Abstract
Testicular tumors include germ cell tumors, sex cord stromal tumors, and ovarian type epithelial tumors. Testicular mucinous tumors belong to ovarian type epithelial tumors and are extremely rare with only 31 cases reported in literature so far. Among those, mucinous adenocarcinoma constitutes only 9 cases. There are no standard treatment guidelines owing to their rarity. We report a case of primary testicular mucinous adenocarcinoma managed by orchidectomy, chemotherapy, and retroperitoneal lymph node dissection. A 44-year-old gentleman presented with right testicular tumor with infiltration and ulceration of scrotal skin. Tumor markers were within normal limits. Patient underwent orchidectomy with excision of involved scrotal skin. HPE suggested mucinous adenocarcinoma of testis. Patient was then administered chemotherapy but had progression of disease and hence taken up for retroperitoneal, bilateral pelvic, and bilateral inguinal lymph node dissection with revision of spermatic cord. Patient recovered uneventfully and is on regular follow-up 6 months now since surgery. There are no standard guidelines for the management of mucinous adenocarcinoma of testis. It is essential to rule out mucinous carcinoma of gastrointestinal tract metastasizing to testis before labeling as primary mucinous adenocarcinoma of testis. Surgery remains the mainstay of treatment in metastasis confined to retroperitoneal and inguinal lymph nodes. Further studies are needed to identify optimal chemotherapy regimen for metastatic and adjuvant scenarios.
Keywords: Testicular mucinous adenocarcinoma, Testicular tumor, Ovarian type epithelial tumor, RPLND, Signet ring cells, Scrotal ulceration, Orchidectomy
Introduction
Testicular tumors include germ cell tumors, sex cord stromal tumors, and ovarian type epithelial tumors. Testicular mucinous tumors belong to ovarian type epithelial tumors and are extremely rare with only 31 cases reported in literature so far. Among those, mucinous adenocarcinoma constitutes only 9 cases. Histologically testicular mucinous tumors resemble their ovarian counterparts. There are no clinical or imaging features specific for epithelial testicular tumors and are often diagnosed after orchidectomy. There are no standard treatment guidelines owing to their rarity. We report a case of primary testicular mucinous adenocarcinoma managed by orchidectomy, chemotherapy, and retroperitoneal lymph node dissection.
Case Report
A 44-year-old gentleman presented with swelling in the scrotum with ulceration. He had history of infertility. Examination revealed a 8 × 7 cm right testicular mass with infiltration and ulceration of scrotal skin. There was thickening of right cord structures and palpable bilateral inguinal nodes. Ultrasound scrotum showed right testicular heterogeneous mass of size 7.6 × 6.5 × 4.6 cm with infiltration into epididymis, vas deferens, and scrotal skin. Enlarged inguinal and paraaortic nodes were found. CT scan of abdomen demonstrated enlargement of precaval and interaortocaval nodes largest 2.2 × 2 cm (Fig. 1).
Fig. 1.
Computed tomography scan showing testicular tumor with ulceration of scrotal skin
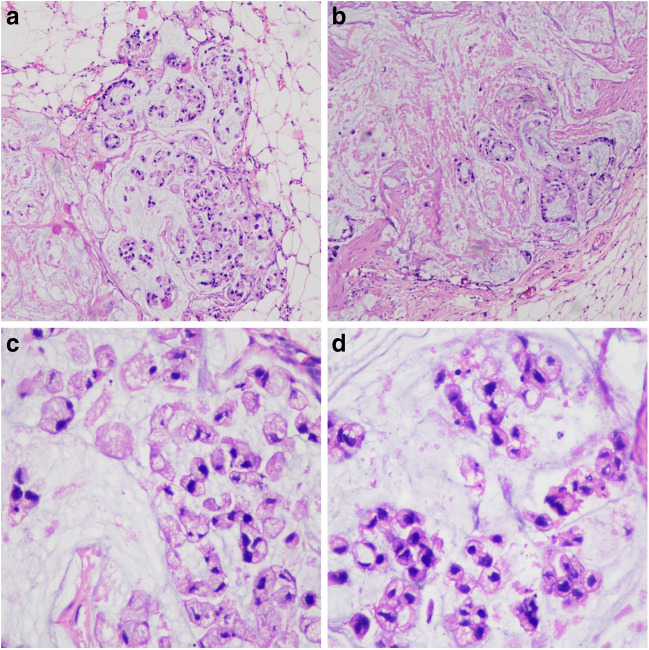
Beta-HCG, AFP, and LDH were within normal limits. VDRL was reactive. CRP was within normal limits. Patient underwent right high inguinal orchidectomy with excision of involved scrotal skin. HPE demonstrated tumor cells with moderate cytoplasm and small hyperchromatic nuclei with inconspicuous nucleoli arranged as singly scattered or nests with extensive extracellular mucin. Few signet ring cells were also seen. Diagnosis of mucinous adenocarcinoma of testis with signet ring cells, infiltration of scrotal skin, and positive spermatic cord margin was made (Fig. 2).
