Abstract
Zinc deficiency occurs in a variety of diseases, including chronic liver disease (CLD). We investigated the correlation between zinc levels and biochemical and hematological tests in CLD and the effect of zinc supplementation with polaprezinc on these values. The first study (Study 1) was a retrospective observational study of 490 patients with CLD not receiving zinc supplementation, with data available from September 2009 to August 2021. Univariate and multiple regression analysis showed that serum zinc levels correlated most strongly with albumin (Alb) and also significantly with prothrombin time activity (PT%) and hemoglobin (Hb). A subsequent study (Study 2) focused on patients with advanced CLD who used polaprezinc for more than 90 days between January 2005 and August 2021. Using a self-controlled design with the 6-month period prior to polaprezinc as the control period, comparisons showed that Alb (p<0.0001), PT% (p<0.0005), and Hb (p<0.01) were significantly improved in the polaprezinc-treated patients compared to the control group. In conclusion, serum zinc levels were correlated with serum Alb, Hb, and PT% in patients with CLD, and zinc supplementation with polaprezinc was associated with improvements in Alb, Hb, and PT% within at least 6 months.
Keywords: zinc, albumin, prothrombin time activity, hemoglobin, chronic liver disease
Introduction
Zinc is one of the essential trace elements for human life, including cell proliferation, differentiation, protein synthesis, and regeneration, and is required for more than 300 enzyme activities in vivo.(1) Zinc deficiency has been reported in a variety of diseases, including inflammatory bowel disease,(2) chronic kidney disease, and diabetes mellitus,(3) and in chronic liver disease (CLD),(4) zinc deficiency has been associated with fibrosis progression,(5) risk of carcinogenesis,(6) risk of hepatic encephalopathy occurrence, and prognosis.(7,8) In cirrhosis, zinc deficiency becomes more pronounced as liver disease progresses, partly due to increased urinary excretion with diuretic administration.(9) Zinc supplementation is therefore expected to be useful in improving disease status, reducing the risk of carcinogenesis, improving hepatic reserve and improving prognosis. Indeed, there are reports that zinc supplementation improves serum zinc levels,(10) ameliorates hepatic encephalopathy,(11) inhibits hepatic carcinogenesis and improves prognosis.(12,13) However, there are no reports of improvement in randomised controlled trials and there is no certainty about the efficacy of zinc supplementation.(14,15)
In the present study, we report our investigation of the involvement of zinc in biochemical and hematological tests in CLD and the effect of polaprezinc, one of zinc preparation, on these tests.
Patients and Methods
Study 1: Correlation between zinc levels and biochemical and hematological tests
The aim of this retrospective observational study was to investigate the association between zinc levels and biochemical and hematological tests. We conducted a retrospective observational study of 490 patients with CLD who were followed up at Nagasaki Medical Center and who did not receive zinc supplementation from September 2009 to August 2021. Biochemical and hematological tests and medications were extracted from the Data Ware House (DWH), an electronic medical record information aggregation system at Nagasaki Medical Center. Univariate and multiple regression analyses were used to examine the association between serum zinc levels and patients’ age, sex, and laboratory values.
Study 2: Association of polaprezinc administration with biochemical and hematological tests
The following retrospective, self-controlled study was conducted to investigate the relationship between the administration of polaprezinc and various laboratory test results. Specifically, patients with advanced CLD [albumin (Alb) <3.5 g/dl, prothrombin time activity (PT%) <80%; hemogrobin (Hb) <13 g/dl, platelet count (Plt) <120 × 103/μl] who used polaprezinc for at least 90 days between January 2005 and August 2021, with data available for 6 months before and after administration, were studied. Patients who started or were prescribed medications affecting Alb, PT%, or Hb within 6 months before or after enrollment were excluded. As a result, the treatment group included patients primarily treated for hypozincemia and those treated for non-bleeding peptic ulcers. Changes in Alb, asparate aminotransferase (AST), alanine aminotransferase (ALT), PT%, Hb, and Plt were analysed using the Wilcoxon signed-rank test. As a comparison control, data from 6 months prior to polaprezinc administration were used. The magnitude of the change from baseline at 3 and 6 months was analysed in the same way using the Mann–Whitney test.
