INTRODUCTION
Isolated diastolic hypertension (IDH) is a blood pressure (BP) phenotype that is less commonly encountered than isolated systolic hypertension (ISH) or combined systolic diastolic hypertension (SDH). While the association of elevated BP with incident cardiovascular disease (CVD) events is unquestionable,1 particularly in the case of elevated systolic BP,2,3 whether isolated elevation in diastolic BP in the presence of normal systolic BP (i.e., IDH) is associated with CVD has been less clear.4,5 This uncertainty has only come more into focus since the release of the 2017 American College of Cardiology/American Heart Association Task Force (ACC/AHA) High BP Guideline, which redefined IDH as a diastolic BP ≥80 mmHg with a systolic BP <130 mmHg.6,7
Establishing a clear and consistent association between IDH and CVD events is important because hypertension guidelines do not distinguish between IDH, ISH, or SDH when providing treatment recommendations. Specifically, guidelines define hypertension as either a systolic BP above threshold or a diastolic BP above threshold or both. As such, they provide the same weight of importance and same treatment recommendation to the following three example BPs; 135/75 mmHg, 125/85 mmHg, and 135/85 mmHg. According to the 2017 ACC/AHA high BP guideline, these three BPs represent ISH, IDH, and SDH, respectively, and all three require antihypertensive drug treatment in the presence of elevated risk for CVD. But are these three BPs the same in terms of their prognostic significance and do they all have the same level of evidence favoring antihypertensive drug treatment from randomized clinical trials? The simple answer is no.
In this con argument, we will review prognostic data describing the associations between the 2017 ACC/AHA definition of IDH and CVD events. We hope to convince readers that the lack of a consistent and clinically meaningful association between this definition of IDH and CVD events challenges the current ACC/AHA guideline, which recommends that clinicians both (a) commence antihypertensive treatment among persons with IDH (i.e., isolated diastolic BP ≥80 mmHg) and 10-year CVD risk of ≥10% and (b) target an on-treatment diastolic BP of <80 mmHg among all persons receiving antihypertensive therapy irrespective of their CVD risk. Indeed, unlike ISH and SDH, there has never been a clinical trial exclusively among adults with IDH that has demonstrated benefit for anti-hypertensive drug treatment.8 As such, the burden of proof informing the need for drug treatment in IDH is based solely on prognostic observational data and expert opinion.
The present con argument is not about the more traditional definition of IDH
The new 2017 ACC/AHA definition of IDH and the more traditional definition of IDH are completely separate entities and, in our opinion, should not be lumped together. The traditional Joint National Committee definition of IDH was a diastolic BP ≥90 mmHg with systolic BP <140 mmHg. The 2018 European Society of Cardiology and European Society of Hypertension Task Force (ESC/ESH)9 and 2019 National Institute for Health and Care Excellence (NICE)10 guidelines continue to use this more conservative IDH definition.
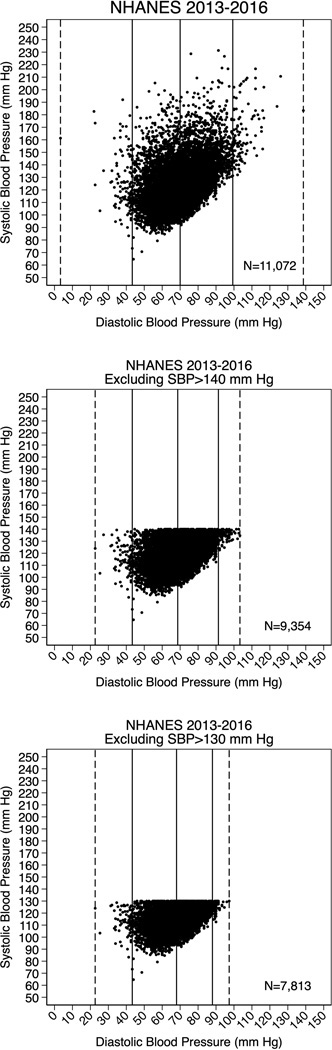
The difference in these two definitions of IDH is not just about semantics. It is much more fundamental and relates to differences in the allowed systolic BPs between both definitions. The implications of these differences in allowed systolic BPs are twofold; first, because systolic BP and diastolic BP are strongly correlated, any definition of IDH that allows systolic BPs up to 140 mmHg will naturally include many more individuals with higher diastolic BPs than a definition of IDH that only allows systolic BPs up to 130 mmHg (see Figure 1).This is important because, when considered in isolation (i.e., without adjusting for systolic BP), elevated diastolic BP is linearly associated with CVD, particularly at very high levels over 100 mmHg.1,11 It is important to emphasize here that our con argument (which states the 2017 ACC/AHA definition of IDH is not clearly associated with incident CVD events) is in large part distinct from any consideration of the overall relationship between diastolic BP and CVD. This might seem contradictory at first; how can we on one hand argue that the 2017 ACC/AHA definition of IDH is not associated with CVD events yet on the other hand declare that diastolic BP is, on average, linearly associated such events? The reason we can do this is because the 2017 definition of IDH required the presence of systolic BP <130 mmHg, which consequently removes most adults with high diastolic BPs >100 mmHg.
