Abstract
Purpose
To ascertain the prevalence and risk factors of gestational diabetes mellitus (GDM) in pregnant women receiving antenatal care (ANC) services within the West Nile subregion of Uganda.
Patients and Methods
An analytical cross-sectional study was conducted on 233 pregnant women who are within 24–28 weeks of gestation and are receiving ANC services in selected hospitals. GDM was diagnosed according to the World Health Organization (WHO) criteria (2013). A questionnaire and anthropometric measurements were used to obtain relevant data. The chi-square test and logistic regression were used to determine the association between GDM and the study variables, including participants’ sociodemographic and medical characteristics.
Results
The prevalence of hyperglycemia first detected in pregnancy among the participants tested was 8%. Overall, 7.45% had GDM and 0.53% had diabetes mellitus in pregnancy. The fasting plasma glucose test alone was positive in 86.7% of the GDM cases. The factors that were significantly associated with GDM included age ≥25 years (p = 0.017, AOR = 3.51) and body mass index (BMI) ≥25 kg/m2 (p = 0.024, AOR = 2.67). Out of the participants diagnosed with GDM, 28.6% did not have a known risk factor. Of the pregnant women with GDM, 57% would have been missed if the selective screening in the national clinical guidelines had been followed. Urinary tract infection (UTI) and Candida were detected in 36.36% and 13.85% of the participants, respectively.
Conclusion
The study provides new data on the prevalence of GDM in rural settings in the West Nile subregion of Uganda. Of the participants, 7.5% were diagnosed with GDM, of which 57% would have been missed based on the selective screening of the national clinical guidelines. The study findings support the universal screening of GDM in pregnant women.
Keywords: prevalence, gestational diabetes mellitus, risk factors, selective screening
Introduction
Gestational diabetes mellitus (GDM) is a type of diabetes with onset or first recognition during pregnancy, where blood glucose levels are higher than normal values (hyperglycemia) but below those diagnostic of overt diabetes.1,2 GDM occurs when less insulin is produced than the body’s needs or when the body cannot adequately use the insulin it produces due to hormonal and metabolic changes during pregnancy.3,4 GDM usually has no symptoms but nonspecific and mild manifestations, including increased frequency or urgency of micturition, increased thirst, fatigue, weight loss, and blurred vision, some of which are consistent with symptoms in normal pregnancy; hence, the recommended diagnosis is through screening rather than clinically.5
If not managed well, GDM can result in serious consequences for the affected pregnant woman and/or the unborn baby or complications later in life for the two. Complications in the pregnant woman include preeclampsia, hypertension, cesarean section, type 2 diabetes later in life, GDM in subsequent pregnancies, induced labor, and antepartum hemorrhage.6–8 Complications in the affected baby include macrosomia, large-for-gestational-age, preterm birth, stillbirth, hypoglycemia, serious breathing problems such as respiratory distress syndrome, shoulder dystocia, neonatal jaundice, obesity or type 2 diabetes later in life, cardiac diseases, and intensive care unit admissions for neonates with outcomes of prenatal mortality.8–10
The incidence of GDM varies by region; however, in general, it is more common in specific risk groups. The risk factors include increased maternal age, obesity or overweight, family history of diabetes, GDM in previous pregnancies, excessive weight gain during pregnancy, a history of stillbirth or abortion, macrosomia, or giving birth to a baby with a congenital abnormality.5,11 However, GDM has also been detected in pregnant women with no known risk factors.12–14 The pooled global standardized prevalence of GDM in a review was 14% (95% CI: 13.97–14.04%) with Africa being one of the regions with the highest prevalence, standing at 14.2% (14.0–14.4%) and the figures for low-, middle-, and high-income countries were 12.7, 9.2 and 14.2%, respectively.15 A systematic review of GDM in Africa gave a pooled prevalence of 13.6% (95% CI: 10.99–16.23%).16 These studies acknowledged limited data from African countries. A study conducted in the urban central Uganda region reported the prevalence of GDM at 30.3%,13 while in another study in Southwestern Uganda, the prevalence of hyperglycemia first detected during pregnancy was 15.64%.17
GDM is preventable and manageable through lifestyle changes such as regular physical activity and a healthy diet, and monitoring blood glucose. Additionally, pharmaceutical interventions such as metformin or insulin can be used where necessary for those who test positive.18 However, for effective management and prevention of adverse outcomes, prompt diagnosis of GDM in pregnancy is needed. The diagnosis of GDM according to the current World Health Organization (WHO) criteria is the one-step oral glucose tolerance test (OGTT), where fasting plasma glucose is measured following 8–14 hours of overnight fasting and then plasma glucose is measured after two (2) hours of administering 75 g of glucose orally.19 The Ugandan clinical guidelines state that pregnant women with a set of selected or predefined risk factors should be screened for GDM; however, this is not routinely done in public health facilities, including the current study sites, as stipulated by the national guidelines.20 In a few instances, screening is done case-by-case based on the clinician’s judgment, and a random glucose test is used instead. Due to inconsistent and selective testing of GDM, the actual epidemiology of GDM is not known in the study region or the entire country. The WHO recommends research on the prevalence of GDM based on the current diagnostic criteria.19 This study therefore aimed to determine the prevalence of GDM, with inclusive diagnosis and the associated risk factors, in pregnant women attending antenatal care services within the West Nile subregion, Uganda.
