Abstract
In situ hybridization is a standard procedure for visualizing mRNA transcripts in tissues. The recent adoption of fluorescent probes and new signal amplification methods have facilitated multiplexed RNA imaging in tissue sections and whole tissues. Here we present protocols for multiplexed hybridization chain reaction fluorescence in situ hybridization (HCR-FISH) staining, imaging, cell segmentation, and mRNA quantification in regenerating axolotl tissue sections. We also present a protocol for whole-mount staining and imaging of developing axolotl limbs.
Keywords: In situ hybridization, Hybridization chain reaction, Axolotl, Limb regeneration, Limb development
1. Introduction
In situ hybridization (ISH) has been the primary technique for imaging DNA and RNA in cells and tissues for over 50 years [1, 2]. Although the original approaches relied upon radiolabeled probes, the most widespread ISH approach in modern research utilizes digoxigenin-tagged probes, which were first demonstrated using antisense DNA probes [3, 4]. Eventually, antisense RNA probes became the dominant method for ISH in tissue sections [5, 6] and whole-mount tissues [7–9] because they showed high specificity and were relatively straightforward and inexpensive to generate [10]. Fluorescent probes have some advantages over digoxigenin-labeled probes, such as their multiplexing capabilities and high resolution but have traditionally been expensive to produce. The cost of oligonucleotide synthesis has decreased in recent years, which could facilitate the widespread adoption of fluorescently tagged oligonucleotide probes and emerge as a solid alternative to digoxigenin-based ISH. Yet, a large variety of FISH methods make it difficult to evaluate the most user-friendly and robust option for any particular animal model [11–18]. Commercial resources generally market mice or human samples, making non-model probe sets prohibitively expensive to commercially design and optimize. Furthermore, colorimetric ISH traditionally does not use quantitative image analysis, so learning new computational image analysis approaches may be intimidating or challenging to implement.
One new method that has shown considerable promise to work in many animal types and large tissues is hybridization chain reaction (HCR). Dirks and Pierce initially developed HCR in 2004 [19], subsequently to be used for multiplexed FISH [20]. The third iteration of HCR (v3.HCR-FISH) has a considerably higher signal to background ratio over the previous methods due to the implementation of split-initiator oligonucleotide probes [18] (Fig. 1a). Two split-initiator probes become whole when the pair hybridizes to their target mRNA, significantly increasing specificity over unsplit initiator probes. Probe pair hybridization then triggers the hybridization chain reaction (Fig. 1b). Five different initiators can be used that pair with five types of fluorescently labeled hairpins, enabling the staining of up to five gene targets in one hybridization, depending upon the microscope’s capabilities (Fig. 1c). The number of genes analyzed with HCR-FISH has also been increased through subsequent rounds of staining [21] (Fig. 1d). Lastly, v3.HCR-FISH has been successful in many different species, corroborating the robustness of the protocol.
Fig. 1.

HCR schematic diagram. Schematic of v3.HCR-FISH on tissue sections. Transcripts of interest are hybridized with pools of split initiator probes containing unique initiators, B1–B5 (a). Following hybridization, initiator-specific fluorescently labeled hairpins are applied to the samples and a hybridization chain reaction is initiated (b). Sections are imaged for each gene of interest (c). Probes with hairpins are removed from the sections with a DNase treatment and probe wash (d). Subsequent rounds of hybridization are performed using new probe pools. (Adapted from “Loop-Mediated Isothermal Amplification (LAMP),” by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates)
ISH has been the primary tool of choice for studying the development and regeneration of amphibians. For example, the axolotl is a powerful model for studying vertebrate development, physiology, and regeneration [22]. Recent advances in transgenics and genomic sequencing are driving new investigation into the molecular basis of axolotl regeneration [23–27]. Although ISH has been utilized for decades in axolotls [28, 29], it has been challenging to implement consistently across tissues and in whole mount. Newer FISH methods are beginning to be used in axolotls [27, 30, 31], including v3.HCR-FISH [32, 33], but broadly applicable probe sets and protocols are not established.
With these considerations in mind, we present here v3.HCR-FISH protocols that have worked well for our lab using axolotl tissues. We provide multiplexed v3.HCR-FISH protocols in axolotl tissue sections and whole-mount samples for visualizing gene expression.
2. Materials
2.1. Axolotls
The supplier for research axolotls in the United States is the Ambystoma Genetic Stock Center, University of Kentucky, USA.