Figure 1-. Scatterplots of Systolic BP and Diastolic BP values in the US Adult Population.
(A) Systolic BP and Diastolic BP are correlated overall.
(B) Among persons with Systolic BP <140 mmHg [who are eligible for a diagnosis of IDH by the traditional JNC criteria] a proportion of these individuals have diastolic BP values ≥100 mmHg.
(C) Among persons with Systolic BP <130 mmHg [who are eligible for a diagnosis of IDH by the 2017 ACC/AHA criteria], nobody has a diastolic BP ≥100 mmHg.
Dashed lines are minimum and maximum diastolic BPs and solid lines are the 5th, 50th, and 95th percentile of diastolic BP value.
Second, compared to a reference group of persons with both normal systolic (<120 mmHg) and normal diastolic BP (<80 mmHg), a definition of IDH that allows the inclusion of persons with systolic BPs up to 140 mmHg will naturally be more likely to be associated with increased risk for CVD events, solely on the basis of differences in systolic BP, than a definition of IDH that only allows the inclusion of persons with systolic BPs up to 130 mmHg. Put another way, the traditional IDH definition includes persons with systolic BPs of between 130 and 140 mmHg, who, relative to normotension, can be expected to be higher risk than the new IDH definition that requires systolic BP be less than 130 mmHg.
For these two reasons, we believe that the traditional definition of IDH is by nature a higher risk BP phenotype that should not be lumped together with the new 2017 ACC/AHA definition of IDH. This belief is borne out in the published data. While historical reports from smaller cohorts did suggest some uncertainty about the prognostic significance of the traditional definition of IDH,12,13 with many finding no association with CVD,4,14–16 recent data from the larger observational cohorts currently available to researchers have, despite some notable exceptions,7 more consistently shown an association between the traditional definition of IDH and CVD events.3,17–25
The mixed results for the traditional IDH definition in older studies and the much more consistent finding of increased risk for CVD with this definition of IDH in more recent cohorts can be explained in large part by differences in power, with the most recent cohorts being large enough to discern statistically significant, though modest in magnitude, relative risk increases in CVD with the traditional definition of IDH. Power is an important consideration in any analysis of IDH, simply because persons with IDH tend to be younger26 and therefore less likely to suffer CVD events, which consequently requires far larger sample sizes to capture the sufficient numbers of events needed to test the prognostic implications of IDH. Importantly, the association between the traditional definition of IDH and CVD events has been consistently demonstrated in the more recent data irrespective of the form of BP measurement applied in these large contemporary studies (e.g., whether the study included less reliable BP measures drawn from routine clinical care3,18,19,25 or more rigorous and standardized BP measures from dedicated study visits17,20–22 or ambulatory BP monitors27).
Therefore, the focus of this con argument is not on the more traditional JNC definition of IDH, which we believe has been sufficiently proven to be associated with increased risk of incident CVD. Rather, our focus is squarely on the new 2017 ACC/AHA definition of IDH. For the latter IDH definition, we believe that the studies to date do not indicate a consistent and clinically meaningful increase in CVD risk (see below).
Why is it important to focus on the new 2017 ACC/AHA definition of IDH?
To start, in redefining IDH, the 2017 ACC/AHA guideline had a major impact on the number of adults newly eligible for a diagnosis of hypertension on the basis of meeting criteria for IDH. For example, among 1.3 million United States (US) outpatients, the prevalence of IDH using the ACC/AHA definition was 6.1% compared with 1.4% when the JNC/ESC/NICE definition was used.3 Almost identical findings were seen in analyses of the National Health and Nutrition Examination Survey (NHANES 2013–2016), where the percent increase in the US population newly eligible for a diagnosis of IDH using 2017 criteria was 5.2%.7 To put this into context, this 5.2% increase translated into absolute numbers of US adults newly eligible for a diagnosis of IDH of approximately 12 million individuals.7 Because these 12 million US adults have IDH by 2017 ACC/AHA criteria but not by the older JNC criteria, some have labelled these persons as having ‘stage 1 IDH’. In a Brazilian cohort using routine BP values from an executive screening clinic, there was a dramatic 8.7-fold increase (from 3.9% by JNC to 34.1% by 2017 ACC/AHA definition) in the prevalence of IDH using the new criteria.28 Increased prevalence of the new definition of IDH has also been reported from a Chinese cohort of adults participating in the Kailuan study (17% of this cohort where newly eligible for a diagnosis of IDH using the 2017 ACC/AHA criteria compared to the older JNC criteria)21 and from less rigorously collected BP data reported in a Japanese insurance-based clinical registry (9.7% of participants were newly eligible for an IDH diagnosis using the 2017 ACC/AHA criteria).19 Therefore, the absolute number of adults around the world newly eligible for a diagnosis of hypertension on the sole basis of meeting criteria for the 2017 ACC/AHA definition of IDH (i.e., adults with stage 1 IDH) is huge.