Fig. 2.
Histopathological examination. a Tumor cells arranged in nest. b Tumor cells showing gland formation. c Extracellular mucin. d Signet ring cell
Immunohistochemistry was positive for CK 20, CDX2, CK 7 (focal), pan CK, and SATB2 (few cells) and negative for SALL4, S-100, PAX8, and TTF-1. FDG PET-CT scan revealed uptake in the retroperitoneal nodes largest in the precaval region (32 × 19 mm) with SUV max of 1.46. Patient was then administered 4 cycles of chemotherapy with 5-fluorouracil and cisplatin. Response assessment FDG PET-CT revealed uptake in b/l inguinal nodes with SUV max of 2.01, precaval nodes 33.5 × 18 mm with SUV max of 2.22 (increased compared to previous scan), and interaortocaval node 7.5 mm × 13.5 mm with SUV max of 2.17 (Fig. 3).
Fig. 3.
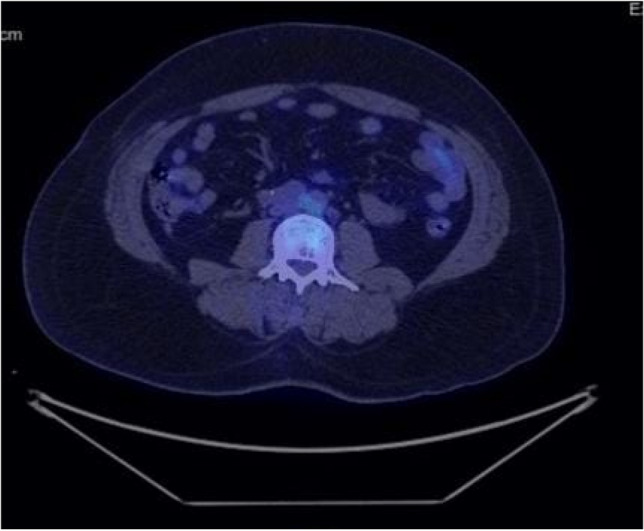
Fused PET-CT scan showing uptake in interaortocaval nodal mass
The case was reviewed again in tumor board and taken up for retroperitoneal, bilateral pelvic, and bilateral inguinal lymph node dissection with revision of spermatic cord. On HPE, 9/11 retroperitoneal nodes, 1/4 right pelvic nodes, 0/6 left pelvic nodes, 2/8 right inguinal nodes, and 4/7 left inguinal nodes showed metastatic tumor deposits. Four retroperitoneal, 1 right pelvic, 1 right inguinal, and 1 left inguinal nodes showed extranodal extension. Patient recovered uneventfully in post-op period and is on regular follow-up 6 months now since surgery.
Discussion
Testicular tumors are classified into germ cell, sex cord stromal, and ovarian type epithelial tumors by WHO. Epithelial testicular tumors constitute serous, mucinous, endometrioid, clear cell, transitional, and squamous subtypes [1]. These tumors are rare and have median age of onset of 60 years. The most common subtype is serous followed by mucinous. They have a spectrum of clinical behaviors ranging from benign to borderline to invasive subtype with potential for metastasis.
