Abstract
Background:
Preoperative anemia is associated with adverse outcomes in cardiac surgery, yet it remains unclear what proportion of this association is mediated through red blood cell (RBC) transfusions.
Methods:
This is a historical observational cohort study of adults undergoing coronary artery bypass grafting or valve surgery on cardiopulmonary bypass at an academic medical center between May 1, 2008, and May 1, 2018. A mediation analysis framework was used to evaluate the associations between preoperative anemia and postoperative outcomes, including a primary outcome of acute kidney injury (AKI). Intraoperative RBC transfusions were evaluated as mediators of preoperative anemia and outcome relationships. The estimated total effect, average direct effect of preoperative anemia, and percent of the total effect mediated through transfusions are presented with 95% confidence intervals and p-values.
Results:
A total of 4,117 patients were included, including 1,234 (30%) with preoperative anemia. Overall, 437/4117 (11%) patients went on to develop AKI, with a greater proportion in patients having preoperative anemia (219/1234 [18%] vs. 218/2883 [8%]). In multivariable analyses, the presence of preoperative anemia was associated with increased postoperative AKI (6.4% [4.2%, 8.7%] absolute difference in percent with AKI, p<0.001), with incremental decreases in preoperative hemoglobin concentrations displaying greater AKI risk (e.g., 11.9% [6.9%, 17.5%] absolute increase in probability of AKI for preoperative hemoglobin of 9 g/dL compared to a reference of 14 g/dL, p<0.001). The association between preoperative anemia and postoperative AKI was primarily due to direct effects of preoperative anemia (5.9% [3.6%, 8.3%] absolute difference, p<0.001) rather than mediated through intraoperative RBC transfusions (7.5% [−4.3%, 21.1%] of the total effect mediated by transfusions, p=0.220). Preoperative anemia was also associated with longer hospital durations (1.07 [1.05, 1.10] ratio of geometric mean length of stay, p<0.001). Of this total effect, 38% (22%, 62%; p<.001) was estimated to be mediated through subsequent intraoperative RBC transfusion. Preoperative anemia was not associated with reoperation or vascular complications.
Conclusions:
Preoperative anemia was associated with higher odds of AKI and longer hospitalizations in cardiac surgery. The attributable effects of anemia and transfusion on postoperative complications are likely to differ across outcomes. Future studies are necessary to further evaluate mechanisms of anemia-associated postoperative organ injury and treatment strategies.
Keywords: Anemia, transfusion, cardiac surgery, mediation, acute kidney injury
Introduction:
Preoperative anemia is associated with adverse outcomes after cardiac surgery, including acute kidney injury (AKI), increased hospital lengths of stay, and perioperative red blood cell (RBC) transfusion.1,2 There are several mechanisms by which preoperative anemia may predispose patients to perioperative organ injuries such as AKI, including impaired oxygen delivery and subsequent anemia-induced tissue ischemia, alterations in iron homeostasis in those with absolute or functional iron deficiency, and inflammation-induced injury secondary to elevations in proinflammatory cytokines in anemias of inflammation, which may be exacerbated by the inflammatory insult of cardiopulmonary bypass. Further, preoperative anemia is often accompanied by chronic illness and may therefore serve as a marker for predisposition for perioperative organ dysfunction.3 Finally, preoperative anemia is a leading risk factor for perioperative RBC transfusion, which in turn is associated with adverse clinical outcomes.2,4–7 To this end, the management of preoperative anemia prior to cardiac surgery has been shown to decrease transfusion exposures,8,9 which in turn may have favorable effects on postoperative outcomes.
While there is an abundance of literature assessing the associations between preoperative anemia and RBC transfusion and/or adverse outcomes in cardiac surgery, our understanding of the potential contributions of preoperative anemia and RBC transfusion to outcomes is limited.10 Specifically, it is unclear if the associations between preoperative anemia and postoperative complications such as AKI are direct effects of anemia, mediated through subsequent perioperative RBC transfusion exposures, or both.11,12 Alternatively, it is possible that the relationship between preoperative anemia and AKI is causally unrelated to the effects of anemia and/or transfusion. Assuming the presence of preoperative anemia and outcome relationships that are primarily mediated through transfusion exposures, then transfusion reductions should be considered a principal metric of preoperative anemia management programs and other patient blood management (PBM) initiatives in cardiac surgery. However, if preoperative anemia and outcome relationships occur independent of transfusion exposures, then preoperative optimization of hemoglobin concentrations in cardiac surgery may translate to clinical benefit irrespective of perceived effects on perioperative transfusion utilization.
In this investigation, we assessed the associations between preoperative anemia and postoperative outcomes in patients undergoing cardiac surgery, with emphasis on the potential mediation of these relationships by RBC transfusion exposures. In doing so, we hoped this study would shed light on the proportion of preoperative anemia and adverse outcome relationships that may be occurring through transfusion exposures.
Methods
This is a retrospective cohort study performed under the appropriate approvals of the local Institutional Review Board (Mayo Clinic, Minnesota) with waived requirement for written informed consent. Study design and conduct were performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.13
The following inclusion criteria were employed: adults ≥ 18 years of age, undergoing elective cardiac surgery with cardiopulmonary bypass between May 1, 2008, and May 1, 2018, at a large academic medical center, and procedures limited to isolated coronary artery bypass grafting (CABG) of any number of bypasses and single- or double-valve surgeries involving the mitral, tricuspid, and/or aortic valves, as previously described.1 Patients also undergoing minor secondary procedures such as patent foramen ovale closure, Maze procedures, and left atrial appendage ligation contemporaneously with the primary procedure were eligible for inclusion. Patients were excluded for the following reasons: non-qualifying cardiac procedures (i.e., off-pump procedures, robotic procedures, combined CABG and valve surgeries, pulmonary valve procedures), preoperative use of mechanical circulatory support (i.e., ventricular assist devices, intra-aortic balloon pumps, extracorporeal membrane oxygenation), American Society of Anesthesiologists (ASA) physical status VI, previous denial of authorization for medical record use in observational research, and previous inclusion in the study, such that patients could only be included once.