Ethics
This was a retrospective, single-center, cohort study. Ethics approval was obtained from the Ethical Review Committee of Nagasaki Medical Center (confirmation no.: 2021068), written informed consent was obtained for the use of their medical records. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical analyses
Differences in baseline characteristics between groups were evaluated using χ2 test for categorical variables and the Mann–Whitney test for continuous variables. Changes over time were analyzed using the Wilcoxon signed rank test, and the Mann–Whitney test was used to compare the treatment and control groups at baseline, 3 months, and 6 months. All analyses were conducted in IBM SPSS ver. 25. A 2-sided p value <0.05 was considered statistically significant.
Results
Study 1: Correlation between zinc levels and biochemical and hematological tests
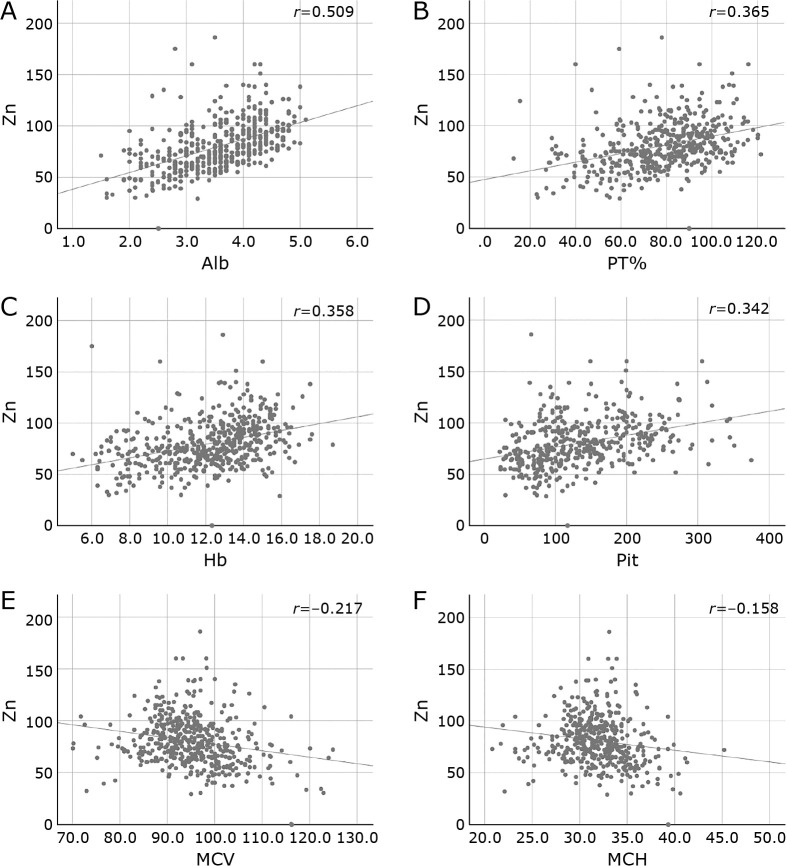
Patient background is shown in Table 1. Table 2 and Fig. 1 show the association between serum zinc and biochemical and hematological tests by univariate analysis. Serum zinc levels were positively correlated with Alb, PT%, Hb, and Plt, and negatively correlated with age, mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) in univariate analysis (Table 2 and Fig. 1). In multiple regression analysis (Table 3), Alb showed the strongest association; PT% and Hb were also involved.
Table 1.
