Abstract
Objectives:
Different methods detecting Plasmodium parasite infection or exposure are available, but systematic comparison of all these methodologies to predict malaria infection is lacking. Understanding the characteristics of respective tests is helpful to choose the most appropriate tests for epidemiological or research purposes.
Methods:
Microscopy, RDT, and PCR was performed for 496 patients presenting with febrile illness in Dakar, Senegal in 2015. Blood samples had laboratory multiplex assays performed for IgG serology and detection of HRP2 antigen. Sensitivity (Se) and specificity (Sp) for different tests were calculated using PCR as the gold standard for detecting active infection. Modeling through latent class analysis (LCA) compared each test to a modeled gold standard for Se/Sp estimates.
Results:
Against PCR, Se/Sp were 95.2%/93.7% for RDT, 90.4%/100.0% for microscopy, and 97.9%/48.1% for lab HRP2 detection. Compared to the modeled gold standard, Se of microscopy was 93.5%, and Se of RDT, PCR, and lab HRP2 detection were all greater than 99%. Se/Sp of IgG serology were substantially for detecting active infection.
Conclusions:
Compared to single tests, a combinatorial LCA approach of multiple biomarkers for detecting malaria infection from patient samples provides greater sensitivity and specificity for epidemiological estimates and research objectives.
Keywords: malaria, diagnostics, PCR, antigen detection, IgG detection, latent class analysis
Introduction
Techniques for detecting the presence of and exposure to malaria parasites have developed rapidly during the last few decades. While expert microscopy has long been considered the gold standard for diagnosis, rapid diagnostic tests (RDTs) that detect the parasite antigens HRP2 and lactate dehydrogenase (LDH) have become widely used at point of care, and the sensitivity of some HRP2 based RDTs rivals or even eclipses that of expert microscopy for diagnosis of Plasmodium falciparum (Abdallah et al., 2019). Due to the relatively long half-life of HRP2 antigen after clearance of an infection, HRP2 based RDTs cannot necessarily distinguish between active and recently cleared infection (Dalrymple et al., 2018). Polymerase chain reaction (PCR) methodologies are usually used in the research context to detect infection and are particularly useful for detecting very low-density infections missed by microscopy or RDT (Lucchi et al., 2011). Detection of malaria antigenemia using a multiplex platform is another tool for the detection of current or very recent infection, as this technique detects HRP2 with greater sensitivity than RDTs (Plucinski et al., 2019a).
Measurement of IgG antibodies has most often been used for detecting exposure in the context of population-based studies, with antibodies of varying half-lives useful for assessing exposure over the long term or more recently. While enzyme-linked immunosorbent assay (ELISA) has traditionally been used, multiplex bead-based assays (MBA) have recently been developed that enable detection of multiple anti-malarial antibodies simultaneously (Sarr, 2011). Multiplex bead-based assays have also been applied to Plasmodium antigen detection and are useful for sensitive detection of antigen-positive individuals with results evaluating performance of point-of-care RDTs (Plucinski et al, 2019b).
Senegal, on the west coast of Africa, is a malaria endemic country with relatively low transmission that is and highly seasonal. With the scale up of malaria control interventions over the last decade, parasite prevalence has fallen considerably. In the capital city of Dakar, cross sectional surveys found malaria parasite prevalence among children under 5 years declined from 1.5% in 2010–2011 to 0.2% in 2017 (Agence Nationale de la Statistique et de la Démographie 2012, 2017). In northern Senegal, five antibodies with short half-lives were found to be useful for assessing short term trends in transmission in low transmission (parasite prevalence < 1%) villages (Sarr et al., 2011). However, the relationship of the seropositivity of malaria antibodies of varying half-lives to malaria diagnostic methods is poorly understood. More information is needed to support interpretation of seroprevalence in febrile patients seeking care, and among patients with active malaria infection.
We present here a facility-based sampling of febrile patients of all ages with suspected malaria, in which RDT, blood smear, and photo-induced electron transfer polymerase chain reaction (PET-PCR) had been conducted. At a later time, blood samples were analyzed in the laboratory for HRP2 antigenemia and a panel of Plasmodium antibodies representing long and short half-life IgG targets.
