Abstract
The nucleocapsid protein (NC) of retroviruses plays a major role in genomic RNA packaging, and some evidence has implicated the matrix protein (MA) of certain retroviruses in viral RNA binding. To further investigate the role of NC in the selective recognition of genomic viral RNA and to address the potential contribution of MA in this process, we constructed chimeric and deletion human immunodeficiency virus type 1 (HIV-1) mutants that alter the NC or MA protein. Both HIV and mouse mammary tumor virus (MMTV) NC proteins have two zinc-binding domains and similar basic amino acid compositions but differ substantially in total length, amino acid sequence, and spacing of the zinc-binding motifs. When the entire NC coding sequence of HIV was replaced with the MMTV NC coding sequence, we found that the HIV genome was incorporated into virions at 50% of wild-type levels. Viruses produced from chimeric HIV genomes with complete NC replacements, or with the two NC zinc-binding domains replaced with MMTV sequences, preferentially incorporated HIV genomes when both HIV and MMTV genomes were simultaneously present in the cell. Viruses produced from chimeric MMTV genomes in which the MMTV NC had been replaced with HIV NC preferentially incorporated MMTV genomes when both HIV and MMTV genomes were simultaneously present in the cell. In contrast, viruses produced from chimeric HIV genomes containing the Moloney NC, which contains a single zinc-binding motif, were previously shown to preferentially incorporate Moloney genomic RNA. Taken together, these results indicate that an NC protein with two zinc-binding motifs is required for specific HIV RNA packaging and that the amino acid context of these motifs, while contributing to the process, is less crucial for specificity. The data also suggest that HIV NC may not be the exclusive determinant of RNA selectivity. Analysis of an HIV MA mutant revealed that specific RNA packaging does not require MA protein.
Incorporation of the RNA genome into a retroviral particle requires participation of cis-acting viral RNA sequences called Ψ, which are typically located at the 5′ end of the genome, together with trans-acting proteins (reference 43 and references therein). A portion of the Gag precursor (GagPr) called the nucleocapsid protein (NC) has been shown to be crucial for this process in a number of retroviruses (reference 12 and references therein). Features common to all the NC proteins include a high content of basic residues and, with the exception of the spumavirus, the presence of zinc-binding motifs of the form CysX2CysX4HisX4Cys (6, 35). NC proteins of most mammalian type C retroviruses have one zinc-binding domain, while all the other retroviral NCs (of type B and D retroviruses, of primate and nonprimate lentiviruses, and of human T-lymphotropic virus types 1 and 2) have two. The role of these features of the NC proteins has been extensively examined in vitro and in vivo (5, 7, 9, 12, 36–38, 40, 45). NC proteins can bind viral RNA in vitro, and alterations in the basic residues as well as the zinc-binding domains can affect RNA binding in vitro (12, 34, 45). Analyses of NC mutants support the role of NC in RNA packaging in vivo (1, 7–9, 12, 20, 21, 36–38, 40). Alteration of the zinc-binding motif(s) can cause loss of packaging of genomic RNA and increased packaging of spliced viral RNA and cellular mRNAs (7, 13, 14, 36–38, 52), while alterations of the basic residues seem to affect the general ability to bind RNA (40, 45).
To study the contribution of NC to specific retroviral RNA packaging, one approach has been to investigate RNA incorporation into chimeric viral particles (5, 7, 13, 52). Substitution of the coding sequences of Rous sarcoma virus (RSV) NC, containing two zinc finger domains, with the coding sequence of Moloney murine leukemia virus (MoMLV) NC, containing only one zinc-binding domain, led to a 50- to 100-fold reduced incorporation of the RSV genome and to the preferential incorporation of the MoMLV genome in competition experiments (13). Similarly, when the MoMLV NC was substituted for the human immunodeficiency virus (HIV) NC in the HIV genome, it preferentially mediated the packaging of RNA containing the MoMLV Ψ element (7). In complementary experiments, substitution of the MoMLV NC coding sequence with an HIV NC sequence in an MoMLV genomic clone led to preferential incorporation of a genome carrying the homologous HIV Ψ sequence (7, 52). These experiments lead to the conclusion that the NC is the major determinant, or one of the major determinants, of affinity and specificity of viral genomic RNA recognition.
The deletion of one of the two zinc-binding domains in NC proteins containing two zinc-binding motifs is sufficient to substantially reduce viral RNA packaging, in some cases to levels similar to that observed with heterologous NC proteins carrying only one zinc-binding domain (8, 20). When two motifs are present, the first generally plays a more important role than the second (8, 20). In RSV, deletion of the first of the two zinc finger motifs abolishes RNA packaging and infectivity, whereas deletion of the second of the two motifs reduces viral infectivity about 100-fold (8). Both RSV zinc fingers are important for 70S RNA dimer formation. In HIV, mutants in which the first and second zinc-binding domain sequences are reversed and mutants containing two second zinc-binding domain sequences package RNA at less than 15% of wild-type levels, while mutants with two first zinc-binding domain sequences package RNA at 70% of wild-type levels but retain only 10% of wild-type infectivity (20).
Here we have extended these studies to determine whether an NC protein with two zinc-binding motifs is adequate for specific HIV RNA packaging independent of the amino acid context of those motifs. For this reason, we replaced either a portion of or the entire HIV NC coding sequence with mouse mammary tumor virus (MMTV) NC coding sequences in an HIV provirus. We also replaced the entire MMTV NC coding sequence with HIV NC coding sequences in an MMTV proviral DNA clone. Both proteins have two zinc-binding domains and similar basic amino acid contents, but they are quite different in total length, amino acid composition, and spacing of the zinc-binding motifs. Both MMTV and HIV use tRNALys as a primer for reverse transcriptase (RT), a process that also involves NC (12).
It is quite possible that the NC domain alone is not sufficient for efficient and specific RNA packaging in vivo. Several observations suggest that portions of the Gag polyprotein other than NC may be involved in efficient and specific RNA packaging. Although the processed NC protein is bound to genomic RNA in the mature viral particle, it is the intact Gag polyprotein that interacts with genomic RNA during HIV assembly. Several studies report poor affinity of NC for specific RNA sequences, suggesting that other portions of the Gag polyprotein may influence specificity (25, 32–34). Matrix proteins (MAs) from a variety of retroviruses have been reported to bind viral RNAs, albeit with widely varying affinities and specificities (11, 32, 33, 46, 49). The observation that incompletely processed bovine leukemia virus (BLV) MA specifically forms a complex with BLV dimer RNA, whereas the mature MA protein lacks this specificity, led to the suggestion that the amino terminus of the Gag polyprotein may cooperate with NC domains to specifically and efficiently package genomic RNA (26). As there are no data that address whether HIV MA is involved in RNA packaging in vivo, we investigated the involvement of HIV MA in this process, using an HIV chimeric virus containing the MoMLV MA and an HIV mutant with the MA protein deleted.
The analysis of HIV NC and MA mutants indicated that the HIV genome is preferentially incorporated when the MMTV NC is present in an HIV background, albeit slightly less efficiently than in the presence of HIV NC, and that the MMTV genome is preferentially incorporated when the HIV participate NC is present in an MMTV background. HIV MA does not participate significantly in RNA packaging.
MATERIALS AND METHODS
Plasmid construction and site-directed mutagenesis.
