Abstract
Purpose
The consumption of highly processed food is often associated with a high intake of inorganic phosphate. Hyperphosphatemia is accompanied by an inflammatory status in patients with chronic kidney disease. However, the immune response to high phosphorus intake in healthy individuals is largely unknown. Therefore, the aim of the present study was to evaluate the effect of a single phosphate-enriched meal on inflammasome activity and plasma levels of inflammatory markers.
Methods
The analysis included 28 participants who received a single dose of either 700 mg phosphorus or a placebo with a test meal. At baseline, 4 and 8 h post-meal, plasma interleukin (IL)-6, IL-1β, IL-10, c-reactive protein (CRP), soluble IL-6 receptor (sIL-6R) and glycoprotein 130 (sgp130) levels were determined. At baseline and 4 h post-meal, peripheral blood mononuclear cells were isolated to assess inflammasome activity. Subsequently, the effect of phosphate with or without glucose on IL-6 and IL-1β gene expression and secretion in U937 monocytes was examined.
Results
While both groups showed a marked postprandial increase in IL-6 plasma levels, neither plasma levels of IL-6, IL-1β, CRP, IL-10, sIL-6R, and sgp130 nor inflammasome activity were affected by phosphate compared to placebo. In U937 cells, there was also no effect of phosphate on IL-6 expression, but the addition of glucose increased it. Phosphate, however, reduced the IL-1β secretion of these cells.
Conclusion
Postprandial inflammatory markers were not affected by dietary phosphate. However, IL-6 plasma levels were markedly increased post-meal, which appears to be a metabolic rather than a pro-inflammatory phenomenon.
Trial registration number
ClinicalTrials.gov, NCT03771924, date of registration: 11th December 2018, retrospectively registered.
Keywords: Clinical trial, Phosphorus, Inflammation, Diet, Nutrition, Monocytes
Introduction
Several studies have noted an association between elevated serum phosphate (Pi) levels and an increased risk of cardiovascular disease (CVD) in patients with chronic kidney disease (CKD) [1, 2]. The higher CVD risk in these patients is suggested to be caused primarily by Pi retention, which can affect vascular and cardiac valvular calcification [3–5]. However, assessment of cross-sectional data from CKD patients has suggested an association between elevated serum Pi concentrations and increased levels of interleukin (IL)-6 and C-reactive protein (CRP), indicating that elevated serum Pi levels may also affect inflammation in patients with CKD [6]. The assumption that phosphate may induce inflammation is supported by observations that vascular smooth muscle cells treated with high-phosphate media show increased expression of inflammatory cytokines [7], and adenine-induced CKD rats fed high-phosphate diets display increased serum and tissue concentrations of tumor necrosis factor-α (TNFα) and oxidative stress markers [8]. Interestingly, increased expression of IL-6 and IL-1β in the liver and increased expression of TNFα and other pro-inflammatory cytokines in bones have also been found in mice without renal impairment that received excessive phosphate [9]. In vitro data obtained from human aortic smooth muscle cells showed that phosphate provoked not only pro-inflammatory responses but also pro-oxidative conditions, which were accompanied by an increased formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [10].
Oxidative stress and the production of mitochondrial ROS might activate inflammasomes [11], molecular complexes that can activate caspase-1 and, in turn, the secretion of IL-1β [12]. IL-1β acts as a primary inflammatory cytokine and induces, among others, the expression of IL-6 [13]. IL-6 mediates its effects either by binding to the membrane-bound IL-6 receptor of cells (classic signaling) or by forming a complex with the soluble form of this receptor (sIL-6R), which subsequently can bind to the membrane-bound glycoprotein 130 (gp130) (trans-signaling) [14]. The membrane-bound IL-6 receptor is expressed only in a few cell types, such as hepatocytes or immune cells [14], and mediates the regenerative and anti-inflammatory effects of IL-6 [15], while ubiquitously expressed gp130 [16] can stimulate pro-inflammatory cascades via IL-6 trans-signaling [17, 18]. Whether phosphate affects classic or trans-signaling or both of these signaling pathways is currently unknown.
However, the importance of phosphate in provoking pro-inflammatory and pro-oxidative situations is relevant not only in the context of concomitant diseases in CKD patients but also for healthy subjects because the rising consumption of processed food is accompanied by a high intake of phosphate additives [19]. In the United States, the consumption of highly processed foods is estimated to account for 50–70% of an individual’s total energy intake [20], and approximately half of these foods contain phosphate additives [21]. As a result, the intake of phosphate in Western populations habitually exceeds the quantity of phosphorus recommended by the Institute of Medicine (700 mg/d) [22]. Trautvetter et al. [23], who analyzed data from 149 subjects in Germany, reported a habitual phosphate intake of more than 1300 mg per day. In contrast to phosphate in natural foods, phosphate from inorganic phosphate additives is more effectively absorbed in the intestine [24]. Although circulating Pi is tightly regulated in healthy subjects [25], our previous data show that compared to placebo, a single dose of 700 mg phosphorus administered orally as sodium dihydrogen phosphate (NaH2PO4, 3.53 g) to healthy subjects results in a significant increase in postprandial plasma Pi levels, which remain elevated over a period of 8 h post-meal (at 480 min: + 13%) [26].
However, the significance of increased plasma Pi levels in healthy subjects in inducing oxidative stress and in turn the stimulation of inflammasome activity, which can trigger the release of IL-1β, IL-6 trans-signaling and the secretion of inflammatory markers, is largely unknown. Since the level of circulating phosphate is not permanently elevated in healthy people but only post-meal, we hypothesized that possible effects of phosphate on inflammasome activity and inflammatory markers are primarily seen postprandially, especially given that inflammation markers can increase within a few hours post-meal [27]. To test this hypothesis, the current study investigated the effect of a single phosphate-enriched test meal on inflammasome activity and the plasma levels of inflammatory markers in healthy subjects.
