Abstract
Introduction
Some clinical studies have shown that music therapy as an adjunctive therapy can improve overall symptoms in patients with schizophrenia. However, the neural mechanisms of this improvement remain unclear due to insufficient neuroimaging evidence.
Methods
In this work, 17 patients with schizophrenia accepted a five-week music therapy (music group) that integrated listening, singing, and composing, and required patients to cooperate in a group to complete music therapy tasks. Meanwhile, 15 patients with schizophrenia received a five-week visual art intervention as the control group including handicraft and painting activities. We collected the Manchester Scale (MS) and Positive and Negative Symptom Scale (PANSS) scores and electroencephalography (EEG) data before and after intervention in two groups.
Results
Behavioral results showed that both interventions mentioned above can effectively help patients with schizophrenia relieve their overall symptoms, while a trend-level effect was observed in favor of music therapy. The EEG results indicated that music therapy can improve abnormal neural oscillations in schizophrenia which is reflected by a decrease in theta oscillation in the parietal lobe and an increase in gamma oscillation in the prefrontal lobe. In addition, correlation analysis showed that in the music group, both reductions in theta oscillations in the parietal lobe and increases in gamma oscillations in the prefrontal lobe were positively correlated with the improvement of overall symptoms.
Discussion
These findings help us to better understand the neural mechanisms by which music therapy improves overall symptoms in schizophrenia and provide more evidence for the application of music therapy in other psychiatric disorders.
Keywords: schizophrenia, music therapy, EEG, power spectral density, neural oscillations
1. Introduction
Schizophrenia is a common and complex psychiatric disorder with overall symptoms (mainly including negative, positive, and emotional symptoms) that may related to deficits in cognitive function (1–3). Currently, antipsychotic medication is the main treatment for schizophrenia, but cognitive impairment and some clinical symptoms may persist after medication (4), so it is urgent to find suitable psychosocial adjunctive therapies to improve overall symptoms and cognitive impairment. Cognitive remediation (5, 6), physical exercise (7), and music therapy (8) have been widely chosen. Music therapy has attracted much attention because of its unique and fun nature, and studies using it to improve clinical symptoms in schizophrenic patients have been reported many times (9, 10).
Music therapy is a psychosocial therapy method in which therapists use various experiences of music and the therapeutic relationship as a therapeutic motive to help the patients recover health (11). Existing studies have shown that music therapy conducted in individual or group form can improve overall symptoms and cognitive functions, such as attention and working memory in schizophrenia (12–16). However, due to the prevalence of symptoms such as social withdrawal in patients with schizophrenia, group music therapy is more widely used. It builds on the cooperation of the patient-patient group that requires group members to collaborate on tasks related to music therapy, helping schizophrenic patients establish social connections with the therapist and other group members, enhancing social cognitive abilities, and improving overall symptoms and cognitive function (17, 18).
EEG as an important tool for understanding neurological disorders, is mainly used to study abnormal neural oscillations in schizophrenic patients. Neural oscillations underlie the cognitive function of the brain and are considered a biomarker of neuropsychiatric disorders (19). Various studies have found that schizophrenic patients have abnormal low- and high-frequency oscillations, especially in theta and gamma bands, compared to healthy individuals (20–22). Higher theta oscillation in schizophrenia is associated with more severe symptoms (23) and poorer verbal memory performance (24). In addition, with the help of psychiatry and neurocognition scales, researchers have demonstrated that the decrease in gamma oscillation of schizophrenia is related to cognitive deficits such as distraction and memory impairment (25, 26). Thus, theta and gamma may be potential biomarkers for patients with schizophrenia.
Notably, the improvement of overall symptoms of music therapy in schizophrenia has been demonstrated by clinical behavioral evidence (27, 28), but the neuroimaging evidence is still insufficient. Therefore, we designed a five-week group music therapy for patients with schizophrenia who participated in our experiment and we explored the neural mechanisms of music therapy on the overall symptoms of schizophrenia with the help of MS (29) scores, PANSS scores, and EEG data.
2. Materials and methods
The study was done with the approval of the Ethics Committee of the University of Electronic Science and Technology of China (No. 1061420210305026). All procedures were carried out in adherence to approved guidelines. Written informed consent was obtained from all participants before the study.
