Abstract
Prostate cancer (PC) treatment has reached a milestone with the introduction of poly(ADP-ribose) polymerase (PARP) inhibitors. PARP inhibitors (PARPi) induce breaks in single-stranded and/or double-stranded DNA, resulting in synthetic lethality in cancer cells lacking functional homologous recombination genes. Around 20% to 25% of patients with metastatic castration-resistant prostate cancer harbor mutations in DNA damage repair genes, either somatic or germline. The success of PARPi in these patients has prompted studies exploring its potential in tumors classified as "BRCAness," which refers to tumors without germline BRCA1 or BRCA2 mutations. Additionally, there is a proposed connection between androgen receptor signaling and synthetic lethality of PARPi. The inclusion of genetic mutation tests in the treatment algorithm for PC is a significant step towards precision and personalized medicine, marking a first in the field. The objectives of this review encompass understanding the mechanism of action of PARPi in both monotherapy and combination therapy, exploring patient selection criteria, discussing pivotal studies that led to its approval, and offering future prospects. However, numerous unanswered questions remain, including the identification of the patient population that could benefit most from PARPi, determining whether to use PARPi as monotherapy or in combination, and finding the optimal timing of PARPi administration in advanced or localized disease. To address these questions, several ongoing clinical trials are being conducted.
Keywords: BRCA1, BRCA2, PARP inhibitors, Prostatic neoplasms
INTRODUCTION
Prostate cancer (PC) is a prevalent malignancy in males. While early-stage disease can be cured through surgery or radiotherapy, metastatic disease development often leads to unfavorable outcomes. Androgen receptor (AR) signaling plays a crucial role in PC, and androgen deprivation therapy (ADT) is the primary treatment for advanced PC [1]. Resistance to ADT can arise through diverse mechanisms [2]. To address the need for more effective treatments in patients with advanced PC who have failed prior therapies, several new drugs have been introduced, showing promising results in delaying disease progression and extending survival [3]. However, more effective treatments are needed for patients with advanced PC for whom previous therapies have failed.
PARP inhibitors (PARPi), a novel class of targeted drugs, have been used in the treatment of various cancers, including metastatic castration-resistant prostate cancer (mCRPC) [4]. The primary mode of action of PARPi is impairing DNA function, leading to inhibition of tumor cell proliferation [5]. Initially approved for breast and ovarian cancer, PARPi has now found application in clinical management of PC [6]. Currently, olaparib and rucaparib are the PARPi globally approved for PC treatment [6].
Advances in clinical research have expanded the use of PARPi from PC cases with BRCA1/2 gene mutations to patients with mutations associated with homologous recombination repair (HRR) [7]. Combining PARPi with novel hormonal therapies has shown potential in enhancing treatment effectiveness against mCRPC [6].
This article provides the latest evidence on the utilization of PARPi in PC treatment, including insights into their mechanisms of action, clinical advancements, various mechanisms of PARPi resistance, and future prospects.
DNA REPAIR AND THE PRINCIPLE OF SYNTHETIC LETHALITY
The preservation of genetic material integrity heavily relies on DNA repair mechanisms. DNA continuously sustains damage from various internal and external factors, such as ultraviolet radiation, reactive oxygen species, and environmental toxins. Failure to repair DNA damage can result in mutations, genomic instability, and ultimately, the onset of cancer or other diseases [8].
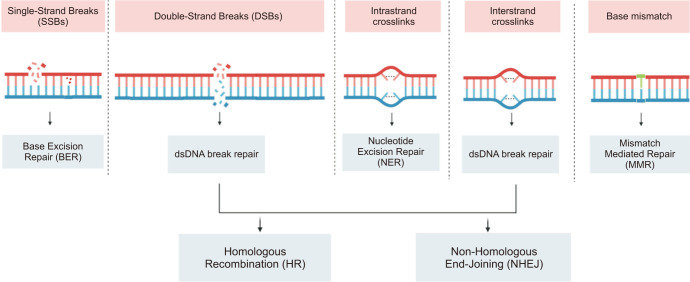
Different types of DNA damage exist, including single-strand breaks (SSBs), double-strand breaks (DSBs), base mismatch, and cross-linking (Fig. 1). Each type necessitates specific repair mechanisms to restore DNA integrity. SSBs are repaired through the base excision repair (BER) pathway, where specialized glycosylases identify and remove damaged bases, and subsequent endonucleases and DNA polymerases process the resulting basic sites to reinstate the accurate nucleotide sequence. DDBs can be repaired through two primary pathways: non-homologous end joining (NHEJ) and homologous recombination (HR). NHEJ involves direct ligation of the broken DNA ends, potentially resulting in small insertions or deletions at the repair site. In contrast, HR requires a homologous DNA template for precise repair without introducing errors. Base damage can be repaired through various pathways, including BER and nucleotide excision repair (NER). NER is particularly vital for repairing bulky adducts induced by environmental carcinogens, whereas BER can address damage caused by reactive oxygen species. Cross-linking damage occurs when two DNA strands become covalently linked and is repaired through the Fanconi anemia pathway, which involves a complex sequence of steps to eliminate crosslinked DNA and restore the correct nucleotide sequence. DNA repair mechanisms are critical for preserving the stability and accuracy of genetic material in living organisms. Deficiencies in DNA repair pathways can contribute to genomic instability, which is associated with the development of cancer and other diseases [9].
Fig. 1. The different DNA damage response pathways. dsDNA: double-stranded DNA. Figure created with BioRender.com.
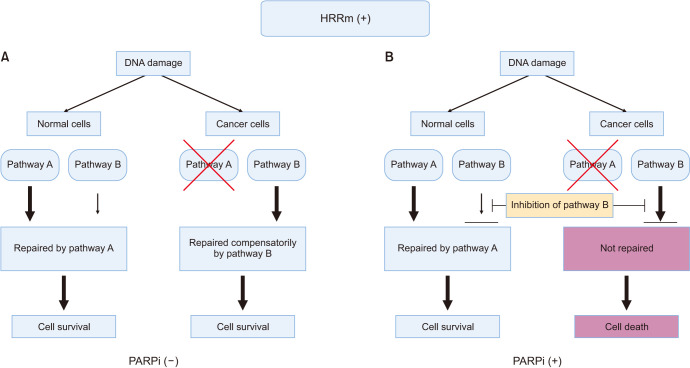
Synthetic lethality is a concept utilized in molecular biology and pharmacology, describing a phenomenon where the simultaneous inactivation of two genes results in cell death, whereas the inactivation of either gene alone is non-lethal. In cancer research, this concept is employed as a therapeutic strategy to selectively eliminate cancer cells with deficiencies in DNA repair pathways. The discovery of synthetic lethality originated from genetic studies conducted on model organisms such as fruit flies and yeast. Researchers observed that mutations in two non-essential genes, which individually did not impact the organism's viability, became lethal when combined. Expanding on this principle, it was later realized that many tumors carry gene mutations that create vulnerabilities exploitable through synthetic lethality-based therapies. The principle of synthetic lethality, in which specific tumor-cell mutations can be exploited to selectively kill cancer cells, has been used in cancer research. This approach involves targeting a pathway or protein that is essential specifically for cancer cell survival only (Fig. 2), with the goal to create a synthetic lethal interaction between a drug and a specific genetic defect or mutation in cancer cells [9,10].
