Abstract
Purpose
This study aimed to explore the existing literature on frailty experienced by patients with prostate cancer (PC) receiving androgen deprivation therapy (ADT).
Materials and Methods
Database and manual searches were conducted to identify relevant studies published in English, with no limitation on the year of publication, according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews guidelines. Four databases—PubMed, Cochrane Library, EMBASE, and CINAHL—were used for database searches and reference lists, related journals, and Google Scholar were used for manual searches.
Results
A total of 12 studies were analyzed for this scoping review. Of these, only 2 were intervention studies, and 1 was a randomized controlled trial. Among the two intervention studies, the multidisciplinary intervention program, including psychological counseling, nutritional coaching, and supervised group physical exercise did not show significant improvement in frailty. In contrast, high-dose vitamin D supplementation significantly decreased frailty. The conceptual and operational definitions of frailty used in each study varied, and the most used one was mainly focused on physical functions. As a result of analyzing the other health-related variables associated with frailty in patients with PC receiving ADT, age, metastases, comorbidities, and incident falls were related to a high frailty level. As for the physiological index, high levels of C-reactive protein, and interleukin-6, and fibrinogen, low levels of total testosterone, lymphocyte count, and creatinine were associated with a high level of frailty. A few studies explored the relationship between psychological and cognitive variables and frailty.
Conclusions
Further research related to frailty in patients with PC receiving ADT should be conducted, and effective interventions to manage frailty should be developed. Additionally, research that considers not only the physical domain of frailty but also the psychological, cognitive, and social domains needs to be conducted.
Keywords: Androgens, Frailty, Hormone replacement therapy, Prostatic neoplasms
INTRODUCTION
Frailty is a complicated condition that affects an individual who experiences one or more geriatric syndromes that influence the dysfunction of several organ systems [1,2]. Frailty is defined as the aging of physical, psychological, and social human function domains that is caused by many variables that influence these domains and increase the risk of negative outcomes [3]. Others define it as a biological syndrome in which resistance to stressors is reduced due to the accumulated degradation of several physiological systems [4,5]. Although there are several definitions of frailty, it is commonly referred to as an increased vulnerability to negative outcomes because of declined function in the physiological system [2].
Research findings have suggested that frailty is related to negative outcomes such as falls, low quality of life (QOL), hospitalization, physical dysfunctions, and death in elderly patients [3,4]. Frailty is strongly associated with sarcopenia which is related to the progressive decrease in muscle mass and is caused by aging [1]. Sarcopenia increases the risk of falls and can cause life-threatening injuries. In particular, fractures, traumatic brain injuries, and organ damage caused by falls are associated with hospitalizations, disability, and death [1,6,7]. Additionally, frailty leads to a decline in physical functions associated with decreased walking speed, low physical activity, and grip strength which in turn increases dependence on activities of daily living [8,9].
Prostate cancer (PC) is a prevalent cancer type among men over the age of 65 and the prevalence is expected to increase [10]. In the United States (US), 248,530 men were diagnosed with PC in 2021, accounting for 26% of all cancer types and the highest incidence among men [11]. Additionally, the 5-year survival rate for patients with local or regional PC is nearly 100%, and for those with metastatic PC, it is 31% in the US [12]. As of 2019, 108,870 men in South Korea were diagnosed with PC, accounting for 11.5% of all cancer types, with the third highest prevalence. The incidence of PC has also increased steadily over the past 20 years, and the annual percentage change significantly increased to 6.7% from 2015 to 2019. The 5-year relative survival rate from 2015 to 2019 was 94.4%, showing the highest survival rate after thyroid cancer [13]. This trend is expected to continue owing to the westernization of lifestyles and population aging. Moreover, with the increase in life expectancy of patients with PC and as the condition is becoming more like a chronic disease, healthcare providers need to help patients maintain a healthy life after PC diagnosis [14].
Recently, androgen deprivation therapy (ADT) that lowers testosterone secretion to below-normal levels has been used not only for the treatment of metastatic PC but also as a conservative treatment for radiation therapy in local or locally advanced cancer [15]. Low testosterone levels due to ADT decrease energy levels, leading to frailty-related falls, osteoporosis, and metabolic syndromes [16,17,18]. In detail, a recent systematic review found that ADT significantly decreased bone mineral density in patients with PC receiving ADT [19]. Loss of muscle mass caused by decreased bone mineral density is related to low muscle strength and physical function [16,20,21,22,23]. Additionally, there is a correlation between ADT and a decrease in lean body mass, which is associated with low physical activity [24,25,26]. Exhaustion/fatigue is also a well-known adverse effect of ADT [24,27].
Despite the evidence that ADT and frailty are related, most studies on frailty in patients with PC in the previous literature explored frailty regardless of treatment methods [10,28,29]. Considering the vulnerability to frailty of patients with PC receiving ADT as well as the significant increase in patients with PC, there is a need to analyze and summarize studies on frailty experienced by patients with PC receiving ADT. Specifically, it is necessary to provide an overall understanding of the evidence by identifying the characteristics of the studies, the factors related to frailty experienced by patients with PC, and the interventions performed. Therefore, this study aimed to explore the existing literature and summarize the related characteristics of frailty experienced by patients with PC receiving ADT.
The specific research questions of this study are as follows:
1) What are the general characteristics of studies on frailty experienced by patients with PC receiving ADT?
2) What conceptual and operational definitions are used in studies on frailty experienced by patients with PC receiving ADT?
3) What are the contents and results of interventions for frailty experienced by patients with PC receiving ADT?
4) What are the other health-related variables associated with frailty in patients with PC receiving ADT?
MATERIALS AND METHODS
1. Study design
Scoping review was adopted in this study. This scoping review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) guidelines, which includes research questions, eligibility, search strategy, selection, data extraction, data analysis, and presentation of results [30].
2. Eligibility
Inclusion criteria were established according to the participant, concept, and context (PCC) recommended by the Joanna Briggs Institute manual for scoping reviews [31]. The study participants were patients with PC receiving ADT, the concept was frailty, and the context was hospitals or communities. Only original peer-reviewed studies were included to establish the reliability of the evidence. The exclusion criteria were: 1) a study on the effect of medical treatments, 2) duplicates, and 3) studies that were not written in English.
3. Search strategy
Four databases, PubMed, Cochrane Library, EMBASE, and CINAHL were used to find relevant studies published in English by November 16, 2022. To select key search terms, MeSH terms were used based on PCC, and the final search terms were determined through discussion with the research team and the professional librarian. The search strategy is described in Supplement Table 1. Manual searches were also conducted by reviewing the references of the selected studies and related journals and a Google Scholar search. The following key terms were used: “prostate cancer”, “ADT” “androgen deprivation therapy”, “androgen suppression therapy”, “hormone therapy”, “hormonal therapy”, and “frailty.”
