This cohort study investigates the risk of major congenital malformations associated with antiseizure medication (ASM) monotherapy in the offspring of mothers taking ASMs during pregnancy.
Key Points
Question
What is the comparative risk of major congenital malformations (MCMs) associated with antiseizure medication (ASM) monotherapy in the offspring of mothers with epilepsy taking ASMs during pregnancy?
Findings
In this cohort study including 9840 pregnancies, offspring exposed to levetiracetam, oxcarbazepine, and lamotrigine had the lowest prevalence of MCMs, compared with other ASMs. An increased risk of MCMs was associated with increasing doses of carbamazepine, phenobarbital, and valproate; the overall prevalence of MCMs decreased by 39% over time, in parallel with declining use of valproate and carbamazepine and greater use of levetiracetam and lamotrigine.
Meaning
Results of this study suggest essential information for safer treatment selection in women of childbearing potential requiring ASM therapy.
Abstract
Importance
Women with epilepsy (WWE) require treatment with antiseizure medications (ASMs) during pregnancy, which may be associated with an increased risk of major congenital malformations (MCMs) in their offspring.
Objective
To investigate the prevalence of MCMs after prenatal exposure to 8 commonly used ASM monotherapies and changes in MCM prevalence over time.
Design, Setting, and Participants
This was a prospective, observational, longitudinal cohort study conducted from June 1999 to October 2022. Since 1999, physicians from more than 40 countries enrolled ASM-treated WWE before pregnancy outcome was known and followed up their offspring until 1 year after birth. Participants aged 14 to 55 years who were exposed to 8 of the most frequently used ASMs during pregnancy were included in this study. Data were analyzed from April to September 2023.
Exposure
Maternal use of ASMs at conception.
Main Outcomes and Measures
MCMs were assessed 1 year after birth by a committee blinded to type of exposure. Teratogenic outcomes across exposures were compared by random-effects logistic regression adjusting for potential confounders and prognostic factors.
Results
A total of 10 121 prospective pregnancies exposed to ASM monotherapy met eligibility criteria. Of those, 9840 were exposed to the 8 most frequently used ASMs. The 9840 pregnancies occurred in 8483 women (mean [range] age, 30.1 [14.1-55.2] years). MCMs occurred in 153 of 1549 pregnancies for valproate (9.9%; 95% CI, 8.5%-11.5%), 9 of 142 for phenytoin (6.3%; 95% CI, 3.4%-11.6%), 21 of 338 for phenobarbital (6.2%; 95% CI, 4.1%-9.3%), 121 of 2255 for carbamazepine (5.4%; 95% CI, 4.5%-6.4%), 10 of 204 for topiramate (4.9%; 95% CI, 2.7%-8.8%), 110 of 3584 for lamotrigine (3.1%; 95% CI, 2.5%-3.7%), 13 of 443 for oxcarbazepine (2.9%; 95% CI, 1.7%-5.0%), and 33 of 1325 for levetiracetam (2.5%; 95% CI, 1.8%-3.5%). For valproate, phenobarbital, and carbamazepine, there was a significant increase in the prevalence of MCMs associated with increasing dose of the ASM. Overall prevalence of MCMs decreased from 6.1% (153 of 2505) during the period 1998 to 2004 to 3.7% (76 of 2054) during the period 2015 to 2022. This decrease over time was significant in univariable logistic analysis but not after adjustment for changes in ASM exposure pattern.
Conclusions and Relevance
Of all ASMs with meaningful data, the lowest prevalence of MCMs was observed in offspring exposed to levetiracetam, oxcarbazepine, and lamotrigine. Prevalence of MCMs was higher with phenytoin, valproate, carbamazepine, and phenobarbital, and dose dependent for the latter 3 ASMs. The shift in exposure pattern over time with a declining exposure to valproate and carbamazepine and greater use of lamotrigine and levetiracetam was associated with a 39% decline in prevalence of MCMs, a finding that has major public health implications.
Introduction
The possibility of prenatal exposure to antiseizure medications (ASMs) leading to increased risk of congenital anomalies has been a concern for more than 5 decades.1 Because most women with epilepsy (WWE) need to continue ASM treatment during pregnancy,2 identifying the safest treatment options for these women is essential. This information could also be applicable to women of childbearing potential who are taking ASMs for nonepilepsy conditions such as psychiatric disorders, migraine, and neuropathic pain.3,4 The assessment of pregnancy outcomes after exposure to different ASMs faces many challenges. Ethical constraints preclude the conduction of randomized studies, leaving observational studies as the sole option. Enrollment of large cohorts is also required to handle the influence of confounding variables.
To address these challenges, independent research groups have established prospective registries to compare the risk of major congenital malformations (MCMs) after prenatal ASM exposure.5 These registries have identified elevated risks after exposure to valproate and possibly topiramate, whereas risks have been lower for lamotrigine and levetiracetam.6,7,8,9,10,11 Some registries have reported a dose-dependent risk for valproate,6,9,10 carbamazepine,9,10 phenobarbital,9,10 and lamotrigine.6,9,10
Studies based on national health databases have confirmed increased risks linked to valproate and topiramate.12,13 Although advantageous due to their large sample size and population-based nature, these databases do not provide information on important details collected in dedicated registries, such as validation and classification of maternal epilepsy diagnosis, seizure control, and precise ASM dosage. Moreover, recording of MCMs in health databases is not based on standardized assessments as in dedicated registries.
The larger epilepsy and pregnancy registries have not published significant updates lately. The latest publication from the International Registry of Antiepileptic Drugs and Pregnancy (EURAP), a large international registry spanning across more than 40 countries, dates back to 2018 and reports data up to 2016.9 Since that time, EURAP has enrolled numerous additional pregnancies. At the same time, changes in ASM prescription patterns have occurred, warranting an updated analysis.
This study provides an updated assessment of the comparative risk of MCMs associated with the 8 most frequently used ASM monotherapies. It also provides new data on dose-outcome associations, changes over time in overall occurrence of MCMs, and the degree to which these changes correlate with changes in ASM prescription patterns.
