazard ratio [95% confidence interval], 0.53 [0.45–0.64], 0.63 [0.52–0.76], and 0.26 [0.18–0.36], or 0.56 [0.48–0.66], 0.62 [0.52–0.74], and 0.26 [0.19–0.37]) after the landmark time. Older studies performed before the current guidance should be interpreted with caution.
Conclusions:
We identified EFS with PSA nadir + 2 ng/ml and rising, PSA >5 ng/ml, or PSADT <6 mo ± ADT initiation and NED as potentially promising early endpoints in HRLPC that should be validated further.
Trial registration:
ClinicalTrials.gov identifiers NCT00004054, NCT00767286, NCT00288080
Keywords: No evidence of disease, Prostate-specific antigen, PSA
Patient summary
We identified novel clinical measure that can expedite development of new medicines for patients with localized prostate cancer at high risk of progression. These measures, which took into account prostate-specific antigen assessments and other clinical characteristics, should be confirmed in future studies. We also defined a novel measure of no evidence of disease that can help treating physicians identify patients with clinically undetectable disease.
Introduction
High-risk localized prostate cancer (HRLPC) is a localized disease at risk of metastasis and death [1], [2] without curative management [3]. Patients would be able to access medicines sooner if earlier-than-traditional survival endpoints are implemented as endpoints in clinical studies. Metastasis-free survival (MFS) has been reported as a strong surrogate endpoint of overall survival (OS) in localized prostate cancer (LPC) [4], but is increasingly confounded by lines of subsequent treatment [5], and its evaluation can require up to 4 yr from study inception in nonmetastatic castration-resistant prostate cancer (nmCRPC) [6], [7]. LPC is an earlier disease state than nmCRPC: the median time from first enrollment to data readout for the primary analysis was assessed at 11.5 yr [8]. This protracted reporting of clinical study results not only delays availability of new therapies, but also requires large patient populations to assess prostate cancer (PC)-specific deaths.
Additionally, divergent approaches are often taken when assessing disease progression in pivotal oncology clinical studies versus real-world physician practice [9], [10]. In clinical studies of LPC, estimates of time-to-event endpoints typically rely on regular tumor imaging in addition to death events [11]. In 28 clinical studies of LPC assessed by the Intermediate Clinical Endpoints in Cancer of the Prostate Working Group (ICECaP), 98% used OS, 77% used prostate-specific antigen (PSA) progression, 72% used MFS or time to metastases, and 58% used PC-specific mortality as endpoints [8]. In contrast, treating physicians are recommended to rely on early indicators of disease progression (eg, PSA progression and clinical symptoms) instead of imaging, resulting in rapid cycling through available treatment options [3]. Using efficacy endpoints that incorporate changes in PSA may help generate clinical data that are more reflective of the data in real-world practice.
Early, reliable indicators of disease progression would address a major challenge in evaluating new therapeutic interventions for HRLPC: most patients are elderly, with an average age of 66 yr at diagnosis [12] and varying degrees of comorbidity and frailty [13]. Competing causes of death or major health events often precede disease-specific death and other disease-specific endpoints [14], [15]. Follow-up that is often years away from the initiation of investigational therapy is required when traditional efficacy endpoints are used, which confounds mortality from non-PC causes, making it difficult to see a treatment effect [5].
Searching for early endpoints, we completed a post hoc analysis of three completed large phase 3 Radiation Therapy Oncology Group (RTOG) studies (9202, 9902, and 0521) in patients with HRLPC treated with primary definitive radiation therapy (RT) [16], [17], [18]. These studies were selected because of similarity in treatment groups, namely, long-term adjuvant androgen-deprivation therapy (ADT) after RT, availability of PSA and testosterone measurements, and date of ADT initiation. Shorter PSA doubling time (PSADT) is associated with worse prognosis in multiple disease stages and treatment scenarios [1], [19], [20], [21]. ADT initiation may indicate clinically significant disease that does not yet meet PSADT criteria but warrants systemic therapy as per the treating physician.
Our first objective was to explore whether modification of early endpoints with PSA kinetics thresholds and other parameters will improve their patient-level association with definitive endpoints (MFS, OS, and PC-specific survival [PCSS]). The early endpoints included in this analysis have been assessed for surrogacy with definitive endpoints previously: event-free survival (EFS) [22], biochemical failure (BCF) [22], [23], and general clinical failure (GCF) [24]. The second objective was to identify early endpoints of progression or clinically undetectable disease state in patients with HRLPC. The latter, similar to the minimal residual disease endpoint used in hematological malignancies [25], [26], [27], describes disease that has responded to investigational interventions but has not yet met the complete response definition. As no comparable early endpoint has been defined in HRLPC, we suggested a term “no evidence of disease” (NED).
Patients and methods
Study design and population
RTOG 9202, 9902, and 0521 enrolled patients from 1992 to 2005. The studies were approved by institutional review boards and followed the principles outlined in the Declaration of Helsinki. The RTOG studies were used to evaluate MFS, disease-free survival (DFS), EFS, BCF, and GCF as surrogate endpoints for OS and PCSS by ICECaP and others [4], [22], [23], [24]. Study designs have been reported [16], [17], [18]; studies included patients with median ages of 66 yr (RTOG 9902 and 0521) and 70 yr (RTOG 9202). Only patients who were treated with 24-mo (long-term) adjuvant ADT after RT were included in this analysis. Patient assessments, including imaging, were performed during follow-up visits every 3 mo during year 1, every 4 mo during year 2, every 6 mo from years 3 to 5, and annually thereafter (RTOG 9202); as clinically indicated and every 6 mo (RTOG 0521); or during follow-up visits every 3 mo in the first 2 yr, every 6 mo in the next 3 yr, and annually thereafter (RTOG 9902; additional information in the Supplementary material).
The analyzed population included 1229 patients (758, 189, and 282 from RTOG 9202, 9902, and 0521, respectively) who had HRLPC and received long-term ADT (Supplementary Table 1). One RTOG 0521 patient had no efficacy data and was excluded from efficacy analyses.