Patiets Characteristics
| Variables | All cases (n = 490) |
|---|---|
| Sex (male), n (%) | 284 (58.0) |
| Age, mean (SD), years | 68 (61–76) |
| Etiology (HCV/HBV/alcohol/nBnC/AIH/PBC) | 212/46/71/124/14/23 |
| Zinc (μg/dl) | 77 (64–94) |
| Total bilirubin (mg/dl) | 0.8 (0.6–1.3) |
| AST (IU/L) | 44 (31–65) |
| ALT (IU/L) | 32 (20–51.3) |
| ALP (U/L) | 306 (222–425.5) |
| Prothrombin time (%) | 80.3 (64.8–92.45) |
| Albumin (g/dl) | 3.7 (3.1–4.2) |
| White blood cell count (103/μl) | 4.5 (3.5–5.9) |
| Hemoglobin (g/dl) | 12.6 (10.6–14.0) |
| MCV (fl) | 94.9 (90.4–99.6) |
| MCH (pg) | 31.8 (30.8–33.8) |
| Platelet count (103/μl) | 122 (82–187) |
| NH3 (μg/dl) | 80.0 (47.0–107.8) |
| Ferritin (ng/ml) | 138.0 (60.0–334.8) |
HCV, hepatitis C virus; HBV, hepatitis B virus; AIH, autoimmune hepatits; PBC, primary birially cholangitis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; MCV, mean corpuscular voloume; MCH, mean corpuscular hemoglobin.
Table 2.
Correlation between serum Zn level and baseline data.
| Variables | r | p value |
|---|---|---|
| Age | −0.09 | <0.05 |
| Total bilirubin (mg/dl) | −0.07 | 0.122 |
| AST (IU/L) | −0.046 | 0.305 |
| ALT (IU/L) | 0.171 | <0.001 |
| ALP (U/L) | −0.031 | 0.489 |
| Prothrombin time (%) | 0.365 | <0.001 |
| Albumin (g/dl) | 0.509 | <0.001 |
| White blood cell count (103/μl) | 0.132 | <0.01 |
| Hemoglobin (g/dl) | 0.358 | <0.001 |
| MCV (fl) | −0.217 | <0.001 |
| MCH (pg) | −0.158 | <0.001 |
| Platelet count (103/μl) | 0.342 | <0.001 |
| NH3 (μg/dl) | 0.091 | 0.710 |
| Ferritin (ng/ml) | −0.065 | 0.236 |
Fig. 1.
Correlation between serum Zn level and baseline data. Serum zinc levels were positively correlated with (A) Alb, (B) PT%, (C) Hb, and (D) platelet count, and negatively correlated with age, (E) MCV, and (F) MCH in univariate analysis.
Table 3.
Multiple linear regression analysis of serum zinc level in chronic liver disease
| Unstandardized Coefficients | SE | Standardized Coefficients | t | p value | |
|---|---|---|---|---|---|
| B | Beta | ||||
| Constant | 1.13 | 22.514 | 0.050 | 0.960 | |
| Age | 0.121 | 0.110 | 0.060 | 1.103 | 0.271 |
| Sex | −1.407 | 2.538 | −0.029 | −0.554 | 0.580 |
| Total bilirubin (mg/dl) | 2.876 | 0.820 | 0.209 | 3.506 | <0.05 |
| AST (IU/L) | −0.042 | 0.062 | −0.063 | −0.678 | 0.498 |
| ALT (IU/L) | 0.046 | 0.047 | 0.091 | 0.974 | 0.331 |
| ALP (U/L) | 0.005 | 0.006 | 0.046 | 0.832 | 0.406 |
| Prothrombin time (%) | 0.199 | 0.098 | 0.148 | 2.040 | <0.05 |
| Albumin (g/dl) | 12.375 | 2.838 | 0.351 | 4.360 | <0.001 |