Besides being eligible for a diagnosis of hypertension, which has psychosocial and numerous other implications, adults newly meeting the 2017 ACC/AHA criteria for IDH are also now exposed to the potential for drug treatment with antihypertensives. However, because half of persons newly eligible for IDH according to the 2017 ACC/AHA guideline are under the age of 50 years and, consequently are low risk for CVD, the problem of overtreatment of these persons is less of a concern than overdiagnosis because the 2017 ACC/AHA guideline stipulates that adults with stage 1 IDH (diastolic BP 80–90 mmHg) must also have a 10 year CVD risk of ≥10% to warrant drug treatment. Nonetheless, analysis of NHANES data suggest that 0.6% of US adults (approximately 1.4 million persons with CVD risk values over 10%) were new candidates for antihypertensive drug treatment solely on the basis of having IDH after the release of 2017 ACC/AHA guidelines.7
The implications of the 2017 ACC/AHA definition of IDH on the diagnosis and treatment of millions of adults around the world is the major reason why a critical focus on this new definition of IDH is, in our opinion, very important and also why a comparison of the new versus older definition of IDH is warranted. In contrast to the convincing increase in CVD risk described above for the older more traditional definition of IDH, if the 2017 ACC/AHA definition of IDH is not actually associated with increased risk of CVD, and given the lack of clinical trial data on the efficacy of antihypertensives for persons with IDH, one should justifiably wonder why we are labelling adults who fulfil the 2017 ACC/AHA criteria for stage 1 IDH with a diagnosis of hypertension and also exposing them to the potential of being prescribed medication.
The ACC/AHA definition of IDH and Target Organ Damage/Atherosclerosis/Systolic Hypertension
If there is an important causal link between the new ACC/AHA definition of IDH and CVD one would expect to find clear evidence of subclinical atherosclerosis and target organ damage in affected individuals. Among 5,104 US participants enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA), IDH by the ACC/AHA definition was not associated with coronary artery calcification on non-contrast chest computed tomography (CT).5 In an analysis of coronary CT angiography data, the ACC/AHA definition of IDH had only a weak and marginally statistically significant adjusted association with any coronary plaque (odds ratio 1.4, 95% CI 1.0–1.8, p=0.04) and, furthermore, this definition of IDH was not associated with coronary plaques of greater than 50% stenosis severity.29 In the Atherosclerosis Risk in Communities (ARIC) Study, the 2017 definition of IDH was not associated with sensitive biomarkers of subclinical myocardial injury in the form of high-sensitivity Troponin T or N terminus-pro Brain Natriuretic Peptide.7 Another cross-sectional analysis of 1,605 French adults in the longitudinal STANISLAS cohort study found that IDH by the ACC/AHA definition was not significantly associated with markers of target organ damage including natriuretic peptides, microalbuminuria, diastolic dysfunction, left ventricular mass, carotid intimal thickness, ankle-brachial indices and pulse-wave velocity.30
While existing evidence does not support an association between IDH and subclinical atherosclerosis or target organ damage, it is worth noting that IDH, by both the old and the new definitions, is a strong risk factor for the future development of systolic hypertension. A landmark analysis of the Framingham Heart Study with 5,209 participants found that those with IDH by JNC/ESC/NICE definition were 23 times more likely to develop combined SDH, compared to participants who were normotensive.31 In a more contemporary analysis of US participants in MESA, individuals with IDH by the ACC/AHA definition were 1.7 times more likely to develop systolic hypertension than those with normotension.5 Therefore, we do not want to give the impression that this new 2017 definition of IDH is entirely benign; it is not, due to its association with future systolic hypertension. As such, persons with IDH by the 2017 definition should be provided with lifestyle and dietary counselling regarding BP control and should have their systolic BPs followed at annual or biennial intervals. Our argument, elaborated in more detail directly below, is simply that the 2017 definition of IDH has not been consistently associated with a clinically meaningful increase in CVD risk, certainly not enough to justify labelling adults meeting 2017 ACC/AHA criteria for stage 1 IDH with the diagnosis of hypertension and potentially also subjecting them to drug treatment.