The biologically active HXB2 HIV-1 proviral clone carried in the plasmid pHXB2gpt (17) was the parental clone used in these studies. A full-length MMTV proviral clone, pUVH (47), was kindly provided by Gregory Shackleford (University of Southern California, Los Angeles). A full-length MoMuLV proviral clone, pMFG-LacZ (44), was kindly provided by R. Mulligan (Children’s Hospital, Boston, Mass.). The oligonucleotides used to generate the mutations present in plasmids pHX/MT-NC (full-length MMTV NC replacing the entire HIV NC sequence in pHXB2gpt), pHX/MT-ZnD (MMTV NC zinc-binding domains replacing HIV NC zinc-binding domains in pHXB2gpt), pHX/MoMA (MoMuLV MA replacing HIV MA in pHXB2gpt), pHX-ΔMA (HIV MA deletion in pHXB2gpt), and pMT/HX-NC (HIV NC replacing MMTV NC in pUVH) are listed in Table 1; standard M13 forward and reverse sequencing primers were used as outer primers. To generate these constructs, oligonucleotide-mediated site-specific mutagenesis by overlap extension was carried out (22). Briefly, a multistep PCR protocol was employed, using either plasmid pUVH or plasmid pMFG-LacZ along with plasmid pHXB2gpt as the template for mutagenesis. Recombinant HIV/MMTV NC PCR products were digested with restriction endonucleases SpeI (bp 1506) and BclI (bp 2428) and inserted into similarly treated pHXB2gpt. In pHX/MT-NC, bp 1923 to 2078 of the HIV sequence (42) were replaced with bp 3121 to 3405 of the MMTV sequence (39). A total of 52 amino acids of the HIV NC protein were replaced by 95 amino acids of the MMTV NC protein. In pHX/MT-ZnD, bp 1961 to 2066 of the HIV sequence (42) were replaced with bp 3211 to 3318 of the MMTV sequence (39). A total of 35 amino acids of the HIV NC protein were replaced by 41 amino acids of the MMTV NC protein. Recombinant MA PCR products were digested with restriction endonucleases ClaI (bp 831) and SpeI (bp 1506) and reinserted into similarly treated pHXB2gpt. These restriction sites are unique within pHXB2gpt and facilitated unidirectional cloning. In plasmid pHX/MoMA, bp 837 to 1145 of the HIV sequence (42) were replaced with bp 669 to 1007 of the MoMLV sequence (48). A total of 103 amino acids of HIV MA were replaced by 113 amino acids of the MoMLV MA protein. This replacement leaves 15 HIV MA amino acids at the NH2 terminus and 13 HIV MA amino acids of the COOH terminus to permit appropriate myristoylation and protease cleavage. In pHX-ΔMA, a deletion of 312 bp that results in the deletion of 104 amino acids (bp 835 to 1146 of the HIV sequence [42]) in the MA protein was introduced. This construct encodes a 28-amino-acid MA, containing 15 amino acids of the HIV MA NH2 terminus joined by a serine to the 12 amino acids of the HIV MA COOH terminus, to permit appropriate myristoylation and protease cleavage of GagPr. To generate plasmid pHX-AD (DNA linker encoding three alanine residues, inserted in the sequence present between the two zinc-binding domains in pHXB2gpt), pHXB2gpt was digested with restriction endonuclease ApaI (bp 2009) and a DNA linker (annealed primer APA/PST [Table 1]) was ligated into this restriction site. To eliminate the production of MMTV virions while maintaining transcription of the full-length viral genomic RNA, we modified plasmid pUVH (47) and constructed plasmid pUVHBf. A nonsense amber mutation (TAG) that replaces codon 76 (ATA) of the MMTV Gag precursor DNA sequence was introduced by a fill-in reaction of the BbsI (bp 1701) (39)-digested DNA. The recombinant MMTV/HIV NC PCR product was digested with restriction endonucleases NcoI and XboI and replaced the corresponding NcoI (bp 2547)/XboI (bp 3502) 965-bp fragment in pUVH. In pMT/HX-NC, bp 3124 to 3387 of the MMTV sequence (39) was replaced with bp 1920 to 2087 of the HIV sequence (42), leaving intact the MMTV NC sequence where one of the two frameshifts necessary for correct Gag-Pol expression occurs. A total of 88 amino acids of the MMTV NC protein was replaced by 56 amino acids of the HIV NC protein. A frameshift mutation in the HIV Gag precursor DNA sequence that prevents expression of the Gag polyprotein was introduced by a fill-in reaction of the ClaI (bp 830) (39)-digested pHXB2gpt DNA. All mutant viral constructs were confirmed by dideoxy sequencing. All DNA manipulations were performed according to standard procedures (4).
TABLE 1.
Oligonucleotide primers used in generation of recombinant virusesa
| Primer | Sequence |
|---|---|
| HMS1 | 5′-ACCAAAGAAAGATTGTTAAGTGTTTTTCCTGTGGTAAGAC |
| HMS2A | 5′-AAATTAGCCTGTCTCTCAGTACACTCACTCTTCCAGTGAT |
| HIVc1961 | 5′-CTTAACAATCTTTCTTTGGT |
| HIV2067 | 5′-ACTGAGAGACAGGCTAATTT |
| HML1 | 5′-AATCAGCTACCATAATGATGGCAGCAGCCATGAGAGGACA |
| HML2A | 5′-ATCTTCCCTAAAAAATTAGCCAAGTTTTTTGAATTTTCAG |
| HIVc1922 | 5′-CATCATTATGGTAGCTGAAT |
| HIV2079 | 5′-GCTAATTTTTTAGGGAAGAT |
| APA/PST | 5′-GCTGCAGCGGCC |
| HMO1 | 5′-AGATCGATGGAAAGATGTCGAGCGGATC |
| HMO2 | 5′-TTGCTGTGTCCTGTGTCAGCGGAGGATCGAGGCGGGGTCG |
| HIV1146 | 5′-GCTGACACAGGACACAGCAA |
| CLA1146 | 5′-AGATCGATCTGACACAGGACACAGC |
| MHL1 | 5′-TTCAGGGTATGGCATATGCAATGCAGAGAGGCAATTTTAG |
| MHL8 | 5′-TACAAGTTTTTTGAATTTTCAAAATTAGCCTGTCTCTCAG |
Cell lines, transfections, viral infections, and assays.
The African green monkey kidney cell line cos-1 was obtained from the American Type Culture Collection and maintained in Dulbecco’s modified Eagle medium (GIBCO/BRL, Bethesda, Md.) supplemented with 10% fetal bovine serum (GIBCO) at 37°C under 5% CO2. The H9 T-lymphoid cell line was maintained as previously described (41). When RNA competition assays were carried out after cotransfection of two plasmid DNAs, approximately 50 μg of pUVHBf was mixed with 10 μg of HXB2gpt DNA, pHX/MT-NC, or pHX/MT-ZnD and transfected into cos-1 cells. Cells were washed 12 h posttransfection, and medium supplemented with 0.1 μM dexamethasone was added to induce transcription of MMTV (47). Transfection of cos-1 cells by calcium phosphate precipitation and analyses of viral mutants were carried out as described previously (2, 3). Supernatants from transfected cos-1 cell cultures were harvested 48 h posttransfection, clarified, and assayed for p24 antigen, using an enzyme-linked immunosorbent assay (ELISA) (p24 core profile kit; DuPont) and for RT activity (10). Viral supernatants derived from three independent transfections per construct were tested in infectivity assays. H9 cells (5 × 105) were exposed to amounts of virus from transfected cells equivalent to 25 ng of p24 in 4 ml of medium. After 4 h of infection, cells were washed, resuspended in 2 ml of cell culture medium, and maintained in T25 culture flasks. Cultures were fed every 4 days by removing 1.5 ml of the 2 ml of cell culture suspension and replacing it with equivalent amounts of fresh medium. Cell density increased from 2.5 × 105/ml just after feeding to 106/ml 4 days later. At each 4-day interval, cleared supernatants were used for p24 ELISA and harvested cells were assayed by immunofluorescence. Each time point of each culture was evaluated in duplicate in both assays. Cultures were carried out for 32 days after infection. Dilutions of the supernatant from pHXB2gpt up to 1:10,000 (virus amount equivalent to 3 pg of p24) were still positive in the infectivity assay. Immunofluorescence assays on H9-infected cells were carried out with a murine monoclonal antibody specific for the p24 Gag protein as previously described (51). Electron microscopy was carried out according to standard procedures.
Western blot analysis.
cos-1 cells were transfected with each mutant, and 72 h posttransfection, the supernatants were collected, filtered through a 0.45-μm-pore-size filter, and centrifuged through a 3-ml cushion of 15% (wt/vol) sucrose at 27,000 rpm for 3 h in an SW28 rotor (Beckman). Each pellet was resuspended in Laemmli buffer (5% glycerol, 1% sodium dodecyl sulfate [SDS], 31.875 mM Tris [pH 6.8], 0.005% bromophenol blue) (28), and the p24 content was determined by ELISA. Samples equivalent to approximately 20 ng of p24 were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose, and probed with HIV-1-positive human serum as described previously (50). 125I-protein A (New England Nuclear, Boston, Mass.) was used to detect HIV-1-specific proteins by autoradiography. In the case of MMTV particles, viral samples from equivalent amounts of clarified supernatants were pelleted, and the protein lysates were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with a polyclonal MMTV positive rabbit serum provided by G. L. Firestone (University of California, Berkeley) as described previously (24). 125I-goat antirabbit (New England Nuclear) was used as secondary antibody to detect MMTV-specific proteins by autoradiography. The intensity of MMTV-specific bands was quantitated by using a Molecular Dynamics PhosphorImager with Image Quant software (Molecular Dynamics, Sunnyvale, Calif.).
Particle-associated RNA analysis.
