Abstract
Accumulation of oxidative damage increases the risk of several disorders. To prevent these diseases, people consume supplements. However, there is little evidence of the impact of supplement intake on cognitive function. Recently, frailty and sarcopenia have become serious issues, and these phenomena include a risk of mild cognitive impairment. In this study, aged mice were fed the combination supplement and cognitive and motor functions were measured. Following 1 month of treatment with the supplement, significant improvements in cognitive function and neuromuscular coordination were observed. Following 2 weeks of treadmill training, treatment with the supplement dramatically increased running distance compared to that in untreated normal aged mice. Serum indices such as triglyceride and total cholesterol were significantly decreased in the supplement-treated aged mice compared to untreated aged mice. These results indicate that the combination supplement may play a role in maintaining cognitive function, coordination ability and improving lipid metabolism.
Keywords: antioxidant, cognitive function, aged mice
Introduction
Aging is a normal physiological phenomenon. With aging, various biological functions decline irreversibly. This age-related decline in physiological function increases the risk of developing various diseases.(1) The most representative diseases are neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and dementia.(2,3) With the advent of an aging society, the number of patients with the above-mentioned neurodegenerative diseases is increasing yearly.(4) Despite the enormous amount of money invested by researchers around the world, there are still no definitive treatments available for these neurodegenerative diseases.(5) The development of effective therapeutic drugs is expected to extend not only the life expectancy but also the healthy life expectancy of humans.
Aging can be divided into physiological aging and pathological aging.(6,7) Physiological aging is inevitable, whereas pathological aging can be delayed. If pathological aging can be reliably slowed, the overall rate of aging progresses through physiological aging alone. As a result, even among people of the same age, it is thought that the risk of developing diseases can be reduced in those whose progression of pathological aging is suppressed.
Oxidative stress plays a major role in the progression of pathological aging.(8,9) Reactive oxygen species (ROS) generated in the body oxidize proteins, lipids, and DNA, resulting in the gradual accumulation of oxides in the body and represents a risk factor for the development of various diseases.(9–11) Thus, if in vivo oxidation can be prevented, it may be possible to delay the progression of pathological aging and reduce the risk of disease. However, the necessity of breathing oxygen and producing ATP results in the risk of oxidative stress being unavoidable.(12) Therefore, the only way to manage this risk is to minimize oxidative stress generated in the body.
The human body contains antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) to remove endogenously generated ROS.(1,13) Furthermore, individuals attempt to protect their bodies from oxidative damage by the dietary intake of antioxidants such as vitamins.(14,15) An optimal scenario would be to achieve a balance between oxidation and reduction, i.e., maintenance of the redox balance; however, if the intake of antioxidants or the amount of antioxidant enzymes is low, oxidation will occur. Therefore, humans may be able to compensate for a lack of antioxidants by taking supplements.
Antioxidants come in many varieties and are readily available in stores. However, some lack scientific evidence for their efficacy, which increases consumer distrust. To address consumer concerns, the use of supplements based on scientific evidence should be encouraged. It is well known that concurrently taking multiple types of antioxidants produces additive effects. However, it is difficult for consumers to understand in which combinations and at what dose supplements should be taken to obtain optimal effects. To address this problem, it is recommended that consumers take supplement mixtures, as opposed to multiple types of supplements. Therefore, there is currently a consumer demand for beneficial combination supplements.
Twendee Mt Control (TwM) is a commercially-available combination supplement that contains 15 substances (coenzyme Q10, ascorbic acid, l-glutamine, l-cysteine, niacin, succinic acid, fumaric acid, riboflavin, pantothenic acid, thiamine, pyridoxamine, biotin, vitamin B12, lactoferrin, and folic acid). Each component has been well researched and evidence of the effects is documented.(16,17) However, there are insufficient data to demonstrate the efficacy of the supplement. Therefore, this study evaluated the effect of TwM on cognitive and motor functions in aged mice.
Materials and Methods
Animals
All animal experiments were approved by the Animal Protection and Ethics Committee of Shibaura Institute of Technology (Approval Number #21005, Approval Date, August 26th, 2021). Eighteen-month-old C57BL/6 mice were gifted by our collaborator, the Division of Anti-oxidant Research, Life Science Center, Gifu University (Gifu, Japan). After 1 week of habituation, the mice were assigned to two groups, which were provided with either TwM-containing drinking water or normal filtered tap water ad libitum for the following month. All mice were provided a control diet (Labo MR Stock; Nosan Corp., Kanagawa, Japan) ad libitum. The number of experimental animals in each group is indicated in each figure caption.
After 1 month of feeding of the experimental drink, the mice were anesthetized with ether and euthanized quickly by decapitation; brain (cerebral cortex) and blood for serum were collected for the corresponding biochemical analyses. During the experimental period, mice were maintained under conditions of controlled temperature (22 ± 2°C) and a 12-h/12-h light/dark cycle. Food and water intake and body weight were measured weekly. Aside from the food, water, and TwM, all chemical reagents were obtained from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan).