The primary exposure of interest was preoperative anemia, which was defined using the closest hemoglobin concentration obtained in the 30 days preceding surgery. Anemia was defined in accordance with World Health Organization (WHO) criteria, as hemoglobin concentration <12 g/dL in women and <13 g/dL in men. Preoperative hemoglobin concentration, assessed in continuous fashion, was employed as a secondary exposure of interest. Intraoperative allogeneic RBC transfusion volume measured in units was evaluated as a potential mediator of the association between preoperative anemia and outcome relationships (primarily) and preoperative hemoglobin and outcome relationships (secondarily). During the study period, institutional guidelines recommended intraoperative RBC transfusion for hemoglobin <8 g/dL during cardiac surgery or at any hemoglobin concentration in presence of rapid bleeding and hemodynamic instability. All perioperative characteristics, including transfusions, laboratory values, surgical characteristics, baseline clinical features, and outcomes were extracted from routinely validated institutional databases, including the Perioperative Data Mart and the Advanced Cohort Explorer (Mayo Clinic, Rochester, Minnesota).
The primary outcome of interest was postoperative AKI in accordance with Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria, which is presented in binary fashion (i.e., presence or absence of AKI) and in accordance with AKI stages (i.e., 1–3, with ascending severity). Secondary outcomes included: hospital length of stay, hospital mortality, reoperation within 7 days, and postoperative vascular complications within 7 days, which was a composite of pulmonary embolism, stroke, or myocardial infarction, as defined previously.1
Statistical Analyses:
The proposed relationships between exposure, mediator, confounding variables, and outcomes are provided in a directed acyclic graph (Figure 1). Patient and surgical characteristics, intraoperative RBC transfusions, and postoperative outcomes are summarized as median (25th percentile, 75th percentile) for continuous variables and frequency (percentage) for categorical variables according to preoperative anemia status (Tables 1 and 2). Absolute standardized differences are presented for comparison.
Figure 1.
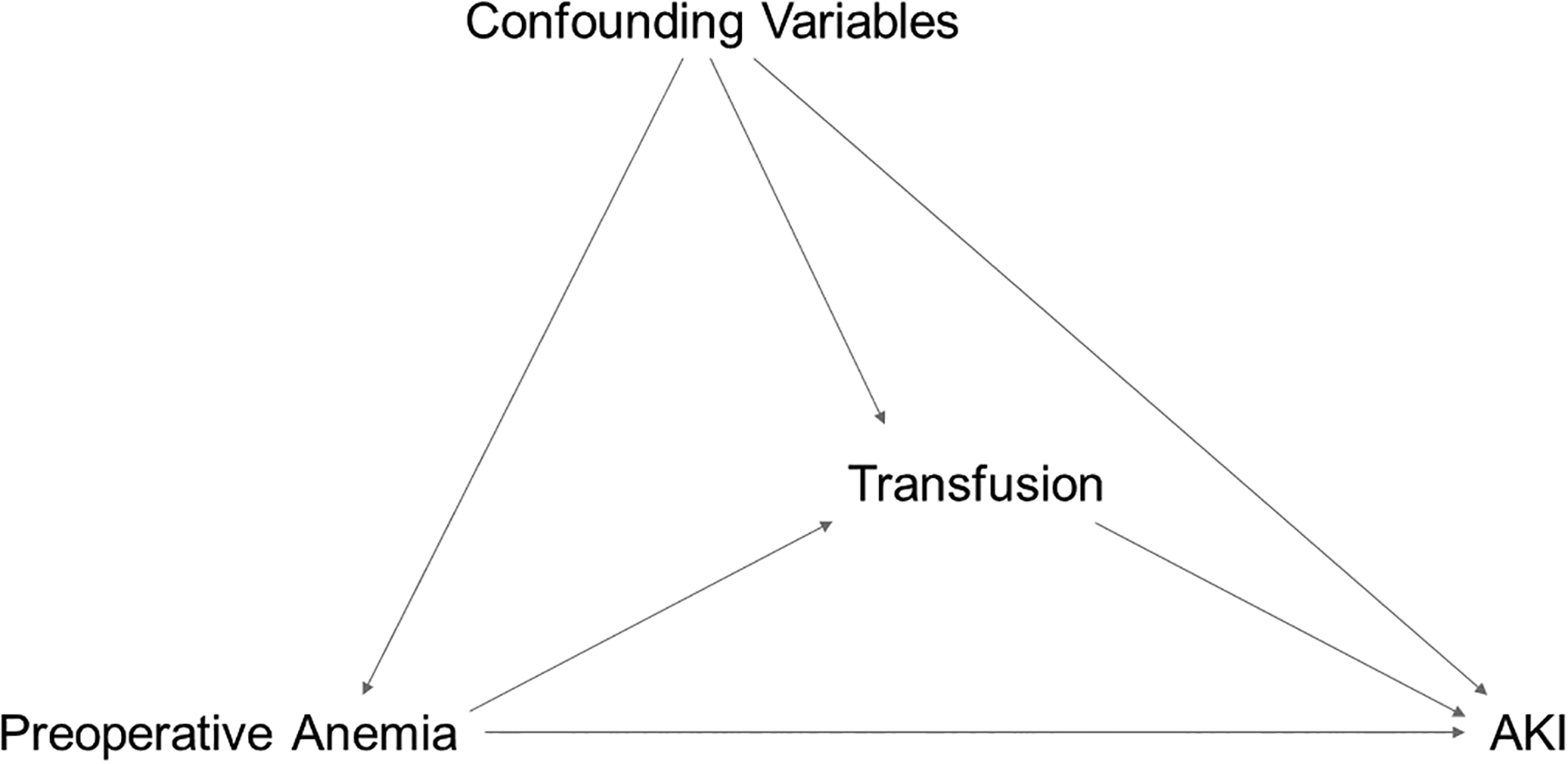
Directed acyclic graph showing the proposed relationships between preoperative anemia, transfusion, and acute kidney injury.
Preoperative anemia, or preoperative hemoglobin concentrations, may have direct effects on acute kidney injury (AKI), or these effects may be mediated through transfusion exposures. Further, a variety of factors may confound the relationships between preoperative anemia, transfusion, and outcomes (e.g., patient age, laboratory values, type of surgery).