RNA was isolated from the virus present in the tissue culture supernatants 48 h after the transfection ended, and quantitative RT-PCR was performed on RNA samples as previously described (40). Quantitative RT-PCR has been used previously to detect minute amounts of viral genomic RNA (27, 40). Briefly, supernatants were cleared of cells or cell debris, and after evaluation of p24 in the medium of all transfectants, virus particles equivalent to 10 ng of p24 present in the supernatants were pelleted. When the RT-PCR assay was carried out to evaluate preferential incorporation of HIV or MMTV RNA, virus was pelleted from supernatants containing virus equivalent to 25 ng of HIV-1 p24, where virions were composed of HIV proteins. MMTV particles, calibrated to contain equivalent amounts of MMTV proteins by Western blot analysis, were pelleted (approximately 1/10 of the supernatant from each transfection). The pellet was resuspended in 0.4 ml of solution D (4.2 M guanidine thiocyanate, 0.1 M sodium citrate, 0.5% SDS, 7.2% 2-mercaptoethanol) containing 120 μg of yeast tRNA per ml as a carrier to monitor final RNA recovery. The RNA was extracted with phenol-chloroform and precipitated with ethanol. To eliminate contaminating transfection or cellular DNA, the RNA was then treated with 16 U of RQ1 DNase I (Promega, Madison, Wis.) in the buffer recommended by the manufacturer (40 mM Tris [pH 8], 10 mM NaCl, 6 mM MgCl2, 10 mM CaCl2) in the presence of 80 U of recombinant RNasin RNase inhibitor (Promega) for 1 h at 37°C. The DNase I was denatured with solution D, and the RNA was precipitated with ethanol. DNase I-treated viral RNAs were resuspended in diethyl pyrocarbonate-treated water, and the yeast tRNA concentration was adjusted to 0.5 mg/ml. A PCR on RNA which did not undergo reverse transcription, and equivalent in amount to the one used for the RT-PCR, was carried out for each sample to exclude incomplete DNase I treatment. An RT-PCR using actin-related primers (ACT1 [5′-ATGGAAGAAGAGATCCGC-3′] and ACTR2 [5′-CCTCGTAGATGGGCACCG-3′]) was carried out to eliminate cellular RNA contamination. Only when a viral RNA sample scored negative in a PCR on RNA which did not undergo reverse transcription, and in an RT-PCR carried out with actin primers, incorporation of viral RNA was investigated by RT-PCR with appropriate viral primers. RNA samples were obtained from three independent transfections of each construct. RNA samples corresponding to 2 ng of p24 (HIV particles) or to an equivalent amount of viral proteins (MMTV particles) were reverse transcribed in a 30-μl reaction with Superscript II (GIBCO/BRL), using a gag-specific primer (HIVc1686 [5′-ACCGGTCTACATAGTCTCTA-3′] or MMTVc2234 [5′-TTCCAATCTGTGGCT-3′]). A third of this reaction was subjected to PCR (94°C for 1 min, 42°C for 1 min, 72°C for 1 min; 18 cycles) with AmpliTaq DNA polymerase (Perkin-Elmer), in the presence of [32P]dCTP, using the same primer as used in the RT reaction and paired with an upstream gag-specific primer (HIV1230 [5′-TCACCTAGAACTTTAAAT-3′] for experiments like the one shown in Fig. 4; HIV979 [5′-TACAACCATCCCTTCAG-3′] for experiments like the one shown in Fig. 6). MMTV gag-specific primer pairs (MMTV1542 [5′-CTGGCTATTATCCAC-3′] and MMTVc2234 [5′-TTCCAATCTGTGGCT-3′]) were used to detect the MMTV genomic RNA. The negative controls included a sample from an RT-PCR lacking input RNA and an RT-PCR with RNA extracted from a mock-transfected supernatant. Equal volumes of RT-PCR or PCR samples were subjected to PAGE and autoradiography. The intensity of each band was quantitated by using a Molecular Dynamics PhosphorImager with ImageQuant software (Molecular Dynamics). To exclude cross-contaminations of samples at the time of DNA transfection, supernatant harvesting, RNA processing, and RT-PCR, the presence of each specific mutation the virion RNA was confirmed by DNA sequencing of the final RT-PCR product.
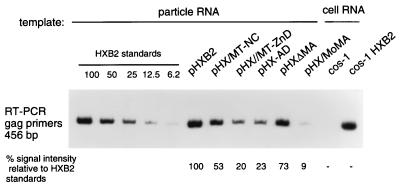
FIG. 4.
Relative nucleic acid content of viral particles determined by RT-PCR. RNA incorporation was measured for all mutant viruses in duplicate in three independent experiments using this assay, and the average values are summarized in Table 3; the results of one representative experiment are shown. RNA samples from pelleted virions containing equal amounts of p24 were reverse transcribed by using primer HIVc1686 and subjected to PCR amplification in the presence of [32P]dCTP, using the HIVc1686 primer paired with primer HIV1230. The negative control included a sample from cos-1 cellular RNA. As a positive control, a sample from an RT-PCR with RNA extracted from cos-1 cells transfected with a wild-type HIV-1 plasmid was included. Equal volumes of RT-PCR samples were subjected to PAGE and autoradiography. The intensity of a band corresponding to the amplification of genomic RNA from mutated viruses was compared to the intensity of bands produced by amplifying serially diluted RNA from the wild-type parental virus HXB2gpt (standards).
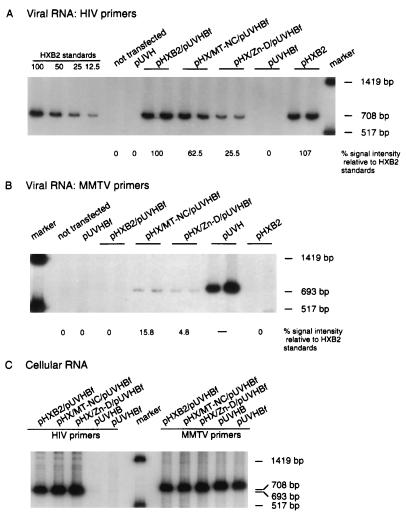
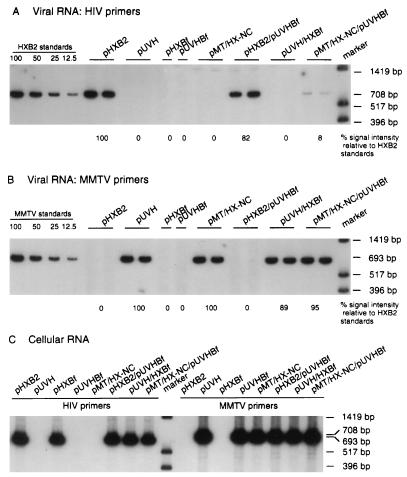
FIG. 6.
Relative homologous and heterologous RNA content of HIV particles determined by RT-PCR. RNA samples corresponding to equal amounts of p24 were reverse transcribed by using primer HIVc1686 (A) or primer MMTVc2234 (B). Equivalent aliquots of the RT reaction were subjected to PCR amplification in the presence of [32P]dCTP. Equal volumes of RT-PCR samples were subjected to PAGE and autoradiography. Two experiments were performed on RNA samples derived from two independent transfections, and the results shown are from one set of RNAs. Each sample was tested in duplicate. (A) Detection of genomic HIV-1 RNAs in a subset of mutant viruses. Primer HIVc1686 was paired with primer HIV979 to amplify a fragment of 708 bp. The intensity of bands was quantitated as described for Fig. 4. (B) Detection of genomic MMTV RNAs in a subset of mutant viruses. Primer MMTVc2234 was paired with primer MMTV1542 to amplify a band of 693 bp. (C) Amplification of cell-derived HIV-1 and MMTV genomic RNAs upon transfection. Primer HIVc1686 was paired with primer HIV979 to amplify a fragment of 708 bp. Primer MMTVc2234 was paired with primer MMTV1542 to amplify a band of 693 bp.
RNase protection assay.
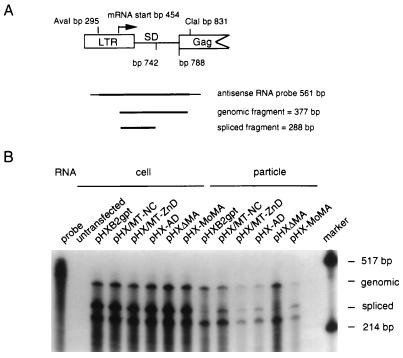
Viral RNA was isolated as described above except that virions equivalent to 100 ng of p24 were centrifuged and the RNA was resuspended in a final volume of 10 μl. Transfected cells from a 100-mm2 dish were lysed with 1 ml of solution D, and cellular RNA was isolated as described above. RNAs were assayed for the presence of viral genomic or spliced species by an RNase protection assay (Ambion Inc., Austin, Tex.). To synthesize a radiolabeled antisense riboprobe, plasmid pGAC was constructed. The AvaI (bp 296)-to-ClaI (bp 830) (39) 534-bp fragment was inserted into the AvaI-to-SphI restriction sites of pGEM4 vector (Promega); restriction endonuclease sites ClaI and SphI were blunted with T4 DNA polymerase prior to ligation. The plasmid was then linearized with AvaI and subjected to in vitro transcription using T7 RNA polymerase (New England Biolabs, Inc., Beverly, Mass.) as recommended by the manufacturer. Approximately one-fifth of the total cellular RNA sample, isolated from each transfection, and the entire viral RNA sample, isolated from virions present in each supernatant, were annealed to 1 ng (4.6 × 104 cpm/ng) of the riboprobe. In these conditions, the riboprobe RNA is in excess of the viral cellular RNA or the viral RNA from virions. The products were digested with RNases provided by the kit, and equivalent volumes were then subjected to denaturing PAGE. The intensity of each band was quantitated as described for the RT-PCR results.
RESULTS
Construction of recombinant viruses.