Endpoints
Early endpoints were event-driven disease progression (DFS, EFS, BCF, and GCF) and binary endpoints of disease progression (EFS [with PSADT <6 mo/ADT]) and undetectable disease (NED). Definitive endpoints were MFS, OS, and PCSS. Definitions and modifications of endpoints, locoregional recurrence, and PSA recurrence are provided in Table 1.
Table 1.
Definitions of endpoints
| Endpoints | Definitions |
|---|---|
|
| |
| Event-driven endpoints | |
| OS | Time from randomization to the date of death from PC or any other cause |
| MFS | Time from randomization to the date of distant metastases or death from any cause |
| PCSS | Time from randomization to the date of death from PC or complications related to the protocol treatment |
| DFS | Time from randomization to the date of locoregional recurrence, distant metastasis, or death |
| EFS (general definition) | Time from randomization to the date of any event of PSA recurrence, locoregional recurrence, distant metastasis, or death |
| Specific EFS definitions | Specific definitions are based on PSA recurrence defined as follows: |
| EFS (PSA nadir + 2 ng/ml) | PSA nadir + 2 ng/ml |
| EFS (PSA nadir + 2 ng/ml and rising) | PSA nadir + 2 ng/ml and rising |
| EFS (PSADT <3 mo) | PSADT <3 mo |
| EFS (PSADT <6 mo) | PSADT <6 mo |
| EFS (PSADT <9 mo) | PSADT <9 mo |
| EFS (PSA >5 ng/ml) | PSA >5 ng/ml |
| EFS (PSA >10 ng/ml) | PSA >10 ng/ml |
| EFS (PSA >25 ng/ml) | PSA >25 ng/ml |
| BCF (general definition) | Time from randomization to the date of PSA recurrence |
| Specific BCF definitions: | Specific definitions are based on PSA recurrence defined as: |
| BCF (PSA nadir+2 ng/ml) | PSA nadir+2 ng/ml |
| BCF (PSA nadir+2 and rising) | PSA nadir+2 and rising |
| BCF (PSADT <3 mo) | PSADT <3 mo |
| BCF (PSADT <6 mo) | PSADT <6 mo |
| BCF (PSADT <9 mo) | PSADT <9 mo |
| BCF (PSA >5 ng/ml) | PSA >5 ng/ml |
| BCF (PSA >10 ng/ml) | PSA >10 ng/ml |
| BCF (PSA >25 ng/ml) | PSA >25 ng/ml |
| GCF (general definition) | Time from randomization to the date of occurrence of locoregional recurrence, distant metastases, PSA recurrence after completion of radiation therapy, or initiation of ADT after completion of protocol treatment |
| Specific GCF definitions | Specific definitions are based on PSA levels and presencea or absence of ADT initiation or presence of death as an event |
| GCF (PSA >5 ng/ml) | PSA >5 ng/ml |
| GCF (PSA >10 ng/ml) | PSA >10 ng/ml |
| GCF (PSA >25 ng/ml) | PSA >25 ng/ml |
| GCF (PSA >5 ng/ml; no ADT) | PSA >5 ng/ml and ADT initiation is excluded |
| GCF (PSA >10 ng/ml; no ADT) | PSA >10 ng/ml and ADT initiation is excluded |
| GCF (PSA >25 ng/ml; no ADT) | PSA >25 ng/ml and ADT initiation is excluded |
| GCF (PSA >5 ng/ml + death) | PSA >5 ng/ml and death |
| GCF (PSA >10 ng/ml + death) | PSA >10 ng/ml and death |
| GCF (PSA >25 ng/ml + death) | PSA >25 ng/ml and death |
| Binary endpoints | |
| NED | Defined as patients meeting all of the following: 1. Alive 2. No biochemical recurrence (defined as PSA >0.5 ng/ml+nadir and rising) 3. No distant metastasis 4. No loco-regional recurrence 5. No subsequent therapy for PC 6. Testosterone recovery to pre-ADT levels (if available) b |
| EFS (PSADT <6 mo/ADT) | Achievement of an event defined as PSADT of <6 mo (PSADT <6 mo), initiation of ADT, locoregional recurrence, distant metastases, or death due to any cause prior to the landmark time |
| Additional definitions | |
| Locoregional recurrence | More than 50% increase in prostate volume compared with the lowest volume by imaging, detection of a new palpable pelvic lesion in the event of previous complete clinical normalization, identification by biopsy or imaging of a new regional lymph node, or biopsy-proven recurrence within the prostate gland, whichever occurred first |
| PSA recurrence | |
| PSA nadir + 2 ng/ml | PSA 2 ng/ml above nadir |
| PSA nadir + 2 ng/ml and rising | PSA nadir + 2 ng/ml and PSA increases at the next visit |
| PSA >5, >10, or >25 ng/ml | PSA values of >5, >10, or >25 ng/ml after nadir |
| PSADT <6 mo | PSA doubling time of <6 mo |
ADT = androgen-deprivation therapy; BCF = biochemical failure; DFS = disease-free survival; EFS = event-free survival; GCF = general clinical failure; MFS = metastasis-free survival; NED = no evidence of disease; OS = overall survival; PC = prostate cancer; PCSS = PC-specific survival; PSA = prostate-specific antigen; PSADT = PSA doubling time.
If data on ADT initiation were not available, patients were considered negative for the endpoints but were included in the total patient number (denominator).
Patients were considered to have testosterone recovery if their serum testosterone increased to be above the level before ADT initiation. If data on testosterone recovery or initiation of ADT were not available, patients were considered negative for NED if all the other criteria are met but were included in the total number of patients (denominator).
Statistical analysis
To evaluate the association between the early and definitive endpoints (primary objective), Spearman’s ρ or Kendall’s τ rank correlation coefficients under a Clayton copula were calculated [28], [29]. Time-to-event distributions were estimated using the Kaplan-Meier method, and the cumulative incidence estimator was used to partition the time to event into individual components over time [30]. Follow-up time started from randomization; patients who did not experience an event were censored on the last date they were known to be event free. An association was considered strong if a correlation coefficient was >0.7, as applied by ICECaP [4].