Fig. 2. The principle of synthetic lethality. The concept of synthetic lethality is based on the idea that DNA damage is often repaired by multiple pathways. In this example, pathways A and B are functional in normal cells, while pathway A is impaired in cancer cells. (A) In the absence of an inhibitor for pathway B, cancer cells can survive because the alternative pathway B compensates for the defect in pathway A. (B) When cancer cells are treated with a PARPi for pathway B, both pathways are blocked, leading to cell death. However, normal cells are not affected because the inhibition of pathway B is compensated by the intact pathway A. PARPi: poly(ADP-ribose) polymerase inhibitors. Figure created with BioRender.com.
PARPi represent a class of drugs capable of creating synthetic lethal interactions in cancer cells with impairments in the HR DNA repair pathway. HR repairs DSBs, and mutations in genes like BRCA1 and BRCA2, involved in this pathway, generate vulnerabilities that can be exploited using PARPi. By blocking the repair of SSBs, PARPi leads to the accumulation of DSBs, proving lethal in cells with HR deficiencies. synthetic lethality demonstrates promise as a cancer therapy strategy due to its selectivity, resulting in fewer adverse effects and improved outcomes for patients [11].
RATIONALE FOR USE OF PARPi IN PC
1. DNA repair pathways and the role of DNA damage repair genes in PC
The integrity of DNA is constantly under threat from various agents and processes, which can directly or indirectly modify its sequence. When DNA is damaged or repaired inaccurately, mutations that initiate and promote tumor formation can arise. To mitigate the effects of DNA damage, healthy cells have developed a set of molecular pathways collectively known as the DNA damage response (DDR). These pathways enable the detection of damage, temporary halting of the cell cycle, and subsequent repair, all crucial for preserving genome stability [12].
The DDR encompasses interconnected pathways responsible for repairing various types of damage. Repair of DNA DSBs can be accomplished through HR or NHEJ. SSB repair involves the BER pathway. Critical proteins such as BRCA1, BRCA2, PALB2, ATM, CHEK1, CHEK2, and RAD51 play roles in HR, while PARP1 and PARP2 are essential for BER [13]. These proteins contribute to DSB repair by promoting HR activation and inhibiting less conservative repair mechanisms like NHEJ. The absence of PARPs can lead to impaired HR, resulting in a prevalence of non-conservative DNA repair pathways [14].
1) DDR and HRR Mutations in PC
Mutations affecting the DDR have been detected in both localized PC and mPC. The most frequently mutated DDR genes in PC include BRCA1, BRCA2, ATM, CHEK2, and RAD51D. Among these mutations, BRCA2 mutations are the most commonly observed (12%–18%), followed by ATM (3%–6%), CHEK2 (2%–5%), and BRCA1 (<2%) in the context of HRR [15,16]. The prevalence of DDR mutations ranges from 5% to 30%, depending on the study population and methodology employed. However, patients with advanced PC and those with a family history of PC tend to exhibit a higher prevalence of DDR mutations. In a study involving 692 patients with mCRPC, 23% exhibited alterations in DDR genes [17].
Numerous studies have established a strong association between frequent deleterious mutations in DDR genes and advanced PC. Specifically, germline mutations in BRCA1/2 have been linked to an increased risk of aggressive PC, as well as a higher likelihood of nodal involvement and distant metastasis at the time of diagnosis [18]. Germline BRCA2 mutations, in particular, elevate the risk of developing PC by eight-fold at the age of 65 [19]. In localized disease, germline BRCA1/2 mutations are associated with disease progression in patients under active surveillance, a high recurrence rate following curative treatment [20], and a more aggressive disease course [21]. The prevalence of germline mutations varies across countries and ethnic groups [22]. The International Stand-Up to Cancer/PC Foundation (SU2C-PCF) team conducted a study involving 150 patients with mPC and identified germline DDR mutations in 8% of cases and somatic DDR mutations in 23% of cases. BRCA2 was the most commonly mutated gene (13%), followed by ATM (7.3%), MSH2 (2%), BRCA1, FANCA, MLH1, RAD51B, and RAD51C [23]. Pritchard et al [24] investigated germline mutations in 692 men with mCRPC without a family history and detected 84 deleterious mutations in 20 DNA repair genes among 82 men (11.8%), with BRCA2 being the most prevalent (5.3%). Nicolosi et al [25] analyzed 3,607 men with PC and identified germline mutations in 620 individuals (17.2%), with 30.7% having BRCA1/2 mutations. Other mutated genes included ATM, PALB2, CHEK2, and mismatch repair genes PMS2 and MLH1/2/6.
Somatic mutations contribute to carcinogenesis [26]. Robinson et al [23] discovered that 23% of patients with mCRPC had somatic mutations in DNA repair pathway genes, and BRCA2 and ATM were the most commonly mutated genes [13]. Several studies reported that 12% of patients with PC carry BRCA1/2 mutations, whereas 8% have ATM mutations, with a higher occurrence in patients with mCRPC [27]. Abida et al [16] observed somatic BRCA2 mutations in tumors before their progression to metastatic disease. Somatic BRCA2 mutations occurred early in tumors of patients who later develop metastatic disease, whereas ATM alterations were more prevalent in CRPC.
2) Mechanism of action of PARPi in PC
Patients with PC with the BRCA2 mutation have a more favorable response to carboplatin-based chemotherapy than those without the BRCA2 mutation. When carboplatin-based chemotherapy is administered in the presence of DNA strand breaks caused by HRR damage, it can generate synergistic lethal effects on tumor cells. These findings elucidate the mechanisms underlying PARPi [28].