2.1. Participants
Patients with schizophrenia recruited in this experiment were from Chengdu Dekang Hospital. Inclusion Criteria: 1) Meet the diagnostic criteria of schizophrenia in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) (30), 2) aged between 20-70 years, 3) elementary school education or above, normal speech ability, normal communication, able to understand and complete the scale, Mini-Mental State Examination (MMSE) score ≥ 24, Montreal Cognitive Assessment (MoCA) score ≥ 25, 4) duration of the disease ≥ 5 years, hospitalization ≥ 6 months, and stable disease condition, 5) informed and consenting to participate. We divided the 38 patients who met the inclusion criteria into the music group and the visual art group. 2 of them were excluded because they voluntarily withdrew from the experiment early due to sudden health issues and family relocation, and 4 because their EEG data were of insufficient quality. The final analysis included 32 individuals. There were 17 participants included in the music group (age range 20-70 years, 10 female), and 15 participants in the visual art group (age range 20-70 years, 7 female). The two groups have similar socio-demographic data and clinical characteristics, including gender, age, marital status, education level, duration of illness, number of antipsychotics, MMSE score, and MoCA score ( Table 1 ).
Table 1.
Sociodemographic and clinical characteristics of study participants.
| Characteristic | Music group | Visual art group | P value |
|---|---|---|---|
| Gendera (male/female) | 7/10 | 8/7 | 0.49 |
| Ageb (years) | 46.12 ± 15.22 | 47.60 ± 9.44 | 0.75 |
| Marital statusa
(married/unmarried) |
1/16 | 1/14 | 0.93 |
| Education levelb (years) | 7.18 ± 4.91 | 6.60 ± 1.20 | 0.67 |
| Duration of illnessb (years) | 21.06 ± 12.83 | 16.93 ± 6.45 | 0.28 |
| Number of antipsychoticsb | 1.94 ± 0.80 | 1.47 ± 0.72 | 0.10 |
| MMSE scoreb | 27.88 ± 1.23 | 28 ± 1.46 | 0.81 |
| MoCA scoreb | 26.53 ± 1.33 | 27 ± 1.32 | 0.34 |
Values are shown as mean ± SD, unless otherwise noted. P values refer to significance between two groups.
a: Chi-square test was used.
b: Two-sample t-tests was used.
2.2. Study design
During the five-week therapy process, the professional therapist who was certified as a U.S. registered Neurological Music Therapist (NMT) and Musical and Imagery Therapist (MIT) conducted twice-weekly 45-minute sessions for the music group. There were 10 topic courses in music therapy based on the aim of assisting patients to develop social skills and express their emotions. Therapy progress began with an assessment to give the therapist a better understanding of the musical ability of the patient. In each course, the therapist would set course activities that integrate singing, songwriting, and listening according to the course targets and ask patients to work together to complete these activities. Every course was divided into 3 or 4 stages, the first stage began with a chorus and then everyone sang their self-introduction song in turn, the activities of the second and third stages were decided by the course target, and the last stage of each course, patients get together to give a chorus named ‘Goodbye song’ ( Table 2 ). Meanwhile, the visual art group learned basic handicraft and painting skills, including independently completing painting works, weaving handbags, or creating bookmarks, notebooks, and other activities.
Table 2.
The music therapy schedule.
| Course topics | Course targets | In-course activities | |||
|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | ||
| Assessment | Musical proficiency assessment | Hello song, self-introduction song | Warm-up activity | Instrumental ensemble | Goodbye song |
| Music experience | Emotional feelings and picture perception of songs | Music sharing | Music experience | ||
| Musical figuration | The perception of musical emotions is concrete and substantial | Music collage | Music creation of the four seasons | ||
| Music speed and mood | The speed of the music matches the emotion, and the body language of the emotion is expressed | Matching music speed and mood | |||
| Music composition |
Music and emotional expression | Music creation | |||
| Re-assessment | Assessment of musical emotional perception | Music sharing | Music handicraft | ||
| Music for self-care | The ego mood matches the musical mood | Music self-care | |||
| Happy mood music | Positive emotions match the music | Happy music session | Music painting | ||
| Sad mood music | Negative emotions match the music | Sad music session | Music handicraft | ||
| End music album |
Mood music matching | Mood music album | Music painting | ||
2.3. Data recording and processing
We recorded EEG signals and behavioral data. The professional doctor evaluated the severity of the clinical symptoms of patients using MS and PANSS scales based on their clinical manifestations before and after intervention. The resting EEG signals were recorded using the actiCHamp plus 64ch electroencephalograph (BrainProducts, Germany) with a 31-lead electrode cap system (Fp1/2, F3/4/7/8, T7/8, Pz/3/4/7/8, Cz/3/4, Oz/1/2, FT9/10, FC1/2/5/6, TP9/10, CP1/2/5/6) according to the 10/20 international electrode placement system. The initial sampling rate was 500Hz, and 31 channels were recorded simultaneously. We collected 15-minute resting EEG from participants in two groups before and after the intervention.
