Abstract
OBJECTIVES
We aimed to review the outcomes of treating incidentally encountered asymptomatic airway stenosis during open-heart surgery conservatively without the use of tracheoplasty.
METHODS
Between January 2002 and October 2022, 25 patients were incidentally diagnosed with tracheal stenosis during open-heart surgery. Intraoperative bronchoscopy and/or laryngoscopy revealed tracheal stenosis; however, this was not consistent with the findings of the preoperative computed tomography. Patients who were diagnosed with a pulmonary artery or vascular sling or had moderate-to-severe respiratory symptoms before open-heart surgery were excluded.
RESULTS
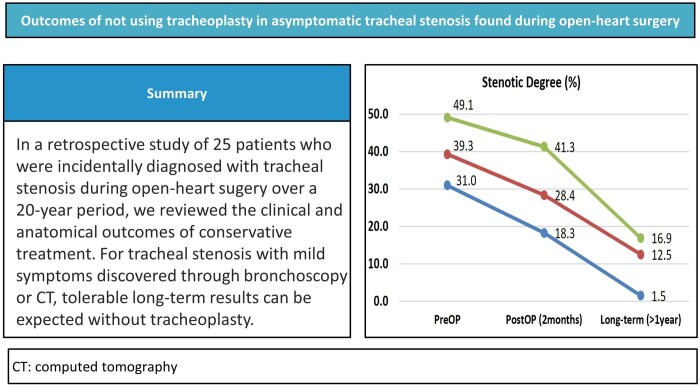
The median age and weight of the patients at operation were 3.0 months and 5.1 kg, respectively. They were categorized as those having tracheal stenosis on preoperative computed tomography (n = 12) or not having tracheal stenosis (n = 13). The narrowest diameter was significantly smaller in the former group (3.0 vs 5.8 mm, P < 0.05). The rates of reintubation and the tracheostomy, and intubation days tended to be higher in former group without statistical significance. Stenotic degree improved 2 months and 1 year or more after the operation (39.3% at operation, 28.4% at 2 months, 12.5% after 1 year). All patients were Ross class 1 or 2 at follow-up (mean, 7.1 years).
CONCLUSIONS
Patients with tracheal stenosis showed tolerable long-term outcomes without using tracheoplasty. Accordingly, if tracheal stenosis, that would cause intubation difficulty, was incidentally revealed, concomitant tracheoplasty may not be required during open-heart surgery if the stenosis did not cause considerable symptoms or signs preoperatively.
Keywords: Congenital tracheal stenosis, Congenital heart disease, Tracheoplasty
Congenital tracheal stenosis (CTS) can be symptomatic in infants and can cause several life-threatening events or developmental delays [1, 2].
INTRODUCTION
Congenital tracheal stenosis (CTS) can be symptomatic in infants and can cause several life-threatening events or developmental delays [1, 2]. The incidence of CTS could be underrepresented because patients may die before the diagnosis is made, and some may not show any significant symptoms or signs. Rania and El-Hakim recommended the conservative treatment of CTS in asymptomatic patients, even if their trachea was revealed to be anatomically stenotic, because the stenotic segment of their trachea may grow and their quality of life could remain unaffected over time [3]. Nonetheless, patients with severe symptoms, such as persistent wheezing, cyanosis, complete tracheal rings, long-segment stenosis or stenosis of <50% of the normal diameter, may require tracheoplasty [4]. Recently, slide tracheoplasty has been accepted as a reliable surgery for managing CTS; however, the overall mortality associated with airway problems during the procedure remains at 10%, and morbidities, such as the ingrowth of granulation tissue and mucosa varus, have been reported in 25% of these patients [5, 6]. The timing of and indications for tracheoplasty remain controversial [7].
Over 50% of patients with CTS have multiple concomitant congenital disorders, the most common being congenital heart disease (CHD) [2]. According to an analysis of the Society of Thoracic Surgeons database, tracheal operations for airway anomalies could increase the mortality and morbidity in paediatric patients who were subjected to cardiac surgery. Further, other studies have shown acceptable outcomes utilizing conservative treatments without using tracheoplasty [8–10].
In this study, we aimed to investigate the long-term outcomes of patients with CHD who showed no or mild symptoms and did not undergo concomitant tracheoplasty, even though their airway stenosis was confirmed by bronchoscopy and/or laryngoscopy during anaesthesia for CHD surgery, after showing difficulty in their intubation.
MATERIALS AND METHODS
Ethical statement
This study was approved by our Institutional Review Board (approval no. 2206-036-1331, 2022-06-09). The requirement for individual consent was waived.
Statistics statement
All data were analysed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). Quantitative variables were expressed as mean values with standard deviations when the data showed normal distribution or median values with interquartile range when the data did not follow normal distribution. Categorical variables were expressed as absolute numbers and relative frequencies. The Mann–Whitney test was used to compare the tracheal diameter, ventilation day, and Ross class between the groups, and the Mantel–Cox test was used to compare the mortality between the 2 groups. The chi-square test was used to analyse the features of each group. In all analyses, a P value of <0.05 was considered statistically significant.