Methods
Study Design and Participants
The EURAP registry is a longitudinal, prospective cohort study established in 1999 and extended to 47 countries with more than 1500 collaborators. The EURAP methodology has been described in detail before.9,14 The study protocol was approved by the ethics committees of participating centers. We obtained informed consent from all the women enrolled. The current analysis included pregnant WWE exposed to ASMs at the time of conception and enrolled within the 16th week of gestation, where fetal outcome had not yet been determined. We excluded pregnancies in women without epilepsy, pregnancies for which physicians did not submit reports within specified deadlines, and those for which follow-up was not yet completed at the current census. We also excluded pregnancies in which ASMs were switched or withdrawn during the first trimester, those exposed to ASM polytherapy or other known teratogenic drugs, and those with comorbidities associated with teratogenic risks (eTable 1 in Supplement 1). Spontaneous abortions, abortions induced for reasons unrelated to fetal abnormalities, pregnancies where fetal outcome could not be determined, and pregnancies leading to offspring with genetic or chromosomal abnormalities were also excluded (eTable 2 in Supplement 1). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Race and ethnicity were ascertained by reporting physicians across participating countries and categorized in the case report form as Aboriginal, Asian, Black, North African, multiracial, Pacific Islander, White, and other or unknown. However, because some categories had minimal representation, this information was recategorized in the database into 3 main categories: Asian, White, and other, which included all other race categories listed in the case report form.
Procedures
We collected information on demographics, epilepsy classification, seizure frequency, comorbidities, family history of MCMs, drug therapy, and other risk factors. Follow-up data were acquired after each trimester, at delivery, and 1 year after delivery. Reporting physicians were responsible for collecting the data, which were transmitted online to the central registry in Milan, Italy. Abnormalities in the offspring were recorded descriptively by reporting physicians. A committee blinded to type of exposure assessed and categorized these abnormalities. When necessary, the committee solicited additional information from the reporting physicians.
Outcomes
Our primary aim was to compare the association of MCMs with the 8 most commonly used monotherapies (carbamazepine, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, topiramate, and valproate) assessed 1 year after birth in offspring exposed to these ASMs in utero and to compare risks across different dose ranges whenever a dose-dependent pattern emerged. We categorized pregnancies based on type and dosage of ASM at the time of conception. Seizures were categorized as either tonic-clonic (including focal to bilateral tonic-clonic or generalized tonic-clonic) or other types. Epilepsies were classified according to International League Against Epilepsy criteria.15 MCMs referred to structural abnormalities with medical, surgical, cosmetic, or functional significance classified according to the European Concerted Action on Congenital Anomalies and Twins (EUROCAT) 2005 criteria.16 The covariates assessed were selected a priori based on their potential relevance, drawing from our previous investigation of pregnancy outcomes.9 Family history of MCMs pertained to the proband’s parents, excluding cerebral malformations causally linked to maternal epilepsy. Folate supplementation was considered appropriate if initiated at least 3 months before conception and continued throughout the first trimester at a dose of 0.4 mg per day or greater.
Statistical Analysis
According to the empirical rule of thumb that the ratio between the number of events and the number of explanatory variables should be 10 or greater, a sample size of 10 000 observations was deemed adequate to minimize the risk of overfitting in a multivariable logistic model, assuming a 5% frequency of teratogenic events in the pooled population and a selected number of plausible predictors no larger than 50. To calculate frequencies of MCMs, the numerator was the sum of all live births plus pregnancy losses with confirmed MCMs, whereas the denominator included live births, pregnancies terminated electively for suspected MCMs, and perinatal deaths. Dose categories were identified by a machine-based algorithm developed to overcome the limits of choosing dose intervals subjectively. The approach consists in splitting each treatment into 3 dose categories by identifying the combination of cutoffs that optimally separates the 3 dose intervals (more than 3 dose categories would add substantial complexity, with doubtful added gain). The process involves testing iteratively by logistic regression all possible cutoff pairs and selecting the combination with the largest C index among those showing a positive trend. If no positive correlation is detected, either because a dose-response association is absent or too weak, or the sample size is inadequate, a further attempt is made by testing 2 dose categories only. Of note, any identified association with only 2 dose categories is less robust and requires confirmation. The Cochrane-Armitage test was used to statistically test a dose-response association across the identified categories. A random-effects logistic model was used to compare treatments after adjusting for potential confounders or prognostic factors and to assess the effect of these factors on outcome. Logistic regression was done by fitting data through a generalized linear mixed model parameterized with logit-link function, binomial distribution, and inclusion of mother-level random effects to adjust for the presence of clustered data (women with more than 1 pregnancy). Results are reported as odds ratios (ORs) with 2-tailed 95% CIs. By setting the type II error at the same severity level as the type I error, multiplicity generated by the 55 comparisons was handled by controlling the false discovery rate through the Benjamini and Hochberg adaptive linear step-up approach17,18 as an alternative to classic Bonferroni type adjustments.19 To analyze changes in prevalence of MCMs over time, pregnancies were categorized based on 4 time periods of conception (1998-2004, 2005-2009, 2010-2014, and 2015-2022), which were defined a priori to ensure reasonably balanced numbers of pregnancies. The multivariable logistic model, which included time periods as independent variables, provided estimates of ORs for comparisons of time periods adjusted for other covariates, including changes in ASM prescription pattern (ASM type and dose category). Partially adjusted and unadjusted ORs were obtained by rerunning the random-effects logistic model parameterized with only the non-ASM covariates (partially adjusted) and time period as the only covariate (unadjusted) respectively. Statistical significance was set at a 2-sided P value <.05. Data were analyzed from April to September 2023 using SAS, version 9.4 (SAS Institute).