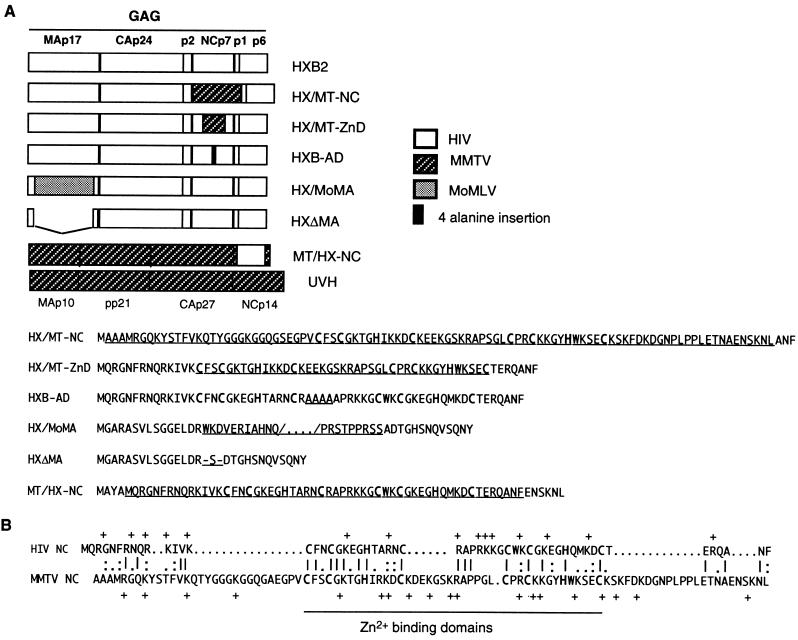
To investigate the role of NC in specific recognition and encapsidation of HIV-1 genomic RNA, we constructed chimeric and other mutant viruses using the HIV-1 or the MMTV genome as the backbone (Fig. 1). The MMTV NC was chosen as a partner for chimera construction for several reasons. Like the HIV-1 NC, the MMTV NC contains two zinc-binding motifs. Deletion of one of the two motifs of HIV has been shown previously to substantially reduce RNA packaging (20), thus making NC proteins that contain only one zinc-binding motif inappropriate for these experiments. Like HIV-1, MMTV utilizes tRNALys as the primer for initiation of reverse transcription. The HIV NC has been shown to participate in the annealing of the tRNALys to the primer-binding site and in the synthesis of proviral DNA, and it is likely that MMTV NC is involved in the same processes. The overall charge properties of the two NC proteins are similar, with MMTV NC having an overall charge of +12 and HIV-1 NC having an overall charge of +11. Despite these similarities, the amino acid sequences of MMTV and HIV-1 NC proteins differ substantially (Fig. 1B). Furthermore, there are significant differences in the length of the peptide that links the two zinc-binding motifs in the MMTV and HIV-1 NC proteins.
FIG. 1.
(A) Schematic representation of Gag mutants and alignment of NC amino acids. The amino acid sequences of HIV-1 and MMTV NC chimeras and the relevant portions of MA protein mutants are indicated for each construct. (B) Sequence alignment for HIV and MMTV NC proteins.
We constructed a virus that expresses full-length MMTV NC p10 in place of the wild-type HIV-1 NC p7 (construct pHX/MT-NC [Fig. 1]) by oligonucleotide-mediated site-specific mutagenesis and overlapping extension (22). The oligonucleotides used in the construction of this and the other mutants are listed in Table 1. We also constructed a chimeric NC HIV-1 in which the wild-type amino acid residues of the two zinc finger motifs and the peptide that links them were replaced with the corresponding domain of MMTV (pHX/MT-ZnD) (Fig. 1). These mutations were introduced into pHXB2gpt, a biologically active HIV-1 DNA clone which produces infectious virus when transfected into cos-1 cells (16). To determine whether the spacing of the two HIV zinc fingers could affect RNA encapsidation, a DNA linker encoding four alanine residues was inserted into pHXB2gpt at the ApaI site located between the sequences encoding the two zinc-binding domains (construct pHX-AD). We also constructed a vector, carrying the full-length MMTV proviral DNA, in which the HIV-1 NC p7 is expressed in place of the wild-type MMTV NC p10 (construct pMT/HX-NC [Fig. 1]).
We investigated whether the HIV-1 MA domain in the Gag precursor polyprotein might be involved in specific viral genomic RNA encapsidation. We generated two MA mutant viruses, a chimeric virus using MA sequences from MoMLV (construct pHX/MoMA) and an MA deletion virus (construct pHX-ΔMA). The MoMLV MA was chosen because this protein is better characterized than, for instance, the MMTV MA. In both of these viruses, the HXB2 MA myristoylation signal was preserved, as it is important for membrane targeting of the GagPr, by leaving the first 15 amino acids of the Gag polyprotein at the N terminus. Amino acids at the MA COOH terminus were also preserved to allow proper protease processing of CA.
Biological activities of recombinant viruses.
To determine whether the mutations introduced in HIV-1 affected the ability of the virus to replicate, viral particles derived from transfection of cells with the wild-type or mutant constructs were tested for infectivity. Culture supernatants adjusted to contain comparable levels of p24 were used to infect H9 cells, which are susceptible to infection and permissive for HIV-1 replication. During the 32-day study, the percentage of HIV-1 antigen-positive cells was monitored and the levels of particle-associated p24 were measured. As expected, wild-type virus replicated efficiently in H9 cells, whereas H9 cultures infected with the recombinant viruses remained p24 antigen free even after 32 days (Table 2). The lack of infectivity of these recombinant viruses was confirmed in a duplicate experiment using independently transfected and calibrated supernatants to infect H9 cells.
TABLE 2.
Analysis of NC and MA recombinant virions from transfected cell supernatants
| Construct | p24 (ng/ml)a | RT activity/particleb | Infectivityc |
|---|---|---|---|
| pHXB2gpt | 31.41 ± 2.16 | 100 | + |
| pHX/MT-NC | 32.01 ± 2.01 | 97.85 ± 3.05 | − |
| pHX/MT-ZnD | 32.19 ± 0.75 | 91.75 ± 14.25 | − |
| pHX-AD | 29.70 ± 2.61 | 99.25 ± 4.15 | − |
| pHX/MoMA | 27.09 ± 0.45 | 87.40 ± 1.70 | − |
| pHXΔMA | 32.01 ± 0.18 | 87.10 ± 1.20 | − |
Average of three independent experiments ± standard error.
Percentage of wild-type activity; average of two experiments ± standard error. All samples used for RT activity had been adjusted to contain equivalent amounts of p24.
Time course analysis of infections by all viruses was carried out for 32 days after infection at every 4 days. Particle-associated p24 was measured by ELISA, and percentages of HIV-1 antigen-positive cells were measured by immunofluorescence using a mouse monoclonal antibody specific for the p24 Gag protein. Each time point of each culture was evaluated in duplicate in both assays. Dilutions of the supernatant from pHXB2gpt up to 1:10,000 (virus amount equivalent to 3 pg of p24) were still positive in the infectivity assay.
Analysis of particle-associated viral proteins and virion morphology.
Since the NC and MA mutants listed in Fig. 1 were not infectious in culture, we examined whether virions released from transfected cells contained particle-associated proteins equivalent to wild-type virions. Mutant viral constructs and the parental wild-type plasmids were independently transfected into cos-1 cells, and culture supernatants were assayed for p24 and RT. The levels of p24, as determined by ELISA, ranged between 27 and 32 ng/ml, and the level of RT activity per particle was equivalent to the wild-type level (Table 2). These data suggest that the mutations did not have a substantial effect on particle release.
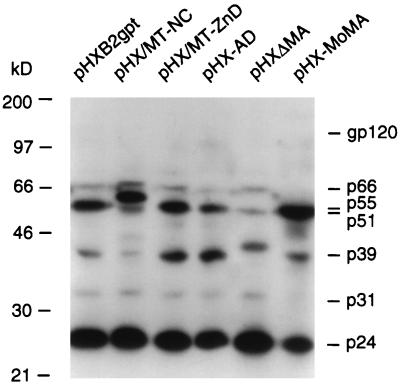
The particle protein content of pelleted virus was also examined. Viral protein lysates were obtained from sedimented supernatants of transfected cos-1 cells. These lysates were normalized to contain equal amounts of p24 and analyzed by Western blotting using a human HIV-1-positive serum (Fig. 2). As expected, the GagPr of virus from mutant construct pHX/MT-NC migrated as a 60-kDa protein, consistent with the presence of a chimeric NC protein that is 43 amino acids longer than the HIV-1 NC. The GagPr of virions produced by pHX-ΔMA is a 45-kDa protein as a consequence of the 104-amino-acid deletion. The addition of 7, 4, and 10 amino acids to GagPr in viral particles from constructs pHX/MT-ZnD, pHX-AD, and pHX/MoMA, respectively, did not cause a noticeable mobility shift of Gag p55 (Fig. 2). A slightly higher amount of p39, one of the intermediate products of Gag processing, was detected for viruses generated from constructs pHX/MT-ZnD and pHX-AD, possibly due to a slower processing rate. The results also show that in particle protein lysates from mutant construct pHX/MoMA, there is a higher amount of unprocessed Gag precursor. As the presence of p24 indicates no major alterations in protease function, the delayed processing of the precursor might be related to the presence of the MoMLV MA protein and its effect on GagPr and Gag-PolPr folding and protease accessibility to cleavage sites. Thus, the analysis confirmed the proper Gag protein profile for each mutant and that proper protein processing occurred, albeit less efficiently for some mutants. The RT activity, measured with equal amounts of particles, also indicates that the mutations have no significant effect on translation of the Gag-Pol precursor, incorporation of this polyprotein into the virion, or cleavage of this polyprotein. Indeed, the 66-kDa subunit of RT and the 31-kDa integrase are present in all the lanes except the one labeled pHX/MoMA (Fig. 2), while the 51-kDa subunit is visible in those lanes where a larger or smaller than wild-type mutated Gag precursor does not comigrate with the 51-kDa RT subunit.