Patient enrolment and operation
Among patients who required intraoperative bronchoscopy and/or laryngoscopy to evaluate their upper airway because there was difficulty in their intubation during anaesthesia prior to cardiovascular surgery for their CHDs, we enrolled those who had not previously shown remarkable respiratory symptoms or signs associated with the airway from January 2002 to October 2022. Bronchoscopic and/or laryngoscopic examinations revealed tracheal stenosis, tracheomalacia or laryngomalacia in all the patients; however, these findings were not completely consistent with the patients’ preoperative computed tomography (CT) images. CT was performed to evaluate their congenital cardiovascular diseases and not to check their airway status; thus, their airway stenosis was incidentally noticed. Therefore, patients who [1] were diagnosed with pulmonary artery sling or vascular ring [2] had moderate-to-severe symptoms, such as persistent wheezing, cough or cyanosis due to known airway problems [3], underwent an intraoperative bronchoscopic and/or laryngoscopic examination for any other reason, such as toileting bronchoscopy or evaluation of the trachea after aortic surgery, and [4] did not undergo preoperative CT were excluded. Finally, we enrolled 25 patients for analysis (Fig. 1).
Figure 1:
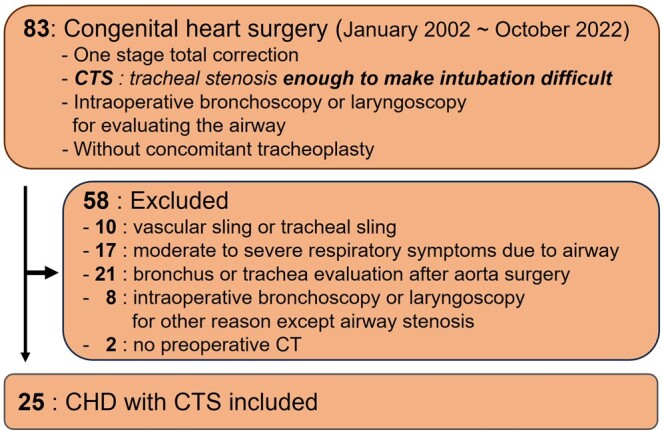
Study’s population diagram. CT: computed tomography; CHD: congenital heart disease; CTS: congenital tracheal stenosis.
Twenty-three patients (92.0%) underwent one-stage total correction of their original CHD through a median sternotomy under cardiopulmonary bypass support, and 2 patients (8.0%) underwent patent ductus arteriosus closure through a left thoracotomy. Although tracheal stenosis was identified owing to the difficulty in their intubation, concomitant tracheoplasty was not performed in any patient.
Additionally, the presence of tracheal stenosis was confirmed by intraoperative bronchoscopy and/or laryngoscopy; nevertheless, compatible findings on the preoperative CT images were found in only 12 patients. The remaining 13 did not show definite tracheal findings on their images. Based on the preoperative CT findings regarding tracheal stenosis, we divided the patients into 2 groups: a stenosis group (S group) and a no-stenosis group (NS group).
Tracheal stenosis measurements made through computed tomography
Preoperative CT was performed in 25 patients and postoperative CT was performed in 17 patients (68.0%) after 1–3 months. Postoperative one-year CT, defined as long-term CT, was performed in 16 patients (64.0%). The narrowest tracheal diameter in each patient was measured in the axial plane of the CT images (mm). The degree of tracheal stenosis was calculated using the following equation.
The average diameter of the normal trachea is the average of the shortest and longest diameters of the proximal and distal tracheas. The total tracheal length was defined as the segmental length from the tracheal cartilage to the inferior edge of the 4th cervical vertebra because the base of the cricoid cartilage in infants is on the opposite side of the spine [11]. In our study, patients with a stenosis degree of >25% were considered to have been diagnosed with tracheal stenosis on CT. The stenotic length ratio was defined as the ratio of the length of the stenotic segment to the full length of the trachea (trachea: from just below the cricoid cartilage to just above the bifurcation) and was measured on the sagittal plane of the CT. The location of the stenotic part of the trachea was determined by dividing the airway into 3 parts: the upper (the cricoid cartilage to the sternoclavicular joint), mid (the sternoclavicular joint to the left innominate vein) and lower parts (the azygos vein to the tracheal bifurcation). Diameter/length ratio was determined to be the ratio of the narrowest diameter (mm) to the stenotic length ratio [7].