Table 1 –
Patient and surgical characteristics according to preoperative anemia status*
| Preoperative Anemia (N=1234) |
No Preoperative Anemia (N=2883) |
Absolute standardized difference | |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 72 (64, 79) | 69 (59, 76) | 0.31 |
| Sex | 0.23 | ||
| Female | 438 (35%) | 1349 (47%) | |
| Male | 796 (65%) | 1534 (53%) | |
| BMI, kg/m2 | 28.5 (25.3, 32.7) | 28.2 (24.7, 32.4) | 0.08 |
| Smoking history | |||
| Current | 96 (8%) | 246 (9%) | 0.03 |
| Former | 554 (45%) | 1083 (38%) | 0.15 |
| Never | 584 (47%) | 1554 (54%) | 0.13 |
| Preoperative lab values | |||
| Hemoglobin, g/dL | 11.5 (10.7, 12.1) | 13.8 (13.2, 14.5) | 2.43 |
| Creatinine, mg/dL | 1.1 (0.9, 1.5) | 1.0 (0.8, 1.1) | 0.45 |
| Platelet count, × 109/L | 206 (165, 263) | 209 (176, 248) | 0.07 |
| Comorbidities | |||
| Charlson score | 6 (4, 8) | 4 (3, 6) | 0.67 |
| Kidney disease | 376 (30%) | 232 (8%) | 0.59 |
| Diabetes with complications | 148 (12%) | 116 (4%) | 0.30 |
| Hypertension | 889 (72%) | 1759 (61%) | 0.24 |
| Surgical characteristics | |||
| Valve surgery | 837 (68%) | 2043 (71%) | 0.07 |
| CPB duration, minutes | 84 (62, 111) | 78 (55, 102) | 0.19 |
| Total intraoperative RBCs, units | 0.74 | ||
| None | 472 (38%) | 2220 (77%) | |
| 1–2 | 460 (37%) | 497 (17%) | |
| 3–4 | 228 (18%) | 133 (5%) | |
| 5+ | 74 (6%) | 33 (1%) |
BMI – body mass index; CPB – cardiopulmonary bypass; RBC – red blood cell.
Continuous and categorical variables are presented as median (25th percentile, 75th percentile) and frequency (percentage) respectively.
Table 2 –
Unadjusted outcomes by preoperative anemia status*
| Preoperative anemia (N=1234) |
No anemia (N=2883) |
|
|---|---|---|
| Acute kidney injury | 219 (17.7%) | 218 (7.6%) |
| AKI stage | ||
| No AKI | 1015 (82.3%) | 2665 (92.4%) |
| Stage 1 | 134 (10.9%) | 159 (5.5%) |
| Stage 2 | 52 (4.2%) | 46 (1.6%) |
| Stage 3 | 33 (2.7%) | 13 (0.5%) |
| Hospital length of stay, days | 6.7 (5.7, 8.5) | 5.8 (4.9, 7.5) |
| Reoperation within 7 days | 232 (18.8%) | 437 (15.2%) |
| Vascular complications | 67 (5.4%) | 99 (3.4%) |
| Hospital mortality | 4 (0.3%) | 4 (0.1%) |
AKI – acute kidney injury.
Continuous and categorical outcomes are presented as median (25th percentile, 75th percentile) and frequency (percentage) respectively. Vascular complications include stroke, myocardial infarction, and pulmonary embolism.
Prior to mediation analysis, univariable and multivariable generalized linear models were used to assess associations between exposure and mediator, exposure and outcome, as well as mediator and outcome. Logistic regression was used for categorical outcomes and estimates represent odds ratios. Poisson regression was used for intraoperative RBCS (i.e., the primary potential mediator) and estimates represent rate ratios (i.e., ratio of mean counts). To satisfy distributional assumptions, hospital length of stay was log-transformed and estimates for association from linear regression assumed normally distributed residuals. As there were very few deaths in the cohort (0.2%), death was not included as a competing risk for length of stay, nor was hospital mortality included as a secondary outcome in mediation analyses. All multivariable models included the same covariates which were selected a priori and included age, sex, body mass index (BMI), smoking status, preoperative creatinine, preoperative platelet count, Charlson comorbidity index, kidney disease, diabetes with complications, hypertension, surgery type (i.e., CABG vs. valve), and total cardiopulmonary bypass time as a marker for surgical complexity.
The mediation analysis estimated the total and direct effects of exposure (e.g. preoperative anemia) on outcome (e.g. postoperative AKI) as well as the mediation effect and proportion of total effect mediated by the proposed mediator (intraoperative RBC transfusion).14 This approach uses a potential outcomes framework implemented in the R mediation package.15,16 In brief, each patient has two potential values for the mediator (RBC transfusions): one potential observation if the patient is anemic and one if not anemic. We observe one value of the mediator corresponding to observed anemic status. The other is unobserved but exists as a conceptual value of what could have happened (RBC transfusions) had the patient had a different anemia status than observed. Similarly, there are potential values of the outcome (AKI) based on anemia and RBC transfusions.17 The mediation effect is obtained by estimating the difference in potential outcomes when patients are treated with RBCs as if anemic versus treated with RBCs as if non-anemic. The direct effect is obtained by estimating the difference in potential outcomes as if anemic and as if non-anemic while holding RBCs constant. The total effect reflects the overall relationship between exposure and outcome – i.e., controlling for confounding but ignoring any mediator variables of interest. This can be partitioned into the portion of the relationship that goes through the mediator (mediation effect, reported as a percentage of the total effect) and the portion that does not go through the mediator (direct effect); that is, the total effect is the sum of mediated and direct effects, subject to rounding error. Further discussion of mediation analysis is available in previous work.14
In our mediation analysis, first, a multivariable generalized linear model was used to estimate the exposure-mediator relationship (i.e., the exposure and covariates described above were included on the right-hand side of the equation). Also, a multivariable generalized linear model was then used to estimate both the exposure-outcome direct effect and the mediator-outcome relationship (i.e., the exposure, mediator, and covariates described above were included on the right-hand side of the equation). We assumed no exposure-mediator interaction. Models in the mediation analysis were fit as described above except in categorical outcomes for which associations were estimated using probit regression given compatibility with existing software. These models were then combined using the potential outcomes methodology as previously outlined.16 For categorical outcomes, results are presented as the absolute increase in probability for the given event associated with preoperative anemia. For hospital length of stay, results represent the estimated ratio of geometric mean hospital lengths of stay. Preoperative hemoglobin quantified as g/dL below 14 was evaluated separately as an exposure in mediation analysis and results are presented by preoperative hemoglobin value compared to the referent value of 14 g/dL.