Preparation of mixed antioxidant supplement
The mixed antioxidant supplement (formula name: Twendee Mt Control) was produced by the Division of Anti-oxidant Research, Gifu University. TwM was developed by TIMA establishment, Balzers, Liechtenstein. Mt Control is also sold by TIMA Tokyo Inc. (Tokyo, Japan) under a product name with a similar formulation; however, it differs from the one used in this experiment. The sample of TwM used in this study is not the commercially-available product, but is produced by Gifu University. The commercially-available TwM is a tablet, but the sample used in this study was a liquid ampoule. TwM comprises 15 substances including coenzyme Q10 (3.6%), ascorbic acid (33.5%), l-glutamine (33.9%), l-cysteine (17.8%), niacin (0.7%), succinic acid (3.6%), fumaric acid (3.6%), riboflavin (1.4%), pantothenic acid (0.36%), thiamine (0.07%), pyridoxamine (0.07%), biotin (0.004%), vitamin B12 (0.0002%), lactoferrin (1.4%), and folic acid (0.01%). The mice were fed TwM (20 mg/kg/day) by oral intake from 18 months of age until euthanasia. Beverages were prepared by taking into account the average body weight and average water intake of mice at 18 months of age.
Behavioral assessment
Morris water maze
Cognitive function such as learning ability was assessed using a Morris water maze apparatus.(18,19) The maze apparatus (120 cm in diameter and 30 cm in height; #MWM-04M; Muromachi Kikai Co. Ltd., Tokyo, Japan) consisted of a pool constructed of acrylic resin. The bottom of the pool was divided into four quadrants using waterproof insulating tape, and visible printed marks (circle, triangle, square, and cross) were placed around the wall. A submerged platform was placed in the center of one quadrant. The water temperature of the pool was maintained at 22 ± 2°C. During an acclimation period prior to the cognitive trials, the mice were allowed to swim freely for 60 s without a platform on each of 3 days. The cognitive trials were performed four times per day and continued for five consecutive days. All trials were performed at the same time of day and were carried out every 3 h (starting at 10:00, 13:00, 16:00, and 19:00). The platform was kept in the same quadrant of the pool for all trials. The escape latency (time to reach the goal), swimming distance, swimming speed, and the proportion of time spent swimming in the quadrant containing the platform were measured using ANY-maze software (ver. 4.98; Stoelting Co., Wood Dale, IL).
Rota-rod test
The rota-rod (Muromachi Kikai Co., Ltd.) test was used to assess coordinated movement ability, and was performed as described previously, with some modifications.(20) The rod speed was 5 rpm for the first 60 s, then accelerated to 50 rpm for 60 s, and then held at a constant speed. The latency to time-to-fall was measured.
Y-maze test
To assess short-term memory, a Y-maze apparatus (Muromachi Kikai Co., Ltd.) was used.(21) The mouse was free to move in the maze for 10 min, and after shooting a video, the ANY-maze software was used to calculate the alternation behavior rate.
Treadmill exercise experiment
Endurance test
The endurance of each mouse was assessed using a treadmill (#MK-690; Muromachi Kikai Co., Ltd.). Prior to the start of the experiment, to acclimate the mice to the treadmill, the mice were allowed to run or walk freely for 10 min (the belt was not active during this phase). Next, the machine was set to accelerate 1 m/min at an incline of 0°, and to habituate the mice to running, it was accelerated from 1 to 10 m/min every 2 min for 20 min. After a 10-min interval, the treadmill was set at an incline of 10° and accelerated from 10 to 30 m/min at a rate of 2 m/min every 4 min, and the mice were allowed to continue running until exhausted. The session was ended when the mouse touched the shock grid for a total of 5 s. The time-to-exhaustion and the running distance were measured for each mouse. The difference in endurance before and after TwM treatment was evaluated by running distance. In this experiment, mice were not subjected to training and were housed in the animal cages for two months.
Treadmill training
One month after each water administration and the endurance test were completed, each group of mice was divided into two groups evenly based on running distance. One group trained on the treadmill three times a week, where the mice ran for 60 min at a 0° incline. The running speed was 15 m/min in the first week and 18 m/min in the second week. A second group was housed normally in individual cages without training for the same period as the first group.
Western blotting
The cerebral cortex was homogenized in radioimmunoprecipitation assay (RIPA) buffer and used in Western blotting as described previously.(13) Protein contents were determined using the Bradford assay (Bio-Rad protein assay, #500-0006JA; Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer’s protocol. Twenty micrograms of each protein extract were separated on 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to cellulose nitrate membranes (CelarTrans, 0.2 μm, #030-25643; FUJIFILM Wako Pure Chemical Corp.). The membranes were blocked by incubation for 1 h at room temperature (RT) in blocking buffer consisting of 2% non-fat skim milk formulated in Tris-HCl-buffered saline (TBS-T, pH 7.6) containing 0.1% Tween 20). The membranes were washed in TBS-T, and then treated overnight at 4°C with each primary antibody, including the following: anti-brain-derived neurotropic factor (BDNF) (N-20) rabbit polyclonal antibody, 1:2,500 (#ab-108319; Abcam plc, Cambridge, UK), anti-nerve growth factor (NGF) (H-20) rabbit polyclonal antibody, 1:4,000 (#sc-548; Santa Cruz Biotechnology (SCBT), Inc., Dallas, TX), anti-tropomyosin receptor kinase A (TrkA) (763) rabbit polyclonal antibody, 1:4,000 (#sc-118; SCBT), anti-TrkB (H-181) rabbit polyclonal antibody, 1:250 (#sc-8316; SCBT). Horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody (Promega Corp., Madison, WI) was used as a secondary antibody at 1:4,000 dilution for 1 h at RT. All Western blotting experiments were performed at least three times. All chemiluminescent signals were generated by incubation with the detection reagents (Immobilon; Merck KGaA, Darmstadt, Germany) according to the manufacturer’s protocol. The relative intensities were determined using an LAS-3000 (FUJIFILM Corp., Tokyo, Japan). Expression ratios were calculated by dividing each protein band intensity value by that of Ponceau S staining in the respective samples, using ImageJ software (1.52a; National Institutes of Health, Bethesda, MD).