4. Selection
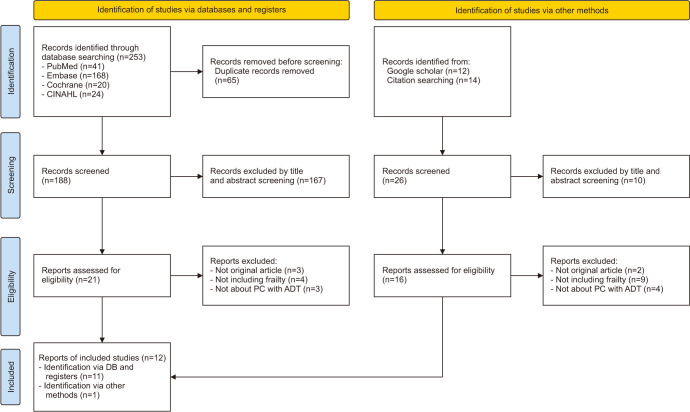
The study selection process is shown in (Fig. 1). A total of 253 studies were searched through a database search, of which 188 titles and abstracts were reviewed, excluding 65 duplicates. After screening based on titles and abstracts, two researchers reviewed the full texts of 21 studies, of which 3 were not original articles, 4 did not include the concept of frailty, and 3 were not about patients with PC receiving ADT. Additionally, 26 studies were identified from citation searching and Google Scholar searches, of which 10 studies were excluded by title and abstract screening. Two researchers reviewed the full texts of 16 studies, of which 2 were excluded because they were not original articles, 9 were excluded because they did not include frailty, and 4 because they were not about PC with ADT. Finally, 12 studies were included.
Fig. 1. PRISMA flowchart for literature selection. PC: prostate cancer, ADT: androgen deprivation therapy.
5. Data charting and analysis
The research team developed a form for data extraction to analyze the identified studies. The data extraction form consisted of the title, author, journal title, year of publication, country, study design, eligibility criteria for sampling, sample size, study period, frailty-related variables, conceptual and operational definitions of frailty, contents of intervention, and findings of the study. Data extraction and analysis were conducted by the two independent researchers according to the data extraction form. In case of discrepancy between the two researchers, a consensus was reached via discussion with a third researcher.
RESULTS
1. General characteristics of the studies
The general characteristics of the 12 studies are shown in Table 1. The main aims related to frailty in the included studies were diverse. There were two experimental studies, one of which examined the effectiveness of dose-dependent vitamin D supplementation on frailty in patients with PC receiving ADT [32], while the other study explored the effectiveness of a multidisciplinary intervention in patients with PC receiving ADT [33]. Among the non-experimental studies, one was a pilot study to evaluate the validity of the ‘Vulnerable Elders Survey (VES)-13’, which is a frailty measurement instrument in older patients with PC who had received ADT [34]. Four studies investigated the effects of ADT on frailty in patients with PC [7,35,36,37]. Two studies explored the relationship between inflammatory markers and frailty of patients with PC who received ADT [38,39]. There was a study conducted to investigate the frailty and insulin resistance in two groups of patients with PC, those who received and those who did not receive ADT [20]. Another study explored the changes in sarcopenia before and after ADT in patients with PC who received ADT [27]. Additionally, there was a study on the correlation between frailty and QOL in patients who were treated for PC [40].
Table 1. General characteristics of the included studies (n=12).
| First author (year) | Country | Study design | Aims | Participants | Sample size (n) | ||
|---|---|---|---|---|---|---|---|
| Inclusion criteria | Exclusion criteria | ||||||
| Mohile (2007) [34] | USA | Cross-sectional study | To identify the prevalence of geriatric impairment in older patients with PC who were receiving ADT and evaluate the validity and reliability of the VES-13 compared with the CGA | - Having started ADT for a rising PSA level after local therapy, or asymptomatic, metastatic disease | - Severe cognitive impairment (SPMSQ: 5 errors) | 50 | |
| - Age ≥70 years | - Less than an 8th-grade education | ||||||
| - Did not have a medical proxy for medical decision making | |||||||
| Bylow (2011) [35] | USA | Case-control prospective study | To examine the impact of ADT to frailty, objective physical performance, and falls in older PC patients with BCR. | - Diagnosis of PC | - Known diagnosis of dementia | 134 | |
| - Age ≥60 years | - Case: 63 | ||||||
| - Taking medications for dementia | - Control: 71 | ||||||
| Cheung (2016) [20] | Australia | Case-control prospective study | To explore the relationships between insulin resistance and frailty with body composition and testosterone in ADT group and not in ADT group | - Diagnosis of localized non-metastatic PC | - Androgen deficiency | 63 | |
| - Significant renal, liver, cardiac, or neuromuscular disease | - Case: 34 | ||||||
| - No prior ADT | - Control: 29 | ||||||
| - Unrestricted activity with a normal ECOG performance statusa of 0 | |||||||
| Mareschal (2017) [33] | Swiss | Quasi-Experimental study | To evaluate the effect of a multidisciplinary intervention program on QOL, the body composition, physical and psychological status of frail patients diagnosed with PC who received ADT combined to RT | - Diagnosis of non-metastatic locally advanced PC or aggressive intermediate disease treated with ADT and EBRT | - Expected survival <16 weeks | 35 | |
| - Incapacity for discernment | |||||||
| - Judgment of the radiation oncologist expert in PC | |||||||
| - Age ≥75 years and/or presenting with at least one out of the following functional or physiological frailty criteria (cardiovascular/pulmonary comorbidities with CCI≥3, VES-13≥3, balance unipedal stance test <5 seconds) | |||||||
| Winters-Stone (2017) [7] | USA | Cross-sectional study | To identify the relationship of ADT and frailty as well as frailty | Diagnosis of PC within the last 10 years (2005–2015) | N/A | 280 | |
| Cheung (2018) [36] | Australia | Case-control prospective study | To identify gains in fat mass and loss of muscle mass improve after cessation of ADT and to compare insulin resistance, frailty, handgrip strength, and QOL of PC patients with ADT with non-ADT | - Diagnosis of localized non-metastatic PC | - Androgen deficiency (baseline total testosterone<10 nmol/L) | 63 | |
| - Case: 34 | |||||||
| - ADT-naïve | - Control: 29 | ||||||
| - ECOG performance statusa of 0 | - Neuromuscular disease | ||||||