In a predefined interaction analysis, mediation analysis was employed to assess the relationship between preoperative anemia and outcomes according to sex. Results were presented according to sex when evidence of any potentially meaningful interaction (p<0.1) was found in the total, average direct, or average mediation effect. In post-hoc analysis, interaction between age expressed as a continuous, linear effect and preoperative anemia and outcome associations was also assessed. As a predefined secondary analysis, we evaluated the lowest intraoperative hemoglobin, measured in g/dL below 14, as a potential mediator of preoperative anemia and outcome relationships. This secondary analysis was done as described above, with the association between preoperative hemoglobin and lowest intraoperative hemoglobin (exposure-mediator) modelled with multivariable linear regression assuming normally distributed residuals. As an additional post-hoc sensitivity analysis, we repeated the primary analysis defining preoperative anemia as hemoglobin < 13 g/dL.
No a priori power analysis was performed for this investigation utilizing an existing cohort of cardiac surgery patients at risk for anemia and AKI, as previously described.1 With 4117 patients, we had 90% power in a Poisson regression model with two-sided alpha = 0.05, to detect a rate ratio of 1.13 intraoperative RBC units associated with preoperative anemia. This assumes a 30% prevalence of anemia and that the mean rate of RBC transfusions overall was 0.79. We also had 90% power in a logistic regression model for the outcome of AKI with two-tailed alpha = 0.05, to detect an odds ratio of 1.12 per RBC unit. This assumes AKI prevalence is 10.6% and the distribution of RBCs has standard deviation 1.43. Two-tailed p-values <0.05 were used to determine statistical significance. No adjustment for multiple comparisons was performed given specification of a single primary outcome with a single exposure and mediator of interest. Additional secondary outcomes were considered without adjustment for multiple comparisons, and as such, these results should not be considered confirmatory. Statistical analyses were performed with R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 4,117 patients were included, including 1,234 (30%) with preoperative anemia (Supplemental Figure 1). Patients with preoperative anemia were older (median 72 [64, 79] vs. 69 [59, 76] years), more likely male (65% vs. 53%), and had higher Charlson comorbidity indices (6 [4, 8] vs. 4 [3, 6]) than those without anemia (Table 1). Further, patients with preoperative anemia had greater intraoperative (62% vs. 23%) and perioperative (80% vs. 40%) RBC transfusion exposure.
In unadjusted analyses, patients with preoperative anemia had higher rates of postoperative AKI (17.7% vs. 7.6%), and greater severity of AKI (Table 2), with an odds ratio of 2.64 (2.16, 3.22), p<0.001 (Table 3). Those with preoperative anemia also experienced longer hospital stays (6.7 [5.7, 8.5] vs. 5.8 [4.9, 7.5] days), more return trips to the OR (18.8% vs. 15.2%), and greater composite postoperative vascular complications (5.4% vs. 3.4%). Mortality was low in both groups (0.3% vs. 0.1%). The primary exposure (i.e., preoperative anemia) was associated with the mediator (i.e., intraoperative RBC transfusion; OR 3.11 [2.88, 3.35], p<0.001) and the primary outcome (i.e., AKI; OR 1.99 [1.58, 2.50], p<0.001) in multivariable analysis. Both preoperative anemia and intraoperative RBCs were associated with hospital length of stay (Table 3).
Table 3 –
Summary of exposure-mediator, mediator-outcome, and exposure-outcome relationships estimated from univariable and multivariable regression*
| Mediator | Exposure | |||||
|---|---|---|---|---|---|---|
| Intraoperative RBCs | Preoperative anemia | Preoperative hemoglobin | ||||
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Univariable analyses | ||||||
| -Mediator | ||||||
| Intraoperative RBCs | NA | - | 3.45 (3.21, 3.70) | <.001 | 1.61 (1.58, 1.65) | <.001 |
| -Outcome | ||||||
| AKI | 1.14 (1.07, 1.21) | <.001 | 2.64 (2.16, 3.22) | <.001 | 1.26 (1.17, 1.35) | <.001 |
| Hospital length of stay | 1.06 (1.05, 1.06) | <.001 | 1.13 (1.11, 1.16) | <.001 | 1.05 (1.05, 1.06) | <.001 |
| Reoperation | 1.12 (1.06, 1.18) | <.001 | 1.30 (1.09, 1.54) | 0.004 | 1.10 (1.04, 1.18) | 0.002 |
| Vascular complications | 1.06 (0.96, 1.17) | 0.222 | 1.62 (1.18, 2.22) | 0.003 | 1.14 (1.02, 1.28) | 0.025 |
| Multivariable analysis | ||||||
| -Mediator | ||||||
| Intraoperative RBCs | NA | - | 3.11 (2.88, 3.35) | <.001 | 1.57 (1.53, 1.60) | <.001 |
| -Outcome | ||||||
| AKI | 1.04 (0.97, 1.12) | 0.229 | 1.99 (1.58, 2.50) | <.001 | 1.24 (1.14, 1.34) | <.001 |
| Hospital length of stay | 1.03 (1.02, 1.04) | <.001 | 1.07 (1.05, 1.10) | <.001 | 1.03 (1.02, 1.04) | <.001 |
| Reoperation | 1.06 (1.00, 1.13) | 0.044 | 1.14 (0.94, 1.38) | 0.183 | 1.05 (0.98, 1.13) | 0.172 |
| Vascular complications | 0.99 (0.88, 1.12) | 0.886 | 1.24 (0.87, 1.77) | 0.232 | 1.07 (0.94, 1.22) | 0.314 |
Results are from univariable and multivariable models assessing the association between the given mediator/exposures (columns) and the given mediator/outcomes (rows). Intraoperative RBCs was modelled with Poisson regression and estimates are rate ratios associated with preoperative anemia (<12 g/dL in women; <13 g/dL in men) or 1 g/dL decrease in pre-operative hemoglobin below 14 g/dL. AKI, reoperation, and vascular complications were modelled with logistic regression and estimates are odds ratios. Hospital length of stay was modelled with linear regression on the log scale and estimates are for the ratio of geometric means. All multivariable models are adjusted for age, sex, BMI, smoking status, preoperative creatinine, preoperative platelet count, Charlson comorbidity index, kidney disease, diabetes with complications, hypertension, surgery type (i.e. CABG vs. valve), and total cardiopulmonary bypass time as a marker for surgical complexity. Multivariable models in the mediator column include adjustment for preoperative anemia. Models in the exposure columns do not include the mediator variable (i.e. intraoperative RBCs).
