Abstract
Background/Objective.
Following aneurysmal subarachnoid hemorrhage (SAH), patients are monitored closely for vasospasm in the intensive care unit (ICU). Conditional vasospasm-free survival describes risk of future vasospasm as a function of time elapsed without vasospasm. Conditional survival has not been applied to this clinical scenario, but could improve patient counseling and ICU utilization. The objective of this study was to characterize conditional vasospasm-free survival following SAH.
Methods.
This was a single institution retrospective cohort study of patients treated for aneurysmal SAH between 1/1/2000–6/1/2020. The primary outcome was the development of vasospasm defined by the first instance of either: (1) radiographic vasospasm on computed tomography angiography, (2) Lindegaard Index >3.0 by transcranial doppler ultrasonography (TCD) or (3) vasospasm-specific intra-arterial therapy. Multivariable Cox regression was performed and conditional vasospasm-free survival curves were constructed.
Results.
528 patients were treated for aneurysmal SAH and 309 (58.5%) developed vasospasm. Conditional survival curves suggest patients that survive to post-bleed day 10 without vasospasm have nearly 90% chance of being discharged without vasospasm. The median onset of vasospasm was post-bleed day 6. Age >50 years, was associated with lower risk (HR=.76, 95%CI 0.64–0.91; p<0.001). Higher initial systolic blood pressure (HR=1.18, 95%CI:1.046–1.350, p=.008), Hunt-Hess grades 4 or 5 (HR=1.304, 95%CI: 1.014–1.676) as well as modified Fisher scale 4 (HR=1.808, 95%CI: 1.198–2.728) were associated with higher vasospasm than the respective lower grades.
Conclusions.
Conditional survival provides a useful framework for counseling patients and making decisions around vasospasm-risk for patients with aneurysmal SAH, while risk factor-stratified plots facilitate a patient-centric evidence-based approach to these conversations and decisions.
MESH terms: survival analysis, intensive care units, intracranial vasospasm, subarachnoid hemorrhage
Introduction
An estimated 70% of patients with aneurysmal subarachnoid hemorrhage (SAH) experience angiographic cerebral vasospasm, and 25% experience clinically symptomatic vasospasm.[1] Vasospasm almost exclusively occurs within the window of 3–14 days after the aneurysm rupture, with a peak at 7–8 days; accordingly, these patients may be hospitalized in the intensive care unit for weeks at a time.[2]
Multiple clinical factors are weakly associated with increased risk of vasospasm, but the time-dependent nature of vasospasm risk has not been rigorously re-assessed since it was originally described by Weir[2] in 1978 and later characterized in a large patient cohort with the International Cooperative Study in 1990.[3,4] Thus, no prognostic scoring systems exist to estimate the vasospasm risk for a patient who has been monitored in an ICU, given that he or she has remained vasospasm-free for any number of days. This type of analysis--known as conditional event-free survival analysis[5]--could allow clinicians to identify low-risk SAH patients who may safely be transferred out of the ICU earlier in the vasospasm window, given that they have not experienced vasospasm up to that point in the hospitalization. Such a tool could reduce ICU utilization, result in cost savings, improve discharge planning, and better inform family discussions about prognosis throughout the duration of the disease course.
We aimed to characterize the conditional vasospasm-free survival curve following aneurysmal subarachnoid hemorrhage. We hypothesized that the conditional probability of vasospasm-free survival increases gradually throughout hospitalization after aSAH, and that clinical characteristics are associated with differing rates of conditional survival.
Methods
Study Design
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author. We designed a retrospective cohort study utilizing patient level data queried from an institutional, fully de-identified data warehouse derived from the electronic medical record.[6] The records are anonymized and date-shifted removing all patient identifiers such that studies using this data are provided an exemption from the local institutional review board (IRB #171624) and the requirement of patient consent is waived. Investigators are only granted access to the portion of the database population which meets the pre-specified inclusion criteria.
Study Population
Included patients were those treated between January 1, 2000 and June 1, 2020 who had a diagnosis of cerebral, aneurysmal SAH determined by ICD-10 codes I60.0 - I60.9 and I67.848, or the associated ICD-9 code 430 for patients treated prior to October 2017. Exclusion criteria were (1) patients admitted with SAH after elective aneurysm surgery and intraoperative aneurysm rupture, (2) no objective evidence of SAH, or (3) no documented radiographic evidence of aneurysm. A sample size was not set a priori.
Clinical Management
Initial Management
Patients presenting to our institution with SAH are evaluated initially in the emergency department or in the neurologic intensive care unit (ICU), in the case of direct transfers from outside medical centers. Initial radiographic evaluation is performed with non-contrast computed tomography of the head (CTH) as well as a CT angiogram of the head and neck. The patient is admitted to the ICU for serial neurologic exams. Those patients with clear radiographic and clinical hydrocephalus as well as those categorized as Hunt-Hess Grade 3–5,[7] typically undergo emergent bedside placement of an external ventricular drain (EVD). All patients receive arterial line placement for hemodynamic monitoring. Systolic blood pressure is maintained at or below 140mmHg prior to securing the aneurysm. All patients are treated with oral nimodipine 60mg every 4 hours from the time of presentation until hospital discharge; for patients who develop hypotension following 60mg nimodipine administration, dosing is changed to 30mg every 2 hours.
Surgical and Post-Surgical Care
All patients undergo a diagnostic cerebral angiogram within 24 hours of admission. The treating surgeon determines whether to proceed with coil embolization or open neurosurgical intervention to secure the aneurysm, usually in the same day or anesthetic period.