To evaluate the association between achievement of binary early endpoints (EFS [with PSADT <6 mo/ADT] or NED) and MFS, OS, and PCSS (secondary objective), a landmark analysis [31], [32] and a Cox proportional-hazard model adjusted for age and PSA at baseline were used. One study showed that patients with HRLPC had approximately 50% chance of remaining disease free at 5 yr after RT and ADT [33]. The periods of 3 and 4 yr after treatment represent the most sensible points in the course of natural disease progression, minimizing a bias from subsequent therapies during long-term follow-up after RT and ADT. In our analysis, patients who were followed until the landmark time of 3 or 4 yr without experiencing the definitive endpoint (OS, MFS, or PCSS) were grouped by whether or not they achieved the binary endpoint at the landmark times. Survival probability estimates of OS, MFS, or PCSS in each group were then computed conditionally on the patient group classification at the landmark times. Patients who experienced an event or discontinued before the landmark were excluded from the landmark analysis.
Results
Across all studies, the median follow-up time from the randomization date in the analyzed population was 12.9 yr, during which 461 patients survived. Death, distant metastases, locoregional recurrence, and PSA data were available for all patients. Subsequent ADT (beyond study arm assigned therapy) was initiated for 411/1228 (33%) patients; testosterone values for analysis were available for 385/1228 (31%) patients.
We first assessed the cumulative incidence function of competing events contributing to MFS, DFS, EFS (PSA nadir + 2 ng/ml), and PCSS over time. Locoregional recurrence, distant metastases, and PSA recurrence contributed to the endpoints primarily in approximately the first 7 yr of follow-up (Fig. 1A–C). PC-specific deaths contributed to PCSS in approximately the first 10 yr (Fig. 1D). Progression events counting toward DFS were MFS and locoregional recurrence that added only a small number of events to DFS (Fig. 1A and 1B). PSA recurrence was the major contributor to EFS (PSA nadir + 2 ng/ml) in the first 7 yr (Fig. 1C). Across all assessed endpoints, death events primarily unrelated to PC were major contributors after 10 yr (Fig. 1A–D).
Fig. 1 – Cumulative incidence of cause-specific failure types in composite endpoints. (A) MFS, (B) DFS, (C) EFS (PSA nadir+2 ng/ml), (D) PCSS.
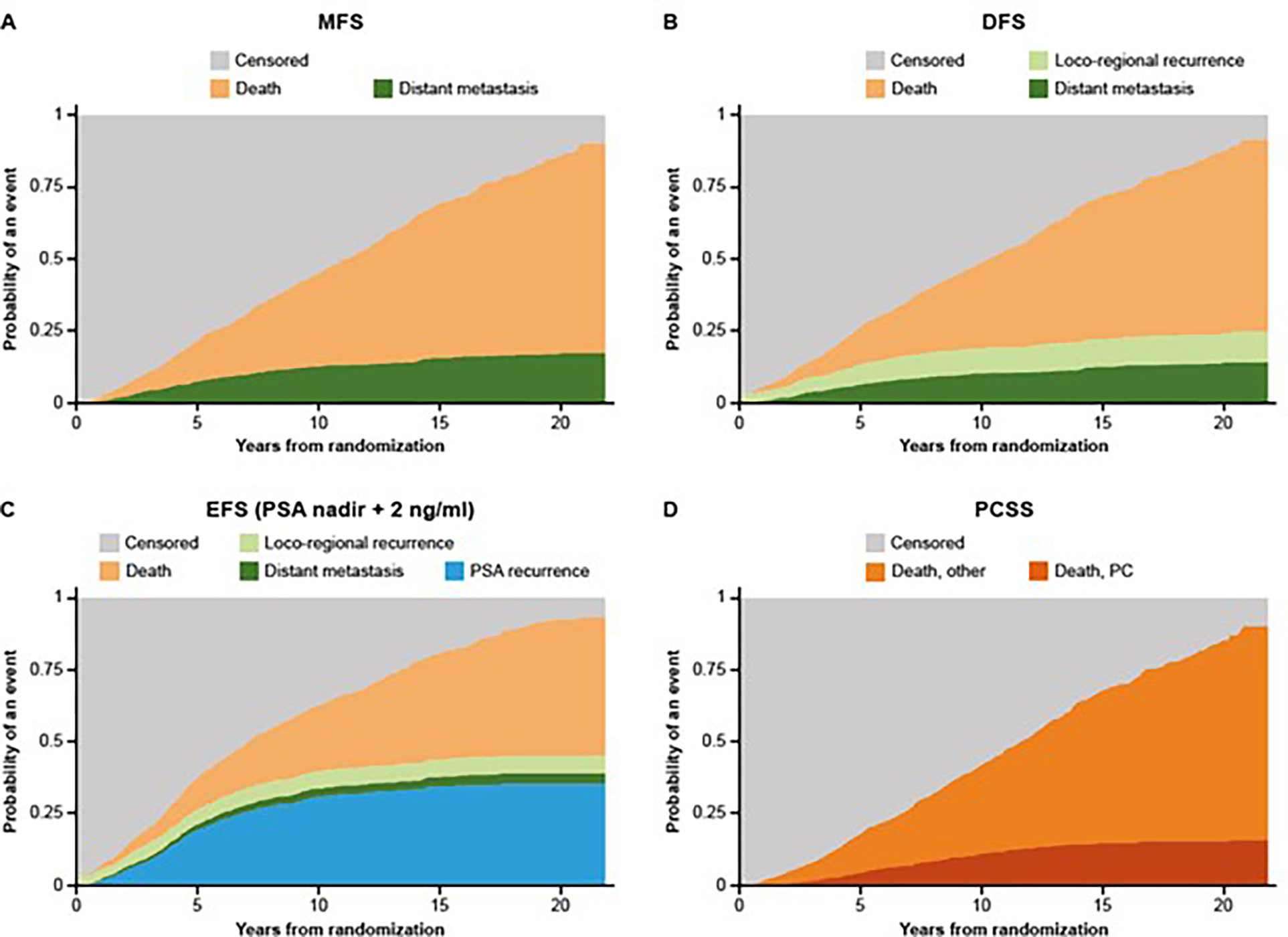
DFS = disease-free survival; EFS = event-free survival; MFS = metastasis-free survival; PCSS = prostate cancer-specific survival; PSA = prostate-specific antigen.