2.2. Equipment and Consumables
Microscope slides.
Water bath sonicator.
Number 1.5 coverslips.
Disposable cryomolds.
Cryostat.
Slide staining boxes (EMS 62010–37).
50-ml conical tubes.
Hybridization chambers (Grace Biolabs 621101).
Incubation oven at 37 °C.
Thermocycler.
Zeiss LSM 880 or equivalent confocal microscope.
Glass capillaries for lightsheet microscopy.
Zeiss Z.1 lightsheet microscope or equivalent.
2.3. Buffers and Solutions
DEPC-treated water: Add 1 ml of 97% pure diethyl pyrocarbonate (DEPC) to 1 L of filtered DI water, mix well, and incubate at room temperature overnight. Autoclave the solution the following day (see Note 1).
1 M KOH: Weigh 2.8 g KOH and add to a 50-ml conical tube. Fill to 50 ml with DEPC water.
Coverslip functionalization solution: Add 1 ml of glacial acetic acid to 18.4 ml of 100% methanol. Then add 600 μl of 3-animopropyltriethoxysilane [34].
Tissue clearing solution: 4% SDS, 200 mM boric acid, pH 8.5. For 1 L, measure 12.37 g of boric acid. Add 500 ml of DEPC-treated water and pH to 8.5 with NaOH. Add 200 ml of 20% SDS and fill to 1 L with DEPC-treated water [35].
5×SSCT: Combine 12.5 ml of 20×SSC and 500 μl of 10% Tween-20 solution in a 50-ml conical tube. Fill with DEPC water to 50 ml.
Purchase HCR-FISH reagents including hybridization buffer, wash buffer, and amplification buffer through Molecular Instruments (https://www.molecularinstruments.com/). Dispose of all waste following appropriate regulations.
Probe solution: 50 μl of probe solution at 5 nM. Resuspend lyophilized oPool oligonucleotide pools in 50 μl TE buffer to make a 1-μM stock probe solution. Dilute stock probe solution 1:200 in hybridization buffer to generate a 5-nM probe solution.
Hairpin solution: 50 μl of hairpins at 60 nM. Pipet 1 μl of each fluorescently labeled H1 hairpin (3 μM) into a 0.2-ml PCR tube. Hairpins with different fluorophores can be combined in the same tube (for example, H1B1-Alexa488 and H1B2-Alexa594). Use a separate tube for the H2 hairpins. Heat the hairpins to 95 °C for 90 s in a thermocycler. Remove from the thermocycler and cool in the dark for 30 min. Combine all hairpins together in 50 μl amplification solution. These amounts can be scaled up as long as the ratio of hairpin to amplification solution is 1:50 vol/vol.
DNase solution: 50 μl of 500 units/ml DNase. Combine 5 μl of 10× DNase buffer and 32.5 μl of DEPC water. Then add 12.5 μl of 2000 units/ml DNase (NEB M0303).
1.5% low melt (LM) agarose: Add 0.15 g of LM agarose to 10 ml 1×PBS. Heat the solution in a heat block or microwave until agarose is completely dissolved. Aliquot into 1.5 ml tubes and store in 4 °C.
2.4. Oligo Probe Design and Ordering
The oPools scale that accommodates 50 pmol/oligo can include up to 36 split-initiator DNA oligonucleotide pairs per mRNA target. Probe pairs are designed to be two bases apart and have half of an HCR initiator sequence appended to the end (Fig. 1). We have developed a web application that includes the axolotl genome for designing custom probe pools (see Note 2). The web app, called Probegenerator, utilizes Oligominer [36] and Bowtie2 [37] to generate probe set pools (see Note 3). First, OligoMiner generates all possible 25 base sequences that fall within specified melting temperature and GC content ranges. Probe candidates are then filtered into pairs of sequences separated by two nucleotides and then aligned to the axolotl genome using Bowtie2 to screen for off-target hits. Qualifying probe pairs are written to separate .csv files for each user specified v3.HCR-FISH initiator sequence. The Probegenerator web application can be found here: probegenerator.herokuapp.com. Sometimes more than 36 probe pairs are available. Probes are selected in the coding sequence first, then 3′UTR, and last the 5′UTR. Once 36 probe pairs are selected, they are ordered as an oPool from Integrated DNA Technologies. oPools contain 50 pmol of each oligonucleotide in a dry pellet. This ordering scheme provides enough probes for 10 ml of hybridization solution (see Note 4).