In mediation analyses, the presence of preoperative anemia was associated with increased postoperative AKI (6.4% [4.2%, 8.7%] absolute increase in probability of AKI, p<0.001; Table 4). Further, incremental decreases in preoperative hemoglobin concentrations were associated with increasing risk for postoperative AKI (e.g., 11.9% [6.9%, 17.5%] absolute increase in probability of AKI for preoperative hemoglobin of 9 g/dL compared to a reference of 14 g/dL, p<0.001). The association between preoperative anemia and postoperative AKI was primarily due to direct effects of preoperative anemia (5.9% [3.6%, 8.3%] absolute increase in probability, p<0.001) rather than mediated through intraoperative RBC transfusions (7.5% [−4.3%, 21.1%] of the total effect mediated through transfusions, p=0.220). Similarly, the presence of preoperative anemia was associated with longer hospitalizations (1.07 [1.05, 1.10] ratio of geometric means, p<0.001). Of this total effect, 37.9% (22.4%, 61.6%) was estimated to be mediated through subsequent intraoperative RBC transfusion. Hospital length of stay increased with incremental decreases in preoperative hemoglobin concentrations (e.g., 1.18 [1.12, 1.24] ratio of geometric mean length of stay for preoperative hemoglobin of 9 g/dL compared to 14 g/dL, p<0.001). Preoperative anemia was not associated with reoperation (1.8% [−0.8%, 4.6%] absolute increase in probability; p=0.185) or vascular complications (0.9% [−0.6%, 2.5%] absolute increase in probability; p=0.254) in adjusted analyses. Results were consistent with the primary analyses when defining preoperative anemia as hemoglobin <13 g/dL for both men and women (Supplemental Table 1).
Table 4 –
Estimated association between preoperative anemia (primary exposure), preoperative hemoglobin, and primary and secondary outcomes accounting for effect mediated by total intraoperative RBC transfusions *
| Total effect | Percent mediated | Direct effect | ||||
|---|---|---|---|---|---|---|
| Est. (95% CI) | p-value | Est. (95% CI) | p-value | Est. (95% CI) | p-value | |
| Acute kidney injury | ||||||
| Preoperative anemia | 6.4 (4.2, 8.7) | <.001 | 7.5 (−4.3, 21.1) | 0.220 | 5.9 (3.6, 8.3) | <.001 |
| Preoperative hemoglobin | ||||||
| 14 g/dL | Referent | ‐ | Referent | ‐ | Referent | ‐ |
| 13 g/dL | 1.7 (1.0, 2.3) | <.001 | 4.1 (−4.6, 18.0) | 0.373 | 1.6 (0.9, 2.3) | <.001 |
| 12 g/dL | 3.6 (2.2, 5.1) | <.001 | 5.1 (−5.9, 21.2) | 0.391 | 3.4 (1.8, 5.0) | <.001 |
| 11 g/dL | 5.9 (3.5, 8.4) | <.001 | 6.3 (−8.1, 25.4) | 0.390 | 5.5 (2.9, 8.3) | <.001 |
| 10 g/dL | 8.6 (5.1, 12.3) | <.001 | 8.9 (−10.8, 32.1) | 0.368 | 7.9 (4.1, 11.8) | <.001 |
| 9 g/dL | 11.9 (6.9, 17.5) | <.001 | 11.2 (−14.3, 38.6) | 0.378 | 10.5 (5.3, 16.0) | <.001 |
| Hospital length of stay | ||||||
| Preoperative anemia | 1.07 (1.05, 1.10) | <.001 | 37.9 (22.4, 61.6) | <.001 | 1.04 (1.02, 1.07) | <.001 |
| Preoperative hemoglobin | ||||||
| 14 g/dL | Referent | ‐ | Referent | ‐ | Referent | ‐ |
| 13 g/dL | 1.02 (1.01, 1.03) | <.001 | 24.9 (11.9, 57.9) | <.001 | 1.02 (1.00, 1.03) | 0.006 |
| 12 g/dL | 1.05 (1.03, 1.07) | <.001 | 30.1 (15.2, 63.8) | <.001 | 1.03 (1.01, 1.06) | 0.003 |
| 11 g/dL | 1.08 (1.05, 1.11) | <.001 | 36.0 (19.2, 69.8) | <.001 | 1.05 (1.01, 1.09) | 0.004 |
| 10 g/dL | 1.12 (1.07, 1.17) | <.001 | 42.3 (23.2, 74.8) | <.001 | 1.07 (1.02, 1.12) | 0.006 |
| 9 g/dL | 1.18 (1.12, 1.24) | <.001 | 49.6 (28.7, 79.9) | <.001 | 1.09 (1.02, 1.15) | 0.004 |
Results are from mediation analyses assessing the associations between preoperative anemia/hemoglobin, intraoperative RBC transfusions, and outcomes. The total effect reflects the overall relationship between exposure (e.g., preoperative anemia) and outcome (e.g., postoperative acute kidney injury). This can be partitioned into the portion of the relationship that goes through the mediator (intraoperative RBC transfusion; mediation effect, reported as percentage of total effect) and the portion that does not go through the mediator (direct effect); that is, the total effect is the sum of mediated effect (not shown, rather reported as percentage of total effect) and direct effects subject to rounding error. “Percent Mediated” describes the size of the average causal mediation effects relative to the total effect and, despite being labeled ‘Percent’ the value does not have to be between 0 and 100. Percent mediated values less than 0% indicate the estimated direct effect is greater than the total effect so that mediation is ‘negative’. Percent mediated values greater than 0% indicate the estimated direct effect is less than the total effect (hypothesized in this study). Percent mediated values can also be >100% indicating that the direct and mediated effects are in opposing directions so that the mediated effect is greater than the total effect. Preoperative hemoglobin was parameterized as g/dL below 14, with all values above 14 defined as 0 g/dL below 14. For acute kidney injury, estimates are for the absolute percentage increase in probability of event associated with the given exposure. For hospital length of stay, estimates are for the ratios of geometric mean hospital lengths of stay.