Results
Of the 28 553 pregnancies entered into the database between June 20, 1999, and October 25, 2022, 10 121 exposed to ASM monotherapy and with complete follow-up data met criteria for inclusion (eFigure 1 in Supplement 1). Of these, 9840 had been exposed to the 8 most frequently used ASMs and were included in the primary analysis (Table 1). Of these, 1108 (11.3%) were pregnancies also entered in other collaborating registries (eTable 3 in Supplement 1). The 9840 pregnancies included in the primary analysis resulted in 9748 live births, 48 perinatal deaths (after 24 weeks of gestation and before 7 completed days), and 44 elective abortions for fetal abnormalities. A total of 1144 women contributed 2 pregnancies, 97 women contributed 3 pregnancies, 5 women contributed 4 pregnancies, and 1 woman contributed 5 pregnancies. Each of the 146 twin pregnancies and the 1 triplet pregnancy were considered separate pregnancies. Thus, the 9840 pregnancies occurred in 8483 women (mean [range] age, 30.1 [14.1-55.2] years). These women self-identified with the following race and ethnicity categories: 797 Asian (9.4%), 7365 White (86.8%), and 309 other (3.6%). For 12 women (0.1%), information on racial group was unavailable.
Table 1. Demographic and Clinical Data of the Study Cohort (N = 9840).
| Characteristic | Mean (range) |
|---|---|
| Maternal age at time of conception, y | 30.1 (14.1-55.2) |
| Duration of pregnancy at time of enrollment, wk | 8 (1-16) |
| Parental history of major congenital malformations, No. (%) | |
| Negative | 9669 (98.3) |
| Positive | 111 (1.1) |
| Information missing | 60 (0.6) |
| Geographical region, No. (%) | |
| North and South America | 105 (1.1) |
| Europe | 8460 (86.0) |
| Eastern Mediterranean | 28 (0.3) |
| Southeast Asia | 410 (4.2) |
| Western Pacific | 837 (8.5) |
| Parity, No. (%) | |
| 0 | 5847 (59.4) |
| 1 | 3225 (32.8) |
| 2 | 608 (6.2) |
| ≥3 | 158 (1.6) |
| Information missing | 2 (0.0) |
| Type of epilepsy, No. (%) | |
| Idiopathic generalized epilepsy | 3959 (40.2) |
| Focal epilepsy | 4865 (49.4) |
| Epilepsy of unknown type | 1016 (10.3) |
| Tonic-clonic seizures during first trimester, No. (%) | |
| No | 9182 (93.3) |
| Yes | 637 (6.5) |
| Information missing | 21 (0.2) |
| Educational level of the father, No. (%)a | |
| Low | 1319 (13.4) |
| Medium or high | 7811 (79.4) |
| Information missing | 710 (7.2) |
| Educational level of the mother, No. (%)a | |
| Low | 1180 (12.0) |
| Medium or high | 8175 (83.1) |
| Information missing | 485 (4.9) |
| Folic acid intake, No. (%) | |
| Appropriate | 3631 (36.9) |
| Inappropriate | 6136 (62.4) |
| Information missing | 73 (0.7) |
| Offspring sex, No. (%) | |
| Female | 4751 (48.3) |
| Male | 5030 (51.1) |
| Information missing | 59 (0.6) |
Parental educational level was categorized into low (≤9 years of education) or medium or high (>9 years of education).
The prevalence of MCMs in the assessed offspring was 9.9% (153 of 1549 pregnancies; 95% CI, 8.5%-11.5%) for valproate, 6.3% (9 of 142 pregnancies; 95% CI, 3.4%-11.6%) for phenytoin, 6.2% (21 of 338 pregnancies; 95% CI, 4.1%-9.3%) for phenobarbital, 5.4% (121 of 2255 pregnancies; 95% CI, 4.5%-6.4%) for carbamazepine, 4.9% (10 of 204 pregnancies; 95% CI, 2.7%-8.8%) for topiramate, 3.1% (110 of 3584 pregnancies; 95% CI, 2.5%-3.7%) for lamotrigine, 2.9% (13 of 443 pregnancies; 95% CI, 1.7%-5.0%) for oxcarbazepine, and 2.5% (33 of 1325 pregnancies; 95% CI, 1.8%-3.5%) for levetiracetam (Table 2). For carbamazepine, phenobarbital and valproate, there was a significant increase in the prevalence of MCMs associated with increasing dose of the ASM (Table 2). Outcomes in the 281 pregnancies exposed to less frequently used ASMs are reported in eTable 4 in Supplement 1, whereas MCM prevalence data among cases lost to follow-up after delivery are summarized in eTable 5 in Supplement 1.
Table 2. Prevalence of Major Congenital Malformations (MCMs) in Offspring Exposed Prenatally to Monotherapy With 1 of 8 Different Antiseizure Medications (ASMs)a.
| ASM treatment (dose range, mg/d) | No. | Prevalence of MCMs (95% CI), % | Dose- dependency P value |
|
|---|---|---|---|---|
| Exposed pregnancies | Pregnancies with MCMs | |||
| Carbamazepine (25-2400) | 2255 | 121 | 5.4 (4.5-6.4) | NA |
| Lamotrigine (5-1300) | 3584 | 110 | 3.1 (2.5-3.7) | |
| Levetiracetam (80-5000) | 1325 | 33 | 2.5 (1.8-3.5) | |
| Oxcarbazepine (75-4500) | 443 | 13 | 2.9 (1.7-5.0) | |
| Phenobarbital (15-300) | 338 | 21 | 6.2 (4.1-9.3) | |
| Phenytoin (30-730) | 142 | 9 | 6.3 (3.4-11.6) | |
| Topiramate (25-600) | 204 | 10 | 4.9 (2.7-8.8) | |
| Valproate (100-3000) | 1549 | 153 | 9.9 (8.5-11.5) | |
| Phenobarbital (≤60) | 76 | 2 | 2.6 (0.3-9.2) | .047 |
| Phenobarbital (>60-≤130) | 197 | 12 | 6.1 (3.2-10.4) | |
| Phenobarbital (>130) | 65 | 7 | 10.8 (4.4-20.9) | |
| Carbamazepine (≤700) | 1506 | 70 | 4.6 (3.6-5.8) | .008 |
| Carbamazepine (>700 -≤1000) | 541 | 32 | 5.9 (4.1-8.2) | |
| Carbamazepine (>1000) | 208 | 19 | 9.1 (5.6-13.9) | |
| Valproate (≤650) | 715 | 43 | 6.0 (4.4-8.0) | <.001 |
| Valproate (>650-≤1450) | 711 | 79 | 11.1 (8.9-13.6) | |
| Valproate (>1450) | 123 | 31 | 25.2 (17.8-33.8) | |
Prevalence at different dose ranges is given for ASMs where a dose dependency was identified.