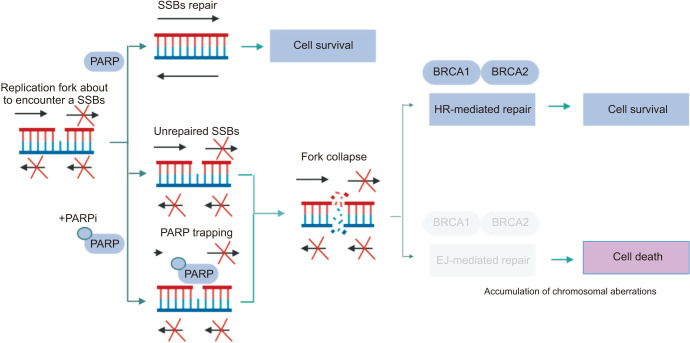
PARPi exert a pharmacologically similar function to nicotinamide and primarily operate through two mechanisms (Fig. 3) [11]. First, they inhibit the catalytic activity of PARP by competitively binding to the active site of NAD+, thereby impeding the repair of SSBs and leading to their conversion into DSBs [29]. Second, PARPi trap PARP-1 on damaged DNA by inhibiting auto-poly(ADP-ribosyl)lation (PARylation) or enhancing DNA affinity for the catalytic site by inducing allosteric changes in the PARP-1 structure [30]. Additionally, PARP-1 contributes to the delay in replication fork progression, impeding DSBs repair and ultimately resulting in cell death [31]. Importantly, PARP trapping cannot occur independently of the catalytic inhibition of PARylation because PARP-1 and PARP-2 cannot be disengaged from DNA until PARPi dissociate from the active site after successful capture [32]. These mechanisms provide the basis for the concept of synthetic lethality, in which the deficiency of BRCA1/2 genes and PARP inhibition synergistically induce tumor cell death [33]. Tumor cells with BRCA mutations are considerably more sensitive to PARPi, exhibiting an approximately 1000-fold higher sensitivity than wildtype BRCA cells [34]. Consequently, the initial focus of PARPi development was on populations harboring BRCA1/2 mutations. However, with advances in molecular biology, PARPi therapy has gradually expanded to include defects in other DDR genes, including ATM, ATR, CHK1, CHK2, DSS1, RPA1, NBSI, FANCD2, FANCA, CDK12, P ALB2, BRI P1, RAD51B, RAD51C, RAD51D, and RAD54 [35]. In the PRIMA trial, PARPi extended the survival of some patients with cancer without HRR-associated genetic alterations. In patients with advanced ovarian cancer who responded to platinum-based chemotherapy, niraparib, a PARPi, as first-line maintenance therapy, has shown significant improvements in progression-free survival (PFS) regardless of patient HRR biomarker status. Consequently, the U.S. Food and Drug Administration (FDA) granted approval for the first PARPi therapy in April 2020 for use in this population without BRCA mutations [36]. Nonetheless, the full therapeutic potential of PARPi for the treatment of tumors requires comprehensive exploration and further investigation.
Fig. 3. Mechanism of action of PARP inhibitors. Initially, it was hypothesized that PARP inhibitors exerted their effects by inhibiting PARylation and inducing cytotoxicity. However, subsequent findings revealed that the primary mechanism underlying tumor cell death was the entrapment of the PARP1 enzyme at sites of DNA damage. When DNA damage occurs, resulting in single-strand breaks (SSBs), PARP1 plays a crucial role in their precise repair. However, when PARP1 becomes entrapped, it poses a significant threat to the progression of replication forks during the S phase of the cell cycle. Consequently, this leads to the collapse of replication forks and the generation of double-strand breaks (DSBs). In cells with intact BRCA genes, these breaks can be accurately repaired through the process of homologous recombination (HR) without introducing errors. Conversely, cells deficient in BRCA1/2 exhibit impaired HR and instead rely on error-prone DNA end-joining (EJ) pathways, such as classical non-homologous EJ or alternative EJ, to mend the DSBs arising from replication fork collapse. This process triggers the accumulation of chromosomal abnormalities and ultimately culminates in cell death through mitotic catastrophe. PARP: poly(ADP-ribose) polymerase, PARylation: poly(ADP-ribosyl) ation. Figure created with BioRender.com.
CLINICAL DEVELOPMENT OF PARPi MONOTHERAPIES IN PC
Olaparib, the initial drug of its kind, was initially developed for breast and ovarian cancer, and subsequently for pancreatic cancer and PC. It became the first PARPi to receive FDA approval for use in PC [37].
The PROfound phase III trial, which was based on two phase II clinical trials (TOPARP-A and TOPARP-B), assessed the effectiveness of olaparib in men with mCRPC who had mutations in DNA repair genes, particularly BRCA1, BRCA2, and ATM, and experienced disease progression after prior treatment with enzalutamide or abiraterone acetate plus prednisone. Patients with mutations were randomly assigned to receive either olaparib or abiraterone/enzalutamide. The trial consisted of two cohorts: cohort A, consisting of patients with mutations in BRCA1, BRCA2, or ATM (245 patients), and cohort B, consisting of patients with alterations in 12 other specified genes (142 patients). The study demonstrated positive results with olaparib, showing improved PFS based on imaging (radiographic PFS) in cohort A. The olaparib group exhibited a longer radiographic PFS compared to the control group (7.4 months vs. 3.6 months, hazard ratio: 0.34, 95% confidence interval [CI]: 0.25–0.47; p<0.001). Moreover, the olaparib group demonstrated a higher objective response rate and overall survival (OS) in cohort A: 33% vs. 2%, and 19.1 months vs. 14.7 months, respectively (hazard ratio: 0.69, 95% CI: 0.50–0.97, p=0.0175). However, 67% of patients in the control arm switched to olaparib after radiographic progression. There was no statistically significant PFS benefit in the combined cohort. The frequency of severe adverse events (AEs) was higher in the olaparib group compared to the control group. Anemia, nausea, and fatigue or weakness were the most common AEs of any severity in the olaparib group. The olaparib group reported a total of 11 cases (4% of patients) of pulmonary embolism, in contrast to 1 case (1%) in the control group, with no resulting fatalities [38]. In May 2020, the FDA-approved olaparib for the treatment of mCRPC that had progressed after AR inhibitor therapy in patients with somatic mutations in any DNA repair gene or germline mutations in BRCA1, BRCA2, or ATM genes. Testing for relevant alterations in germline and somatic DNA is now considered standard care for these patients [39]. In Europe, the approval by the European Medicines Agency (EMA) is limited to patients with alterations in BRCA1 or BRCA2 genes.
Rucaparib is another PARPi that has shown promise in the treatment of PC. A single-arm phase II trial called TRITON-2 assessed the efficacy of rucaparib in patients with mCRPC who had mutations in DNA repair genes and experienced disease progression after receiving 1–2 AR inhibitors and paclitaxel. The cohort with BRCA1/2 mutations demonstrated an objective response rate of 43.5% and a prostate-specific antigen (PSA) response rate of 54.8%. The most common grade ≥3 AEs were anemia (25.2%). Rucaparib exhibited significant anti-tumor activity and an acceptable safety profile in treating mCRPC patients with deleterious BRCA gene mutations [40]. Consequently, the FDA granted accelerated approval for rucaparib in May 2020 for use in adult patients with mCRPC associated with deleterious BRCA mutations (germline and/or somatic) who had previously received AR inhibitors and paclitaxel. Rucaparib is currently being evaluated in the phase III TRITON3 trial (NCT02975934), which compares rucaparib to the physician's choice of abiraterone or enzalutamide in patients with mCRPC and deleterious BRCA1, BRCA2, or ATM mutations.