Serum parameters
Total protein (TP), albumin (ALB), iron (Fe), lactic acid dehydrogenase (LDH), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (T-CHO), free cholesterol (F-CHO), esterified cholesterol (E-CHO), aspartate aminotransferase (AST), alanine aminotransferase (ALT), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured by an external vendor (Oriental Yeast Co., Ltd., Tokyo, Japan).
Statistical analysis
Data are expressed as mean ± SE, and were analyzed using Prism 9.2.0 (GraphPad Software, San Diego, CA). P values of less than 0.05 were considered statistically significant. The detailed statistical methods are described in the individual figure captions.
Results
Twendee Mt Control does not change body weight in aged mice
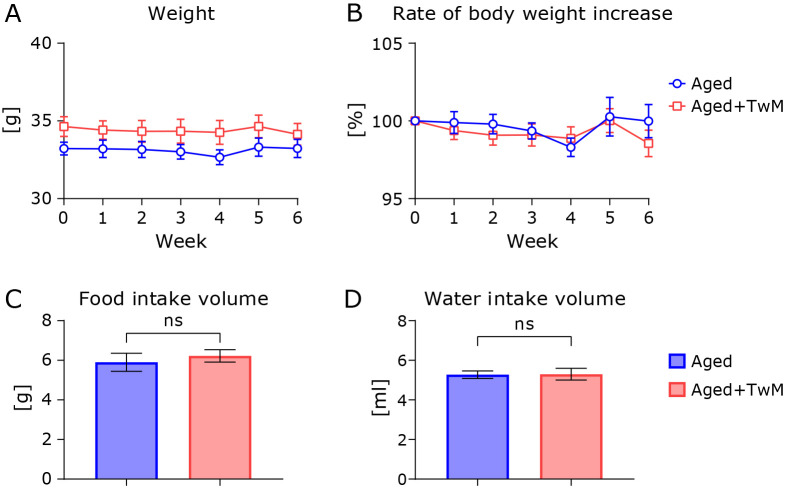
Eighteen-month-old mice were provided with free access to TwM-containing water (or normal filtered tap water) for the subsequent 1.5 month (one month for treatment only, and half a month for the experimental period). The body weights of both mouse groups did not increase in a time-dependent manner (Fig. 1A and B). There was no significant difference in body weight between the two aged mouse groups. Food and water intake were not significantly different between the aged and TwM-treated aged mice (Fig. 1C and D).
Fig. 1.
The effect of Twendee Mt Control on body weight and food and water intake in aged mice. Body weight of the two mouse groups from 18 to 19.5 months of age (A, B). Aged, n = 8; Twendee Mt Control-treated aged mice (Aged + TwM), n = 8. Food and water intake of the final week for each mouse group (C, D). Aged, n = 4; Twendee Mt Control-treated aged mice (Aged + TwM), n = 4. Data were analyzed using a two-way analysis of variance (A, B). Differences of each week’s values and the food and water intake of the final week were analyzed by a two-tailed non-paired Student’s t test (C, D).
Twendee Mt Control significantly improves cognitive dysfunction in aged mice
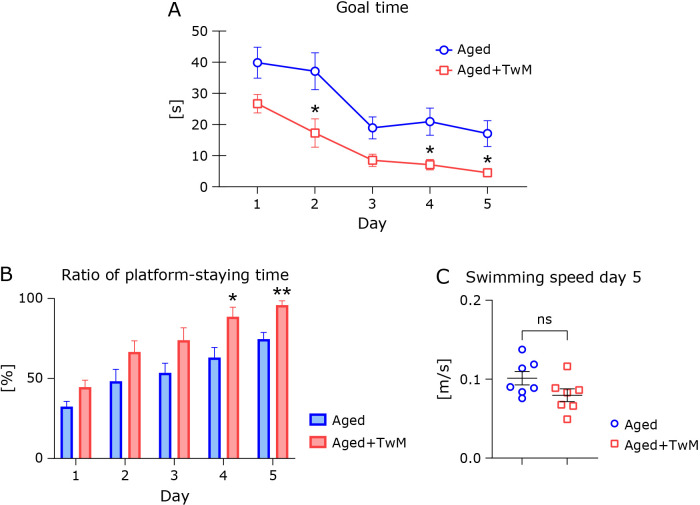
To clarify the biological benefit of the TwM mixed antioxidant supplement, we assessed cognitive function using the Morris water maze test. The daily mean goal time gradually decreased in both aged mouse groups, confirming that all mice learned the location of the escape platform (Fig. 2A). The goal time on each trial day of the TwM-treated aged group was significantly faster (except for Trial Days 1 and 3) than that of the untreated aged group. The ratio of platform-staying time in the TwM-treated aged mice was apparently (nominally) higher than that of the untreated aged group on all trial days (Fig. 2B). The mean swimming speed of the TwM-treated group (on Day 5) was nominally lower (but not significantly) than that of the untreated aged group (Fig. 2C).
Fig. 2.
Twendee Mt Control improves cognitive function in aged mice, as assessed by the Morris water maze. The time-to-goal (escape latency) in the Morris water maze test is shown in (A). The ratio of platform-staying time in the platform quadrant is shown in (B). The swimming speed (Day 5) is shown in (C). Twendee Mt Control-treated aged mice (Aged + TwM; n = 7) were tested, and age-matched control mice (Aged; n = 7) were used as a normal aged group. *p<0.05, **p<0.01, vs the age-matched control group. The data are shown as mean ± SE. Statistical analysis of goal time was performed using a two-way analysis of variance (A). Statistical analyses of the swimming speed, and the ratio of staying time in the platform quadrant were performed using two-tailed non-paired Student’s t tests (B, C).