| - Independently living in the community | - Limitation in their exercise tolerance | ||||||
| - Active cardiac, respiratory, or joint disease | |||||||
| - Requirements for a walking aid | |||||||
| Navarro-Martínez (2019) [39] | Spain | Case-control prospective study | To explore the contribution of peripheral inflammation in frailty syndrome in patients with PC receiving ADT | - Diagnosis of PC | - Severe cognitive impairment (MMSE score<21), severe psychiatric disorders | 92 | |
| - Prescription of ADT | - Case: 46 | ||||||
| - Control: 46 | |||||||
| - Blindness | |||||||
| - Acute infections | |||||||
| Buigues (2020) [38] | Spain | Prospective observational study | To determine the relationship between progression (1 year follow up) of frailty syndrome and inflammatory markers | - Diagnosis of PC | - Severe cognitive impairment (MMSE score<21), severe psychiatric disorders | 39 | |
| - Prescription of ADT | |||||||
| - Blindness | |||||||
| - Acute infections | |||||||
| Couderc (2020) [27] | France | Prospective observational study | To examine the prevalence of sarcopenia in older PC patients before initiation of ADT and RT and identify the impact of ADT on the occurrence or aggravation of sarcopenia in this population | - Age ≥70 years | N/A | 31 | |
| - Referred for a CGA before initiation of ADT and RT for a localized or locally advanced PC | |||||||
| Momota (2020) [37] | Japan | Prospective observational study | To explore the relationship between frailty and RARP, radiotherapy, ADT alone, and metastatic diseases | - Localized PC patients treated by RARP, RT, or ADT-alone depending on eligibility for surgery or patient preference | - Not applicable for frailty evaluation using the G8 | 540 | |
| - Insufficient treatment information | |||||||
| - Metastatic PC patients with mHNPC or mCRPC were given a SOC treatment | |||||||
| Hamaya (2021) [40] | Japan | Retrospective study | To compare G8 with QOL scores between the localized diseases (M0 group) and mCSPC and examine the association of G8 and QOL scores in each group and the effect of frailty on worse QOL | - Diagnosis of Localized PC or mCSPC | N/A | 409 | |
| - Fulfilled frailty screening and HRQOL questionnaires with untreated status | - M0 group: 369 | ||||||
| - mCSPC: 40 | |||||||
| Inglis (2021) [32] | USA | RCT | To evaluate the impact of vitamin D supplementation in a dose-dependent way on phase angle and physical function in PC patients receiving ADT | - Age ≥60 years | - Adequate vitamin D levels | 59 | |
| - Diagnosis of PC with no bone metastases | - Hypercalcemia, osteoporosis, stage IV kidney disease, or myocardial infarction within the past year | - Experimental: 29 | |||||
| - Control: 30 | |||||||
| - Being within 6 months of starting ADT with an additional six more months planned | |||||||
| - Suboptimal vitamin D levels (<32 ng/mL) | |||||||
| - Total serum calcium ≤10.5 mg/dL | |||||||
| - No contraindications for fitness testing | |||||||
PC: prostate cancer, ADT: androgen deprivation therapy, VES: vulnerable elders survey, CGA: comprehensive geriatric assessment; PSA: prostate specific antigen; SPMSQ: short portable mental status questionnaire, BCR: biochemical recurrence, QOL: quality of life, EBRT: external beam radiotherapy, CCI: charlson comorbidity index, N/A: not applicable, MMSE: mini-mental state examination, RT: radiotherapy, RARP: robot assisted radical prostatectomy, mHNPC: metastatic hormone-naïve prostate cancer, mCRPC: metastatic castration-resistant prostate cancer, SOC: standard of care, G8: geriatric 8 screening tool, mCSPC: metastatic castration-sensitive prostate cancer, HRQOL: health related quality of life, M0: localized prostate cancer, RCT: randomized controlled trial.
aECOG performance status: a scale for evaluating a person’s level of ability to care for oneself, daily activity, and physical ability.
The inclusion criteria for each study are presented in Table 1. Five studies limited by age, as the inclusion criteria for participants ranged from 60 to 75 years and older [27,32,33,34,35]. Four studies restricted the participants to patients with localized PC [20,33,36,37] and one study included patients who did not have bone metastasis [32]. Five studies included participants regardless of the clinical stage [7,34,35,38,39].
In each of the 12 included studies, exclusion criteria were presented according to the purpose of the study. Cognitive function [34,38,39], diagnosis of dementia [35], ability of decision-making [33,34], and educational level [34] were used for exclusion criteria. Two studies examined baseline testosterone levels and excluded patients with androgen deficiency to evaluate eugonadism before ADT and as a reference to the post-castration levels [20,36]. Five studies excluded participants based on expert judgments regarding severe disease, expected survival time, and life-threatening medical conditions [20,33,36,38,39]. Exercise tolerance was also used to exclude participants in one study [36].
2. Conceptual and operational definitions of frailty
The conceptual and operational definitions of frailty used in the 12 included studies are presented in Table 2. Among these, 2 studies described the conceptual definition of frailty for patients with PC receiving ADT [33,34]. One study applied the definition of the vulnerable elderly proposed by Saliba et al [41] which defined a frail person as “a person over 65 years of age who is at an increased risk of functional diminish or death within 2 years.” Another study used cancer-specific definitions of frailty suggested by Hurria et al [42], in which frailty was measured using age and/or functional and physiological frailty criteria based on the perspective of geriatric oncology [33]. Hurria et al [42] defined frail people as “older individuals who are at higher risk for cancer treatment toxicity because of age-associated conditions, such as functional losses, cognitive impairment, or physiologic changes”.
Table 2. Frailty related characteristics, contents of intervention, and results (n=12).