In interaction analyses, there were no significant interactions of sex on the associations between preoperative anemia and postoperative AKI (direct effect interaction p=0.560), hospital length of stay (p=0.464), or vascular complications (p=0.757). The total, direct, and mediated effects of preoperative anemia and postoperative AKI associations are displayed separately for men and women (Supplemental Table 2), with higher rates of AKI in the presence of preoperative anemia in both men and women. These associations were primarily driven by direct effects of anemia rather than mediated through transfusion exposures. There was a significant interaction of sex with preoperative anemia and reoperation relationships (p=0.015). In women, the presence of preoperative anemia was associated with a 6.1% (1.8%, 10.5%) absolute increase in the probability of reoperation (p=0.005), while there was no significant relationship between preoperative anemia and reoperation in men (−1.0% [−4.2%, 2.4%], p=0.534; Supplemental Table 3). Like AKI, the association between preoperative anemia and reoperation was primarily driven by direct effects of anemia. There were no significant interactions of age on the associations between preoperative anemia and postoperative AKI (direct effect interaction p=0.731), hospital length of stay (p=0.992), or vascular complications (p=0.127). While there was no evidence of a total effect of anemia on reoperation in older patients (0.1% [−2.8%, 3.1%], p=0.959 at age 75 [3rd quartile of age distribution]) there was a significant increase in probability of reoperation associated with anemia in younger patients (total effect 4.9% [1.7%, 8.2%], p=0.003; direct effect 4.1% [0.8%, 7.4%], p=0.012 at age 60 [1st quartile of age distribution]; direct effect interaction p=0.027).
In secondary analyses evaluating potential mediation of preoperative anemia and outcome relationships by the lowest intraoperative hemoglobin concentration, the association between preoperative anemia and AKI was primarily a direct effect of preoperative hemoglobin rather than mediated through nadir intraoperative hemoglobin (Supplemental Table 4). Similarly, the association between preoperative anemia and hospital length of stay was primarily a direct effect of preoperative hemoglobin with only modest mediation through the nadir hemoglobin concentration.
Discussion
In adults undergoing elective valve replacement or CABG, preoperative anemia was associated with increased rates of AKI and longer hospitalizations. The association between preoperative anemia and AKI was primarily mediated through direct effects of preoperative hemoglobin concentrations rather than mediated through RBC transfusion exposures. However, the association between preoperative anemia and prolonged hospitalization was both a direct effect of preoperative hemoglobin and mediated through transfusion exposures. These relationships were consistent in women and men. Additionally, in women but not men, preoperative anemia was associated with higher reoperation rates, which was primarily a direct effect of preoperative anemia rather than mediated through transfusion exposures. Similarly, in younger (i.e., age 60) but not older adults (i.e., age 75) preoperative anemia was associated with higher reoperation rates, with minimal mediation through transfusions. These findings suggest that preoperative anemia and outcome relationships in cardiac surgery are not uniformly attributable to the effects of anemia and/or transfusion. Rather, the attributable effects of anemia and transfusion are likely to differ across outcomes. As demonstrated in this study, preoperative hemoglobin concentrations were the prominent driver of the association between preoperative anemia and AKI, while both preoperative anemia and transfusion exposures contributed to prolonged hospitalization associations.
Previous studies have demonstrated that preoperative anemia is associated with adverse effects in cardiac surgery.1,2,5,18,19 Similarly, numerous studies have demonstrated inferior outcomes in those with perioperative RBC transfusion exposures.4,6,7,10,20,21 However, there is limited evidence evaluating the relative contributions of preoperative anemia and RBC transfusions on outcomes in cardiac surgery. In a multicenter study of more than 30,000 patients undergoing isolated CABG, the authors created independent multivariable models of preoperative hematocrit and RBC transfusion exposures, respectively, on postoperative outcomes, noting that both models were associated with hospital mortality and AKI.10 However, the transfusion model had modestly higher predictive performance as evaluated through area under the curve (AUC) parameters, leading the authors to conclude that RBC transfusion, rather than the preoperative hematocrit, is the more important driver of risk-adjusted morbidity and mortality. However, evaluating transfusion and preoperative hematologic status in isolation provides an incomplete picture of exposure-outcome relationships. Preoperative hematocrit is intricately linked to subsequent transfusion exposures,10 such that transfusions may fall along a possible causal pathway between anemia and outcomes. Indeed, our results highlight primarily direct effects of preoperative anemia on some postoperative outcomes (i.e., AKI) and effects mediated, in part, through perioperative RBC transfusion exposures on others (i.e., hospital duration). Importantly, mediation analyses in non-cardiac surgery have also shown that preoperative anemia is associated with adverse post-surgical outcomes, and many of these associations are driven through mediation factors such as transfusion.14,22 However, as shown with the primary outcome of AKI in the current investigation, anemia-outcome associations are not wholly attributable to transfusion exposures. As such, it is possible that augmentation of preoperative hemoglobin concentrations, even in the absence of transfusion reductions, may be associated with improvements in some postoperative outcomes. However, it is important to note that this observational study, even though employing a mediation framework, characterizes associations between anemia and outcomes and does not confirm the presence or nature of causal pathways. Future clinical trials are necessary to evaluate the proposed pathways and establish causality.
Nadir intraoperative hemoglobin concentrations have been associated with higher morbidity and mortality in cardiac surgery.23–25 Further, animal models of induced-anemia during cardiopulmonary bypass show sustained reductions in renal cortical and medullary oxygenation despite enhanced renal blood flow, with greatest impairments observed in the renal medulla.26 This suggests that medullary hypoxia may be a key driver for postoperative AKI in cardiac surgery. Recognizing that intraoperative hemoglobin concentrations may reside on a potential causal pathway between preoperative anemia and postoperative AKI, we performed a pre-defined secondary analysis evaluating nadir intraoperative hemoglobin concentrations as potential mediators of preoperative anemia and outcome relationships. To that end, the associations between preoperative anemia and outcomes of AKI and hospital duration were both primarily direct effects of preoperative rather than intraoperative hemoglobin concentrations. Hence, only a small portion of preoperative anemia and outcome relationships appear to be mediated through nadir intraoperative hemoglobin concentrations. This does not imply that intraoperative hemoglobin concentrations are unimportant for postoperative outcomes such as AKI, but rather that they do not mediate preoperative anemia and outcome relationships. We hypothesize that some patients presenting for surgery with anemia may already be experiencing renal oxygenation deficits or other subclinical kidney injury, predisposing them to heightened risk of AKI. This risk is likely to be exacerbated by the effects of cardiopulmonary bypass, including inflammation, neurohormonal activation, microembolization, increased free hemoglobin exposure secondary to native RBC injury and/or transfusion, ischemia-reperfusion injury, and other types of oxidative stress. Alternatively, anemia is associated with acute and chronic disease states that heighten risk for perioperative renal injury, and it is possible that preoperative anemia may primarily serve as a marker for greater preoperative disease burden and inherent risk of AKI. However, it is important to recognize that 1) the associations between lower preoperative hemoglobin concentrations and AKI persist despite pre-specified adjustment for preoperative renal risk factors and chronic disease severity, and 2) animal models suggest the presence of anemia-mediated oxygen deficits during and after cardiopulmonary bypass that occur independent of underlying disease severity.26 Certainly, it is possible that preoperative anemia may be both a marker of underlying renal risk and a driver of adverse renal outcomes. Additionally, the association between preoperative anemia and AKI may be occurring through other mediating factors besides nadir hemoglobin concentrations and transfusion exposures. As one example, low oxygen delivery (DO2) during cardiopulmonary bypass has been associated with higher risk of AKI,24,25 and strategies designed to maintain higher DO2 may reduce the incidence of postoperative AKI.27,28 It is likely that patients with preoperative anemia may be at heightened risk for impaired DO2 during cardiopulmonary bypass, which contributes to higher rates of postoperative AKI. Regardless of the underlying mechanism(s), the presence of preoperative anemia should be considered a clinically important risk factor for AKI after cardiac surgery. Future trials should specifically evaluate whether pharmacological anemia treatment may reduce organ injury, with hypoxia-mediated injury being a key mechanism of interest.