EEG preprocessing was done by the toolbox ‘EEGLAB’ and the ‘zero-reference’ code, utilizing the Reference Electrode Standardization Technique (https://www.neuro.uestc.edu.cn/name/shopwap/do/index/content/96). A Finite Impulse Response (FIR) band-pass filter (0.5-100Hz) was applied and the sampling rate was reduced to 100Hz. Independent Component Analysis (ICA) was used to remove ocular artifacts, following which the data was segmented into 2-second intervals, with a threshold of ±100 UV selected to exclude other artifacts.
Power spectral density (PSD), which describes the power distribution of signals in the frequency domain, is widely used to analyze the neural oscillation of schizophrenia (26, 31, 32). We calculated the theta and gamma power spectral densities using Welch’s method of the two groups at pre- and post-therapy.
2.4. Statistic analysis
IBM SPSS Statistics 26.0 was used to perform the statistical analysis. Numerical variables were given as means (standard deviation, SD), while categorical variables were described as numbers. The Chi-square (χ2) test was used for categorical variables, and the Student’s t-test or Mann-Whitney test was used for continuous variables according to their normal distribution and variance homogeneity. We compared the changes in PSD within the group using the paired t-test. Subsequently, to observe the difference in intervention effect between the two groups, we performed the two-sample t-test on the PSD. Besides, to explore the relationship between changes in theta and gamma oscillations and the improvement of overall symptoms in the music group, Pearson’s correlation analysis between the PSD and MS/PANSS scores was used.
3. Results
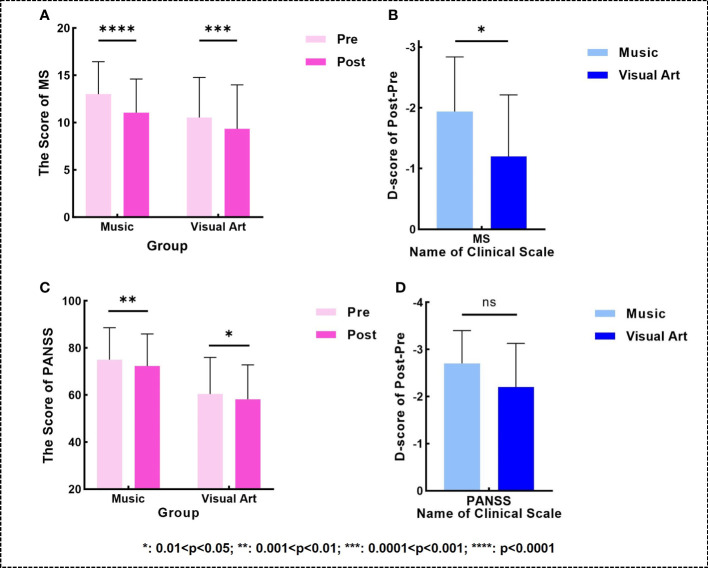
After the intervention, the MS and PANSS scores of the two groups significantly decreased (music group: pMS < 0.0001, tMS = -8.899, pPANSS = 0.0013, tPANSS = -3.891; visual art group: pMS = 0.0004, tMS = -4.583, pPANSS = 0.0325, tPANSS = -2.374; Figures 1A, C ). Moreover, the decline in MS scores in the music group was greater than that in the visual art group (p = 0.0363, t = 2.192; Figure 1B ). However, no significant between-group difference was observed in the decrease in PANSS scores ( Figure 1D ).
Figure 1.
(A, C) Changes in the MS/PANSS scores (higher scores indicating more severe overall symptoms) within two groups. (B, D) comparison of post-pre differences in the MS/PANSS scales between two groups.