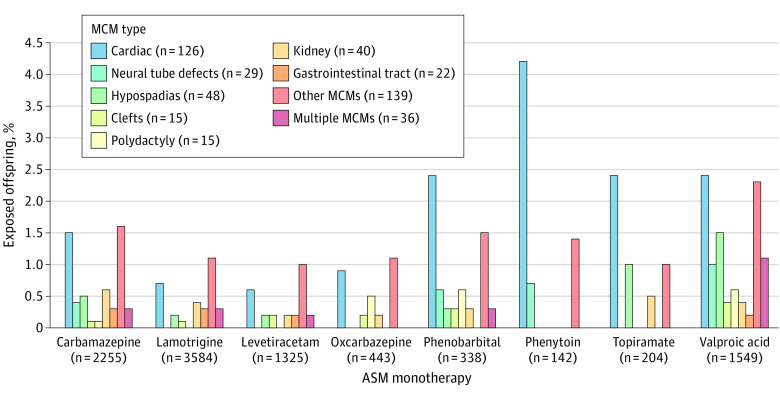
Cardiac malformations were the most frequent abnormality across ASMs, particularly after exposure to phenytoin, phenobarbital, topiramate, and valproate (Figure 1). Valproate exposure was associated with a wide range of MCMs, which also included hypospadias, neural tube defects, and multiple defects. There were no specific patterns associated with levetiracetam or lamotrigine (Figure 1).
Figure 1. Prevalence of Different Categories of Major Congenital Malformations (MCMs) After Prenatal Exposure to Monotherapy With 8 Antiseizure Medications (ASMs).
Prevalence (expressed as percentage of exposed offspring) of different categories of MCMs after prenatal exposure to monotherapy with the 8 most commonly used ASMs.
Table 3 summarizes the results of the multivariable analysis where the teratogenic risk of individual ASMs was compared with that of the ASM associated with the lowest prevalence of MCMs (levetiracetam). Comparisons across other ASMs and risks associated with non-ASM covariates are also shown.
Table 3. Multivariable Analysis of Comparative Risk of Major Congenital Malformations in the Study Cohort.
| Comparison | OR (95% CI) | Adjusted P valuea |
|---|---|---|
| Drug comparisons with levetiracetam (≥80 mg/d to 5000 mg/d) | ||
| Carbamazepine (≤700 mg/d) | 1.73 (1.10-2.72) | .02 |
| Carbamazepine (>700 mg/d to ≤1000 mg/d) | 2.23 (1.32-3.78) | .007 |
| Carbamazepine (>1000 mg/d) | 3.74 (2.01-6.96) | <.001 |
| Phenobarbital (≤60 mg/d) | 0.96 (0.22-4.14) | .96 |
| Phenobarbital (>60 mg/d to ≤130 mg/d) | 2.44 (1.21-4.92) | .02 |
| Phenobarbital (>130 mg/d) | 5.11 (2.09-12.45) | .001 |
| Valproate (≤650 mg/d) | 2.53 (1.54-4.16) | .001 |
| Valproate (>650 mg/d to ≤1450 mg/d) | 5.22 (3.32-8.20) | <.001 |
| Valproate (>1450 mg/d) | 14.27 (8.06-25.28) | <.001 |
| Lamotrigine (≥5 mg/d to 1300 mg/d) | 1.26 (0.84-1.89) | .26 |
| Oxcarbazepine (≥75 mg/d to 4500 mg/d) | 1.09 (0.56-2.13) | .80 |
| Phenytoin (≥30 mg/d to 730 mg/d) | 2.39 (1.08-5.31) | .04 |
| Topiramate (≥25 mg/d to 600 mg/d) | 2.02 (0.97-4.21) | .06 |
| Within-drug comparisons | ||
| Carbamazepine (>700 mg/d to ≤1000 mg/d vs ≤700 mg/d) | 1.29 (0.83-1.99) | .26 |
| Carbamazepine (>1000 mg/d vs ≤700 mg/d) | 2.16 (1.26-3.71) | .01 |
| Carbamazepine (>1000 mg/d vs >700 mg/d to ≤1000 mg/d) | 1.68 (0.92-3.07) | .09 |
| Phenobarbital (>60 mg/d to ≤130 mg/d vs ≤60 mg/d) | 2.54 (0.55-11.74) | .23 |
| Phenobarbital (>130 mg/d vs ≤60 mg/d) | 5.32 (1.04-27.06) | .05 |
| Phenobarbital (>130 mg/d vs >60 mg/d to ≤130 mg/d) | 2.10 (0.77-5.68) | .15 |
| Valproate (>650 mg/d to ≤1450 mg/d vs ≤650 mg/d) | 2.06 (1.38-3.07) | .001 |
| Valproate (>1450 mg/d vs ≤650 mg/d) | 5.63 (3.31-9.58) | <.001 |
| Valproate (>1450 mg/d vs >650 mg/d to ≤1450 mg/d) | 2.74 (1.68-4.44) | <.001 |
| Other comparisons across ASMs | ||
| Carbamazepine (≤700 mg/d) vs lamotrigine (5-1300 mg/d) | 1.37 (0.99-1.90) | .06 |
| Oxcarbazepine (75-4500 mg/d) vs lamotrigine (5-1300 mg/d) | 0.86 (0.48-1.56) | .63 |
| Topiramate (25-600 mg/d) vs lamotrigine (5-1300 mg/d) | 1.60 (0.82-3.13) | .17 |
| Valproate (≤650 mg/d) vs lamotrigine (5-1300 mg/d) | 2.01 (1.36-2.97) | .001 |
| Carbamazepine (>700 mg/d to ≤1000 mg/d) vs lamotrigine (5-1300 mg/d) | 1.77 (1.16-2.70) | .02 |
| Phenobarbital (>60 mg/d to ≤130 mg/d) vs lamotrigine (5-1300 mg/d) | 1.93 (1.03-3.62) | .049 |
| Oxcarbazepine (75-4500 mg/d) vs carbamazepine (≤700 mg/d) | 0.63 (0.34-1.16) | .14 |
| Topiramate (25-600 mg/d) vs carbamazepine (≤700 mg/d) | 1.16 (0.58-2.33) | .67 |
| Valproate (≤650 mg/d) vs carbamazepine (≤700 mg/d) | 1.46 (0.95-2.24) | .08 |
| Other comparisons across ASMs | ||
| Phenobarbital (>60 mg/d to ≤130 mg/d) vs carbamazepine (≤700 mg/d) | 1.40 (0.74-2.68) | .30 |
