Abstract
Purpose:
Intermediate-risk prostate cancer is a heterogeneous disease state with diverse treatment options. The 22-gene Decipher genomic classifier (GC) retrospectively has shown to improve risk stratification in these patients. We assessed the performance of the GC in men with intermediate-risk disease enrolled in NRG Oncology/RTOG 01–26 with updated follow-up.
Methods and Materials:
After National Cancer Institute approval, biopsy slides were collected from NRG Oncology/RTOG 01–26, a randomized phase 3 trial of men with intermediate-risk prostate cancer randomized to 70.2 Gy versus 79.2 Gy of radiation therapy without androgen deprivation therapy. RNA was extracted from the highest-grade tumor foci to generate the locked 22-gene GC model. The primary endpoint for this ancillary project was disease progression (composite of biochemical failure, local failure, distant metastasis, prostate cancer-specific mortality, and use of salvage therapy). Individual endpoints were also assessed. Fine-Gray or cause-specific Cox multivariable models were constructed adjusting for randomization arm and trial stratification factors.
Results:
Two-hundred fifteen patient samples passed quality control for analysis. The median follow-up was 12.8 years (range, 2.4–17.7). On multivariable analysis, the 22-gene GC (per 0.1 unit) was independently prognostic for disease progression (sub-distribution hazard ratio [sHR], 1.12; 95% confidence interval [CI], 1.00–1.26; P = .04), biochemical failure (sHR, 1.22; 95% CI, 1.10–1.37; P < .001), distant metastasis (sHR, 1.28; 95% CI, 1.06–1.55; P = .01), and prostate cancer-specific mortality (sHR, 1.45; 95% CI, 1.20–1.76; P < .001). Ten-year distant metastasis in GC low-risk patients was 4% compared with 16% for GC high-risk patients. In patients with lower GC scores, the 10-year difference in metastasis-free survival rate between arms was −7%, compared with 21% for higher GC patients (P-interaction = .04).
Conclusions:
This study represents the first validation of a biopsy-based gene expression classifier, assessing both its prognostic and predictive value, using data from a randomized phase 3 trial of intermediate-risk prostate cancer. Decipher improves risk stratification and can aid in treatment decision-making in men with intermediate-risk disease.
Introduction
Intermediate-risk prostate cancer represents a heterogeneous disease state. Treatment ranges from observation, active surveillance, radical prostatectomy or radiation therapy, and the combination of radiation therapy with androgen-deprivation therapy (ADT). Currently clinicopathologic risk stratification methods have modest ability to discriminate prognosis accurately, especially within a given risk group. This has led to the long-standing dichotomy of over- and undertreatment.
In 2013, the Memorial Sloan Kettering Cancer Center intermediate-risk subclassification system was developed to improve risk stratification for these patients. Subsequently in 2018, Spratt and colleagues developed and validated the clinical-genomic risk groups that incorporated the Decipher 22-gene genomic classifier (GC) into NCCN risk stratification.1 The clinical-genomic risk groups had superior discrimination for long-term outcomes compared with both the 3-tier NCCN and Zumsteg intermediate-risk subclassification. This work was later validated by Berlin et al using a prospective registry of patients treated with dose-escalated external beam radiation therapy without ADT.2 The improvement in risk stratification from use of the GC in men with intermediate-risk disease identified patients at sufficiently low risk of developing metastatic disease or death from prostate cancer to safely avoid the use of ADT.
The 22-gene GC subsequently was validated to improve prognostic performance in randomized phase 3 trials in high-risk localized, postprostatectomy, metastatic hormone-sensitive, and nonmetastatic castration-resistant prostate cancer.3 However, there remains no comparable validation of any tissue-based genomic biomarker in men with intermediate-risk disease. We hypothesized that the biopsy GC would be independently prognostic for long-term clinically meaningful endpoints, and to test this we submitted for National Cancer Institute approval to analyze patients enrolled in NRG Oncology/RTOG 01–26, a randomized phase 3 trial of men with intermediate-risk prostate cancer. We present the results of the first phase 3 trial validation of any gene expression biomarker, to our knowledge, from pretreatment biopsy samples in men with intermediate-risk prostate cancer treated with radiation therapy without ADT.
Methods and Materials
Translational ancillary project and GC assessment
Approval for this translational science project and statistical analysis plan was granted from the Cancer Therapy Evaluation Program Core Correlative Sciences Committee (CTEP CCSC) through the National Cancer Institute to access archival biopsy specimens from men treated on NRG Oncology/RTOG 01–26.4 Briefly, RTOG 01–26 was a randomized phase 3 trial of men with intermediate-risk prostate cancer randomized to receive external beam radiation therapy alone without ADT to 70.2 Gy versus 79.2 Gy that was initially reported with an 8-year median follow-up. An additional 4 years of follow-up has been updated for this ancillary project.