2.5. Ordering Hairpins
We order fluorescently labeled hairpins directly from Molecular Instruments (https://www.molecularinstruments.com/). Be sure to match the hairpins (B1–B5) with the initiator on the mRNA of interest. It is up to the investigator to best match initiators with fluorophores (for example, our common ordering scheme is 600 pmol B1 Alexa Fluor 647, 600 pmol B2 Alexa Fluor 594, and 600 pmol B3 Alexa Fluor 488).
3. Methods
3.1. Coverslip Functionalization
Multiround HCR-FISH is performed on a functionalized coverslip with a hybridization chamber adhered to the coverslip. Coverslips need to be functionalized to promote tissue adhesion. Coverslip functionalization protocol is based upon a previous protocol [34]. All steps are performed at room temperature.
Submerge coverslips into 1 M KOH solution in a 50-ml conical tube. Float the tube in a water bath sonicator and sonicate for 20 min.
Wash coverslips with DEPC-treated water 3 × 5 min.
Submerge coverslips in 100% MeOH for 10 min.
Pipet the coverslip functionalization solution onto the coverslips and incubate for 2 min. This step can be done in a petri dish or slide staining box.
Wash coverslips with DEPC-treated water 3 × 5 min.
Dry coverslips and store at room temperature in a slide box or conical tube with silica for up to 2 weeks.
3.2. Tissue Cryosectioning and Section Dehydration
Tissue collection: Anesthetize axolotl in 0.01% benzocaine. Surgically remove tissue from animal, mount immediately in cryomold filled with 100% OCT, and flash freeze in dry ice/isopentane bath.
Tissue sectioning: For multiround HCR, sections are collected on a coverslip rather than a microscope slide (see Note 5). Draw a template grid on a microscope slide that matches with the hybridization chambers (Fig. 2). This ensures that cryosections line up with the hybridization chamber that is used in the protocol. Adhere the coverslip to the microscope slide by adding a small drop of water on the microscope slide and placing the coverslip on top of the templated slide at room temperature. Cryosection the tissue at 10 μm and adhere to the coverslip that is on top of the templated slide. Once the tissue section is on the coverslip, do not remove it from the cryostat. Let it dry in the cryostat for 30 min. Immediately perform tissue fixation after cryosectioning.
Fixation and tissue clearing: Without letting the slide thaw, apply 4% PFA to cover sections and incubate for 15 min. Wash sections with 2×SSC, 3 × 5 min by placing solutions directly on the coverslip. Apply 4% SDS, 200 mM boric acid solution to sections, 2 × 5 min to clear the tissue. Wash sections with 2SSC, 3 × 5 min. All steps are performed at room temperature.
Dehydration: Incubate sections in 100% EtOH for 10 min at room temperature and then allow sections to dry completely. Once dry, peel the plastic cover off of the hybridization chamber and adhere the chamber onto the coverslip on top of the tissue sections. Wash sections with 50 μl 2×SCC 2 × 5 min.
Fig. 2.