| Topiramate (25-600 mg/d) vs oxcarbazepine (75-4500 mg/d) | 1.85 (0.79-4.35) | .16 |
| Valproate (≤650 mg/d) vs oxcarbazepine (75-4500 mg/d) | 2.32 (1.20-4.51) | .02 |
| Carbamazepine (>700 mg/d to ≤1000 mg/d) vs oxcarbazepine (75-4500 mg/d) | 2.04 (1.05-3.97) | .045 |
| Phenobarbital (>60 mg/d to ≤130 mg/d) vs oxcarbazepine (75-4500 mg/d) | 2.23 (0.99-5.06) | .06 |
| Valproate (≤650 mg/d) vs topiramate (25-600 mg/d) | 1.26 (0.61-2.59) | .54 |
| Phenobarbital (>60 mg/d to ≤130 mg/d) vs topiramate (25-600 mg/d) | 1.21 (0.50-2.90) | .67 |
| Phenobarbital (>60 mg/d to ≤130 mg/d) vs valproate (≤650 mg/d) | 0.96 (0.49-1.90) | .91 |
| Effect of non-ASM covariates | ||
| Parental history of major congenital malformations | 3.43 (1.94-6.05) | <.001 |
| Idiopathic generalized epilepsy vs focal epilepsy | 0.81 (0.64-1.03) | .08 |
| Epilepsy of unknown type vs focal epilepsy | 0.81 (0.58-1.15) | .25 |
| North and South America vs Europe | 1.06 (0.45-2.51) | .89 |
| Eastern Mediterranean vs Europe | 1.16 (0.15-8.97) | .89 |
| Southeast Asia vs Europeb | 1.79 (1.17-2.73) | .01 |
| Western Pacific vs Europe | 1.05 (0.75-1.46) | .77 |
| Maternal age at conception | 1.01 (0.99-1.04) | .15 |
| Tonic-clonic seizures during first trimester of pregnancy | 0.89 (0.59-1.33) | .56 |
| Folic acid (appropriate use vs no use or inappropriate use) | 1.17 (0.96-1.44) | .12 |
| Parity 1 vs 0 | 0.80 (0.64-0.99) | .049 |
| Parity ≥2 vs 0 | 0.71 (0.48-1.06) | .10 |
| Offspring sex | 1.02 (0.84-1.23) | .84 |
| Period 2005-2009 vs 1998-2004 | 0.97 (0.76-1.23) | .78 |
| Period 2010-2014 vs 1998-2004 | 0.91 (0.69-1.21) | .51 |
| Period 2015-2022 vs 1998-2004 | 1.00 (0.72-1.37) | .99 |
Abbreviations: ASM, antiseizure medication; OR, odds ratio.
Multiplicity generated by the 55 comparisons was handled by controlling false discovery rate, using the Benjamini and Hochberg adaptive linear step-up.
The higher prevalence of major congenital malformations is limited to cardiac malformations and is explained by the fact that the Kerala register routinely perform cardiac ultrasound examinations of all children in their cohort.
Phenytoin, the 2 highest dose categories of phenobarbital, and all dose categories of carbamazepine and valproate were associated with significantly higher risk of MCMs compared with levetiracetam. The low dose of valproate and the medium dose of carbamazepine and phenobarbital were associated with higher risk than lamotrigine. The low dose of valproate and the medium dose of carbamazepine were associated with higher risk than oxcarbazepine.
A parental history of MCMs was associated with a greater than 3-fold increase in risk. Neither epilepsy type nor occurrence of tonic-clonic seizures were associated with significantly increased risk of MCMs. The odds of MCMs were not reduced in pregnancies with periconceptional folate supplementation (Table 3).
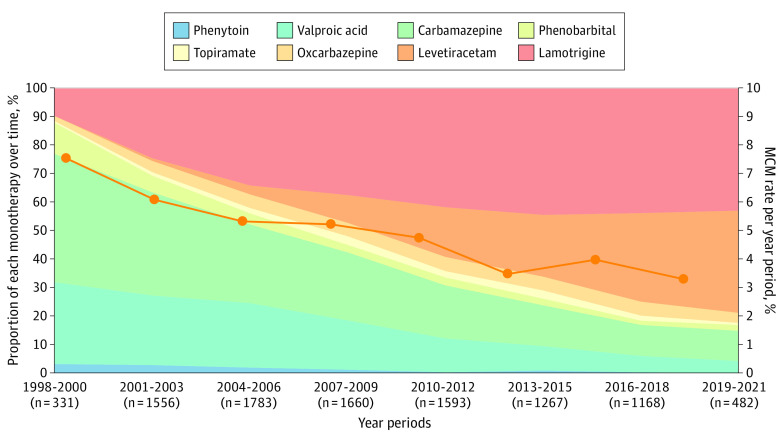
The prevalence of MCMs decreased over time from 6.1% (153 of 2505) during 1998 to 2004 to 5.1% (143 of 2825) during 2005 to 2009, 4.0% (98 of 2456) during 2010 to 2014, and 3.7% (76 of 2054) in 2015 to 2022. As shown in eTable 6 in Supplement 1, the decrease in MCM prevalence was significant at univariable logistic analysis (OR between first and last period, 0.59; 95% CI, 0.45-0.79) and at the multivariable logistic analysis, which includes only non-ASM covariates (OR between first and last period, 0.56; 95% CI, 0.42-0.75) but not after adjustment for changes in type of ASM exposure (Table 3). As shown in Figure 2, prescription patterns changed markedly over time, with a declining use of valproic acid and carbamazepine and greater use of lamotrigine and levetiracetam.