Materials and methods
Study population
The participants included in the present study were part of the clinical trial entitled “Postprandial Response of Individuals to Dietary Inorganic Phosphate”. This study focused on the acute effects of dietary phosphate versus placebo on the postprandial plasma levels of Pi, urinary Pi excretion, regulators of mineral homeostasis such as fibroblast growth factor 23 (FGF23) and cardiometabolic risk factors and was published in 2022 [26]. The study was approved by the Ethics Committee of the Medical Faculty at Martin Luther University Halle-Wittenberg and was carried out in accordance with the Declaration of Helsinki. The study was conducted as a double-blind interventional trial with a crossover design at the Department of Internal Medicine II and the Institute of Agricultural and Nutritional Sciences at Martin Luther University Halle-Wittenberg (clinical trials.gov; ID: NCT03771924). For the current analysis, only blood of the first trial sequence was analyzed because the effects of phosphate in comparison to placebo were not different for any of the parameters measured between the two treatment sequences [26]. Thus, the present analysis was treated as a double-blind study with a parallel design.
Inclusion and exclusion criteria
The inclusion and exclusion criteria as well as the characteristics of the study participants were previously described in detail [26]. In brief, the inclusion criteria were as follows: body mass index (BMI) between 18.5 and 29.9 kg/m2; normal kidney function as assessed by plasma creatinine, cystatin C and protein excretion levels and rated by an experienced nephrologist; and plasma 25-hydroxy vitamin D (25(OH)D) levels > 30 nmol/l to ensure that no vitamin D-deficient subjects were included in the study. The exclusion criteria were the presence of allergies or intolerances to the test meal, pregnancy, lactation, chronic disease, medication, smoking, blood donation within the last two months, and participation in other clinical studies.
Sample size
As already reported by Volk et al. [26], the sample size was calculated (G*Power 3.1.9.2) to receive a significant difference (p < 0.05; Power 90%) in the postprandial plasma Pi concentrations between the phosphate and the placebo groups. Based on these specifications, the calculated sample size was 16. To consider possible differences in the effect of phosphate caused by the trial sequence, the sample size was increased to 32. Assuming a drop-out rate of 10%, 36 individuals were included in the study and randomly assigned to either the placebo or phosphate group (also see [26]). During the study, seven participants dropped out for personal reasons, and one person had to be excluded due to missing IL-6 values. Thus, the data for 28 subjects were included in the present analysis.
Experimental treatment
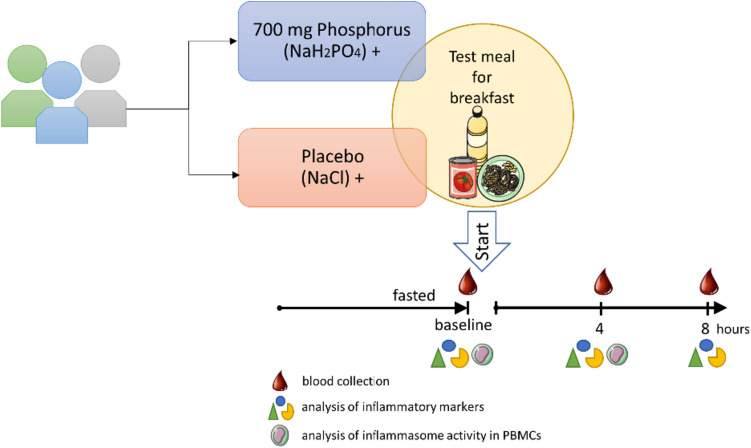
The study design was previously described in detail [26]. To ascertain whether the inclusion criteria were met, BMI, kidney function, and 25(OH)D levels were assessed 2–3 weeks prior to the beginning of the study. Therefore, all subjects had to complete a questionnaire to assess demographic and anthropometric data, and blood and urine samples were collected. Participants enrolled in the study were randomly assigned to two groups and received a single dose of either 700 mg phosphorus (3.53 g NaH2PO4) or a placebo containing NaCl (to keep sodium intake the same in both groups) together with a test meal (150 g boiled pasta, 180 g tomato sauce, 20 g corn oil) (Fig. 1). NaH2PO4 and NaCl were administered as capsules of identical appearance. The intervention started in the morning after a 12 h overnight fast. A blood sample taken 15 min before the intervention was used to analyze baseline levels.
Fig. 1.
Study design. A test meal together with phosphate or a placebo was served after a 12 h overnight fast. Analyses of plasma were conducted at baseline (− 15 min before the test meal) and 4 and 8 h post-meal, and analyses of isolated peripheral blood mononuclear cells (PBMCs) were conducted at baseline and 4 h post-meal
The figure was created with images adapted and modified from Servier Medical Art by Servier licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/; https://creativecommons.org/licenses/by/3.0/legalcode).
Anthropometric evaluation, blood sampling and laboratory procedures
BMI, blood pressure, glucose and lipids were assessed as described recently [26]. In brief, weight and height to calculate BMI were self-reported by participants. Blood pressure was assessed on the dominant arm in triplicate with a 1-min interval using automated portable upper-arm blood pressure monitors, and plasma concentrations of glucose and lipids were assessed at the central laboratory of the university hospital of Martin Luther University Halle-Wittenberg. To assess whether BMI, blood pressure, and blood lipids were in the normal or pathological ranges, the classification of the World Health Organization (WHO) was used for BMI, the European Society of Hypertension (ESH) guidelines for blood pressure [28], and “The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS)” guidelines for lipids [29].
Venous blood samples collected at baseline and 4 and 8 h post-meal were used to analyze the plasma levels of IL-6, IL-1β, CRP, IL-10, sIL-6R and sgp130. Peripheral blood mononuclear cells (PBMCs) for inflammasome activity measurements were isolated from blood obtained at baseline and 4 h postprandial, the time point with the highest Pi levels [26]. The 8 h postprandial levels were analyzed to consider the well-known time-delayed release of pro-inflammatory cytokines into the plasma [30, 31]. Plasma levels of IL-6, high sensitivity IL-1β (hsIL-1β), sIL-6R (all from IBL international GmbH, Tecan group), IL-10 (R&D Systems Inc.), soluble gp130 (sgp130) (RayBiotech Life, Inc.) and CRP (DRG Diagnostics GmbH) were determined using commercially available ELISA kits according to the manufacturer’s instructions.