The 2017 ACC/AHA definition of IDH and CVD Outcomes
A 2019 analysis of 60,866 participants aged 40–79 years in the Korean National Health Insurance Service (KNHIS) Screening Cohort reported that IDH by the newer ACC/AHA guideline (i.e., stage 1 IDH) was not associated with CVD (HR 1.12 [95% CI 0.95–1.31]].32 A second report from KNHIS of participants over 40 years of age corroborated this finding.33 In 2020, BP and cardiovascular outcomes data from 8,703 ARIC Study participants (with follow-up from 1990 through 2017) were analysed. IDH by the ACC/AHA definition was not associated with CVD events in ARIC (HR 1.06 [95% CI 0.89–1.26]).7 These results were corroborated in two further cohorts; NHANES III and the Give Us a Clue to Cancer and Heart Disease (CLUE) II cohort.7,34
A further study in 2020 analysed data from 151,831 participants enrolled in the United Kingdom (UK) Biobank.20 After a median follow-up of 10 years, IDH by the ACC/AHA definition was not associated with CVD (HR 1.08 [95% CI 0.98–1.18]). In 2021, a large Chinese cohort from the Kailuan Study was used to evaluate the association between the ACC/AHA definition of IDH and CVD.21 Although the top-line results included a borderline increased risk for CVD events with the ACC/AHA definition (HR 1.13 [95% CI: 1.02–1.26]), once adjusted for baseline systolic BP, no association was seen (HR 1.05 [95% CI: 0.94–1.17]). In 2021, a study using data from 5,104 participants in MESA also reported no association between IDH by ACC/AHA definition CVD (HR 1.19 [95% CI 0.77–1.84]) over 13 years of follow-up.5
Therefore, most of the studies published since 2017 have not found IDH by the ACC/AHA definition to be associated with an increased risk of CVD. An obvious explanation for this is that, among persons with systolic BP <130 mmHg, there are too few individuals with high enough diastolic BPs to cause excess incidence of CVD events (Figure 1). Another explanation for this null association could be the regression to the mean phenomenon. 35 Specifically, because persons with IDH have elevated diastolic BP in the presence of normal systolic BP (which is unexpected given that diastolic BP is usually correlated with systolic BP), it is likely that if the diastolic BP measurement where to be repeated again in some of these individuals that it would be closer to the mean diastolic BP (i.e., it would be lower) and that the person would be redesignated as normotensive instead of having IDH. For this reason, some adults classified as having IDH in published cohorts to date might have been found to be actually normotensive with repeated measures of diastolic BP.
However, the data on a null association between the 2017 ACC/AHA definition of IDH and CVD have been admittedly inconsistent. For example, 2 analyses of large databases from Japan and Korea have reported a statistically significant association between this IDH definition and CVD. The first of these used data from 1,746,493 individuals in a large Japanese nationwide claims database (the JMDC) and found IDH by the ACC/AHA definition to be associated with increased risk of CVD (HR 1.17 [95% CI 1.14–1.20]).19 Of note BP data in this very large study were taken from a routine annual health check-ups and cardiovascular outcomes were captured using insurance claims on insured individuals. The second study used data from 6,424,090 participants from the KNHIS and, importantly, only analyzed participants aged 20 to 39 years who were not taking antihypertensive medication. Again, BP data in this KNHIS study were taken from routine check-ups and outcomes were captured using insurance claims. After a median follow-up of 13.2 years, IDH by ACC/AHA definition (N=1,271,505) was found to be associated with an increased risk of CVD in this young sample (HR 1.36 [95% CI 1.29–1.43]).18 A further KNHIS analysis reported that the 2017 ACC/AHA definition of IDH was also associated with CVD among adults aged 40–64 years (HR 1.11 [95% CI 1.10–1.13]).36 Of interest, these two reports appear to contradict two previously published analysis, noted above,32,33 of the exact same KNHIS dataset that found no association between the 2017 ACC/AHA definition of IDH and CVD.
Accordingly, there are, to our knowledge, 4 separate reports of the 2017 ACC/AHA definition of IDH using the KNHIS dataset, 2 of which show no association with CVD and 2 of which do suggest a weak association. The reasons behind these discrepancies are not entirely clear but may relate to differences in selection criteria (e.g., age cut-offs and baseline calendar year of the cohort chosen) and to the approaches used in the analysis (e.g., reference groups and adjustment variables chosen for the regression models). Indeed, a salient example of how inappropriate use of both selection criteria and references groups can adversely affect analyses testing the association between IDH and CVD can be found in analyses of the UK Biobank. Our group found no association of the 2017 ACC/AHA definition of IDH and CVD events in UK Biobank.20 However, published a year later, another group reported increased risk for this IDH definition in the exact same UK Biobank dataset.37 Closer inspection of the latter analysis reveals that the authors used normotension (BP <120/80 mmHg) as the reference group and did not exclude outlier participants with spurious elevated biologically implausible diastolic BP values. All of these contradictory reports bely the weakness of the association between the 2017 ACC/AHA definition of IDH and CVD events and point to the statistical fragility of any association that may exist.