Figure 2. Proportion of Monotherapies per Year Groups (N = 9840).
Changes in exposure to different antiseizure medication (ASM) monotherapies over time in the population included in the study and corresponding prevalence (%) of major congenital malformations (MCMs).
Discussion
This updated analysis includes 2485 more prospective pregnancies (34%) than our 2018 analysis9 and adds 8 more years of follow-up for treatment changes and related risk implications.14 To our knowledge, this was the largest published cohort among epilepsy and pregnancy registries, permitting more precise estimation of the teratogenic risk associated with commonly used ASMs and other variables. The risk was lowest for levetiracetam, oxcarbazepine, and lamotrigine, and a dose dependency was identified for valproate, phenobarbital, and carbamazepine. In contrast with our earlier analysis, which included fewer lamotrigine-exposed pregnancies (2514 vs 3584 currently), we did not detect any dose dependency for lamotrigine.
Our multivariable analysis included 13 comparisons of the risk of MCMs between levetiracetam and other ASM exposures, 9 within-drug comparisons, 17 other comparisons across ASMs, and 16 comparisons with non-ASM covariates (Table 3). One important finding was that even at the lowest doses (≤650 mg per day) valproate was associated with a higher teratogenic risk compared with levetiracetam, lamotrigine, and oxcarbazepine—but not topiramate. The lowest doses of carbamazepine (≤700 mg per day) were associated with a higher risk than levetiracetam, whereas intermediate doses of carbamazepine (>700-1000 mg per day) were also associated with higher risk compared with lamotrigine and oxcarbazepine. Phenobarbital doses up to 60 mg per day were not associated with higher risk than levetiracetam, but the CIs were wide.
With respect to earlier studies, a network meta-analysis did not find increased risks with exposure to lamotrigine or levetiracetam20 nor did the Medicines and Healthcare Products Regulatory Agency UK Public Assessment Report.21 A population-based study using Nordic health databases found no increase in MCM risk with lamotrigine, levetiracetam, and oxcarbazepine but also no increased risk with carbamazepine.12 In contrast to our findings, the Nordic study found a dose-dependent increase in MCM risk with topiramate, similar to that of valproate.12 The latter study included ASM-exposed pregnancies regardless of indication, and dose estimates were derived from dispensing data rather than direct information from the treating physicians. The prevalence of MCMs after exposure to ASM monotherapies was further evaluated by a recent Cochrane review.22 Outcomes in cohort studies, such as EURAP and database-derived assessments, were evaluated separately. Of the approximately 25 000 pregnancies included in the Cochrane review, our previous report contributed 7355.9 Our current analysis of almost 10 000 pregnancies enabled 55 comparisons to be made between different ASM exposures and associations with non-ASM covariates. For comparisons made in both the current analysis and the Cochrane review (levetiracetam vs lamotrigine, levetiracetam vs oxcarbazepine, levetiracetam vs topiramate, oxcarbazepine vs lamotrigine, and lamotrigine vs topiramate) results were substantially similar.
The importance of accounting for the effect of covariates in multivariable analysis is demonstrated by our finding that parental history of MCMs was associated with a 3.4-fold increase in risk of MCMs in the offspring. This may imply a genetic predisposition to teratogenic effects of ASMs, as suggested by a higher risk of MCMs in the offspring of ASM-treated women who had a child with MCMs in a prior pregnancy while taking the same medication.23,24 Similar to earlier studies,25,26,27,28,29 we failed to identify a protective effect of folate supplementation against MCMs in ASM-exposed offspring. Periconceptional folate supplementation, however, has other benefits, including a decreased risk of preterm birth,30 improved cognitive development and verbal abilities in the offspring,31,32,33 and decreased risk of developing autistic traits postnatally.34 We did not analyze the precise doses of folate and, thus, could not contribute to the ongoing debate on optimal supplementation doses.35,36
A remarkable finding of our extended analysis, now spanning 24 years, is the continuous decline in overall prevalence of MCMs in offspring exposed to ASM monotherapies, from 6.1% in 1998 to 2004 to 3.7% (−39%) in 2015 to 2022. This decline is most likely related to a declining exposure to valproate and carbamazepine and an increased use of levetiracetam and lamotrigine (Figure 2). The fact that the decline in MCM rates was no longer significant after adjusting for changes in type of ASM exposure strongly suggests that these variables were causally related. Moreover, the categories of MCMs that declined most markedly (by at least 50% from first to last period) were neural tube defects, hypospadias, oral clefts, and polydactyly, some of which are associated to a greater extent with valproate and carbamazepine (Figure 1 and eFigure 2 in Supplement 1).
Strengths and Limitations
Strengths of our study include a large sample size collected prospectively with rigorous methodology over many years, including 1-year follow-up of the offspring and blinded outcome assessment using standardized criteria. The assessment was not based on review of medical records but on direct reporting from the physician who was in charge of the clinical treatment of the women. Further strengths are the availability of accurate information on ASM doses at time of conception, seizure control, and other covariates, such as parental history of MCMs, all of which could be included in the multivariable analysis. Comparisons were based on data collected according to a standardized protocol, unlike the Cochrane review and other meta-analyses where comparisons included heterogeneous data from different sources.
Compared with studies based on national health databases, our cohort was not population based, which is an acknowledged limitation. EURAP pregnancies are likely to be enrolled by physicians with a special interest in epilepsy and pregnancy and, as such, possibly include women with more severe epilepsies but also more specialized management. Parental race was not included as a covariate in the analysis, but only a minority of pregnancies occurred in non-White women. A further limitation is the lack of a control group of pregnancies in women with untreated epilepsy. However, the question that EURAP aims to answer is which are the safest treatment options when ASM therapy needs to be continued during pregnancy. Although not directly comparable, the Cochrane review found that the prevalence of MCMs in the offspring of women without epilepsy was 2.1% in cohort studies and 3.3% in health record studies.22 These estimates are within the range found for levetiracetam, oxcarbazepine, and lamotrigine in our study.