We then evaluated the strength of patient-level associations between early endpoints and MFS, OS, and PCSS. All associations between OS and MFS, OS and DFS, and MFS and DFS were strong (per ρ > 0.7), with ρ = 0.96, 0.85, and 0.88, respectively, and narrow 95% confidence intervals (CIs; Fig. 2). Associations between PCSS and MFS or DFS had ρ = 0.85 or 0.71, but 95% CIs were wide (Supplementary Fig. 1C). Next we assessed EFS-based early endpoints with different thresholds for a PSA recurrence event: PSA nadir + 2 ng/ml (standard definition), PSA nadir + 2 and rising, and four progressively rigorous PSA thresholds (PSA >5, >10, and >25 ng/ml after nadir). Associations of these endpoints with OS or MFS had ρ > 0.7, with the lowest ρ for EFS with PSA nadir + 2 ng/ml. Correlation coefficients increased with >5, >10, and >25 ng/ml PSA thresholds (Fig. 2). The association of EFS-based endpoints with PCSS had ρ around 0.7 and wide 95% CIs (Supplementary Fig. 1C).
Fig. 2 – Estimates of associations between time-to-event early and definitive endpoints. Spearman’s rank correlation coefficients for (A) OS, (B) MFS, and (C) PCSS with variations of EFS-based points.
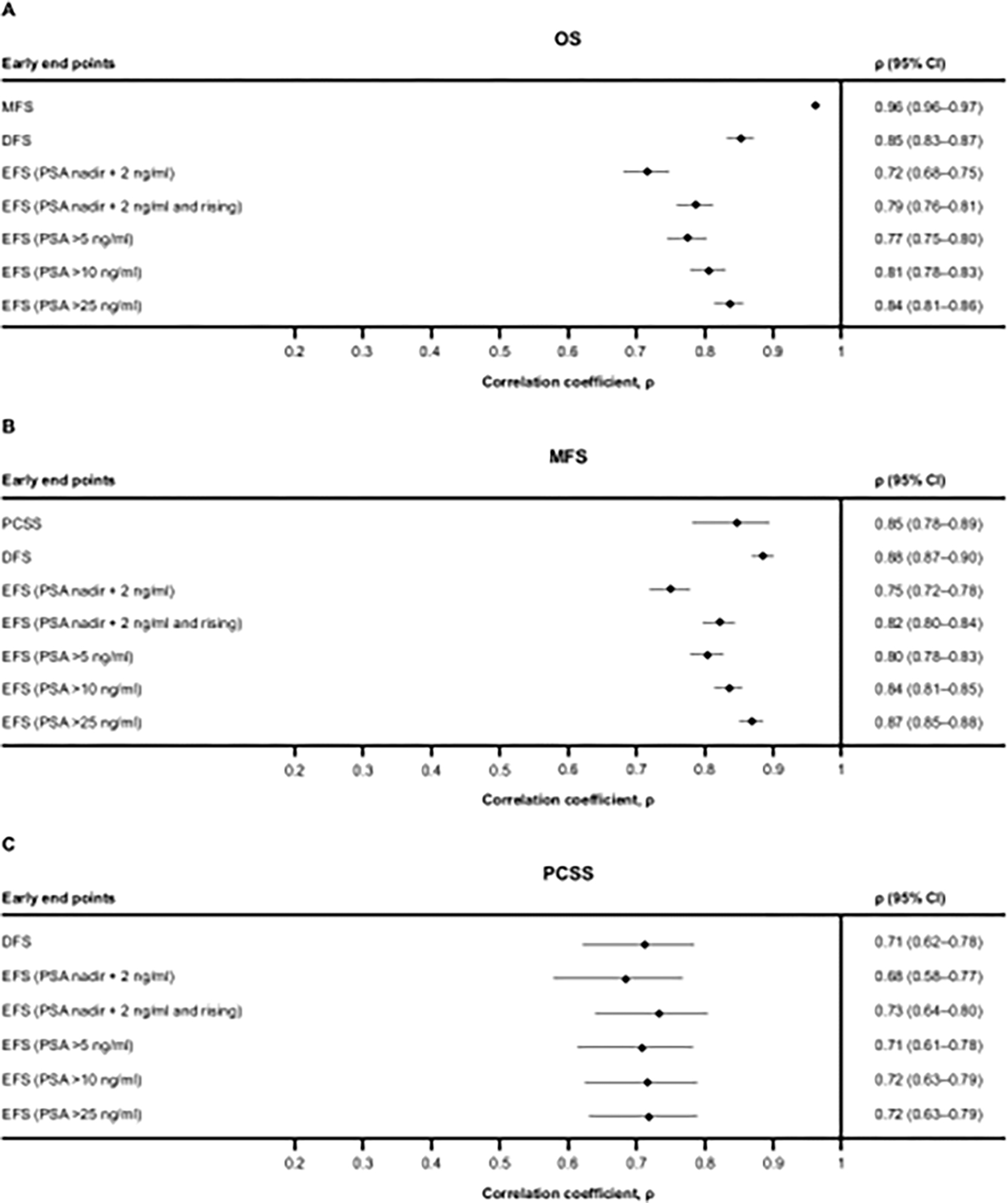
CI = confidence interval; DFS = disease-free survival; EFS = event-free survival; MFS = metastasis-free survival; OS = overall survival; PCSS = prostate cancer-specific survival; PSA = prostate-specific antigen.
None of the associations between BCF- or GCF-based early endpoints and OS or MFS were strong (ρ ≤ 0.7), except between GCF (PSA >25 ng/ml + death) and MFS (ρ = 0.72; Supplementary Fig. 1A and B). Associations between BCF-based early endpoints and PCSS had ρ > 0.7 and wide 95% CIs (Supplementary Fig. 1C). Associations between GCF-based endpoints and PCSS were weak (ρ ≤ 0.7), except endpoints with ADT removed as an event that had ρ > 0.7. Kendall’s rank correlation analysis (Supplementary Fig. 2) showed similar results for all associations.