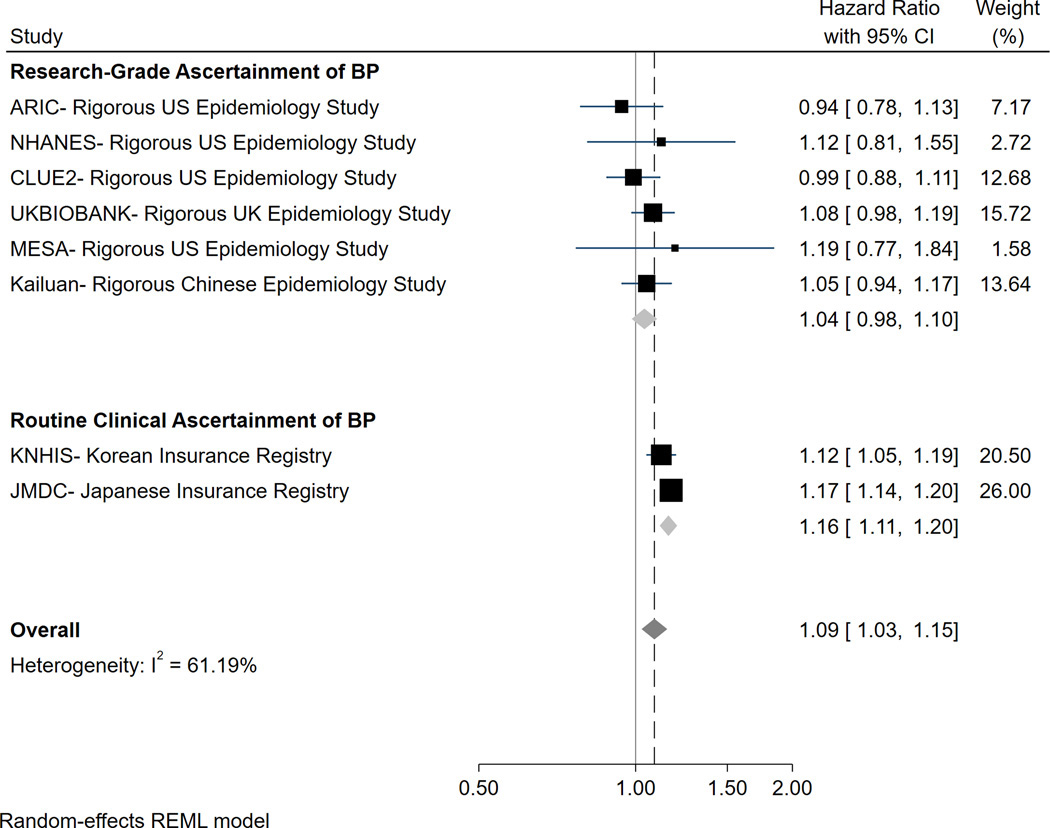
Even if we were to accept the methodological limitations of the registry studies that found a statistically significant association between the 2017 ACC/AHA definition of IDH and CVD events, it is not clear that this association is of sufficient magnitude to be clinically relevant. For example, in a stratified meta-analysis, the reported hazard ratio for CVD was just 1.16 (95% CI 1.11–1.20) in the registry studies5 (Figure 2). Because these registry studies included several million participants who were followed for upward of 10 years, they have the power to detect even the weakest of statistical associations. In our opinion, the weak magnitude of any association and the inconsistent statistical significance of these associations do not suggest that persons meeting the 2017 ACC/AHA criteria for IDH have a clinically meaningful increase in risk for CVD, particularly among those with stage 1 IDH. In particular, we do not feel that any increase in CVD risk is meaningful enough to justify labelling these adults as hypertensive and potentially exposing them to antihypertensive medications.
Figure 2-. Meta-analysis of the association between the 2017 ACC/AHA definition of IDH and CVD, stratified according to the form of BP measurement used in each study.
When rigorous data are examined, the association of the ACC/AHA definition of IDH and CVD does not appear to be significant. Even when less rigorous data from massive registries are included, any association is weak and unlikely of sufficient clinical significance to warrant labelling these individuals with a disease (hypertension) and exposing them to potential drug treatment. N.B. The exception to this appears to be in younger adults, where the relative risk increase in CVD among those with the 2017 ACC/AHA definition of IDH may be more significant; however, because the absolute risk of CVD is so low in these young adults, the actual risk of CVD remains very low.
Figure 2 adapted from Eur Heart J. 2021;42(21):2119–2129.