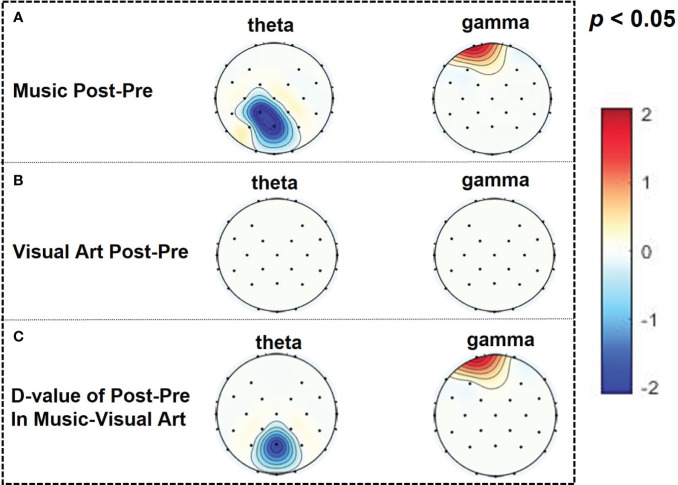
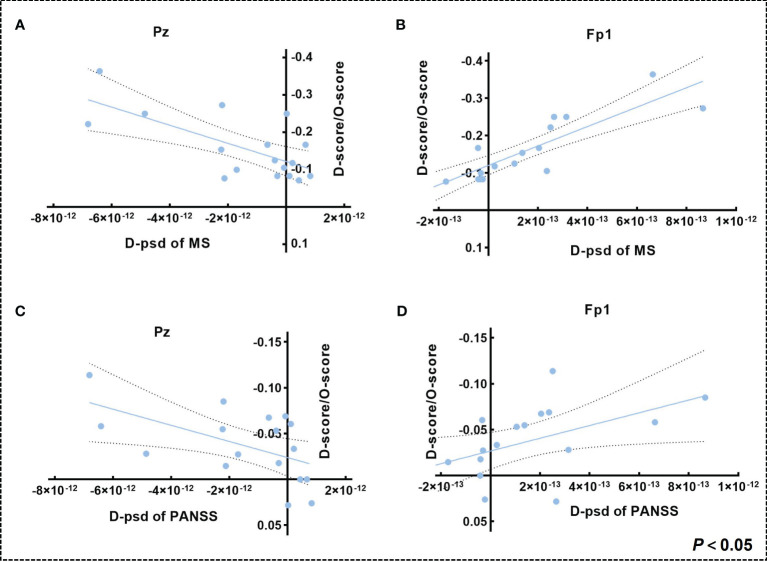
In the music group, the parietal theta PSD decreased and the prefrontal gamma PSD increased (CP1: p = 0.0148, t = -2.7314; Pz: p = 0.0208, t = -2.5651; Fp1: p = 0.0480, t = 2.1415; Figure 2A ), while there was no significant difference in the visual art group ( Figure 2B ). Subsequent results on the difference of post-pre PSD between the two groups showed that the music group has a significant decrease in theta PSD in the parietal lobe and an increase in gamma PSD in the prefrontal lobe compared with the visual art group (Pz: p = 0.0465, t = -2.0762; FP1: p = 0.0478, t = 2.0640; Figure 2C ). Pearson’s correlation analysis results showed that changes in MS and PANSS scale scores were linearly correlated with the change of theta PSD in the parietal lobe and gamma PSD in the prefrontal lobe (Pz: pMS = 0.0025, rMS = 0.6837, pPANSS = 0.0225, rPANSS = 0.5490; Fp1: pMS < 0.0001, rMS = -0.8606, pPANSS = 0.0375, rPANSS = -0.5078; Figure 3 ).
Figure 2.
(A) The difference of post-pre PSD in theta (5-7Hz) and gamma (30-45Hz) bands within the music group (t-plot) and (B) the difference of post-pre PSD in theta and gamma bands within the visual art group (t-plot). (C) Comparison of post-pre PSD differences between two groups.
Figure 3.
(A, B) Correlation between (The change of MS score/Original MS score) and the change of theta PSD in the parietal lobe and gamma PSD in the prefrontal lobe in the music group. (C, D) Correlation between (The change of PANSS score/Original PANSS score) and the change of theta PSD in the parietal lobe and gamma PSD in the prefrontal lobe in the music group (dashed intervals indicate 95% confidence intervals for the best-fit straight line).
4. Discussion
Consistent with previous studies (33, 34), we found that both music and visual art interventions can effectively help patients with schizophrenia relieve their overall symptoms. However, the decline in MS scores was greater in the music group than in the visual art group, and a trend-level effect observed for PANSS scores also favored the music group. This may be because compared with visual art intervention, which involves independent handicraft and painting activities, music therapy can provide a more interactive and collaborative therapeutic environment for patients with schizophrenia, helping them to become more engaged and build social connections (35, 36), alleviating negative and positive symptoms (9, 37), thereby improving their overall symptoms more effectively.