| First author | Conceptual/operational definitions of frailty | Instruments of frailty | Frailty related variable | Contents of intervention/Results related to frailty | |||
|---|---|---|---|---|---|---|---|
| Mohile (2007) [34] | Vulnerable older people by Saliba et al [41] | · VES-13 | N/A | - The reliability of the VES-13 was 0.92 (Pearson correlation coefficient). | |||
| · CGA | |||||||
| - ADL, IADL | - The cut-off score of 3 on the VES-13 had 72.7% sensitivity and 85.7% specificity for CGA deficits and was highly predictive for identifying impairment. | ||||||
| - SPPB | |||||||
| - Comorbidity score | |||||||
| - Number of medications | |||||||
| - MOS Social Support Scale | |||||||
| - SPMSQ | |||||||
| Bylow (2011) [35] | The frailty phenotype by Fried et al & Modified “obese” frailty based on the definition by Fried et al [4] | · Fried’s 5 criteria | · General & Clinical characteristics | - Men with BCR on ADT are frailer with lower performance status and more falls (p=0.02). | |||
| - Unintentional weight loss: self-reported weight loss>10 pounds over the last year | - ADT | ||||||
| - Self-reported comorbidities | |||||||
| - Exhaustion: self-reported exhaustion based on two items from CES-D | - Comorbidity significantly increased the likelihood of “obese” frailty (p=0.01) and falls (p=0.01). | ||||||
| - Weakness/grip strength: using hand dynamometer | · Physiological index | ||||||
| - Hemoglobin | |||||||
| - Walking speed: using a timed 15-foot walk, | · Physical function | - Mild anemia, which has been associated with both androgen depletion and frailty, is more prevalent in men on ADT (p=0.01). | |||||
| - Falls | |||||||
| - Physical activity: self-reported physical activity based on weighted score of kilocalories expended per week | |||||||
| - When controlling for age, clinical characteristics, and comorbidities, the men with BCR on ADT group showed significant trends in “obese” frailty (p=0.01) and falls (p=0.01). | |||||||
| · Modified “obese” frailty criteria (substituting obesity [BMI>30.0 kg/m2] for the ‘weight loss’ criteria of Fried’s 5 criteria) | |||||||
| Cheung (2016) [20] | The frailty phenotype by Fried et al [4] | · Fried’s 5 criteria | · General & Clinical characteristics | Within 12 months of commencement, frailty increased with ADT (p<0.001), which was related to decreased testosterone (p=0.028), and less to fat mass (p=0.056) or lean mass (p=0.79). | |||
| - Unintentional weight loss: self-reported weight loss>10 pounds in the last year | |||||||
| - ADT | |||||||
| - Exhaustion: self-reported exhaustion based on two items from CES-D | · Physiological index | ||||||
| - Total testosterone | |||||||
| - Weakness/grip strength: three times in the dominant and non-dominant hand with hand dynamometer | |||||||
| - Walking speed: time to walk 4 m | |||||||
| - Physical activity: Minnesota Leisure Time Physical Activity Questionnaire | |||||||
| Mareschal (2017) [33] | A cancer-specific definition of frailty by Hurria et al [42] | Old (age≥75 years) and/or presenting with at least one out of the following functional or physiological frailty criteria | N/A | (1) Contents of intervention: nutritional coaching, supervised group physical training, psychological counseling. | |||
| - Cardiovascular/pulmonary comorbidities with CCI≥3 | |||||||
| (2) Follow-up period: start of ADT and 3, 6, 9, 12, 18, and 24 months or at discharge, thereafter (patients were discharged from the study when total testosterone blood levels turned back to normal. The last follow-up was planned 12 months after study discharge). | |||||||
| - VES-13≥3 | |||||||
| - Balance unipedal stance test<5 seconds | |||||||
| (3) Results: no significant change over the two years, including post-study follow-up. Means of QOL, nutritional, physical, and psychological variables remained stable over more than 2 years. | |||||||
| Winters-Stone (2017) [7] | Positive by the FRAIL scale which was developed by a European, Canadian, and American GAP & Modified “obese” frailty based on the definition by the GAP | · Self-reported FRAIL scale | · General & Clinical characteristics | - Current (43%) or past (40%) ADT users were more likely to be classified as prefrail or frail than never users (15%) (p<0.001), and the prevalence of combined obese frailty and prefrailty was even greater in current (59%) or past (62%) ADT users than never users (25%) (p<0.001). | |||
| · Modified “obese” frailty criteria (substituting obesity (BMI>30.0 kg/m2) for the ‘shrinking’ criteria of the FRAIL scale) | |||||||
| - ADT | |||||||
| Cheung (2018) [36] | The frailty phenotype by Fried et al [4] | · Fried’s 5 criteria | N/A | - Two years after cessation of ADT, participants had no difference in frailty score with those who did not (p=0.51). | |||
| Specific measurement methods are not mentioned. | |||||||
| Navarro-Martínez (2019) [39] | The frailty phenotype by Fried et al [4] | · Fried’s 5 criteria | · Physiological index | - The severity of frailty syndrome and lymphocyte count were significantly negatively correlated (p<0.01). The concentration of IL-6 (p<0.05), CRP (p<0.05), and fibrinogen (p<0.01) were significantly associated with frailty syndrome. | |||
| - Unintentional weight loss: self-reported weight loss which was 5% or 4.5 kg or more in the last year | - Lymphocyte count | ||||||
| - IL-6 | |||||||
| - CRP | |||||||
| - Exhaustion: self-reported exhaustion based on one item from CES-D | - Fibrinogen | ||||||
| - Creatinine | |||||||
| - Weakness/grip strength: three times in each hand alternately with a hand dynamometer | |||||||
| - Creatinine had a significantly higher mean serum concentration in robust individuals compared to prefrail and frail patients (p=0.037) | |||||||
| - Walking speed: time to walk 4.6 m | |||||||
| - Physical activity: IPAQ, the total amount of energy spent on activities for 1 week | |||||||
| Buigues (2020) [38] | The frailty phenotype by Fried et al [4] | · Fried’s 5 criteria | · General & Clinical characteristics | - At baseline, older patients were more frailer than younger patients (p=0.009) and there were significant differences in the CCI between robust, prefrail and frail individuals at follow-up (p=0.002). | |||
| - Unintentional weight loss: self-reported weight loss of 4.5 kg or more in the last year | - Age | ||||||
| - ADT | |||||||
| - CCI adjusted for age | |||||||
| - Exhaustion: self-reported exhaustion based on one item from CES-D | · Physiological index | ||||||
| - Lymphocyte count | |||||||
| - Weakness/grip strength: three times in each hand alternately with a hand dynamometer | - IL-6 | - At the baseline, a high IL-6 was associated with a significantly increased OR of being frail (p=0.013), as was a high IL-8 (p=0.014) compared to being non-frail. A higher lymphocyte count associated with a significant OR of being frailty (p=0.036). | |||||
| - IL-8 | |||||||
| - CRP | |||||||
| - Monocyte count | |||||||
| - Walking speed: time to walk 4.6 m | - Lymphocyte count | ||||||
| - Physical activity: IPAQ, the total amount of energy spent on activities for 1 week | |||||||
| - At baseline, there was a significant difference in CRP concentration in blood between robust, prefrail, and frail individuals (p=0.04). | |||||||
| - At the follow up, there were significant differences between robust, prefrail and frail individuals for IL-6 (p<0.001), and the monocyte count (p=0.04). A higher IL-6 was associated with a significantly increased OR of being frail (p=0.011). | |||||||
| - A higher log (IL-6) was associated with a significantly increased risk of frailty progression at 1 year follow-up (p<0.05), as was a lower lymphocytes count (p<0.05). | |||||||
| Couderc (2020) [27] | The frailty phenotype by Fried et al [4] | · CGA | N/A | Compared with older PC patients scheduled to receive RT or ADT without sarcopenia, those with probable sarcopenia had more geriatric frailties, although the differences did not reach significance. | |||