Airway examination by bronchoscopy and/or laryngoscopy
Anaesthesiologists usually prepare an endotracheal tube (E-tube) of a standardized size based on the patient’s age and weight [12]. As mentioned above, intubation was difficult in all the patients in this study not only with a standardized E-tube but also with smaller E-tubes. Therefore, the patients’ airways were evaluated using intraoperative bronchoscopy and/or laryngoscopy to perform the intubations. Flexible bronchoscopic examination was performed on all the patients by anaesthesiologists, and additional laryngoscopic examinations by otolaryngologists were required in 11 (44%) patients to explore the supraglottic, glottic and subglottic areas.
Postoperative management strategy and long-term functional status evaluation
After surgery, the intensive care unit team attempted to wean the patients from ventilation as early as possible. We also tried effective lung care after extubation through physiotherapy in the left and right side-up positions using a vibrator and nebulizer therapy every 6 h by an expert nursing team. We used acetylcysteine (Mucomyst), budesonide inhaler (Pulmicort), albuterol inhaler (Ventolin), epinephrine or aminophylline infusion to improve airway stability in the intensive care unit. If the patients showed acidosis with pH <7.2, hypercapnia with PaCO2 > 60 mmHg or an increase in the number of breaths of ≥20, indicating that they would not tolerate self-respiration after extubation, we considered reintubation. However, we still attempted to minimize the intubation period to prevent mechanical irritation of the airway. Dexamethasone was administered 3 times at 6-h intervals before extubation and once more after extubation in patients requiring prolonged (≥7 days) ventilation support or repeated reintubation (Fig. 2). Long-term functional status was evaluated at the last outpatient clinic visit using the Ross classification [13].
Figure 2:
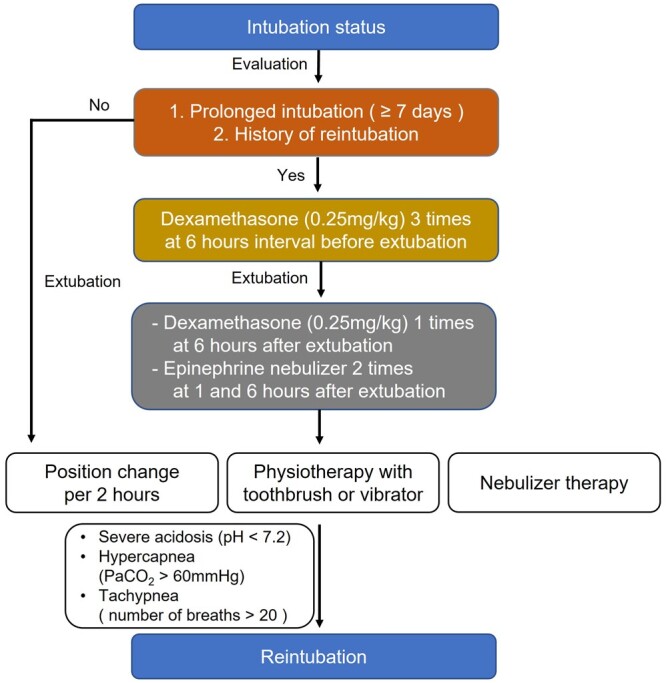
Postoperative airway management strategy.
RESULTS
Baseline characteristics and perioperative data
None of the patients underwent concomitant tracheoplasty during their congenital heart surgery and all were discharged without additional surgical management of their airways. Three patients ultimately underwent tracheostomy at a median of 11 months after cardiac surgery, whereas the other 22 patients did not require any additional procedures for the trachea. Patient characteristics are described in Table 1.
Table 1:
Patient characteristics
| Variables | n = 25 (%) |
|---|---|
| Sex (male), n (%) | 17 (68.0) |
| Age (months), median (IQR) | 3 (2–6) |
| Bwt (kg), median, (IQR) | 5.1 (3.2–7.6) |
| Preoperative symptom, n (%) | |
| Dyspnoea due to cardiac origin | 13 (52.0) |
| Tachycardia | 4 (16.0) |
| No symptom | 6 (24.0) |
| Others | 2 (8.0) |
| Congenital heart disease diagnosis, n (%) | |
| Interrupted aortic arch | 6 (24.0) |
| Tetralogy of Fallot | 5 (20.0) |
| Coarctation of the aorta | 4 (16.0) |
| Ventricular septal defect | 3 (12.0) |
| Atrial septal defect | 2 (8.0) |
| Others | 5 (20.0) |
| Preoperative CT evaluation, n (%) | |
| Tracheal stenosis | 12 (0.48) |
| No tracheal stenosis | 13 (0.52) |
| Ventilation (day), median (IQR) | 5 (3–9) |
| Reintubation | 4 (16.0) |
| Tracheal stenosis evaluation in operating room, n (%) | |
| Flexible bronchoscopy | 25 (100.0) |
| Laryngoscopy | 11 (44.0) |
| Tracheal stenosis diagnosis by bronchoscopy ± laryngoscopy, n (%) | |
| Tracheal stenosis | 14 (56.0) |
| Tracheomalacia | 7 (28.0) |
| Laryngomalacia | 4 (16.0) |
| Tracheostomy, n (%) | 3 (12.0) |
| Follow-up duration (month), median (IQR) | 115 (49.0–144.0) |
| Survival, n (%) | 23 (92.0) |
| Functional status of last outpatient clinic, n (%) | |
| Ross class I | 21 (84.0) |
| Ross class 2 | 2 (8.0) |
Bwt: body weight; CT: computed tomography; IQR: interquartile range.