Materials and Methods
Study design
This study utilized blood samples from patients seeking care for fever under a protocol designed to validate a malaria diagnosis technique using loop mediated isothermal amplification (LAMP) technology (Alethia Malaria Meridian Bioscience Inc. Cincinnati, OH) (Lucchi et al., 2013, Lucchi et al., 2016). Patients of all ages with suspected malaria who sought care at two health facilities in peri-urban Dakar (Youssou Mbargane hospital in Rufisque and Deggo health post in Pikine) were enrolled during the malaria transmission season from September to November 2015. During the consultation, patients presenting with fever or reported fever during the preceding 48 hours were tested using a malaria RDT (Carestart Malaria Pan, Access Bio, United Kingdom), with treatment according to Ministry of Health guidelines.
Ethics Statement
The study was explained to the participant or the relative for children in French or in the local language. All patients were enrolled after giving their informed consent. The study protocol was reviewed and approved by National Ethics Committee of the Senegal Ministry of Health and Social Welfare. U.S. Centers for Disease Control and Prevention staff were not considered to be engaged in human subjects research.
Data and sample collection
Febrile patients were recruited regardless of RDT result. Age, sex, the time of sampling, and date were collected from patients who provided informed consent. Thick and thin smears were created, and 4 ml of venous blood was drawn into a tube containing EDTA as an anticoagulant. For each patient, 50 μl of venous blood were spotted in each of 5 circles onto a 903 Whatman filter paper, allowed to dry in ambient air (dried blood spots; DBS), and stored at room temperature in individual resealable bags with desiccant.
Laboratory assays
Thick and thin blood smears were prepared for all patients onsite. The thin and thick films were performed using 1 drop and 3 drops of blood, respectively, then stained with Giemsa 10% for 20 minutes. Expert microscopists at the Parasitology Laboratory at LeDantec Hospital read thick smears for diagnosis and performed species identification using the thin smears. All slides were read by two microscopists and in case of discrepancy of 20 % or more in reported parasite density, the slides were read by a third reader, whose reading was considered final. A total of one hundred fields were read before declaring a thick film negative. The parasite density (PD) was determined in the thick film by counting 200 (if the number of parasites was equal to or greater than 100 after counting 200 WBC) or 500 (if the number of parasites was less than 100 after counting 200 WBC) white blood cells (WBC) trophozoites. The PD was calculated by multiplying the number of trophozoites by 8000 WBCs (the average number of WBCs per μl of blood) divided by number of WBCs counted.
PET-PCR was carried out at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, USA on the DBS samples after DNA extraction. To run the PET-PCR, total DNA was extracted from two dried blood spots using the QIAamp blood kit (QIAGEN, Inc., Chatsworth, CA), and PET-PCR was run according to the protocol multiplexed for Plasmodium genus and P. falciparum species primers (Lucchi et al., 2013, Lucchi et al., 2016). A sample was considered positive if the Ct ≤ 40 cycles.
Antibody testing of the DBS elutions was performed at CDC. Whole blood was eluted from a 6 mm diameter circle of DBS with a solution of phosphate buffered saline (PBS), 0.05% Tween20, 0.5% BSA and 0.02% NaN3 to a final 1:20x dilution of whole blood. Presence of anti-P. falciparum IgG antibodies was determined using the Luminex multiplex bead-based assay (Rogier et al., 2015). The IgG antibodies assayed for were against the MSP1, AMA1, LSA1, CSP, SALSA, StarpR, and GLURP-Ro P. falciparum antigens. Recombinant proteins or peptides were coupled to seroMap beads (Luminex Corp) and multiplex IgG detection assay run as previously described with an approximate serum dilution of 1:400 (Rogier et al., 2017). Plates were read the same day on the Bio-Plex 200 instrument (BioRad, Hercules, CA). The median fluorescence signal for a target of 50 beads/analyte were generated, and the median fluorescence intensity (MFI) final was generated for each analyte after subtracting MFI values from blank background beads that were included on each plate (Rogier et al., 2015). This MFI-bg value was used for analyses. The HRP2 antigen was detected using a multiplex bead-based assay as described previously (Rogier et al., 2017). The 1:20 dilution of whole blood was used as sample type, and plates read on the Bio-Plex 200 instrument under the same conditions as described above, and MFI-bg data was used for analyses.
Data analysis
The threshold for positivity to HRP2 antigen was determined by running the assay on 92 negative blood samples (assayed at 1:20 dilution) from U.S. residents who had never traveled to malaria-endemic countries. The lognormal mean and standard deviation were generated for the assay signal from this sample set, and the log mean + 3 standard deviation (SD) assay signal was used as the HRP2 positivity threshold. In the same manner for each of the IgG targets, the same 92 blood samples were assayed with the multiplex IgG detection assay (at serum dilution of 1:400), and same distribution calculated for seropositivity threshold assay signals for each of the antigen targets.