| - G8 score | |||||||
| - ECOG-PS | |||||||
| - ADL, IADL | |||||||
| Momota (2020) [37] | Geriatric frailties by G8 score & The frailty phenotype by Fried et al [4] | · Fried’s 5 criteria | · General & Clinical characteristics | - The number of patients with frailty was significantly lower among the patients treated with RARP than those treated with RT (p<0.001) and ADT alone (p<0.001), and those with M1 disease (p<0.001). | |||
| Specific measurement methods are not mentioned. | - Metastases | ||||||
| - Treatment modalities | |||||||
| · G8 score | |||||||
| - The G8 score was significantly higher in the M0 group than in the M1 group (p<0.001). | |||||||
| - The G8 score was significantly higher in the RARP group compared with the RT and ADT-alone groups (p<0.001) | |||||||
| Inglis (2021) [32] | A phase angle value <5.7 in older men | Phase angle value from BIA | · Body composition | (1) Contents of intervention: high-dose vitamin D supplementation | |||
| - Phase angle value | |||||||
| - Experimental group: high dose vitamin D | |||||||
| - Control group: low dose vitamin D | |||||||
| (2) Follow-up period: week 12 and week 24 | |||||||
| - Subjects took the supplements for 24 weeks. | |||||||
| (3) Results | |||||||
| - The experimental group had significantly wider phase angle values at week 12 (p=0.014) and at week 24 (p=0.018) | |||||||
| Hamaya (2021) [40] | Geriatric frailties by G8 score | G8 score | · General & Clinical characteristics | - The G8 scores of the patients with ADT alone were significantly lower than the patients with RARP and RT in the M0 group (p<0.001). | |||
| - ADT | |||||||
| - Metastases | |||||||
| - A significant difference was observed between patients with M0 and mCSPC groups in the G8 score (p=0.002). | |||||||
VES: vulnerable elders survey, CGA: comprehensive geriatric assessment, ADL: activities daily living, IADL: instrumental activities daily living, SPPB: short physical performance battery, MOS: medical outcomes study, SPMSQ: short portable mental status questionnaire, N/A: not applicable, CES-D: center for epidemiological studies-depression, ADT: androgen deprivation therapy, BMI: body mass index, BCR: biochemical recurrence, CCI: charlson comorbidity index, QOL: quality of life, FRAIL: fatigue, resistance, ambulation, illnesses, and loss of weight, GAP: geriatric advisory panel, IPAQ: international physical activity questionnaire, IL: Interleukin, CRP: C-reactive protein, OR: odds ratio, ECOG-PS: European cooperative oncology group-performance status, PC: prostate cancer, G8: geriatric 8 screening tool, RT: radiotherapy, RARP: robot assisted radical prostatectomy, M1: metastatic prostate cancer, M0: localized prostate cancer, mCSPC: metastatic castration-sensitive prostate cancer, BIA: bioelectrical impedance analysis.
Regarding the operational definition of frailty, seven studies used the frailty phenotype proposed by Fried et al [4], which defines frailty as weakness, slowness, exhaustion, low physical activity, and unintentional weight loss [4,20,27,35,36,37,38,39]. Two studies used geriatric frailty as defined by the G8 score [37,40]. The G8 score consists of age, body mass index (BMI), weight loss, food intake, medication, neuropsychological condition, subjective health status, and motor skills. The score ranges from 0 to 17, with a score of 14 or less indicating frailty [43]. One study defined a frail person if scored positive by the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight (FRAIL) scale which was proposed by a European, Canadian, and American geriatric advisory panel, and a modified FRAIL definition where 'shrinking' criteria was replaced with obesity was also used in this study [7]. There was also a study where a person with a phase angle of less than 5.7 was defined as frail [32]. The phase angle is measured noninvasively using bioelectrical impedance analysis, which indicates nutrition and hydration status.
3. Contents and results of interventions
Mareschal et al [33] provided a multidisciplinary intervention including psychological counseling, nutritional coaching, and supervised group physical exercise to 35 patients at the start of ADT. The psychologists provided psychological counseling through individual or couple sessions with emotional support. The dietician checked the body composition and unwanted weight gain. The physiotherapist supervised combined aerobic and resistance exercises to maintain the appropriate level of lean body mass and decrease fatigue level. In this study, participants were assessed for depression, QOL, treatment effectiveness, anxiety, cognition, nutritional parameters including BMI, fat-free mass index, fat mass index (FMI), and physical parameters including Borg scale, Timed Up and Go (TUG), handgrip strength (HGS), Six-Minute Walk Test (6MWT). In the study by Inglis et al [32], they identified the effect of dose-dependent vitamin D supplementation on physical function and phase angle in patients with PC receiving ADT. The multidisciplinary intervention had no effect on improving frailty, but vitamin D supplementation significantly reduced frailty.
4. Variables related to frailty
We classified variables for which each study explored the relationship between frailty into general and clinical characteristics, physiological index, body composition, physical function, psychological, cognitive, and social factors, and QOL.
The general and clinical characteristics related to frailty were age, treatment modalities, metastases, and comorbidities. Among the general characteristics, older patients were frailer than younger patients [38]. Five studies showed that ADT, a clinical characteristic, significantly increased frailty in patients with PC [7,20,35,38,40]. In a study that identified the frailty level 2 years after cessation of ADT, it was found that the frailty was not significantly different from those who did not receive ADT [36]. Additionally, the frailty level of those who received robotic-assisted radical prostatectomy was significantly lower than that of those who received radiotherapy or ADT alone, and those with metastatic PC [37]. Two studies found that metastases significantly correlated with a high level of frailty [37,40]. In the study in which participants were followed-up 1 year after starting ADT, there was no significant difference in the Charlson Comorbidity Index according to frailty level at baseline, but there was a significant difference after 1 year [38]. Similarly, in the study comparing patients with PC who experienced biochemical recurrence (BCR) on ADT to patients with PC not receiving ADT, self-reported comorbidities significantly increased falls and "obese" frailty [35].
Of the 12 studies, 4 studies identified the relationship between the physiological index and frailty [20,35,38,39]. Hemoglobin level, which was slightly lower than normal, was more prevalent in men on ADT [35]. The decrease in total testosterone levels was related to an increase in frailty [20]. Two studies found that high interleukin (IL)-6 levels, high C-reactive protein (CRP) levels, and low lymphocyte count were related to a high level of frailty [38,39]. One study reported that the higher the IL-8 level, the more severe the frailty [38], but this was not significant in another study [39]. High fibrinogen and low creatinine levels were also associated with frailty [39]. Also, a study found a significant difference between robust, prefrail, and frail individuals for monocyte count at 1 year follow-up [38].
Body composition, BMI, FMI, and lean mass were not related to frailty [33]. In a randomized controlled trial (RCT) to examine the effect of dose-dependent vitamin D supplementation on frailty of patients with PC, the high-dose vitamin D supplementation group’s phase angle value was wider than the low-dose group’s [32].