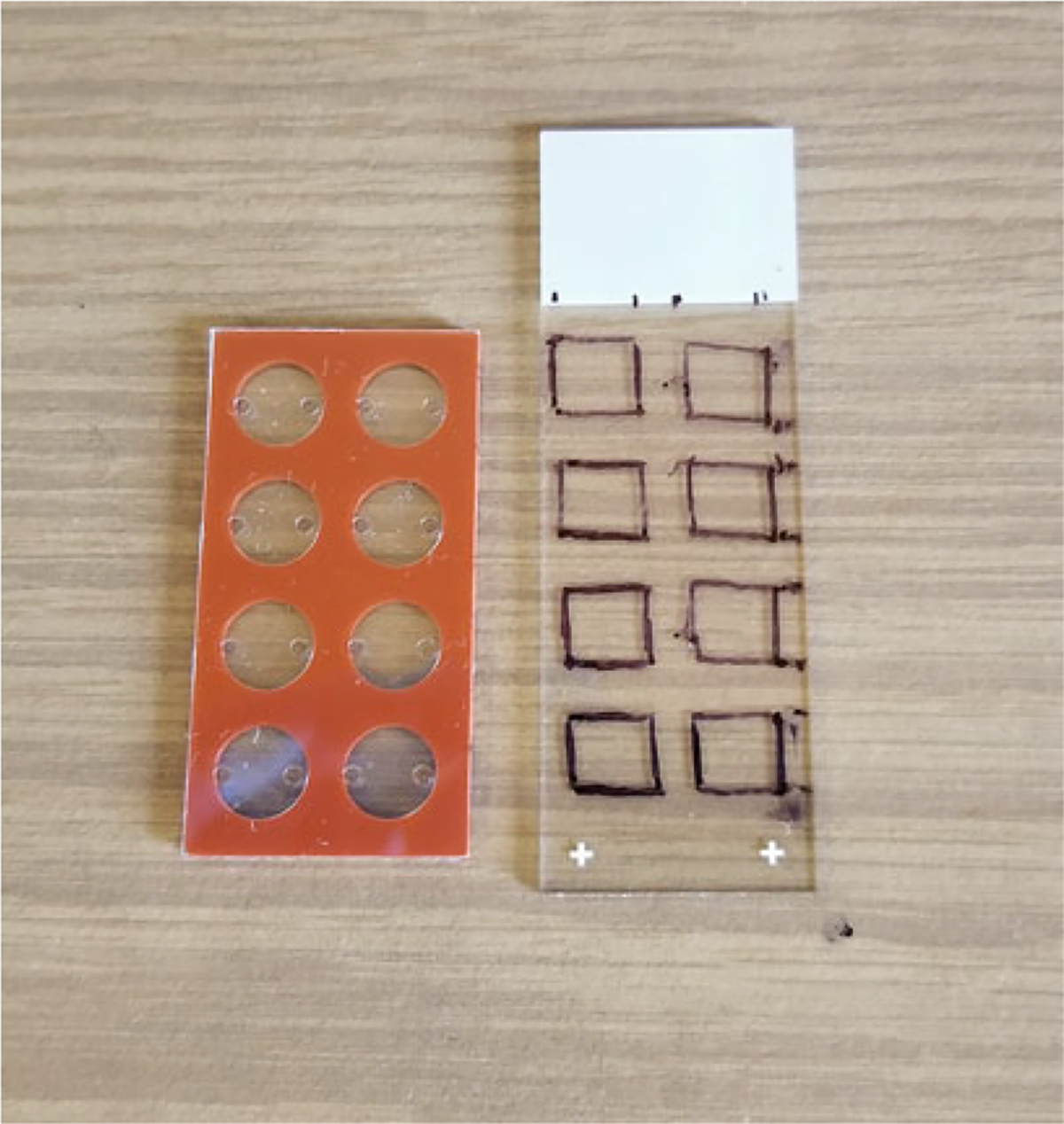
Hybridization chamber-well locations. Areas on the slide correlating to the hybridization chambers are marked with a sharpie. Sections are collected onto a functionalized coverslip placed on top of the marked slide. This ensures proper placement of the sections for the hybridization chamber
3.3. v3.HCR-FISH on Tissue Sections
Prehybridization: Pipet 50 μl of hybridization buffer into a hybridization chamber and incubate at 37 °C for 15 min. Place the prepared probe solution at 37 °C to preheat during prehybridization.
Hybridization: Remove the hybridization buffer from the chamber and pipet 50 μl of preheated probe solution into the hybridization chamber. Hybridize overnight at 37 °C in an incubator using a coverslip to seal the chambers.
Probe washing: Preheat probe wash solution at 37 °C. Remove the probe solution from the chamber and pipet 50 μl of preheated probe wash solution to the hybridization chamber 3 × 15 min at 37 °C. Then wash sections with 5×SSCT for 15 min at 37 °C followed by a 5-min 5SSCT wash at room temperature.
Amplification: Remove 5×SSCT and pipet 50 μl of amplification buffer to the hybridization chamber. Incubate for 30 min at room temperature. Remove preamplification solution and pipet 50 μl of hairpin solution into the chamber. Cover hybridization chambers with a coverslip and leave for 3 h at room temperature in a humidified slide staining box.
Amplification washing and mounting: Remove hairpin solution and wash sections with 50 μl 5×SSCT, 2 × 30 min at room temperature. Apply a DNA stain such as DAPI or Hoechst to the chambers for 5 min at room temperature and then wash with PBS for 5 min. Fill the hybridization chamber with anti-fade mounting media and image immediately or store at 4 °C for later imaging.