Of the anti-falciparum antibodies, MSP1 and AMA1 were categorized as long half-life antibodies and LSA1, CSP, SALSA, StarpR, and GlurpRo were categorized as short half-life antibodies (Ondigo et al., 2014, Yman et al., 2019). If the MFI-bg signal of either of the long half-life antibodies was categorized as seropositive, long half-life antibody status was categorized as positive; similarly, if the MFI-bg signal of any short half-life antibody was categorized as seropositive, short half-life antibody status was categorized as positive.
We conducted a latent class analysis (LCA) to calculate sensitivity and specificity of each test compared to a modeled gold standard as described previously (Liu et al., 2016). We considered one class to represent disease positive and the other as diseased negative. We performed two analyses: one LCA including RDT, blood smear, PET-PCR, and HRP2 antigenemia (four-test model), and one LCA with RDT, blood smear, PET-PCR, HRP2 antigenemia, long half-life antibodies, and short half-life antibodies (six-test model). We used the Hui-Walter latent class method (Hui et al., 1980) to estimate disease class membership probabilities and assess the diagnostic test characteristics. The number of classes for the four- and six-test models were specified by choosing the smallest Bayesian Information Criterion (BIC) (Schwarz, 1978). The basic latent class model assumes that manifest variables are independent of each other within the latent classes, termed conditional independence (locally independent). That is, it is assumed that the diagnostic tests are independent within a latent class. Conditional independence of the models was assessed using a modified version of Garrett and Zeger’s approach (Garrett et al., 2000). Log odds ratios for both the observed and expected two-way tables associated with a pair of tests were calculated and compared with a z-test. Extreme z-values indicated conditional independence. In such cases the model was refit by replacing the variables for the two dependent tests with a joint 4 category variable ((positive, positive); (positive, negative); (negative, positive); and (negative, negative)).
We performed logistic regression analysis using stepwise backwards elimination to explore the relationship between antibody levels (logMFI-bg) and HRP2 antigen positivity, considering HRP2 antigenemia as evidence of current or recent infection in the past two months (Plucinski et al., 2018).
Results
In total, 496 patients were enrolled, 60.1% male and 39.9% female. 16.6% were < 15 years of age, 22.3% were 15–19 years, 28.3% were 20–29 years, 18.0% were 30–44 years, and 14.8% were 45 years and older. Results for RDT and HRP2 antigenemia were available for all 496 patients, and antibody (long and short half-life panel) results were available for 494 patients, 298 of whom had blood smear results, 284 of whom had PET-PCR results, and 224 of whom had results for all six tests.
RDTs were positive in 67.3% (95% CI 63.0–71.5) of those for whom results were available, HRP2 antigenemia in 81.9% (95% CI 78.2–85.2), blood smear in 61.4% (95% CI 55.6–67.0), and PET-PCR was positive in 68.7% (95% CI 62.9–74.0). These proportions were not significantly different among the 225 for whom all four results were available. Overall, one or more long half-life antibodies were present in 89.4% (95% CI 86.6–92.0) and one or more short half-life antibodies were present in 80.6% (95% CI 77.1–84.1) (Table 1).
Table 1.
Test positivity rate among patients presenting with febrile illness, by test, among samples with results available
| Test | Results available (N) | Positivity % (95% CI) |
|---|---|---|
| RDT | 496 | 67.3% (63.0–71.5 |
| HRP2 antigenemia | 496 | 81.9% (78.2–85.2) |
| Blood smear | 298 | 61.4% (55.6–67.0) |
| PET-PCR | 284 | 68.7% (62.9–74.0) |
| ≥ 1 long half-life antibody + | 494 | 89.4% (86.6–92.0) |
| ≥ 1 short half-life antibody + | 494 | 80.6% (77.1–84.1) |
Results for the concordance of test results for the 225 samples for whom RDT, HRP2 antigenemia, blood smear, and PET-PCR are shown in Figure 1. In total, 188 of the febrile patients (83.7%) had a positive result for one or more tests, and 130 (57.8%) were positive for all four diagnostic tests (RDT, blood smear, PET-PCR, and HRP2 antigenemia). Using PET-PCR as the gold standard, RDT was 95.2% sensitive and 93.7% specific, expert microscopy was 90.4% sensitive and 100.0% specific, and HRP2 antigenemia was 97.9% sensitive and 48.1% specific. Four patients (1.8%) had a positive RDT and HRP2 antigenemia, but negative PCR and blood smear, suggesting recently cleared parasitemia. Three infections (1.3%) were detected by PET-PCR but not RDT or HRP2 antigenemia; one of these was also detected by blood smear, and two had very low amounts of DNA by PET-PCR. While HRP2 antigenemia was identified in 37 samples (16.6%) not positive by blood smear, RDT, or PET-PCR, it was identified in three samples positive by PCR that were negative by RDT and blood smear.