Isolation of peripheral blood mononuclear cells and analysis of inflammasome complexes
To test whether the single oral intake of the phosphate-rich meal leads to elevated mitochondrial stress and consequently to the activation of inflammasome complexes, mitochondrial ROS, caspase-1 activity and pyroptosis were assessed in PBMCs at baseline and 4 h post-meal. The isolation of PBMCs and the analysis of inflammasome complexes were performed as described previously [32]. In brief, PBMCs were isolated from whole-blood samples by ficollization (GE Healthcare, Solingen, Germany). The viability of PBMCs was tested with 7-aminoactinomycin D (7-AAD) staining (Thermo Fisher Scientific, Darmstadt, Germany). The viability of PBMCs was > 99% for all participants. Representative of nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome activation, the caspase-1 activity was determined under basal and stimulated conditions. For stimulation of cells, lipopolysaccharide (LPS from E. coli 0111:B4; 1 µg/ml, Sigma‒Aldrich, Steinheim, Germany) and nigericin (5 µg/ml, Sigma‒Aldrich) were used to induce a high caspase-1 response. In contrast to LPS, nigericin (acting as a K+ ionophore) was applied for the last 15 min of the incubation period. In brief, 0.25 × 106 PBMCs were incubated at 37 °C in a 5% CO2 atmosphere. After an incubation period of 4 h, caspase-1 was detected by flow cytometry (MACS Quant analyzer, Miltenyi Biotec, Bergisch-Gladbach, Germany) with the FAM (fluorescent carboxyfluorescein)-YVAD (Tyr-Val-Ala-Asp)-FLICA (fluorescent-labeled inhibitors of caspases) inhibitor probe (Bio-Rad, Feldkirchen, Germany). One hour before ending the regular incubation period, the cells were pelleted and resuspended in sterile PBS/0.5% human serum albumin (HAS) containing the FAM-YVAD-FLICA inhibitor probe. The incubation was continued for 1 h. Samples without caspase-1 inhibitor were used as negative controls. Cells were counterstained using labeled anti-monocyte- (cluster of differentiation 14; CD14) and anti-lymphocyte- (cluster of differentiation 3; CD3) specific antibodies (Thermo Fisher Scientific). The caspase-1 activity of the cells was expressed as the mean fluorescence intensity (MFI). Inflammatory cell death (pyroptosis) was measured by analysis of 7-AAD and caspase-1 double-positivity (%).
Mitochondrial oxidative stress
Mitochondrial ROS were determined by flow cytometry with the fluorogenic indicator probe MitoSOX™ (Thermo Fisher). Therefore, 0.25 × 106 PBMCs were cultivated for a total of 2 h. For the last 15 min of the incubation period, cells were labeled with 2 µM MitoSOX™. Cells without MitoSox™ staining served as negative controls. The data are expressed as MFI.
Cell culture, RNA isolation and real-time reverse-transcription polymerase chain reaction
To investigate the effects of phosphate on IL-6 and IL-1β expression and to dissect the effects of phosphate and nutrients, particularly glucose, on the observed postprandial increase in circulating IL-6, cell culture studies using U937 monocytes (Leibniz-Institut DSMZ GmbH, Braunschweig, Germany) were carried out. Glucose was chosen for cell culture experiments to mimic the postprandial state because the test meal was rich in carbohydrates (116 g) but low in fat (24 g) [33]. For this purpose, cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 10% fetal bovine serum (FBS) and 0.5% gentamicin at 37 °C and 5% CO2. For the experiments, 1 × 106 cells were seeded per well of 24-well plates. After a rest of 2 h, cells were treated with either glucose-free RPMI 1640 media with 10% FBS alone (negative control) or with LPS (1 µg/ml, positive control) or with 10, 25, or 50 mM glucose, with or without the addition of 10 mM phosphate (ratio: Na2HPO4/NaH2PO4, 4:1). For analysis of mRNA expression, cells were treated with the corresponding substances for 2 h. For analysis of secreted ILs, cells were treated for 24 h.
Following the 2 h treatment, cells were harvested, and total RNA was isolated using Tri-Reagent (Sigma‒Aldrich) according to the manufacturer’s instructions. Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV reverse transcriptase) (Promega Corp.) was used for cDNA synthesis. The relative mRNA abundance of IL-6 and IL-1β was analyzed using real-time reverse-transcription polymerase chain reaction (real-time RT‒PCR). GoTaq Flexi DNA Polymerase (Promega Corp.) was used to amplify a total of 1 µl cDNA template with the Rotorgene 6000 system (Corbett Research Ltd.). The comparative threshold cycle (CT) method [34] was used to determine the relative abundance of target gene mRNA normalized to the endogenous reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer sequences were as follows (GAPDH: forward: GACCACAGTCCATGCCATCAC, reverse: TCCACCACCCTGTTGCTGTAG, NM_001357943.2; IL-6: forward: GCAGAAAAAGGCAAAGAATC, reverse: CTACATTTGCCGAAGAGC, NM_001371096.1; IL-1β: forward: CTAAACAGATGAAGTGCTCC, reverse: GGTCATTCTCCTGGAAGG, NM_000576.3). Following the 24 h incubation, the cell culture supernatant was collected for quantification of secreted IL-6 (R&D Systems Inc.) and IL-1β protein (IBL international GmbH, Tecan group) using commercially available ELISA kits according to the manufacturer’s protocol.