Once the aneurysm is secured, the patient is returned to the ICU for continued monitoring. Systolic blood pressure parameters are liberalized to 90–180 mmHg initially, and maintenance intravenous fluids are administered to maintain euvolemia. Beginning 72 hours post-rupture, the patient undergoes serial transcranial doppler ultrasound (TCD) assessment every 24–72 hours. If the Lindegaard index (Li) rises above 3,[8] suggesting early vasospasm, there may be no direct immediate interventions, but close attention is given to the patient’s blood pressure and neurologic exam. If patients demonstrate spontaneous blood pressure elevations without developing neurologic deficits, these parameters are further liberalized as this may indicate developing vasospasm.[9] If the patient develops a focal neurologic deficit, or mental status decline of 1–2 points on the Glasgow Coma Scale without an obvious underlying cause (e.g. seizure, fever, hydrocephalus) a repeat CT angiogram (CTA) is performed to screen for vasospasm.
If detected, initial management of symptomatic vasospasm includes vasopressor support in conjunction with elevating the systolic blood pressure parameters. If these interventions do not improve the patient’s neurologic status, the patient undergoes intra-arterial (IA) treatment with angioplasty or directed IA calcium-channel blocker therapy (e.g. verapamil).
Patients are continuously monitored in the ICU for 14 days or longer (for patients with clinical vasospasm or other critical conditions). Strict contraindications for transfer out of the ICU at our institution include ongoing symptomatic vasospasm or radiographic vasospasm early in the hospitalization (i.e. post-bleed day [PBD] <14), ongoing ventilator support, vasoactive infusions (vasopressors, intravenous antihypertensive drips), or an EVD. Patients are transitioned to a ‘step down’ unit once no contraindications exist, his or her neurological examination is stable, and hourly neurologic checks are not indicated.
Data Collection
Explanatory Variables
Data were extracted from provider documentation. The bleed date was determined by reviewing the initial neurosurgical consult notes and emergency department documentation. Time to any event was defined in days with the bleed date considered day 0. Patient and radiologic variables were selected due to previous reports suggesting their role in vasospasm risk.[10–12]
Patient factors at presentation included sex, age, race, hypertension, current tobacco use, initial systolic blood pressure at presentation, and Hunt-Hess grade.[7] Imaging data were not directly available; radiographic variables of interest were derived from radiologists’ reports. Radiologic factors included the Fisher scale,[13] modified Fisher scale,[11] aneurysm location, and presence of intraparenchymal (IPH) or intraventricular (IVH) hemorrhage. Hunt-Hess grade and radiologic factors are routinely documented in neurosurgical admission and progress notes; when not clearly documented, Hunt-Hess scores were assigned based on the documented physical examination and radiological scores were determined by review of the radiologist’s interpretation of admission CT imaging.
Data related to aneurysm treatment or indications for ICU admission were collected including the need for mechanical ventilation, vasopressors, and hypertonic saline. Surgical treatment was categorized as endovascular or open.
Outcomes
The primary outcome was conditional vasospasm-free survival. Onset of vasospasm was defined by the first instance of any one of three available measures: (1) CTA presence of radiographic vasospasm according to neuroradiologist interpretation, (2) Li >3.0 determined by TCDs or mention of TCDs demonstrating vasospasm when quantitative reports were unavailable or (3) vasospasm treatment with IA therapy. Time-to-vasospasm was measured in days from initial hemorrhage. Patients without vasospasm were censored at the time of death or hospital discharge. Patients discharged before day 21 without signs of vasospasm were assumed to be vasospasm free through day 21.
Secondary outcomes included in-hospital mortality, discharge disposition (i.e. home, inpatient rehab [IPR], skilled nursing facility [SNF], long-term acute care facility [LTAC], or hospice), modified Rankin Scale at discharge (mRS), delayed cerebral ischemia (DCI). Patients with documented magnetic resonance imaging (MRI) interpretations indicating ischemia were considered to have DCI.
Statistical Analysis
Descriptive univariate analyses were performed using Wilcoxon rank-sum test (continuous variables) and Pearson chi-square test (categorical variables). Variable redundancy analysis was performed before fitting multivariable models. No redundant variables were detected. Missing covariate data were handled using multiple imputation (R package “mi”).
Multivariable Cox regression was used to assess the relationship between time-to-vasospasm and a priori selected factors including modified Fisher scale (0–2, 3, or 4) adjusted for age, systolic blood pressure (SBP), sex (male/female), race (Caucasian, African American, other), history of hypertension, tobacco use (yes/no), Hunt-Hess grade (1–3 versus 4–5), intraparenchymal hemorrhage (yes/no), and intraventricular hemorrhage (yes/no). The last day of follow-up was the date of discharge, vasospasm, or death. In these multivariable models, continuous variables (age and SBP) were initially treated as a nonlinear continuous term with the restricted cubic spline function. Partial effect plots were created to describe the independent nonlinear effect of age adjusted for other covariates in the model.
Conditional survival curves were created to estimate vasospasm-free survival for patients who have already survived a time period (e.g., 14 days) following subarachnoid hemorrhage. Conditional survival was stratified by previously described risk factors for vasospasm including modified Fisherscale,[14] tobacco use,[12] sex,[12] and age groups (<50 vs ≥50).[12,15] All statistical analyses were performed in R 4.0.0.
Results
Study Population
Within the de-identified data warehouse 562 records met the ICD-based inclusion criteria. Thirty-four records represented patients treated electively for unruptured aneurysms and were excluded, leaving 528 patients treated for aneurysmal SAH between the years 2000 and 2020. Demographics and baseline clinical information for the study population are shown in TABLE 1.
Table 1.