Stenosis group versus no-stenosis group
The findings of the preoperative CT and intraoperative bronchoscopy and/or laryngoscopic examination of the airway in the S and NS groups are described in Table 2. The patients’ age and weight were higher in the S group than in the NS group, although the difference was not statistically significant, and the preoperative symptoms were not significantly different between the groups. Laryngomalacia was only diagnosed in the NS group, whereas tracheomalacia was diagnosed in the S group. Among several factors, only the narrowest diameter and narrowest diameter according to body weight were significantly smaller in the S group than in the other group.
Table 2:
Characteristics of S and NS group
| Variables | S group (n = 12) | NS group (n = 13) | P-value |
|---|---|---|---|
| Sex (male), n (%) | 8 (66.7) | 9 (69.2) | 0.89 |
| Age (months), median (IQR) | 4.4 (2.0–6.3) | 3.4 (2.0–4.0) | 0.34 |
| Body weight (kg), median (IQR) | 5.7 (3.1–7.9) | 4.5 (3.2–5.1) | 0.21 |
| Cardiac disease, n (%) | 0.12 | ||
| Interrupted aortic arch | 3 (25.0) | 3 (23.1) | |
| Tetralogy of Fallot | 4 (33.3) | 1 (7.7) | |
| Coarctation of aorta | 4 (30.8) | ||
| Others | 5 (41.7) | 5 (38.5) | |
| Preoperative symptom, n (%) | 0.39 | ||
| Dyspnoea due to heart failure | 6 (50.0) | 7 (53.8) | |
| Tachycardia | 3 (25.0) | 1 (7.7) | |
| Others (tachypnoea, poor oral intake) | 2 (15.4) | ||
| No symptom | 3 (25.0) | 3 (23.1) | |
| Preoperative trachea, median (IQR) | |||
| Narrowest diameter (mm) | 2.8 (2.2–3.2) | 5.8 (5.2–6.5) | <0.001 |
| Narrowest diameter/body weight (mm/kg) | 0.6 (0.4–0.7) | 1.4 (1.1–1.7) | <0.001 |
| Broadest diameter (mm) | 6.6 (5.6–7.0) | 7.0 (6.3–7.9) | 0.61 |
| Total tracheal length (mm) | 38.3 (30.2–46.5) | 36.5 (33.8–38.5) | 0.62 |
| Total tracheal length/body weight (mm/kg) | 7.4 (5.8–7.9) | 9.2 (6.9–11.1) | 0.14 |
| Bronchoscopy and/or laryngoscopy, n (%) | 0.07 | ||
| Tracheal stenosis | 7 (58.3) | 7 (53.8) | |
| Tracheomalacia | 5 (41.7) | 2 (15.4) | |
| Laryngomalacia | 4 (30.8) |
IQR: interquartile range; NS: no-stenosis; S: stenosis.
Rate of reintubation and ventilation duration were longer in the S group than in the other group, but the differences were not statistically significant. Postoperative follow-up duration was longer in the NS group than in the other group; however, in terms of functional status, patients in both groups were all classified as Ross class I or II at their most recent outpatient visit. Nevertheless, the functional status of the NS group was significantly better than that of the S group. (Table 3, Fig. 3).
Table 3:
Short- and long-term clinical outcomes
| Variables | S group (n = 12) | NS group (n = 13) | P-value |
|---|---|---|---|
| Perioperative outcome | 0.60 | ||
| Reintubation, n (%) | 3 (25.0) | 1 (7.7) | |
| Intubation day (day), median (IQR) | 7 (2.8–11.5) | 4 (3–7) | |
| Early mortality (≤30 days), n (%) | 0 (0.0) | 0 (0.0) | |
| Long-term outcome | 0.78 | ||
| Follow-up duration (month), median (IQR) | 90.4 (22.8–130.0) | 115.8 (63.0–163.0) | |
| Tracheostomy, n (%) | 2 (16.7) | 1 (7.7) | |
| Late mortality (>30 days), n (%) | 1 (8.3) | 1 (7.7) | |
| Functional outcome | <0.01 | ||
| Ross class I | 9 | 12 | |
| Ross class II | 2 | 0 |
IQR: interquartile range; NS: no-stenosis; S: stenosis.