| White blood cell count (103/μl) | 1.016 | 0.733 | 0.085 | 1.386 | 0.167 |
| Hemoglobin (g/dl) | 1.838 | 0.788 | 0.175 | 2.333 | <0.05 |
| MCV (fl) | 0.315 | 0.501 | 0.094 | 0.630 | 0.529 |
| MCH (pg) | −1.692 | 1.303 | −0.208 | −1.299 | 0.195 |
| Platelet count (103/μl) | 0.012 | 0.024 | 0.034 | 0.475 | 0.635 |
| Ferritin (ng/ml) | −5.40E − 06 | 0.004 | 0.000 | −0.001 | 0.999 |
Study 2: Association of polaprezinc administration with biochemical and hematological tests
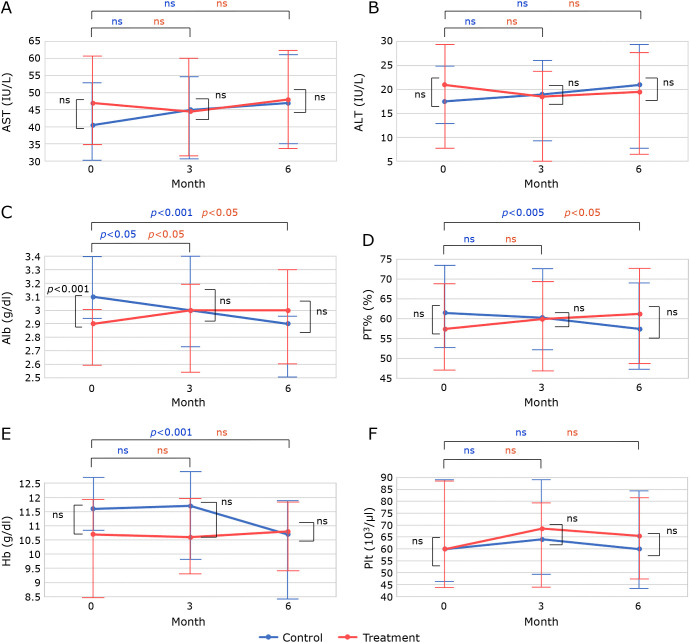
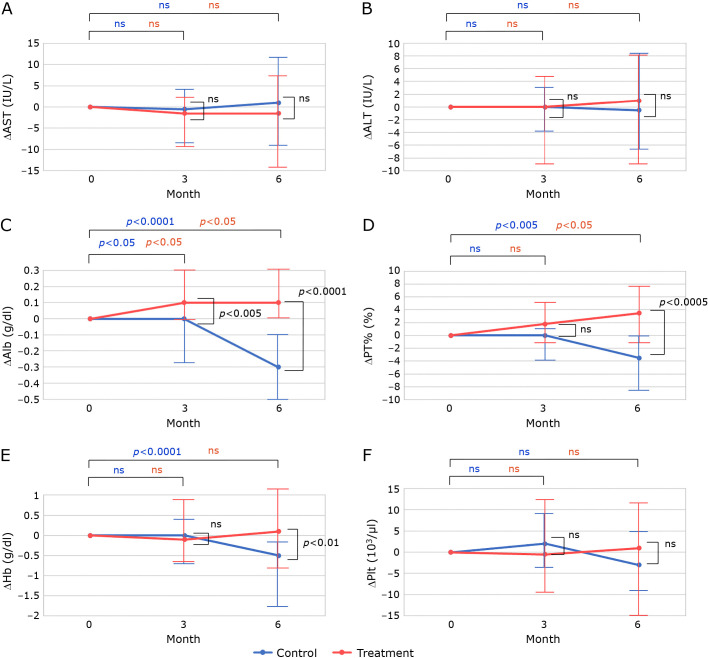
There were 187 patients with CLD who had taken polaprezinc for more than 90 days. To include patients with advanced CLD, patients with Alb<3.5 g/dl, PT%<80%, Hb<13 g/dl, and Plt<120 × 103 at the start of polaprezinc administration were selected. Patients with complete medical records for 6 months before and after polaprezinc administration were also included. Exclusion criteria were: 1). Branched-chain amino acid (BCAA) started within 6 months before or after polaprezinc administration; 2). use of iron, Interferon (IFN), Direct acting antivirals (DAA), warfarin or Direct oral anticoagulant (DOAC) within 6 months before or after polaprezinc administration; 3). blood transfusion received around 6 months before or after polaprezinc administration; 4). patients with sustained virological response (SVR) of hepatitis C. 35 patients were enrolled (Fig. 2). Polaprezinc was administered at the discretion of the treating physician for hypozincemia and gastrointestinal symptoms in a patient with CLD. The treatment and control groups were originally the same patients, and there were no significant differences in patient background except that Alb was significantly better in the control group (Table 4). There were no significant differences, but the treatment group was lower for Hb and higher for MCV. The control group showed a worsening trend in Alb (p<0.001), PT% (p<0.01) and Hb (p<0.001) (Fig. 3C, 3D, and 3E). In the treatment group, Alb improved at 3 months (p<0.05) and PT% improved at 6 months (p<0.05). In addition, changes in biochemistry and hematological tests were compared (Fig. 4). No significant differences were found for AST, ALT, and Plt. While, change from baseline was significantly better in Alb (p<0.001), PT% (p<0.001), and Hb (p<0.01) in the treatment group than in the control group after 6 months (Fig. 4C–E). As in previous reports, serum zinc levels improved in the treatment group, but were not shown in this study because of the large number of missing data.