There has also been some concern about the validity of the results from the above large registries as they comprised BP data collected from routine clinic recordings that are frequently unreliable (in particular for diastolic BP measurement)38 and are often measured in a non-standardised manner.5 The use of claims data for CVD outcomes can also be problematic as these data are fashioned to obtain reimbursement rather than for the purpose of research with the potential for coding errors, lack of detail in claims, omitted claims and selection biases.39 Indeed, in the aforementioned stratified meta-analysis,5 we found that there was no association between the 2017 ACC/AHA definition of IDH and CVD events in studies using only research grade measures of BP and adjudicated outcomes (HR 1.04, 95% CI 0.98–1.10) (Figure 2).
Therefore, we believe that the totality of high-quality data published to date do not support an overall statistically robust increase in CVD risk among all adults who meet the 2017 ACC/AHA criteria for IDH, and most certainly not among all adults with stage 1 IDH by this definition. This belief applies irrespective of the individual’s baseline anti-hypertensive medication use status. This is important because persons with IDH who are treatment naïve may represent a different cohort to persons who meet IDH criteria but are on antihypertensive medications at baseline. Several analyses have found no prognostic association between IDH and CVD even when the analytic samples were stratified on the basis of baseline antihypertensive medication status.7,20 Furthermore, one analysis adjusted for antihypertensive medication use during follow-up as a time updated variable and continued to find no association between IDH and CVD7, implying that even those with treatment naïve IDH at baseline who subsequently end up on BP medications are not at increased risk compared to normotension.
Remaining Uncertainties about the significance of the 2017 ACC/AHA definition of IDH
The KNHIS and JMDC datasets have admittedly hinted to a potential increase in risk for CVD among two subgroups who meet the 2017 ACC/AHA definition of IDH; (1) Asian adults and (2) adults of any race who are younger than 40 years. Recent data have shed further light on these two outstanding areas of uncertainty. First, rigorously collected BP data were recently analyzed from 39,545 participants in a prospective study in rural Liaoning Province, China. By the ACC/AHA definition, IDH was found to be weakly associated with increased risk of CVD (HR 1.18 [95% CI 1.04–1.34]).40 Whether this weak 18% relative risk increase in CVD justifies consideration of anti-hypertensive therapies among Asian adults with IDH is currently unknown and would require evidence from randomized controlled trials.
Second, a recent age-stratified analysis has further investigated whether there are differences in the prognostic significance of IDH among young vs older adults. This is important because the BP phenotype displayed by an individual can be transient and can change over the life course. For example, whereas systolic BP tends to increase with age41, diastolic BP often falls. This increase in systolic BP and reduction in diastolic BP results in a lower prevalence of IDH and higher prevalence of systolic hypertension among the elderly. Using data from 11,135 participants in the IDACO database over a median follow-up of 13.8 years, IDH by the ACC/AHA definition using ambulatory BP criteria (24-hour mean systolic BP <125 mmHg and diastolic BP ≥75 mmHg) was not found to be associated with an increased risk of CVD in the sample overall (HR 1.14 [95% CI 0.94–1.40]). However, when participants younger than 50 years of age were analyzed, there was a significant increase in relative risk for CVD (HR 2.87 [95% CI 1.72–4.80]) with significant statistical evidence for effect modification on the basis of age (P interaction of <0.001).27
Therefore, it is fair to conclude that IDH may have more prognostic significance in the young on a relative risk scale. However, the decision to initiate antihypertensive therapy should be based on the absolute reductions in CVD.42 The increased relative risk of CVD events seen in young individuals with IDH does not necessarily equate to an elevated absolute risk of CVD events that is of sufficient magnitude to justify the initiation of drug therapy. Instead, this is an area that deserves to be the focus of further clinical trial research, particularly with regards to the efficacy, safety, tolerability, and cost-effectiveness of antihypertensive therapy in young adults with stage 1 IDH. Until such trial data emerge, we do not think that the small increase in absolute risk for CVD among young adults with stage 1 IDH is enough to warrant drug therapy.
Conclusion
Our interpretation of the totality of data published to date allows for the following conclusions to be drawn regarding IDH. First, the traditional JNC/ESC/NICE definition of IDH does appear to be associated with risk of CVD. Second, by changing the definition of IDH in 2017 ACC/AHA guidelines, many millions of adults around the world are newly eligible for a diagnosis of hypertension and some may be considered for drug treatment. Third, these individuals with stage 1 IDH by 2017 ACC/AHA criteria do not, however, appear to be consistently at higher risk for CVD than persons with normotension. Finally, fourth, there may be some exceptions to the overall null association between the 2017 definition of IDH and CVD, namely among Asian adults and young adults. However, even in both of these exceptions (and because of the small relative risk increase CVD among in the former group [Asian adults] and the low absolute risk for CVD in the latter group [young adults]), the absolute increase in risk for CVD with the 2017 ACC/AHA definition of IDH is very small in both groups and unlikely to be clinically meaningful enough to warrant labelling these individuals with a disease or providing them drug therapy. Rather, because the 2017 ACC/AHA definition is associated with the development of future elevations in systolic BP, we believe that persons with stage 1 IDH do not warrant drug treatment and should instead be provided lifestyle and dietary counselling and undergo interval BP checks to screen for the development of systolic hypertension or stage 2 IDH. Should systolic hypertension or stage 2 IDH (i.e., diastolic BPs ≥90 mmHg) develop, only then do we feel should the patient be considered for antihypertensive drug treatment, taking into account their other CVD risk factors and treatment preferences.