Imaging: Acquire images using a Zeiss LSM 880 confocal microscope with a 63×/1.4 oil Plan Apochromat objective or similar microscope. The laser lines and associated gating that are recommended for each fluorophore are as follows: DAPI/405 (378–497), 488 (493–520), 514 (580–624), 561 (612–661), and 647 (668–755).
3.4. Probe Wiping and Rehybridization of New Probes
The first probe set can be removed and new probes added if you plan to image more genes than can be imaged in a single-staining experiment (more than 4).
Remove mounting media and pipet 50 μl of 2×SSC, 3 × 15 min at room temperature.
Wipe DNA probes and hairpins with DNase solution for 1 h at room temperature. Then pipet 50 μl of probe wash solution 3 × 5 min at 37 °C followed by 3 × 5 min 2×SSC washes at room temperature.
Repeat steps in Subheading 3.3 with new probes. Skip the nuclear staining step in subsequent rounds of v3.HCR-FISH.
Repeat steps in Subheading 3.4 for each subsequent round of staining.
3.5. Multiround v3. HCR-FISH Image Analysis: Segmentation
Images of multiround experiments are aligned using Zen software. We provide an example data analysis pipeline for counting fluorescent foci for two rounds of HCR-FISH. This analysis consists broadly of two major steps: cell segmentation and dot counting. We perform cell segmentation using the deep-learning method, Cellpose [38] (Fig. 3). Cellpose can be utilized in a web app (www.cellpose.org) or through local installation (https://github.com/mouseland/cellpose).
Fig. 3.

Multiplexed FISH. Two sequential rounds of v3.HCR-FISH performed on a regenerating axolotl limb tissue section. The DAPI image is shown for reference (a) along with four genes stained in round 1: Midkine (Mdk) (b), Keratin 5 (Krt5) (c), Axolotl anterior gradient (Aag) (d), Thrombospondin 1 (Thbs1) (e) and an overlay of all four genes (f). Four genes stained in round 2: Hyaluronan And Proteoglycan Link Protein 3 (g), Keratin 17 (h), Methyltransferase-like (i), Laminin beta 1 (j) and an overlay of all 4 genes (k). 50-μm scale bar is included on DAPI image and a dotted line represents the epithelium/mesenchyme boundary
To open the installed Cellpose on your local machine, first open Anaconda.
Open command prompt through base directory.
Type -run “conda activate cellpose.”
Type -run “python -m cellpose.”
Drag the image into Cellpose window.
Change cell diameter based on sample type and image acquisition.
Change parameters if needed for optimal segmentation.
Once desired mask is generated, save mask as a .png.
It may be necessary to convert this mask file to a .tif depending on what program you wish to use for your analysis.
It may be necessary to hand annotate images if settings do not catch every cell, this is easily accomplished in the cellpose GUI.
3.6. Multiround v3. HCR-FISH Image Analysis: Dot Counting
Perform dot detection and counting using CellProfiler [39]. This pipeline consists of identifying cell regions of interest from the Cellpose mask, isolating puncta from raw FISH images, counting dots, and assigning dots to a cell. The CellProfiler pipeline used is provided here (https://github.com/Lovelya-NEU/Hybridization-chain-reaction-fluorescence-in-situ-hybridization-in-Ambystoma-mexicanum-tissue). Raw image files were put through a gaussian filter of sigma radius 1 prior to uploading into the pipeline (Fig. 4).
Fig. 4.

Graphical depiction of data analysis workflow. Major steps and outputs from HCR analysis pipeline. First, nuclei/cells are segmented using Cellpose (a). This mask is saved and then imported into CellProfiler where it is converted into regions of interest (b). HCR-FISH puncta are then identified in CellProfiler and assigned to a parent cell (c). The data can be outputted into a spreadsheet for further analysis
3.7. Whole-Mount v3. HCR-FISH
Sample collection and preparation: For developmental limb buds, cut the embryo in half and collect only the trunk and limb bud. Immediately fix the tissue in at least 10× the volume of 4% PFA in a 1.5-ml tube overnight at 4 °C. Wash tissue 3 × 5 min in 1 PBST at room temperature.
Sample dehydration: Dehydrate samples on ice in an increasing methanol series for 5 min at each step: 25% MeOH (diluted in 1×PBST), 50% MeOH, 75% MeOH, and 100% MeOH. Replace the 100% MeOH and store at −20 °C overnight (see Note 6).