Fig. 2.
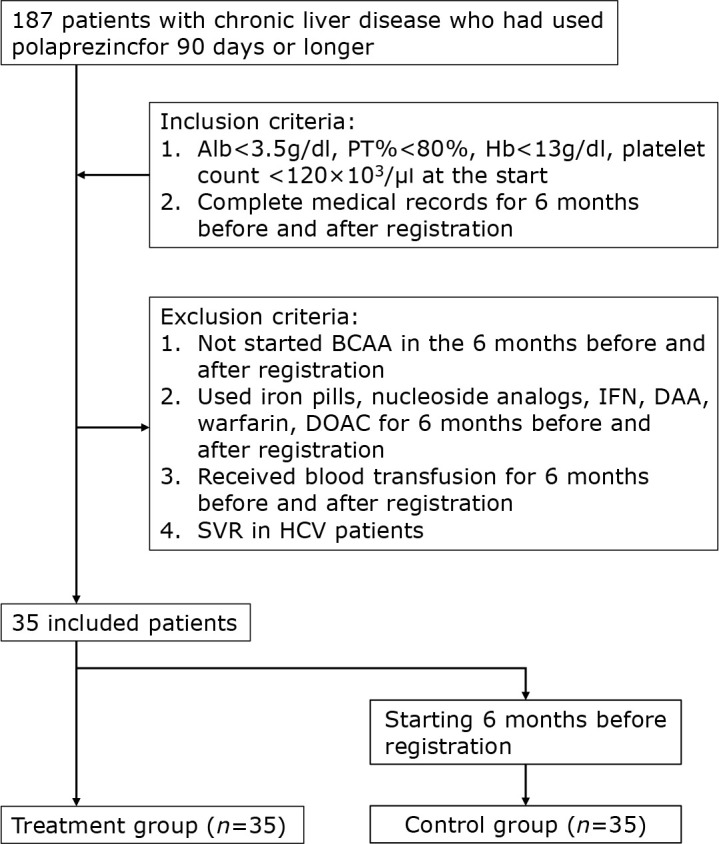
Flow chart for the selection of patients.
Table 4.
Comparison of clinical background factors of the study patients
| Variables | Treatment group (n = 35) | Control group (n = 35) | p value |
|---|---|---|---|
| Sex (male), n (%) | 21 (60.0) | 21 (60.0) | 1 |
| Age, years (IQR) | 65.4 (59.5–73.6) | 64.9(59.0–73.1) | 1 |
| Etiology (HCV/HBV/alcohol/nBnC/PBC) | 19/2/8/4/2 | 19/2/8/4/2 | 1 |
| Total bilirubin, mg/dl (IQR) | 1.8 (1.15–2.4) | 1.5 (1.05–2.1) | 0.46 |
| AST, IU/L (IQR) | 47 (35.0–62.5) | 40.5 (30.0–54.5) | 0.73 |
| ALT, IU/L (IQR) | 21 (7.5–29.5) | 17.5 (7.5–25.0) | 0.56 |
| Prothrombin time, % (IQR) | 57.4 (47.03–68.98) | 61.5 (52.48–73.38) | 0.2 |
| Albumin, g/dl (IQR) | 2.9 (2.6–3.0) | 3.1 (2.93–3.40) | <0.001 |
| Hemoglobin, g/dl (IQR) | 10.7 (8.48–11.90) | 11.60 (10.08–12.68) | 0.07 |
| MCV, fl (IQR) | 98.6 (94.03–101.9) | 97.9 (92.83–102.38) | 0.07 |
| Platelet count, 103/μl (IQR) | 60.0 (43.0–84.0) | 60.0 (45.5–84.5) | 0.82 |
| Diuretics administration, n (%) | 25 (71.4) | 23 (65.7) | 0.6 |
| BCAA administration, n (%) | 28 (80.0) | 28 (80.0) | 1.0 |
HCV, hepatitis C virus; HBV, hepatitis B virus; PBC, primary birially cholangitis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BCAA, branched-chain amino acid.