Sources of Funding and Disclosures
The authors report no funding or disclosures relevant to this manuscript.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Collaboration PS. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 2.Whelton SP, McEvoy JW, Shaw L, et al. Association of Normal Systolic Blood Pressure Level With Cardiovascular Disease in the Absence of Risk Factors. JAMA Cardiol. 2020;5(9):1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flint AC, Conell C, Ren X, et al. Effect of Systolic and Diastolic Blood Pressure on Cardiovascular Outcomes. N Engl J Med. 2019;381(3):243–251. [DOI] [PubMed] [Google Scholar]
- 4.Quinn S, McEvoy JW. Systolic and Diastolic Blood Pressure and Cardiovascular Outcomes. N Engl J Med. 2019;381(17):1690–1691. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen AP, Al Rifai M, Arps K, et al. A cohort study and meta-analysis of isolated diastolic hypertension: searching for a threshold to guide treatment. Eur Heart J. 2021;42(21):2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):2199–2269. [DOI] [PubMed] [Google Scholar]
- 7.McEvoy JW, Daya N, Rahman F, et al. Association of Isolated Diastolic Hypertension as Defined by the 2017 ACC/AHA Blood Pressure Guideline With Incident Cardiovascular Outcomes. JAMA. 2020;323(4):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351(9118):1755–1762. [DOI] [PubMed] [Google Scholar]
- 9.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 10.NICE. National Institute for Health and Care Excellence: Hypertension in adults: diagnosis and management NG136. 2019. https://www.nice.org.uk/guidance/ng136, accessed March 14, 2022
- 11.Arvanitis M, Qi G, Bhatt DL, et al. Linear and Nonlinear Mendelian Randomization Analyses of the Association Between Diastolic Blood Pressure and Cardiovascular Events: The J-Curve Revisited. Circulation. 2021;143(9):895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannel WB, Wilson PW, Nam BH, D’Agostino RB, Li J. A likely explanation for the J-curve of blood pressure cardiovascular risk. Am J Cardiol. 2004;94(3):380–384. [DOI] [PubMed] [Google Scholar]
- 13.Niiranen TJ, Mäki J, Puukka P, Karanko H, Jula AM. Office, home, and ambulatory blood pressures as predictors of cardiovascular risk. Hypertension. 2014;64(2):281–286. [DOI] [PubMed] [Google Scholar]
- 14.Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med. 2002;162(5):577–581. [DOI] [PubMed] [Google Scholar]
- 15.Strandberg TE, Salomaa VV, Vanhanen HT, Pitkala K, Miettinen TA. Isolated diastolic hypertension, pulse pressure, and mean arterial pressure as predictors of mortality during a follow-up of up to 32 years. J Hypertens. 2002;20(3):399–404. [DOI] [PubMed] [Google Scholar]
- 16.Hozawa A, Ohkubo T, Nagai K, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self-measurement of blood pressure at home: the Ohasama study. Arch Intern Med. 2000;160(21):3301–3306. [DOI] [PubMed] [Google Scholar]
- 17.Sheriff HM, Tsimploulis A, Valentova M, et al. Isolated diastolic hypertension and incident heart failure in community-dwelling older adults: Insights from the Cardiovascular Health Study. Int J Cardiol. 2017;238:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Yano Y, Cho SMJ, et al. Cardiovascular Risk of Isolated Systolic or Diastolic Hypertension in Young Adults. Circulation. 2020;141(22):1778–1786. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko H, Itoh H, Yotsumoto H, et al. Association of Isolated Diastolic Hypertension Based on the Cutoff Value in the 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines With Subsequent Cardiovascular Events in the General Population. J Am Heart Assoc. 2020;9(19):e017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath BP, Kundu P, Daya N, et al. Isolated Diastolic Hypertension in the UK Biobank: Comparison of ACC/AHA and ESC/NICE Guideline Definitions. Hypertension. 2020;76(3):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Ji C, Shi J, Chen S, Huang Z, Jonas JB. Isolated diastolic hypertension as defined by the 2017 American College of Cardiology/American Heart Association blood pressure guideline and incident cardiovascular events in Chinese. J Hypertens. 2021;39(3):519–525. [DOI] [PubMed] [Google Scholar]
- 22.Kelly TN, Gu D, Chen J, et al. Hypertension subtype and risk of cardiovascular disease in Chinese adults. Circulation. 2008;118(15):1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arima H, Murakami Y, Lam TH, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia-Pacific Region. Hypertension. 2012;59(6):1118–1123. [DOI] [PubMed] [Google Scholar]