We next assessed the association between achievement of a binary early endpoint of progression, that is, EFS with failure events defined as PSADT <6 mo or ADT initiation (PSADT <6 mo/ADT) with definitive endpoints (MFS, OS, and PCSS) using landmark analyses. Patients failing with PSADT <6 mo or starting ADT at the 3-yr landmark had median OS of 6.1 yr and median MFS of 7.1 yr after the landmark time, while patients without either of these failures had median OS of 9.9 yr and median MFS of 9.7 yr (Fig. 3A and 3C, and Supplementary Table 2). Hazard ratios (HRs) (95% CI) for OS and MFS were 0.53 (0.45–0.64) and 0.63 (0.52–0.76), favoring no achievement of EFS (PSADT <6 mo/ADT) at 3 yr. HR (95% CI) for PCSS was 0.26 (0.18–0.36), also favoring no achievement of EFS (PSADT <6 mo/ADT) at 3 yr; the median times to PCSS were not reached (Fig. 3E, and Supplementary Table 2). Similar results were observed for EFS (PSADT <6 mo/ADT) achieved at the 4-yr landmark analysis (Fig. 3B, 3D, and 3F and Supplementary Table 2).
Fig. 3 – Landmark analysis of outcomes by achievement of EFS (PSADT <6 mo/ADT). Kaplan-Meier estimates for (A, B) OS, (C, D) MFS, and (E, F) PCSS by EFS (PSADT <6 mo/ADT) achieved at (A, C, E) 3 yr or at (B, D, F) 4 yr of treatment.
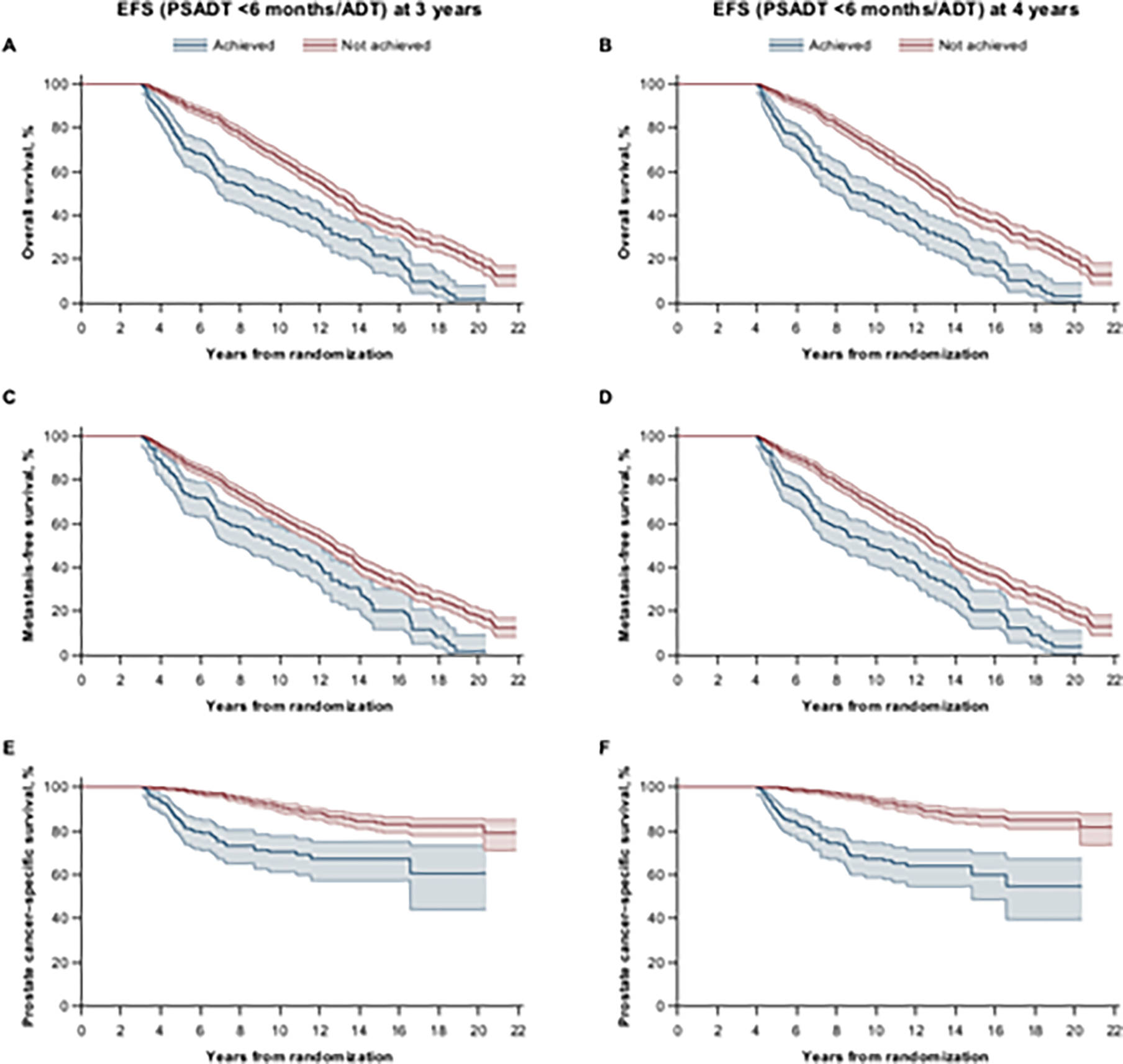
Shading represents 95% confidence interval. ADT = androgen-deprivation therapy; EFS = event-free survival; MFS = metastasis-free survival; OS = overall survival; PSADT, prostate-specific antigen doubling time.
Finally, we assessed the association between achievement of NED (a binary classification endpoint that assessed meeting all available criteria) with definitive endpoints of OS, MFS, and PCSS in the landmark analyses. Patients failing to achieve NED at the 3-yr landmark time had median OS of 7.1 yr and median MFS of 7.1 yr after the landmark time, while patients achieving NED at 3 yr had median OS of 10.2 yr and median MFS of 9.8 yr. HRs (95% CIs) for OS and MFS were 0.56 (0.48–0.66) and 0.62 (0.52–0.74), favoring achievement of NED at 3 yr (Fig. 4A and 4C, and Supplementary Table 3). The HR (95% CI) for PCSS was 0.26 (0.19–0.37), also favoring achievement of NED at 3 yr; the median time to PCSS was not reached (Fig. 4E and Supplementary Table 3). Similar results were observed for NED achieved at 4 yr (Fig. 4B, 4D, and 4F, and Supplementary Table 3).