After CTEP CCSC approval, the NRG biobank retrieved available pretreatment diagnostic biopsy samples. Central pathology review was conducted (J.P.S.) and the biopsy specimen with highest-grade tumor focus was selected for microdissection. RNA extraction from formalin-fixed paraffin embedded tumor tissue, cDNA amplification, oligonucleotide microarray hybridization, and microarray quality control were all conducted in a Clinical Laboratory Improvement Amendments−certified laboratory (Decipher Biosciences, a subsidiary of Veracyte Inc, San Diego, CA) as previously described.5,6 Samples that passed all criteria were included in the final analysis.
GC scores were calculated based on the locked GC model and were generated on a scale of 0 to 1. The continuous GC scores were generated and then linked to the clinical trial database at the NRG Oncology Statistics and Data Management Center. Categorical GC analysis used the previously locked cut points for the commercial assay of 0.45 and 0.60 to define low, intermediate, and high GC risk groups.7 However, the age of the tissue in this cohort (13–20 years) naturally depresses the GC scores.5,8 To account for this, the GC score distributions observed in a large cohort of NCCN intermediate-risk with prospectively collected GC (n = 14,130) were used to adjust the thresholds for categorical GC analysis appropriate for archival tissue samples, with cut points of 0.27 and 0.40 to define low, intermediate, and high-risk tissue sample age-adjusted GC.9,10
Treatment
Full details of the treatment received can be found in the study publication or in the trial protocol (NCT00033631).4 Briefly, men were treated with dose-escalated (79.2 Gy) or standard dose (70.2 Gy) 3-dimensional conformal radiation therapy or intensity modulated radiation therapy without any neoadjuvant, concurrent, or adjuvant ADT. Use of brachytherapy was not permitted.
Study objectives and endpoints
The primary objective of this project was to evaluate the independent associations of GC with oncologic outcomes on multivariable analysis, and the secondary objective was to explore whether associations and interactions between GC and radiation therapy dose could be identified. The primary endpoint of this study was time to the disease progression (DP), which was defined as biochemical failure (BF; Phoenix definition), local failure, distant metastasis (DM), prostate cancer-specific mortality (PCSM), or receipt of salvage therapy. Secondary endpoints included time to biochemical failure (Phoenix and American Society for Radiation Oncology definitions), time to distant metastasis, time to PCSM, and time to receipt of salvage therapy. Exploratory endpoints included time to distant metastasis or death of any cause (metastasis-free survival; MFS) and overall survival (OS). All individual endpoints were defined per the trial protocol.
Statistical analysis
A statistical analysis plan was prespecified and approved by the NRG Oncology Statistics and Data Management Center. All patients with available samples were analyzed as randomized. Summary statistics were reported as medians and interquartile ranges (IQRs) and counts and proportions for continuous and categorical clinical or pathologic variables, respectively. Comparative analyses between groups were performed using the Wilcoxon rank-sum test for continuous variables. The Fisher exact test or χ2 test was used for categorical variables. The event rates and event-free rates at given times within randomization arms or GC risk groups were estimated by the cumulative incidence method and compared using Gray’s test, when accounting for competing risks, or the log-rank test, otherwise. Univariable and multivariable analyses (UVA, MVA) of Fine-Gray or Cox proportional hazards models for GC were constructed, where death without events were treated as competing risks, as appropriate. MVAs were adjusted by the trial stratification variables of clinical risk group (defined as Gleason score of 5–6 with prostate-specific antigen (PSA) ≥10 but <20 ng/mL or Gleason score of 7 and PSA <15 ng/mL) and treatment modality (3-dimensional conformal radiation therapy or intensity modulated radiation therapy), as well as randomization arms (as a main effect in the Fine-Gray models or as strata in the Cox PH models) to account for treatment heterogeneity. Categorical GC was analyzed using the cut points as previously described.