Twendee Mt Control significantly improves age-related coordination disability
To further clarify TwM benefits, we measured mouse coordination using a rota-rod apparatus. Time-to-fall from the rod in the untreated aged mice was significantly faster than for the TwM-treated aged mice (Fig. 3A). At the same time, we measured the rod speed-to-fall. The score of TwM-treated aged mice was significantly faster than that of the untreated aged mice (Fig. 3B).
Fig. 3.
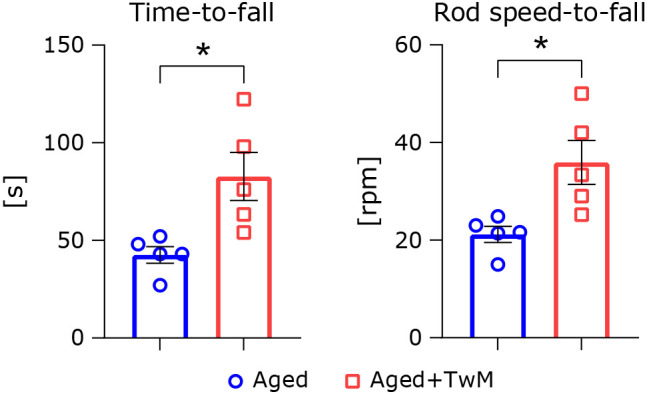
Time-to-fall and rod speed-to-fall in the rota-rod test. The time-to-fall and rod speed-to-fall are shown on the left and right side, respectively. Aged mice (Aged), n = 5; Twendee Mt Control-treated aged mice (Aged + TwM), n = 5. *p<0.05. The data are shown as mean ± SE. Statistical analysis of the data was performed using a two-tailed non-paired Student’s t test.
Twendee Mt control did not affect exploratory behavior in the Y-maze
Exploratory behavior was measured using the Y-maze task. There were no significant differences in the number of arm entries (Fig. 4A). The alternation ratio of TwM-treated aged mice was nominally higher (but not significantly) compared to the untreated aged group (Fig. 4B).
Fig. 4.
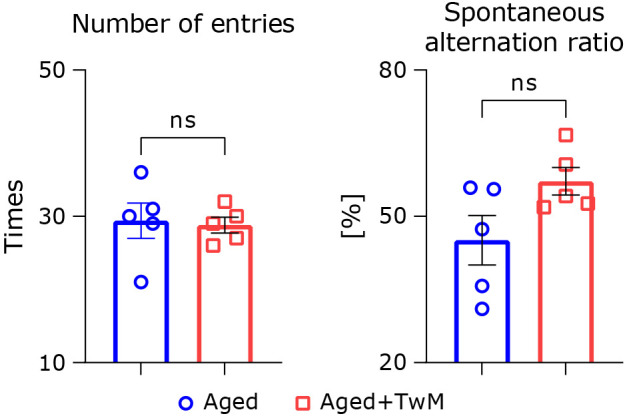
Alternation score in the Y-maze test. Total number of entries and spontaneous alteration ratio are shown on the left and right sides, respectively. Aged mice (Aged), n = 5; Twendee Mt Control-treated aged mice (Aged + TwM), n = 5. *p<0.05. The data are shown as mean ± SE. Statistical analysis of the data was performed using a two-tailed non-paired Student’s t test.
Effect of Twendee Mt Control treatment on running distance in aged mice
To compare the effect of TwM treatment, we compared running distance before and 1 month after treatment with TwM or filtered tap water in aged mice (Fig. 5). Prior to the start of the feeding experiment, the running distance of mice on the treadmill was assessed and did not differ in both aged mouse groups (blue circle vs red circle). One month after treatment with normal filtered tap water, the running distance was significantly lower than that of the before treatment (blue circle vs blue square) (Fig. 5A). However, the running distance in TwM-treated mice did not between differ before and after 1 month of treatment (red circle vs red square). The increase in relative running distance was significantly greater in TwM-treated aged mice compared to untreated aged mice (Fig. 5B).
Fig. 5.
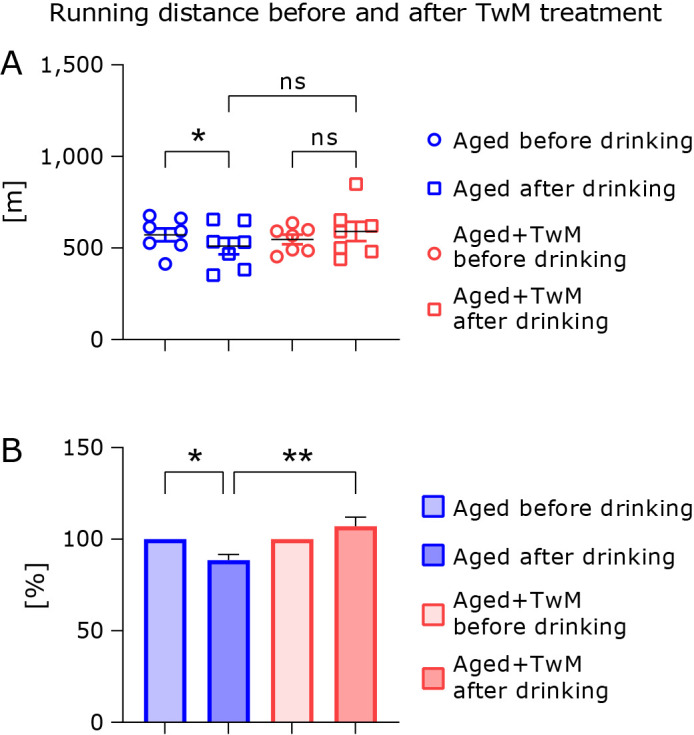
Changes in treadmill running distance of aged mice before and after Twendee Mt Control treatment. The circles indicate the running distance before starting treatment with TwM or filtered tap water, and squares indicate the running distance 1 month after treatment with each drinking water (A). Relative running distance (B). Before starting treatment with each water, running distance was set to 100% (B). Aged (n = 7), Aged + Twendee Mt Control (n = 7). * p<0.05. ** p<0.01. The data are shown as means ± SE. Data were analyzed using a one-way analysis of variance followed by Tukey–Kramer’s test.