Fig. 4 – Landmark analysis of outcomes by achievement of NED. Kaplan-Meier estimates for (A, B) OS, (C, D) MFS, and (E, F) PCSS by NED achieved at (A, C, E) 3 yr or at (B, D, F) 4 yr of treatment.
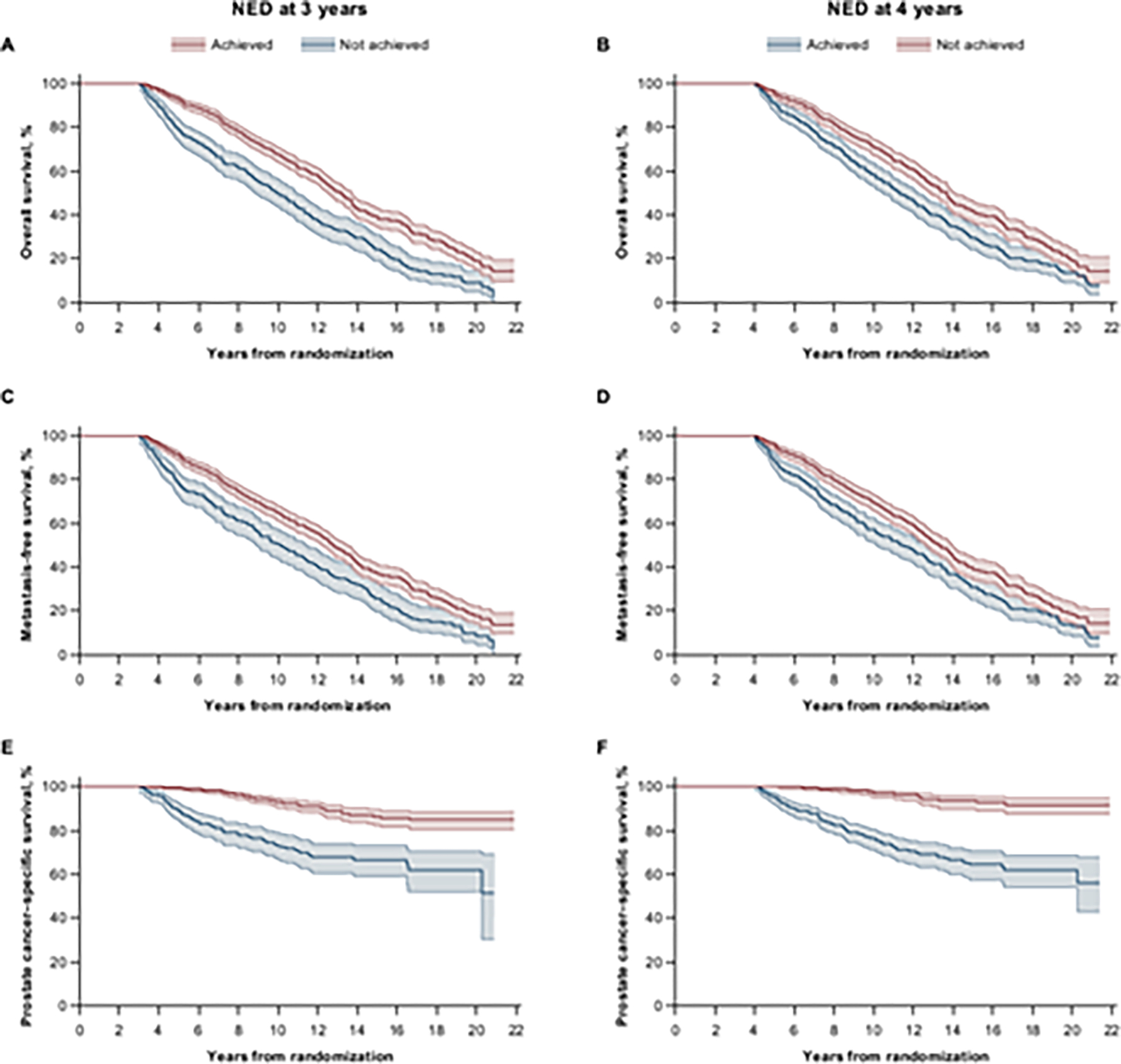
Shading represents 95% confidence interval. MFS = metastasis-free survival; NED = no evidence of disease; OS = overall survival; PCSS = prostate cancer-specific survival.
Discussion
This post hoc analysis of the long-term ADT treatment groups from three phase 3 RTOG studies in HRLPC identified several early clinical endpoints associated with the definitive endpoints of MFS, OS, and PCSS. The cumulative incidence function assessment showed disease progression events mostly occurring within the first 7 yr of diagnosis, with competing causes of deaths dominating events after 10 yr (Fig. 1). Therefore, early endpoints assessed in this analysis were those that incorporated progression events with higher PSA levels, initiation of ADT, locoregional recurrence, and distant metastatic disease, along with deaths. The absence of progression events as well as testosterone recovery constituted the state of undetectable disease called no evidence of disease or NED.
The correlation analyses showed a strong patient-level association between MFS and OS, and a weaker association between DFS and OS, consistent with the previous strong correlation of MFS and weaker correlation of DFS with OS [4]. These findings reassured us that the selected statistical method worked appropriately in this data set. Locoregional recurrence events contributed very little to the number of events over distant metastatic disease or deaths. Therefore, a weaker association between DFS and OS is not surprising.