The interaction effect of randomization arm and GC risk group was assessed, with and without adjusting for the trial stratification clinical variables. Similarly, subgroup analyses of randomization arm and GC risk (with GC intermediate and high groups combined due to the similarity in outcomes and sample size constraints) were performed to estimate survival rates at 10 years. Due to the exploratory nature, no multiple testing adjustment was used. All testing was performed at a significance level of .05 with 2-sided tests. Statistical analyses were performed using R, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
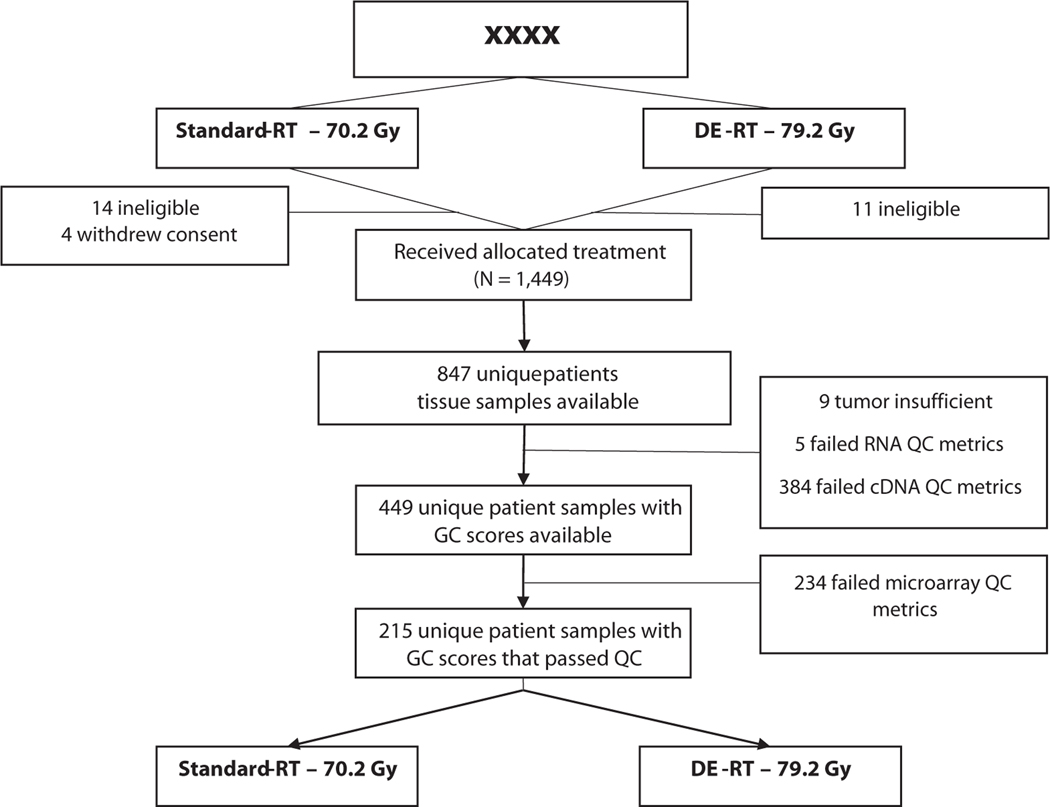
Out of 1532 patients randomized in NRG Oncology/RTOG 01–26, 1499 patients were eligible and analyzable, of which 847 had archived tissues from 2002 to 2008 for genomic analysis (Fig. 1). Of these patients, there was sufficient tumor sample and RNA to generate GC scores for 449 unique patients (53%). Of these 449 available patient samples, 215 (48%) passed GC assay quality control and formed the primary analytical cohort (median age, 70 years [IQR, 65–74], Table 1). Median PSA at study entry was 7.2 ng/mL (IQR, 5–10.2), 50% had a cT2 stage, 14% had Gleason score ≤6, 61% with 3+4, and 24% with 4+3 disease. Median follow-up for censored patients was 12.8 years (IQR, 11.7–14).
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram of the patient sample availability and sample quality from the NRG Oncology/RTOG 01–26-Decipher ancillary project. Abbreviations: DE = dose-escalated; GC = genomic classifier; QC = quality control; RT = radiation therapy.
Table 1.
Demographic and clinical characteristics among the GC analytic cohort, standard-RT (70.2Gy) arm, and dose-escalated (79.2 Gy) arm in the NRG Oncology/RTOG 0126 ancillary project.