Statistical analysis
All data were analyzed using the Statistical Analysis System (SAS) 9.4 software package (SAS Institute Inc., Cary, NC, USA). The data were tested for normal distribution or, in the absence of normal distribution, for lognormal distribution (plasma IL-6, CRP, IL-6 mRNA expression and secretion in U937 monocytes). Baseline characteristics were analyzed using a t test. For the analysis of cytokine concentrations, pyroptosis and cell culture studies, the mixed-model procedure (PROC MIX) was used. The effects of phosphate treatment, time of intervention and their interaction were considered fixed, and participants were considered random. Due to the differences between the groups at baseline, the baseline values were also included in the model as covariates for plasma sIL-6R and IL-10. For the cell culture studies, glucose treatment, phosphate treatment and their interaction were considered fixed, and the repetition was considered random in the model. Due to missing normally distributed data, mitochondrial ROS and caspase activity were analyzed using the Wilcoxon rank-sum test with the SAS procedure npar1way as a nonparametric alternative to the two-sample t test. The plasma parameter data for 4 h post-meal for one subject were missing. The results are presented as total numbers, boxplots (using the Tukey method) and means ± standard deviations (SD). Differences with p values < 0.05 were considered significant. GraphPad Prism 9 (San Diego, CA, USA) was used to create figures.
Results
Characteristics of study participants
The participants enrolled in the study were aged between 18 and 35 years (Table 1). At baseline, the two groups did not differ in BMI, blood pressure, blood glucose or lipid levels (Table 1). The majority (~ 80%) of all subjects had a BMI within the normal range of 19–25 kg/m2, while five individuals had a BMI between 25 and 28 kg/m2 (three in the placebo group and two in the phosphate group). Fasting blood concentrations of glucose and triglycerides were within normal ranges. According to ESH [28] and ESC/EAS [29] guidelines, one person in the placebo group had an elevated systolic blood pressure (148 mmHg), and one person in this group was hypercholesterolemic (5.8 mmol/l). In addition, six participants (three in each group) had blood LDL cholesterol concentrations above the cutoff value for individuals at low cardiovascular risk (> 3 mmol/l) [29].
Table 1.
Characteristics of the study participants at baseline
| Placebo group | Phosphate group | |
|---|---|---|
| Number of participants | 14 | 14 |
| Sex (male/female) | 4/10 | 3/11 |
| Age (years) | 22.2 ± 4.6 | 22.2 ± 2.6 |
| BMI (kg/m2) | 22.1 ± 3.2 | 22.5 ± 1.9 |
| SBP (mmHg) | 117.8 ± 11.9 | 111.9 ± 10.3 |
| DBP (mmHg) | 72.1 ± 6.2 | 71.1 ± 5.1 |
| Plasma glucose (mmol/l) | 4.8 ± 0.3 | 4.7 ± 0.3 |
| Plasma triglycerides (mmol/l) | 0.9 ± 0.3 | 0.8 ± 0.4 |
| Plasma total cholesterol (mmol/l) | 4.0 ± 0.8 | 3.7 ± 0.6 |
| HDL cholesterol (mmol/l) | 1.6 ± 0.4 | 1.4 ± 0.2 |
| LDL cholesterol (mmol/l) | 2.5 ± 0.6 | 2.4 ± 0.5 |
BMI body mass index, DPB diastolic blood pressure, HDL high-density lipoprotein, LDL low-density lipoprotein, SPB systolic blood pressure; the data are shown as the mean ± SD
Effect of phosphate intake on postprandial plasma levels of inflammatory markers
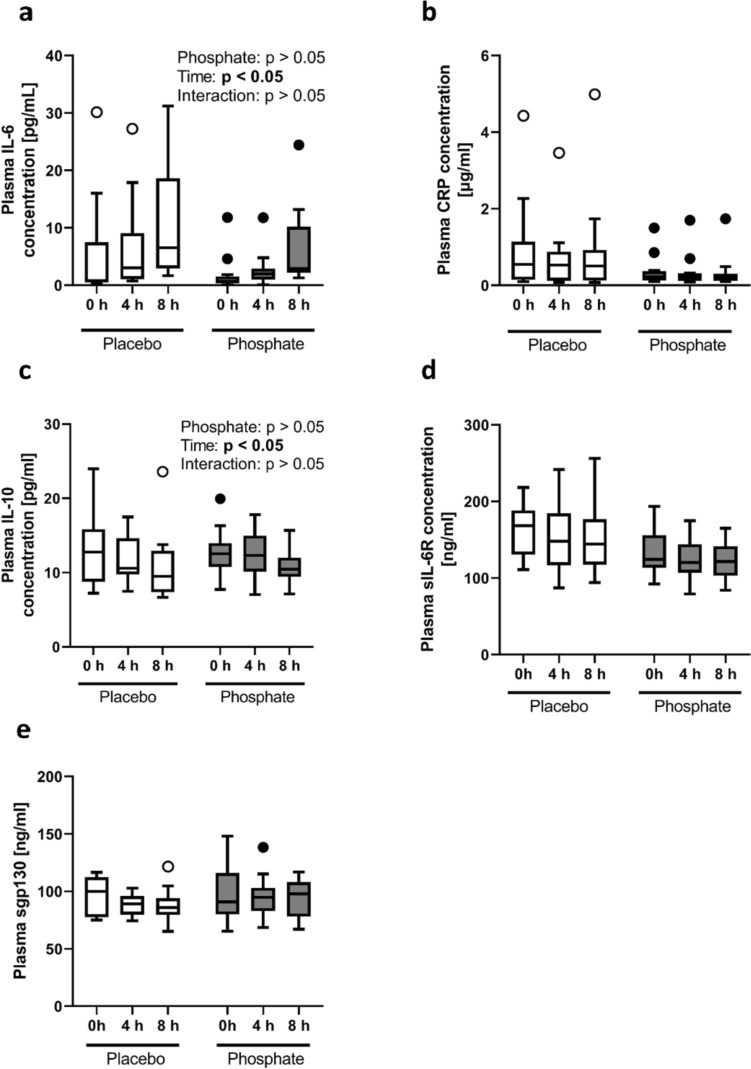
The most important findings were that the postprandial concentrations of IL-6, CRP, IL-10, sIL-6R, and sgp130 did not differ between the phosphate and placebo groups (Fig. 2). Circulating IL-1β was not measurable at baseline or postprandial in the majority of subjects. Interestingly, both groups showed a significant postprandial increase in IL-6 that reached values above the upper limit of normal ranges (> 10 pg/ml) in 40% of the individuals 8 h post-meal (Fig. 2a).