Demographic and baseline clinical data.
| Variable | (−) Vasospasm N=219 | (+) Vasospasm N=309 | Combined N=528 | P-value |
|---|---|---|---|---|
| Age | 56 (46–66) | 53 (45–61) | 55 (45–64) | 0.0031 |
| Sex | 0.362 | |||
| Male | 64 (29%) | 102 (33%) | 166 (31%) | |
| Female | 155 (71%) | 207 (67%) | 362 (69%) | |
| Race | 0.582 | |||
| Caucasian | 167 (76%) | 249 (81%) | 416 (79%) | |
| African American | 37 (17%) | 41 (13%) | 78 (15%) | |
| Other | 8 (4%) | 8 (3%) | 16 (3%) | |
| Missing | 7 (3%) | 11 (4%) | 18 (3%) | |
| Hypertension | 129 (59%) | 192 (62%) | 321 (61%) | 0.452 |
| Tobacco use | 118 (54%) | 185 (60%) | 303 (57%) | 0.172 |
| Aneurysm location | 0.0242 | |||
| ICA | 19 (9%) | 34 (11%) | 53 (10%) | |
| ACA | 12 (6%) | 18 (6%) | 30 (6%) | |
| MCA | 32 (15%) | 43 (14%) | 75 (14%) | |
| PCA | 5 (2%) | 3 (1%) | 8 (2%) | |
| ACOM | 63 (29%) | 96 (31%) | 159 (30%) | |
| PCOM | 63 (29%) | 53 (17%) | 116 (22%) | |
| Basilar Tip | 7 (3%) | 27 (9%) | 34 (6%) | |
| PICA | 7 (3%) | 19 (6%) | 26 (5%) | |
| Vertebral/Vertebrobasilar | 5 (2%) | 9 (3%) | 14 (3%) | |
| Other | 3 (1%) | 6 (2%) | 9 (2%) | |
| Missing | 3 (1%) | 1 (0%) | 4 (1%) | |
| Hunt Hess Grade | 0.0082 | |||
| 1 | 35 (16%) | 23 (7%) | 58 (11%) | |
| 2 | 68 (31%) | 82 (27%) | 150 (28%) | |
| 3 | 59 (27%) | 88 (28%) | 137 (28%) | |
| 4 | 33 (15%) | 73 (24%) | 106 (20%) | |
| 5 | 24 (11%) | 42 (14%) | 66 (12%) | |
| Missing | 0 (0%) | 1 (0%) | 1 (0%) | |
| Fisher Grade | 0.0012 | |||
| 1 | 15 (7%) | 6 (2%) | 21 (4%) | |
| 2 | 29 (13%) | 27 (9%) | 56 (11%) | |
| 3 | 47 (21%) | 48 (16%) | 95 (18%) | |
| 4 | 128 (58%) | 227 (73%) | 355 (67%) | |
| Missing | 0 (0%) | 1 (0%) | 1 (0%) | |
| Modified Fisher Grade | <0.0012 | |||
| 0 | 19 (9%) | 7 (2%) | 26 (5%) | |
| 1 | 32 (15%) | 31 (10%) | 63 (12%) | |
| 2 | 15 (7%) | 9 (3%) | 24 (5%) | |
| 3 | 73 (33%) | 92 (30%) | 165 (31%) | |
| 4 | 80 (37%) | 170 (55%) | 250 (47%) | |
| IPH | 61 (28%) | 80 (26%) | 141 (27%) | 0.612 |
| IVH | 106 (48%) | 199 (64%) | 305 (58%) | <0.0012 |
| SBP (n=522) | 142 (126–155) | 145 (130–165) | 144 (128–160) | 0.0781 |
| Admission Day | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.511 |
Continuous variables listed as median (IQR), categorical variables as n (%).
Tests used:
Wilcoxon test;
Pearson test.
ICA: internal carotid artery; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; ACOM: anterior communicating artery; PCOM: posterior communicating artery; IPH: intraparenchymal hemorrhage; IVH: intraventricular hemorrhage; SBP: systolic blood pressure
Patients were admitted on PBD 0 most frequently (373/528, 70.6%). The majority of patients received definitive treatment within 1 day of presentation (452/528, 85.6%). The aneurysm treatment modality was predominantly endovascular (444/528, 84%). Of the remaining patients, 73 (13.8%) underwent open surgical clipping, 8 (1.5%) did not undergo any procedures, and definitive treatment information was unavailable for 3 (0.6%). Among the 8 patients not undergoing any procedures, 5 (0.9%) had a devastating neurological injury at presentation, 2 (0.4%) had aneurysms that were deemed unable to be treated due to technical reasons, and 1 (0.02%) patient declined all interventions.
Development of Vasospasm
Among the 528 patients, 309 (58.5%) developed vasospasm by our proxy definition. Vasospasm was identified by CTA in 154 patients, TCDs in 100 patients, and IA therapy in 21 patients; the remaining 34 patients met more than one of the three criteria simultaneously for vasospasm.
The median vasospasm-free survival for the entire population was 9 days. Among patients who developed vasospasm, the median onset was PBD 6 (range 0–17). A smoothed estimate of the hazard function for the development of vasospasm based on this patient population is shown in FIGURE 1A. The Kaplan-Meier curve for vasospasm-free survival is depicted in FIGURE 1B.