Two studies identified the relationship between physical function and frailty [33,35]. In the longitudinal interventional study that evaluated the effect of a multidisciplinary intervention program to manage ADT side effects in frail patients with PC, participants were assessed for the Borg scale, TUG, HGS, and 6MWT [33]. The Borg scale measures self-rated perceived exertion, TUG measures mobility and balance, HGS measures upper extremity strength, and the 6MWT measures walking endurance. When the patients were observed at 12-month follow-up after study discharge, the four measures remained stable without significant differences. In the study comparing patients with PC who experienced BCR on ADT to patients with PC not receiving ADT, Short Physical Performance Battery and incident falls over 6 months were used to assess physical function [35]. In this study, men with BCR on ADT showed significant trends in "obese" frailty and falls when controlling for age, clinical characteristics, and comorbidities [35].
Regarding psychological and cognitive variables, the multidisciplinary intervention study measured Mini-Mental State Examination and Hospital Anxiety and Depression Scale (HAD-A&D) scores, and no significant changes were seen during the intervention and post-study follow-up period [33]. In this study, there was no significant change in the QOL before and after the intervention. Two studies measured the participants’ QOL but didn’t investigate the relationship between frailty and QOL of patients with PC receiving ADT [36,40].
DISCUSSION
This study aimed to explore the literature on frailty of patients with PC receiving ADT and to summarize their findings to provide an overview of the evidence. The major findings of this study were as follows: 1) there were a few intervention studies for frailty of patients with PC receiving ADT; 2) most studies used the operational definitions of Fried et al [4], which mainly focused on physical functions; and 3) there was limited research exploring the relationship between frailty and psychological, cognitive, and social factors of patients with PC receiving ADT.
First, although frailty is prevalent in patients with PC receiving ADT and adversely affects their health outcomes, few interventional studies have been conducted. Particularly, there was only one RCT [32]. In a study that provided a multidisciplinary intervention program, it was found that the program had no effect on body composition, physical function, psychological factors, and QOL including frailty [33]. Since this study had a one-group pre-post test design, attention should be paid to the interpretation of the study results. Therefore, RCTs for the development and evaluation of an integrated intervention that considers the characteristics of frailty affecting various aspects need to be conducted.
Additionally, no qualitative study has comprehensively explored the experience of frailty among patients with PC receiving ADT. According to a qualitative study that was not included in the current study, the self-management experiences of PC survivors who received ADT were classified into seven categories. The seven categories were further divided into subcategories, and among them, “weight loss”, “low energy”, and “coping with deterioration of physical abilities” overlapped with frailty properties [44]. As in this study, the symptom experience of patients with PC receiving ADT is related to frailty but is not fully recognized and clearly understood in their care [7,24,38]. Therefore, studies that identify the relationship between ADT and frailty of patients with PC receiving ADT should be conducted, which ultimately contributes to raising awareness about maintaining and improving QOL after survival of them.
Second, although the most commonly used definition in the included studies was the frailty phenotype proposed by Fried et al [4], the methods used to measure the Fried’s five criteria differed in each study. Two studies using the Fried’s five criteria did not describe specific measurement methods for each of the five items [36,37]. Walking speed [20], weight loss [39], and physical activity [38,39] were measured differently from the guideline proposed by Fried et al [4]. Moreover, there was a case in which no definite instrument according to the definition used, and frailty was measured according to only age and functional or physiological frailty criteria [33]. In two studies, the researchers performed a comprehensive geriatric assessment (CGA) [27,34], and the CGA items performed in the two studies were different. One study argued that the cut-off score should be different when applying the G8 score to Japanese patients with PC [37].
No conceptual or operation definition comprehensively considered the multidimensional characteristics of frailty of patients with PC receiving ADT. Although ADT is recommended for locally advanced PC, metastatic PC, BCR after treatment with curative intent, it causes various side effects that can be conceptualized as frailty [44]. In particular, patients with PC receiving ADT experienced side effects in various aspects, such as physical, psychological, cognitive, and social functions, regardless of aging [16,37]. Therefore, a conceptual definition and corresponding operational definition that comprehensively considers the multidimensional characteristics of frailty experienced by PC patients receiving ADT should be developed.
Finally, a few studies have examined the relationships between psychological, cognitive, and social factors and frailty among patients with PC receiving ADT. Among the 12 included studies, only one explored the relationship between psychological and cognitive variables and frailty [33]. No study has explored the relationship between social factors and frailty. ADT is a treatment for lowering testosterone levels, which can be closely related to psychological side effects, cognitive impairment, and difficulties with masculinity and self-image, which lead to social isolation [17,45,46,47]. Therefore, it is difficult to define and characterize frailty experienced by patients with PC receiving ADT through the frailty phenotype described by Fried et al [4] which does not include psychological, cognitive, and social domains. Recently, attempts have been made to understand frailty not only in the physical domain, but also in the psychological, cognitive, and social domains [48]. According to ‘an integral conceptual model of frailty’, frailty is determined through the interaction between the physical, psychological, and social dimensions [49]. In this model, physical frailty includes sensory functions, nutritional status, activity level, and physical functions such as mobility, endurance, and balance; psychological frailty includes coping, mood, and cognitive function; and social frailty includes relationships with others and social support [49]. Therefore, to comprehensively understand the frailty of patients with PC receiving ADT, more research needs to be conducted to explore their frailty not only in the physical domain, but also in the psychological, cognitive, and social domains.
Among 12 included studies, 10 studies were conducted in Western countries and 2 were conducted in Japan. This result might be due to the higher incidence of PC in the United States and Europe than in Asian countries [50,51]. In recent years, the number of patients with PC in Asian countries such as Japan, Singapore, and South Korea is increasing due to environmental factors such as a higher intake of dietary fat and meat and reduced physical activity, and increased exposure to prostate specific antigen testing [50,52,53]. Therefore, more studies on the frailty of patients with PC receiving ADT need to be conducted in Asian countries. Furthermore, considering paucity of research on the management of symptoms associated with ADT, more studies adopting multidisciplinary approaches are needed to provide patients with PC receiving ADT with individualized care.
This scoping review has some strengths. First, it reviewed all published studies without limiting the publication year and study design. Second, to the best of our knowledge, this is the first scoping review on this topic and can be used by researchers and healthcare providers. Third, the screening and selection of relevant studies were conducted by two independent researchers; in case of disagreement between the two researchers, consensus was reached by discussing with a third researcher. This study also had some limitations. First, we included articles from peer-reviewed journals that were published in English. Relevant articles may be published in other languages as well. Furthermore, since we excluded grey literature, we could not be certain that we had identified all relevant available literature. Second, we did not classify the differences according to ADT type. ADT can be classified into drugs that stop androgens from working, other androgen-suppressing drugs, treatment to lower androgen levels from the adrenal glands, and treatment to lower testicular androgen levels [12]. Nonetheless, most of the 12 included studies did not describe the type of ADT used, and the purpose of the four types of ADT was the same. Consequently, we are sure that it would be more meaningful to identify the characteristics of frailty experienced by patients with PC receiving ADT than to classify ADT treatment types.
