Abstract
Several groundbreaking clinical trials with the potential to transform the management paradigm of both locally advanced and persistent, recurrent, or metastatic cervical cancers have been presented in 2023. This review describes the reported data from INTERLACE and KEYNOTE-A18 in the locally advanced setting, as well as BEATcc, innovaTV 301 and DESTINY-PanTumor02 for advanced disease. The practice implications of their positive results are interpreted in the context of global health considerations, and updated treatment algorithms are proposed. Furthermore, emerging trends in drug development for cervical cancer are discussed. As the routine use of immune checkpoint inhibitors (ICIs) for curative and palliative indications increases in the foreseeable future, patients whose cervical cancers which persist, relapse or progress after prior ICI exposure will represent an area of unmet clinical need and form the key target population for next-generation trials. Future research will help shape oncologists’ approaches in the optimal selection, sequencing and re-treatment or rechallenge of immuno-oncology agents and/or antibody-drug conjugates in women with cervical cancer.
Keywords: Cervical Cancer, Chemoradiotherapy, Immune Checkpoint Inhibitors, Immunoconjugates, Adoptive Immunotherapy
Synopsis
This review highlights the practice-changing trials of cervical cancer systemic therapies in 2023. New standards of care in locally advanced and persistent, recurrent, or metastatic disease are presented. Lastly, we outline future research directions which can inform selection and sequencing of emerging therapeutics.
INTRODUCTION
Cervical cancer is the fourth most common cancer among females worldwide, contributing to substantial morbidity and mortality [1,2]. While breakthroughs in cervical cancer management have occurred over past decades, 2023 was annus mirabilis because of multiple reported impactful studies which alter the systemic treatment paradigms for locally advanced cervical cancer (LACC) and persistent, recurrent, or metastatic disease—henceforth referred to as advanced cervical cancer (aCC). As the standards of care shift ensuing from fresh evidence, clinicians treating patients with cervical cancer start to face unprecedented challenges in the sequencing and optimal choice of different therapeutic classes, whereas the perennial conundrum surrounding disparities in drug access deepens. This review summarizes and contextualizes the data presented in this momentous year. We further aim to identify and discuss future perspectives for research and unmet needs in cervical cancer pharmacotherapy.
LACC
It has been nearly a quarter-century ago when the US National Cancer Institute issued a clinical announcement for the last advancement in LACC, recommending concurrent cisplatin-based chemoradiation (CCRT) over radiotherapy (RT) alone, based on 5 pivotal randomized phase III trials [3,4,5,6,7,8]. The positive readouts from the long-awaited Gynecologic Cancer InterGroup (GCIG) INTERLACE and KEYNOTE-A18 (ENGOT-cx11/GOG-3047) trials [9,10] are poised to rejuvenate treatment standards for LACC.
INTERLACE was an open-label academic study which accrued 500 subjects with International Federation of Gynecology and Obstetrics (FIGO) 2009 stage IB2–IVA (except stage IIIA) and stage IB1 node-positive squamous cell carcinoma, adenocarcinoma and adenosquamous carcinoma of the cervix with nodal involvement up to the aortic bifurcation over a decade between November 2012 and November 2022 [9]. They were randomized 1:1 to receive induction dose dense paclitaxel 80 mg/m2 plus carboplatin area under the curve (AUC) 2 once weekly for 6 weeks followed by CCRT or standard CCRT alone. The UK and Mexico contributed to a vast majority of subjects at 76% and 20%, respectively. Owing to RT quality assurance across trial centers, high proportions of patients received protocol-mandated external beam RT and brachytherapy, and median overall RT duration was 45 days. RT techniques had improved over that decade [11,12,13] and 3-dimensional image-guided adaptive brachytherapy was encouraged within this trial.
After a median follow-up of 64 months, induction chemotherapy significantly improved progression-free survival (PFS) (73% vs. 64% at 5 years, hazard ratio [HR]=0.65; 95% confidence interval [CI]=0.46–0.91; p=0.013) and overall survival (OS) (80% vs. 72% at 5 years, HR=0.61; 95% CI=0.40–0.91; p=0.04) compared to CCRT alone. Distant relapses were reduced by 8% (12% vs. 20%) with induction chemotherapy, suggesting that priming chemotherapy eliminates micrometastases, thereby altering the natural history of LACC and enhancing survival. Despite a greater incidence of hematological adverse events (AEs), predominantly neutropenia (19% vs. 5%), which one would expect from additional doublet chemotherapy, completion rates of the induction and CCRT phases were high, and RT delivery was not compromised in the experimental arm. INTERLACE is the first and largest randomized study in LACC to demonstrate an OS advantage from induction chemotherapy before CCRT in the intention-to-treat population, regardless of response to induction chemotherapy [14,15,16].
Adopting a different approach, KEYNOTE-A18 evaluated the addition of the anti-programmed death-1 (anti-PD-1) immune checkpoint inhibitor (ICI), pembrolizumab, concurrently to conventional CCRT followed by maintenance pembrolizumab [10]. The trial enrolled 1,060 patients with FIGO 2014 stage IB2–IIB node-positive and stage III–IVA any nodal status LACC. In the experimental arm, pembrolizumab was administered at 200 mg every 3 weeks for 5 cycles concomitantly with chemoradiation then a further 15 cycles of 400 mg dosed at 6-week intervals. Median follow-up for the first interim analysis was 17.9 months. Compared to placebo, the pembrolizumab arm showed statistically significant improvement in a co-primary end point, PFS (67.8% vs. 57.3% at 24 months, HR=0.70; 95% CI=0.55–0.89; p=0.002). The other co-primary end point, OS, remained immature at this analysis although numerically in favor of the pembrolizumab arm. The safety profile of pembrolizumab plus CCRT was predictable and manageable; in particular, there was no significant excess of diarrhea and colitis AEs. The superior efficacy of combining pembrolizumab in a concurrent-adjuvant fashion to CCRT contrasts with the inability of the anti-programmed death-ligand 1 (anti-PD-L1), durvalumab, when investigated as such to meet the PFS primary end point in the CALLA trial [17]. Notwithstanding the inherent flaws of cross-trial comparison, differences between KEYNOTE-A18 and CALLA (Table 1) [18,19,20] might still contribute to their discrepant findings. The KEYNOTE-A18 cohort appeared to be of overall higher intrinsic risk than CALLA’s, as supported by a lower 24-month PFS rate in the placebo-controlled arm.