Sample rehydration: Rehydrate samples on ice in a decreasing methanol series for 5 min at each step: 75% MeOH (diluted in 1×PBST), 50% MeOH, 25% MeOH, followed by two 5-min washes in PBST.
Proteinase K treatment: Treat samples with 10 μg/ml of proteinase K for 15 min at room temperature (see Note 7). Terminate proteinase K activity by fixing samples in 4% PFA at room temperature for 3 × 20 min followed by 3 × 5 min washes with 1PBST at room temperature.
Hybridization: Briefly incubate tissue in hybridization buffer at 37 °C for 5 min in a 1.5-ml tube. Prehybridize tissue in preheated hybridization buffer at 37 °C for 30 min. Apply enough hybridization probe solution to cover the tissues (usually 50 μl) in a 1.5-ml tube and incubate overnight at 37 °C.
Probe washing: Wash the sample in preheated probe wash solution for 4 × 15 min in a 37 °C incubator. Wash samples 2 × 5 min in 5×SSCT at room temperature. Pre-amplify tissue in amplification buffer for 30 min at room temperature.
Amplification: Prepare the hairpin amplification solution as described in Subheading 2.3. Make enough hairpin to submerge the tissue (usually 25–50 μl). Apply hairpin amplification solution to sample and incubate overnight at room temperature in the dark.
Amplification washing: Wash samples with 1 ml of 5×SSCT at room temperature for 5, 30, 30, and 5 min each. Sample can be stored at 4 00boC prior to imaging.
3.8. Imaging with Lightsheet Fluorescence Microscopy (LSFM)
Mount sample in LSFM capillary with 1.5% low-boiling point agarose (see Note 8).
Once the sample solidifies, place agarose-embedded sample in 1×PBS for 5 min.
Whole-mount imaging is performed on a Zeiss Lightsheet Z.1 microscope using a 10× water objective with laser lines 488, 561, and 647 (Fig. 5) (see Note 9). The imaging chamber is filled with 1×PBS.
Fig. 5.

Whole-mount FISH. Whole-mount, 3D visualization of Shh (magenta), Prrx1 (yellow), and Fgf8 (cyan) in a stage 46 axolotl limb bud (a). A single z-slice of the limb bud (b). Scale bar = 100 μm
4. Notes
Perform the protocol using RNase-free techniques. Clean work area with RNase AWAY and use fresh gloves. Use disposable plasticware or RNase-free glassware for all the solutions. Prepare all solutions using Diethyl pyrocarbonate (DEPC)-treated water or water from a high-end water purification system that generates 18-megohm water. When handling DEPC, make sure you are wearing gloves and only open the bottle inside a fume hood.
Probes can also be designed through Molecular Instruments. They will design and synthesize the probe pools and ship hybridization-ready pools.
For further description of the website design, see https://github.com/davidfstein/probegenerator.
Alternatively, plates of individual oligos that contain a single oligo in each well can be purchased, which will provide a large amount of oligo (4 nM per oligo for example).
If one round of HCR is going to be performed, sections can becollected on Superfrost Plus microscope slides instead of coverslips and the hybridization chambers are not used.
Samples can be left at −20 °C for long-term storage.
The concentration and duration of proteinase K treatmentshould be optimized for each tissue type. Our lab has found that this treatment is sufficient for mild clearing of tissue without significant tissue damage.
It is important to orient the sample such that no tissue obscuresthe region of interest, such as the body cavity. Forceps can be inserted into the LSFM capillary as the agarose is solidifying to manipulate the tissue into position.
Refractive index matching can be performed by embedded tissue in EasyIndex overnight at 4 °C and imaging with a 5× or 20× clearing-enabled objective in EasyIndex.
Acknowledgements
All images were acquired in the Northeastern Chemical Imaging of Living Systems facility. We thank the Institute for Chemical Imaging of Living Systems at Northeastern University for consultation and imaging support. The authors would like to thank Harry Choi at Molecular Instruments for feedback during protocol development. Grant sponsors include NSF grants 1558017 and 1656429 to JRM. NIH grant HD099174 to JRM. Material and information obtained from the Ambystoma Genetic Stock Center funded through NIH grant: P40-OD019794.
Footnotes
Competing Interests Authors declare no competing interests.