Figure 3:
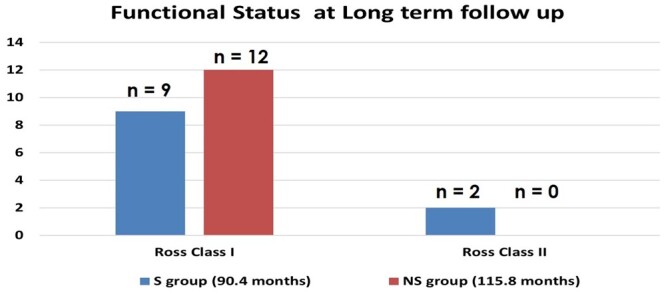
This figure shows the functional status at long-term follow-up of the Stenosis group (S group) and No-stenosis (NS group). In the S group, 9 patients were Ross class I and 2 patients were Ross class II. In the NS group, all patients were Ross class I. Although, the functional status of the 2 groups were all Ross class I or II, they were significantly different (P < 0.01).
Short- and long-term computed tomography outcomes of the stenosis group
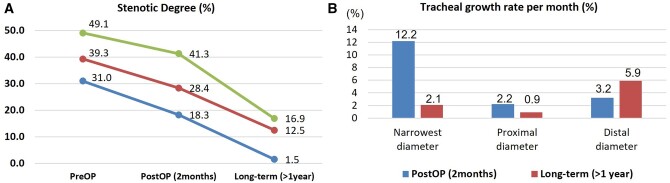
In the group S, tracheal stenosis in the 9 patients (75.0%) who underwent short-term (1–3 month) postoperative CT and 6 patients (50.0%) who underwent long-term (>1 year) postoperative CT were compared (Table 4, Fig. 4A). The median long-term CT follow-up duration was 113 (interquartile range 62–140) months. The most common location of the stenosis was the lower part of the airway. The stenotic degree and narrowest diameter were shown to have improved in the postoperative and long-term CT (39.3%, 2.8 mm at operation, 28.4%, 4.0 mm at 2 months, 12.5%, 8.8 mm at ≥1 year). Moreover, the patients’ diameter/length ratio values improved (7.2 at operation, 11.6 at 2 months, 30.7 at ≥1 year) but, the length of the stenotic lesions did not change markedly over time (14 mm at operation, 12 mm at 2 months, 14 mm at ≥1 year).
Table 4:
Short- and long-term CT evaluation of stenosis patients’ trachea
| In the S group | Pre-OP (n = 12) | Post-OP (n = 9) | Long-term (n = 6) |
|---|---|---|---|
| Location of stenosis, n (%) | |||
| Upper, mid, lower | 2 (16.7) | 1 (11.1) | 1 (16.7) |
| Mid, lower | 2 (16.7) | 1 (11.1) | |
| Mid | 2 (16.7) | 2 (22.2) | 1 (16.7) |
| Lower | 6 (50.0) | 5 (55.6) | 4 (66.7) |
| CT finding, mean (IQR) | |||
| Narrowest diameter (mm) | 2.8 (2.2–3.2) | 4.0 (3.2–4.7) | 8.8 (6.75–10.45) |
| Stenotic degree (%) | 39.3 (31.6–49.1) | 28.4 (18.3–41.3) | 12.5 (1.5–16.9) |
| Length of stenotic lesion (mm) | 14.0 (12.0–16.3) | 12.0 (10.0–16.0) | 14.0 (2.5–23.1) |
| SLR (%) | 32.0 (24.5–37.4) | 31.0 (23.0–36.0) | 24.8 (4.3–25.7) |
| DLR value (= diameter/SLR) | 7.2 (5.6–15.5) | 11.6 (7.8–12.4) | 30.7 (21.9–44.6) |
CT: computed tomography; DLR: diameter length ratio; IQR: interquartile range; post-OP: postoperative; pre-OP: preoperative; S: stenosis; SLR: stenotic length ratio.
Figure 4:
(A) Stenotic degree in the S group has improved over time. Red line represents the median value of the stenotic degree. Green and blue lines represent the interquartile line of the stenotic degree. (B) Two months after operation, the growth rate was highest (12.2%) in the narrowest part of the trachea. One year after operation, the growth rate was highest (5.9%) in the distal part of the trachea. post-OP: postoperative; pre-OP: preoperative.
Based on the narrowest part of the airway, the growth rates per month of the trachea in the proximal, narrowest and distal parts of the airway were compared (Fig. 4B). The growth rates of the narrowest parts were high on postoperative CT; however, the distal part showed the highest growth rate 1 year after operation.
Tracheostomy and late mortality during the follow-up
Three female patients ultimately underwent tracheostomy during follow-up. A six-month-old patient (body weight: 7.6 kg) who underwent surgery for an interrupted aortic arch was discharged after only 2 days of ventilator care. However, 11 months after surgery, her tracheomalacia aggravated, and she underwent tracheostomy. Another one-month-old patient (body weight: 2.4 kg) who underwent surgery for coarctation of the aorta was discharged after 7 days of ventilator care. One month after surgery, an iatrogenic tracheal injury occurred during bronchoscopy, and an urgent tracheostomy was performed. Another four-month-old patient (body weight: 8.7 kg) who underwent surgery for an interrupted aortic arch was discharged after 3 days of ventilator care. Thirty-three months after the surgery, she was diagnosed with spinal muscular atrophy, and a tracheostomy was required because of respiratory difficulty. None of the patients showed left bronchial stenosis after arch repair with extended end-to-end anastomosis and arch surgeries did not affect their airway structure.