Table 1. Differences in study design, baseline characteristics and control arm PFS of patients between CALLA and KEYNOTE-A18 trials.
| Characteristic or result | CALLA [17] | KEYNOTE-A18 [10] | ||
|---|---|---|---|---|
| Number of randomized patients | 770 | 1,060 | ||
| Eligible population* | Stages IB2–IIB N+ or IIIA–IVA any node | Stages IB2–IIB N+ or III–IVA any node | ||
| Definition of N+ by CT/MR axial imaging | ≥1 LN ≥10 mm SAD | ≥2 LN ≥15 mm SAD | ||
| Immune checkpoint inhibitor | Anti–PD-L1, durvalumab | Anti–PD-1, pembrolizumab | ||
| Primary end point(s) | PFS | PFS and OS | ||
| Proportion of SCC (%) | 83.3 | 83.3 | ||
| Proportion of PD-L1 “positive”/missing (%) | 91.9 (SP263 TAP ≥1%)/4.5 | 94.3 (22C3 CPS ≥1)/0.9 | ||
| Proportion of stage III–IVA (%) | All: 65.6 | All: 56.4 | ||
| Durva arm: 64.9 | Placebo arm: 66.2 | Pembro arm: 55.6 | Placebo arm: 57.3 | |
| Proportion of N+ (%) | All: 74.0 | All: 83.3 | ||
| Durva arm: 72.5 | Placebo arm: 75.6 | Pembro arm: 84.1 | Placebo arm: 82.5 | |
| Proportion of PALN+ (%) | All: 11.0 | All: 22.0 | ||
| Durva arm: 12.2 | Placebo arm: 9.9 | Pembro arm: 22.4 | Placebo arm: 21.5 | |
| PFS of the placebo + CCRT arm | 62.1% at 24 months | 57.3% at 24 months | ||
CCRT, concurrent chemoradiotherapy; CT/MR, computed tomography/magnetic resonance; Durva, durvalumab; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; N+, node positive; OS, overall survival; PALN+, para-aortic lymph node positive; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; Pembro, pembrolizumab; PFS, progression-free survival; SAD, short axis dimension; SCC, squamous cell carcinoma; SP263 TAP, Tumor Area Positivity score by Ventana SP263 assay; 22C3 CPS, Combined Positive Score by Dako 22C3 assay.
*The FIGO 2014 staging system for cervical cancer [18] used in KEYNOTE-A18 is identical to the FIGO 2009 system [19] used in CALLA. These 2 trials had the discretion to define nodal positivity surgically or by radiological criteria. Nodal positivity in cervical cancer was first designated as stage III by FIGO in 2018 [20].
Juxtaposed against INTERLACE and KEYNOTE-A18 were the final publications of 2 other trials in 2023 which did not supplant CCRT as the standard of care for LACC. In OUTBACK, 4 cycles of adjuvant paclitaxel 155 mg/m2 plus carboplatin AUC 5 dosed 3-weekly did not improve 5-year OS compared to CCRT alone (72% vs. 71%, HR=0.90; 95% CI=0.70–1.17; p=0.81) [21]. This implies that sequencing of platinum-taxane chemotherapy after completion of CCRT is not beneficial to patients with LACC, as opposed to induction before CCRT. Conversely, neoadjuvant platinum-based chemotherapy before radical hysterectomy (RH) for FIGO 2009 stages IB2–IIB disease in the EORTC-55994 study was non-superior to CCRT [22], mirroring the results of the similar Tata Memorial Center study [23].
1. Evolving standards of care in LACC
In 2023, induction paclitaxel-carboplatin chemotherapy before CCRT and separately, concurrent-maintenance pembrolizumab plus CCRT, have emerged as twin—and possibly complementary—new treatment standards for LACC. The INTERLACE protocol is applicable to patients of FIGO 2018 stages IB3–IVA (except IIIA and those with nodal disease above the aortic bifurcation) whereas the eligibility of KEYNOTE-A18 trial approximates to FIGO 2018 stages IIIA–IVA.
Not only is INTERLACE more translatable to a broader segment of the LACC population but induction chemotherapy is also more widely available, affordable, and feasible in diverse healthcare systems around the world. The global burden of LACC lies disproportionately within low- and middle-income countries (LMIC) [1,2]. Prefacing radical RT with 6 weeks of active treatment may also serve to ameliorate waitlists for RT facilities in resource-constrained settings. Crucially, induction chemotherapy has already demonstrated a 39% relative risk reduction of all-cause mortality compared to the usual standard CCRT alone. Conversely, maturity of OS data in KEYNOTE-A18 and regulatory approval for pembrolizumab for this indication are both awaited. For these reasons, INTERLACE potentially has far-reaching impact to women with LACC worldwide and to a larger extent than KEYNOTE-A18.
Still, unanswered questions from the initial report of INTERLACE include:
• Are there any patient subgroups which do not benefit as much from induction chemotherapy?
• Are these results extrapolatable to patients who are ineligible for cisplatin (e.g. renal impairment)? Can we safely assume that patients who are intended for concurrent carboplatin-RT will benefit from induction paclitaxel-carboplatin too?
Some key opinion leaders have already advocated sequencing the INTERLACE followed by KN-A18 regimens to treat higher risk LACC. However, the clinical benefit of doing so is at best inferred from the presented data of the individual studies. It is unlikely that a prospective trial for such an intercalated strategy will be conducted although real-world evidence will accumulate over time. Moreover, the best way to sequence ICI with definitive RT for LACC, including as induction or priming before RT, is still under active investigation.
aCC
Moving to the aCC setting, KEYNOTE-826 recently established the combination of pembrolizumab with paclitaxel-platinum chemotherapy as the preferred frontline treatment because of positive dual primary end points of PFS and OS compared to the placebo-controlled arm [24]. Per protocol, pembrolizumab was dosed at 200 mg every 3 weeks for up to 35 doses, and use of bevacizumab was discretionary. The sustained OS benefit with extended follow-up in KEYNOTE-826 which was updated in 2023 reaffirms the primacy of a chemoimmunotherapy regimen [25]. However, the contemporaneously published BEATcc trial offers another viable alternative.