Effect of Twendee Mt Control treatment on the training effect of exercise in aged mice
At the end of the 1-month treatment with each drinking water in aged mice, the endurance of all mice was measured. Training was conducted three times a week, and the mice ran for 60 min at a given treadmill speed (details are described in the Materials and Methods). Two weeks after the training, all mice were subjected to the endurance test again. Both groups of aged mice showed significantly increased running distance compared to that before the start of training (Fig. 6). The increase in relative running distance after training in the TwM-treated mice was significantly higher than that of the untreated group.
Fig. 6.
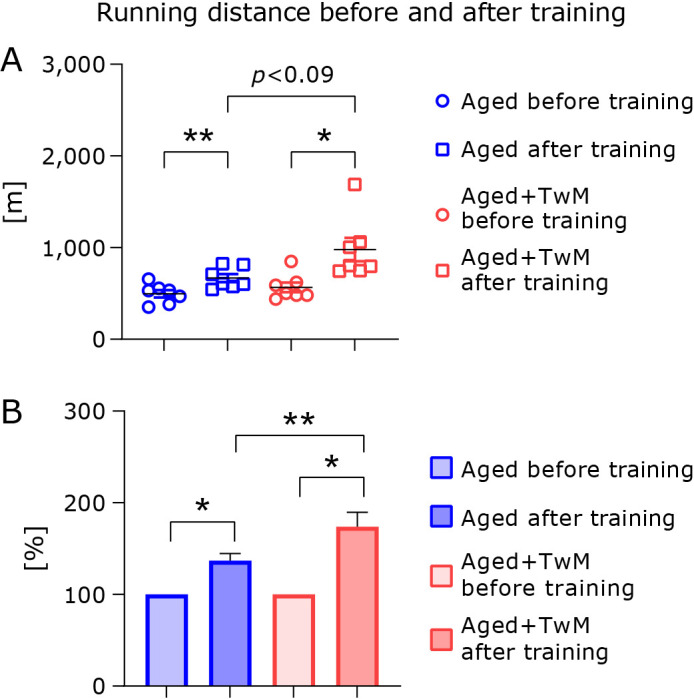
Changes in treadmill running distance of aged mice with or without Twendee Mt Control treatment before and after two weeks of training. The circles indicate the running distance before the start of training, and the squares indicate the running distance two weeks after training (A). Relative running distance (B). Before starting the treatment with each water, running distance was set to 100% (B). Aged (n = 7), Aged + Twendee Mt Control (n = 7). *p<0.05, **p<0.01. The data are shown as means ± SE. Data were analyzed using a one-way analysis of variance followed by Tukey–Kramer’s test.
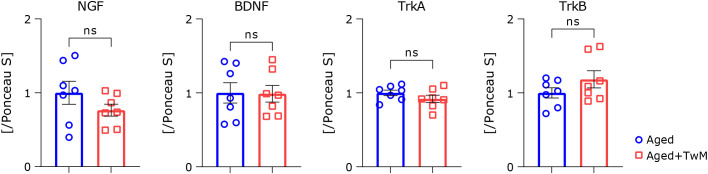
Ingestion of Twendee Mt Control does not change neurotrophic factor secretion in the aged mouse brain
Treatment with TwM improved cognitive dysfunction in aged mice. To clarify the possible mechanism of this change, we measured brain levels of neurotrophic factors such as BDNF and NGF and their cognate receptors (TrkB and TrkA, respectively) (Fig. 7). Contrary to expectation, there were no significant differences in all indices.
Fig. 7.
Western blotting analysis of the levels of neurotrophic factor-related proteins in the brains of aged and Twendee Mt Control-treated aged mice. All experiments were performed using the cerebral cortex region. The relative ratio of each protein band intensity to Ponceau S-staining intensity is shown, with ratios of aged samples set to 1. Aged mice (Aged), n = 7; Twendee Mt Control-treated aged mice (Aged + TwM) n = 7. The data are shown as mean ± SE. Comparisons were performed using a two-tailed non-paired Student’s t test.
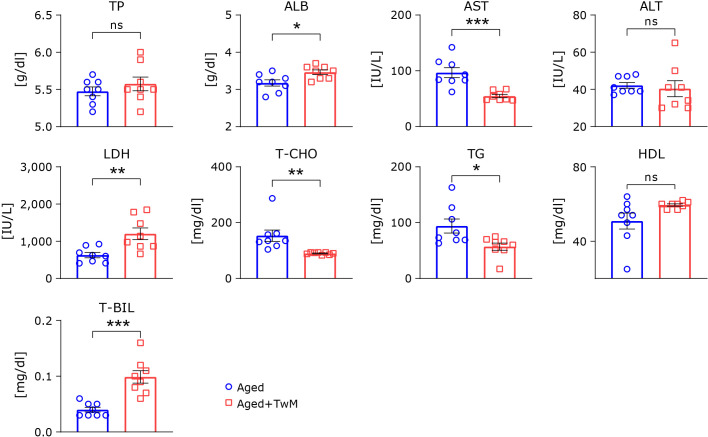
Changes in serum parameters by Twendee Mt Control treatment of aged mice
After the Morris water maze and rota-rod tests were performed, we collected blood and measured serum parameters (Fig. 8). Serum levels of albumin, lactic acid dehydrogenase, and total bilirubin were significantly higher in the TwM-treated aged group compared to the age-matched control. Aspartate aminotransferase, total cholesterol and triglyceride were significantly lower in the TwM-treated aged group compared to the age-matched controls. Other serum parameters did not show significant differences between the treated and untreated aged mice.