Previous studies have shown that theta and gamma oscillations appear to be associated with a wide range of perceptual and cognitive processes of schizophrenia (38, 39). We found that music therapy significantly decreased the theta oscillation in the parietal lobe and increased the gamma oscillation in the prefrontal lobe, analogous to the effects of antipsychotics or deep-brain stimulation (40–42), which may indicate the therapeutic effect of music therapy as a psychosocial intervention for patients with schizophrenia. These changes in theta and gamma oscillations in brain regions associated with cognitive function may reflect greater attention and better verbal memory capacity (24, 43–45). Patients with schizophrenia whose verbal memory capacity significantly declined manifested an increase of theta oscillations in the parietal lobe (45, 46). After taking medication, theta oscillations decreased (41). Our results are also consistent with these studies. In addition, schizophrenic patients with attention deficits are prone to symptoms such as fantasies and cognitive disorders, which may be related to their lower gamma oscillations (47, 48). Increased prefrontal gamma oscillations may be associated with the fact that musical rhythms and melodies increase patients’ interest and enhance auditory attention (49). Notably, the existing studies have shown that the presence of an active and trained therapist has an important influence on cognitive function in patients with schizophrenia (5, 50). In this paper, patients in the music group accepted a five-week therapy under the guidance of a professional music therapist who was certified as a U.S. registered Neurological Music Therapist and Musical and Imagery Therapist. This therapy was effective in improving theta and gamma oscillations, which may indicate a positive impact on cognitive performance but this must be further demonstrated by specialized measurements.
Correlation results showed that a decrease in parietal theta oscillations and an increase in prefrontal gamma oscillations were positively correlated with changes in the PANSS and Manchester scales. That is to say, after the music therapy, patients experienced a reduction in positive symptoms, negative symptoms, depressive symptoms, and anxiety symptoms, as well as a recovery in theta and gamma oscillations. These results suggested that these two types of neural oscillations may be bio-indicators to improve overall symptoms in schizophrenic patients and may also be targets for intervention.
5. Limitations
This study has some limitations. Firstly, the sample size of our participants was small, and future studies could increase the sample size to improve the reliability of the study. Secondly, further long-term follow-up studies could be conducted to assess the lasting effects of music therapy. Thirdly, we have not explored the neurological mechanisms underlying certain specific symptoms, which could be further investigated in the future to provide better music therapy for schizophrenics with specific symptoms. Fourthly, our assessment of overall symptoms relies on subjective measurement tools such as the Manchester and PANSS scores. Although these tools are widely used in psychiatric research, there are problems with subjective assessments, including the influence of patient expectations and the therapeutic relationship. Multiple assessment tools, including objective physiologic indicators and the establishment of a positive therapeutic relationship, should be used in future studies to gain a more comprehensive understanding of the patient’s symptomatic status and to improve the accuracy and credibility of findings.
6. Conclusion
Our study provides evidence that music therapy can be an effective adjunctive therapy to help patients with schizophrenia improve overall symptoms. This improvement may be related to the fact that music therapy helps patients with schizophrenia to establish social connections and increase engagement, which in turn alleviates overall symptoms. The EEG results indicated that music therapy can improve abnormal neural oscillations in patients with schizophrenia which is reflected by a decrease in theta oscillation in the parietal lobe and an increase in gamma oscillation in the prefrontal lobe. In addition, both reductions in theta oscillations in the parietal lobe and increases in gamma oscillations in the prefrontal lobe were positively correlated with the improvement of overall symptoms, suggesting that music therapy may improve overall symptoms by modulating neural oscillations in theta and gamma. Our findings may help us better understand the neural mechanisms of music therapy for schizophrenia and provide more evidence for the application of music therapy in other psychiatric disorders.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was done with the approval of the Ethics Committee of the University of Electronic Science and Technology of China (No. 1061420210305026). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. LW: Methodology, Writing – original draft, Writing – review & editing, Investigation. JC: Investigation, Methodology, Writing – original draft. CQ: Writing – review & editing. TL: Data curation, Resources, Writing – review & editing. YW: Project administration, Resources, Writing – review & editing. YL: Investigation, Methodology, Writing – review & editing. PZ: Visualization, Writing – review & editing. SG: Conceptualization, Writing – review & editing. JL: Conceptualization, Data curation, Methodology, Writing – review & editing, Writing – original draft.