Figure 1. Concordance among tests for active P. falciparum infection for 225 patients with results for all tests.
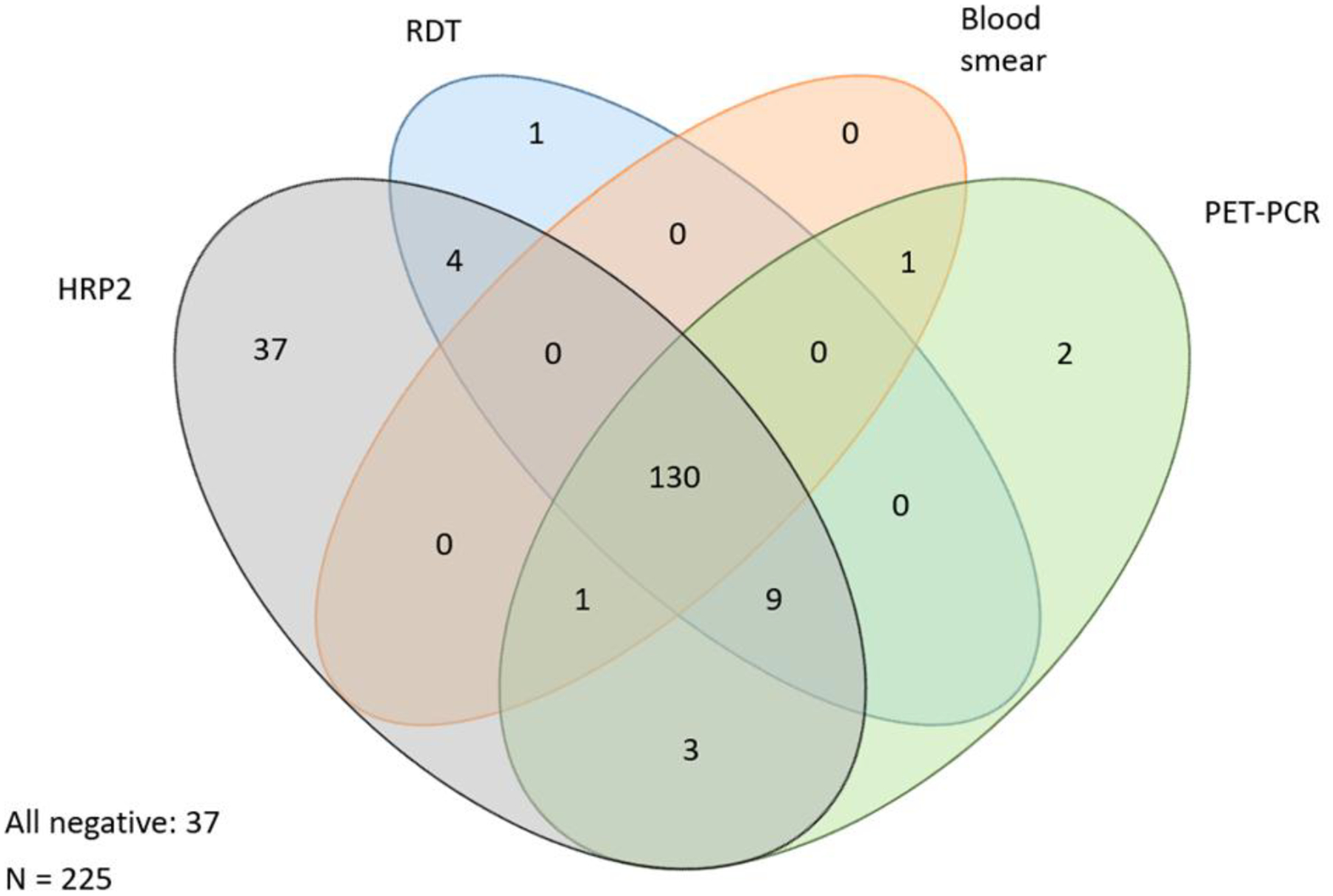
Ovals display number of positives for each test either by itself, or concordance with other tests.