Niraparib is a highly selective oral inhibitor of PARP1 and PARP2 with superior trapping potency and cytotoxicity compared to olaparib [41]. The GALAHAD trial, a single-arm study, evaluated the safety and efficacy of niraparib in patients who experienced disease progression after prior treatment with paclitaxel and an AR inhibitor. As of May 23, 2019, interim findings from the study indicated that niraparib treatment resulted in an overall response rate (ORR) of 41%, a complete response rate of 63%, and median radiographic PFS (rPFS) and OS of 8.2 and 12.6 months, respectively, in patients with BRCA1/2 mutations [42]. In October 2019, the FDA designated niraparib as a breakthrough therapy for the treatment of mCRPC patients with BRCA1/2 mutations who had previously been treated with paclitaxel and an AR inhibitor.
Talazoparib is a potent PARPi that exhibits high catalytic enzyme inhibition and effective trapping of PARP1 DNA errors [43]. The drug was assessed in the open-label phase II trial TALAPRO-1, which enrolled patients with metastatic mCRPC and mutations in DNA damage response-homologous recombination repair (DDR-HRR) genes (ATM, ATR, BRCA1, BRCA2, CHEK2, FANCA, MLH1, MRE11A, NBN, PALB2, and RAD51C). The study demonstrated a radiological response rate of 29.8%, with a higher response rate observed in patients with BRCA1/2 mutations. The most common grade 3–4 AEs requiring emergency treatment included anemia, thrombocytopenia, and neutropenia. TALAPRO-1 trial provided evidence of sustained anti-tumor activity of talazoparib in heavily pretreated patients with mCRPC and DDR-HRR mutations [44].
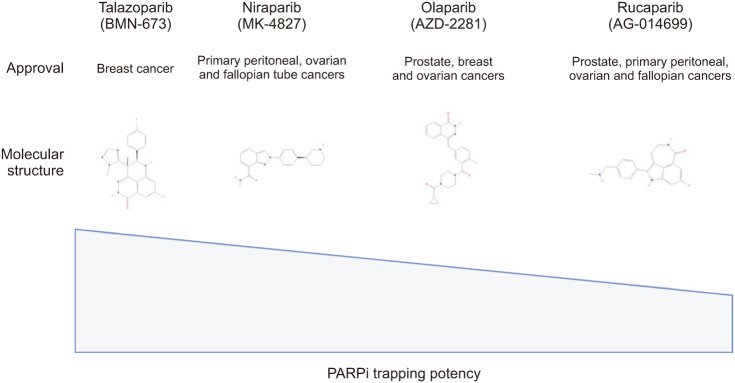
While there have been no clinical trials directly comparing different PARPi, preclinical studies indicate variations in the ability of PARPi to trap PARP enzymes across different tumor cells, including PC cells. Among the tested inhibitors, talazoparib exhibited the highest PARP trapping capacity, followed by niraparib, olaparib, and rucaparib (Fig. 4) [45].
Fig. 4. Molecular structure of PARPi and their capacity of trapping PARP. PARP: poly(ADP-ribose) polymerase, PARPi: PARP inhibitors. Figure created with BioRender.com.
Currently, PARPi is approved as a monotherapy and demonstrates effectiveness only in a small population of PC patients with BRCA1/2 gene mutations or mutations in HRR-related genes. The incidence of these mutations in mCRPC patients is low (only 8.8% for BRCA1/2) [46]. Therefore, it is crucial to urgently investigate the efficacy of PARPi in other PC patients without BRCA1/2 mutations. Additionally, similar to other targeted therapies, advanced PC patients may develop resistance to PARPi. Hence, combining PARPi with other therapies could be a valuable strategy to enhance efficacy or overcome resistance.
PARPi COMBINATION THERAPIES IN PC
Combining PARPi treatments serves two primary objectives: firstly, to extend the efficacy of PARPi therapy by delaying resistance development, and secondly, to broaden the scope of patients who can derive benefits from PARPi treatment by overcoming potential resistance associated with monotherapy [47].
1. Combinations with AR-signaling inhibitors
The primary focus of PC treatment revolves around targeting the AR pathway [48]. Previous investigations have explored the synergistic potential between the AR and DDR pathways, supported by preclinical evidence. These studies have revealed three key mechanisms through which these drug groups interact. Firstly, PARPi enhance the anti-androgenic effect by inhibiting PARP, thereby promoting AR transcription [49]. Secondly, ADT can enhance sensitivity to PARPi by inducing PARP overexpression [50]. Lastly, anti-androgen therapy can suppress the expression of DDR genes, leading to genomic instability and an increased likelihood of DDR mutations. Consequently, this phenomenon is referred to as the BRCAness phenotype [50].
Numerous combinations of PARPi and anti-androgen agents have been investigated, yielding varied outcomes. A phase II trial evaluating the combination of veliparib and abiraterone found no discernible differences in outcomes [51]. However, a double-blind, placebo-controlled study comparing olaparib plus abiraterone to abiraterone alone demonstrated a statistically significant increase in rPFS among patients with mCRPC [52]. Retrospective analysis of genomic profiles indicated that both HRR mutation carriers and non-carriers derived benefits from this combination therapy [53].
In 2022, Kim N Chi et al [54] presented the preliminary findings of the MAGNITUDE trial (NCT03748641) at the ASCO-GU Conference. This phase III trial was a randomized, double-blind study that aimed to evaluate the efficacy of niraparib in combination with abiraterone acetate and prednisone as a first-line therapy for patients with mCRPC. The trial enrolled patients who tested positive or negative for HRR biomarkers. Eligible participants were mCRPC patients who had received up to four months of prior abiraterone treatment. The enrolled patients were divided into two groups: those with specific gene alterations related to HRR biomarkers (ATM, BRCA1, BRCA2, BRIP1, CDK12, CHEK2, FANCA, HDAC2, and PALB2) and those without these gene alterations. They were randomly assigned in a 1:1 ratio to receive either niraparib 200 mg once daily in combination with abiraterone or placebo in combination with abiraterone. The primary endpoint of the study was rPFS, and the secondary endpoints included time to initiation of cytotoxic chemotherapy, time to symptomatic progression, OS, time to PSA progression, and ORR. A total of 423 HRR biomarker-positive patients were randomized to receive either niraparib+abiraterone (n=212) or placebo+abiraterone (n=211). The median age of the patients was 69 years, with 23% having received prior treatment with abiraterone, 21% having visceral metastases, and 53% having BRCA1/2 mutations. The median follow-up period was 18.6 months. In the subgroup of patients with BRCA1/2 mutations, the combination of niraparib + abiraterone showed a significant 47% improvement in rPFS compared to the placebo + abiraterone group (16.6 vs. 10.9 months). Similarly, in all HRR biomarker-positive patients, the combination therapy demonstrated a 26% improvement in rPFS (16.5 vs. 13.7 months; hazard ratio 0.74, 95% CI: 0.57–0.97). The results of rPFS assessed by investigators were consistent with those of a blinded, independent central review. However, the planned analysis of 233 HRR biomarker-negative patients showed no benefit from adding niraparib to abiraterone for the composite endpoint (first occurrence of PSA progression or rPFS; hazard ratio: 1.09, 95% CI: 0.75–1.59). Among HRR biomarker-positive patients, 67% and 46.4% experienced grade 3/4 AEs in the niraparib+abiraterone and placebo+abiraterone groups, respectively, and the treatment discontinuation rates were 9% and 3.8%, respectively. There were no significant differences in overall quality of life between the two treatment groups, as assessed using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) scale [54].