The open-label, randomized BEATcc (ENGOT-cx10/GEICO 68-C/JGOG1084/GOG-3030) trial compared the anti-PD-L1, atezolizumab, paclitaxel–platinum plus bevacizumab to chemotherapy–bevacizumab for first-line treatment of aCC [26]. The main differences in study design from KEYNOTE-826 are the lack of placebo control, use of atezolizumab (1,200 mg every 3 weeks) until progressive disease (PD) or unacceptable toxicity, mandatory bevacizumab with contraindications thereof precluding eligibility, and discontinuation of chemotherapy after at least 6 cycles only in cases of complete response. The addition of atezolizumab to the chemotherapy–bevacizumab backbone extended both PFS (median 13.7 vs. 10.4 months, HR=0.62; 95% CI=0.49–0.78; p<0.0001) and OS (median 32.1 vs. 22.8 months, HR=0.68; 95% CI=0.52–0.88; p=0.0046). The benefits in PFS and OS were generally consistent across all protocol-specified subgroups. No new safety signals from the quadruplet regimen were observed either. Thus, BEATcc conclusively proves synergism between ICI and anti-angiogenic therapy in previously untreated aCC, but also dispels the notion that may arise from CALLA that anti-PD-L1 antibodies are non-efficacious in cervical cancer. Exploratory biomarker analyses, including for PD-L1 status, are planned in BEATcc, and have yet to be reported.
As these 2 confirmatory trials reinforce ICIs’ position in frontline management of aCC, the search for more agents to the armamentarium in later lines has intensified. Cytotoxic monotherapies such as gemcitabine, vinorelbine, pemetrexed and topoisomerase I inhibitors remain valid options, but are of limited efficacy and lack evidence of conferring OS benefit over best supportive care in this population [27]. Antibody-drug conjugates (ADCs) have superseded cytotoxics as treatment of choice of aCC after previous platinum-based chemotherapy with or without bevacizumab and ICI.
Tissue factor is almost ubiquitously expressed in cervical cancer tissue. Tisotumab vedotin comprises a human monoclonal antibody which is directed against tissue factor covalently linked to the microtubule inhibitor, monomethyl auristatin E. Tisotumab vedotin earned accelerated approval from the US Food and Drug Administration in 2021 for the treatment of aCC with PD on or after chemotherapy, based on the single-arm phase II innovaTV 204 (ENGOT-cx6/GOG-3023) trial with 101 subjects. In innovaTV 204, tisotumab vedotin attained an objective response rate (ORR) of 24% and median duration of response (DOR) of 8.3 months [28]. The open-label phase III innovaTV301 (ENGOT-cx12/GOG-3057) was a confirmatory trial, randomizing patients 1:1 to tisotumab vedotin or physician’s choice chemotherapy (topotecan, vinorelbine, gemcitabine, irinotecan, pemetrexed) [29]. 502 patients with progressive recurrent/metastatic cervical cancer on or after doublet chemotherapy ± bevacizumab and anti-PD-(L)1 ICI, if eligible and available, and not exceeding 2 prior lines of treatment were recruited. Tissue factor expression was analyzed as part of the study but did not restrict trial enrollment. More than a quarter of patients on both arms had prior ICI, and a third of the study participants were Asians. At interim analysis after a median follow-up of 10.8 months, tisotumab vedotin was superior to chemotherapy in terms of OS (median 11.5 vs. 9.5 months, HR=0.70; 95% CI=0.54–0.89; p=0.0038), investigator-assessed PFS (median 4.2 vs. 2.9 months, HR=0.67; 95% CI=0.54–0.82; p≤0.0001) and ORR (17.8% vs. 5.2%, odds ratio=4.0; p<0.0001). AEs of special interest associated with tisotumab vedotin are ocular toxicities, peripheral neuropathy, and hemorrhage. Dose discontinuation due to ocular and peripheral neuropathy events occurred at a rate of 5.6% for each. To date, innovaTV301 is the second trial to demonstrate OS improvement over chemotherapy beyond first line in aCC since EMPOWER-Cervical 1 (ENGOT-cx9/GOG-3016) which investigated the anti-PD-1 inhibitor, cemiplimab, in ICI-naïve patients irrespective of PD-L1 status [30].
Another ADC with available data in 2023 for previously treated aCC is trastuzumab deruxtecan. This is a human epidermal growth factor 2 (HER2)-directed ADC with a potent topoisomerase I inhibitor payload. The single-arm, multi-cohort phase II DESTINY-PanTumor02 basket trial evaluated trastuzumab deruxtecan (5.4 mg/kg once every 3 weeks) for HER2-expressing (immunohistochemistry [IHC] 3+/2+ as per ASCO/CAP gastric cancer scoring) advanced cancers after at least 1 line of systemic treatment or without standard options [31]. Within the cohort of 40 aCC subjects, 5 patients with IHC 1+ status were included after a protocol-specified interim analysis. The overall investigator-assessed ORR was 50% and median DOR was 14.2 months. By central testing, 8 and 20 patients had HER2 IHC 3+ and 2+ tumors, respectively. Their corresponding ORR were 75% and 40%; median DOR not reached and 3.8 months. Patients with retrospective centrally determined IHC 1+/0 tumors were few in numbers, but they showed encouraging anti-tumor activity signals too.
DESTINY-PanTumor01 was a similar phase II trial also testing trastuzumab deruxtecan but for solid tumors harboring activating HER2 mutations. Out of 3 aCC patients enrolled, 2 (66.7%) had objective responses according to independent central review [32].
1. Evolving standards of care in aCC
It is reasonable to conclude from KEYNOTE-826 and BEATcc trials that the latest first-line standard of care in ICI-naïve patients should be:
• Paclitaxel-platinum + bevacizumab combined with pembrolizumab or atezolizumab in patients who are candidates for bevacizumab, or
• Platinum doublet chemotherapy + pembrolizumab in those have contraindications to bevacizumab.