| Variable | Total | 70.2 Gy | 79.2 Gy | P value |
|---|---|---|---|---|
| Total | 215 (100) | 107 (50) | 108 (50) | |
| Age, y | ||||
| Median (range) | 70 (39, 86) | 64–74 | 65–74 | .659 |
| IQR (Q1, Q3) | 65–74 | 70 (39, 86) | 70 (57, 83) | |
| Race, no. (%) | ||||
| African American | 16 (7) | 5 (5) | 11 (10) | .313 |
| White | 190 (88) | 98 (92) | 92 (85) | |
| Other/unknown | 9 (4) | 4 (4) | 5 (5) | |
| Ethnicity, no. (%) | ||||
| Hispanic or Latino | 5 (2) | 2 (2) | 3 (3) | 1.000 |
| Non-Hispanic/Latino | 207 (96) | 104 (97) | 103 (95) | |
| Unknown | 3 (1) | 1 (1) | 2 (2) | |
| Zubrod performance status, no. (%) | ||||
| 0 | 198 (92) | 98 (92) | 100 (93) | .806 |
| 1 | 17 (8) | 9 (8) | 8 (7) | |
| PSA level at study entry (ng/mL) | ||||
| Median (range) | 7.2 (0.1, 19.0) | 4.8–10.2 | 5.2–10.3 | .894 |
| IQR (Q1, Q3) | 5.0–10.2 | 7.5 (0.1, 18.9) | 7.1 (0.6, 19.0) | |
| <10 | 154 (72) | 76 (71) | 78 (72) | .095 |
| 10 to <15 | 51 (24) | 29 (27) | 22 (20) | |
| 15–20 | 10 (5) | 2 (2) | 8 (7) | |
| Gleason score, no. (%) | ||||
| ≤(3+3) | 31 (14) | 13 (12) | 18 (17) | .341 |
| (3+4) | 132 (61) | 64 (60) | 68 (63) | |
| (4+3) | 52 (24) | 30 (28) | 22 (20) | |
| GS × PSA at study entry, no. (%) | ||||
| GS <7 and PSA (10–20 ng/mL) | 31 (14) | 13 (12) | 18 (17) | .454 |
| GS 7 and PSA <15 ng/mL | 184 (86) | 94 (88) | 90 (83) | |
| T stage, no. (%) | ||||
| T1 | 107 (50) | 50 (47) | 57 (53) | 0.453 |
| T2 | 108 (50) | 57 (53) | 51 (47) | |
| Number of NCCN I-R features, no. (%) | ||||
| 1 | 156 (73) | 76 (71) | 80 (74) | .341 |
| 2 | 52 (24) | 29 (27) | 23 (21) | |
| 3 | 7 (3) | 2 (2) | 5 (5) | |
| RT modality, no. (%) | ||||
| 3D-CRT | 137 (64) | 69 (64) | 68 (63) | .928 |
| IMRT | 78 (36) | 38 (36) | 40 (37) | |
| Follow-up for OS status (y) | ||||
| Median (range) | 12.8 (2.4, 17.7) | 11.6–14.0 | 11.8–14.0 | .988 |
| IQR (Q1, Q3) | 11.7–14.0 | 12.8 (3.7, 17.7) | 13.0 (2.4, 15.8) | |
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy; GS = Gleason score; IMRT = intensity modulated radiation therapy; IQR = interquartile range; I-R = intermediate-risk; NCCN = National Comprehensive Cancer Network; OS = overall survival; PSA = prostate-specific antigen; Q1, Q3 = first quartile, third quartile; RT = radiation therapy.
All 215 patients with samples that passed assay quality control had complete baseline clinical and outcome information. P values were calculated using the Wilcoxon rank-sum test for continuous variables and the χ2 test or Fisher exact test for categorical variables.
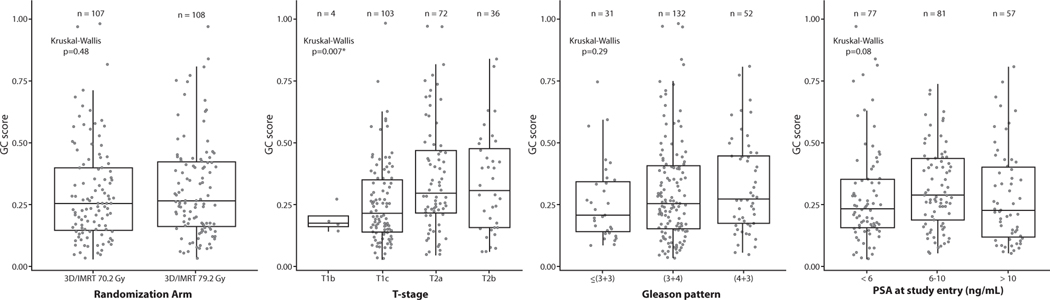
The analytical cohort was well balanced for all patient and clinicopathologic characteristics between the 2 randomization arms (Table 1) and was representative of the overall trial (Table E1). Box dot plots show the distribution of GC scores by randomization arm, T stage, Gleason score, and PSA at study entry (Fig. 2), as well as by clinical risk group and radiation therapy modality (Fig. E1). There was substantial heterogeneity of GC scores within the arms, clinical stages, and Gleason scores. The median GC score was 0.26 (IQR, 0.16–0.41), with 81%, 10%, and 9% being classified as GC low-, intermediate-, and high-risk, respectively. Reweighting based on the age of tissues yielded a distribution more similar to commercial clinical use of 54%, 19%, and 27%, respectively (Fig. E2).
Fig. 2.
Distribution of GC within clinical subgroups: (A) by randomization arm, (B) by T stage, (C) by Gleason pattern, and (D) by PSA at study entry. Abbreviations: 3D/IMRT = 3-dimensional/intensity modulated radiation therapy; GC = genomic classifier; PSA = prostate-specific antigen.