Fig. 3.
The graphs were drawn using the median and interquartile range values obtained from the SPSS analysis. The points in the graph show the median change over time in (A) AST, (B) ALT, (C) Alb, (D) PT%, (E) Hb, and (F) platelet count. Error bars indicate interquartile range. Blue dots indicate change in control group and red dots indicate change in treatment group. Changes over time were analyzed using the Wilcoxon signed-rank test, and significant differences for each group are indicated at the top of the graph. Comparisons between the treatment and control groups at baseline, 3 months, and 6 months were analyzed using the Mann–Whitney test, and significant differences for each group are shown within the graph.
Fig. 4.
It shows the change from baseline in biochemical and haematological tests, presented in the same format as Fig. 3.
Discussion
In this study, zinc levels were associated with Alb, PT%, and Hb in CLD. In advanced CLD, Alb, PT%, and Hb also showed a decreasing trend at 6 months. Polaprezinc treatment was associated with improved Alb, PT%, and Hb, but not with improved AST, ALT, and Plt at 6 months.
Recently, zinc was reported to transiently bind to ERp44, a chaperone protein that monitors the quality of secreted proteins, inducing major conformational changes in ERp44 and facilitating its interaction with its binding partner proteins.(16) This report indicates that proteins are secreted in an incomplete state during zinc deficiency. It has been shown that zinc is an essential trace element for the organism and that its deficiency leads to detrimental situations for the human body. In liver disease, hypozincemia has been reported to be associated with hepatic carcinogenesis,(6) prognosis, and the development of hepatic encephalopathy.(7,8) In addition, as CLD progresses, the frequency of fluid retention and the need for diuretics increases.(9) The use of diuretics further aggravates hypozincemia. Zinc supplementation is therefore expected to correct zinc deficiency in liver disease and improve the condition. However, previous reports on zinc supplementation have not adequately demonstrated its usefulness.(14) In other words, there are few reports of improvements in Alb, PT%, etc., and the improvement in prognosis is not satisfactory.(17) In advanced CLD, thrombocytopenia and anemia are seen, as well as decreased Alb and PT%. Thrombocytopenia has been associated with reduced thrombopoietin production and increased splenic function in portal hypertension,(18) but anemia in CLD is rarely mentioned.(19) Anemia in CLD is most commonly caused by gastrointestinal bleeding (25%) and iron deficiency (9%), with 53% of cases reported to have an unknown cause.(20) On the other hand, the involvement of macrocytic anemia has also been reported in cirrhosis, with reports of an association with the severity of liver impairment in patients with hepatitis B virus (HBV)-related decompensated cirrhosis and with folic acid and vitamin B12 deficiency in cirrhosis.(21,22) The present study suggests that macrocytic anemia in CLD is correlated with zinc deficiency and might be partially improved by zinc supplementation. Past research reports have examined the effects of zinc supplementation with polaprezinc, zinc sulfate, and zinc acetate. The effects of zinc supplementation were reported as identical. On closer inspection, it appears that many papers have reported improved prognosis in trials using polaprezinc.(13,23,24) Zinc acetate has been shown to improve zinc levels, but has not been shown to have a sufficient effect on liver reserve.(25,26) The amount of zinc preparations used differs among polaprezinc, zinc sulfate, and zinc acetate.(15) The highest daily dose of zinc used is zinc acetate, 50–200 mg/day, administered 2–3 times daily. Polaprezinc, on the other hand, contains 34 mg/day of zinc and is administered twice daily. Zinc preparations were originally used in Wilson’s disease. The main effect is to provide a therapeutic effect by reducing copper absorption in Wilson’s disease,(27) where excess copper is a problem. In other words, the aim was to reduce the body’s copper stores through the oral use of zinc preparations. Hypocupremia with zinc preparations has also been reported in Wilson’s disease, a copper overload disorder.(28) Poor prognosis has also been reported in cases where serum zinc has not improved after zinc supplementation.(26) Although zinc has been studied for many years, there is still a lack of evidence about effective ways to improve zinc deficiency.(17) Zinc preparations are also associated with iron absorption and have been reported to have antagonistic effects with iron.(29) Zinc has been reported to be competitive with divalent trace elements and may also have effects on divalent ions other than copper and iron, such as selenium, manganese and chromium.(30) The lack of expected effects with the use of zinc preparations may be due in part to inadequate consideration of the dosage, frequency of administration, and timing of oral administration, taking into account the effects of the preparation on other divalent ions. Polaprezinc has been reported to improve long-term prognosis,(13,23,24) and the present study may indicate why. We believe that zinc supplements should be evaluated not only for improvement in serum zinc levels, but also for improvement in Alb, PT%, and Hb.