Conclusions
This cohort study provided essential information for physicians considering treatment options for WWE of childbearing potential, taking into consideration dose as well as type of ASM. Results suggest that exposure to levetiracetam, lamotrigine, and oxcarbazepine was associated with a low risk of MCMs and, in particular, a risk lower than that associated with the lowest dose category of valproate. We also identified a dose-dependent teratogenic risk associated with valproate, carbamazepine, and phenobarbital but not for lamotrigine. Although small numbers call for caution, the observation that a low dose of phenobarbital (≤60 mg per day) was not associated with an increased risk compared with any other ASM is particularly relevant for resource-limited settings, where phenobarbital may be the only affordable ASM. Most importantly, we observed major changes in the pattern of ASM prescription over time, resulting in a prominent decline in the overall prevalence of MCMs. Considering that there are up to 12 million WWE of childbearing potential worldwide and that additional women are exposed to ASMs for other indications,4,37,38,39 a 39% decline in the prevalence of MCMs in their offspring has major public health implications. Continued monitoring of teratogenic outcomes by registries and health databases is needed to assess the safety of prenatal exposure to newer generation ASMs. In addition to MCMs, other potential adverse consequences of prenatal ASM exposure, such as neurodevelopmental disorders,32,37,40,41 are equally important for treatment decisions and also require further investigation in future studies.
eTable 1. Exclusions for Comorbidities and Comedications Associated With Teratogenic Risks
eTable 2. Exclusions for Chromosomal or Genetic Abnormalities in Offspring
eFigure 1. Flowchart Describing Study Population and Selection Process
eTable 3. Overlapping Data Among Other Pregnancies Registries
eTable 4. Outcomes of Pregnancies Exposured to Monotherapy With Less Frequently Used ASMs
eTable 5. MCM Prevalence Among the Cases Lost to Follow-Up After Delivery
eTable 6. Tables With Time Trends in MCM Univariable and Multivariable Versions
eFigure 2. MCM Types by Time Periods
Nonauthor Collaborators. EURAP Collaborators.
Data Sharing Statement.
References
- 1.Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet. 1968;2(7581):1296. doi: 10.1016/S0140-6736(68)91781-9 [DOI] [PubMed] [Google Scholar]
- 2.Edey S, Moran N, Nashef L. SUDEP and epilepsy-related mortality in pregnancy. Epilepsia. 2014;55(7):e72-e74. doi: 10.1111/epi.12621 [DOI] [PubMed] [Google Scholar]
- 3.Lawal OD, Meador KJ, Hume AL, Wen X. Utilization of antiseizure medications in women of childbearing age with epilepsy and nonepilepsy indications: a retrospective cohort study. Neurology. 2023;101(10):e1083-e1096. doi: 10.1212/WNL.0000000000207580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madley-Dowd P, Rast J, Ahlqvist VH, et al. Trends and patterns of antiseizure medication prescribing during pregnancy between 1995 and 2018 in the UK: a cohort study. BJOG. 2024;131(1):15-25. doi: 10.1111/1471-0528.17573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomson T, Battino D, Craig J, et al. ; ILAE Commission on Therapeutic Strategies . Pregnancy registries: differences, similarities, and possible harmonization. Epilepsia. 2010;51(5):909-915. doi: 10.1111/j.1528-1167.2010.02525.x [DOI] [PubMed] [Google Scholar]
- 6.Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry. 2014;85(9):1029-1034. doi: 10.1136/jnnp-2013-306318 [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Díaz S, Smith CR, Shen A, et al. ; North American AED Pregnancy Registry; North American AED Pregnancy Registry . Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692-1699. doi: 10.1212/WNL.0b013e3182574f39 [DOI] [PubMed] [Google Scholar]
- 8.Mawhinney E, Craig J, Morrow J, et al. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80(4):400-405. doi: 10.1212/WNL.0b013e31827f0874 [DOI] [PubMed] [Google Scholar]
- 9.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530-538. doi: 10.1016/S1474-4422(18)30107-8 [DOI] [PubMed] [Google Scholar]
- 10.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP study group . Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10(7):609-617. doi: 10.1016/S1474-4422(11)70107-7 [DOI] [PubMed] [Google Scholar]
- 11.Vajda FJE, Graham JE, Hitchcock AA, Lander CM, O’Brien TJ, Eadie MJ. Antiepileptic drugs and fetal malformation: analysis of 20 years of data in a pregnancy register. Seizure. 2019;65:6-11. doi: 10.1016/j.seizure.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 12.Cohen JM, Alvestad S, Cesta CE, et al. Comparative safety of antiseizure medication monotherapy for major malformations. Ann Neurol. 2023;93(3):551-562. doi: 10.1002/ana.26561 [DOI] [PubMed] [Google Scholar]
- 13.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014;261(3):579-588. doi: 10.1007/s00415-013-7239-x [DOI] [PubMed] [Google Scholar]
- 14.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Declining malformation rates with changed antiepileptic drug prescribing: an observational study. Neurology. 2019;93(9):e831-e840. doi: 10.1212/WNL.0000000000008001 [DOI] [PubMed] [Google Scholar]
- 15.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521. doi: 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EUROCAT . EUROCAT Guide 1.3 and reference documents: instructions for the registration and surveillance of congenital anomalies: Newtownabbey EUROCAT Central Registry, 2005. Accessed September 30, 2023. https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/EUROCAT-Guide-1.3.pdf
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 18.Hsueh HM, Chen JJ, Kodell RL. Comparison of methods for estimating the number of true null hypotheses in multiplicity testing. J Biopharm Stat. 2003;13(4):675-689. doi: 10.1081/BIP-120024202 [DOI] [PubMed] [Google Scholar]