The phase III double-blind PROpel trial (NCT03732820) presented notable findings at the 2022 ASCO-GU Conference. The trial enrolled men with mCRPC who had not previously received abiraterone and had discontinued another androgen receptor pathway inhibitor more than a year before enrollment. Participants were categorized based on the location of distant metastasis and prior receipt of docetaxel for metastatic hormone-sensitive PC (mHSPC). They were then randomly assigned to receive either full-dose abiraterone (1,000 mg daily) with placebo or full-dose olaparib (300 mg BID). The primary endpoint of the trial was the time until disease progression, as assessed by the investigator rPFS. The analysis included 399 patients in the abiraterone plus olaparib group and 397 patients in the abiraterone plus placebo group. The incidence of HRR mutations was comparable between the two groups (28% in the olaparib group and 29% in the placebo group). The trial demonstrated a 34% reduction in progression or death when olaparib was added to abiraterone (rPFS hazard ratio=0.66, 95% CI: 0.54–0.81; p<0.0001). The addition of olaparib extended the median rPFS by 8.2 months (24.8 months vs. 16.6 months). These findings were confirmed by a blinded independent central review, which showed a 39% improvement in rPFS and an 11.2-month improvement in rPFS with olaparib treatment. The results were consistent across all predefined subgroups, with no significant differences observed. However, patients with HRR mutations had a hazard ratio of 0.50 (95% CI: 0.34–0.73), indicating greater benefit, while those without HRR mutations had a hazard ratio of 0.76 (95% CI: 0.60–0.97). Preliminary data suggested a trend towards improved OS when olaparib was added to abiraterone, with a hazard ratio of 0.86 (95% CI: 0.66–1.12; p=0.29). The combination of abiraterone and olaparib resulted in a toxicity profile similar to previous reports. The occurrence of AEs or AE-related deaths was comparable between the two groups. However, a higher percentage of patients receiving abiraterone plus olaparib (47%) experienced grade 3 or higher AEs compared to those receiving abiraterone plus placebo (38%). The most common AE observed in patients receiving olaparib was anemia, which occurred in 46% of patients and was grade 3 or higher in 15% of patients [55]. The phase III PROpel study, presented at the 2023 ASCO GU Cancers Symposium, revealed the final results indicating that the addition of olaparib to standard care abiraterone as a first-line treatment for mCRPC resulted in longer PFS compared to abiraterone alone, and there was a tendency towards improved median OS. Although the OS data were still premature, in the intention-to-treat population, the median OS was 42.1 months with olaparib plus abiraterone, while it was 34.7 months with abiraterone plus placebo (with a maturity rate of 47.9%). The hazard ratio was 0.81 (95% CI: 0.67–1.00, p=0.0544), suggesting a potential survival advantage with the combination therapy. Notably, the greatest survival benefits were observed in patients who tested positive for the BRCA mutation [56].
These two combination trials exhibit significant differences in their outcomes. The efficacy of olaparib seems to be independent of the patients' HRR status, whereas niraparib demonstrates benefits limited to cancers with HRR mutations. These findings carry several immediate implications: firstly, olaparib is currently recommended as the preferred PARPi for mCRPC: secondly, these results position PARPi as a first-line treatment for mCRPC; and thirdly, the results demonstrate a therapeutic advantage for patients regardless of their HRR status, which distinguishes it from other PARPi. Presently, numerous ongoing clinical trials are investigating these combinations in other clinical populations, such as mHSPC, non-mCRPC, and high-risk, non-metastatic/localized PC. The outcomes of these trials will become available in the coming years, as summarized in Table 1.
Table 1. Ongoing trials of PARPi combination therapies with NHTs in PC.
| PARPi | Trial, NCT No. | Study phase/design | Population | Combined agent & grouping | Primary outcomes | |
|---|---|---|---|---|---|---|
| Olaparib | PROact, NCT05167175 | Phase 2/Single Group Assignment | mHSPC (HRR+) | Olaparib+AA | rPFS | |
| NU_16U05/NCT03012321 | Phase 2/RCT | mCRPC | AA vs. Olaparib vs. Olaparib+AA | Objective PFS | ||
| D081SC00001Sub/NCT05171816 | Phase 3/RCT | mCRPC | Olaparib+AA vs. Placebo+AA | rPFS | ||
| PROpel/NCT03732820 | Phase 3/RCT | mCRPC | Olaparib+AA vs. Placebo+AA | rPFS | ||
| Rucaparib | CASPAR/NCT04455750 | Phase 3/RCT | mCRPC (HRR+) | Rucaparib+Enzalutamide vs. Placebo+Enzalutamide | rPFS; OS | |
| Niraparib | ASCLEPIuS/NCT04194554 | Phase 1, 2/Single Group Assignment | PC | AA+Leuprolide+100 mg/200 mg Niraparib but held for 5 days (+/– 2 days) prior to RT, during SBRT, and 5 days (+/– 2 days) after last fraction of SBRT AA+Leuprolide+200 mg Niraparib without breaks during SBRT until completion of 6 cycles | DLT; Proportion of patients experiencing biochemical failure | |
| AMPLITUDE/NCT04497844 | Phase 3/RCT | mHSPC (HRR+) | Niraparib+AA vs. Placebo+AA | rPFS | ||
| MAGNITUDE/NCT03748641 | Phase 3/RCT | mCRPC | Treatment: | rPFS | ||
| Phase RCT: Niraparib+AA vs. Placebo+AA; | ||||||
| Phase OLE: all receive Niraparib+AA | ||||||
| Cohort 1: Participants with mCRPC and HRR Gene Alteration; | ||||||
| Cohort 2: Participants with mCRPC and No HRR Gene Alteration; | ||||||
| Cohort 3 (Open-label): Participants with mCRPC | ||||||
| Talazoparib | ZZ-First/NCT04332744 | Phase 2/RCT | mHSPC | Talazoparib+Enzalutamide+ADT vs. Enzalutamide+ADT | PSA-CR | |
| TALAPRO-2/NCT03395197 | Phase 3/RCT | mCRPC | Talazoparib+Enzalutamide vs. Placebo+Ezalutamide | Confirm the dose of Talazoparib (part 1); rPFS (part 2) | ||
| TALAPRO-3/NCT04821622 | Phase 3/RCT | mHSPC (DDR mutated) | Talazoparib+Enzalutamide vs. Placebo+Enzalutamide | rPFS | ||
mHSPC: metastatic hormone-sensitive PC, HRR: homologous recombination repair, rPFS: radiographic progression-free survival, mCRPC: metastatic castration-resistant prostate cancer, RCT: randomized controlled trial, OS: overall survival, PC: prostate cancer, DLT: dose limiting toxicities, ADT: androgen deprivation therapy, PSA-CR: prostate specific antigen complete response, DDR: DNA damage response.