Fig. 2.
Plasma concentrations of a interleukin-6 (IL-6), b C-reactive protein (CRP), c interleukin-10 (IL-10), d soluble IL-6 receptor (sIL-6R) and e soluble glycoprotein 130 (sgp130) at baseline (0 h) and 4 h and 8 h after intake of the test meal with either a placebo (Placebo) (white bars) or 700 mg phosphorus (Phosphate) (gray bars). N = 14 per group; one value is missing in the placebo group at 4 h post-meal. The data are presented as boxplots [using the Tukey method]
Effect of phosphate intake on the inflammasome activity of peripheral blood mononuclear cells
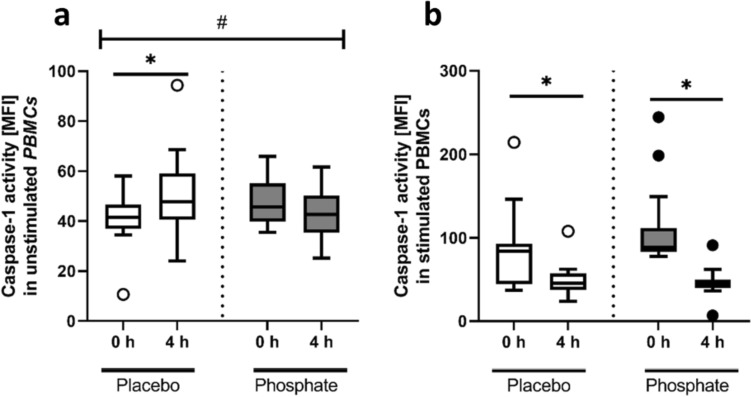
In unstimulated PBMCs (without added LPS) isolated 4 h post-meal, the quantity of mitochondrial ROS and pyroptosis did not differ between the two groups (Table 2), and there were no post-meal changes (4 h post-meal compared to baseline) in mitochondrial ROS (Table 2). Compared to baseline, PBMCs of both groups were characterized by a small decline in postprandial pyroptosis (Table 2). Interestingly, the postprandial activity of caspase-1 in unstimulated cells remained unchanged in the phosphate group, while it increased in the placebo group (25%; p < 0.05; Fig. 3a). In contrast, PBMCs stimulated with LPS showed a decline in caspase-1 activity 4 h post-meal compared to baseline (− 42% for placebo; − 59% for phosphate; both: p < 0.05), which was, by trend, more pronounced in stimulated PBMCs of the phosphate group than in those of the placebo group (p = 0.08; Fig. 3b).
Table 2.
Mitochondrial ROS and pyroptosis in unstimulated PBMCs of participants receiving the test meal with either a placebo or 700 mg phosphorus at baseline and 4 h post-meal
| Placebo group | Phosphate group | |||
|---|---|---|---|---|
| Baseline | 4 h | Baseline | 4 h | |
| Number of participants | 14 | 14 | 14 | 14 |
| Mitochondrial ROS (MFI) | 14.7 ± 19.5 | 10.0 ± 16.6 | 15.2 ± 16.7 | 10.6 ± 14.8 |
| Pyroptosis (AAD + caspase-1 + frequency, %)* | 7.4 ± 2.9 | 5.0 ± 3.6 | 6.3 ± 3.0 | 4.6 ± 2.7 |
MFI mean fluorescence intensity, PBMC peripheral blood mononuclear cells, ROS reactive oxygen species
*Effect of phosphate: not significant, effect of time: p < 0.05, interaction: not significant. The data are presented as the mean ± SD
Fig. 3.
Caspase-1 activity expressed as the mean fluorescence intensity [MFI] in a unstimulated and b lipopolysaccharide (LPS)-stimulated peripheral blood mononuclear cells (PBMCs) of participants at baseline (0 h) and 4 h after intake of the test meal with either a placebo (Placebo) (white bars) or 700 mg phosphorus (Phosphate) (gray bars). The data are presented as boxplots [using the Tukey method]. *p < 0.05 compared to baseline within a group, #p < 0.05 comparing changes between the groups
Effects of phosphate and glucose on IL-6 and IL-1β expression and secretion in vitro
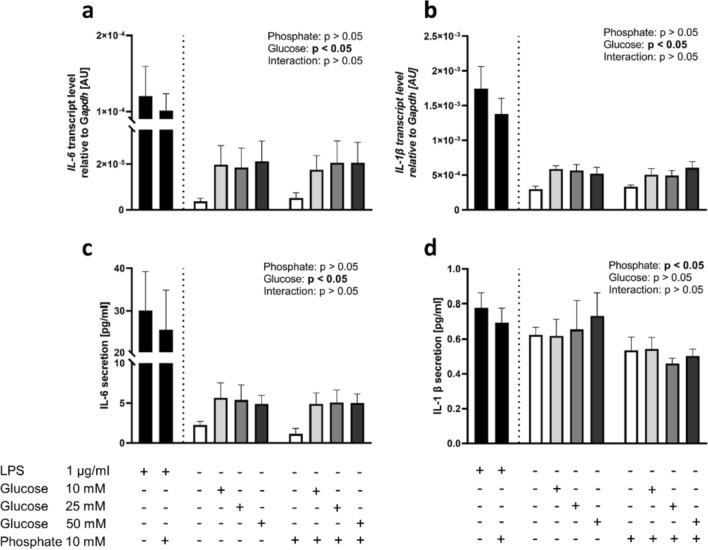
Treatment of U937 cells with 10 mM phosphate did not affect the mRNA abundance of IL-6 or IL-1β or the IL-6 secretion of these cells (Fig. 4a–c). More importantly, cells treated with phosphate showed lower secretion of IL-1β than cells treated without phosphate (p < 0.05, Fig. 4d), indicating that phosphate had no pro-inflammatory effect. However, the treatment of cells with glucose resulted in a significant increase in the mRNA abundance of IL-6 and IL-1β and IL-6 secretion (p < 0.05, Fig. 4a–c) but not in IL-1β secretion, suggesting that the postprandial IL-6 increase observed in the study subjects was the result of carbohydrate intake as part of the test meal.