FIGURE 1:
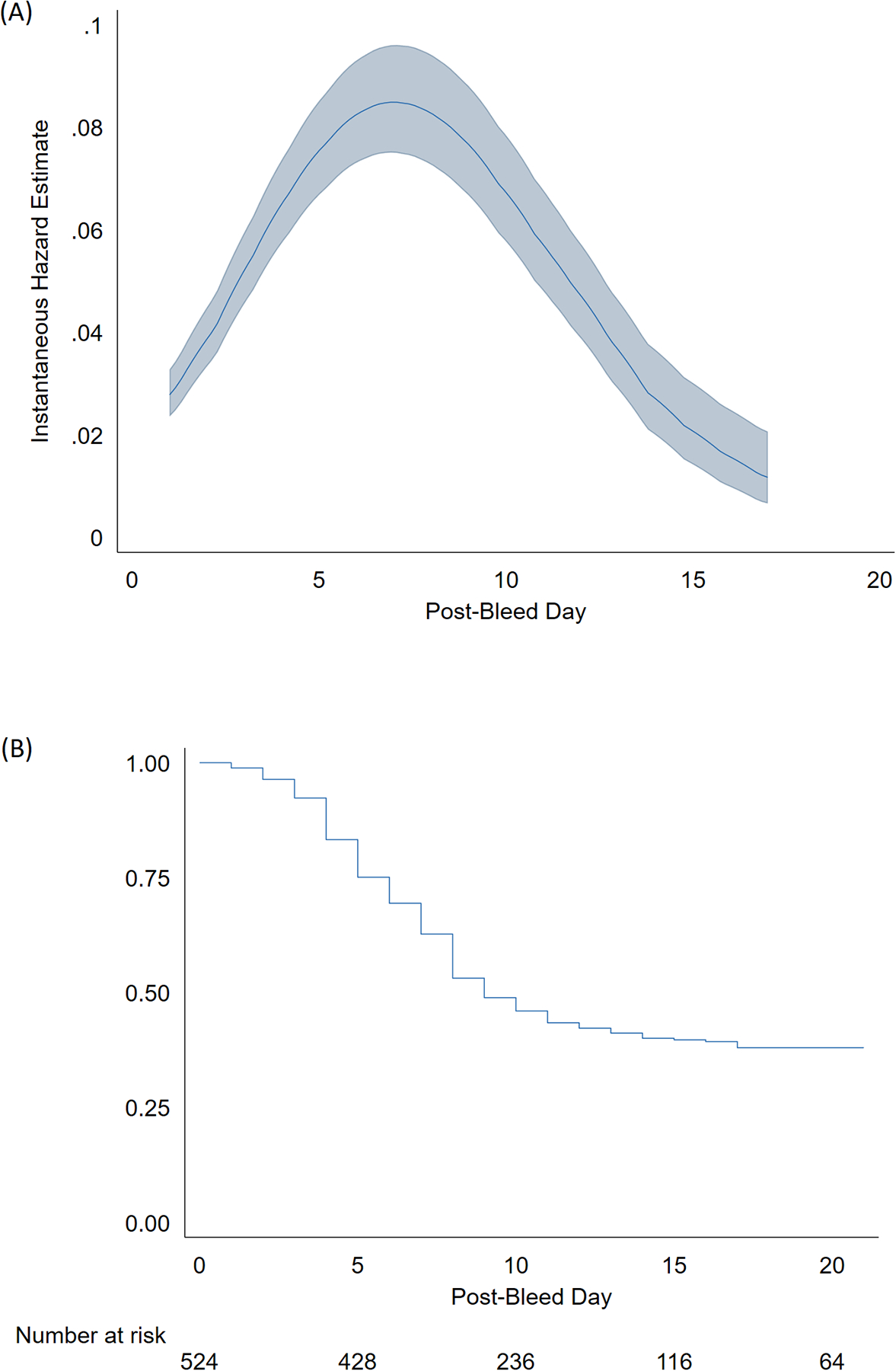
Vasospasm risk profile. (A) Estimated hazard function for the development of vasospasm, (B) Kaplan-Meier plot of vasospasm-free survival.
Secondary outcomes of mortality, DCI, discharge mRS, and discharge disposition are summarized in TABLE 2. Vasospasm was associated with increased risk of DCI, greater neurologic disability as measured by mRS, and higher likelihood of discharge to facility.
Table 2.
Clinical outcomes following aneurysmal subarachnoid hemorrhage, according to development of radiographic spasm.
| Variable | (−) Vasospasm N=219 | (+) Vasospasm N=309 | Combined N=528 | P-value |
|---|---|---|---|---|
| Censored (days) | 21 (21–21) | 6.0 (4–8) | 9 (5–21) | <0.0011 |
| Hospital Mortality | 33 (15%) | 40 (13%) | 73 (14%) | 0.492 |
| DCI | 12 (5%) | 32 (10%) | 44 (8%) | 0.0272 |
| MRI not obtained | 196 (89%) | 250 (81%) | 446 (84%) | |
| Discharge mRS | <0.0012 | |||
| 1 | 36 (16%) | 21 (7%) | 57 (11%) | |
| 2 | 33 (15%) | 32 (10%) | 65 (12%) | |
| 3 | 49 (22%) | 67 (22%) | 116 (22%) | |
| 4 | 49 (22%) | 122 (39%) | 171 (32%) | |
| 5 | 14 (6%) | 22 (7%) | 36 (7%) | |
| 6 | 33 (15%) | 40 (13%) | 73 (14%) | |
| Missing | 5 (2%) | 5 (2%) | 10 (2%) | |
| Discharge Disposition | 0.0472 | |||
| Home | 108 (49%) | 122 (29%) | 230 (44%) | |
| Rehabilitation Facility | 49 (22%) | 93 (30%) | 142 (27%) | |
| Skilled Nursing Facility | 20 (9%) | 36 (12%) | 56 (11%) | |
| Long-term Acute Care | 3 (1%) | 10 (3%) | 13 (2%) | |
| Hospice | 0 (0%) | 3 (1%) | 3 (1%) | |
| NA | 39 (18%) | 45 (15%) | 84 (16%) |
Continuous variables listed as median (IQR), categorical variables as n (%).
Tests used:
Wilcoxon test;
Pearson test.
DCI: delayed cerebral ischemia; mRS: modified Rankin scale; NA: not applicable
Clinical Predictors of Vasospasm
Univariate associations between population characteristics and vasospasm risk are shown in TABLE 1. The multivariable Cox regression model for assessing time-to-vasospasm based on pre-specified clinical predictors is described in TABLE 3. Increasing age was associated with significantly lower risk (HR=.76, 95%CI 0.64–0.91; p<0.001) among older patients and higher risk among younger patients (HR=1.09, 95% CI 0.90 – 1.31; p=0.39), with the turning point around 50 years. Higher initial systolic blood pressure (HR=1.18, 95%CI 1.05–1.35, p=.008) was associated with a greater risk of vasospasm. Hunt-Hess grades 4 or 5 (HR=1.30, 95%CI 1.01–1.68) as well as modified Fisher scale 4 (HR=1.81, 95%CI 1.20–2.73) were associated with higher risk of vasospasm compared to the lower respective grades. Aneurysm location was associated with vasospasm risk on univariate analysis but was not included in the multivariable model. IVH was not independently associated with vasospasm in the multivariable model potentially due to the inclusion of IVH in the calculation of the modified Fisher scale.