- 19.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850-857. doi: 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 20.Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95. doi: 10.1186/s12916-017-0845-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medicines & Healthcare Products Regulatory Agency . MHRA public assessment report January 2021. Accessed September 30, 2023. https://www.gov.uk/government/publications/public-assesment-report-of-antiepileptic-drugs-review-of-safety-of-use-during-pregnancy/antiepileptic-drugs-review-of-safety-of-use-during-pregnancy
- 22.Bromley R, Adab N, Bluett-Duncan M, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2023;8(8):CD010224. doi: 10.1002/14651858.CD010224.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell E, Devenney E, Morrow J, et al. Recurrence risk of congenital malformations in infants exposed to antiepileptic drugs in utero. Epilepsia. 2013;54(1):165-171. doi: 10.1111/epi.12001 [DOI] [PubMed] [Google Scholar]
- 24.Vajda FJ, O’Brien TJ, Lander CM, Graham J, Roten A, Eadie MJ. Teratogenesis in repeated pregnancies in antiepileptic drug-treated women. Epilepsia. 2013;54(1):181-186. doi: 10.1111/j.1528-1167.2012.03625.x [DOI] [PubMed] [Google Scholar]
- 25.Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10(7):e0131130. doi: 10.1371/journal.pone.0131130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meador KJ, Pennell PB, May RC, et al. ; MONEAD Investigator Group . Fetal loss and malformations in the MONEAD study of pregnant women with epilepsy. Neurology. 2020;94(14):e1502-e1511. doi: 10.1212/WNL.0000000000008687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow JI, Hunt SJ, Russell AJ, et al. Folic acid use and major congenital malformations in offspring of women with epilepsy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2009;80(5):506-511. doi: 10.1136/jnnp.2008.156109 [DOI] [PubMed] [Google Scholar]
- 28.Thomas SV, Jose M, Divakaran S, Sankara Sarma P. Malformation risk of antiepileptic drug exposure during pregnancy in women with epilepsy: results from a pregnancy registry in South India. Epilepsia. 2017;58(2):274-281. doi: 10.1111/epi.13632 [DOI] [PubMed] [Google Scholar]
- 29.Vajda FJE, O’Brien TJ, Graham JE, et al. Folic acid dose, valproate, and fetal malformations. Epilepsy Behav. 2021;114(pt A):107569. doi: 10.1016/j.yebeh.2020.107569 [DOI] [PubMed] [Google Scholar]
- 30.Alvestad S, Husebye ESN, Christensen J, et al. Folic acid and risk of preterm birth, preeclampsia, and fetal growth restriction among women with epilepsy: a prospective cohort study. Neurology. 2022;99(6):e605-e615. doi: 10.1212/WNL.0000000000200669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husebye ESN, Gilhus NE, Riedel B, Spigset O, Daltveit AK, Bjørk MH. Verbal abilities in children of mothers with epilepsy: association to maternal folate status. Neurology. 2018;91(9):e811-e821. doi: 10.1212/WNL.0000000000006073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meador KJ, Baker GA, Browning N, et al. ; NEAD Study Group . Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244-252. doi: 10.1016/S1474-4422(12)70323-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meador KJ, Pennell PB, May RC, et al. ; NEAD Investigator Group . Effects of periconceptional folate on cognition in children of women with epilepsy: NEAD study. Neurology. 2020;94(7):e729-e740. doi: 10.1212/WNL.0000000000008757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjørk M, Riedel B, Spigset O, et al. Association of folic acid supplementation during pregnancy with the risk of autistic traits in children exposed to antiepileptic drugs in utero. JAMA Neurol. 2018;75(2):160-168. doi: 10.1001/jamaneurol.2017.3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vegrim HM, Tomson T, Bjørk MH. Benefits and risks of periconceptional folic acid supplementation-Reply. JAMA Neurol. 2023;80(4):421-422. doi: 10.1001/jamaneurol.2023.0092 [DOI] [PubMed] [Google Scholar]
- 36.Voinescu PE, Pennell PB, Meador K. Benefits and risks of periconceptional folic acid supplementation. JAMA Neurol. 2023;80(4):420-421. doi: 10.1001/jamaneurol.2023.0095 [DOI] [PubMed] [Google Scholar]
- 37.Bjørk MH, Zoega H, Leinonen MK, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672-681. doi: 10.1001/jamaneurol.2022.1269 [DOI] [PubMed] [Google Scholar]
- 38.Cohen JM, Cesta CE, Furu K, et al. Prevalence trends and individual patterns of antiepileptic drug use in pregnancy 2006-2016: a study in the 5 Nordic countries, US, and Australia. Pharmacoepidemiol Drug Saf. 2020;29(8):913-922. doi: 10.1002/pds.5035 [DOI] [PubMed] [Google Scholar]
- 39.Hurault-Delarue C, Morris JK, Charlton R, et al. ; EUROmediSAFE consortium . Prescription of antiepileptic medicines including valproate in pregnant women: a study in three European countries. Pharmacoepidemiol Drug Saf. 2019;28(11):1510-1518. doi: 10.1002/pds.4897 [DOI] [PubMed] [Google Scholar]
- 40.Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696-1703. doi: 10.1001/jama.2013.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meador KJ, Cohen MJ, Loring DW, et al. ; MONEAD Investigator Group . Cognitive outcomes at age 3 years in children with fetal exposure to antiseizure medications (MONEAD study) in the USA: a prospective, observational cohort study. Lancet Neurol. 2023;22(8):712-722. doi: 10.1016/S1474-4422(23)00199-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Exclusions for Comorbidities and Comedications Associated With Teratogenic Risks
eTable 2. Exclusions for Chromosomal or Genetic Abnormalities in Offspring
eFigure 1. Flowchart Describing Study Population and Selection Process
eTable 3. Overlapping Data Among Other Pregnancies Registries
eTable 4. Outcomes of Pregnancies Exposured to Monotherapy With Less Frequently Used ASMs
eTable 5. MCM Prevalence Among the Cases Lost to Follow-Up After Delivery
eTable 6. Tables With Time Trends in MCM Univariable and Multivariable Versions
eFigure 2. MCM Types by Time Periods
Nonauthor Collaborators. EURAP Collaborators.
Data Sharing Statement.