FIG. 2.
Analysis of the protein content of HIV-1 mutant particles by Western blotting. Viral protein lysates, normalized to contain comparable amounts of p24, were subjected to SDS-PAGE (10% gel), transferred to nitrocellulose, and probed with HIV-1-positive human serum. The construct used to generate each lysate is indicated above the relevant lane. Viral proteins were visualized after 12 h of autoradiography.
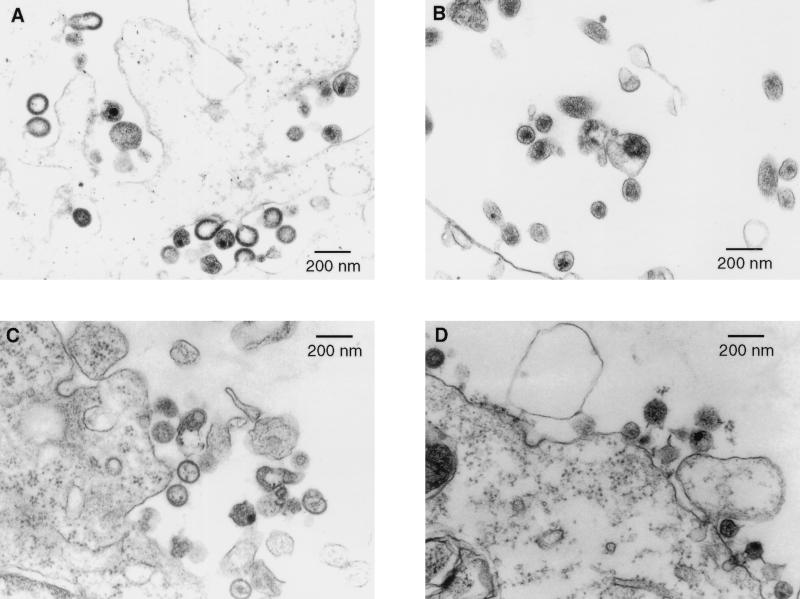
The morphology of wild-type virus and recombinant particles was examined by electron microscopy (Fig. 3). Immature and mature particles were present among the cells transfected with the plasmid producing wild-type virus (pHXB2gpt), consistent with ongoing virus production 48 h after transfection. Immature and mature particles were also present among the cells transfected with plasmids pHXΔMA, confirming previous observations (15, 30, 31), and pHX/MT-NC (Fig. 3B), although in the case of virus from pHX/MT-NC, the mature particles were not as frequent as with the wild-type virus. Predominantly, and in some cases exclusively, immature particles were present among the cells transfected with the plasmids pHX/MT-ZnD (Fig. 3C), pHX-AD, and pHX/MoMA (data not shown). Some core condensation was visible in particles produced by pHX-AD, although it was a less dense core than in the case of the wild-type virus and was not localized at the center of the particle. Particles produced by pMT/HX-NC were of both the mature and immature phenotypes, of normal size, and of type B morphology (Fig. 3D).
FIG. 3.
Electron micrographs of transfected cos-1 cells. (A) Particles derived from construct pHXB2gpt; (B) particles derived from construct pHX/MT-NC; (C) particles derived from construct pHX/MT-ZnD; (D) particles derived from construct pMT/HX-NC. Pictures were taken at a magnification of ×18,000 for all panels.
In summary, the analyses of particle morphology and particle-associated viral proteins indicate that the mutations introduced in the viruses did not significantly affect viral particle release and Gag protein composition, but virion morphology was substantially altered.
Viral genomic RNA content of mutated viral particles.
To assess the RNA packaging properties of these mutant viruses, RT-PCR was performed on RNA samples derived from wild-type and mutant viral particles containing equivalent amounts of p24. To determine the percentage of RNA incorporation by mutant viruses relative to the parental wild type, the intensity of a band corresponding to amplified genomic RNA from mutated viruses was compared to the intensity of bands produced by amplifying serially diluted RNA from the wild-type parental virus pHXB2gpt. An RT-PCR using actin-related primers was carried out to exclude the presence of cellular RNA contamination (data not shown). A PCR with RNA which did not undergo reverse transcription was carried out for each sample to ensure complete DNase I treatment (data not shown). The results, expressed as the average of three independent experiments in which each sample was tested in duplicate, are summarized in Table 3, and a representative experiment is shown in Fig. 4. When viral genomic RNA was amplified with gag-specific primers, the recombinant virus in which the HIV NC had been replaced by the full-length MMTV NC (construct pHX/MT-NC) packaged twofold less RNA (50% of the wild-type level) than did the wild type. The recombinant virus expressing the HIV NC with replacement of its zinc-binding domains with MMTV zinc-binding domains (construct pHX/MT-ZnD) packaged fivefold less RNA (19% of the wild-type level) than did the wild type. Similar results were obtained when this evaluation was carried out with an RNase protection assay (Fig. 5 and Table 3).
TABLE 3.
Quantitative analysis of HIV-1 genomic RNA in virions derived from NC and MA recombinant virusesa
| Construct | Viral genomic RNA incorporation (% of wild-type level)
|
Ratio of genomic to spliced RNA determined by RNase protectiond | |
|---|---|---|---|
| RT-PCRb | RNase protectionc | ||
| pHXB2gpt | 100 | 100 | 1:0.10 |
| pHX/MT-NC | 49 ± 3 | 56 ± 14 | 1:1.10 |
| pHX/MT-ZnD | 19 ± 3 | 17 ± 1 | 1:1.06 |
| pHX-AD | 27 ± 6 | 20 ± 3 | 1:1.31 |
| pHX/Mo-MA | 11 ± 3 | 10 ± 4 | 1:4.31 |
| pHXΔMA | 72 ± 2 | 97 ± 5 | 1:0.12 |
Relative nucleic acid content of viral particles was measured by RT-PCR and RNase protection.
RT-PCRs using gag-specific primers (HIV1230 and HIVc1686) were carried out to detect viral genomic RNA incorporation (Fig. 4). The average of values ± standard error from three independent experiments is reported for each mutant.
RNase protection analysis was carried out with the HIV-1-specific probe indicated in Fig. 5. The average of values ± standard error from two independent experiments is reported for each mutant. All percentage estimates are rounded to the closest integer.
Ratios were evaluated by dividing the number of counts detected in the bands representing spliced messengers by the number of counts detected in the bands representing genomic messengers, after these counts had been normalized to compensate for the difference in size of the two bands (288 bp versus 377 bp).
FIG. 5.
RNase protection assay on cellular and viral RNAs. (A) Schematic representation of the radiolabeled antisense riboprobe obtained from plasmid pGAC linearized with AvaI. It extends from the ClaI site through the first splice donor (SD) site and beyond the viral transcription initiation site to the AvaI site. The predicted protected fragment sizes are also indicated. LTR, long terminal repeat. (B) RNase protection assay on cellular and viral RNAs to simultaneously detect spliced and unspliced messengers. Ten-microgram aliquots of the total cellular RNA sample isolated from each transfection and of a viral RNA sample equivalent to 100 ng of p24, isolated from virions present in each supernatant, were annealed to 1 ng (4.6 × 104 cpm/ng) of the riboprobe. The fastest-migrating band appearing in the panel is an artifact of the assay. The intensity of each band was quantitated as described in Materials and Methods.
Since one of the major differences between HIV NC and MMTV is the spacing of the two zinc fingers, we investigated whether the results observed with pHX/MT-ZnD were dependent on the different spacings of the zinc-binding domains. Lengthening the peptide that links the two zinc-binding motifs by four amino acids (construct pHX-AD) reduced the RNA packaging efficiency fourfold (27% of the wild-type level), to levels similar to that shown by pHX/MT-ZnD. The levels of RNA incorporation for the chimeric NC viruses from pHX/MT-ZnD and pHX-AD were similar to that of the virus from the pBR construct in which all of the basic residues of the protein sequence linking the two zinc finger motifs had been changed to alanine (40). These data suggest that the effect of this intervening peptide on the conformation and function of the zinc-binding motifs is critical.
As some reports have suggested that the retroviral MA protein might be involved in RNA packaging, the effect of replacing the MA domain with the corresponding domain of the MoMLV MA on RNA packaging was investigated. Virus produced by construct pHX/MoMA packaged 10-fold less RNA (11.5% of the wild-type level) than did the wild type. Since this result might be a consequence of the altered folding structure of the GagPr due to the presence of MoMLV MA, we also examined the effect of deleting the majority of the MA domain of HIV-1 GagPr on RNA packaging. Virus produced by construct pHXΔMA incorporated genomic RNA at 72.5% of the wild-type level. This result supports the conclusion that the HIV-1 MA domain does not have a critical role in recognition and packaging of viral genomic RNA.