Based on the findings of this scoping review, we would recommend that more studies on frailty of patients with PC receiving ADT should be conducted. Particularly, RCTs need to be conducted for developing effective interventions for them. Furthermore, a definition and appropriate instrument that comprehensively consider the characteristics of frailty in patients with PC receiving ADT should be developed.
CONCLUSIONS
Frailty of patients with PC receiving ADT can be characterized by older age, having metastases and comorbidities. Low lymphocyte counts, low creatinine and total testosterone levels, and high CRP, IL-6, fibrinogen levels were associated with frailty of patients with PC receiving ADT. Further research should be undertaken to develop an integrated intervention that considers multidimensional aspects of frailty in patients with PC receiving ADT. Additionally, an appropriate definition and instrument for frailty in patients with PC receiving ADT should be developed and used to improve effective communication and awareness of frailty among healthcare providers.
Acknowledgements
None.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2020R1A6A1A03041989) and Brain Korea 21 FOUR Project funded by National Research Foundation (NRF) of Korea, Yonsei University College of Nursing. This work was also supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1F1A1062769 and 2022R1A2C1092084).
- Conceptualization: JP, YK.
- Data curation: JP, GWR, HL, YK.
- Formal analysis: JP, HL, YK.
- Funding acquisition: JP, YDC.
- Investigation: GWR, HL, YK.
- Methodology: JP, GWR, YK.
- Project administration: JP, YDC.
- Resources: JP, YDC.
- Software: HL, YK.
- Supervision: JP, GWR, YK.
- Validation: HL, YDC.
- Visualization: HL, YK.
- Writing – original draft: JP, GWR, YK.
- Writing – review & editing: JP, YK.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220280.
Search queries and strategies by the electronic databases
References
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. Erratum in: Lancet 2013;382:1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ožić S, Vasiljev V, Ivković V, Bilajac L, Rukavina T. Interventions aimed at loneliness and fall prevention reduce frailty in elderly urban population. Medicine (Baltimore) 2020;99:e19145. doi: 10.1097/MD.0000000000019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gobbens RJ, van Assen MA. The prediction of quality of life by physical, psychological and social components of frailty in community-dwelling older people. Qual Life Res. 2014;23:2289–2300. doi: 10.1007/s11136-014-0672-1. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. doi: 10.1093/ageing/afz046. Erratum for: Age Ageing 2019;48:16-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winters-Stone KM, Moe E, Graff JN, Dieckmann NF, Stoyles S, Borsch C, et al. Falls and frailty in prostate cancer survivors: current, past, and never users of androgen deprivation therapy. J Am Geriatr Soc. 2017;65:1414–1419. doi: 10.1111/jgs.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tornero-Quiñones I, Sáez-Padilla J, Espina Díaz A, Abad Robles MT, Sierra Robles Á. Functional ability, frailty and risk of falls in the elderly: relations with autonomy in daily living. Int J Environ Res Public Health. 2020;17:1006. doi: 10.3390/ijerph17031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahat G, Ozkok S, Kilic C, Karan MA. SARC-F questionnaire detects frailty in older adults. J Nutr Health Aging. 2021;25:448–453. doi: 10.1007/s12603-020-1543-9. [DOI] [PubMed] [Google Scholar]
- 10.Della Pepa C, Cavaliere C, Rossetti S, Di Napoli M, Cecere SC, Crispo A, et al. Predictive comprehensive geriatric assessment in elderly prostate cancer patients: the prospective observational scoop trial results. Anticancer Drugs. 2017;28:104–109. doi: 10.1097/CAD.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. Erratum in: CA Cancer J Clin 2021;71:359. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society. Cancer facts & figures 2021 [Internet] American Cancer Society; c2021. [cited 2022 Nov 16]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html. [Google Scholar]
- 13.Korea Central Cancer Registry. Annual report of cancer statistics in Korea in 2019. National Cancer Center; 2021. Dec, Report No.: 117044. [Google Scholar]
- 14.Schmitz-Dräger BJ, Bismarck E, Grammenos D, Ebert T, Starlinger R, Ottillinger B, et al. Lifestyle aspects in a contemporary middle-European cohort of patients undergoing androgen deprivation therapy for advanced prostate cancer: data from the non-interventional LEAN study. Br J Nutr. 2023;130:495–502. doi: 10.1017/S0007114522003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaeffer E, Srinivas S, Antonarakis ES, Armstrong AJ, Bekelman JE, Cheng H, et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Canc Netw. 2021;19:134–143. doi: 10.6004/jnccn.2021.0008. [DOI] [PubMed] [Google Scholar]
- 16.Brown JE, Handforth C, Compston JE, Cross W, Parr N, Selby P, et al. Guidance for the assessment and management of prostate cancer treatment-induced bone loss. A consensus position statement from an expert group. J Bone Oncol. 2020;25:100311. doi: 10.1016/j.jbo.2020.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casado E, Borque-Fernando A, Caamaño M, Graña J, Muñoz-Rodríguez J, Morote J. Multidisciplinary consensus on the prevention and treatment of osteoporosis and fragility fractures in patients with prostate cancer receiving androgen-deprivation therapy. World J Mens Health. 2022;40:74–86. doi: 10.5534/wjmh.210061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129–140. doi: 10.1080/13685538.2018.1482487. [DOI] [PubMed] [Google Scholar]
- 19.Kim DK, Lee JY, Kim KJ, Hong N, Kim JW, Hah YS, et al. Effect of androgen-deprivation therapy on bone mineral density in patients with prostate cancer: a systematic review and meta-analysis. J Clin Med. 2019;8:113. doi: 10.3390/jcm8010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung AS, Hoermann R, Dupuis P, Joon DL, Zajac JD, Grossmann M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur J Endocrinol. 2016;175:229–237. doi: 10.1530/EJE-16-0200. [DOI] [PubMed] [Google Scholar]
- 21.Cheung AS, Zajac JD, Grossmann M. Muscle and bone effects of androgen deprivation therapy: current and emerging therapies. Endocr Relat Cancer. 2014;21:R371–R394. doi: 10.1530/ERC-14-0172. [DOI] [PubMed] [Google Scholar]
- 22.Galvão DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009;12:198–203. doi: 10.1038/pcan.2008.51. [DOI] [PubMed] [Google Scholar]
- 23.Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012;30:3271–3276. doi: 10.1200/JCO.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: a conceptual review. Cancer. 2007;110:2604–2613. doi: 10.1002/cncr.23084. [DOI] [PubMed] [Google Scholar]