Materials and Methods
Study Design and Setting
This quantitative cross-sectional study was conducted in the West Nile subregion of Northwestern Uganda at two major health facilities. The study sites included Arua Regional Referral Hospital (ARRH) and Oli Health Center IV. ARRH is located in Arua city with (3.0303° N, 30.9073° E) coordinates, approximately 498 km by road northwest of the capital city, Kampala, and it is a regional public hospital receiving referrals from the districts in the region and also receiving patients from the neighboring countries of South Sudan and the Democratic Republic of Congo. Oli Health Center IV is a lower-level public health facility, which is the largest in that category in the region, and serves the local population of the Arua Central Division in Arua city with approximately 71,900 people. The West Nile subregion has a predominantly rural population of over three (3) million people, and this region contributes to 2.2% of the national GDP. The West Nile subregion is a host community for refugees from neighboring countries.
Study Population
The target population included all pregnant women seeking ANC services in the regional referral hospital and Oli Health Center IV, and the study included all those who were within 24–28 weeks of gestation and willingly consented to participate in the study. The exclusion criteria included pregnant women who were severely ill due to other disease conditions or unconscious to give a response to complete our questionnaire, and those who were known to have diabetes mellitus.
Sampling Method and Estimation
A total of 233 participants were enrolled in the study from August 2022 to October 2022, using convenience sampling. The formula Z2 × p(1-p)/e2 was used to estimate the sample size.21 Where Z is the statistics corresponding to the 95% confidence interval, p is the expected proportion, taken to be 15.64%17 and e is the margin of error equal to 0.05.
Data Collection
All pregnant women attending antenatal services at the ARRH and Oli Health Center IV were screened for eligibility for enrolment in the study. Informed consent was sought from those found eligible. For participants who consented to participate in the study, independent variable data consisting of the demographic and behavioral characteristics and vital physical measurements were collected on the day of enrolment, while blood samples were collected on enrolment day or second visit for laboratory analysis. Clean-catch midstream urine samples were also collected from the participants on the day of enrolment. Trained research assistants were used for data collection.
Demographic and Medical Characteristics
Upon obtaining consent, the first step was the collection of data on demographic, behavioral, or medical characteristics through face-to-face interviews using a researcher-administered semi-structured and pretested questionnaire. Each participant was asked about their age, educational background, date of last normal menstrual period, gravidity, occupation status, physical activity, history of raised pressure, family history of diabetes, alcohol consumption, smoking habits, history of macrosomia, history of miscarriage or stillbirth, and previous GDM. This information was obtained from the participants, their antenatal cards, and the hospital registers after obtaining ethical and administrative approval.
Anthropometric Measurements
The second step included physical measurements, such as height and weight, required to determine body mass index (BMI). Weight in kilograms divided by height in meters squared yielded the BMI. In addition, a measurement of blood pressure was taken.
Laboratory Measurements
After obtaining the demographics and physical measurements, participants were asked on the day of enrolment if they had an overnight fast of 8–12 hours (those reporting to the antenatal clinic minus taking breakfast). Those who did not have an overnight fast on the day of enrolment were requested to return within three days (and instructed not to take breakfast on the day of return) while continuing with their routine duties. A whole blood sample was collected from each participant in a gray-topped fluoride-oxalated vacutainer tube by venipuncture after the overnight fast (8–12 hours). The fasting whole blood samples collected from the participants were centrifuged for 5 minutes at 5000 rpm within 30 minutes of collection to obtain plasma. Fasting plasma glucose level was measured spectrophotometrically using a Cobas C 111 analyzer (Roche Diagnostics, Germany) at the Oak Diagnostic Center, Arua City, which is located next to ARRH. After collecting the fasting blood sample from each participant, a two (2) hour oral glucose tolerance test was performed according to the recent WHO guidelines19 as follows: a) Participants were given a lemon-flavored solution of glucose (75 g of glucose in 300 mL of water) to drink and were asked to relax for 2 hours without ingesting any feed. b) After two hours, a whole blood sample was collected from each participant in a gray-topped fluoride-oxalated vacutainer tube, processed, and plasma glucose measured as described above. Routine quality control was assessed using the glucose controls from the manufacturer.
The plasma glucose level was interpreted according the WHO guidelines as follows;
I. Normoglycemic
<5.1 mmol/l (92 mg/dl) for fasting plasma glucose and/or <8.5 mmol/l (153 mg/dl) for the 2-hour OGTT plasma glucose.