PSA recurrence is an early indicator of disease progression occurring with a high probability (Fig. 1). However, an early endpoint of EFS with PSA recurrence defined as PSA nadir + 2 ng/ml, the definition previously assessed in relation to EFS [22] and BCF [22], [23], has not been shown to be a surrogate for OS [22]. To better understand whether PSA-based progression events can improve the patient-level association between early and definitive endpoints, we counted higher PSA levels (>5, >10, or >25 ng/ml after nadir) and increasing PSA (PSA nadir + 2 ng/ml and rising or PSADT <6 mo) as disease progression events. Consistent with a weak surrogacy for OS [22], EFS (PSA nadir + 2 ng/ml) showed the weakest association among all assessed EFS-based endpoints in both Spearman’s and Kendall’s rank correlation analyses. However, when PSA recurrence in the EFS definition included rising PSA (PSA nadir + 2 ng/ml and rising), the association improved. EFS with PSA >5, >10, or >25 ng/ml was associated progressively strongly with OS and MFS: the higher the PSA cutoff, the stronger the association. It should be noted that patients with LPC are treated before their PSA levels reach >10 or >25 ng/ml [3]. Given the strength of association with definitive endpoints, EFS (PSA nadir + 2 ng/ml and rising) and EFS (PSA >5 ng/ml) are promising early endpoints for further validation. However, with the increasing use of prostate-specific membrane antigen positron-emission tomography as an imaging tool for rising PSA [3], physicians will likely initiate treatment before PSA rises to >5 ng/ml. Therefore, these endpoints need continuing refinement based on current recommendations and practices. EFS (PSADT <6 mo/ADT) demonstrated a good patient-level association with MFS and OS in the landmark analysis, suggesting that further validation of this early progression endpoint is warranted. Interestingly, PSADT <6 mo has been shown to be associated with HRLPC [19], [21], supporting our findings.
Times to BCF and GCF have been identified as candidate surrogate endpoints in HRLPC [23], [24]. In our analysis, the association between BCF-based endpoints and definitive endpoints (OS or MFS) followed the pattern described for EFS with PSA cutoffs of nadir + 2 ng/ml and rising, >5 ng/ml, >10 ng/ml, or >25 ng/ml, although associations were weak. The association between GCF-based endpoints with PSA cutoffs of >5, >10, or >25 ng/ml and OS or MFS was also weak; removing ADT initiation as a qualifying event did not improve the association. Adding death to the GCF definition strengthened the association, but correlation coefficients were still <0.7 in general.
Endpoints describing a treatment response may have utility in LPC. We defined NED using important clinical (no locoregional recurrence and distant metastasis) and biochemical (no PSA >0.5 ng/ml + nadir and rising and testosterone recovery to pre-ADT levels) parameters and lack of subsequent therapy for PC to identify disease-free patients. Achievement of NED at 3 yr was associated with prolonged OS, MFS, and PCSS. It should be noted that data on testosterone recovery and ADT initiation were not available for all assessed patients and that baseline testosterone levels may be confounded by prior therapies. Therefore, testosterone recovery to a predetermined level, for example, >200 ng/dl, is a good alternative for future refinement of NED. Bone scans were also collected with different frequency across RTOG studies; hence, validity of NED in HRLPC should be assessed in future studies.
Our analysis is limited by its post hoc nature. The data set included older studies initiated two to three decades ago, when physicians waited for higher PSA levels before initiating therapy [34], [35] and there was less awareness about the importance of ethnic and racial diversity in health equity. Recruitment diversity challenges existing for current studies performed in North America also existed when the RTOG studies were performed. Bone scans for assessing metastases were collected with different frequencies across the studies. Some clinically relevant parameters, such as testosterone measurements or date of ADT initiation, which are now recognized as important, were not robustly collected in these studies; therefore, the true rate of NED, EFS (PSADT <6 mo/ADT), and GCF with no ADT initiation event may be lower. Clinical symptoms and signs of disease progression without an accompanying rise in PSA or increased use of pain medications considered important for ADT initiation may be less well documented. Our findings advance early endpoint development but should be generalized in studies with diverse populations and uniform data collection. The treatment landscape of HRLPC has changed dramatically since the last of the three assessed studies was completed. Second-generation hormonal agents, such as abiraterone acetate plus prednisone, enzalutamide, apalutamide, or darolutamide, in combination with ADT [6], [7], [36], [37], [38], [39] have prolonged the time to metastases or death of patients with PC and rising PSA significantly. These achievements further highlight the need for early endpoints.
CONCLUSIONS
This analysis of early endpoints from RTOG patients with HRLPC treated with long-term ADT following primary definitive RT identified EFS with BCF, defined as PSA nadir + 2 ng/ml and rising, PSA >5 ng/ml, or PSADT <6 mo with or without ADT initiation, as promising early endpoints suitable for further validation. NED may serve to measure clinically undetectable disease in LPC, and its utility should be assessed further in prospective clinical studies.
Supplementary Material
Twitter statement.
Clinical and PSA failure plus absence of disease may be promising early endpoints in high-risk localized prostate cancer
Take home message.
In high-risk localized prostate cancer, event-free survival, with higher thresholds of prostate-specific antigen counting as events and a new definition of no evidence of disease, are promising early endpoints.
ACKNOWLEDGMENTS
Writing assistance was provided by Larissa Belova, PhD, of Parexel, and was funded by Janssen Global Services, LLC.
Funding/Support and role of the sponsor: This analysis was funded by Janssen Research & Development. The sponsor was involved in the interpretation of the data and preparation and review of the manuscript. All the authors had access to the data, drafted the manuscript with input from the sponsor (Janssen), reviewed and approved the manuscript before submission, and made the decision to submit the manuscript for publication. Janssen Global Services, LLC provided funding for editorial assistance.
Footnotes
Financial Disclosures: Felix Feng certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Felix Feng reports consulting or advisory role for Astellas Pharma, Artera, Bayer, Bristol Myers Squibb, BlueStar Genomics, Exact Sciences, Foundation Medicine, Janssen Biotech, Myovant Sciences, Novartis, Roivant, SerImmune, Tempus, and Varian Medical Systems; stock and other ownership interests from Artera; and research funding from Zenith Epigenetics. James J. Dignam reports a membership in the data monitoring committee for Celgene and Merck, and institutional research support from the U.S. National Cancer Institute. Howard Sandler reports serving as a member of a clinical trial steering committee with Janssen. Branko Miladinovic, Ke Zhang, Daniel Wang, and Margaret Yu are employees of Janssen Research & Development, and may hold stock in Johnson & Johnson.