Table 3.
Multivariable cox regression for prediction of vasospasm-free survival.
| Variable | Coefficient | Standard Error | Wald Z | p-value |
|---|---|---|---|---|
| Age | 0.0091 | 0.0097 | 0.94 | 0.3481 |
| Nonlinear | −0.0309 | 0.0119 | −2.6 | 0.0094 |
| SBP | 0.0054 | 0.002 | 2.65 | 0.008 |
| Female sex | −0.0291 | 0.1242 | −0.23 | 0.8146 |
| Hypertension | 0.0826 | 0.123 | 0.67 | 0.5016 |
| Tobacco Use | 0.0501 | 0.1188 | 0.42 | 0.6733 |
| Hunt Hess Grade 4–5 | 0.2653 | 0.128 | 2.07 | 0.0383 |
| Modified Fisher Grade 3 | 0.3444 | 0.181 | 1.9 | 0.0571 |
| Modified Fisher Grade 4 | 0.592 | 0.2101 | 2.82 | 0.0048 |
| IPH | −0.0908 | 0.135 | −0.67 | 0.5012 |
| IVH | 0.1349 | 0.1727 | 0.78 | 0.4348 |
IPH: intraparenchymal hemorrhage; IVH: intraventricular hemorrhage
Conditional Vasospasm-Free Survival
The conditional vasospasm-free survival curve for the study population is depicted in FIGURE 2. This graph depicts the probability of surviving to hospital discharge without developing vasospasm (Y-axis), as a function of the time already elapsed without evidence of vasospasm (X-axis). The sigmoid relationship corresponds to a gradual increase in the probability of vasospasm-free survival to discharge throughout the hospitalization period. Patients who remain vasospasm-free at PBD 10 have an approximately 87% probability of survival to discharge without vasospasm.
FIGURE 2:
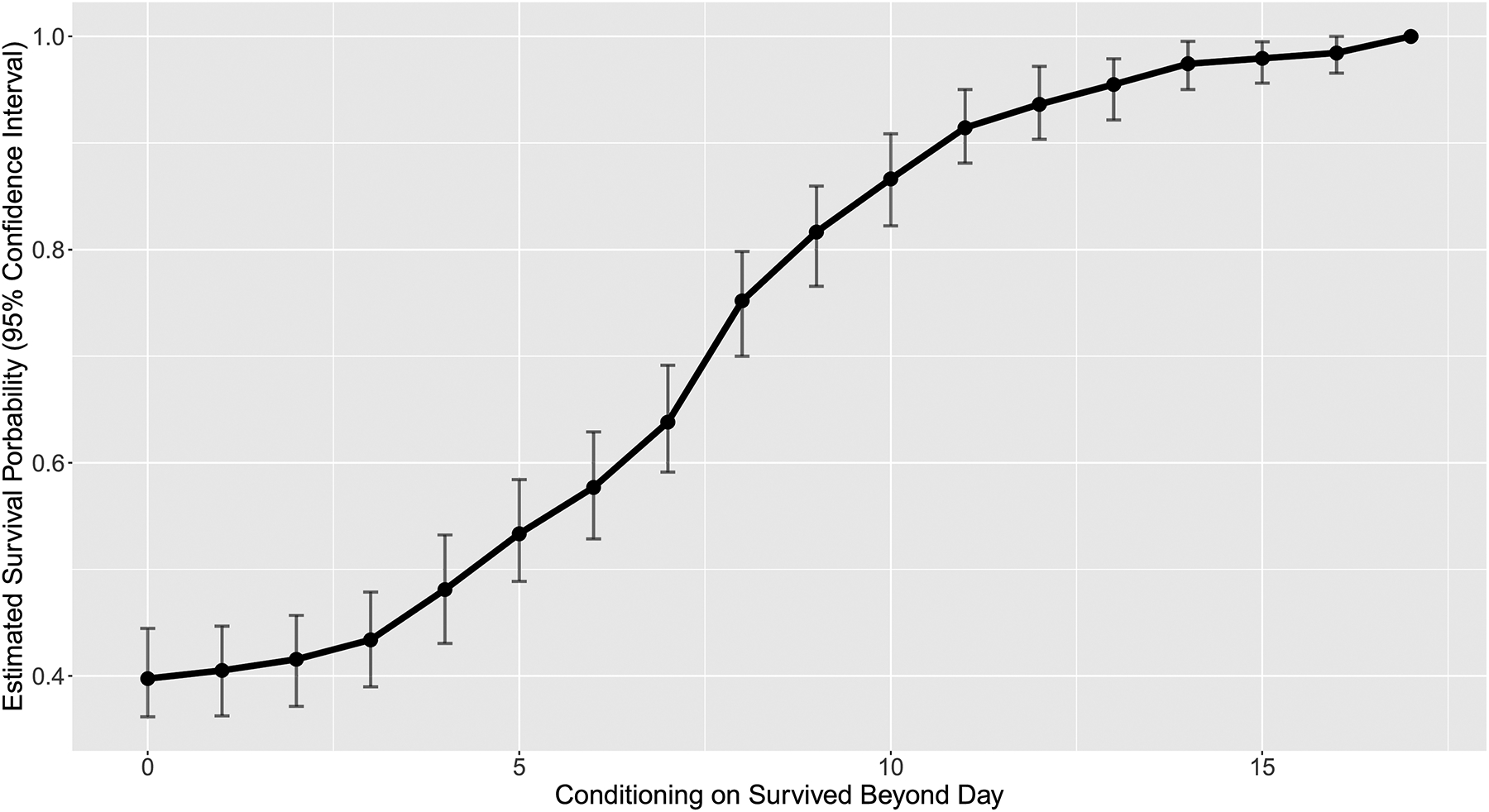
Conditional vasospasm-free survival curve. The Y-axis represents the estimated probability of survival to hospital discharge without developing vasospasm; the X-axis represents the time already elapsed without vasospasm.