II. Gestational diabetes mellitus
5.1–6.9 mmol/l (92 −125 mg/dl) for fasting plasma glucose and/or 8.5–11.0 mmol/l (153 −199 mg/dl) for the 2-hour OGTT plasma glucose.
III. Diabetes mellitus in pregnancy
≥7.0 mmol/l (126 mg/dl) for fasting plasma glucose and/or ≥11.1 mmol/l (200 mg/dl) for the 2-hour OGTT plasma glucose.
Urinalysis, including microscopy (with 50µL of uncentrifuged urine) and semiquantitative urine chemistry analysis using 10-parameter urine strips (Cypress Diagnostics, Belgium), was performed immediately after urine sample collection. The analytes in urine chemistry included protein and glucose as potential risk markers and other components in urine, such as leukocyte esterase, nitrites, blood, ketones, urobilinogen, bilirubin, specific gravity, and pH. Results were recorded. Urinary tract infection (UTI) was considered when there was significant pyuria (≥3 pus cells per high-power field) and a positive Gram staining result on uncentrifuged urine in conjunction with findings of leukocyte esterase, nitrites, proteinuria, or hematuria, which were consistent with the reported acute signs and symptoms of UTI.20,22
Data Management and Analysis
The questionnaires were coded for reference and double-checked to ensure complete and correct responses were obtained. Access to the collected data was restricted, and after data entry into Excel, it was imported into STATA version 14, which was used for the analysis. The data were displayed using graphs, pie charts, and tables. Means (±standard deviation), modes, and frequencies were used to describe numeric variables, whereas frequencies and percentages were used to describe categorical variables. The prevalence of GDM is presented as the proportion of those who tested positive out of the total number of participants tested. Bivariate and multivariate logistic regression, as well as the chi-square test, were used to analyze the risk factors associated with GDM. An increased association between independent and dependent variables was indicated by higher odds ratio (>1), whereas p-value of <0.05, at 95% confidence interval, was used to determine the statistical significance.
Ethics Approval and Informed Consent
The study was approved by a local research ethics committee (REC), Lacor Hospital REC (Ref number: Lacor-2022-99), which is the nearest REC in the region. Administrative clearance was obtained from the different study sites. This study was performed with strict adherence to the approved protocol. Written or thumb-printed informed consent was obtained from all participants. Pregnant mothers 15–17 years old were considered emancipated minors who could consent for themselves to a study that would benefit them, in accordance with the national guidelines for research ethics. Participation was voluntary and willing, and confidentiality was maintained. The results of plasma glucose measurements were provided to the attending clinician for the management of the patient, and the principal investigator with support from the site clinicians provided treatment for those diagnosed with UTI and Candida. The ethical guidelines and regulations of the Declaration of Helsinki were followed when conducting the study.
Results
Participants’ Socio-Demographic and Medical Characteristic
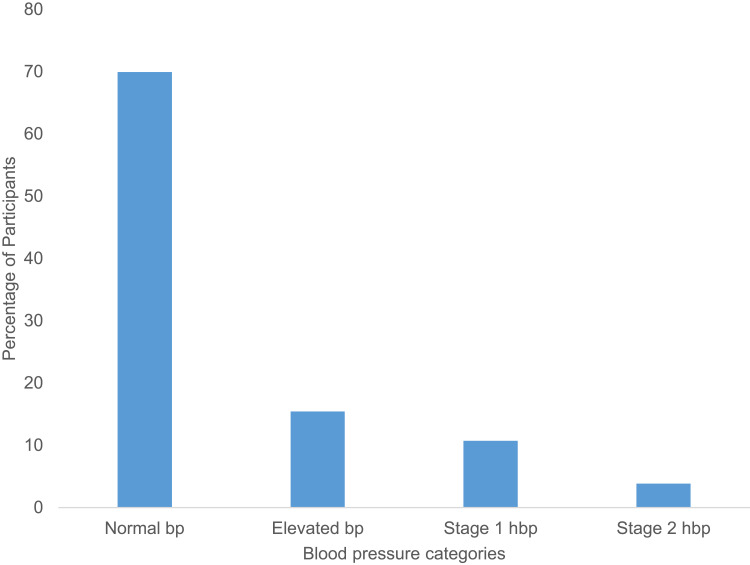
A total of 233 participants were recruited for this study after providing consent, and all participants reported for the first study visit. Table 1 presents the sociodemographic and medical characteristics of the study participants. The participants’ ages ranged from 15 to 39 years old with mean of 25.09+5.196, whereas only 38.63% were employed. Majority of the participants (57.94%) had primary education as the highest level of education. Participants’ BMI ranged from 16.60 to 39.14 Kg/m2 with mean of 24.85+4.12. Most of the pregnant women (70.82%) were multiparous. More than half (55.36%) of the participants had at least 30 minutes of exercise and substance use was observed in 6.01% of the participants, of which the common one was alcohol (Table 1) (see Supplementary Table 1). Macrosomia and abortion in the previous pregnancy were reported by 18.03% and 16.31% of participants, respectively. Only one (1) participant had previous GDM, and 29.61% had relatives with diabetes mellitus (of which 43.48% were first-degree relatives). Excessive weight gain just before pregnancy was reported by 32.62% of the pregnant women (Table 1). Majority of the participants (69.96%) had normal blood pressure (Figure 1).