22-gene GC risk stratification
Patients with GC high (>0.6) had 5-year DP of 40% (95% CI, 18–62) compared with 20% (95% CI, 14–26) in GC low (Fig. E3). Similar estimated rates were observed in the GC high versus GC low risk groups for the secondary endpoints (5-year BF Phoenix of 40% [95% CI, 18–62] vs 15% [95% CI, 10–21]; 5-year BF American Society for Radiation Oncology of 56% [95% CI, 33–79] vs 33% [95% CI, 26–40]; 5-year receipt of salvage therapy of 30% (95% CI, 9–51] vs 13% [95% CI, 8–18]; 10-year DM of 16% [95% CI, 0–33] vs 4% [95% CI, 1–6]; and 10-year PCSM of 21% [95% CI, 2–40] vs 4% [95% CI, 1–6]) and exploratory endpoints (event-free rates; 10-year MFS of 48% [95% CI, 24–71] vs 63% [95% CI, 56–70] and 10-year OS of 53% [95% CI, 30–76] vs 65% [95% CI, 58–72]). Estimated rates at median follow-up time of 12 years are provided in Table E2. Consistent results were seen with GC risk groups using the age-adjusted thresholds appropriate for analysis of archival tissue specimens (Fig. E4).
MVA
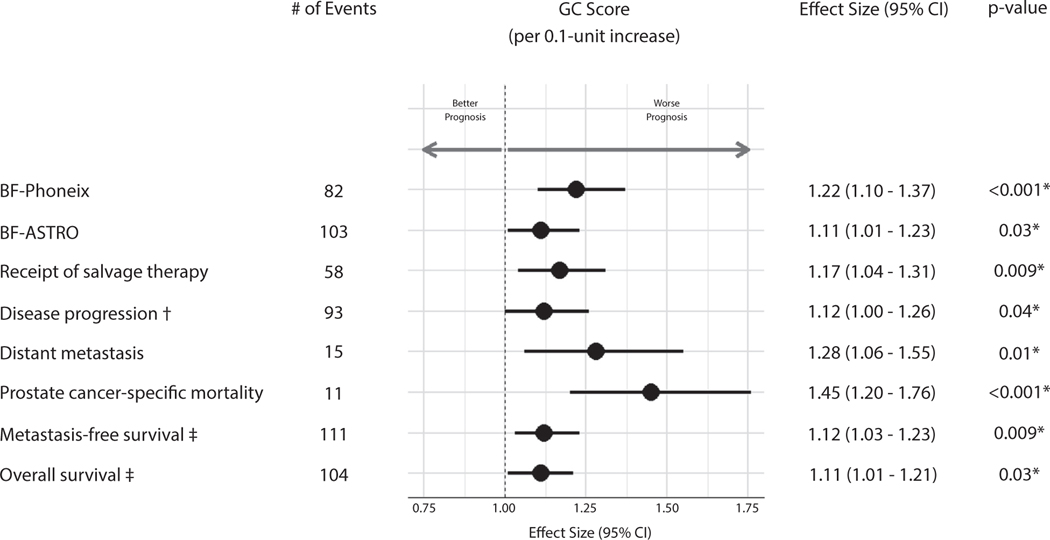
In MVA, accounting for competing risks of death where appropriate, and adjusting for randomization arm and the trial stratification variables, GC as a continuous score was independently prognostic for all primary (DP) and secondary endpoints (BF, receipt of salvage therapy, DM, and PCSM), as well as exploratory endpoints, MFS and OS (Fig. 3, Table E3). GC score (per 0.1-unit increase) had an sHR of 1.12 (95% CI, 1.0–1.26; P = .04) for the primary endpoint of the study, DP, as well as an sHR of 1.22 (95% CI, 1.1–1.37; P < .001) for BF Phoenix, HR of 1.12 (95% CI, 1.03–1.23; P = .009) for MFS, and sHR of 1.45 (95% CI, 1.2–1.76; P < .001) for PCSM. Similar results were seen when adjusting for standard clinical variables on MVA (Table E4). UVA results are available in Table E5.
Fig. 3.
Prognostic performance of GC score in multivariable Fine-Gray or Cox proportional hazards models for all endpoints. Effect sizes of GC were reported per 0.1-unit increases and are reported for each endpoint from the appropriate model. Multivariable models were adjusted for the trial stratification variables and randomization arm (as main effect in Fine-Gray and as strata in Cox; see Table E3 for full model). *Indicates P < .05. †Indicates primary endpoint. ‡Indicates Cox PH model. Abbreviations: ASTRO = American Society for Radiation Oncology; BF = biochemical failure; CI = confidence interval; GC = genomic classifier.