Conditional survival curves were also stratified by age, sex, tobacco use, and modified Fisher scale (FIGURE 3A–D, respectively). The conditional survival curves were similar for age, sex, and tobacco use groups; patients with modified Fisher scale of 4 had lower probability of vasospasm-free survival compared to patients with modified Fisher scale of 0–2 until approximately PBD 8.
FIGURE 3:
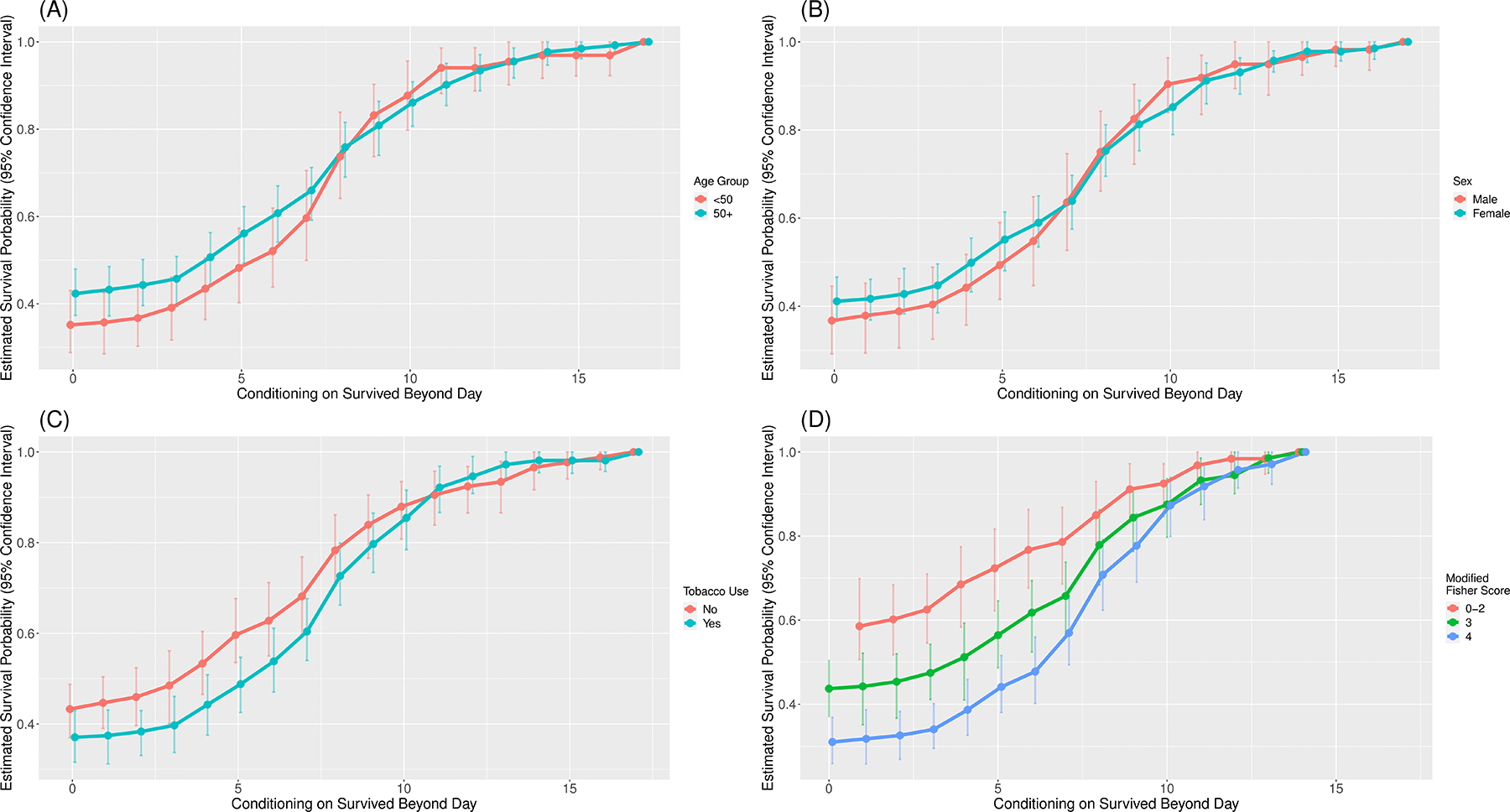
Stratified conditional vasospasm-free survival curves for age (A), sex (B), tobacco use (C), and modified Fisher grade (D).
Discussion
Over the past decade, conditional survival has become a more widely accepted approach to prognostication in the oncologic literature.[16–19] As classic survival analysis estimates prognosis from the time of diagnosis, conditional survival allows for a more practical, real-time appraisal of prognosis which may inform clinical decision-making. While conditional survival analysis is more commonplace in the systemic oncology literature there have only been a few explorations of conditional survival in neuro-oncology.[20–22] One recent study addressed conditional survival of an unselected cohort of critically ill patients,[23], but apart from this investigation conditional survival has not been used for more acute cerebrovascular pathologies or specifically in neurocritical care.
Key Results
Through this retrospective pilot study of patients treated over 20 years at a single institution, we characterize conditional vasospasm-free survival following aneurysmal subarachnoid hemorrhage. Traditional survival analysis demonstrates the probability of being without vasospasm at a particular time (e.g. “at day 10 about 50% of patients have experienced vasospasm”), while conditional survival describes the probability that a patient who has been vasospasm-free up to a particular day will continue without vasospasm (e.g. “you have been vasospasm-free for 10 days, so the chance of experiencing vasospasm before discharge is ~10%.”). The conditional survival plots provide neurosurgeons and critical care physicians with a real-time risk estimator.