Table 1.
Socio-Demographic and Medical Characteristics of the Study Participants
| Variable | Category | Frequency (n=233) | Percentage (%) |
|---|---|---|---|
| Maternal Age (years) | 15–19 | 33 | 14.16 |
| 20–24 | 79 | 33.91 | |
| 25–29 | 73 | 31.33 | |
| 30–34 | 37 | 15.88 | |
| 35–39 | 11 | 4.72 | |
| Education level | None | 6 | 2.58 |
| Primary | 135 | 57.94 | |
| Secondary | 70 | 30.04 | |
| Tertiary | 22 | 9.44 | |
| Working status | Employed | 90 | 38.63 |
| Housewife | 143 | 61.37 | |
| BMI (Kg/m2) | <25 | 138 | 59.23 |
| ≥25 | 95 | 40.77 | |
| Gravidity | Prime gravida | 68 | 29.18 |
| Multiparous | 165 | 70.82 | |
| <3 | 118 | 50.64 | |
| ≥3 | 115 | 49.36 | |
| Physical activity (≥30 minutes daily) | Yes | 129 | 55.36 |
| No | 104 | 44.64 | |
| Social history of substance use | Yes | 14 | 6.01 |
| No | 219 | 93.99 | |
| Macrosomia | Yes | 42 | 18.03 |
| No | 191 | 81.97 | |
| Previous abortion | Yes | 38 | 16.31 |
| No | 195 | 83.69 | |
| History of gestational diabetes | Yes | 1 | 0.43 |
| No | 158 | 67.81 | |
| Do not know | 74 | 31.76 | |
| Family history of diabetes | Yes | 69 (30 first degree) | 29.61 |
| No | 107 | 45.92 | |
| Do not know | 57 | 24.46 | |
| Excessive weight gain just before pregnancy | Yes | 76 | 32.62 |
| No | 134 | 57.51 | |
| Do not know | 23 | 9.87 |
Abbreviation: BMI, body mass index.
Figure 1.
A bar graph showing the variation in blood pressure of the study participants.
Notes: Proportion of the pregnant mothers in the study with the four categories of blood pressure (normal blood pressure, elevated blood pressure, stage 1 and stage 2 hypertension) expressed as a percentage of total number of participants. Key; bp, blood pressure and hbp, hypertension.
Prevalence of Gestational Diabetes Among Pregnant Women Receiving Antenatal Care Services Within Arua City
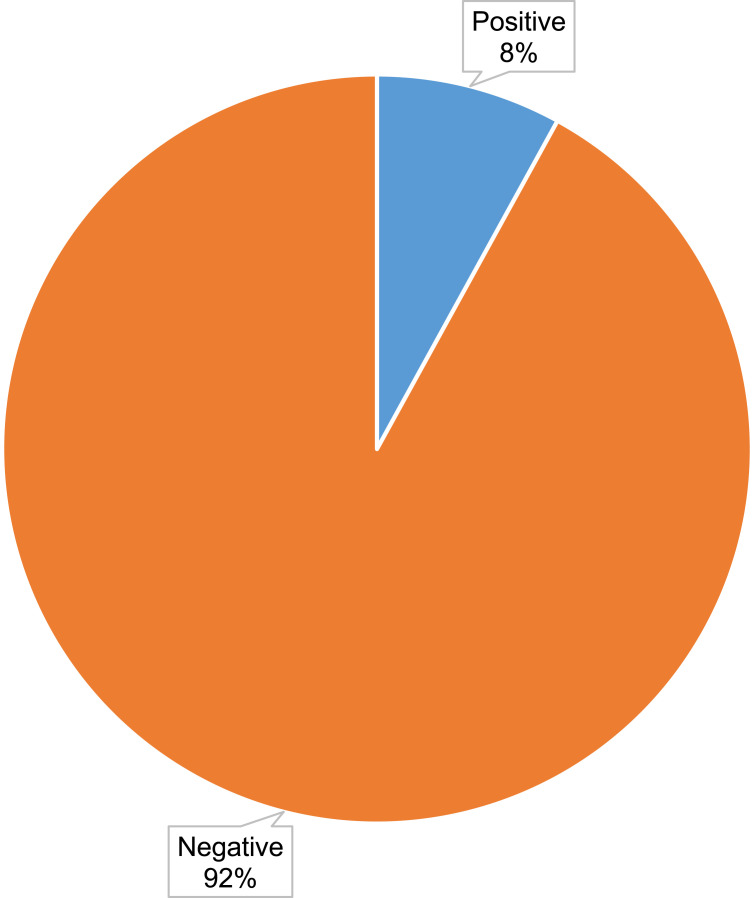
Of the 233 enrolled participants, urine samples were collected from 231 on the first visit, with urine examinations performed on all the samples collected, while 188 participants reported for the oral glucose tolerance test (OGTT) on the second visit. The mean fasting plasma glucose was 4.38±0.51, whereas that of the 2-hour OGTT plasma glucose was 5.26±1.11. Out of the 188 participants, 15 (8%) had hyperglycemia first detected during pregnancy (Figure 2), and in 13 (86.7%) of these cases, only the fasting plasma glucose test is positive. Of the 15, one (1) met the WHO criteria for diabetes mellitus in pregnancy (WHO, 2013), while the remaining 14 were GDM cases, making 7.45% of the total. With reference to the selective screening of the Uganda national clinical guidelines based on selected risk factors, 57.14% of the participants diagnosed with GDM in our study would have been missed, and the prevalence of GDM would be 3.19% instead of 7.45%.