In the 4-test LCA, HRP2 antigenemia and RDT were found to be conditionally dependent, and in the 6-test LCA, long and short half-life antibodies were found to be conditionally dependent; the model was refit by replacing the variables for the two dependent tests with a joint 4 category variable ((positive, positive); (positive, negative); (negative, positive); and (negative, negative))(Table 2). Positivity of the modeled gold standard was 62.4% for the 4-test LCA and 62.3% for the 6-test LCA. Sensitivity and specificity for RDT, blood smear, PET-PCR, and HRP2 antigen was very similar whether or not antibody positivity was included in the model. Sensitivity was greater than 99% for RDT, PET-PCR, and HRP2 antigen detection, but only 93.6% for blood smear, for both the 4-test and 6-test LCA. Comparing RDT, blood smear, PET-PCR, and antigen detection, specificity was highest for blood smear (99.6% and 99.5% for the 4-test and 6-test LCA, respectively), and lowest for HRP2 antigenemia (47.3% in the 6-test LCA). In the 6-test LCA, the sensitivity and specificity of antibody detection were both lower than the other tests and (87.3% and 15.4%, respectively) compared to the modeled gold standard.
Table 2.
Sensitivity and specificity of each test compared to modeled gold standard using 4-test and 6-test latent class analysis
| 4 tests: RDT, blood smear, PCR, HRP-2 antigenemia | 6 tests: RDT, blood smear, PCR, HRP-2 antigenemia, long and short half-life antibody | |||
|---|---|---|---|---|
| Test | sensitivity | specificity | sensitivity | specificity |
| Blood smear | 93.59 (89.44–97.74) |
99.63 (98.28–100.00) |
93.61 (89.46–97.77) |
98.48 (95.79–100.00) |
| PET | 99.87 (99.25–100.00) |
93.43 (87.88–98.98) |
99.86 (99.18–100.00) |
92.21 (86.27–98.15) |
| RDT +, HRP-2 + | 98.37 (96.21–100.00) |
-- | -- | -- |
| RDT −, HRP-2 − | -- | 45.47 (34.77–6.17) |
-- | -- |
| RDT | -- | -- | 99.10 (97.43–100.00) |
93.33 (87.83–98.83) |
| HRP-2 | -- | -- | 99.91 (99.33–100.00) |
47.27 (36.60–57.93) |
| Antibodies: long +, short + | 87.26 (81.71–92.82) |
-- | ||
| Antibodies: long −, short − | -- | 15.47 (07.75–23.19) |
||
Seropositivity was highest for IgG antibodies with known longer half-lives, at 85.5% and 78.3% for MSP1 and AMA1, respectively (Table 3). IgG positivity was lowest for antigens inducing more short-term IgG responses, with StarpR and SALSA at 17.7% and 10.7%, respectively. Seropositivity reported for patients with positive vs. negative HRP2 antigenemia was also highly similar to that reported for patients with positive vs. negative PCR. Except for IgG antibodies against HRP2, overall antibody positivity was associated with both HRP2 antigen positivity and PET-PCR positivity for each IgG target. In the multivariate model, logMFI-bg of AMA1 (Pr Chi-sq 0.0046), LSA1 (Pr Chi-sq 0.0372), StarpR (Pr Chi-sq 0.0077), and GlurpRo (Pr Chi-sq <0.0001) were positively associated with HRP2 antigenemia, logMFI-bg of HRP2 was negatively associated with HRP2 antigenemia (Pr Chi-sq <0.0001), and CSP, SALSA and MSP1 were non-significant.
Table 3.