The current treatment sequencing after the first line is less clear. We suggest that for patients who have not received ICI before, cemiplimab regardless of PD-L1 status [30] or pembrolizumab in tumors of PD-L1 combined positive score ≥1 [33] should be used earlier to avoid the emergence of immune exhaustion, taking note of the OS advantage which second-third line cemiplimab has over chemotherapy, and reserving ADCs for later use. Otherwise, in ICI-exposed patients, tisotumab vedotin is preferred before trastuzumab deruxtecan because the former has level I evidence of OS benefit, and virtually all patients are eligible when biomarker selection is not as critical. HER2 expression level is subject to interobserver variability as seen in DESTINY-PanTumor02 [31], and its predictive role in aCC is not well understood. Still, the US NCCN guidelines recommend that trastuzumab deruxtecan be reserved for patients whose tumors are HER2 IHC 3+/2+ [34] as per the trial’s original eligibility criteria.
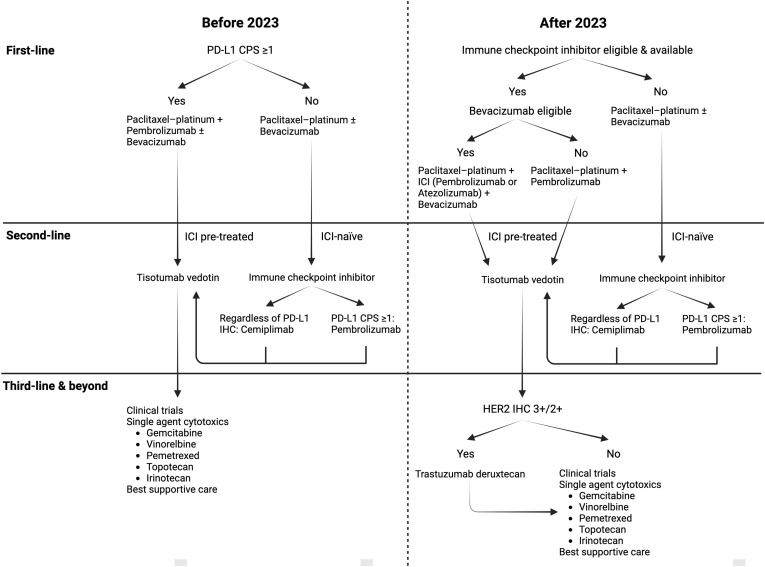
Fig. 1 shows our proposed changes to the treatment algorithms after 2023 for aCC patients who are not amenable to curative-intent surgery or RT.
Fig. 1. Proposed changes to treatment algorithms for advanced cervical cancer after 2023.
Changes are necessitated by the introduction of new treatment options in first line (atezolizumab) and late-line (trastuzumab deruxtecan). Furthermore, results of the confirmatory phase III trial for second- to third-line tisotumab vedotin versus chemotherapy were announced in 2023.
CPS, combined positive score; HER2, human epidermal growth factor 2; ICI, immune checkpoint inhibitor; IHC, immunohistochemistry; PD-L1, programmed death-ligand 1.
Practical considerations influencing the individual patient’s access to the above drugs relate to drug regulatory approval, availability, affordability, and reimbursement/funding which vary across territories. For instance, second- or later-line cemiplimab is approved in Europe and Japan but not in the US. Again, patients in LMIC are disadvantaged by their diminished access to ICIs and ADCs outside of clinical trials than those in developed economies.
FUTURE DIRECTIONS
After 2023, the adoption of induction paclitaxel-carboplatin and/or pembrolizumab among patients with LACC is anticipated to increase over time. Those whose tumors are resistant or refractory to these agents will soon represent an area of unmet clinical need. While GOG-240 and phase II studies showed that patients with persistent or recurrent tumors after concurrent cisplatin-RT retain sensitivity towards platinum (when combined with paclitaxel ± bevacizumab) [35,36,37], post-INTERLACE patients cannot be assumed to respond favorably to paclitaxel–carboplatin re-treatment in the palliative setting, except probably those with long treatment-free intervals. In this post-INTERLACE/KEYNOTE-A18 paradigm, trials evaluating ADCs, passive immunotherapies (including anti-PD-L1 inhibitors and bispecific antibodies) or active immunotherapies (e.g. adoptive cell therapy with tumor infiltrating lymphocytes [TILs]) as the initial salvage therapy will be important in informing future practice. These modalities, either individually or in combinations, are predicted to form the mainstay of pre-treated aCC treatment before sequential cytotoxic monotherapies.
Looking forward, research hypotheses which will become clinically relevant soon are whether tumors which persist, relapse or progress on or after curative-intent pembrolizumab plus CCRT will respond to treatments incorporating atezolizumab and/or ADCs such as tisotumab vedotin or trastuzumab deruxtecan with or without bevacizumab. Likewise, will the patients with aCC whose tumors progress on or after prior anti-PD-1 ICI benefit from PD-L1 inhibition in a subsequent line, or vice versa? To our knowledge, there is no proof-of-concept prospective trial for anti-PD(L)1 ICI re-treatment or rechallenge upon relapse or PD, respectively, in solid tumor oncology although retrospective experiences for other cancer types suggest feasibility of this approach, with greater clinical benefit in patients who derived disease control from a preceding course of ICI and less so in those who received intervening treatment(s) between 2 ICI courses [38,39,40]. These are questions which are best clarified by well-designed clinical trials, ideally with preplanned biospecimen collection for translational research.
Even though most trials in aCC have so far followed a “one drug, one trial” model, cooperative groups may work on creating adaptive platform trials to test multiple agents in the post-ICI setting. Traditionally, drug development in cervical cancer has concentrated on the discovery and evaluation of new drug classes with distinct mechanisms of action from existing standard therapies. This framework is likely to endure longer but a better understanding of the resistance mechanisms of emerging agents can potentially lead to innovative ways to prevent or overcome drug resistance.
In the aCC setting, ICIs have been or are being evaluated together with another ICI, TILs, cancer vaccine or multi-targeted tyrosine kinase inhibitors, or as bispecific antibodies or bifunctional fusion proteins, with varying degrees of success reported of completed studies (Table 2). Almost exclusively, these studies select for ICI-naïve patients. On the other hand, the efficacy signal from cohort 2 of the phase II C-145-04 will be of immense interest. In this cohort, patients previously treated with an anti-PD-(L)1 ICI undergo nonmyeloablative lymphodepletion and are then infused with their autologous TILs (lifileucel) followed by interleukin-2 administration.