The clinical presentation of epithelial testicular tumors is usually testicular mass similar to germ cell tumors. Ultrasound examination of scrotum is the modality of choice to characterize testicular tumors while CT scan is used to screen for metastases. There are no specific clinical or imaging features specific to epithelial tumors and hence are often diagnosed after orchidectomy. Mucinous adenocarcinoma from colon, stomach, appendix, pancreas, and prostate can metastasize to testis. The possibility of such metastatic tumor should be ruled out before labeling the tumor as primary mucinous adenocarcinoma of testis. Histologically metastatic testicular mucinous adenocarcinoma has prominent vascular area involvement and tumor multifocality in testicular interstitium. The distinction can also be made by IHC analysis. Metastatic tumors from GI tract stain positively with CK, CEA, EMA, S-100, CA-125, and CA 19-9 whereas primary testicular mucinous adenocarcinoma stain with CK 7, MUC2, MUC5AC, and MUC6. They can be positive for CK7 and CK20 or positive to CK20 but negative to CK7 [2].
The origin of epithelial testicular tumors is debatable. The existing hypotheses are that these tumors may arise from mullerian remnants within testicular tissue or that they arise as a result of metaplasia of mesothelium of tunica vaginalis which corresponds to pelvic mesothelium in females [3].
There are no standard guidelines for the management of mucinous adenocarcinoma of testis as only 9 cases are reported in literature so far. The initial step in all cases is high inguinal orchidectomy as a part of diagnostic procedure. Few cases have been spared of chemotherapy as no metastases were detected [3–5]. In cases with nodal or distant metastases, various chemotherapy regimens were tried that include regimens for gastrointestinal cancers and ovarian cancers [2, 6, 7]. In our case, 5-fluorouracil with cisplatin was used since signet ring cells were present resembling GI tract epithelium. But since disease progressed patient underwent retroperitoneal, pelvic, and inguinal lymph nodal dissection as a part of curative intent treatment and is kept under follow-up.
Conclusion
The clinical features, imaging characteristics, and tumor markers lack specificity to differentiate testicular epithelial tumors from germ cell tumors. Testicular mucinous tumors are almost always diagnosed after orchidectomy. It is essential to rule out mucinous carcinoma of gastrointestinal tract metastasizing to testis before labeling as primary mucinous adenocarcinoma of testis. Surgery remains the mainstay of treatment in metastasis confined to retroperitoneal and inguinal lymph nodes. Further studies are needed to identify optimal chemotherapy regimen for metastatic and adjuvant scenarios.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Declarations
Ethical Approval
This is a case report and hence exempted from ethical approval by institutional ethical committee.
Informed Consent
Informed consent was obtained from the participant included in this study.
Consent for Publication
Informed consent was obtained from participant for publication of data in the journal.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moch H, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Tanriverdi O, Tarimer ML, Pak CD, Uylas S, Alkan A, Dere Y, Yazici A, Sen S, Sahin H. Management of a patient with primary mucinous testicular adenocarcinoma as a rare case with adjuvant and metastatic sequential treatments. J Oncol Pharm Pract. 2020;26(6):1520–1523. doi: 10.1177/1078155220903374. [DOI] [PubMed] [Google Scholar]
- 3.Iuga AC, Mull J, Batra R, Miller W. Mucinous cystadenocarcinoma of the testis: a case report. Hum Pathol. 2011;42(9):1343–1347. doi: 10.1016/j.humpath.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Di Franco C, Porru D, Viglio A, Paulli M, Rovereto B. Primary paratesticular mucinous “ovarian-type” adenocarcinoma: a rare case of scrotal tumor in a patient with history of bilateral cryptorchidism. World J Nephrol Urol. 2015;4(4):260–263. doi: 10.14740/wjnu235w. [DOI] [Google Scholar]
- 5.Lei H, Lai L, Xu H, Bai S, Shi M, Yang L (2020) Primary mucinous adenocarcinoma of the testis: a case report and review of the literature. 10.21203/rs.3.rs-67135/v1
- 6.Taga H, Hosokawa Y, Manabe T, Hagiwara N, Yamada T, Iwata T, Miyashita H. A case of primary testicular mucinous carcinoma. Hinyokika Kiyo Acta Urologica Japonica. 2019;65:87–91. doi: 10.14989/ActaUrolJap_65_3_87. [DOI] [PubMed] [Google Scholar]
- 7.Azuma T, Matayoshi Y, Nagase Y (2012, 685946) Primary mucinous adenocarcinoma of the testis. Case Rep Med 2012. 10.1155/2012/685946 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.