2. Combinations with immunotherapy
Studies have also explored the combination of PARPi with immunotherapy. It is important to note that currently, there is no FDA approval for immune checkpoint inhibitors (ICI) specifically for PC, except for pembrolizumab's tissue-agnostic approval in tumors with a high tumor mutational burden (TMB) or microsatellite instability. However, emerging data on ICI suggest that certain patient populations may benefit from incorporating them into their treatment strategies. The combination of PARPi and ICI has been investigated in several studies, with the hypothesis that PARPi-induced DNA damage may influence the tumor immune microenvironment [47].
Studies on tumors with high TMB have indicated that TMB can serve as a surrogate marker for neoantigen load and potentially predict the response to ICI [57,58]. There is also evidence suggesting a potential association between high TMB and HRR [59]. Therefore, combining PARPi with ICI appears to be a logical approach for targeting the responses of patients with HRR deficiency. Additionally, it is hypothesized that the DNA repair disruption caused by PARPi could enhance the neoantigen load, leading to increased TMB and potentially making tumors more susceptible to ICI therapy.
The KEYNOTE-365 study is a phase Ib/II trial investigating pembrolizumab in combination with other agents in patients with mCRPC who had previously received docetaxel chemotherapy [60]. HRR alterations were not mandatory for enrollment, but there were challenges in determining the HRR status due to issues with the circulating tumor DNA assay used. In cohort A, patients received pembrolizumab and olaparib. The study's primary endpoints were safety, PSA response rate of 50% (PSA50), and ORR as evaluated by an independent review. Out of the 102 treated patients, 29% were PD-L1 positive. A PSA50 response was observed in 15% of patients, with an ORR of 8.5% and a disease control rate of 26%. The median rPFS was 4.5 months, and the median OS was 14 months. Immune-mediated AEs occurred in 12 patients (12%), with approximately 4% experiencing grade 3–5 toxicity.
Currently, the JAVELIN PARP Medley trial is underway, investigating the combination of talazoparib and avelumab [61]. This trial follows a phase Ib/II basket design and includes patients with advanced solid tumors, including mCRPC, irrespective of HRR status. Patients enrolled in the trial receive a combination of avelumab and talazoparib. In the phase II trial's mCRPC cohort, no confirmed objective responses (OR) were reported; however, two out of 21 patients showed PSA responses. Among the HRR-positive mCRPC subgroup, the ORR was 11.1%. These preliminary findings lay the groundwork for future clinical trials in this area.
3. Combinations with chemotherapy
The combination of PARPi and cytotoxic chemotherapy has been investigated to capitalize on the cytotoxic effects of chemotherapy in synthetic lethality. A small study involving 25 patients with mCRPC explored the combination of veliparib and temozolomide [62]. Eligible patients had experienced disease progression after at least one docetaxel-based chemotherapy regimen. The treatment regimen consisted of cycles of veliparib and temozolomide. The trial results indicated tolerability but limited efficacy. No OR were observed, and only two out of 25 patients had a confirmed PSA decline of 30% or more. The study did not assess HRR status, and the lack of activity could be attributed to the absence of patient selection based on HRR status, the use of veliparib (which is relatively less potent compared to other PARPi), or the selection of temozolomide (which is not commonly used as a cytotoxic agent in PC management). Currently, an ongoing trial is recruiting participants to evaluate the combination of talazoparib and temozolomide for the treatment of mCRPC (NCT04019327).
The use of a combination of chemotherapy and a PARPi in clinical studies is uncommon due to concerns about increased toxicity. However, the role of PARPi as maintenance therapy following cytotoxic treatment is currently being investigated. Two ongoing studies (NCT03442556 and NCT03263650) specifically address this question.
RESISTANCE TO PARPi
Despite the favorable treatment outcomes observed, a significant number of patients eventually develop resistance to PARPi. Acquired resistance mechanisms vary and include frame shift or nonsense mutations, multiple reversion mutations in HRR genes such as BRCA-1, BRCA-2, RAD51C, RAD51D, and PALB2, protection of DNA replication fork, expression of different BRCA-1 variants, and demethylation of promoter regions of BRCA-1 and RAD51C [63,64,65] Resistance to PARPi can also arise from mechanisms that promote the phosphorylation of PARP-1, leading to a reduction in PARP trapping [66]. Additionally, the presence of ABC transporters can diminish the effectiveness of PARPi [67]. Understanding these resistance mechanisms, particularly PARPi's involvement in processes unrelated to DNA repair, is crucial for enhancing the efficacy of PARPi as anticancer agents and developing strategies to overcome resistance and enhance sensitivity to PARPi. Numerous studies have demonstrated that combining PARPi with other agents can improve therapeutic efficacy and overcome drug resistance. For instance, in the phase Ib/II KEYNOTE-365 study, the combination of olaparib and pembrolizumab exhibited anticancer efficacy and showed promising safety profiles in patients with mCRPC [68]. Another phase II trial revealed that patients who received the combination of olaparib and abiraterone had improved survival outcomes compared to those who received placebo plus abiraterone [69]. Moving forward, it will be crucial to focus on evaluating the potential of combining PARPi with additional drugs, creating more opportunities for the treatment of mCRPC.
CONCLUSION AND FUTURE PERSPECTIVE
PARPi represent the first therapeutic agent based on the synthetic lethal concept. The initial studies involving PARPi in PC marked the first biomarker-driven phase II-III trials in this field. Over the years, evidence-based investigations have consistently demonstrated the efficacy and safety of PARPi, particularly in patients with mCRPC harboring HRR-related genetic mutations, with a particular emphasis on BRCA1/2 mutations. As the first FDA-approved targeted therapy for biomarker-selected advanced PC patients, PARPi is currently indicated as monotherapy in the second-line setting or beyond.