Selectivity of RNA incorporation in mutated viral particles.
Viruses expressing chimeric HIV-1/MoMuLV NC have been reported to have reduced genomic RNA incorporation but increased incorporation of spliced viral mRNA (7, 13, 52). We investigated whether our recombinant viruses packaged spliced viral RNAs at levels different from the wild-type level. We performed an RNase protection assay on cellular and viral RNAs to simultaneously detect spliced and unspliced messengers, using a radiolabeled antisense riboprobe that extends from the viral transcription initiation site beyond the first splice donor site (Fig. 5). All cell-associated viral RNA derived from wild-type and mutant constructs exhibited a 2-to-1 ratio of spliced to genomic RNA, in agreement with previously published observations (7). Quantification by PhosphorImager of the percentage of genomic RNA present in wild-type and mutant particles yielded similar estimates, whether the investigation was carried out by RT-PCR or RNase protection assay (Fig. 4, Fig. 5B, and Table 3). As expected, the prevalent species present in wild-type viral particles was genomic RNA. This was also true for particles derived from construct pHX-ΔMA. On average, our results show a 10-fold preference for genomic viral RNA over spliced viral RNA for these two constructs. Virus produced from construct pHX/MT-NC packaged approximately half as much unspliced viral RNA as did the wild type, and it packaged spliced RNA at levels equivalent to unspliced RNA levels. Thus, in this mutant, the ability to preferentially package genomic viral RNA is reduced although the total amount of viral RNA incorporated in the particle is not. Mutant constructs pHX/MT-ZnD and pHX-AD also packaged spliced RNA at the same levels as unspliced RNA, while construct pHX/MoMA showed a threefold preference for spliced RNA over unspliced RNA, indicating a substantial modification of the ability of its GagPr to interact specifically with the genomic RNA. The substitution of the entire NC domain appears to generate a more functional NC protein than substitution of the zinc-binding domains only, as virus generated from pHX/MT-ZnD incorporates two- to threefold less RNA than virus generated from construct pHX/MT-NC. In viral particles produced from pHX/MT-ZnD, the levels of both genomic and spliced mRNAs were reduced to a similar extent (Fig. 5 and Table 3).
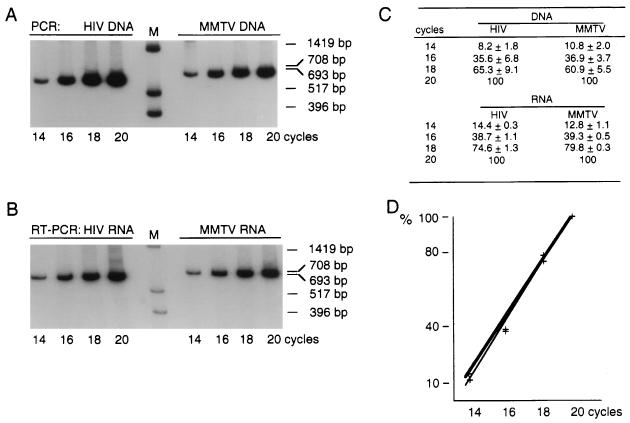
To test whether NC chimeras would preferentially package HIV or MMTV viral genomic RNA, we cotransfected cells with pHX/MT-NC and pHX/MT-ZnD together with plasmid pUVHBf (Fig. 6). This latter construct produces full-length MMTV transcripts from which GagPr proteins cannot be translated because an amber mutation was introduced at codon 76 of the Gag precursor gene. Because of the relatively low transcription efficiency of the MMTV construct relative to the HIV construct, even in the presence of 0.1 mM dexamethasone, we used five times more MMTV DNA than HIV wild-type (pHXB2gpt) or chimera DNAs in the transfection experiment. This strategy resulted in equivalent amounts of HIV and MMTV RNAs present within transfected cells as determined by RT-PCR (Fig. 6C) and by slot blot (data not shown). The HIV and MMTV fragments targeted for amplification allowed direct quantitative analysis of HIV and MMTV RNA incorporation. These two fragments were chosen to have approximately the same size and a similar dCTP content. Rates of reverse transcription, PCR amplification, and isotope incorporation were similar for both sets of primers and templates, as indicated by PCR and RT-PCR analysis performed on known amounts of plasmid DNAs and RNAs carrying the HIV and MMTV target sequences (Fig. 7A and B). Amplification after 18 cycles occurs still in the linear range of amplification of each fragment (Fig. 7C and D). Therefore, the intensity of the MMTV-related bands can be compared to the intensity of the HIV-related bands. The results indicated that the MMTV NC present in the HIV background did not favor the incorporation of the MMTV genomic RNA (Fig. 6A and B). No detectable MMTV RNA was incorporated into wild-type HIV particles when constructs pHXB2gpt and pUVHBf were cotransfected in cos-1 cells. When construct pHX/MT-NC was cotransfected with construct pUVHBf, the virus produced had incorporated five times more HIV genomic RNA than MMTV RNA (Table 4). Similarly, when construct pHX/MT-ZnD was cotransfected with construct pUVHBf, the virus produced had incorporated approximately five times more HIV genomic RNA than MMTV RNA (Table 4). We conclude that the NC chimeric viruses produced by pHX/MT-NC and pHX/MT-ZnD preferentially package HIV genomic RNA.
FIG. 7.
Amplification of HIV and MMTV fragments selected for quantitative analysis of HIV and MMTV RNA incorporation. (A) Representative PCR amplification using equimolar amounts of HIV and MMTV plasmid DNA templates. The intensity of bands after 14, 16, 18, and 20 cycles is shown. (B) Representative RT-PCR amplification using equimolar amounts of HIV and MMTV in vitro-transcribed RNA templates. The intensity of bands after 14, 16, 18, and 20 cycles is shown. Each sample was tested in duplicate, and the PhosphorImager values were for HIV 569,824 (14 cycles), 1,525,234 (16 cycles), 2,937,182 (18 cycles), and 3,932,910 (20 cycles) and for MMTV 546,690 (14 cycles), 1,664,639 (16 cycles), 3,373,286 (18 cycles), and 4,224,588 (20 cycles). (C) Estimates of the intensity of the bands representing amplified fragments after various amplification cycles. The level of signal detected in each fragment after 20 cycles was defined as 100%. Values detected at 18, 16, and 14 cycles are expressed as the percentage of the value at 20 cycles. The percentages and their standard errors are the average of four independent experiments. Percentage, instead of scanned values, was used to avoid variability in numbers due to the use of isotopes with different specific activities in different experiments. (D) Curves representing the percentage of incorporated radioactivity at 14, 16, 18, and 20 cycles of RT-PCR (the thin line represents the HIV curve; the thick line represents the MMTV curve).
TABLE 4.
Quantitative analysis of HIV-1 and MMTV genomic RNAs in virions derived from cotransfection of recombinant virusesa
| Construct | Viral genomic RNA incorporation (% of wt level)
|
Ratio of HIV to MMTV (1,b 3, 4) or MMTV to HIV (2, 5) genomic RNA | |
|---|---|---|---|
| HIV RT-PCR | MMTV RT-PCR | ||
| 1 (pHXB2/pUVHBf) | 90 ± 5 | 0 | ∞ |
| 2 (pUVH/pHXBf) | 0 | 94 ± 5 | ∞ |
| 3 (pHX/MT-NC/pUVHBf) | 66 ± 3 | 13 ± 3 | 5 |
| 4 (pHX/MT-ZnD/pUVHBf) | 22 ± 3 | 5 ± 0.1 | 4.4 |
| 5 (pMT/HX-NC/pHXBf) | 7 ± 1 | 99 ± 4 | 14 |
Relative nucleic acid content of viral particles was measured by RT-PCR. RT-PCRs using HIV (HIV979 and HIVc1686) and MMTV (MMTV1542 and MMTVc2234) gag-specific primers were carried out to detect viral genomic RNA incorporation as described in Materials and Methods. The set of primers used for the HIV RNA detection shown here is different from the set used for experiments reported in Table 3. The average of values ± standard error from two independent experiments rounded to the closest integer is reported for each transfection. Constructs pHXBf and pUVHBf do not generate particles because of a frameshift mutation in the Gag open reading frame. wt, wild type.
Construct number.
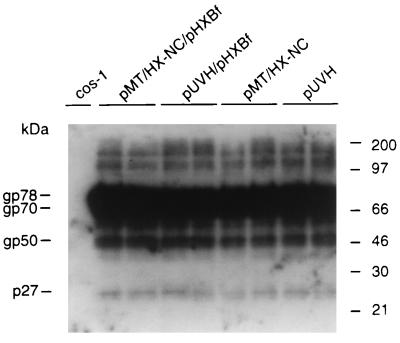
A reciprocal experiment was carried out with a construct producing MMTV particles, where the HIV-1 NC p7 is expressed in place of the wild-type MMTV NC p10 (construct pMT/HX-NC). Particle content in supernatants from cells transfected with vectors producing MMTV particles was evaluated by Western blotting and scanning analysis (Fig. 8). Volumes of viral supernatants containing equal amounts of MMTV particles were used to pellet the virions and to evaluate RNA incorporation into viral particles. The results indicated that the HIV NC present in the MMTV background did not favor the incorporation of the HIV genomic RNA (Fig. 9A and B). No detectable HIV RNA was incorporated into wild-type MMTV particles when constructs pUVH and pHXBf were cotransfected in cos-1 cells. When construct pMT/HX-NC was cotransfected with construct pHXBf, the virus produced incorporated 11 times more MMTV genomic RNA than HIV RNA (Table 4).