A nine-month-old patient (body weight: 8.2 kg) underwent surgery for a double-outlet right ventricle. The patient died 18 years after the surgery for unknown reasons. Another one-month-old patient (body weight: 2.7 kg) underwent tetralogy of Fallot surgery. Nine months after surgery, the patient was hospitalized with a suspected aspiration pneumonia and died of septic shock.
DISCUSSION
Although 3 patients (12.0%) eventually underwent tracheostomy, and 2 died (8.0%), this was not related to the airway problems that were incidentally found in the operating room. All the survivors’ functional statuses were Ross 1 or 2 at the long-term follow-up after the conservative treatment.
CTS can present with a variety of clinical manifestations and is associated with other anomalies, particularly CHD. Congenital heart surgery with concomitant tracheoplasty is usually requested for some patients, but it could increase the possibility of postoperative morbidity and mortality [1]. A recent study reported that mortality rates increase when airway surgery is performed alongside open-heart surgery [9]. However, some authors recommend concomitant tracheoplasty with open-heart surgery in patients with tracheal stenosis [14, 15]. We adopted a conservative strategy for airway problems, especially those incidentally found, followed by observation for airway problems. We reviewed the outcomes of this strategy for the treatment of airway problems associated with congenital cardiovascular diseases. Short-term effects were evaluated based on the anatomical changes noted on CT, and long-term effects were evaluated based on changes in anatomical and functional statuses.
In our study, patients with preoperatively identified vascular or complete tracheal rings that could directly affect the trachea were excluded because they may require surgical treatment. Nevertheless, we attempted to maintain our conservative strategy in these patients [8]. Previous studies have reported controversial results regarding whether tracheoplasty should be performed for patients with pulmonary artery slings. Some have argued that pulmonary artery implantation is sufficient to reduce or alleviate tracheal stenosis; conversely, others have argued that pulmonary artery implantation is not sufficient and tracheoplasty should be performed to completely resolve tracheal stenosis to result in a better quality of life [16–18]. Therefore, we excluded patients with these diseases; only those with CHD, that did not directly affect the trachea, were included, which seemed reasonable for this study.
According to previous studies, tracheoplasty has been proposed for the treatment of severe CTS, with tolerable outcomes being reported. Although the mortality rate of slide tracheoplasty is almost 10–15% [14, 19, 20], recently, it has been widely used for the surgical management of airway problems. It allows tracheal growth and provides stable cartilaginous walls with normal epithelial cell lining. However, it has some disadvantages, including shortening of the trachea, prolonged use of cardiopulmonary bypass, anastomotic failure and high reoperation or reintervention rates associated with intimal granulation tissue growth [14, 21]. Problems at the anastomotic site, including restenosis and dehiscence, are the most frequent and hazardous complications of tracheoplasty [14, 19]. Chung et al. reported that ∼2/18 (11%) patients underwent reoperation for tracheal restenosis, and Antón-Pacheco et al. reported that 3/14(21%) patients underwent reinterventions, such as reoperation, laser division or stent placement, because of severe postoperative complications [14, 19].
Generally, tracheal stenosis can be evaluated by CT or bronchoscopy, but the diagnostic criteria are unclear because of variability in the growth, weight, and height of paediatric patients. In this study, significant tracheal stenosis was defined as tracheal stenosis diagnosed based on the findings of bronchoscopic and/or laryngoscopic examination, which was performed because there was difficulty in intubating the patients. Bronchoscopic and/or laryngoscopic evaluation can reflect both dynamic and static elements of the trachea, whereas CT mainly presents static aspects of the structures. Although muscle relaxants are used during intubation, direct visible evaluation through bronchoscopy and/or laryngoscopy is used to evaluate the dynamic components of the airway, including the supraglottis, glottis, subglottis, trachea and bronchus [22]. According to Morshed et al., CT and bronchoscopy have been reported to have ∼80–90% sensitivity and specificity for intraoperative findings in adults with tracheal stenosis [23]. Filauro et al. reported that there were limitations in evaluating the trachea in paediatric patients using only imaging studies, such as CT [24]. Therefore, a comprehensive preoperative assessment of the airway is important, and careful treatments are required in cases of tracheal stenosis, that are detected on both CT and bronchoscopy. In our study, half of the patients were categorized as having airway stenosis based on preoperative CT findings, although they did not exhibit airway-related symptoms or signs.