In addition, we provide early evidence that the conditional survival plot morphology is unchanged across several previously identified risk factors, with the exception of modified Fisher scale. Even in this case, conditional vasospasm-free survival irrespective of modified Fisher scale converges to 90% chance at PBD 10, suggesting the modified Fisher scale is most useful for prognostication early in the vasospasm window. These risk factor-stratified conditional survival plots have potential to facilitate a patient-centric evidence-based approach to these conversations and decisions regarding vasospasm risk.
Secondary Outcomes
Development of vasospasm was associated with DCI, higher mRS at discharge, and rates of discharge to facility, but not with mortality, in this study, consistent with the findings of other studies.[14] Although vasospasm may only represent a surrogate of a more complex interaction of pathologic mechanisms [24], it nonetheless represents a useful intermediary between these mechanisms and multiple outcome measures.
Implications
To our knowledge there is no practice guideline or consensus which stipulates how long a patient should remain in the ICU or in the hospital following aneurysmal SAH, even in the absence of clinical vasospasm.[25] Some authors have described institutional initiatives for early discharge to a ‘step down’ unit[26] or even home,[27] for select low-risk patients, but practice variation among institutions has not been surveyed. Individual providers, then, are left to risk stratify patients based on his or her clinical judgement alone.
Daily assessment of a patient’s conditional probability of vasospasm-free survival, on the other hand, may allow for routine risk-stratification at the bedside to inform earlier discharge from the ICU. Importantly, older patients had a lower risk of vasospasm; given the high rate of ICU delirium and downstream morbidity in the elderly population,[28] it may be even more important to transfer these patients out of the ICU earlier.
There are, of course, other indications for ICU management other than vasospasm risk such as presence of an EVD, intubation, or ongoing use of vasopressors. Among the 528 patients in this study, 278 (53%) were intubated at some point during their hospitalization, and 71% of these patients were extubated on or before PBD 10. Seventy-nine percent (416/528) of patients received vasopressors, and 49% of these received vasopressors beyond PBD 10. In our study, EVD and ICU status were not available for review. However, based on conditional survival, if a patient has not developed signs or symptoms suggestive of vasospasm prior to PBD 10, the clinical team may be able to focus on de-escalating care.
Generalizability
The conditional survival curves constructed in this study are likely generalizable to at least other ICU settings in the United States where aneurysmal subarachnoid hemorrhage is treated. Our population containing about 70% female patients with a median age in the sixth decade is consistent with previously published studies over the last 20 years.[12,14,15,29,30] The overall rate of vasospasm as determined by CTA, angiogram, or TCD was approximately 60%, which is congruent with literature utilizing similar proxy definitions.[30] Our definition of vasospasm was intentionally highly sensitive as our goal was to provide conservative conditional vasospasm-free survival estimates. Conservative criteria are paramount if the data are used to determine patients that may be transferred out of the ICU earlier. Finally, we were able to recapitulate several important risk factors for developing vasospasm including younger age[12,15,29,31] and higher clinical and radiographic grades of SAH,[11,12,15,29,30] providing further external validity of our results.
Limitations
There are limitations to this study. Primarily, the data was obtained from our single institutional de-identified medical record. While the use of this data in clinically-oriented research has been widely accepted,[6,32–35] billing and diagnosis codes are required for record identification and it is likely that not all eligible patients treated in the timeframe were included. Based on Stroke Center Certification data, our institution currently treats approximately 60 aneurysmal SAH patients per year—far greater than the 26 per year rate in the study cohort. However, we have no reason to believe that any subgroups were systematically missed.
Although clinical management of SAH patients was mostly consistent throughout the lengthy study period, some elements have evolved. While angiography and treatment within 24hrs of presentation is the current norm at our institution, time from bleed day to treatment did decrease slightly over the years of the study (p=0.02, data not shown). Additionally, IA therapy was included in the proxy definition of vasospasm, yet evidence supporting this procedure mounted gradually during the 21st century [36,37]. In our study, the rate of vasospasm was not associated with the year of presentation (p=0.85, data not shown), suggesting that gradual adoption of IA therapy did not introduce substantial ascertainment bias.
As mentioned previously, a very inclusive proxy definition of vasospasm was used in this study. This definition includes both clinical, radiographic, and TCD criteria, some of which may not be clinically meaningful.[15] Future studies using prospectively-collected clinical data may allow for more precise conditional survival curves based on a clinically-relevant definition of vasospasm.
Due to limitations in the follow-up documentation available in the data warehouse, data on DCI using consensus criteria [38] could not be accurately collected, and an inexact definition was used. For the purposes of in-hospital prognostication and early discharge from the ICU, vasospasm more conservative proxy outcome since only a portion of patients with vasospasm develop DCI.[39]
Lastly, actual radiographic images are unavailable in this data warehouse, so all radiographic variables were exclusively extracted from radiologist reports without independent imaging review. Nonetheless, any potential differences in neurosurgeon and neuroradiologist interpretations would likely have a negligible effect on our conservative proxy definition of vasospasm. Despite these limitations, the de-identified dataset provides many benefits including enhanced data query capabilities and a much lower risk of exposing patient protected health information in a pilot study.
Conclusion
The risk of cerebral vasospasm following aneurysmal subarachnoid hemorrhage may dictate management decisions such as continued monitoring in the ICU or high frequency neurologic exams. Conditional survival provides a clinically useful improvement over classical survival analysis for counseling patients and making decisions around vasospasm-risk for patients with aneurysmal SAH, while risk factor-stratified plots facilitate a patient-centric evidence-based approach to these conversations and decisions.
Sources of Funding:
REDCap, used for study data management, is supported by NCATS/NIH grant UL1 TR000445. One author (PDK) is supported by a training grant from the National Cancer Institute of the NIH under award number T32CA106183.
Footnotes
The authors have no conflicts of interest to disclose.