When analyzed as a categorical variable (GC low as reference group), GC was independently prognostic for all endpoints, except DM and OS. GC high (>0.6) had an sHR of 2.41 (95% CI, 1.28–4.57; P = .007) for DP, sHR of 3.34 (95% CI, 1.68–6.66; P < .001) for BF Phoenix, HR of 1.82 (95% CI, 1.0–3.31; P = .05) for MFS, and sHR of 7.37 (95% CI, 1.96–27.73; P = .003) for PCSM (Table E6).
Interaction of GC with radiation therapy dose
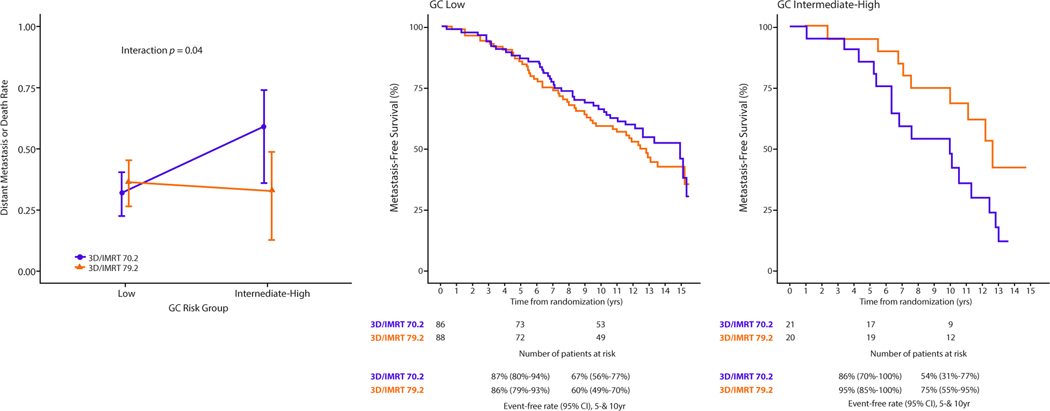
In exploratory analysis, we investigated whether patients with higher GC scores received greater clinical benefit from dose-escalation. As in the results reported for the NRG Oncology/RTOG 01–26 trial, a clinical benefit was observed for patients in the dose-escalated arm for all endpoints except for MFS, PCSM, and OS (Fig. E5). The dose-escalated and standard arms in the analytical cohort had similar 10-year MFS (62% [95% CI, 53–72] and 64% [95% CI, 55–74], respectively). Similar results were observed among GC low patients, with 10-year MFS of 60% (95% CI, 49–70) and 67% (95% CI, 56–77), respectively (Table E7). However, among GC intermediate-high patients, dose-escalation showed greater absolute benefit, with 10-year MFS of 75% (95% CI, 55–95) compared with 54% (95% CI, 31–77) for standard dose (UVA interaction P = .04, MVA interaction P = .03; Fig. 4, Table E8). The direction of the interaction effect was similar for PCSM and OS but there were no other significant interaction effects (P > .05) observed (E8).
Fig. 4.
Interaction plot for GC risk group and randomization arm for the metastasis-free survival endpoint. Predicted 10-year metastasis-free survival from the Fine-Gray analysis of the interaction between GC risk group and randomization arm, with reported interaction term P value and (1-cumulative incidence) plots by GC risk group (see Table E8 for complete interaction model). Abbreviations: 3D/IMRT = 3-dimensional/intensity modulated radiation therapy; CI = confidence interval; GC = genomic classifier.
Discussion
The present study represents the first validation, to our knowledge, of any tissue-based gene expression biomarker in the context of a randomized phase 3 trial from pretreatment biopsy tissue in intermediate-risk localized prostate cancer. In a relatively homogenous cohort treated with radiation therapy alone without ADT, the Decipher GC was independently prognostic on multivariable analysis for all oncologic endpoints assessed even after accounting for highly prognostic factors such as the Gleason score (ie, grade group 3 vs 2). Furthermore, patients with a low GC score had very low rates of death from prostate cancer (4%) with long-term follow-up. However, patients with high GC scores had unacceptably high rates of disease progression (>60%) and distant metastasis (>15%) with radiation therapy alone and warrant discussion of treatment intensification.