Fig. 8.
Serum parameters in aged and Twendee Mt Control-treated aged mice. Aged mice (Aged), n = 7; Twendee Mt Control-treated aged mice (Aged + TwM), n = 7. The data are shown as means ± SE. *p<0.05, **p<0.01, ***p<0.001. Comparisons were performed using a two-tailed non-paired Student’s t test. TP, total protein; ALB, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactic acid dehydrogenase; T-CHO, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; T-BIL, total bilirubin.
Discussion
Twendee Mt Control improves cognitive function and coordinated movement in aged mice
Although ROS accelerate aging, the most prominent age-related physiological decline is in cognitive function.(18) The Morris water maze task is one of the major tools used to assess cognitive function in rodent models.(18) In our previous study, the cognitive function in normal aged mice was significantly impaired compared to the 6-month-old mice.(1) In the present study, the goal time of TwM-treated aged mice was significantly shorter than that of the untreated aged mice (Fig. 2). Notably, the ratios of platform-staying time on training days 4 and 5 were almost 100%. However, the swimming speed was not significantly different with or without TwM treatment; in fact, the TwM group was slightly slower. These results indicate that treatment with TwM for 1 month in aged mice improved cognitive function. Although oxidation in the brain increases with age, post-aging combination supplement intake may be beneficial for cognitive function. This is similar to our previous results, in which the administration of tocotrienols, forms of vitamin E, to aged rats significantly improved cognitive function.(22) In the normal aged mice, the lipid peroxidative products such as thiobarbituric reactive substances significantly increased in cortex, cerebellum and hippocampus compared to the normal 6-month-old mice, and SOD activity decreased significantly.(1) Although the amount of lipid peroxidative products and antioxidant enzyme activities were not measured in this study, our previous report showed that aged-mice and rats increased brain oxidations and the administration of antioxidant vitamin such as tocotrienols to aged rats did not decrease the amount of lipid peroxide in the cerebral cortex and hippocampal regions.(1,22) The reason for this observation is not well understood, but it is possible that non-antioxidant neuroprotective effects were involved. Nishimura et al.(23) reported that the intake of antioxidant vitamins may enhance neuronal functions or stimulating neuronal generation. However, further research is required to clarify the mechanism underlying the observed effect.
In the Rota-rod test score, the time and rod speed until falling were significantly longer and faster in TwM-treated aged mice compared to the untreated aged mice (Fig. 3). Time-to-fall scores are usually negatively correlated to animal weight.(24) However, body weight was not significantly different between the TwM treated and untreated groups. This result indicates that treatment with the mixed antioxidant enhances coordination ability. Several lines of evidence have demonstrated that deficiency of antioxidant substances, such as vitamin C and coenzyme Q10, is closely connected with the Rota-rod test score.(25,26) In addition, although vitamin E was not included in our sample of TwM, it has been reported that vitamin E deficiency causes gait disturbance.(27) The reason for this is suggested to be that vitamin E deficiency causes abnormalities in the Purkinje cell layer of the cerebellum. It has also been reported that vitamin B12 (which is contained in TwM) also stimulates neuronal excitability.(28) The antioxidants contained in TwM may stimulate nerves in the brain by acting on the stability of the membranes of brain nerve cells and by promoting nerve transmission. This point may be due to the fact that TwM was effective in recognition function, and that there may be some common mechanism, such as enhancement of neural function and stimulation of neurogenesis.(23)
Unfortunately, the Y-maze test did not show a significant difference between aged mice with or without TwM treatment (Fig. 4). These results indicate that while treatment with TwM in aged mice may improve cognitive and coordination functions, the effect was not observable in all types of tests. Thus, several behavioral tests may need to be assessed to reveal the effects of substances such as natural products and vitamins.
Twendee Mt Control enhances running distance in aged mice
Frailty and sarcopenia are important problems among the elderly and reduce healthy life expectancy. Furthermore, worsening symptoms increase the risk of developing various neurodegenerative diseases, including dementia. This highlights the importance of daily exercise. If daily exercise is not maintained, exercise levels will continue to decrease as a result of the age-related decline in muscle strength. Therefore, we examined changes in endurance before and after administration of TwM using the treadmill test.