Figure 2.
A pie chart showing the prevalence of hyperglycemia first detected in pregnancy among the study participants.
Notes: A percentage representation of the pregnant women in the study with or without hyperglycemia first detected in pregnancy. Key; blue section, participants who tested positive for hyperglycemia and orange section, participants who tested negative for hyperglycemia.
The results of urine glucose tests indicated that all the participants diagnosed with GDM had normal glucose levels, while only one (1) participant with normal plasma glucose results had trace levels of glucose. No analysis was performed for urine glucose as a potential risk marker. Of the 231 urine samples analyzed, 84 were found to have UTI, with a prevalence of 36.36%, while 32 had Candida, with a prevalence of Candida as 13.85%.
The Risk Factors Associated with Gestational Diabetes Mellitus
Bivariate logistic regression was performed to assess factors associated with hyperglycemia first detected in pregnancy among the participants. The assessed factors included age, education, work status, BMI, gravidity, physical activity, and macrosomia in previous pregnancies (Table 2). Some variables that were not significant were not included. Pregnant women aged 25 years and above were 4.3 times more likely to develop hyperglycemia with onset in pregnancy than those below 25 years, and this was statistically significant (p-value = 0.017). Those pregnant women with BMI ≥25 Kg/m2 were more likely (OR 3.41, 95% CI 1.11–10.41) to get GDM than those with BMI below 25 Kg/m2 (p-value = 0.024). Education level, working status, gravidity, physical activity, and macrosomia in previous pregnancies were not associated with hyperglycemia first detected in pregnancy among the study participants. Of the participants with GDM, 28.57% did not have any of the known risk factors for GDM.
Table 2.
Summary of Bivariate Logistic Regression Analysis of Risk Factors Associated with Gestational Diabetes, n=188
| Variable | Hyperglycemic | Normoglycemic | Chi-Square | p-value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Age | |||||
| 15–24 | 3 | 90 | 5.66 | 0.017 | Ref |
| 25–39 | 12 | 83 | 4.34 (1.18–15.91) | ||
| Education level | |||||
| None | 0 | 4 | 0.95 | 0.813 | Ref |
| Primary | 8 | 98 | 1.47 (0.17–12.47) | ||
| Secondary | 6 | 53 | 2.04 (0.23–18.09) | ||
| Tertiary | 1 | 18 | 1 (omitted) | ||
| Working status | |||||
| Housewife | 6 | 106 | 2.5934 | 0.107 | Ref |
| Employed | 9 | 67 | 2.37 (0.81–6.97) | ||
| BMI | |||||
| <25 | 5 | 109 | 5.0917 | 0.024 | Ref |
| ≥25 | 10 | 64 | 3.41 (1.11–10.41) | ||
| Gravidity | |||||
| Prime gravida | 4 | 54 | 0.1338 | 0.715 | Ref |
| Multiparous | 11 | 119 | 0.80 (0.24–2.63) | ||
| Physical activity | |||||
| Yes | 5 | 99 | 3.1878 | 0.074 | Ref |
| No | 10 | 74 | 0.37 (0.12–1.14) | ||
| Macrosomia | |||||
| No | 11 | 144 | 0.9355 | 0.333 | Ref |
| Yes | 4 | 29 | 1.81 (0.54–6.07) | ||
Abbreviations: BMI, body mass index; Ref, reference; CI, confidence interval.
In multivariate analysis, neither age nor BMI had an effect modification on each other. The adjusted odds ratio differed from the crude ratio by more than 10%, and the two variables confound each other (Table 3). Age greater than or equal to 25 years and BMI ≥25 are risk factors for gestational diabetes mellitus.
Table 3.