Fig. 4.
Transcript levels of a interleukin-6 (IL-6) and b interleukin-1 β (IL-1β) in U937 cells after 2 h treatment and concentrations of c IL-6 and (d) IL-1β in supernatant after 24 h incubation with medium alone (white bars), with added lipopolysaccharide (LPS) (black bars), and with addition (+) of different glucose concentrations [10 mM, 25 mM, 50 mM] (gray bars), with (+) or without (–) addition of phosphate [10 mM]. The data are presented as the mean ± SEM. The LPS data are shown as a positive control and were not included in statistical analyses
Discussion
The current study investigated the acute effects of a single phosphate-enriched meal on inflammation, assessed by pro-inflammatory cytokines and soluble cytokine receptors in plasma as well as inflammasome activity in PBMCs. The data showed that phosphate in comparison to placebo altered neither the levels of pro-inflammatory cytokines nor inflammasome activity. These findings were corroborated by the observed postprandial decline in caspase activity in isolated PBMCs of the phosphate group and results from cell culture studies that showed no pro-inflammatory response after phosphate treatment. Thus, the current results, which are not indicative of any inflammatory stress following phosphate intake, are in contrast to data linking phosphate to inflammation in CKD patients with hyperphosphatemia and animal models incorporating excessive Pi feeding [6, 8].
Data on the underlying mechanisms of Pi in inflammation come primarily from animal and cell studies and suggest increased ROS formation following Pi treatment [8, 10]. Wei et al. [35] showed that high Pi levels can induce the transcriptional activity of NF-E2-related factor 2 (NRF 2) and promote its translocation into the nucleus in vascular smooth muscle cells (VSMCs). These authors concluded that the activation of NRF 2, which results in increased expression of anti-oxidant and anti-inflammatory genes, is a mechanism that counteracts the oxidative stress induced by excessive Pi [35]. Conversely, the current human study did not indicate any differences in mitochondrial ROS between the phosphate and placebo groups. However, notably, we did not use an excessive dose of phosphate but rather administered an acute dose of the entire recommended daily amount [22], which resulted in moderately higher plasma Pi levels and a higher incremental area under the curve (iAUC) of phosphate 8 h post-meal when compared to placebo (mean difference in iAUC phosphate: 139 mmol/l × 480 min) [26]. Therefore, we conclude that a single oral dose of moderate amounts of phosphate in healthy subjects leading to a slight but significant increase in Pi plasma levels might not promote mitochondrial oxidative stress or inflammation in healthy subjects.
The absence of an inflammatory response in phosphate-treated healthy individuals in contrast to patients with CKD [6] might result from the fact that CKD patients, at least in the later stages of the disease, suffer from permanent highly elevated serum Pi levels [36]. Hyperphosphatemia is usually accompanied by the secretion of FGF23 [37], a phosphaturic hormone that is not only inducible by acute and chronic inflammatory factors [38] but also associated with inflammation, as shown in several clinical studies [39–41]. Additionally, in vitro and animal studies have shown that FGF23 stimulates the formation and release of cellular IL-6 and TNF α [42], and genome-wide analysis has revealed that FGF23 is a regulator of pro-inflammatory genes such as TNF α, IL-2, IL-4 and IL-6 in different cell types, such as mast cells or T cells [43]. Importantly, the phosphate-treated subjects in the current study did not show any increase in postprandial FGF23 levels [26], which may have been due to the short study period or the low calcium intake with the meal. Thus, it is tempting to speculate that the noninflammatory effect of phosphate in the current study could result from the absence of an increase in FGF23 levels. Since long-term studies have found that high phosphate intake is associated with elevated FGF23 plasma levels in healthy subjects [44, 45], it would be interesting to investigate inflammation biomarkers in subjects treated with phosphate for several weeks.
As expected, the absent effect of phosphate on inflammasome activity in the current study was accompanied by an absent increase in inflammatory markers such as IL-6, IL-1β, sIL-6R or sgp130. These findings, together with the data obtained from the cell culture study, which are not indicative of any inflammatory effect of phosphate, suggest that an acute oral dose of phosphate did not induce inflammation. Interestingly, the pronounced postprandial increase in IL-6 without any difference between the two groups was not caused by phosphate. Although the IL-6 levels remained elevated for at least 8 h postprandial and were even in the pathological range in 40% of the subjects, this phenomenon is probably not the result of an inflammatory process because other inflammatory markers remained within normal ranges. It can be speculated that the rise in plasma IL-6 levels was a metabolic rather than an inflammatory response. A postprandial increase in IL-6 was also found in other studies with healthy subjects [27, 33, 46]. Recent data suggest that IL-6, which is also released during exercise, is necessary to maintain energy homeostasis [47]. In addition to nutrient intake, IL-6 is known to display a diurnal secretory pattern in humans with low levels of IL-6 in the morning hours and high levels at night [48]. It can, therefore, not be ruled out that the diurnal pattern of IL-6 was, at least in part, responsible for the observed increase in IL-6.
Strengths and limitations of this study
This study investigated a series of classical pro-inflammatory cytokines and CRP, inflammasome activity and soluble cytokine receptors. Additionally, in contrast to other intervention studies on phosphate, the current trial included a relatively high number of participants, and the effect of phosphate on pro-inflammatory cytokines was also tested in a separate cell culture study.