In the present study, detailed data were extracted to understand the influence of zinc supplements in liver disease. The exclusion criteria were also strict to exclude the influence of the drug. However, as this was a retrospective observational study, selection bias and confounding effects may not have been completely eliminated. In addition, STUDY 2 did not examine changes in serum zinc levels due to an insufficient number of samples for zinc measurement. It would have been desirable to examine the relationship between changes in zinc levels and changes in Alb, PT%, and Hb, but the small number of samples did not allow us to examine this relationship. Prospective studies on the relationship between changes in zinc and changes in other markers and on a larger number of patients would be desirable in the future.
Conclusion
Serum zinc levels were correlated with serum Alb, Hb, and PT% in patients with CLD, and zinc supplementation with polaprezinc was associated with improvements in Alb, Hb, and PT% within at least 6 months.
Acknowledgments
This study was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number 21fk0210069h0002. In addition, we thank Rumiko Hamada, Clinical Research Center, Nagasaki Medical Center, for her support of our research.
Abbreviations
- AIH
autoimmune hepatits
- Alb
albumin
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
asparate aminotransferase
- BCAA
branched-chain amino acid
- CLD
chronic liver disease
- DAA
direct acting antivirals
- DOAC
direct oral anticoagulant
- Hb
hemoglobin
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- IFA
interferon
- MCH
mean corpuscular hemoglobin
- MCV
mean corpuscular volume
- PBC
primary birially cholangitis
- Plt
platelet count
- PT%
prothrombin time activity
- SVR
sustained virological response
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Prasad AS. Zinc: an overview. Nutrition 1995; 11 (1 Suppl): 93–99. [PubMed] [Google Scholar]
- 2.Miyaguchi K, Tsuzuki Y, Ichikawa Y, et al. Positive zinc intake and a Japanese diet rich in n-3 fatty acids induces clinical remission in patients with mild active ulcerative colitis: a randomized interventional pilot study. J Clin Biochem Nutr 2023; 72: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakatani S, Mori K, Shoji T, Emoto M. Association of zinc deficiency with development of CVD events in patients with CKD. Nutrients 2021; 13: 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katayama K, Kawaguchi T, Shiraishi K, et al. The prevalence and implication of zinc deficiency in patients with chronic liver disease. J Clin Med Res 2018; 10: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MC, Lee JI, Kim JH, et al. Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PLoS One 2020; 15: e0240195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shigefuku R, Iwasa M, Katayama K, et al. Hypozincemia is associated with human hepatocarcinogenesis in hepatitis C virus-related liver cirrhosis. Hepatol Res 2019; 49: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 7.Miwa T, Hanai T, Toshihide M, et al. Zinc deficiency predicts overt hepatic encephalopathy and mortality in liver cirrhosis patients with minimal hepatic encephalopathy. Hepatol Res 2021; 51: 662–673. [DOI] [PubMed] [Google Scholar]
- 8.Hiraoka A, Nagamatsu K, Izumoto H, et al. Zinc deficiency as an independent prognostic factor for patients with early hepatocellular carcinoma due to hepatitis virus. Hepatol Res 2020; 50: 92–100. [DOI] [PubMed] [Google Scholar]
- 9.Chiba M, Katayama K, Takeda R, et al. Diuretics aggravate zinc deficiency in patients with liver cirrhosis by increasing zinc excretion in urine. Hepatol Res 2013; 43: 365–373. [DOI] [PubMed] [Google Scholar]