Although defects in HRR genes have been identified in approximately 20% to 25% of advanced PC patients, the therapeutic implications of these defects are not yet fully elucidated. Research on PARPi has shown significant anti-tumor activity, but the optimal set of genetic markers to consider for patient selection remains unclear. Despite concerns regarding PARPi resistance, combination strategies have emerged as a potential means to circumvent or delay resistance development. Furthermore, combination therapies offer a way to incorporate PARPi into patient management, even in the absence of underlying HRR alterations.
Future research endeavors should prioritize addressing crucial questions, such as identifying patient subgroups that can derive the greatest benefits from PARPi treatment, determining the optimal treatment stages for its implementation, and refining combination approaches. With ongoing clinical trials producing additional results, PARPi holds substantial promise as a treatment strategy that can be potentially employed across various stages of cancer progression.
Acknowledgements
None.
Footnotes
Conflict of Interest: The author has nothing to disclose.
Funding: None.
References
- 1.Uo T, Sprenger CC, Plymate SR. Androgen receptor signaling and metabolic and cellular plasticity during progression to castration resistant prostate cancer. Front Oncol. 2020;10:580617. doi: 10.3389/fonc.2020.580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrasekar T, Yang JC, Gao AC, Evans CP. Mechanisms of resistance in castration-resistant prostate cancer (CRPC) Transl Androl Urol. 2015;4:365–380. doi: 10.3978/j.issn.2223-4683.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Zhou Q, Hankey W, Fang X, Yuan F. Second generation androgen receptor antagonists and challenges in prostate cancer treatment. Cell Death Dis. 2022;13:632. doi: 10.1038/s41419-022-05084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99. doi: 10.1016/j.eururo.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Wang L, Wang Y, Zhang C, Hong Z, Han Z. The synthetic lethality of targeting cell cycle checkpoints and PARPs in cancer treatment. J Hematol Oncol. 2022;15:147. doi: 10.1186/s13045-022-01360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Diao L, Zhang C, Wang F, Guan X, Yao X. Use of PARP inhibitors in prostate cancer: from specific to broader application. Front Endocrinol (Lausanne) 2023;14:1164067. doi: 10.3389/fendo.2023.1164067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pezaro C. PARP inhibitor combinations in prostate cancer. Ther Adv Med Oncol. 2020;12:1758835919897537. doi: 10.1177/1758835919897537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clancy S. DNA damage & repair: mechanisms for maintaining DNA integrity. Nature Educ. 2008;1:103 [Google Scholar]
- 9.Cannan WJ, Pederson DS. Mechanisms and consequences of double-strand DNA break formation in chromatin. J Cell Physiol. 2016;231:3–14. doi: 10.1002/jcp.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 11.Rose M, Burgess JT, O'Byrne K, Richard DJ, Bolderson E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol. 2020;8:564601. doi: 10.3389/fcell.2020.564601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhmoud JF, Woolley JF, Al Moustafa AE, Malki MI. DNA damage/repair management in cancers. Cancers (Basel) 2020;12:1050. doi: 10.3390/cancers12041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirza MR, Pignata S, Ledermann JA. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann Oncol. 2018;29:1366–1376. doi: 10.1093/annonc/mdy174. [DOI] [PubMed] [Google Scholar]
- 15.Nombela P, Lozano R, Aytes A, Mateo J, Olmos D, Castro E. BRCA2 and other DDR genes in prostate cancer. Cancers (Basel) 2019;11:352. doi: 10.3390/cancers11030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017:PO.17.00029. doi: 10.1200/PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo J, Giri VN. Germline testing and genetic counselling in prostate cancer. Nat Rev Urol. 2022;19:331–343. doi: 10.1038/s41585-022-00580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Congregado B, Rivero I, Osmán I, Sáez C, Medina López R. PARP inhibitors: a new horizon for patients with prostate cancer. Biomedicines. 2022;10:1416. doi: 10.3390/biomedicines10061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37:490–503. doi: 10.1200/JCO.18.00358. [DOI] [PubMed] [Google Scholar]
- 22.Rebbeck TR. Prostate cancer genetics: variation by race, ethnicity, and geography. Semin Radiat Oncol. 2017;27:3–10. doi: 10.1016/j.semradonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. Erratum in: Cell 2015;162:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolosi P, Ledet E, Yang S, Michalski S, Freschi B, O'Leary E, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019;5:523–528. doi: 10.1001/jamaoncol.2018.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenschein C, Soto AM. Somatic mutation theory of carcinogenesis: why it should be dropped and replaced. Mol Carcinog. 2000;29:205–211. doi: 10.1002/1098-2744(200012)29:4<205::aid-mc1002>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013;63:920–926. doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pomerantz MM, Spisák S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer. 2017;123:3532–3539. doi: 10.1002/cncr.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min A, Im SA. PARP inhibitors as therapeutics: beyond modulation of PARylation. Cancers (Basel) 2020;12:394. doi: 10.3390/cancers12020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30:1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pommier Y, O'Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8:362ps17. doi: 10.1126/scitranslmed.aaf9246. Erratum in: Sci Transl Med 2016;8:368er7. [DOI] [PubMed] [Google Scholar]
- 33.Topatana W, Juengpanich S, Li S, Cao J, Hu J, Lee J, et al. Advances in synthetic lethality for cancer therapy: cellular mechanism and clinical translation. J Hematol Oncol. 2020;13:118. doi: 10.1186/s13045-020-00956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santiago-O'Farrill JM, Weroha SJ, Hou X, Oberg AL, Heinzen EP, Maurer MJ, et al. Poly(adenosine diphosphate ribose) polymerase inhibitors induce autophagy-mediated drug resistance in ovarian cancer cells, xenografts, and patient-derived xenograft models. Cancer. 2020;126:894–907. doi: 10.1002/cncr.32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adashek JJ, Jain RK, Zhang J. Clinical development of PARP inhibitors in treating metastatic castration-resistant prostate cancer. Cells. 2019;8:860. doi: 10.3390/cells8080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 37.Nizialek E, Antonarakis ES. PARP inhibitors in metastatic prostate cancer: evidence to date. Cancer Manag Res. 2020;12:8105–8114. doi: 10.2147/CMAR.S227033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 39.Hernando Polo S, Moreno Muñoz D, Rosero Rodríguez AC, Silva Ruiz J, Rosero Rodríguez DI, Couñago F. Changing the history of prostate cancer with new targeted therapies. Biomedicines. 2021;9:392. doi: 10.3390/biomedicines9040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abida W, Campbell D, Patnaik A, Sautois B, Shapiro J, Vogelzang NJ, et al. Preliminary results from the TRITON2 study of rucaparib in patients (pts) with DNA damage repair (DDR)-deficient metastatic castration-resistant prostate cancer (mCRPC): updated analyses. Ann Oncol. 2019;30(Suppl 5):v325–v325. [Google Scholar]