Our approach uses a novel potential outcomes framework for mediation analysis rather than the product method for mediation proposed by Baron and Kenny offering flexibility for our mediation analysis that includes both count and continuous mediators (RBC transfusions, lowest intraoperative hemoglobin) and continuous and dichotomous outcomes (length of stay, AKI) under a single framework.14,29 There are several limitations to this study. First, this data is observational, and the possibility for residual confounding persists despite prespecified adjustment of variables that may confound exposure-outcome, mediator-outcome, and exposure-mediator relationships. Second, although we present a mediation framework, this investigation does not establish causality. Third, although we adjusted for surgery type (i.e., CABG vs. valve) and markers of surgical complexity, we included both single and double-valve surgeries, and it is possible that there may be outcome differences across these. Further, we did not include off-pump CABG or combined CABG-valve procedures, which limits generalizability. Fourth, we did not have information on the causes of preoperative anemia in this cohort. It is possible that clinical outcomes may differ based on the underlying causes of preoperative anemia. Fifth, patients in this cohort did not receive evaluation or management of preoperative anemia. Future studies are needed to evaluate how anemia management strategies may influence anemia and postoperative outcome relationships. Sixth, while we assessed potential mediation of preoperative anemia outcome and outcome relationships by intraoperative transfusions and nadir hemoglobin concentrations, we were unable to assess other potential mediation factors such as DO2, which should be considered in future studies. Additionally, we did not include postoperative RBC transfusions as proposed mediators, as this could lead to mediator-outcome (i.e., cause-effect) inversion (e.g., postoperative transfusions may have occurred after postoperative AKI, and hence would not be valid under a mediation framework). Future work is necessary to characterize the potential mediation of preoperative anemia and postoperative outcome relationships by postoperative transfusions. Finally, this data is derived from a single academic medical center, which may limit external validity.
In summary, preoperative anemia was associated with higher rates of postoperative AKI and longer hospital durations after elective cardiac surgery. The association between preoperative anemia and AKI appears to be primarily driven by direct effects of preoperative anemia, while both anemia and subsequent RBC transfusion exposures contribute to prolonged hospitalizations. Future clinical trials are needed to further evaluate mechanisms of anemia-associated postoperative organ injury and test whether pharmacological anemia treatment may reduce AKI and other adverse events after cardiac surgery.
Supplementary Material
Key Points:
Question: How much of the association of preoperative anemia and adverse outcomes in cardiac surgery is mediated through RBC transfusion exposures?
Findings: The association between preoperative anemia and AKI is largely related to direct effects of anemia rather than mediated through transfusions, while the association between preoperative anemia and prolonged hospitalizations is both a direct effect of anemia and mediated through transfusions.
Meaning: Preoperative anemia is associated with adverse outcomes in cardiac surgery, which are not wholly attributable to effects of transfusion.
Funding:
Dr. Warner is supported by K23HL153310 from the National Heart Lung and Blood Institute (NHLBI). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflicts of Interest:
Matthew Warner: Dr. Warner is a non-remunerated member of the Board of Directors of the Society for the Advancement of Patient Blood Management (SABM). He has received research support from the NIH.
Andrew Hanson: No conflicts of interest.
Phillip Schulte: No conflicts of interest.
Juan Ripoll Sanz: No conflicts of interest.
Mark Smith: Dr. Smith received funding from CSL Behring for an investigator-initiated research study.
Marissa Kauss: No conflicts of interest.
Juan Crestanello: No conflicts of interest.
Daryl Kor: Dr. Kor has received research support from the NIH. He also receives consulting fees from NIH, UpToDate, and Dynocardia.