- 10.Katayama K, Hosui A, Sakai Y, et al. Effects of zinc acetate on serum zinc concentrations in chronic liver diseases: a multicenter, double-blind, randomized, placebo-controlled trial and a dose adjustment trial. Biol Trace Elem Res 2020; 195: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen YC, Chang YH, Fang CJ, Lin YS. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr J 2019; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosui A, Kimura E, Abe S, et al. Long-term zinc supplementation improves liver function and decreases the risk of developing hepatocellular carcinoma. Nutrients 2018; 10: 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumura H, Nirei K, Nakamura H, et al. Zinc supplementation therapy improves the outcome of patients with chronic hepatitis C. J Clin Biochem Nutr 2012; 51: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diglio DC, Fernandes SA, Stein J, Azeredo-da-Silva A, de Mattos AA, Tovo CV. Role of zinc supplementation in the management of chronic liver diseases: a systematic review and meta-analysis. Ann Hepatol 2020; 19: 190–196. [DOI] [PubMed] [Google Scholar]
- 15.Tan HK, Streeter A, Cramp ME, Dhanda AD. Effect of zinc treatment on clinical outcomes in patients with liver cirrhosis: a systematic review and meta-analysis. World J Hepatol 2020; 12: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe S, Amagai Y, Sannino S, et al. Zinc regulates ERp44-dependent protein quality control in the early secretory pathway. Nat Commun 2019; 10: 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloom A, Bloom S, Silva H, Nicoll AJ, Sawhney R. Zinc supplementation and its benefits in the management of chronic liver disease: an in-depth literature review. Ann Hepatol 2021; 25: 100549. [DOI] [PubMed] [Google Scholar]
- 18.Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int 2017; 37: 778–793. [DOI] [PubMed] [Google Scholar]
- 19.Dawidowski J, Pietrzak A. Rare causes of anemia in liver diseases. Adv Clin Exp Med 2022; 31: 567–574. [DOI] [PubMed] [Google Scholar]
- 20.Scheiner B, Semmler G, Maurer F, et al. Prevalence of and risk factors for anaemia in patients with advanced chronic liver disease. Liver Int 2020; 40: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Yan B, Yang L, et al. Macrocytic anemia is associated with the severity of liver impairment in patients with hepatitis B virus-related decompensated cirrhosis: a retrospective cross-sectional study. BMC Gastroenterol 2018; 18: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama S, Hirayama C, Yamamoto S, et al. Red blood cell status in alcoholic and non-alcoholic liver disease. J Lab Clin Med 2001; 138: 332–337. [DOI] [PubMed] [Google Scholar]
- 23.Moriya K, Nishimura N, Namisaki T, et al. Zinc administration and improved serum markers of hepatic fibrosis in patients with autoimmune hepatitis. J Clin Med 2021; 10: 2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuoka S, Matsumura H, Nakamura H, et al. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J Clin Biochem Nutr 2009; 45: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozeki I, Nakajima T, Suii H, et al. Evaluation of treatment with zinc acetate hydrate in patients with liver cirrhosis complicated by zinc deficiency. Hepatol Res 2020; 50: 488–501. [DOI] [PubMed] [Google Scholar]
- 26.Horiguchi S, Naganuma A, Tateyama Y, et al. Efficacy of zinc acetate treatment for patients with decompensated liver cirrhosis complicated by hypozincemia. Biol Trace Elem Res 2022; 200: 497–504. [DOI] [PubMed] [Google Scholar]
- 27.Członkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers 2018; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai S, Gong JY, Yang J, Wang JS. Anemia following zinc treatment for Wilson’s disease: a case report and literature review. BMC Gastroenterol 2019; 19: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondaiah P, Yaduvanshi PS, Sharp PA, Pullakhandam R. Iron and zinc homeostasis and interactions: does enteric zinc excretion cross-talk with intestinal iron absorption? Nutrients 2019; 11: 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jomova K, Makova M, Alomar SY, et al. Essential metals in health and disease. Chem Biol Interact 2022; 367: 110173. [DOI] [PubMed] [Google Scholar]