- 41.Bruin MAC, Sonke GS, Beijnen JH, Huitema ADR. Pharmacokinetics and pharmacodynamics of PARP inhibitors in oncology. Clin Pharmacokinet. 2022;61:1649–1675. doi: 10.1007/s40262-022-01167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith MR, Sandhu SK, Kelly WK, Scher HI, Efstathiou E, Lara PN, et al. Pre-specified interim analysis of GALAHAD: a phase 2 study of niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD) Ann Oncol. 2019;30(Suppl 5):v851–v934. [Google Scholar]
- 43.Boussios S, Abson C, Moschetta M, Rassy E, Karathanasi A, Bhat T, et al. Poly (ADP-ribose) polymerase inhibitors: talazoparib in ovarian cancer and beyond. Drugs R D. 2020;20:55–73. doi: 10.1007/s40268-020-00301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bono JS, Mehra N, Scagliotti GV, Castro E, Dorff T, Stirling A, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22:1250–1264. doi: 10.1016/S1470-2045(21)00376-4. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins TA, Shi Y, Rodriguez LE, Solomon LR, Donawho CK, DiGiammarino EL, et al. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res. 2015;13:1465–1477. doi: 10.1158/1541-7786.MCR-15-0191-T. [DOI] [PubMed] [Google Scholar]
- 46.Tukachinsky H, Madison RW, Chung JH, Gjoerup OV, Severson EA, Dennis L, et al. Genomic analysis of circulating tumor DNA in 3,334 patients with advanced prostate cancer identifies targetable BRCA alterations and AR resistance mechanisms. Clin Cancer Res. 2021;27:3094–3105. doi: 10.1158/1078-0432.CCR-20-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peyraud F, Italiano A. Combined PARP inhibition and immune checkpoint therapy in solid tumors. Cancers (Basel) 2020;12:1502. doi: 10.3390/cancers12061502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Attard G, Richards J, de Bono JS. New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clin Cancer Res. 2011;17:1649–1657. doi: 10.1158/1078-0432.CCR-10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weaver AN, Yang ES. Beyond DNA repair: additional functions of PARP-1 in cancer. Front Oncol. 2013;3:290. doi: 10.3389/fonc.2013.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel M, Nowsheen S, Maraboyina S, Xia F. The role of poly(ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: a review. Cell Biosci. 2020;10:35. doi: 10.1186/s13578-020-00390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hussain M, Daignault-Newton S, Twardowski PW, Albany C, Stein MN, Kunju LP, et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol. 2018;36:991–999. doi: 10.1200/JCO.2017.75.7310. Erratum in: J Clin Oncol 2018;36:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:975–986. doi: 10.1016/S1470-2045(18)30365-6. [DOI] [PubMed] [Google Scholar]
- 53.Saad F, Chi KN, Shore ND, Graff JN, Posadas EM, Freeman S, et al. Interim results of a phase Ib study of niraparib plus androgen receptor-targeted therapy in men with metastatic castration-resistant prostate cancer. Ann Oncol. 2018;29(Suppl 8):VIII292 [Google Scholar]
- 54.Chi KN, Rathkopf DE, Smith MR, Efstathiou E, Attard G, Olmos D, et al. Phase 3 MAGNITUDE study: first results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40(Suppl 6):abstr 12 [Google Scholar]
- 55.Saad F, Armstrong AJ, Thiery-Vuillemin A, Oya M, Loredo E, Procopio G, et al. PROpel: phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2022;40(Suppl 6):abstr 11 [Google Scholar]
- 56.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore ND, Procopio G, et al. Final overall survival (OS) in PROpel: abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2023;41(Suppl 6):abstr LBA16 [Google Scholar]
- 57.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 58.Lee CH, Yelensky R, Jooss K, Chan TA. Update on tumor neoantigens and their utility: why it is good to be different. Trends Immunol. 2018;39:536–548. doi: 10.1016/j.it.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Wilpe S, Tolmeijer SH, Koornstra RHT, de Vries IJM, Gerritsen WR, Ligtenberg M, et al. Homologous recombination repair deficiency and implications for tumor immunogenicity. Cancers (Basel) 2021;13:2249. doi: 10.3390/cancers13092249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu EY, Piulats JM, Gravis G, Fong PCC, Todenhöfer T, Laguerre B, et al. Corrigendum to "Pembrolizumab plus olaparib in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort a study” [Eur Urol 83 (2023) 15-26] Eur Urol. 2023;83:e87. doi: 10.1016/j.eururo.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Yap TA, Bardia A, Dvorkin M, Galsky MD, Beck JT, Wise DR, et al. Avelumab plus talazoparib in patients with advanced solid tumors: the JAVELIN PARP medley nonrandomized controlled trial. JAMA Oncol. 2023;9:40–50. doi: 10.1001/jamaoncol.2022.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hussain M, Carducci MA, Slovin S, Cetnar J, Qian J, McKeegan EM, et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2014;32:904–912. doi: 10.1007/s10637-014-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229:422–429. doi: 10.1002/path.4140. [DOI] [PubMed] [Google Scholar]
- 64.Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, et al. TOPARP-A Investigators. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017;7:1006–1017. doi: 10.1158/2159-8290.CD-17-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ter Brugge P, Kristel P, van der Burg E, Boon U, de Maaker M, Lips E, et al. Mechanisms of therapy resistance in patient-derived xenograft models of BRCA1-deficient breast cancer. J Natl Cancer Inst. 2016;108:djw148. doi: 10.1093/jnci/djw148. Erratum in: J Natl Cancer Inst 2020;112:1075. [DOI] [PubMed] [Google Scholar]
- 66.Gogola E, Duarte AA, de Ruiter JR, Wiegant WW, Schmid JA, de Bruijn R, et al. Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell. 2018;33:1078–1093.e12. doi: 10.1016/j.ccell.2018.05.008. Erratum in: Cancer Cell 2019;35:950-2. [DOI] [PubMed] [Google Scholar]
- 67.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu EY, Piulats JM, Gravis G, Fong PCC, Todenhöfer T, Laguerre B, et al. Pembrolizumab plus olaparib in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort a study. Eur Urol. 2023;83:15–26. doi: 10.1016/j.eururo.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Saad F, Thiery-Vuillemin A, Wiechno P, Alekseev B, Sala N, Jones R, et al. Patient-reported outcomes with olaparib plus abiraterone versus placebo plus abiraterone for metastatic castration-resistant prostate cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2022;23:1297–1307. doi: 10.1016/S1470-2045(22)00498-3. Erratum in: Lancet Oncol 2022;23:e446. [DOI] [PubMed] [Google Scholar]