However, the present study also had some limitations. The calculated sample size was not designed to detect possible differences in inflammatory markers. The study was rather descriptive, and the underlying mechanisms for the postprandial IL-6 increase could not fully be explained. Additionally, the current study investigated only the acute phosphate effects. Thus, any conclusions about the long-term effects of phosphate on inflammation and higher phosphate doses are not possible.
Conclusion
The data from the current study did not show an inflammatory response of acute phosphate intake because neither pro-inflammatory cytokines nor inflammasome activity in PBMCs were affected in subjects who consumed a test meal with a phosphate additive. However, it cannot be excluded that long-term intake of diets rich in phosphate may lead to inflammatory conditions, e.g., via FGF23. The observed increase in postprandial IL-6 is induced by other nutrients, such as glucose, rather than by phosphate.
Acknowledgments
The authors thank all study participants for their participation in the study and the German Federal Ministry of Education and Research (BMBF) (No. 01EA1808C) for financial support.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the German Federal Ministry of Education and Research (BMBF) (No. 01EA1808C).
Data availability
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical standards
The study was performed in accordance with the principles of the ethical standards recorded in the Declaration of Helsinki of 1964 and its later amendments and approved by the local responsible Ethics Committee of the Medical Faculty at Martin Luther University Halle-Wittenberg (ethical approval number: 2018-83). Before study inclusion, all participants gave written informed consent.
Footnotes
Matthias Girndt and Gabriele I. Stangl have contributed equally to this work.
References
- 1.McGovern AP, de Lusignan S, van Vlymen J, et al. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community based cohort study. PLoS One. 2013;8:e74996. doi: 10.1371/journal.pone.0074996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozic M, Diaz-Tocados JM, Bermudez-Lopez M, et al. Independent effects of secondary hyperparathyroidism and hyperphosphataemia on chronic kidney disease progression and cardiovascular events: an analysis from the NEFRONA cohort. Nephrol Dial Transplant. 2022;37:663–672. doi: 10.1093/ndt/gfab184. [DOI] [PubMed] [Google Scholar]
- 3.Shang D, Xie Q, Ge X, et al. Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol. 2015;16:107. doi: 10.1186/s12882-015-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu M, Fujii H, Kono K, et al. Clinical implication of consistently strict phosphate control for coronary and valvular calcification in incident patients undergoing hemodialysis. JAT. 2023;30:1568–1579. doi: 10.5551/jat.64159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Górriz JL, Molina P, Cerverón MJ, et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10:654–666. doi: 10.2215/CJN.07450714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarro-González JF, Mora-Fernández C, Muros M, et al. Mineral metabolism and inflammation in chronic kidney disease patients: a cross-sectional study. Clin J Am Soc Nephrol. 2009;4:1646–1654. doi: 10.2215/CJN.02420409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D, Bi X, Liu Y, et al. High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-κb signaling pathway. Kidney Blood Press Res. 2017;42:1205–1215. doi: 10.1159/000485874. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Tokumoto M, Tatsumoto N, et al. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am J Physiol Renal Physiol. 2014;306:F1418–F1428. doi: 10.1152/ajprenal.00633.2013. [DOI] [PubMed] [Google Scholar]
- 9.Czaya B, Heitman K, Campos I, et al. Hyperphosphatemia increases inflammation to exacerbate anemia and skeletal muscle wasting independently of FGF23-FGFR4 signaling. Elife. 2022;11:1059. doi: 10.7554/eLife.74782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Moreno JM, Herencia C, de Oca AM, et al. High phosphate induces a pro-inflammatory response by vascular smooth muscle cells and modulation by vitamin D derivatives. Clin Sci (Lond) 2017;131:1449–1463. doi: 10.1042/CS20160807. [DOI] [PubMed] [Google Scholar]
- 11.Long Y, Liu X, Tan X-Z, et al. ROS-induced NLRP3 inflammasome priming and activation mediate PCB 118- induced pyroptosis in endothelial cells. Ecotoxicol Environ Saf. 2020;189:109937. doi: 10.1016/j.ecoenv.2019.109937. [DOI] [PubMed] [Google Scholar]
- 12.Bo N, Yilin H, Chaoyue Y, et al. Acrylamide induces NLRP3 inflammasome activation via oxidative stress- and endoplasmic reticulum stress-mediated MAPK pathway in HepG2 cells. Food Chem Toxicol. 2020;145:111679. doi: 10.1016/j.fct.2020.111679. [DOI] [PubMed] [Google Scholar]
- 13.Tahtinen S, Tong A-J, Himmels P, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022;23:532–542. doi: 10.1038/s41590-022-01160-y. [DOI] [PubMed] [Google Scholar]
- 14.Baran P, Hansen S, Waetzig GH, et al. The balance of interleukin (IL)-6, IL-6·soluble IL-6 receptor (sIL-6R), and IL-6·sIL-6R·sgp130 complexes allows simultaneous classic and trans-signaling. J Biol Chem. 2018;293:6762–6775. doi: 10.1074/jbc.RA117.001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeh H, Rudolph N, Billing U, et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: fusing experimental insights and dynamic modelling. Cell Commun Signal. 2019;17:46. doi: 10.1186/s12964-019-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choy E, Rose-John S. Interleukin-6 as a multifunctional regulator: inflammation, immune response, and fibrosis. J Scleroderma Relat Disord. 2017;2:S1–S5. doi: 10.5301/jsrd.5000265. [DOI] [Google Scholar]
- 17.Valle ML, Dworshak J, Sharma A, et al. Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells. Exp Eye Res. 2019;178:27–36. doi: 10.1016/j.exer.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S, Tanaka T, Inoue H, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci USA. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidou S, Christodoulou A, Frank K, et al. A study of ultra-processing marker profiles in 22,028 packaged ultra-processed foods using the Siga classification. J Food Compos Anal. 2021;99:103848. doi: 10.1016/j.jfca.2021.103848. [DOI] [Google Scholar]