References
- 1.Gall JG, Pardue ML (1969) Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A 63(2):378–383. 10.1073/pnas.63.2.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John HA, Birnstiel ML, Jones KW (1969) RNA-DNA hybrids at the cytological level. Nature 223(5206):582–587. 10.1038/223582a0 [DOI] [PubMed] [Google Scholar]
- 3.Herrington CS, Burns J, Graham AK, Evans M, McGee JO (1989) Interphase cytogenetics using biotin and digoxigenin labelled probes I: relative sensitivity of both reporter molecules for detection of HPV16 in CaSki cells. J Clin Pathol 42(6):592–600. 10.1136/jcp.42.6.592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tautz D, Pfeifle C (1989) A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98(2):81–85. 10.1007/bf00291041 [DOI] [PubMed] [Google Scholar]
- 5.Aigner S, Pette D (1990) In situ hybridization of slow myosin heavy chain mRNA in normal and transforming rabbit muscles with the use of a nonradioactively labeled cRNA. Histochemistry 95(1):11–18. 10.1007/bf00737222 [DOI] [PubMed] [Google Scholar]
- 6.Komminoth P, Merk FB, Leav I, Wolfe HJ, Roth J (1992) Comparison of 35S- and digoxigenin-labeled RNA and oligonucleotide probes for in situ hybridization. Expression of mRNA of the seminal vesicle secretion protein II and androgen receptor genes in the rat prostate. Histochemistry 98(4):217–228. 10.1007/bf00271035 [DOI] [PubMed] [Google Scholar]
- 7.Hemmati-Brivanlou A, Frank D, Bolce ME, Brown BD, Sive HL, Harland RM (1990) Localization of specific mRNAs in Xenopus embryos by whole-mount in situ hybridization. Development 110(2):325–330 [DOI] [PubMed] [Google Scholar]
- 8.Herrmann BG (1991) Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development 113(3):913–917 [DOI] [PubMed] [Google Scholar]
- 9.Schulte-Merker S, Ho RK, Herrmann BG, Nüsslein-Volhard C (1992) The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116(4):1021–1032 [DOI] [PubMed] [Google Scholar]
- 10.Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR (1984) Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res 12(18):7035–7056. 10.1093/nar/12.18.7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S (2008) Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods 5(10):877–879. 10.1038/nmeth.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishi JY, Beliveau BJ, Lapan SW et al. (2018) SABER enables highly multiplexed and amplified detection of DNA and RNA in cells and tissues. bioRxiv. 10.1101/401810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouhanifard SH, Mellis IA, Dunagin M et al. (2018) ClampFISH detects individual nucleic acid molecules using click chemistry-based amplification. Nat Biotechnol. 10.1038/nbt.4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codeluppi S, Borm LE, Zeisel A et al. (2018) Spatial organization of the somatosensory cortex revealed by osmFISH. Nat Methods 15(11):932–935. 10.1038/s41592-018-0175-z [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Flanagan J, Su N et al. (2012) RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14(1):22–29. 10.1016/j.jmoldx.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagendran M, Riordan DP, Harbury PB, Desai TJ (2018) Automated cell-type classification in intact tissues by single-cell molecular profiling. Elife 7. 10.7554/eLife.30510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyllborg D, Langseth CM, Qian X et al. (2020) Hybridization-based in situ sequencing (HybISS) for spatially resolved transcriptomics in human and mouse brain tissue. Nucleic Acids Res 48(19):e112. 10.1093/nar/gkaa792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi HMT, Schwarzkopf M, Fornace ME et al. (2018) Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145(12). 10.1242/dev.165753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirks RM, Pierce NA (2004) Triggered amplification by hybridization chain reaction. Proc Natl Acad Sci U S A 101(43):15275–15278. 10.1073/pnas.0407024101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi HM, Chang JY, Trinh LA, Padilla JE, Fraser SE, Pierce NA (2010) Programmable in situ amplification for multiplexed imaging of mRNA expression. Nat Biotechnol 28(11):1208–1212. 10.1038/nbt.1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah S, Lubeck E, Zhou W, Cai L (2016) In situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron 92(2):342–357. 10.1016/j.neuron.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voss SR, Epperlein HH, Tanaka EM (2009) Ambystoma mexicanum, the axolotl: a versatile amphibian model for regeneration, development, and evolution studies. Cold Spring Harb Protoc 2009(8). 10.1101/pdb.emo128 [DOI] [PubMed] [Google Scholar]