Positivity of each antibody among all samples, and by HRP-2 antigenemia and PET-PCR status
| By HRP-2 antigenemia result | By PET-PCR result | ||||||
|---|---|---|---|---|---|---|---|
| Antibody Target | Percent positive (n=496) |
HRP-2 positive (n=406) |
HRP-2 negative (n= 90) |
Chi-SQ* | PET-PCR positive (n = 195) |
PET-PCR negative (n = 89) |
Chi-SQ* |
| GlurpRo | 69.6 % (65.5–73.6) |
77.1% (72.7–81.1) |
35.6% (25.7–46.4) |
P <0.001 | 80.0% (73.6–85.4) |
49.4% (38.7–60.3) |
P <0.001 |
| HRP2 | 9.5 % (7.1–12.4) |
9.4% (6.7–12.6) |
10.0% (4.7–18.1) |
P = 0.85 | 9.7% (6.0–14.8) |
10.1% (4.0–17.0) |
P = 0.84 |
| LSA1 | 37.2 % (32.9–41.7) |
42.9% (38.0–47.9) |
11.4% (5.6–19.9) |
P <0.001 | 45.6% (38.5–52.9) |
18.6% (11.0–28.5) |
P <0.001 |
| AMA1 | 78.3 % (74.4–81.9) |
82.5% (78.5–86.1) |
59.1% (48.1–69.5) |
P <0.001 | 87.7% (82.2–92.0) |
54.7% (43.6–65.4) |
P <0.001 |
| CSP | 59.3 % (54.8–63.7) |
64.8% (59.9–69.5) |
34.1% (24.3–45.0) |
P <0.001 | 74.4% (67.6–80.3) |
32.6% (22.8–43.5) |
P <0.001 |
| MSP1 | 85.5 % (82.0–88.5) |
89.5% (86.1–92.4) |
67.0% (56.2–76.7) |
P <0.001 | 91.8% (87.0–95.2) |
72.1% (61.4–81.2) |
P <0.001 |
| StarpR | 17.7 (14.5–21.4) |
21.2% (17.3–25.2) |
2.2% (0.3–7.8) |
P <0.001 | 23.1% (17.4–29.6) |
5.6% (1.9–12.6) |
P =0.0003 |
| SALSA | 10.7 (8.1–13.7) |
12.3% (9.3–15.9) |
3.3% (0.7–9.4) |
P = 0.005 | 12.8% (8.5–18.3) |
2.3% (0.3–7.9) |
P =0.005 |
Exact confidence limits
Discussion
This clinic-based study enabled a comparison of multiple tests used for determining P. falciparum infection as well as assessment of IgG seropositivity status among patients with suspected malaria. Both RDTs and lab-detected HRP2 antigenemia detection by multiplex bead-based assay had high sensitivity for detection of parasite infection, approaching that of PET-PCR, and surpassing that of expert microscopy. Microscopy was more specific than either PCR or the modeled gold standard, as RDTs and particularly HRP2 antigenemia detect residual antigenemia long after an infection has cleared (Plucinski et al., 2018). None of the IgG tests, either singly or in combination, approached the performance of currently available diagnostic tests, making it clear that serology for IgG antibodies has low value as a tool for detection of active infection.
The modeled gold standard by latent class analysis found consistent results, regardless of whether IgG positivity was included in the analysis (4-test LCA vs 6-test LCA). Compared to the modeled gold standard, PET-PCR, RDT, and HRP2 antigenemia each had a sensitivity of greater than 99%, while blood smear had a sensitivity of 93.6% in both the 4-test and 6-test LCA. In comparison, an analysis using LCA to compare sensitivity of PCR, blood smear, and RDT to a modeled gold standard among children under five years in a cross-sectional population-based survey in the Democratic Republic of Congo found sensitivities for blood smear, RDT, and PCR of 76.7%, 86.9%, and 94.6%, respectively, and specificities of 97.2%, 88.1%, and 88.3%, respectively (Doctor et al., 2016). In this DRC study population, parasite prevalence by PCR was 38.6%. While the values differed substantially from this population of clinically ill older children and adults, both found PCR to be highly sensitive and blood smear to be highly specific compared to a modeled gold standard.
Historical parasite prevalence among children < 5 years in the study area has been estimated at 0.2% (Agence Nationale de la Statistique et de la Démographie, 2015), antibody prevalence among this older symptomatic population, even among those who were antigen negative, was substantially higher. Seroprevalence estimates by an IgG-detecting MBA in a population-based survey (of all ages) conducted in 2013 in a zone of central Senegal with slightly higher transmission found similar results for antibodies against CSP, GLURP, and MSP1, but substantially higher seroprevalence for AMA1 and substantially lower seroprevalence of LSA1 and SALSA (Perraut et al., 2017). In this current study, detection of long half-life IgG (against AMA1 and MSP1) and short half-life IgG (CSP, GLURP-Ro, LSA1, SALSA, and StarpR) antibodies was significantly associated with presence of parasite (by PCR) and parasite antigen (by HRP2 antigenemia). The only IgG antibody not correlated with parasite positivity was that against the HRP2 antigen.
There are important limitations to our study: while the sampling of a population seeking care for clinical illness was appropriate for an evaluation of diagnostic modalities, this population is not representative of all persons residing in this area, and therefore findings cannot be generalized to the overall population and may be different from those seen in a cross-sectional survey among mostly asymptomatic individuals. Additionally, these results are from a single study site in Senegal, and the use of these same biomarkers and the LCA may not provide the same results in other human and P. falciparum populations.