Acknowledgments
We thank all the participants in our study.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Research Unit of NeuroInformation, Chinese Academy of Medical Sciences 2019RU035 (M112019-I2M-5-039-1), and the Music and Digital Intelligence, Key Laboratory of Sichuan Province (SZ2023ZDXM03 and SZ2023ZDXM02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Bozikas VP, Kosmidis MH, Kioperlidou K, Karavatos A. Relationship between psychopathology and cognitive functioning in schizophrenia. Compr Psychiatry. (2004) 45:392–400. doi: 10.1016/j.comppsych.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 2. Fan X, Goff DC, Henderson DC. Inflammation and schizophrenia. Expert Rev Neurother. (2014) 7:789–96. doi: 10.1586/14737175.7.7.789 [DOI] [PubMed] [Google Scholar]
- 3. Sun X, Yue S, Duan M, Yao D, Luo C. Psychosocial intervention for schizophrenia. Brain-Apparatus Communication: A J Bacomics. (2023) 2:2178266. doi: 10.1080/27706710.2023.2178266 [DOI] [Google Scholar]
- 4. Joshi YB, Thomas ML, Braff DL. Anticholinergic medication burden–associated cognitive impairment in schizophrenia. Am J Psychiatry. (2021) 178:838–47. doi: 10.1176/appi.ajp.2020.20081212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vita A, Barlati S, Ceraso A, Nibbio G, Ariu C, Deste G, et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia. JAMA Psychiatry. (2021) 78:848–58. doi: 10.1001/jamapsychiatry.2021.0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solmi M, Croatto G, Piva G, Rosson S, Fusar-Poli P, Rubio JM, et al. Efficacy and acceptability of psychosocial interventions in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. Mol Psychiatry. (2022) 28:354–68. doi: 10.1038/s41380-022-01727-z [DOI] [PubMed] [Google Scholar]
- 7. Fernández-Abascal B, Suárez-Pinilla P, Cobo-Corrales C, Crespo-Facorro B, Suárez-Pinilla M. In- and outpatient lifestyle interventions on diet and exercise and their effect on physical and psychological health: a systematic review and meta-analysis of randomised controlled trials in patients with schizophrenia spectrum disorders and first episode of psychosis. Neurosci Biobehav Rev. (2021) 125:535–68. doi: 10.1016/j.neubiorev.2021.01.005 [DOI] [PubMed] [Google Scholar]
- 8. Honda S, Matsushita K, Noda Y, Tarumi R, Nomiyama N, Tsugawa S, et al. Music rhythm perception and production relate to treatment response in schizophrenia. Schizophr Res. (2023) 252:69–76. doi: 10.1016/j.schres.2022.12.040 [DOI] [PubMed] [Google Scholar]
- 9. Pedersen IN, Bonde LO, Hannibal NJ, Nielsen J, Aagaard J, Gold C, et al. Music therapy vs. Music listening for negative symptoms in schizophrenia: randomized, controlled, assessor- and patient-blinded trial. Front Psychiatry. (2021) 12. doi: 10.3389/fpsyt.2021.738810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ivanova E, Panayotova T, Grechenliev I, Peshev B, Kolchakova P, Milanova V. A complex combination therapy for a complex disease–neuroimaging evidence for the effect of music therapy in schizophrenia. Front Psychiatry. (2022) 13. doi: 10.3389/fpsyt.2022.795344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shoemark H, Hanson-Abromeit D. Music Therapy Handbook. J Music Therapy. (2015) 52(2):319–21. [Google Scholar]
- 12. Chambliss C MH, Tyson K. Motor performance of schizophrenics after mellow and frenetic antecedent music. Perceptual motor skills. (1996) 82:153–4. doi: 10.2466/pms.1996.82.1.153 [DOI] [PubMed] [Google Scholar]
- 13. Glicksohn J, Cohen Y. Can music alleviate cognitive dysfunction in schizophrenia? Psychopathology. (2000) 33:43–7. doi: 10.1159/000029118 [DOI] [PubMed] [Google Scholar]
- 14. Yao Y, He H, Duan M, Li S, Li C, Chen X, et al. The effects of music intervention on pallidum-DMN circuit of schizophrenia. BioMed Res Int. (2020) 2020:1–10. doi: 10.1155/2020/4107065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silverman MJ. The influence of music on the symptoms of psychosis: a meta-analysis. J music Ther. (2003) 40:27–40. doi: 10.1093/jmt/40.1.27 [DOI] [PubMed] [Google Scholar]