- 25.Mitsuzuka K, Arai Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int J Urol. 2018;25:45–53. doi: 10.1111/iju.13473. [DOI] [PubMed] [Google Scholar]
- 26.Nilsen TS, Johansen SH, Thorsen L, Fairman CM, Wisløff T, Raastad T. Does androgen deprivation for prostate cancer affect normal adaptation to resistance exercise? Int J Environ Res Public Health. 2022;19:3820. doi: 10.3390/ijerph19073820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couderc AL, Muracciole X, Nouguerede E, Rey D, Schneider S, Champsaur P, et al. HoSAGE: sarcopenia in older patients before and after treatment with androgen deprivation therapy and radiotherapy for prostate cancer. J Nutr Health Aging. 2020;24:205–209. doi: 10.1007/s12603-019-1294-7. [DOI] [PubMed] [Google Scholar]
- 28.Levy I, Finkelstein M, Bilal KH, Palese M. Modified frailty index associated with Clavien-Dindo IV complications in robot-assisted radical prostatectomies: a retrospective study. Urol Oncol. 2017;35:425–431. doi: 10.1016/j.urolonc.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Molina-Garrido MJ, Guillén-Ponce C. Use of geriatric assessment and screening tools of frailty in elderly patients with prostate cancer. Review. Aging Male. 2017;20:102–109. doi: 10.1080/13685538.2016.1277516. [DOI] [PubMed] [Google Scholar]
- 30.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 31.Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. In: JBI manual for evidence synthesis. Aromataris E, Munn Z, editors. JBI; 2020. Scoping reviews. [Google Scholar]
- 32.Inglis JE, Fernandez ID, van Wijngaarden E, Culakova E, Reschke JE, Kleckner AS, et al. Effects of high-dose vitamin D supplementation on phase angle and physical function in patients with prostate cancer on ADT. Nutr Cancer. 2021;73:1882–1889. doi: 10.1080/01635581.2020.1819348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mareschal J, Weber K, Rigoli P, Biason E, Frambati L, Gotteland C, et al. The ADAPP trial: a two-year longitudinal multidisciplinary intervention study for prostate cancer frail patients on androgen deprivation associated to curative radiotherapy. Acta Oncol. 2017;56:569–574. doi: 10.1080/0284186X.2016.1273545. [DOI] [PubMed] [Google Scholar]
- 34.Mohile SG, Bylow K, Dale W, Dignam J, Martin K, Petrylak DP, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109:802–810. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 35.Bylow K, Hemmerich J, Mohile SG, Stadler WM, Sajid S, Dale W. Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: a case-control study. Urology. 2011;77:934–940. doi: 10.1016/j.urology.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung AS, Tinson AJ, Milevski SV, Hoermann R, Zajac JD, Grossmann M. Persisting adverse body composition changes 2 years after cessation of androgen deprivation therapy for localised prostate cancer. Eur J Endocrinol. 2018;179:21–29. doi: 10.1530/EJE-18-0117. [DOI] [PubMed] [Google Scholar]
- 37.Momota M, Hatakeyama S, Soma O, Tanaka T, Hamano I, Fujita N, et al. Geriatric 8 screening of frailty in patients with prostate cancer. Int J Urol. 2020;27:642–648. doi: 10.1111/iju.14256. [DOI] [PubMed] [Google Scholar]
- 38.Buigues C, Navarro-Martínez R, Sánchez-Martínez V, Serrano-Carrascosa M, Rubio-Briones J, Cauli O. Interleukin-6 and lymphocyte count associated and predicted the progression of frailty syndrome in prostate cancer patients undergoing antiandrogen therapy. Cancers (Basel) 2020;12:1716. doi: 10.3390/cancers12071716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarro-Martínez R, Serrano-Carrascosa M, Buigues C, Fernández-Garrido J, Sánchez-Martínez V, Castelló-Domenech AB, et al. Frailty syndrome is associated with changes in peripheral inflammatory markers in prostate cancer patients undergoing androgen deprivation therapy. Urol Oncol. 2019;37:976–987. doi: 10.1016/j.urolonc.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Hamaya T, Hatakeyama S, Momota M, Narita T, Iwamura H, Kojima Y, et al. Association between the baseline frailty and quality of life in patients with prostate cancer (FRAQ-PC study) Int J Clin Oncol. 2021;26:199–206. doi: 10.1007/s10147-020-01798-4. [DOI] [PubMed] [Google Scholar]
- 41.Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 42.Hurria A, Dale W, Mooney M, Rowland JH, Ballman KV, Cohen HJ, et al. Cancer and Aging Research Group. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32:2587–2594. doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 44.Chien CH, Huang XY. Self-care experiences of advanced prostate cancer survivors who underwent androgen deprivation therapy. Cancer Nurs. 2022;45:190–200. doi: 10.1097/NCC.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 45.European Association of Urology (EAU) EAU guidelines. EAU; 2023. [Google Scholar]
- 46.Benedict C, Dahn JR, Antoni MH, Traeger L, Kava B, Bustillo N, et al. Positive and negative mood in men with advanced prostate cancer undergoing androgen deprivation therapy: considering the role of social support and stress. Psychooncology. 2015;24:932–939. doi: 10.1002/pon.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JW, Kim DK, Lee HS, Park JY, Ahn HK, Ha JS, et al. Androgen deprivation therapy in patients with prostate cancer is associated with the risk of subsequent Alzheimer's disease but not with vascular dementia. World J Mens Health. 2022;40:481–489. doi: 10.5534/wjmh.210019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara Y, Kondo K, Koyano W, Murayama H, Shinkai S, Fujita K, et al. Social frailty as social aspects of frailty: research, practical activities, and prospects. Geriatr Gerontol Int. 2022;22:991–996. doi: 10.1111/ggi.14492. [DOI] [PubMed] [Google Scholar]
- 49.Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook. 2010;58:76–86. doi: 10.1016/j.outlook.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Kitagawa Y, Namiki M. Prostate-specific antigen-based population screening for prostate cancer: current status in Japan and future perspective in Asia. Asian J Androl. 2015;17:475–480. doi: 10.4103/1008-682X.143756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhee H, Gunter JH, Heathcote P, Ho K, Stricker P, Corcoran NM, et al. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2015;115 Suppl 5:3–13. doi: 10.1111/bju.12964. [DOI] [PubMed] [Google Scholar]
- 52.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taitt HE. Global trends and prostate cancer: a review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am J Mens Health. 2018;12:1807–1823. doi: 10.1177/1557988318798279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search queries and strategies by the electronic databases