Each of the testing modalities examined has different characteristics that make it suited for particular applications. RDT demonstrates remarkable sensitivity and very good specificity for point of care use. Even expert microscopy, while highly specific, is not as sensitive as the other testing modalities, and may be best used in reference centers and research, though PCR continues to be the most appropriate tool for identification and characterization of parasites in research settings. HRP2 antigen detection using the laboratory assay is highly sensitive and may be a useful tool for identifying samples that are RDT negative but may be PCR positive in research settings. While multiplex seroprevalence is not a useful diagnostic modality for acute diagnosis, MFI assay signal and seropositivity classification gives far greater nuance and serology may be very useful for research and surveillance, particularly in low transmission settings in which parasite prevalence is so low that massive sample sizes are needed for accurate and precise estimates. These findings provide guidance for study and surveillance design, considering test characteristics, to choose the most appropriate tools for the desired measurement.
Acknowledgements
We would like to thank Lamine Ndiaye, Tolla Ndiaye, Amy Gaye, and Mamadou Samb Yade for their contributions in patient enrollment, sample collection, and preparation of blood smears.
Funding
For this secondary analysis, the authors declare no funding sources.
List of Abbreviations
- CSP
circumsporozoite protein
- DBS
dried blood spot
- ELISA
enzyme-linked immunosorbent assay
- GLURP-Ro
glutamate-rich protein Ro fragement
- HRP2
histidine-rich protein 2
- IgG
immunoglobulin G
- LAMP
loop mediated isothermal amplification
- LCA
latent class analysis
- LDH
lactate dehydrogenase
- LSA1
liver stage antigen 1
- MBA
multiplex bead assay
- MFI-bg
median fluorescence intensity minus background
- MSP1
merozoite surface protein 1
- PBS
phosphate buffered saline
- PET-PCR
photo-induced electron transfer polymerase chain reaction
- PD
parasite density
- RDT
rapid diagnostic test
- SALSA
sporozoite and liver stage antigen
Footnotes
Ethics approval and consent to participate
All patients were enrolled after giving their informed consent. The study protocol was reviewed and approved by National Ethics Committee of the Senegal Ministry of Health and Social Welfare. U.S. Centers for Disease Control and Prevention staff were not considered to be engaged in human subjects research.
Competing interests
The authors declare that they have no competing interests
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Abdalla ZA, Rahma NA, Hassan EE, Abdallah TM, Hamad HE, Omer SA, Adam I. The diagnostic performance of rapid diagnostic tests and microscopy for malaria diagnosis in eastern Sudan using a nested polymerase chain reaction assay as a reference standard. Trans R Soc Trop Med Hyg. 2019; 113(11):701–705. [DOI] [PubMed] [Google Scholar]
- 2.Agence Nationale de la Statistique et de la Démographie (ANSD) [Sénégal], and ICF International. 2012. Senegal Demographic and Health and Multiple Indicator Cluster Survey (EDS-MICS) 2010–2011. Rockville, Maryland, USA: ANSD and ICF International. [Google Scholar]
- 3.Agence Nationale de la Statistique et de la Démographie (ANSD) [Sénégal], et ICF International. 2015. Sénégal : Enquête Démographique et de Santé Continue (EDS-Continue 2014). Rockville, Maryland, USA: : ANSD et ICF International. [Google Scholar]
- 4.Agence Nationale de la Statistique et de la Démographie (ANSD) [Sénégal], et ICF. 2018. Sénégal : Enquête Démographique et de Santé Continue (EDS-Continue 2017). Rockville, Maryland, USA: : ANSD et ICF. [Google Scholar]
- 5.Dalrymple U, Arambepola R, Gething PW, Cameron E. How long do rapid diagnostic tests remain positive after anti-malarial treatment? Malar J. 2018; 17(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doctor SM, Liu Y, Whitesell A, Thwai KL, Taylor SM, Janko M, Emch M, Kashamuka M, Muwonga J, Tshefu A, Meshnick SR. Malaria surveillance in the Democratic Republic of the Congo: comparison of microscopy, PCR, and rapid diagnostic test. Diagn Microbiol Infect Dis. 2016; 85(1):16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrett ES, Zeger SL. Latent class model diagnosis. Biometrics. 2000; 56, 1055–1067. [DOI] [PubMed] [Google Scholar]