- 23.Khattak S, Tanaka EM (2015) Transgenesis in axolotl (Ambystoma mexicanum). Methods Mol Biol 1290:269–277. 10.1007/978-1-4939-2495-0_21 [DOI] [PubMed] [Google Scholar]
- 24.Flowers GP, Timberlake AT, McLean KC, Monaghan JR, Crews CM (2014) Highly efficient targeted mutagenesis in axolotl using Cas9 RNA-guided nuclease. Development 141(10):2165–2171. 10.1242/dev.105072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fei JF, Schuez M, Knapp D, Taniguchi Y, Drechsel DN, Tanaka EM (2017) Efficient gene knockin in axolotl and its use to test the role of satellite cells in limb regeneration. Proc Natl Acad Sci U S A 114(47):12501–12506. 10.1073/pnas.1706855114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowoshilow S, Schloissnig S, Fei JF et al. (2018) The axolotl genome and the evolution of key tissue formation regulators. Nature 554(7690):50–55. 10.1038/nature25458 [DOI] [PubMed] [Google Scholar]
- 27.Smith JJ, Timoshevskaya N, Timoshevskiy VA, Keinath MC, Hardy D, Voss SR (2019) A chromosome-scale assembly of the axolotl genome. Genome Res 29(2):317–324. 10.1101/gr.241901.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardiner DM, Blumberg B, Komine Y, Bryant SV (Jun 1995) Regulation of HoxA expression in developing and regenerating axolotl limbs. Development 121(6):1731–1741 [DOI] [PubMed] [Google Scholar]
- 29.Sun HB, Neff AW, Mescher AL, Malacinski GM (1995) Expression of the axolotl homologue of mouse chaperonin t-complex protein-1 during early development. Biochim Biophys Acta 1260(2):157–166. 10.1016/0167-4781(94)00187-8 [DOI] [PubMed] [Google Scholar]
- 30.Freitas PD, Lovely AM, Monaghan JR (2019) Investigating Nrg1 signaling in the regenerating axolotl spinal cord using multiplexed FISH. Dev Neurobiol. 10.1002/dneu.22670 [DOI] [PubMed] [Google Scholar]
- 31.Woodcock MR, Vaughn-Wolfe J, Elias A et al. (2017) Identification of mutant genes and introgressed tiger salamander DNA in the laboratory axolotl, Ambystoma mexicanum. Sci Rep 7(1):6. 10.1038/s41598-017-00059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leigh ND, Sessa S, Dragalzew AC et al. (2020) von Willebrand factor D and EGF domains is an evolutionarily conserved and required feature of blastemas capable of multitissue appendage regeneration. Evol Dev 22(4):297–311. 10.1111/ede.12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schloissnig S, Kawaguchi A, Nowoshilow S et al. (2021) The giant axolotl genome uncovers the evolution, scaling, and transcriptional control of complex gene loci. Proc Natl Acad Sci U S A 118(15). 10.1073/pnas.2017176118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goh JJL, Chou N, Seow WY et al. (2020) Highly specific multiplexed RNA imaging in tissues with split-FISH. Nat Methods 17(7):689–693. 10.1038/s41592-020-0858-0 [DOI] [PubMed] [Google Scholar]
- 35.Chung K, Deisseroth K (2013) CLARITY for mapping the nervous system. Nat Methods 10(6):508–513. 10.1038/nmeth.2481 [DOI] [PubMed] [Google Scholar]
- 36.Beliveau BJ, Kishi JY, Nir G et al. (2018) OligoMiner provides a rapid, flexible environment for the design of genome-scale oligonucleotide in situ hybridization probes. Proc Natl Acad Sci U S A 115(10):e2183–e2192. 10.1073/pnas.1714530115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stringer C, Wang T, Michaelos M, Pachitariu M (2021) Cellpose: a generalist algorithm for cellular segmentation. Nat Methods 18(1):100–106. 10.1038/s41592-020-01018-x [DOI] [PubMed] [Google Scholar]
- 39.McQuin C, Goodman A, Chernyshev V et al. (2018) CellProfiler 3.0: next-generation image processing for biology. PLoS Biol 16(7):e2005970. 10.1371/journal.pbio.2005970 [DOI] [PMC free article] [PubMed] [Google Scholar]