Table 2. Selected phase I–III clinical trials of immune checkpoint inhibitor combinations in advanced cervical cancer.
| Trial registration number | Trial name | Phase | Line of therapy | Drugs under investigation | Trial status | Key findings of reported trials† | |
|---|---|---|---|---|---|---|---|
| Anti-PD-1 + anti-CTLA-4 | |||||||
| NCT02488759 | CheckMate 358 | I/II | 2–3 | Nivo ± Ipi | Completed | Nivo: ORR 26%, mDOR N.R. | |
| Nivo 3 + Ipi 1: ORR 31%, mDOR 24.4 mo | |||||||
| Nivo 1 + Ipi 3: ORR 38%, mDOR 34.1 mo [S1] | |||||||
| NCT03495882 | - | I/II | 2 | Bal + Zal | Completed | ORR 25.6%, mDOR N.R. [S2] | |
| NCT03894215 | RaPiDS/GOG-3028 | II | 2+ | Bal ± Zal | Active, not recruiting | - | |
| NCT03852251 | COMPASSION-03 | Ib/II | 2+ | Cadonilimab* | Active, not recruiting | ORR 32.3%, mDOR N.R. [S3] | |
| NCT04982237 | Study 303 | III | 1 | Cadonilimab* + Paclitaxel-platinum ± Bev | Recruiting | - | |
| Anti-PD-(L)1 + anti-TIGIT | |||||||
| NCT02964013 | KEYVIBE-001 | I | 2+ | Pembro + Vibo | Active, not recruiting | Pembro + Vibo 200: ORR 15%, mDOR N.R. | |
| Pembro + Vibo 700: ORR 23%, mDOR N.R. [S4] | |||||||
| NCT04693234 | AdvanTIG-202 | II | 2+ | Tis ± Oci | Completed | Tis + Oci: ORR 22.5%, mDOR 17.3 mo | |
| Tis: ORR 32.5% [S5] | |||||||
| NCT04300647 | SKYSCRAPER-04 | II | 2–3 | Atezo ± Tira | Active, not recruiting | Atezo + Tira: ORR 19.0%, mPFS 2.8 mo | |
| Atezo: ORR 15.6%, mPFS 1.9 mo [S6] | |||||||
| Bifunctional anti-PD-L1/TGF-βRII agents | |||||||
| NCT02517398 | INTR@PID 001 | I | 2+ | Bintrafusp alfa | Completed | ORR 28.2%, mDOR 11.7 mo [S7] | |
| NCT03427411 | INTR@PID 012 | II | 2+ | Bintrafusp alfa | Completed | ||
| NCT04246489 | INTR@PID 017 | II | 2+ | Bintrafusp alfa | Completed | - | |
| NCT04551950 | INTR@PID 046 | I | 1 | Bintrafusp alfa + Paclitaxel-platinum (cohort 1A) | Completed | Cohort 1A: grade ≥3 AEs 50.0% | |
| Bintrafusp alfa + Paclitaxel-platinum + Bev (cohort 1B) | Cohort 1B: grade ≥3 AEs 44.4% [S8] | ||||||
| NCT03774979 | Study I-102 | I | 2+ | Retlirafusp alfa | Active, not recruiting | ORR 15.6%, mDOR N.R. [S9] | |
| NCT05179239 | Study III-309 | III | 1 | Retlirafusp alfa + Paclitaxel–platinum ± Bev biosimilar | Recruiting | - | |
| Anti-PD-1 + TILs | |||||||
| NCT03108495 | C-145-04 | II | 1 | Pembro + Lifileucel (cohort 3) | Recruiting | Cohort 3: ORR 57.1% [S10] | |
| NCT05342506 | XHKT-SCT006-2022 | II | 2+ | Toripalimab + genetically modified TILs | Not yet recruiting | - | |
| Anti-PD-L1 + cancer vaccine | |||||||
| NCT04800978 | ESR-18-14325 | II | 2 | Durvalumab + BVAC-C | Not yet recruiting | - | |
| Anti-PD-1 + multi-TKI | |||||||
| NCT04680988 | Study II-217 | II | 2+ | Camre ± Fam | Active, not recruiting | Camre + Fam: ORR 41.0%, mDOR 16.0 mo | |
| Camre: ORR 24.1%, mDOR N.R. [S11] | |||||||
| NCT03816553 | CLAP | II | 2+ | Camre + Apatinib | Completed | ORR 55.6%, mDOR N.R. [S12] | |
| - | - | II | 2+ | Sintilimab + Anlotinib | - | ORR 54.8%, mPFS 9.4 mo [S13] | |
| - | - | II | 2+ | Tis + Anlotinib | - | ORR 35.3%, mPFS N.R. [S14] | |
| NCT04337463 | HX-001 | I/II | 2+ | Toriparlimab + Onatasertib | Unknown | - | |
| NCT04865887 | Study 3849 | II | 2+ | Pembro + Lenvatinib | Recruiting | - | |
| ChiCTR2200062897 | - | II | 1 | Penpulimab + Anlotinib + Paclitaxel-platinum 2 cycles | Completed | ORR 85.7%, mPFS N.R. [S15] | |
2+, second- or later-line; AEs, adverse events; Atezo, atezolizumab; Bal, balstilimab; Bev, bevacizumab; Camre, camrelizumab; CTLA-4, cytotoxic T-lymphocyte associated protein 4; Fam, famitinib; Ipi 1, ipilimumab 1 mg/kg every 6 weeks; Ipi 3, ipilimumab 3 mg/kg every 3 weeks; mDOR, median duration of response; mPFS, median progression-free survival; multi-TKI, multi-targeted tyrosine kinase inhibitor; Nivo (1/3), nivolumab (dose in mg/kg every 2 weeks); N.R., not reached; Oci, ociperlimab; ORR, objective response rate; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; Pembro, pembrolizumab; TGF-βRII, transforming growth factor beta receptor II; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TILs, tumor infiltrating lymphocytes; Tira, tiragolumab; Tis, tislelizumab; Vibo, vibostolimab; Zal, zalifrelimab.
*Cadonilimab is an anti-PD-1/CTLA-4 bispecific antibody.