- 8.Hui S, Walter S Estimating the error rates of diagnostic tests. Biometrics. 1980; 36, 167–171. [PubMed] [Google Scholar]
- 9.Liu Y, Mwapasa V, Khairallah C, Thwai KL, Kalilani-Phiri L, Ter Kuile FO, Meshnick SR, Taylor SM. Rapid Diagnostic Test Performance Assessed Using Latent Class Analysis for the Diagnosis of Plasmodium falciparum Placental Malaria. Am J Trop Med Hyg. 2016; 95(4):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, et al. Molecular Diagnosis of Malaria by Photo-Induced Electron Transfer Fluorogenic Primers: PET-PCR. PLoS ONE. 2013; 8(2): e56677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucchi NW 1, Karell MA, Journel I, Rogier E, Goldman I, Ljolje D, Huber C, Mace KE, Jean SE, Akom EE, Oscar R, Buteau J, Boncy J, Barnwell JW, Udhayakumar V. PET-PCR method for the molecular detection of malaria parasites in a national malaria surveillance study in Haiti, 2011. Malar J. 2014; 13:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucchi NW, Gaye M, Diallo MA, Goldman IF, Ljolje D, Deme AB, Badiane A, Ndiaye YD, Barnwell JW, Udhayakumar V, Ndiaye D, Lucchi NW, et al. Evaluation of the Illumigene Malaria LAMP: A Robust Molecular Diagnostic Tool for Malaria Parasites. Sci Rep. 2016; 6:36808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ondigo BN, Hodges JS, Ireland KF, Magak NG, Lanar DE, Dutta S, Narum DL, Park GS, Ofulla AV, John CC. Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis. 2014; 210(7):1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perraut R, Varela ML, Loucoubar C, Niass O, Sidibé A, Tall A, Trape JF, Wotodjo AN, Mbengue B, Sokhna C, Vigan-Womas I, Touré A, Richard V, Mercereau-Puijalon O, Perraut R, et al. Serological signatures of declining exposure following intensification of integrated malaria control in two rural Senegalese communities. PLoS One. 2017; 12(6):e0179146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plucinski MM, Dimbu PR, Fortes F, Abdulla S, Ahmed S, Gutman J, Kachur SP, Badiane A, Ndiaye D, Talundzic E, Lucchi N, Aidoo M, Udhayakumar V, Halsey E, Rogier E. Posttreatment HRP2 Clearance in Patients With Uncomplicated Plasmodium Falciparum. Malaria J Infect Dis. 2018; 217(5):685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plucinski MM, Dimbu PR, Fortes F, Murphy SC, Smith NT, Cruz KR, Seilie AM, Halsey ES, Aidoo M, Rogier E. Malaria Parasite Density in Individuals with Different Rapid Diagnostic Test Results and Concentrations of HRP2 Antigen. Am J Trop Med Hyg. 2019a; 100(5):1202–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plucinski MM, Herman C, Jones S, Dimbu R, Fortes F, Ljolje D, Lucchi N, Murphy SC, Smith NT, Cruz KR, Seilie AM, Halsey ES, Udhayakumar V, Aidoo M, Rogier E. Screening for Pfhrp2/3-Deleted Plasmodium falciparum, Non-falciparum, and Low-Density Malaria Infections by a Multiplex Antigen Assay. J Infect Dis. 2019b; 219(3):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogier E, Wiegand R, Moss D, Priest J, Angov E, Dutta S, Journel I et al. 2015. Multiple comparisons analysis of serological data from an area of low Plasmodium falciparum transmission. Malar J. 2015; 14:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogier E, Plucinski M, Lucchi N, Mace K, Chang M, Lemoine JF, Candrinho B, Colborn J, Dimbu R, Fortes F, Udhayakumar V, Barnwell J. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS One. 2017; 12(2):e0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarr JB, Orlandi-Pradines E, Fortin S, Sow C, Cornelie S, Rogerie F, Guindo S, Konate L, Fusaï T, Riveau G, Rogier C, Remoue F. Assessment of exposure to Plasmodium falciparum transmission in a low endemicity area by using multiplex fluorescent microsphere-based serological assays. Parasit Vectors. 2011; 4:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz G Estimating the dimension of a model. Annals of Statistics. 1978; 6:461–464. [Google Scholar]
- 22.Yman V, White MT, Asghar M, Sundling C, Sondén K, Draper SJ, Osier FHA, Färnert A. Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: kinetics and longevity in absence of re-exposure. BMC Med. 2019;17(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