- 24.Lotfaliany M, Akbarpour S, Mozafary A, Boloukat RR, Azizi F, Hadaegh F. Hypertension phenotypes and incident cardiovascular disease and mortality events in a decade follow-up of a Middle East cohort. J Hypertens. 2015;33(6):1153–1161. [DOI] [PubMed] [Google Scholar]
- 25.Hisamatsu T, Miura K, Ohkubo T, et al. Isolated systolic hypertension and 29-year cardiovascular mortality risk in Japanese adults aged 30−-49 years. J Hypertens. 2020;38(11):2230–2236. [DOI] [PubMed] [Google Scholar]
- 26.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37(3):869–874. [DOI] [PubMed] [Google Scholar]
- 27.McEvoy JW, Yang WY, Thijs L, et al. Isolated Diastolic Hypertension in the IDACO Study: An Age-Stratified Analysis Using 24-Hour Ambulatory Blood Pressure Measurements. Hypertension. 2021;78(5):1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cesena FHY, Nary FC, Santos RD, Bittencourt MS. The contribution of the systolic and diastolic components for the diagnosis of arterial hypertension under the 2017 ACC/AHA Guideline and metabolic heterogeneity among individuals with Stage 1 hypertension. J Clin Hypertens (Greenwich). 2020;22(7):1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon YH, Park GM, Lee JY, et al. Association of Stage 1 Hypertension Defined by the ACC/AHA 2017 Guideline With Asymptomatic Coronary Atherosclerosis. Am J Hypertens. 2021;34(8):858–866. [DOI] [PubMed] [Google Scholar]
- 30.Monzo L, Ferreira JP, Lamiral Z, et al. Isolated diastolic hypertension and target organ damage: Findings from the STANISLAS cohort. Clin Cardiol. 2021;44(11):1516–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin SS, Pio JR, Wong ND, et al. Predictors of new-onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation. 2005;111(9):1121–1127. [DOI] [PubMed] [Google Scholar]
- 32.Son JS, Choi S, Lee G, et al. Blood Pressure Change from Normal to 2017 ACC/AHA Defined Stage 1 Hypertension and Cardiovascular Risk. J Clin Med. 2019;8(6):820. doi: 10.3390/jcm8060820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YJ, Kim SH, Kang SH, et al. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: a nationwide population-based study from Korea. Eur Heart J. 2019;40(9):724–731. [DOI] [PubMed] [Google Scholar]
- 34.Bae E, Rocco MV, Lee J, et al. Impact of DBP on all-cause and cardiovascular mortality: results from the National Health and Nutrition Examination survey, 1999–2014. J Hypertens. 2022;40(1):108–116. [DOI] [PubMed] [Google Scholar]
- 35.Salam A, Atkins E, Sundstrom J, et al. Effects of blood pressure lowering on cardiovascular events, in the context of regression to the mean: a systematic review of randomized trials. J Hypertens. 2019;37(1):16–23. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Yano Y, Cho SMJ, Park S, Lloyd-Jones DM, Kim HC. Cardiovascular Risk of Isolated Diastolic Hypertension Defined by the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline: A Nationwide Age-Stratified Cohort Study. Hypertension. 2020;76(6):e44–e46. [DOI] [PubMed] [Google Scholar]
- 37.Li FR, He Y, Yang HL, et al. Isolated systolic and diastolic hypertension by the 2017 American College of Cardiology/American Heart Association guidelines and risk of cardiovascular disease: a large prospective cohort study. J Hypertens. 2021;39(8):1594–1601. [DOI] [PubMed] [Google Scholar]
- 38.Blank SG, Mann SJ, James GD, West JE, Pickering TG. Isolated elevation of diastolic blood pressure. Real or artifactual? Hypertension. 1995;26(3):383–389. [DOI] [PubMed] [Google Scholar]
- 39.McEvoy JW. Letter by McEvoy Regarding Article, “Cardiovascular Risk of Isolated Systolic or Diastolic Hypertension in Young Adults”. Circulation. 2021;143(3):e20–e21. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Liu S, Jiao Y, Zheng L, Sun Y, Sun Z. Association of isolated diastolic hypertension based on different guideline definitions with incident cardiovascular risk in a Chinese rural cohort. J Clin Hypertens (Greenwich). 2022. Jan;24(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duprez DA. Systolic hypertension in the elderly: addressing an unmet need. Am J Med. 2008;121(3):179–184 e173. [DOI] [PubMed] [Google Scholar]
- 42.Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet. 2005;365(9457):434–441. [DOI] [PubMed] [Google Scholar]