†Reported trials are listed in Supplementary References.
As alluded earlier in this paper, studies of ICIs in the curative space are focused on the sequencing question: as neoadjuvant or induction strategies before radical surgery or CCRT respectively; concurrently with CCRT; maintenance after priming or concomitant administration with CCRT; adjuvant after confirmed response to CCRT or even after RH (Fig. 2) [41].
Fig. 2. The sequencing of immune checkpoint inhibitors with relation to concurrent chemoradiation or RH in clinical trials of locally advanced cervical cancers.
A number of Phase I–III trials investigate anti-PD-(L)1 alone or together with anti-CTLA-4 ICIs timed before, during and/or after CCRT. Postulated mechanisms of action of ICIs and synergism with CCRT are presented in the topmost panel. Separately, the single-arm phase II NACI trial [41] explored the neoadjuvant application of an anti-PD-1 (camrelizumab) with carboplatin; responders were considered for radical surgery. To our knowledge, no trial has been designed yet for adjuvant ICI therapy after definitive surgery for LACC.
CCRT, concurrent chemoradiation; ChT, cytotoxic chemotherapy; CR, complete remission; ICI, immune checkpoint inhibitor; CTLA-4, cytotoxic T-lymphocyte associated protein 4; LACC, locally advanced cervical cancers; PD, progressive disease; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PR, partial response; RH, radical hysterectomy; RT, radiation therapy; SD, stable disease; TME, tumor microenvironment.
*Bars are not drawn to scale according to the duration of per-protocol ICI therapy but are intended to illustrate the sequencing of ICI vis-à-vis CCRT. Studies which investigated ICI in the same phase are grouped together.
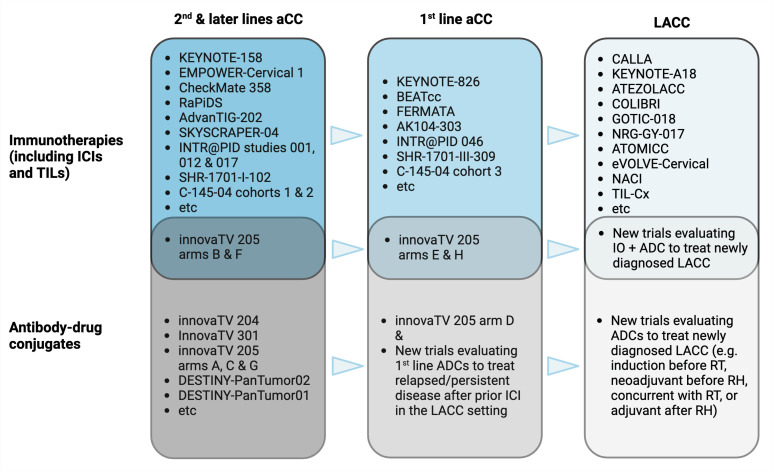
The clinical development of ADCs in cervical cancer is beginning to parallel that of ICIs: transiting from second- or later lines to the first line in advanced disease, then into the LACC setting. Combinatorial trials of both drug classes are underway and may be planned across the different settings of cervical cancer (Fig. 3) [42]. One such example in the first line for aCC is the ongoing arm H of the phase Ib/II innovaTV 205 (ENGOT-cx8/GOG-3024) trial wherein tisotumab vedotin-pembrolizumab combination is given in conjunction with carboplatin ± bevacizumab. The safety signal from this cohort will inform whether a larger randomized trial of the same or similar component drugs in patients previously untreated for recurrent/metastatic disease should proceed. In LACC, ADCs may also potentially be tested as neoadjuvant or induction strategies, concomitantly with RT, or adjuvant treatment after RH.
Fig. 3. Prospective evolution of the clinical development of immunotherapies and antibody-drug conjugates in cervical cancer.
After successes in later lines in aCC, immunotherapies, mainly ICIs, have moved forward to the first line and for curative indications. Autologous TILs, a type of adoptive T cell therapy, has also made headway from metastatic disease into the LACC setting [42]. ADCs are likely to follow this path of drug development. There is potential for combination trials across the disease spectrum as ICI resistance emerges with increasing routine clinical use for aCC and soon, LACC.
aCC, advanced cervical cancers; ADCs, antibody-drug conjugates; ICI, immune checkpoint inhibitor; IO, immuno-oncology agents; LACC, locally advanced cervical cancers; RH, radical hysterectomy; RT, radiation therapy; TILs, tumor infiltrating lymphocytes.
It must be added that there are several other investigational ADCs or immunoconjugates for aCC which target the following tumor-associated antigens:
• Tissue factor – XB002 (ClinicalTrials.gov identifier NCT04925284);
• HER2 – A166 (NCT03602079) and a first-in-human immune-stimulating antibody conjugate, BDC-1001 (NCT04278144);
• Trophoblast cell surface antigen 2 (TROP2) – sacituzumab govitecan (NCT01631552, NCT05838521), LCB84 (NCT05941507), and MK-2870 (NCT06049212);
• B7-H4 – HS-20093 (NCT06112704); and
• AXL – enapotamab vedotin (NCT02988817).
Lastly, unmet clinical needs still exist despite recent therapeutic advances. Patients with rare or uncommon aggressive cervical cancer histological subtypes, namely neuroendocrine carcinoma, carcinosarcoma and gastric type endocervical adenocarcinoma, as well as those living with human immunodeficiency virus are typically excluded from or underrepresented in large randomized clinical trials, including those reviewed in this paper. To fill these gaps in knowledge, the gynecologic oncology community must rely on case series and registry studies for retrospective review of the effectiveness of novel therapeutics for such patient subpopulations. The Neuroendocrine Cervical Tumor Registry (NeCTuR), which has generated valuable insights into the management of high-grade neuroendocrine cervical cancers [43,44], originated from a single institution but has expanded to an international catchment with voluntary participation [45]. Efforts to build and maintain trans-geographical tumor registries with prospective data collection for rare tumors and underserved patient groups should be encouraged and can complement the activities of GCIG’s Cervical Cancer Research Network to promote research and trials in LMIC.