Adjusted Odds Ratio in Multiple Logistic Regression Analysis
| Variable | Crude Odds Ratio | Adjusted Odds Ratio |
|---|---|---|
| Age | ||
| 15–24 | Ref | Ref |
| 25–39 | 4.34 | 3.51 (95% CI 0.93–13.23) |
| BMI | ||
| <25 | Ref | Ref |
| ≥25 | 3.41 | 2.67 (95% CI 0.85–8.4) |
Abbreviations: BMI, body mass index; Ref, reference; CI, confidence interval.
Pregnancy Complications Associated with Gestational Diabetes Mellitus
Pregnancy complications observed in the study were assessed as outcomes of GDM. UTI, Candida, and raised blood pressure (composed of elevated blood pressure, stage 1 and stage 2 high blood pressure as presented in Figure 1) were compared in participants with or without hyperglycemia first detected in pregnancy. Pregnant women diagnosed with hyperglycemia were 2.57 times more likely to develop UTI compared to those who were normoglycemic; however, this was not statistically significant (95% CI 0.87–7.56, χ2 = 3.12, p-value=0.077). Gestational diabetes mellitus was not associated with increased chances of raised blood pressure or Candida infections during pregnancy.
Discussion
In the current study, the prevalence of hyperglycemia with onset in pregnancy was 8%, and the overall prevalence of gestational diabetes mellitus was 7.45%. The prevalence of GDM in this study is in agreement with a systematic review of GDM in sub-Saharan Africa, with a pooled prevalence of 9% (95% CI, 7–12%).11 However, the prevalence of GDM in the present study was lower compared to the 14.0% pooled global standardized prevalence of GDM as well as the 14.2% in Africa,15 and similarly for the 13.6% pooled prevalence in Africa from another systematic review.16 These systematic reviews and meta-analyses have acknowledged limited data from sub-Saharan countries. Studies in East Africa showed varying results; for example, a study in Rwanda had a contrastingly lower prevalence of 3.2%,23 while in Tanzania, a prevalence of 27.5% was noted in the urban Dodoma region24 but a lower prevalence of 4.3% in a rural region of Tanzania.25 Similar studies in Uganda found a 30.3% prevalence of GDM in Central Uganda13 and a 15.64% prevalence of hyperglycemia with onset in pregnancy in Western Uganda.17 The differences could be due to variations in study populations’ socio-demographic characteristics, like lifestyle and age, genetic factors, and socio-economic status. The study region of the West Nile subregion has a predominantly rural population, contributing only 2.2% of the national GDP. Urban regions have an increased likelihood of risks like obesity, a sedentary lifestyle or diabetes (relatives). Notably, the mean BMI in the current study was lower than that in the two studies conducted in Uganda.
This study aimed to diagnose GD in all consenting pregnant women in order to determine its prevalence since it is not a routine procedure in health facilities in Uganda. Accordingly, if the selective screening was performed instead based on the indications (selected risk factors) as per the national clinical guidelines,20 57.14% of the participants diagnosed with GD in the current study would be missed, lowering the prevalence to 3.19%. This is mainly due to the selectiveness, but also because some cases of GDM have no known risk factors (discussed later).
The variables studied as risk factors included maternal age, education level, working status, BMI, gravidity, GDM in previous pregnancy, physical activity, macrosomia in previous pregnancy, substance use, history of abortion or stillbirth, and family history of diabetes mellitus. In the current study, maternal age ≥25 years and BMI ≥25 kg/m2 were found to be significantly associated with GDM (adjusted OR 3.51 and 2.67, respectively), while other variables were not. A systematic review in sub-Saharan Africa showed similar findings where age greater than 25 and BMI greater than 25 were associated with GDM.11 In a systematic review that assessed studies all over the globe, increasing maternal age was found to be associated with GDM, where the risk increased with successive age groups,26 while Mdoe et al, 2021 found that age above 35 years was significantly associated with GDM (AOR, 3.1).24 Studies in Africa have demonstrated that being overweight or BMI ≥ 25 is a risk factor for GDM,16,25,27 while a systemic study in Asia concurred with the fact that BMI ≥ 25 was associated with GDM.28 The association between maternal age and GDM can be explained by the decrease in sex hormone-binding globulin with age, which has been linked to an increase in insulin resistance and therefore elevated blood glucose levels.29,30 Meanwhile, the relationship between GDM and BMI ≥ 25 can be attributed to overweight women having high levels of fat, which is thought to increase insulin resistance and consequently raise glucose levels in the blood.4 Other variables, including macrosomia, family history of diabetes mellitus, GDM in previous pregnancy, physical inactivity, substance use, and history of stillbirth or abortion, were not significantly associated with GDM in the present study, but at least two or more were proven to be risk factors in various studies.11,16,24,27 There is possible variation in the population studied, as discussed above. In the current study, 28.57% of participants with GDM did not have any of the known risk factors for GD. A similar observation was noted in a study in Uganda, where 23.8% had no known risk factor,13 while a study in France reported a much lower number (16.7%) with no risk factors because a set of preselected risk factors was used.12 The findings in the present study do not support the current practice of selective screening but rather encourage universal screening.