FIG. 8.
Analysis of the protein content of MMTV-1 particles by Western blotting. Viral protein lysates, normalized to contain comparable amounts of MMTV particles, were subjected to SDS-PAGE (10% gel), transferred to nitrocellulose, and probed with an MMTV-positive polyclonal rabbit serum. The construct used to generate each lysate is indicated above the relevant lane. Viral proteins were visualized after 24 h of autoradiography. The intensity of the bands representing gp78, gp70, gp50, and p27 was quantitated by using a Molecular Dynamics PhosphorImager with ImageQuant software (Molecular Dynamics).
FIG. 9.
Relative homologous and heterologous RNA content of MMTV particles determined by RT-PCR. RNA samples corresponding to equal amounts of p24 (in the case of HIV particles) or equal amounts of protein lysates (in the case of MMTV particles) were reverse transcribed by using primer HIVc1686 (A) or primer MMTVc2234 (B). Equivalent aliquots of the RT reaction were subjected to PCR amplification in the presence of [32P]dCTP. Equal volumes of RT-PCR samples were subjected to PAGE and autoradiography. Two experiments were performed on RNA samples derived from two independent transfections, and the results shown are from one set of RNAs. Each sample was tested in duplicate. (A) Detection of genomic HIV-1 RNAs in a subset of mutant viruses. Primer HIVc1686 was paired with primer HIV979 to amplify a fragment of 708 bp. The intensity of bands was quantitated as described for Fig. 4. (B) Detection of genomic MMTV RNAs in a subset of mutant viruses. Primer MMTVc2234 was paired with primer MMTV1542 to amplify a band of 693 bp. (C) Amplification of cell-derived HIV-1 and MMTV genomic RNAs upon transfection. Primer HIVc1686 was paired with primer HIV979 to amplify a fragment of 708 bp. Primer MMTVc2234 was paired with primer MMTV1542 to amplify a band of 693 bp.
When the pΔMA construct was cotransfected with pUVHBf, no MMTV RNA was incorporated in the HIV mutated particles (data not shown), suggesting that the MA deletion does not affect substantially the affinity of HIV GagPr for the HIV RNA or the specificity of this interaction.
DISCUSSION
Retroviral NC proteins appear to have roles at multiple stages of the virus life cycle (reference 12 and references therein). As part of Gag precursor molecules, they are involved in particle assembly, genomic viral RNA packaging and positioning of the tRNA primer. The processed NCs may be involved in interactions with both RNA and DNA in the early steps of infection as cofactors for reverse transcription (12, 29). The molecular mechanisms by which NC is involved in these aspects of the viral life cycle are of considerable interest. The results described here extend our understanding of NC domain function in vivo, in that they indicate that an NC protein with two zinc-binding domains is necessary, but not sufficient, for selective HIV RNA packaging.
The Gag55Pr is a critical determinant of particle formation, and considerable experimental evidence has implicated the HIV NC domain in this process. For example, the 14 residues at the NH2 terminus of NC and at least one of the Zn-binding domains are necessary for particle formation (5, 18, 19, 23). In the experiments described here, the HIV NC domain was altered by replacing the entire domain with the MMTV NC, by replacing the two zinc-binding domains with MMTV zinc-binding domains, or by increasing the size of the linker segment between the two zinc-binding domains. These NC chimeras, while producing noninfectious viral particles, did not substantially affect the efficiency of particle formation, nor did they eliminate packaging of HIV genomic RNA. All of the NC mutations did, however, affect particle morphology. It is possible that the altered morphology reflects structural modifications of the viral proteins and that these modifications are incompatible with infectivity. Early steps in the virus life cycle such as uncoating and reverse transcription might not occur appropriately in these viruses.
The contribution of HIV NC to selective HIV genomic RNA packaging has been the subject of much study (7, 12, 52). One model that has emerged from these studies is that the NC domain of the HIV Gag55Pr binds specifically to HIV genomic RNA to effect packaging, and that after processing of Gag55Pr during virus maturation, NC binds nonspecifically to various portions of the HIV genome. Recent studies have suggested that the NC domain of Gag55Pr might be entirely responsible for specific HIV genomic RNA packaging. When the MoMLV NC was substituted for the HIV NC in the HIV genome, it preferentially mediated the packaging of RNA containing the MoMLV Ψ element (7). Replacement of the MoMLV NC coding sequence with an HIV NC sequence in an MoMLV genomic clone led to preferential incorporation of a genome carrying the homologous HIV Ψ sequence (7, 52). One interpretation of these experiments is that the NC domain is the dominant determinant of specific viral genomic RNA recognition. Another possibility is that two zinc-binding domains are necessary, but not sufficient, for specific HIV RNA packaging and that the presence of a single zinc-binding domain in the MoMLV NC protein is adverse to HIV RNA packaging. It is this latter hypothesis that is supported by evidence presented here. Our results demonstrate that HIV genomic RNA is preferentially packaged by MMTV NC chimeras in HIV backgrounds, indicating that two zinc-binding domains are required for specific HIV RNA packaging. Similarly, replacement of MMTV NC with the HIV NC led to preferential incorporation of the MMTV genome.
Combined with previously published results, the observations described here allow us to postulate that the RNA-protein interactions that occur in retroviral genome packaging can be separated into three different classes according to the number of NC zinc-binding domains that are necessary for selective RNA packaging function. HIV-1 is among the retroviruses that require two zinc-binding domains in the NC protein. MoMLV is an example of a retrovirus that requires one zinc-binding domain for selective genome packaging. The spumavirus lacks a requirement for a known zinc-binding domain. Since the NC domains contribute to RNA binding, it would be interesting to ascertain whether there is a relationship between the number of zinc-binding motifs in NC and the secondary and tertiary structures of the Ψ site in genomic RNAs for various retroviruses. In this case, proteins with two zinc-binding domains should more effectively substitute for one another than proteins with only one motif, as suggested by the results described here.
Reasonably efficient HIV genomic RNA packaging can occur with viruses in which the NC sequence has been replaced with the MMTV NC. This result suggests that viral components other than NC make important contributions to specific RNA packaging. Several studies have reported that the affinity of NC for specific RNA sequences is poor and have suggested that additional portions of the Gag polyprotein may influence specificity (27, 32, 34). MA proteins from a variety of retroviruses have been reported to bind viral RNAs, albeit with widely varying affinities and specificities, and BLV MA in particular forms a complex with BLV dimer RNA (11, 25, 32, 33, 46, 49). These observations, and the presence of positively charged domains in HIV MA that could be involved in nucleic acid interactions, prompted our investigation of the MA role in HIV packaging. Our initial experiments with a chimeric virus containing the MoMLV MA protein in an HIV background appeared to support a role for HIV MA in RNA incorporation, as RNA incorporation was reduced approximately 10-fold. However, the analysis of an MA deletion mutant virus (from plasmid pHX-ΔMA) did not support such a conclusion, as this virus incorporated genomic RNA almost as efficiently as the wild type and the ratio of packaged genomic RNA to spliced RNA was identical to that for the wild type. To the contrary, this result demonstrates that the HIV MA domain is not necessary for efficient and specific genomic RNA packaging. It may be that the MoMLV MA domain in the HIV Gag chimeric precursor produced from pHX/MoMA has an adverse effect on the structure of the chimeric precursor and that reduced RNA incorporation by this mutant is due to this defect. The absence of a requirement for the HIV MA domain in specific RNA packaging, taken together with the observation that HIV/MMTV-NC chimeras preferentially incorporate HIV genomic RNA, suggests that the CA domain of Gag or other HIV viral proteins may contribute to RNA packaging.
ACKNOWLEDGMENT
This work was supported by NIH grant AI36060.