Prior to the start of TwM treatment, the running distance did not differ between both aged-mouse groups (Fig. 5). After 1 month of maintenance in the animal cages, the untreated aged mice had significantly reduced running distance compared to the first trial. However, the TwM-treated mice did not show reduced running distance between trials. The relative running distance in TwM-treated mice significantly increased compared to the untreated group. After 2 weeks of treadmill training, the relative running distance significantly increased compared to the untreated mice (Fig. 6). These results indicate that treatment with TwM might mitigate age-related muscle weakness and enhance training effects. Although the detailed mechanisms are unknown, there are several previous studies on the individual components contained in TwM. Maintenance of coenzyme Q10 plasma levels reduces the risk of sarcopenia and frailty in the elderly,(29) and treatment with coenzyme Q10 prevents cardiovascular events by affecting the heart muscle.(30) Coenzyme Q10 plays an important role in mitochondrial function and could be a mechanism contributing to muscle weakness and enhanced training effects.(31) In addition, it has been reported that levels of vitamins B1, B2, and B6 decrease with exercise.(32) TwM contains many kinds of vitamin B, and their main function as co-enzymes for energy metabolism. In addition, succinate and fumarate play important roles in the citric acid cycle to generate nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2). NADH and FADH2 are closely related to ATP generation in mitochondrial oxidative phosphorylation. From the above, it is suggested that the intake of these compounds plays an important role when energy is depleted during exercise. Many commercial energy drinks are also high in B vitamins. This is thought to be one of the reasons why the intake of TwM enhanced the training effect.
Neurotrophic factors and serum index values after administration of TwM to aged mice
To clarify the mechanism of cognitive improvement in TwM-treated aged mice, the expression of neurotrophic factors, such as BDNF and NGF, and their receptors, were measured by Western blotting. However, no significant differences in neurotrophic factor expression were observed (Fig. 7). Before starting the experiment, we hypothesized that TwM intake and exercise training would increase the expression of neurotrophic factors. This is because many publications have demonstrated the relationship between BDNF expression levels in the brain and exercise.(33) Not only is BDNF expression increased by stimulation of the brain during exercise, but many reports have also indicated it effectiveness for depression and neurodegenerative diseases.(34,35) Given that there was no change in neurotrophic factor expression, it remains to be clarified why TwM intake significantly improved learning performance in the water maze. There was also no change in swimming speed with or without TwM treatment. It is also possible that it may have an effect on muscle tissue and fat cells via mitochondria, but further investigation is required to elucidate these issues.
Serum test results showed that AST, triglyceride, and total cholesterol levels were significantly improved by TwM treatment. These results indicate that TwM intake may be effective in improving liver function. Certain vitamins and amino acids are well known to be beneficial for the liver. For example, vitamin C was reported to exert an anti-inflammatory effect in the liver against heat stress.(36) Since the mice in the present study were subjected to training, heat stress may play a major role in triggering liver inflammation. It has also been reported that dipeptides of l-glutamine and l-alanine exert antioxidant and anti-inflammatory effects in the liver.(37) It is possible that aged mice have considerable liver inflammation due to the accumulation of oxidative damage. In addition, since the experimental animals ingested nutrient-rich food ad libitum, it is possible that even normal diets provide excess nutrients. In calorie-restricted mice, it is clear that not only the longevity-associated gene SIRT1 but also the mTOR pathway is enhanced, and oxidative stress and inflammatory responses are reduced, leading to increased lifespan.(38) In this study, the intake of TwM did not have an anti-obesity effect; however, it is conceivable that it exerted beneficial lipid metabolism effects and antioxidant effects. In the future, we plan to conduct experiments using other model animals, such as obesity and diabetes models, and further investigate the effect of TwM on improving liver function and lipid metabolism. In addition to these, in this experiment, various beneficial effects were obtained by ingesting TwM, so it is possible that it has some kind of effect on the intestinal flora. Therefore, there is room to consider these as well.
In our study, treatment with TwM counteracted cognitive dysfunction and coordination disability in aged mice. Given that oxidative products in the human body gradually accumulate during senescence, chronic intake of multiple antioxidant supplements may prevent further oxidation and impede the onset of several severe clinically and relevant diseases.
Author Contributions
Conceptualization, YF, HI, and KF; Data curation, YKato, SY, AK, TH, YKanome, and KF; Formal analysis, KF; Investigation, YT and KF; Resources, FY, YH, and HI; Project administration, TY and KF; Supervision, KF; Writing-review and editing, KF; All other contributions to the research, KF. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted under a joint research agreement between Shibaura Institute of Technology and Gifu University. The research funding was provided by Division of Anti-oxidant Research, Life Science Research Center, Gifu University.
Conflict of Interest
FY, YH, and HI are employees of Gifu University. The Division of Anti-oxidant Research is a laboratory that has been established in the Life Science Research Center, Gifu University based on a research fund from the TIMA establishment. A product is sold by the TIMA Tokyo Inc. under the name of TwM as a product with a similar formulation, but it is different from the one used in this experiment. The TwM used in the experiment was not the commercially available product itself, but one produce by Gifu University. FY, YH, and HI receive payment for consultation or expert testimony provided to, and do not have stock or stock options from, TIMA Group. Some author photos are posted on the homepage. But they haven’t received all the payments. TY is a developer of TwM un collaboration with the TIMA Foundation and TIMA Tokyo Inc. The sponsor had no control over the interpretation, writing, or publication of this work. The other authors declare that they have no conflicts of interest to disclose.