Currently, there are no predictive biomarkers in clinical use to guide the use of radical treatment or addition of ADT to radiation therapy for men with intermediate-risk disease. Instead, prognostic biomarkers have been developed with the goal of identification of patients with sufficiently indolent disease to avoid futile treatment intensification. This concept is at the core of what defines the utility of a prognostic biomarker. If there is similar relative benefit from treatment intensification across prognostic risk groups, there will be smaller and smaller absolute benefits with improved prognosis. Fundamentally, this is the concept used to justify the omission of ADT in low and favorable intermediate-risk patients, given NCCN risk groups and number of intermediate-risk factors were shown to not predict relative benefit from ADT in NRG Oncology/RTOG 94–08 and 08–15 randomized trials of radiation therapy with or without short-term ADT.11,12
We demonstrate that the 22-gene GC is independently prognostic for clinically meaningful endpoints, including DM, MFS, and PCSM, and can identify a subset of intermediate-risk patients with a low risk of distant progression or death from prostate cancer with radiation therapy alone. Based on the MARCAP consortium’s meta-analysis, there is an estimated 40% relative reduction in 10-year DM from the addition of short-term ADT to radiation therapy (HR, 0.60).13 Thus, the 10-year rate of DM of patients enrolled in RTOG 01–26 was 4% for GC low patients, and a relative reduction of 40% would translate into a 1.6% absolute reduction in 10-year DM. We previously demonstrated that most surveyed experts would themselves not take short-term ADT for a <2.5% 10-year absolute benefit.14 Thus, improved prognostication can improve the ability to perform more accurate shared decision-making with patients regarding the absolute benefit and harm of treatment intensification.
Currently, the Decipher GC is available in the United States for the majority of prostate cancer patients at initial diagnosis and postprostatectomy. The present study adds high level biomarker evidence to the prior 8 reported randomized phase 3 trials demonstrating the prognostic ability of the 22-gene GC. The results from this study further support the current active and enrolling parallel phase 3 trial, GUIDANCE (NRG-GU010, NCT04484818)) of men with unfavorable intermediate-risk disease. Importantly, NRG-GU010, as well as NRG-GU009, are not testing the validity of the GC, as this has been demonstrated in more than 40 studies to date. Rather, these trials are assessing that in less versus more aggressive disease states defined by clinical-genomic risk can better personalize treatment paradigms to minimize over- and undertreatment.
A similar concept to better guide the use of ADT with radiation therapy was recently reported from a retrospective observational registry study using a different gene expression test (Prolaris).15 However, in this study they were unable to identify a subset of intermediate-risk patients, regardless of their biomarker score, who benefited from ADT. This is concerning given the proven benefit of ADT in numerous randomized trials. This may be secondary to the lack of prognostic performance of the genomic component of the biomarker, termed the cell-cycle progression score, which had a c-index of only 0.52, or near identical to a coin flip. Currently, the 22-gene GC represents the only gene expression biomarker validated in multiple randomized trials in localized prostate cancer.3,5,10,16 Thus, gene expression tests do not at present time appear interchangeable, and caution should be used with biomarkers validated in more simplistic “studies of convenience” that lack validation from previously conducted prospective randomized trials to determine their validity and utility.17
This study has limitations. Only a subset of patients banked tissue on the trial, of which there was insufficient or inadequate quality tissue available for the majority of patients for gene expression analyses. This is likely due to the tissue being approximately 20 years old and stored at room temperature. Regardless, the sample size limited power, especially analyses with fewer events, and largely prohibited adequately powered subset analyses. Despite this, on multivariable analysis there was consistent improvement in prognostication with the addition of the GC. Although the study had a National Cancer Institute−approved protocol and analysis plan, it was not specified a priori before reporting of the initial trial results. The exploratory analysis of the interaction between GC and radiation therapy dose is hypothesis generating, meets the minority of subset analysis credibility criteria, and requires validation. Finally, other variables, such as percent Gleason pattern 4 and percent positive biopsy cores were not collected for patients in this trial.
Conclusion
The Decipher 22-gene GC represents, to our knowledge, the only gene expression biomarker with randomized phase 3 validation in men with intermediate-risk prostate cancer. The GC is independently prognostic for DP, BCR, DM, MFS, and PCSM on multivariable analysis treated with radiation therapy alone on NRG Oncology/RTOG 01–26. Men with low GC scores had low rates of metastatic progression or death from prostate cancer with long-term follow-up, and the use of the GC can assist with more accurate estimates of absolute benefit from treatment intensification for better personalized shared decision-making.
Supplementary Material
Acknowledgments—
This study was conducted with support from University Hospitals Seidman Cancer Center. We thank the support of the National Cancer Institute for the conduct of this randomized trial (U10CA180868, NRG Oncology Operations; U10CA180822, NRG Oncology Statistics and Data Management Center; U24CA196067, NRG Specimen Bank; and UG1CA189867, NCORP). We thank Leslie Longora and Sandy De Vries (NRG Biobank) and James A. Proudfoot and Jason Hughes (Veracyte) for all their assistance in this study.