- 20.Baldridge AS, Huffman MD, Taylor F, et al. The healthfulness of the US packaged food and beverage supply: a cross-sectional study. Nutrients. 2019 doi: 10.3390/nu11081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvo MS, Dunford EK, Uribarri J. Industrial use of phosphate food additives: a mechanism linking ultra-processed food intake to cardiorenal disease risk? Nutrients. 2023 doi: 10.3390/nu15163510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Academies Press (US) (1997) Dietary reference intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride, Washington (DC) [PubMed]
- 23.Trautvetter U, Ditscheid B, Jahreis G et al (2018) Habitual intakes, food sources and excretions of phosphorus and calcium in three German study collectives. Nutrients. 10.3390/nu10020171 [DOI] [PMC free article] [PubMed]
- 24.Pendón-Ruiz de Mier MV, Vergara N, Rodelo-Haad C, et al. Assessment of inorganic phosphate intake by the measurement of the phosphate/urea nitrogen ratio in urine. Nutrients. 2021 doi: 10.3390/nu13020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner ME, Paynter AS, White CA, et al. Sex differences in phosphate homeostasis: females excrete more phosphate and calcium after an oral phosphate challenge. J Clin Endocrinol Metab. 2023;108:909–919. doi: 10.1210/clinem/dgac616. [DOI] [PubMed] [Google Scholar]
- 26.Volk C, Schmidt B, Brandsch C, et al. Acute effects of an inorganic phosphorus additive on mineral metabolism and cardiometabolic risk factors in healthy subjects. J Clin Endocrinol Metab. 2022;107:e852–e864. doi: 10.1210/clinem/dgab635. [DOI] [PubMed] [Google Scholar]
- 27.Mazidi M, Valdes AM, Ordovas JM, et al. Meal-induced inflammation: postprandial insights from the Personalised REsponses to DIetary Composition Trial (PREDICT) study in 1000 participants. Am J Clin Nutr. 2021;114:1028–1038. doi: 10.1093/ajcn/nqab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groha P, Schunkert H. Management der arteriellen Hypertonie. CME. 2016;13:49–57. doi: 10.1007/s11298-016-5552-2. [DOI] [PubMed] [Google Scholar]
- 29.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Park E, Edirisinghe I, et al. Maximizing the health effects of strawberry anthocyanins: understanding the influence of the consumption timing variable. Food Funct. 2016;7:4745–4752. doi: 10.1039/c6fo00995f. [DOI] [PubMed] [Google Scholar]
- 31.Phillips LK, Peake JM, Zhang X, et al. Postprandial total and HMW adiponectin following a high-fat meal in lean, obese and diabetic men. Eur J Clin Nutr. 2013;67:377–384. doi: 10.1038/ejcn.2013.49. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich C, Wildgrube S, Fiedler R, 2020. NLRP3 inflammasome activation in hemodialysis and hypertensive patients with intact kidney function. Toxins (Basel) [DOI] [PMC free article] [PubMed]
- 33.Volk C, Brandsch C, Schlegelmilch U, et al. Postprandial metabolic response to rapeseed protein in healthy subjects. Nutrients. 2020 doi: 10.3390/nu12082270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 35.Wei R, Enaka M, Muragaki Y. Activation of KEAP1/NRF2/P62 signaling alleviates high phosphate-induced calcification of vascular smooth muscle cells by suppressing reactive oxygen species production. Sci Rep. 2019 doi: 10.1038/s41598-019-46824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freethi R (2019) Study of serum levels of calcium, phosphorus and alkaline phosphatase in chronic kidney disease. Univ J Pre Paraclin Sci 5
- 37.Kritmetapak K, Losbanos L, Berent TE, et al. Hyperphosphatemia with elevated serum PTH and FGF23, reduced 1,25(OH)2D and normal FGF7 concentrations characterize patients with CKD. BMC Nephrol. 2021;22:114. doi: 10.1186/s12882-021-02311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Ortiz ME, Díaz-Tocados JM, Muñoz-Castañeda JR, et al. Inflammation both increases and causes resistance to FGF23 in normal and uremic rats. Clin Sci (Lond) 2020;134:15–32. doi: 10.1042/CS20190779. [DOI] [PubMed] [Google Scholar]
- 39.El-Hodhod MA-A, Hamdy AM, Abbas AA, et al. Fibroblast growth factor 23 contributes to diminished bone mineral density in childhood inflammatory bowel disease. BMC Gastroenterol. 2012;12:44. doi: 10.1186/1471-230X-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanks LJ, Casazza K, Judd SE, et al. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One. 2015;10:e0122885. doi: 10.1371/journal.pone.0122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallquist C, Mansouri L, Norrbäck M, et al. Associations of fibroblast growth factor 23 with markers of inflammation and leukocyte transmigration in chronic kidney disease. Nephron. 2018;138:287–295. doi: 10.1159/000485472. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Grabner A, Yanucil C, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90:985–996. doi: 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czaya B, Faul C. The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci. 2019 doi: 10.3390/ijms20174195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrari SL, Bonjour J-P, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 45.Vervloet MG, van Ittersum FJ, Büttler RM, et al. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011;6:383–389. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emerson SR, Sciarrillo CM, Kurti SP, et al. High-fat meal-induced changes in markers of inflammation and angiogenesis in healthy adults who differ by age and physical activity level. Curr Dev Nutr. 2019;3:nzy098. doi: 10.1093/cdn/nzy098. [DOI] [Google Scholar]
- 47.Ghanemi A, St-Amand J. Interleukin-6 as a "metabolic hormone". Cytokine. 2018;112:132–136. doi: 10.1016/j.cyto.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Bixler EO, Lin H-M, et al. IL-6 and its circadian secretion in humans. NeuroImmunoModulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ulrich C, Wildgrube S, Fiedler R, 2020. NLRP3 inflammasome activation in hemodialysis and hypertensive patients with intact kidney function. Toxins (Basel) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.