Data sharing: The RTOG 0521, 9202, and 9902 trial data are available at https://nctn-data-archive.nci.nih.gov/. Requests for additional supporting data should be directed to NRG Oncology at APC@nrgoncology.org.
References
- [1].Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005;294:433–9. [DOI] [PubMed] [Google Scholar]
- [2].Katz MH, McKiernan JM. High-risk, clinically localized prostate cancer: is monotherapy adequate? Rev Urol 2007;9:S19–27. [PMC free article] [PubMed] [Google Scholar]
- [3].National Comprehensive Cancer Network. Prostate cancer. Version 1.2023. —September 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- [4].Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017;35:3097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med 2019;380:1235–46. [DOI] [PubMed] [Google Scholar]
- [7].Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 2018;378:1408–18. [DOI] [PubMed] [Google Scholar]
- [8].ICECaP Working Group. The development of intermediate clinical endpoints in cancer of the prostate (ICECaP). JNCI. J Natl Cancer Inst 2015;107:djv261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Haslam A, Gill J, Prasad V. The frequency of assessment of progression in randomized oncology clinical trials. Cancer Rep (Hoboken) 2021;5:e1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang Bartlett C, Mardekian J, Cotter MJ, et al. Concordance of realworldversus conventional progression-free survival from a phase 3 trial of endocrine therapy as first-line treatment for metastatic breast cancer. PLoS One 2020;15:e0227256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), et al. Nonmetastatic castration resistant prostate cancer: Considerations for metastasis-free survival endpoint in clinical trials guidance for industry. 2021. https://www.fda.gov/media/117792/download.
- [12].Cancer.Net. Prostate cancer: statistics. 2022. https://www.cancer.net/cancer-types/prostate-cancer/statistics.
- [13].Boyle HJ, Alibhai S, Decoster L, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer 2019;116:116–36. [DOI] [PubMed] [Google Scholar]
- [14].Elmehrath AO, Afifi AM, Al-Husseini MJ, et al. Causes of death among patients with metastatic prostate cancer in the US from 2000 to 2016. JAMA Netw Open 2021;4:e2119568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu-Yao G, Stukel TA, Yao SL. Changing patterns in competing causes of death in men with prostate cancer: a population based study. J Urol 2004;171:2285–90. [DOI] [PubMed] [Google Scholar]
- [16].Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92–02. J Clin Oncol 2003;21:3972–8. [DOI] [PubMed] [Google Scholar]
- [17].Rosenthal SA, Hu C, Sartor O, et al. Effect of chemotherapy with docetaxel with androgen suppression and radiotherapy for localized high-risk prostate cancer: the randomized phase III NRG Oncology RTOG 0521 trial. J Clin Oncol 2019;37:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rosenthal SA, Hunt D, Sartor AO, et al. A phase 3 trial of 2 years of androgen suppression and radiation therapy with or without adjuvant chemotherapy for high-risk prostate cancer: final results of Radiation Therapy Oncology Group phase 3 randomized trial NRG oncology RTOG 9902. Int J Radiat Oncol Biol Phys 2015;93:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jackson WC, Johnson SB, Li D, et al. A prostate-specific antigen doubling time of <6 months is prognostic for metastasis and prostate cancer-specific death for patients receiving salvage radiation therapy post radical prostatectomy. Radiat Oncol 2013;8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin YC, Lin PH, Shao IH, et al. Prostate-specific antigen kinetics effects on outcomes of low-volume metastatic prostate cancer patients receiving androgen deprivation therapy. J Oncol 2021;2021:9648579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Suzman DL, Cullen D, Trock BJ, et al. PSA doubling time (PSADT) 6 months to identify a subgroup of men with biochemically-recurrent prostate cancer (BRPC) after prostatectomy (RP) at highest risk of distant metastasis (dMET). J Clin Oncol 2016;34:e16587. [Google Scholar]
- [22].Xie W, Regan MM, Buyse M, et al. Event-free survival, a prostate-specific antigen-based composite end point, is not a surrogate for overall survival in men with localized prostate cancer treated with radiation. J Clin Oncol 2020;38:3032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dignam JJ, Hamstra DA, Lepor H, et al. Time interval to biochemical failure as a surrogate end point in locally advanced prostate cancer: analysis of randomized trial NRG/RTOG 9202. J Clin Oncol 2019;37:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ray ME, Bae K, Hussain MH, Hanks GE, Shipley WU, Sandler HM. Potential surrogate endpoints for prostate cancer survival: analysis of a phase III randomized trial. J Natl Cancer Inst 2009;101:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].De Angelis F, Breccia M. Molecular monitoring as a path to cure acute promyelocytic leukemia. Rare Cancers Ther 2015;3:119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Savona MR. Molecular monitoring and minimal residual disease in the management of chronic myelogenous leukemia. J Community Support Oncol 2014;12:171–8. [DOI] [PubMed] [Google Scholar]
- [27].van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood 2015;125:3996–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schemper M, Kaider A, Wakounig S, Heinze G. Estimating the correlation of bivariate failure times under censoring. Stat Med 2013;32:4781–90. [DOI] [PubMed] [Google Scholar]
- [29].Xie W, Halabi S, Tierney JF, et al. A systematic review and recommendation for reporting of surrogate endpoint evaluation using meta-analyses. JNCI Cancer Spectr 2019;3:pkz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med 1992;11:813–29. [DOI] [PubMed] [Google Scholar]
- [31].Mi X, Hammill BG, Curtis LH, Lai EC, Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med 2016;35:4824–36. [DOI] [PubMed] [Google Scholar]
- [32].Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983;1:710–9. [DOI] [PubMed] [Google Scholar]
- [33].Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997;337:295–300. [DOI] [PubMed] [Google Scholar]
- [34].Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol 2013;11:14–23. [PMC free article] [PubMed] [Google Scholar]
- [35].Shipley WU, Thames HD, Sandler HM, et al. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA 1999;281:1598–604. [DOI] [PubMed] [Google Scholar]
- [36].Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 2019;37:2974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019;381:13–24. [DOI] [PubMed] [Google Scholar]
- [38].Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–60. [DOI] [PubMed] [Google Scholar]
- [39].Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med 2018;378:2465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