Disclosures:
D.E.S. received personal fees from AstraZeneca, Bayer, Varian, Janssen, Boston Scientific, Blue Earth, and AstraZeneca and is the principal investigator of NRG GU006, a trial using the Decipher biomarker to assess PAM50 luminal/basal status. V.Y.T.L. was an employee of Veracyte Inc. E.D. is an employee of Veracyte Inc. P.T.T. receives personal fees for consulting from RefleXion, Noxopharm, Janssen-Taris Biomedical, Myovant, and AstraZeneca outside the submitted work and has a patent (9114158; Compounds and Methods of Use in Ablative Radiation Therapy licensed to Natsar Pharm). D.J.S.T. is a contractor for Veracyte Inc. F.Y.F is a stock holder in ARTERA.AI and has received personal fees from Astellas Pharma, Bayer, Bristol Myers Squibb, Exact Sciences, Foundation Medicine, Janssen Biotech, Myovant Sciences, Novartis, Roivant, SerImmune and Varian Medical Systems and research funding from Zenith Epigenetics.
Footnotes
This protocol is registered with ClinicalTrials.gov and may be viewed online at https://clinicaltrials.gov/ct2/show/NCT00033631.
Research data will be made available per the NCTN Data Archiving Rules https://nctn-data-archive.nci.nih.gov/.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ijrobp.2023.04.010.
References
- 1.Spratt DE, Zhang J, Santiago-Jiménez M, et al. Development and validation of a novel integrated clinical-genomic risk group classification for localized prostate cancer. J Clin Oncol 2018;36:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlin A, Murgic J, Hosni A, et al. Genomic classifier for guiding treatment of intermediate-risk prostate cancers to dose-escalated image guided radiation therapy without hormone therapy. Int J Radiat Oncol Biol Phys 2019;103:84–91. [DOI] [PubMed] [Google Scholar]
- 3.Jairath NK, Dal Pra A, Vince R Jr., et al. A Systematic Review of the Evidence for the Decipher Genomic Classifier in Prostate Cancer. Eur Urol 2021;79:374–383. [DOI] [PubMed] [Google Scholar]
- 4.Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol 2018;4 e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng FY, Huang HC, Spratt DE, et al. Validation of a 22-gene genomic classifier in patients with recurrent prostate cancer: an ancillary study of the NRG/RTOG 9601 randomized clinical trial [published correction appears in JAMA Oncol. 2021 Apr 1;7(4):639]. JAMA Oncol 2021; 7: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen PL, Martin NE, Choeurng V, et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis 2017;20:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross AE, Johnson MH, Yousefi K, et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur Urol 2016;69:157–165. [DOI] [PubMed] [Google Scholar]
- 8.Abdueva D, Wing M, Schaub B, Triche T, Davicioni E. Quantitative expression profiling in formalin-fixed paraffin-embedded samples by affymetrix microarrays. J Mol Diagn 2010;12:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muralidhar V, Zhang J, Wang Q, et al. Genomic validation of 3-tiered clinical subclassification of high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2019;105:621–627. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen PL, Huang HR, Spratt DE, et al. Analysis of a biopsy-based genomic classifier in high-risk prostate cancer: meta-analysis of the NRG oncology/radiation therapy oncology group 9202, 9413, and 9902 phase 3 randomized trials. IntJ Radiat Oncol Biol Phys 2023;116:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. New Engl J Med 2011;365:107–118. [DOI] [PubMed] [Google Scholar]
- 12.Krauss DJ, Karrison T, Martinez AA, et al. Dose-Escalated Radiotherapy Alone or in Combination With Short-Term Androgen Deprivation for Intermediate-Risk Prostate Cancer: Results of a Phase III Multi-Institutional Trial [published online ahead of print, 2023 Apr 27]. J Clin Oncol. 2023;JCO2202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishan AU, Sun Y, Hartman H, et al. Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: An individual patient data meta-analysis [published correction appears in Lancet Oncol. 2022;23:e319]. Lancet Oncol. 2022;23:304–316. [DOI] [PubMed] [Google Scholar]
- 14.Spratt DE, Tward JD. Absolute versus Relative Benefit of Androgen Deprivation Therapy for Prostate Cancer: Moving Beyond the Hazard Ratio to Personalize Therapy. Int J Radiat Oncol Biol Phys 2020;108:899–902. [DOI] [PubMed] [Google Scholar]
- 15.Tward J, Lenz L, Flake II DD, et al. The Clinical Cell-Cycle Risk (CCR) score is associated with metastasis after radiation therapy and provides guidance on when to forgo combined androgen deprivation therapy with dose-escalated radiation. Int J Radiat Oncol Biol Phys 2022;113:66–76. [DOI] [PubMed] [Google Scholar]
- 16.Dal Pra A, Ghadjar P, Hayoz S, et al. Validation of the Decipher genomic classifier in patients receiving salvage radiotherapy without hormone therapy after radical prostatectomy - an ancillary study of the SAKK 09/10 randomized clinical trial. Ann Oncol 2022;33:950–958. [DOI] [PubMed] [Google Scholar]
- 17.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009;101:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.