Pregnancy complications such as UTI, Candida, and hypertension have been studied as outcomes of GDM. In the present study, the risk of UTI was 2.57 times higher for hyperglycemic pregnant women than for those who were normoglycemic; however, the difference was not statistically significant. A study in South India showed that pregnant women with GDM were three (3) times more likely to develop UTI than their counterparts who did not have GDM (P = 0.051, OR = 3.2),31 which was consistent with a systemic review that showed similar results where there was a significant association between GDM and UTI but not vaginal candidiasis.32 The explanation for this could be that gestational diabetes compromises the immune system, predisposing it to infections such as UTI.33,34
One of our limitations was the lack of funding, as we were unable to follow our participants to study maternal and newborn outcomes. The study was conducted in a hospital setting; however, the participants visited the hospitals on scheduled days, and the two health facilities have a big catchment area serving the local population and the entire region, which is a representation of the community. Data on pre-pregnancy BMI were not available, as this is not a routine practice in our setting, and we used BMI at the time of the visit.
Conclusion
This study provides new data on the prevalence of GDM in rural settings in the West Nile subregion of Uganda. The study found that the prevalence of hyperglycemia with onset in pregnancy was 8%, with an overall prevalence of GDM of 7.45%. Maternal age of ≥25 years and BMI ≥25 kg/m2 were significantly associated with GDM, and 28.6% of pregnant women with GDM had no known risk factors, whereas 57% of those diagnosed with GDM would have been missed according to the selective screening of national clinical guidelines. The findings support the universal screening of pregnant women within gestation weeks of 24–28 for GDM during antenatal care.
Acknowledgments
Our heartfelt gratitude goes to the pregnant mothers who volunteered to participate in this study. We are grateful to the antenatal clinic and maternity ward staff at Oli Health Center IV and Arua Regional Referral Hospital for their support in this study. We thank the Oak Diagnostic Center for their support during the sample analysis. The abstract of this paper was presented at the annual Muni research dissemination symposium and the biennial conference of the African Society for Laboratory Medicine (ASLM 2023) as a poster presentation.
Abbreviations
ANC, antenatal care; AOR, adjusted odds ratio; ARRH, Arua Regional Referral Hospital; BMI, body mass index; GD, gestational diabetes; GDP, gross domestic product; OGTT, oral glucose tolerance test; UTI, urinary tract infection; WHO, World Health Organization; BMI, body mass index; Ref, reference; CI, confidence interval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.World Health Organization. Classification of Diabetes Mellitus; 2019. [Google Scholar]
- 2.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement_1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 3.Baz B, Riveline JP, Gautier JF. Endocrinology of pregnancy: gestational diabetes mellitus: definition, aetiological and clinical aspects. Eur J Endocrinol. 2016;174(2):R43–R51. doi: 10.1530/EJE-15-0378 [DOI] [PubMed] [Google Scholar]
- 4.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342. doi: 10.3390/ijms19113342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Global Report on Diabetes; 2016. Available from: https://www.who.int/publications/i/item/9789241565257. Accessed March 7, 2024.
- 6.Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: global comparisons. Int J Womens Health. 2011;3:367. doi: 10.2147/IJWH.S26094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sreelakshmi PR, Nair S, Soman B, Alex R, Vijayakumar K, Kutty VR. Maternal and neonatal outcomes of gestational diabetes: a retrospective cohort study from Southern India. J Family Med Prim Care. 2015;4(3):395. doi: 10.4103/2249-4863.161331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377. doi: 10.1136/BMJ-2021-067946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray SR, Reynolds RM. Short‐and long‐term outcomes of gestational diabetes and its treatment on fetal development. Prenat Diagn. 2020;40(9):1085–1091. doi: 10.1002/pd.5768 [DOI] [PubMed] [Google Scholar]
- 10.Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes-a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12(1):1–13. doi: 10.1186/1471-2393-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natamba BK, Namara AA, Nyirenda MJ. Burden, risk factors and maternal and offspring outcomes of gestational diabetes mellitus (GDM) in sub-Saharan Africa (SSA): a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):1–11. doi: 10.1186/s12884-019-2593-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosson E, Benbara A, Pharisien I, et al. Diagnostic and Prognostic Performances Over 9 Years of a Selective Screening Strategy for Gestational Diabetes Mellitus in a Cohort of 18,775 Subjects. Diabetes Care. 2013;36(3):598–603. doi: 10.2337/DC12-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakabuye B, Bahendeka S, Byaruhanga R. Prevalence of hyperglycaemia first detected during pregnancy and subsequent obstetric outcomes at St. Francis Hospital Nsambya. BMC Res Notes. 2017;10(1):1–10. doi: 10.1186/s13104-017-2493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polska E, Katarzyna Cypryk A, Cypryk K, et al. Gestational diabetes mellitus - an analysis of risk factors. Endokrynol Pol. 2008;59(5):393–397. [PubMed] [Google Scholar]