CONCLUSION
The year 2023 has undisputedly been a historic year for cervical cancer considering the multiple positive studies presented, notably with OS gains over the standard-of-care control arms in INTERLACE, BEATcc, and innovaTV 301. innovaTV 301 is commendable on 2 accounts: for rapidly adapting to the advent of ICI in first and second lines in its eligibility criteria, and for being a bona fide global study by recruiting more than half of its participants from outside of Europe and the US. innovaTV 301 provides an exemplar of inclusivity and diversity for future clinical trials in cervical cancer—and gynecological cancers at large—to emulate, yielding results which can be applicable to more patients beyond the Western world.
With new data changing the treatment landscape of cervical cancer, it is timely that GCIG is holding its inaugural Cervical Cancer Consensus Conference to discuss these in October 2024. By then, updated survival data from KEYNOTE-A18 and BEATcc may have been reported. GCIG consensus meetings aim to harmonize the design including reference arms of upcoming trials, and to identify research opportunities and unmet needs [46]. Consensus is expected to be reached readily on the reference arms of future trials such as induction chemotherapy followed by CCRT in LACC, and first-line anti-PD(L)1 ICI and taxane-platinum ± bevacizumab for de novo metastatic cervical cancer. The reference arm of choice for trials focusing on ICI-treated patients with persistent/recurrent tumors may be more controversial because further evidence is awaited from ongoing studies.
Certainly, quality research will better inform clinicians of the optimal therapeutic selection, sequencing and re-treatment or rechallenge of immuno-oncology agents and/or ADCs to improve outcomes for women with cervical cancer.
ACKNOWLEDGEMENTS
Figures 1, 2 and 3 were created with BioRender.com.
Footnotes
Conflict of Interest: A.D.J.M. has no conflict of interest to declare.
C.J.J. received honoraria from Merck Sharp & Dohme, GlaxoSmithKline and AstraZeneca; research funding from Bristol-Myers Squibb (institution); travel, accommodations, expenses from AstraZeneca, Merck KGaA and Roche; and held a consulting or advisory role in GlaxoSmithKline, Merck Sharp & Dohme and AstraZeneca.
- Conceptualization: C.J.J.
- Project administration: C.J.J.
- Supervision: C.J.J.
- Writing - original draft: A.D.J.M., C.J.J.
- Writing - review & editing: A.D.J.M., C.J.J.
SUPPLEMENTARY MATERIAL
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023;11:e197–e206. doi: 10.1016/S2214-109X(22)00501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCI Press Office. NCI issues clinical announcement on cervical cancer: chemotherapy plus radiation improves survival [Internet] Bethesda, MD: National Cancer Institute; 1999. [cited 2023 Dec 13]. Available from: http://www.nih.gov/news/pr/feb99/nci-22.htm. [Google Scholar]
- 4.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 6.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 7.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 8.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 9.McCormack M, Gallardo Rincón D, Eminowicz G, Diez P, Farrelly L, Kent C, et al. LBA8 - A randomised phase III trial of induction chemotherapy followed by chemoradiation compared with chemoradiation alone in locally advanced cervical cancer: the GCIG INTERLACE trial. Ann Oncol. 2023;34:S1276. [Google Scholar]
- 10.Lorusso D, Xiang Y, Hasegawa K, Scambia G, Leiva Galves MH, Ramos Elias P, et al. LBA38 - Pembrolizumab plus chemoradiotherapy for high-risk locally advanced cervical cancer: a randomized, double-blind, phase III ENGOT-cx11/GOG-3047/KEYNOTE-A18 study. Ann Oncol. 2023;34:S1279–S1280. [Google Scholar]
- 11.Lin Y, Chen K, Lu Z, Zhao L, Tao Y, Ouyang Y, et al. Intensity-modulated radiation therapy for definitive treatment of cervical cancer: a meta-analysis. Radiat Oncol. 2018;13:177. doi: 10.1186/s13014-018-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pötter R, Tanderup K, Schmid MP, Jürgenliemk-Schulz I, Haie-Meder C, Fokdal LU, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021;22:538–547. doi: 10.1016/S1470-2045(20)30753-1. [DOI] [PubMed] [Google Scholar]
- 13.Sagae S, Toita T, Matsuura M, Saito M, Matsuda T, Sato N, et al. Improvement in radiation techniques for locally advanced cervical cancer during the last two decades. Int J Gynecol Cancer. 2023;33:1295–1303. doi: 10.1136/ijgc-2022-004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Costa, SCS, Bonadio RC, Gabrielli FCG, Aranha AS, Dias Genta, MLN, Miranda VC, et al. Neoadjuvant chemotherapy with cisplatin and gemcitabine followed by chemoradiation versus chemoradiation for locally advanced cervical cancer: a randomized phase II trial. J Clin Oncol. 2019;37:3124–3131. doi: 10.1200/JCO.19.00674. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi A, Rawat S. Comparative study of neoadjuvant chemotherapy followed by definitive chemoradiotherapy versus definitive chemoradiotherapy alone in locally advanced carcinoma of cervix. J Obstet Gynaecol India. 2019;69:546–552. doi: 10.1007/s13224-019-01236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Li Y, Wang H, Shen L, Wang Q, Shao S, et al. Neoadjuvant chemotherapy with weekly cisplatin and paclitaxel followed by chemoradiation for locally advanced cervical cancer. BMC Cancer. 2023;23:51. doi: 10.1186/s12885-023-10517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monk BJ, Toita T, Wu X, Vázquez Limón JC, Tarnawski R, Mandai M, et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1334–1348. doi: 10.1016/S1470-2045(23)00479-5. [DOI] [PubMed] [Google Scholar]
- 18.FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125:97–98. doi: 10.1016/j.ijgo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145:129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- 21.Mileshkin LR, Moore KN, Barnes EH, Gebski V, Narayan K, King MT, et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24:468–482. doi: 10.1016/S1470-2045(23)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenter GG, Greggi S, Vergote I, Katsaros D, Kobierski J, van Doorn H, et al. Randomized phase III study comparing neoadjuvant chemotherapy followed by surgery versus chemoradiation in stage IB2-IIB cervical cancer: EORTC-55994. J Clin Oncol. 2023;41:5035–5043. doi: 10.1200/JCO.22.02852. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36:1548–1555. doi: 10.1200/JCO.2017.75.9985. [DOI] [PubMed] [Google Scholar]