- 16. Lu S-F, Lo C-HK, Sung H-C, Hsieh T-C, Yu S-C, Chang S-C. Effects of group music intervention on psychiatric symptoms and depression in patient with schizophrenia. Complementary Therapies Med. (2013) 21:682–8. doi: 10.1016/j.ctim.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 17. Hayashi N, Tanabe Y, Nakagawa S, Noguchi M, Iwata C, Koubuchi Y, et al. Effects of group musical therapy on inpatients with chronic psychoses: A controlled study. Psychiatry Clin Neurosci. (2002) 56:187–93. doi: 10.1046/j.1440-1819.2002.00953.x [DOI] [PubMed] [Google Scholar]
- 18. Peng S-M, Koo M, Kuo J-C. Effect of group music activity as an adjunctive therapy on psychotic symptoms in patients with acute schizophrenia. Arch Psychiatr Nurs. (2010) 24:429–34. doi: 10.1016/j.apnu.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 19. Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. (2005) 9:474–80. doi: 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 20. Rutter L, Carver FW, Holroyd T, Nadar SR, Mitchell-Francis J, Apud J, et al. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp. (2009) 30:3254–64. doi: 10.1002/hbm.20746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garakh Z, Zaytseva Y, Kapranova A, Fiala O, Horacek J, Shmukler A, et al. EEG correlates of a mental arithmetic task in patients with first episode schizophrenia and schizoaffective disorder. Clin Neurophysiol. (2015) 126:2090–8. doi: 10.1016/j.clinph.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 22. Newson JJ, Thiagarajan TC. EEG frequency bands in psychiatric disorders: A review of resting state studies. Front Hum Neurosci. (2019) 12. doi: 10.3389/fnhum.2018.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull. (2008) 35:826–39. doi: 10.1093/schbul/sbn021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirihara K, Rissling AJ, Swerdlow NR, Braff DL, Light GA. Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol Psychiatry. (2012) 71:873–80. doi: 10.1016/j.biopsych.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spencer KM, Nestor PG, Perlmutter R. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci. (2004) 101:17288–93. doi: 10.1073/pnas.0406074101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grent-'T-Jong T, Gross J, Goense J, Wibral M, Gajwani R, Gumley AI, et al. Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. eLife. (2018) 7:e37799. doi: 10.7554/eLife.37799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D S. Book Review: The Dynamics of Music Psychotherapy. British J Music Therapy. (2000) 14(2):102–4. [Google Scholar]
- 28. Geretsegger M, Mössler KA, Bieleninik Ł., Chen X-J, Heldal TO, Gold C. Music therapy for people with schizophrenia and schizophrenia-like disorders. Cochrane Database Systematic Rev. (2017) 2017:CD004025. doi: 10.1002/14651858.CD004025.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatrica Scandinavica. (1977) 55:299–308. doi: 10.1111/j.1600-0447.1977.tb00174.x [DOI] [PubMed] [Google Scholar]
- 30. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, text revision DSM-5-TR. American Psychiatric Association Publishing. (2022). doi: 10.1176/appi.books.978089042 [DOI] [Google Scholar]
- 31. Gambini O, Colombo C, Macciardi F, Locatelli M, Calabrese G, Sacchetti E, et al. EEG power spectrum profile and structural CNS characteristics in schizophrenia. Biol Psychiatry. (1990) 27:1331–4. doi: 10.1016/0006-3223(90)90504-U [DOI] [PubMed] [Google Scholar]
- 32. Thilakavathi B, Shenbaga Devi S, Malaiappan M, Bhanu K. EEG power spectrum analysis for schizophrenia during mental activity. Australas Phys Eng Sci Med. (2019) 42:887–97. doi: 10.1007/s13246-019-00779-w [DOI] [PubMed] [Google Scholar]
- 33. Talwar N, Crawford MJ, Maratos A, Nur U, Mcdermott O, Procter S. Music therapy for in-patients with schizophrenia. Br J Psychiatry. (2018) 189:405–9. doi: 10.1192/bjp.bp.105.015073 [DOI] [PubMed] [Google Scholar]
- 34. Maurus I, Röll L, Keeser D, Schmitt A, Hasan A, Hirjak D, et al. Effects of exercise in people with severe mental illness and recommendations for its implementation as add-on therapy. Eur Psychiatry. (2022) 65:S21–1. doi: 10.1192/j.eurpsy.2022.80 [DOI] [Google Scholar]