Glossary of Terms:
- AKI
acute kidney injury
- ASA
American Society of Anesthesiologists
- AUC
area under the curve
- CABG
coronary artery bypass grafting
- DO2
oxygen delivery
- KDIGO
Kidney Disease Improving Global Outcomes
- NYHA
New York Heart Association
- RBCs
red blood cells
- WHO
World Health Organization
References:
- 1.Ripoll JG, Smith MM, Hanson AC, et al. Sex-Specific Associations Between Preoperative Anemia and Postoperative Clinical Outcomes in Patients Undergoing Cardiac Surgery. Anesth Analg. Published online February 4, 2021. doi: 10.1213/ANE.0000000000005392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padmanabhan H, Siau K, Curtis J, et al. Preoperative Anemia and Outcomes in Cardiovascular Surgery: Systematic Review and Meta-Analysis. Ann Thorac Surg. 2019;108(6):1840–1848. doi: 10.1016/j.athoracsur.2019.04.108 [DOI] [PubMed] [Google Scholar]
- 3.Warner MA, Shore-Lesserson L, Shander A, Patel SY, Perelman SI, Guinn NR. Perioperative Anemia. Anesth Analg. Published online March 2020:1. doi: 10.1213/ane.0000000000004727 [DOI] [PubMed] [Google Scholar]
- 4.Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. Ann Thorac Surg. 2021;112(3):981–1004. doi: 10.1016/J.ATHORACSUR.2021.03.033 [DOI] [PubMed] [Google Scholar]
- 5.Ranucci M, Di Dedda U, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G. Impact of preoperative anemia on outcome in adult cardiac surgery: A propensity-matched analysis. Ann Thorac Surg. 2012;94(4):1134–1141. doi: 10.1016/j.athoracsur.2012.04.042 [DOI] [PubMed] [Google Scholar]
- 6.Paone G, Brewer R, Theurer PF, Bell GF, Cogan CM, Prager RL. Preoperative predicted risk does not fully explain the association between red blood cell transfusion and mortality in coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2012;143(1):178–185. doi: 10.1016/J.JTCVS.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 7.Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Cardiol. 2008;23(6):607–612. doi: 10.1097/HCO.0B013E328310FC95 [DOI] [PubMed] [Google Scholar]
- 8.Spahn DR, Schoenrath F, Spahn GH, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. 2019;6736(18):1–12. doi: 10.1016/S0140-6736(18)32555-8 [DOI] [PubMed] [Google Scholar]
- 9.Song JW, Soh S, Shim JK, et al. Effect of Perioperative Intravenous Iron Supplementation for Complex Cardiac Surgery on Transfusion Requirements: A Randomized, Double-blinded Placebo-controlled Trial. Ann Surg. 2022;275(2):232–239. doi: 10.1097/SLA.0000000000005011 [DOI] [PubMed] [Google Scholar]
- 10.LaPar DJ, Hawkins RB, McMurry TL, et al. Preoperative anemia versus blood transfusion: Which is the culprit for worse outcomes in cardiac surgery? J Thorac Cardiovasc Surg. 2018;156(1):66–74.e2. doi: 10.1016/J.JTCVS.2018.03.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paone G. Anemia, transfusion, and outcome: Both are bad…does it really matter which is worse? J Thorac Cardiovasc Surg. 2018;156(1):75–76. doi: 10.1016/j.jtcvs.2018.03.051 [DOI] [PubMed] [Google Scholar]
- 12.Shander A, Javidroozi M, Ozawa S, Hare GMT. What is really dangerous: Anaemia or transfusion? Br J Anaesth. 2011;107(SUPPL. 1):i41–i59. doi: 10.1093/bja/aer350 [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med. 2007;147(8):573. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 14.Mascha EJ, Dalton JE, Kurz A, Saager L. Understanding the mechanism: Mediation analysis in randomized and nonrandomized studies. Anesth Analg. 2013;117(4):980–994. doi: 10.1213/ANE.0B013E3182A44CB9 [DOI] [PubMed] [Google Scholar]
- 15.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59(5):1–38. doi: 10.18637/JSS.V059.I0526917999 [DOI] [Google Scholar]
- 16.Imai K, Keele L, Tingley D. A General Approach to Causal Mediation Analysis. Published online 2010. doi: 10.1037/a0020761 [DOI] [PubMed] [Google Scholar]
- 17.Schulte PJ, Mascha EJ. Propensity Score Methods: Theory and Practice for Anesthesia Research. Anesth Analg. 2018;127(4):1074–1084. doi: 10.1213/ANE.0000000000002920 [DOI] [PubMed] [Google Scholar]
- 18.Karkouti K, Wijeysundera DN, Beattie WS. Risk associated with preoperative anemia in cardiac surgery : A multicenter cohort study. Circulation. 2008;117(4):478–484. doi: 10.1161/CIRCULATIONAHA.107.718353 [DOI] [PubMed] [Google Scholar]
- 19.Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116(5):471–479. doi: 10.1161/CIRCULATIONAHA.106.653501 [DOI] [PubMed] [Google Scholar]
- 20.Loor G, Rajeswaran J, Li L, et al. The least of 3 evils: Exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg. 2013;146(6):1480–1487.e6. doi: 10.1016/J.JTCVS.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 21.Pattakos G, Koch CG, Brizzio ME, et al. Outcome of Patients Who Refuse Transfusion After Cardiac Surgery: A Natural Experiment With Severe Blood Conservation. Arch Intern Med. 2012;172(15):1154–1160. doi: 10.1001/ARCHINTERNMED.2012.2449 [DOI] [PubMed] [Google Scholar]
- 22.Saager L, Turan A, Reynolds LF, Dalton JE, Mascha EJ, Kurz A. The association between preoperative anemia and 30-day mortality and morbidity in noncardiac surgical patients. Anesth Analg. 2013;117(4):909–915. doi: 10.1213/ANE.0B013E31828B347D [DOI] [PubMed] [Google Scholar]
- 23.Hogervorst E, Rosseel P, Van Der Bom J, et al. Tolerance of intraoperative hemoglobin decrease during cardiac surgery. Transfusion. 2014;54(10 Pt 2):2696–2704. doi: 10.1111/TRF.12654 [DOI] [PubMed] [Google Scholar]
- 24.Ranucci M, Romitti F, Isgrò G, et al. Oxygen Delivery During Cardiopulmonary Bypass and Acute Renal Failure After Coronary Operations. Ann Thorac Surg. 2005;80(6):2213–2220. doi: 10.1016/J.ATHORACSUR.2005.05.069 [DOI] [PubMed] [Google Scholar]
- 25.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125(6):1438–1450. doi: 10.1016/S0022-5223(02)73291-1 [DOI] [PubMed] [Google Scholar]
- 26.Darby PJ, Kim N, Hare GMT, et al. Anemia increases the risk of renal cortical and medullary hypoxia during cardiopulmonary bypass. Perfusion. 2013;28(6):504–511. doi: 10.1177/0267659113490219 [DOI] [PubMed] [Google Scholar]
- 27.Ranucci M, Johnson I, Willcox T, et al. Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J Thorac Cardiovasc Surg. 2018;156(5):1918–1927.e2. doi: 10.1016/J.JTCVS.2018.04.045 [DOI] [PubMed] [Google Scholar]
- 28.Brown JR, Baker RA, Shore-Lesserson L, et al. The Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists/American Society for Extracorporeal Technology Clinical Practice Guidelines for the Prevention of Adult Cardiac Surgery-Associated Acute Kidney Injury. Anesth Analg. 2023;136(1):176–184. doi: 10.1213/ANE.0000000000006286 [DOI] [PubMed] [Google Scholar]
- 29.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