- 24.Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 25.Monk BJ, Colombo N, Tewari KS, Dubot C, Caceres MV, Hasegawa K, et al. First-line pembrolizumab + chemotherapy versus placebo + chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. J Clin Oncol. 2023;41:5505–5511. doi: 10.1200/JCO.23.00914. [DOI] [PubMed] [Google Scholar]
- 26.Oaknin A, Gladieff L, Martínez-García J, Villacampa G, Takekuma M, De Giorgi U, et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial. Lancet. 2024;403:31–43. doi: 10.1016/S0140-6736(23)02405-4. [DOI] [PubMed] [Google Scholar]
- 27.Valdivia A, Grau-Béjar JF, García-Durán C, Oaknin A. Treatment strategies in cervical cancer: treatment of advanced disease. J Cancer Metastasis Treat. 2022;8:35. [Google Scholar]
- 28.Coleman RL, Lorusso D, Gennigens C, González-Martín A, Randall L, Cibula D, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:609–619. doi: 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]
- 29.Vergote IB, González-Martín A, Fujiwara K, Kalbacher E, Bagameri A, Ghamande S, et al. LBA9 - innovaTV 301/ENGOT-cx12/GOG-3057: a global, randomized, open-label, phase III study of tisotumab vedotin vs investigator’s choice of chemotherapy in 2L or 3L recurrent or metastatic cervical cancer. Ann Oncol. 2023;34:S1276–S1277. [Google Scholar]
- 30.Tewari KS, Monk BJ, Vergote I, Miller A, de Melo AC, Kim HS, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med. 2022;386:544–555. doi: 10.1056/NEJMoa2112187. [DOI] [PubMed] [Google Scholar]
- 31.Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024;42:47–58. doi: 10.1200/JCO.23.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li BT, Meric-Bernstam F, Bardia A, Naito Y, Siena S, Aftimos PG, et al. 654O Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with solid tumors harboring specific HER2-activating mutations (HER2m): primary results from the international phase II DESTINY-PanTumor01 (DPT-01) study. Ann Oncol. 2023;34:S459–S460. [Google Scholar]
- 33.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Cervical Cancer. Version: 1.2024 [Internet] Plymouth Meeting, PA: National Comprehensive Cancer Network; 2023. [cited 2023 Dec 13]. Available from: https://www.nccn.org/guidelines/ [Google Scholar]
- 35.Tewari KS, Sill MW, Birrer MJ, Penson RT, Huang H, Moore DH, et al. Final survival analysis of topotecan and paclitaxel for first-line treatment of advanced cervical cancer: an NRG oncology randomized study. Gynecol Oncol. 2023;171:141–150. doi: 10.1016/j.ygyno.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redondo A, Colombo N, McCormack M, Dreosti L, Nogueira-Rodrigues A, Scambia G, et al. Primary results from CECILIA, a global single-arm phase II study evaluating bevacizumab, carboplatin and paclitaxel for advanced cervical cancer. Gynecol Oncol. 2020;159:142–149. doi: 10.1016/j.ygyno.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Tanigawa T, Takeshima N, Ishikawa H, Nishio S, Usami T, Yamawaki T, et al. Paclitaxel-carboplatin and bevacizumab combination with maintenance bevacizumab therapy for metastatic, recurrent, and persistent uterine cervical cancer: an open-label multicenter phase II trial (JGOG1079) Gynecol Oncol. 2022;165:413–419. doi: 10.1016/j.ygyno.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Plazy C, Hannani D, Gobbini E. Immune checkpoint inhibitor rechallenge and resumption: a systematic review. Curr Oncol Rep. 2022;24:1095–1106. doi: 10.1007/s11912-022-01241-z. [DOI] [PubMed] [Google Scholar]
- 39.Perdyan A, Sobocki BK, Balihodzic A, Dąbrowska A, Kacperczyk J, Rutkowski J. The effectiveness of cancer immune checkpoint inhibitor retreatment and rechallenge – a systematic review. Cancers (Basel) 2023;15:3490. doi: 10.3390/cancers15133490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitagawa S, Hakozaki T, Kitadai R, Hosomi Y. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: case series and literature review. Thorac Cancer. 2020;11:1927–1933. doi: 10.1111/1759-7714.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li K, Chen J, Hu Y, Wang YZ, Shen Y, Chen G, et al. Neoadjuvant chemotherapy plus camrelizumab for locally advanced cervical cancer (NACI study): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2024;25:76–85. doi: 10.1016/S1470-2045(23)00531-4. [DOI] [PubMed] [Google Scholar]
- 42.Huang H, Nie CP, Liu XF, Song B, Yue JH, Xu JX, et al. Phase I study of adjuvant immunotherapy with autologous tumor-infiltrating lymphocytes in locally advanced cervical cancer. J Clin Invest. 2022;132:e157726. doi: 10.1172/JCI157726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frumovitz M, Chisholm GB, Jhingran A, Ramalingam P, Flores-Legarreta A, Bhosale P, et al. Combination therapy with topotecan, paclitaxel, and bevacizumab improves progression-free survival in patients with recurrent high-grade neuroendocrine cervical cancer: a Neuroendocrine Cervical Tumor Registry (NeCTuR) study. Am J Obstet Gynecol. 2023;228:445.e1–445.e8. doi: 10.1016/j.ajog.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Flores Legarreta A, Salvo G, Gonzales NR, Chisholm G, Hillman RT, Frumovitz M. RB1 alteration and poor prognosis in women with high-grade neuroendocrine carcinoma of the uterine cervix: a NeCTuR study. J Gynecol Oncol. 2023;34:e50. doi: 10.3802/jgo.2023.34.e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuroendocrine Cervical Tumor Registry. About the registry [Internet] Houston, TX: MD Anderson Cancer Center Gyncologic Oncology & Reproductive Medicine NECTUR study; 2023. [cited 2023 Dec 13]. Available from: https://necervix.com/registry/ [Google Scholar]
- 46.Vergote I, González-Martín A, Lorusso D, Gourley C, Mirza MR, Kurtz JE, et al. Clinical research in ovarian cancer: consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 2022;23:e374–e384. doi: 10.1016/S1470-2045(22)00139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.