Some studies have reported that conservative management of selected patients with CTS could result in better outcomes than those of surgical treatment. Cheng et al. reported that mortality was lower in the observation group than that in the operation group (9% vs 27%, respectively), but the symptoms in the operation group were more severe, and the patients were ∼12 months younger [10]. In this study, regardless of the cause of CTS or the location of the stenotic lesions, the lesions showed growth, and in some cases, the stenotic portion grew faster than the normal portion.
Postoperative airway management is important for patients who require endotracheal intubation during perioperative cardiac surgery because stenotic problems can be aggravated by tracheal irritation due to the E-tube or repeated intubation trials. In our study, none of the patients underwent additional airway interventions for tracheal stenosis after their open-heart surgery or before their discharge.
A comparison of tracheal stenosis on CT after 1–3 months and >1 year in the S group showed that several parameters of tracheal stenosis improved over time, although the length of the stenotic lesions hardly changed. Their high growth rate was more predictable than those of the proximal and distal trachea. Cheng et al. also applied conservative therapy for tracheal stenosis for ∼2 decades and reported that the growth rate of the stenotic trachea was higher than normal; however, the reason for the unexpected accelerated growth of the stenotic portion of the trachea remains unclear [10]. We presented the growth of the stenotic lesions as a long-term functional outcome. Both groups of patients were classified as Ross class I or II 90–115 months after cardiovascular surgery, indicating that their functional status was tolerable. Even if the functional status of the NS group was found to be significantly better (P < 0.05), the number of tracheostomies was lower than those of the S group.
Limitations
Our study had several limitations. First, this was a retrospective study, and its drawbacks, including some missing data, could not be avoided. Second, the sample size of this single-centre study was too small to allow the use of powerful statistical tools. Third, some CT scans did not cover the glottic area and the total tracheal length was slightly shorter than the reported one in some patients. Despite these limitations, our study shows that concomitant tracheoplasty may not always be mandatory for patients with CTS and CHD if they do not show remarkable symptoms or signs related to respiratory difficulty.
CONCLUSION
Our study showed that the long-term clinical outcomes of the management for patients with tracheal asymptomatic tracheal stenosis, which was incidentally revealed during intubation difficulty prior to open-heart surgery, were acceptable without the need of tracheoplasty. This suggests that a conservative treatment, one not employing tracheoplasty, could be considered for these patients, which would help avoid the complications associated with tracheoplasty. Larger prospective studies are needed to validate our results.
ACKNOWLEDGEMENT
None declared.
Glossary
ABBREVIATIONS
- CHD
Congenital heart disease
- CT
Computed tomography
- CTS
Congenital tracheal stenosis
- E-tube
Endotracheal tube
- NS group
No-stenosis group
- S group
Stenosis group
Contributor Information
Seon Yong Bae, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Republic of Korea.
Jae Hong Lee, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Republic of Korea.
Hye Won Kwon, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Republic of Korea.
Sungkyu Cho, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Republic of Korea; Department of Thoracic and Cardiovascular Surgery, College of Medicine, Seoul National University, Seoul, Republic of Korea.
Chiheon Kwon, Department of Radiology, Seoul National University Hospital, Seoul, Republic of Korea.
Woong-Han Kim, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Republic of Korea; Department of Thoracic and Cardiovascular Surgery, College of Medicine, Seoul National University, Seoul, Republic of Korea.
Jae Gun Kwak, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Republic of Korea; Department of Thoracic and Cardiovascular Surgery, College of Medicine, Seoul National University, Seoul, Republic of Korea.
FUNDING
None declared.