References
- 1.Yoshida N, Kato Y, Takatsu H, Fukui K. Relationship between cognitive dysfunction and age-related variability in oxidative markers in isolated mitochondria of Alzheimer’s disease transgenic mouse brains. Biomedicines 2022; 10: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: cause and Treatment. Molecules 2020; 25: 5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartsch T, Wulff P. The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience 2015; 309: 1–16. [DOI] [PubMed] [Google Scholar]
- 4.Ohno S, Chen Y, Sakamaki H, Matsumaru N, Yoshino M, Tsukamoto K. Humanistic burden among caregivers of patients with Alzheimer’s disease or dementia in Japan: a large-scale cross-sectional survey. J Med Econ 2021; 24: 181–192. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association. 2020 Alzheimer’s diseases facts and figures. Alzheimers Dement 2020; 16: 391–460. [Google Scholar]
- 6.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153: 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest 2018; 128: 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11: 298–300. [DOI] [PubMed] [Google Scholar]
- 9.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997; 82: 291–295. [DOI] [PubMed] [Google Scholar]
- 10.Davalli P, Mitic T, Caporali A, Lauriola A, D'Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev 2016; 2016: 3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pao PC, Patnaik D, Watson LA, et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat Commun 2020; 11: 2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014; 94: 909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukui K, Okihiro S, Ohfuchi Y, et al. Proteomic study on neurite responses to oxidative stress: search for differentially expressed proteins in isolated neurites of N1E-115 cells. J Clin Biochem Nutr 2019; 64: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukui K. Neuroprotective and anti-obesity effects of tocotrienols. J Nutr Sci Vitaminol (Tokyo) 2019; 65 (Supplement): S185–S187. [DOI] [PubMed] [Google Scholar]
- 15.Fukui K, Shirai M, Ninuma T, Kato Y. Anti-obesity effects of tocotrienols and bran in high-fat diet-treated mice. Nutrients 2019; 11: 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang FF, Barr SI, McNulty H, Li D, Blumberg JB. Health effects of vitamin and mineral supplements. BMJ 2020; 369: m2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2013; 159: 824–834. [DOI] [PubMed] [Google Scholar]
- 18.Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann NY Acad Sci 2001; 928: 168–175. [DOI] [PubMed] [Google Scholar]
- 19.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984; 11: 47–60. [DOI] [PubMed] [Google Scholar]
- 20.Kato Y, Aoki Y, Fukui K. Tocotrienols influence body weight gain and brain protein expression in long-term high-fat diet-treated mice. Int J Mol Sci 2020; 21: 4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato Y, Uchiumi H, Usami R, et al. Tocotrienols reach the brain and play roles in the attenuation of body weight gain and improvement of cognitive function in high-fat diet-treated mice. J Clin Biochem Nutr 2021; 69: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneai N, Sumitani K, Fukui K, Koike T, Takatsu H, Urano S. Tocotrienol improves learning and memory deficit of aged rats. J Clin Biochem Nutr 2016; 58: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishihira J, Nishimura M, Kurimoto M, et al. The effect of 24-week continuous intake of quercetin-rich onion on age-related cognitive decline in healthy elderly people: a randomized, double-blind, placebo-controlled, parallel-group comparative clinical trial. J Clin Biochem Nutr 2021; 69: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukui K, Suzuki Y, Kato Y, Takeuchi N, Takenaka H, Kohno M. Effect of extract-added water derived from deep-sea water with different hardness on cognitive function, motor ability and serum indexes of obese mice. Nutrients 2022; 14: 1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce MR, Diasio DL, Rodrigues LM, Harrison FE, May JM. Combined vitamin C and E deficiency induces motor defects in gulo(−/−)/SVCT2(+/−) mice. Nutr Neurosci 2013; 16: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta V, Dhull DK, Joshi J, Kaur S, Kumar A. Neuroprotective potential of azilsartan against cerebral ischemic injury: possible involvement of mitochondrial mechanisms. Neurochem Int 2020; 132: 104604. [DOI] [PubMed] [Google Scholar]
- 27.Aristotelous P, Stefanakis M, Pantzaris M, et al. The effects of specific omega-3 and omega-6 polyunsaturated fatty acids and antioxidant vitamins on gait and functional capacity parameters in patients with relapsing-remitting multiple sclerosis. Nutrients 2021; 13: 3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang A, Ackley BD, Yan D. Vitamin B12 regulates glial migration and synapse formation through isoform-specific control of PTP-3/LAR PRTP expression. Cell Rep 2020; 30: 3981–3988.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Bella-Garzón R, Fernández-Portero C, Alarcón D, Amián JG, López-Lluch G. Levels of plasma coenzyme Q10 are associated with physical capacity and cardiovascular risk in the elderly. Antioxidants (Basel) 2022; 11: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei Z, Ma L, Li Y, et al. CoQ10 improves myocardial damage in doxorubicin-induced heart failure in C57BL/6 mice. Front Biosci (Landmark Ed) 2022; 27: 244. [DOI] [PubMed] [Google Scholar]
- 31.Hargreaves I, Heaton RA, Mantle D. Disorders of human coenzyme Q10 metabolism: an overview. Int J Mol Sci 2020; 21: 6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manore MM. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am J Clin Nutr 2000; 72(2 Suppl): 598S–606S. [DOI] [PubMed] [Google Scholar]
- 33.Mahalakshmi B, Maurya N, Lee SD, Bharath Kumar V. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci 2020; 21: 5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a promising therapeutic agent in Parkinson’s diseases. Int J Mol Sci 2020; 21: 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szuhany KL, Otto MW. Assessing BDNF as a mediator of the effects of exercise on depression. J Psychiatr Res 2020; 123: 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du J, Shi Y, Zhou C, et al. Antioxidative and anti-inflammatory effects of vitamin C on the liver of laying hens under chronic heat stress. Front Vet Sci 2022; 9: 1052553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, Ying H, Zheng Y, Ma H, Li L, Zhao Y. Alanyl-glutamine protects against lipopolysaccharide-induced liver injury in mice via alleviating oxidative stress, inhibiting inflammation, and regulating autophagy. Antioxidants (Basel) 2022; 11: 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahl D, Solon-Biet SM, Wang QP, et al. Comparing the effects of low-protein and high-carbohydrate diets and calorie restriction on brain aging in mice. Cel Rep 2018; 25: 2234–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]