- 35. Campillo M, Vates T, Pratdesava A, Vallve M, Casals A, Ortega Vallve J, et al. Music therapy in psychiatric units: evaluating its effectiveness. Eur Psychiatry. (2023) 66:S902–3. doi: 10.1192/j.eurpsy.2023.1912 [DOI] [Google Scholar]
- 36. Mi Y, Lei X. Sleep loss and lack of social interaction: a summary interview. Brain-Apparatus Communication: A J Bacomics. (2023) 2:2163593. doi: 10.1080/27706710.2022.2163593 [DOI] [Google Scholar]
- 37. Tseng P-T, Chen Y-W, Lin P-Y, Tu K-Y, Wang H-Y, Cheng Y-S, et al. Significant treatment effect of adjunct music therapy to standard treatment on the positive, negative, and mood symptoms of schizophrenic patients: a meta-analysis. BMC Psychiatry. (2016) 16:1–11. doi: 10.1186/s12888-016-0718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho Ry KR, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. (2006) 103:19878–83. doi: 10.1073/pnas.0609440103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bloch B RA, Vadas L. The effects of music relaxation on sleep quality and emotional measures in people living with schizophrenia. J music Ther. (2010) 47:27–52. doi: 10.1093/jmt/47.1.27 [DOI] [PubMed] [Google Scholar]
- 40. Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, et al. N-methyl-d-aspartic acid receptor antagonist–induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. (2009) 158:705–12. doi: 10.1016/j.neuroscience.2008.10.031 [DOI] [PubMed] [Google Scholar]
- 41. Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Structure Funct. (2011) 217:395–409. doi: 10.1007/s00429-011-0351-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zepeda NC, Crown LM, Medvidovic S, Choi W, Sheth M, Bergosh M, et al. Frequency-specific medial septal nucleus deep brain stimulation improves spatial memory in MK-801-treated male rats. Neurobiol Dis. (2022) 170:105756. doi: 10.1016/j.nbd.2022.105756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wichniak A OŁ., Linke M. Electroencephalographic theta activity and cognition in schizophrenia: preliminary results. World J Biol Psychiatry. (2015) 16:206–10. doi: 10.3109/15622975.2014.966145 [DOI] [PubMed] [Google Scholar]
- 44. Pan F, Wang J-Y, Xu Y, Huang M-L. Abnormal parietal encephalomalacia associated with schizophrenia. Medicine. (2017) 96:e6310. doi: 10.1097/MD.0000000000006310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coffman BA, Murphy TK, Haas G, Olson C, Cho R, Ghuman AS, et al. Lateralized evoked responses in parietal cortex demonstrate visual short-term memory deficits in first-episode schizophrenia. J Psychiatr Res. (2020) 130:292–9. doi: 10.1016/j.jpsychires.2020.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cao Y, Han C, Peng X, Su Z, Liu G, Xie Y, et al. Correlation between resting theta power and cognitive performance in patients with schizophrenia. Front Hum Neurosci. (2022) 16. doi: 10.3389/fnhum.2022.853994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bygrave AM, Jahans-Price T, Wolff AR, Sprengel R, Kullmann DM, Bannerman DM, et al. Hippocampal–prefrontal coherence mediates working memory and selective attention at distinct frequency bands and provides a causal link between schizophrenia and its risk gene GRIA1. Trans Psychiatry. (2019) 9:142. doi: 10.1038/s41398-019-0471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perrottelli A, Giordano GM, Brando F, Giuliani L, Pezzella P, Mucci A, et al. Unveiling the associations between EEG indices and cognitive deficits in schizophrenia-spectrum disorders: A systematic review. Diagnostics. (2022) 12:2193. doi: 10.3390/diagnostics12092193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding R, Tang H, Liu Y, Yin Y, Yan B, Jiang Y, et al. Therapeutic effect of tempo in Mozart’s “Sonata for two pianos” (K. 448) in patients with epilepsy: An electroencephalographic study. Epilepsy Behav. (2023) 145:109323. doi: 10.1016/j.yebeh.2023.109323 [DOI] [PubMed] [Google Scholar]
- 50. Vita A, Gaebel W, Mucci A, Sachs G, Barlati S, Giordano GM, et al. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur Psychiatry. (2022) 65:e57. doi: 10.1192/j.eurpsy.2022.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.