Conflict of interest: None declared.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Seon Yong Bae: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing—original draft. Jae Hong Lee: Resources. Hye Won Kwon: Resources. Sungkyu Cho: Resources. Chiheon Kwon: Data curation; Formal analysis; Investigation; Methodology. Woong-Han Kim: Resources. Jae Gun Kwak: Conceptualization; Data curation; Project administration; Resources; Writing—review and editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Ichiro Kashima and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Hofferberth SC, Watters K, Rahbar R, Fynn-Thompson F.. Management of congenital tracheal stenosis. Pediatrics 2015;136:e660–9. [DOI] [PubMed] [Google Scholar]
- 2. Yokoi A. Congenital tracheal stenosis: what should we look at for successful tracheoplasty? Transl Pediatr 2018;7:229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rania Y, El-Hakim H.. Congenital tracheal stenosis managed conservatively: systematic review of the literature. J Otolaryngol Head Neck Surg 2012;41:288–302. [PubMed] [Google Scholar]
- 4. Herrera P, Caldarone C, Forte V, Campisi P, Holtby H, Chait P. et al. The current state of congenital tracheal stenosis. Pediatr Surg Int 2007;23:1033–44. [DOI] [PubMed] [Google Scholar]
- 5. Butler CR, Speggiorin S, Rijnberg FM, Roebuck DJ, Muthialu N, Hewitt RJ. et al. Outcomes of slide tracheoplasty in 101 children: a 17-year single-center experience. J Thorac Cardiovasc Surg 2014;147:1783–9. [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Wang S, Lu Z, Zhu L, Du X, Wang H. et al. Slide tracheoplasty in 81 children: improved outcomes with modified surgical technique and optimal surgical age. Medicine (Baltimore) 2017;96:e8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harada A, Shimojima N, Shimotakahara A, Azuma S, Ishizuka Y, Tomita H. et al. Surgical indication for congenital tracheal stenosis complicated by pulmonary artery sling. J Thorac Dis 2019;11:5474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwak JG, Kim WH, Min J, Lee C, Jang W, Lee CH.. Is tracheoplasty necessary for all patients with pulmonary artery sling and tracheal stenosis? Pediatr Cardiol 2013;34:498–503. [DOI] [PubMed] [Google Scholar]
- 9. Riggs KW, Zafar F, Jacobs ML, Jacobs JP, Thibault D, Guleserian KJ. et al. Tracheal surgery for airway anomalies associated with increased mortality in pediatric patients undergoing heart surgery: Society of Thoracic Surgeons Database analysis. J Thorac Cardiovasc Surg 2021;161:1112–21.e7. [DOI] [PubMed] [Google Scholar]
- 10. Cheng W, Manson DE, Forte V, Ein SH, MacLusky I, Papsin BC. et al. The role of conservative management in congenital tracheal stenosis: an evidence-based long-term follow-up study. J Pediatr Surg 2006;41:1203–7. [DOI] [PubMed] [Google Scholar]
- 11. Adewale L. Anatomy and assessment of the pediatric airway. Paediatr Anaesth 2009;19Suppl 1:1–8. [DOI] [PubMed] [Google Scholar]
- 12. Cole F. Pediatric formulas for the anesthesiologist. AMA J Dis Child 1957;94:672–3. [DOI] [PubMed] [Google Scholar]
- 13. Ross RD. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol 2012;33:1295–300. [DOI] [PubMed] [Google Scholar]
- 14. Chung SR, Yang JH, Jun TG, Kim WS, Kim YH, Kang IS. et al. Clinical outcomes of slide tracheoplasty in congenital tracheal stenosis. Eur J Cardiothorac Surg 2015;47:537–42; discussion 542. [DOI] [PubMed] [Google Scholar]
- 15. Sengupta A, Murthy RA.. Congenital tracheal stenosis & associated cardiac anomalies: operative management & techniques. J Thorac Dis 2020;12:1184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hraska V, Photiadis J, Haun C, Schindler E, Schneider M, Murin P. et al. Pulmonary artery sling with tracheal stenosis. Multimed Man Cardiothorac Surg 2009;2009:mmcts.2008.003343. [DOI] [PubMed] [Google Scholar]
- 17. Torre M. Left pulmonary artery sling and congenital tracheal stenosis: to slide or not to slide? J Thorac Dis 2017;9:4881–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong X, Zhou G, Liu Y, Liu Y, Wang H, Feng Z.. Management of pulmonary artery sling with tracheal stenosis: LPA re-implantation without tracheoplasty. Int J Clin Exp Med 2015;8:2741–7. [PMC free article] [PubMed] [Google Scholar]
- 19. Antón-Pacheco JL, Comas JV, Luna C, Benavent MI, López M, Ramos V. et al. Treatment strategies in the management of severe complications following slide tracheoplasty in children. Eur J Cardiothorac Surg 2014;46:280–5; discussion 285. [DOI] [PubMed] [Google Scholar]
- 20. Li X, Cheng LC, Cheung YF, Lun KS, Chau KT, Chiu SW.. Management of symptomatic congenital tracheal stenosis in neonates and infants by slide tracheoplasty: a 7-year single institution experience. Eur J Cardiothorac Surg 2010;38:609–14. [DOI] [PubMed] [Google Scholar]
- 21. Speggiorin S, Gilbert TW, Broadhead M, Roebuck DJ, McLaren CA, Elliott MJ.. Do tracheas grow after slide tracheoplasty? Ann Thorac Surg 2012;93:1083–6. [DOI] [PubMed] [Google Scholar]
- 22. Paradis TJ, Dixon J, Tieu BH.. The role of bronchoscopy in the diagnosis of airway disease. J Thorac Dis 2016;8:3826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morshed K, Trojanowska A, Szymański M, Trojanowski P, Szymańska A, Smoleń A. et al. Evaluation of tracheal stenosis: comparison between computed tomography virtual tracheobronchoscopy with multiplanar reformatting, flexible tracheofiberoscopy and intra-operative findings. Eur Arch Otorhinolaryngol 2011;268:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Filauro M, Mazzola F, Missale F, Canevari FR, Peretti G.. Endoscopic preoperative assessment, classification of stenosis, decision-making. Front Pediatr 2019;7:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.