REFERENCES
- 1.Aldovini A, Young R A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990;64:1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldovini A, Feinberg M B. Transfection of molecularly cloned HIV genomes. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press, Inc.; 1990. pp. 147–175. [Google Scholar]
- 3.Aldovini A, Young R A. Construction and analysis of HIV and SIV mutants. Methods Mol Genet. 1994;4A:3–17. [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1987. [Google Scholar]
- 5.Bennet R P, Nelle T D, Willis J W. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1993;67:6487–6498. doi: 10.1128/jvi.67.11.6487-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg J. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986;232:485–486. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz R D, Ohagen A, Hoglund S, Goff S P. Retroviral nucleocapsid domains mediate the specific recognition of genomic viral RNAs by chimeric polyproteins during RNA packaging in vivo. J Virol. 1995;69:6445–6456. doi: 10.1128/jvi.69.10.6445-6456.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles N E, Damay P, Spahr P F. Effect of rearrangements and duplications of the Cys-His motifs of Rous sarcoma virus nucleocapsid protein. J Virol. 1993;67:623–631. doi: 10.1128/jvi.67.2.623-631.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell S, Vogt V M. Assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel M D, Letvin N L, King N W, Kannagi P M, Sehgal P K, Hunt R D, Kanki P J, Essex M, Desrosiers R C. Isolation of a T cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 11.Darlix J L, Spahr P F. Binding sites of viral protein p19 onto Rous sarcoma virus RNA and possible controls of viral function. J Mol Biol. 1982;160:147–161. doi: 10.1016/0022-2836(82)90172-3. [DOI] [PubMed] [Google Scholar]
- 12.Darlix J L, Lapadat-Tapolski M, De Rocquigny H, Roques B P. First glimpses at structure function relationships of the nucleocapsid protein of retroviruses. J Mol Biol. 1995;254:523–537. doi: 10.1006/jmbi.1995.0635. [DOI] [PubMed] [Google Scholar]
- 13.Dupraz P, Spahr P F. Specificity of Rous sarcoma virus nucleocapsid protein in genomic RNA packaging. J Virol. 1992;66:4662–4670. doi: 10.1128/jvi.66.8.4662-4670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Embretson J E, Temin H M. Lack of competition results in efficient packaging of heterologous murine retroviral RNAs and reticuloendotheliosis virus encapsidation-minus RNAs by the reticuloendotheliosis virus helper cell line. J Virol. 1987;61:2675–2683. doi: 10.1128/jvi.61.9.2675-2683.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fäcke M, Janetzko A, Shoeman R L, Kräusslich H-G. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J Virol. 1993;67:4972–4980. doi: 10.1128/jvi.67.8.4972-4980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg M B, Jarrett R F, Aldovini A, Gallo R C, Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986;46:807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- 17.Fisher A G, Collalti E, Ratner E, Gallo R C, Wong-Staal F. A molecular clone of HTLV-III with biological activity. Nature. 1985;316:262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- 18.Franke E K, Yuan H E H, Bossolt K L, Goff S P, Luban J. Specificity and sequence requirements for interactions between various retroviral Gag proteins. J Virol. 1994;68:5300–5305. doi: 10.1128/jvi.68.8.5300-5305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gheysen D, Jacobs E, de Foresta F, Thiriat C, Francotte M, Thines D, DeWilde M. Assembly and release of HIV-1 precursor Pr55gag virus like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 20.Gorelick R J, Chabot D J, Rein A, Henderson L E, Arthur L O. The two zinc fingers in the human immunodeficiency virus type 1 nucleocapsid protein are not functionally equivalent. J Virol. 1993;67:4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorelick R J, Chabot D J, Ott D E, Gagliardi T D, Rein A, Henderson L E, Arthur L O. Genetic analysis of the zinc finger in the Moloney murine leukemia virus nucleocapsid domain: replacement of zinc-coordinating residues with other zinc-coordinating residues yields noninfectious particles containing genomic RNA. J Virol. 1996;70:2593–2597. doi: 10.1128/jvi.70.4.2593-2597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton R M, Ho S N, Pullen J K, Hunt H D, Cai Z, Pease L R. Gene splicing by overlap extension. Methods Enzymol. 1993;217H:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- 23.Jowett J B, Hockley D J, Nermut M V, Jones I M. Distinct signals in human immunodeficiency virus type 1 Pr55 necessary for RNA binding and particle formation. J Gen Virol. 1992;73:3079–3086. doi: 10.1099/0022-1317-73-12-3079. [DOI] [PubMed] [Google Scholar]
- 24.Kain S R, Jen T I, Firestone G L. Glucocorticoid regulated trafficking of mouse mammary tumor virus proteins in permeabilized hepatoma cells. J Biol Chem. 1993;268:19640–19649. [PubMed] [Google Scholar]
- 25.Karpel R L, Henderson L E, Orolszlan S. Interactions of retroviral structural proteins with single-stranded nucleic acids. J Biol Chem. 1987;262:4961–4967. [PubMed] [Google Scholar]
- 26.Katoh I, Kyushiki H, Sakamoto Y, Ikawa Y, Yoshinaka Y. Bovine leukemia virus matrix-associated protein MA(p15): further processing and formation of a specific complex with the dimer of the 5′-terminal genomic RNA fragment. J Virol. 1991;65:6845–6855. doi: 10.1128/jvi.65.12.6845-6855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima E, Shirasaka T, Anderson B D, Chokekijchai S, Steinberg S M, Broder S, Yarchoan R, Mitsuya H. Human immunodeficiency virus type 1 (HIV-1) viremia changes and development of drug-related mutations in patients with symptomatic HIV-1 infections receiving alternating or simultaneous zidovudine and didanosine therapy. J Infect Dis. 1995;171:1152–1158. doi: 10.1093/infdis/171.5.1152. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lapadat-Tapolsky M, De Rocquigny H, Van Gent D, Roques B, Plasterk R, Darlix J L. Interactions between HIV-1 nucleocapsid protein and viral DNA may have important functions in the viral life cycle. Nucleic Acids Res. 1993;21:831–839. doi: 10.1093/nar/21.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee P P, Linial M L. Efficient particle formation can occur if the matrix domain of human immunodeficiency virus type 1 Gag is substituted by a myristylation signal. J Virol. 1994;68:6644–6654. doi: 10.1128/jvi.68.10.6644-6654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee Y M, Tang X B, Cimakasky L M, Hildreth J E, Yu X F. Mutations in the matrix protein of human immunodeficiency virus type 1 inhibit surface expression and virion incorporation of viral envelope glycoproteins in CD4+ T lymphocytes. J Virol. 1997;71:1443–1452. doi: 10.1128/jvi.71.2.1443-1452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leis J P, McGinnis J, Green R W. Rous sarcoma virus p19 binds to specific double stranded regions of viral RNA: effect of p19 on cleavage of viral RNA by RNase III. Virology. 1978;84:87–98. doi: 10.1016/0042-6822(78)90220-9. [DOI] [PubMed] [Google Scholar]
- 33.Leis J P, Scheible P, Smith R E. Correlation of RNA binding affinity of avian oncornavirus p19 proteins with the extent of processing of virus genome RNA in cells. J Virol. 1980;35:722–731. doi: 10.1128/jvi.35.3.722-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luban J, Goff S P. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 Gag polyprotein. J Virol. 1991;65:3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurer B, Bannaret H, Darai G, Flugel R M. Analysis of the primary structure of the long terminal repeat and the gag and pol genes of the human spumaretrovirus. J Virol. 1988;62:1590–1597. doi: 10.1128/jvi.62.5.1590-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meric C, Spahr P F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol. 1986;60:450–459. doi: 10.1128/jvi.60.2.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meric C, Gouilloud E, Spahr P-F. Mutations in Rous sarcoma virus nucleocapsid protein p12(NC): deletions of Cys-His boxes. J Virol. 1988;62:3328–3333. doi: 10.1128/jvi.62.9.3328-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Méric C, Goff S P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989;63:1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore R, Dixon M, Smith R, Peters G, Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987;61:480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poon D T K, Wu J, Aldovini A. Charged amino acid residues of human immunodeficiency virus type 1 nucleocapsid p7 protein involved in RNA packaging and infectivity. J Virol. 1996;70:6607–6616. doi: 10.1128/jvi.70.10.6607-6616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popovic M, Sarngadharan M G, Read E, Gallo R C. Detection isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 42.Ratner L, Haseltine W, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, Ivanoff L, Petteway S R, Jr, Pearson M L, Lautenberg J A, Papas T S, Ghrayeb J, Chang N T, Gallo R C, Wong-Staal F. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 43.Rein A. Retroviral RNA packaging: a review. Arch Virol Suppl. 1994;9:513–522. doi: 10.1007/978-3-7091-9326-6_49. [DOI] [PubMed] [Google Scholar]
- 44.Riviere I, Brose K, Mulligan R C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmalzbauer E, Strack B, Dannull J, Guehmann S, Moelling K. Mutations of basic amino acids of NCp7 of human immunodeficiency virus type 1 affect RNA binding in vitro. J Virol. 1996;70:771–777. doi: 10.1128/jvi.70.2.771-777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen A, Todaro G. The genome associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977;10:91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- 47.Shackleford G M, Varmus H E. Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc Natl Acad Sci USA. 1988;85:9655–9659. doi: 10.1073/pnas.85.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinnick T M, Lerner R A, Sutcliffe J G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981;293:543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- 49.Steeg C M, Vogt V M. RNA-binding properties of the matrix protein (p19gag) of avian sarcoma and leukemia viruses. J Virol. 1990;64:847–855. doi: 10.1128/jvi.64.2.847-855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Travers K, Kanki P J. HIV antibody detection in serum. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press, Inc.; 1990. pp. 3–14. [Google Scholar]
- 51.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1 (HIV-1) J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Barklis E. Nucleocapsid protein effects on the specificity of retrovirus RNA encapsidation. J Virol. 1995;69:5716–5722. doi: 10.1128/jvi.69.9.5716-5722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]