- 15.Wang H, Li N, Chivese T, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabet Res Clin Pract. 2022;183:109050. doi: 10.1016/j.diabres.2021.109050 [DOI] [PubMed] [Google Scholar]
- 16.Muche AA, Olayemi OO, Gete YK. Prevalence and determinants of gestational diabetes mellitus in Africa based on the updated international diagnostic criteria: a systematic review and meta-analysis. Arch Public Health. 2019;77(1):1–20. doi: 10.1186/s13690-019-0362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiiza F, Kayibanda D, Tumushabe P, et al. Frequency and Factors Associated with Hyperglycaemia First Detected during Pregnancy at Itojo General Hospital, South Western Uganda: a Cross-Sectional Study. J Diabetes Res. 2020;2020:1–9. doi: 10.1155/2020/4860958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Association AD. Standards of Care in Diabetes—2023 Abridged for Primary Care Providers. Clin Diabetes. 2023;41(1):4–31. doi: 10.2337/CD23-AS01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization WH. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. World Health Organization. 2013. [PubMed] [Google Scholar]
- 20.Ministry of Health Uganda. Uganda Clinical Guidelines 2016: national Guidelines for Management of Common Condtions. Ministry of Health, Uganda. 2016;1–1142. [Google Scholar]
- 21.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121–126. doi: 10.4103/0253-7176.116232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health England. UK Standards for Microbiology Investigations: investigation of Urine; 2019. Available from: https://www.gov.uk/uk-standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical-laboratories. Accessed June 27, 2023.
- 23.Meharry PM, Tengera O, Rulisa S, et al. Prevalence of gestational diabetes mellitus among women attending antenatal care at public health centers in Rwanda. Diabet Res Clin Pract. 2019;151:252–259. doi: 10.1016/j.diabres.2019.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mdoe MB, Kibusi SM, Munyogwa MJ, Ernest AI. Prevalence and predictors of gestational diabetes mellitus among pregnant women attending antenatal clinic in Dodoma region, Tanzania: an analytical cross-sectional study. BMJ Nutr Prev Health. 2021;4(1):69. doi: 10.1136/bmjnph-2020-000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mghanga F, Maduhu E, Nyawale H. Prevalence and associated factors of gestational diabetes mellitus among rural pregnant women in southern Tanzania. Ghana Med J. 2020;54(2):82–87. doi: 10.4314/gmj.v54i2.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Ren X, He L, Li J, Zhang S, Chen W. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabet Res Clin Pract. 2020;162:108044. doi: 10.1016/J.DIABRES.2020.108044 [DOI] [PubMed] [Google Scholar]
- 27.Beyene FY, Kassa BG, Mihretie GN, Ayele AD. Gestational diabetes mellitus and its associated factors in Ethiopia: a systematic review and meta-analysis. Eur J Med Res. 2023;28(1):1–14. doi: 10.1186/S40001-023-01088-5/FIGURES/11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):1–20. doi: 10.1186/S12884-018-2131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottarsdottir K, Hellgren M, Bock D, Nilsson AG, Daka B. Longitudinal associations between sex hormone-binding globulin and insulin resistance. Endocr Connect. 2020;9(5):418–425. doi: 10.1530/EC-20-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggio M, Lauretani F, Basaria S, et al. Sex hormone binding globulin levels across the adult lifespan in women - The role of body mass index and fasting insulin. J Endocrinol Invest. 2008;31(7):597. doi: 10.1007/BF03345608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhat M, Ramesha KN, Sarma SP, Menon S, Sowmini CV, Ganesh Kumar S. Determinants of gestational diabetes mellitus: a case control study in a district tertiary care hospital in south India. Int J Diabetes Dev Ctries. 2010;30(2):91. doi: 10.4103/0973-3930.62599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yefet E, Bejerano A, Iskander R, Kimhi TZ, Nachum Z. The Association between Gestational Diabetes Mellitus and Infections in Pregnancy—Systematic Review and Meta-Analysis. Microorganisms. 2023;11(8):1956. doi: 10.3390/MICROORGANISMS11081956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McElwain CJ, McCarthy FP, McCarthy CM. Gestational Diabetes Mellitus and Maternal Immune Dysregulation: what We Know So Far. Int J Mol Sci. 2021;22(8):4261. doi: 10.3390/IJMS22084261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoikou M, Grimolizzi F, Giaglis S, et al. Gestational diabetes mellitus is associated with altered neutrophil activity. Front Immunol. 2017:8(JUN):248780. doi: 10.3389/FIMMU.2017.00702/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]