This study was performed in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist, including the RECORD (The REporting of studies Conducted using Observational Routinely-collected health Data) statement.
References
- 1.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–72. [DOI] [PubMed] [Google Scholar]
- 2.Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978;48:173–8. [DOI] [PubMed] [Google Scholar]
- 3.Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18–36. [DOI] [PubMed] [Google Scholar]
- 4.Kassell NF, Torner JC, Jane JA, Haley EC Jr, Adams HP. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2: Surgical results. J Neurosurg. 1990;73:37–47. [DOI] [PubMed] [Google Scholar]
- 5.Hieke S, Kleber M, König C, Engelhardt M, Schumacher M. Conditional Survival: A Useful Concept to Provide Information on How Prognosis Evolves over Time. Clin Cancer Res. 2015;21:1530–6. [DOI] [PubMed] [Google Scholar]
- 6.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20. [DOI] [PubMed] [Google Scholar]
- 8.Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl. 1988;42:81–4. [DOI] [PubMed] [Google Scholar]
- 9.Hosmann A, Klenk S, Wang W-T, Koren J, Sljivic S, Reinprecht A. Endogenous arterial blood pressure increase after aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2020;190:105639. [DOI] [PubMed] [Google Scholar]
- 10.Inagawa T Risk Factors for Cerebral Vasospasm Following Aneurysmal Subarachnoid Hemorrhage: A Review of the Literature. World Neurosurg. 2016;85:56–76. [DOI] [PubMed] [Google Scholar]
- 11.Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–7; discussion 21–7. [DOI] [PubMed] [Google Scholar]
- 12.Rumalla K, Lin M, Ding L, Gaddis M, Giannotta SL, Attenello FJ, et al. Risk Factors for Cerebral Vasospasm in Aneurysmal Subarachnoid Hemorrhage: A Population-Based Study of 8346 Patients. World Neurosurg. 2021;145:e233–41. [DOI] [PubMed] [Google Scholar]
- 13.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–8. [DOI] [PubMed] [Google Scholar]
- 15.Charpentier C, Audibert G, Guillemin F, Civit T, Ducrocq X, Bracard S, et al. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke. 1999;30:1402–8. [DOI] [PubMed] [Google Scholar]
- 16.Mertens AC, Yong J, Dietz AC, Kreiter E, Yasui Y, Bleyer A, et al. Conditional survival in pediatric malignancies: analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program. Cancer. 2015;121:1108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamboni BA, Yothers G, Choi M, Fuller CD, Dignam JJ, Raich PC, et al. Conditional survival and the choice of conditioning set for patients with colon cancer: an analysis of NSABP trials C-03 through C-07. J Clin Oncol. 2010;28:2544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabor EC, Gonen M, Chapman PB, Panageas KS. Dynamic prognostication using conditional survival estimates. Cancer. 2013;119:3589–92. [DOI] [PubMed] [Google Scholar]
- 19.Haydu LE, Scolyer RA, Lo S, Quinn MJ, Saw RPM, Shannon KF, et al. Conditional Survival: An Assessment of the Prognosis of Patients at Time Points After Initial Diagnosis and Treatment of Locoregional Melanoma Metastasis [Internet]. Journal of Clinical Oncology. 2017. p. 1721–9. Available from: 10.1200/jco.2016.71.9393 [DOI] [PubMed] [Google Scholar]
- 20.Porter KR, McCarthy BJ, Berbaum ML, Davis FG. Conditional survival of all primary brain tumor patients by age, behavior, and histology. Neuroepidemiology. 2011;36:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85:485–91. [PubMed] [Google Scholar]
- 22.Farah P, Blanda R, Kromer C, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Conditional survival after diagnosis with malignant brain and central nervous system tumor in the United States, 1995–2012. J Neurooncol. 2016;128:419–29. [DOI] [PubMed] [Google Scholar]
- 23.Marshall DC, Hatch RA, Gerry S, Young JD, Watkinson P. Conditional Survival With Increasing Duration of ICU Admission: An Observational Study of Three Intensive Care Databases. Crit Care Med. 2020;48:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–63. [DOI] [PubMed] [Google Scholar]
- 25.Diringer MN, Bleck TP, Claude Hemphill J, Menon D, Shutter L, Vespa P, et al. Critical Care Management of Patients Following Aneurysmal Subarachnoid Hemorrhage: Recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference [Internet]. Neurocritical Care. 2011. Available from: 10.1007/s12028-011-9605-9 [DOI] [PubMed] [Google Scholar]
- 26.Chartrain AG, Awad AJ, Sarkiss CA, Feng R, Liu Y, Mocco J, et al. A step-down unit transfer protocol for low-risk aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2017;43:E15. [DOI] [PubMed] [Google Scholar]
- 27.Collins CI, Hasan TF, Mooney LH, Talbot JL, Fouraker AL, Nelson KF, et al. Subarachnoid Hemorrhage “Fast Track”: A Health Economics and Health Care Redesign Approach for Early Selected Hospital Discharge. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 2020;4:238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65. [DOI] [PubMed] [Google Scholar]
- 29.Foreman PM, Chua MH, Harrigan MR, Fisher WS 3rd, Tubbs RS, Shoja MM, et al. External validation of the Practical Risk Chart for the prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J. Neurosurg 2017. p. 1530–6. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Perry JJ, English SW, Alkherayf F, Joseph J, Nobile S, et al. Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2018;1–8. [DOI] [PubMed] [Google Scholar]
- 31.Darkwah Oppong M, Iannaccone A, Gembruch O, Pierscianek D, Chihi M, Dammann P, et al. Vasospasm-related complications after subarachnoid hemorrhage: the role of patients’ age and sex. Acta Neurochir. 2018;160:1393–400. [DOI] [PubMed] [Google Scholar]
- 32.Dennis J, Yengo-Kahn AM, Kirby P, Solomon GS, Cox NJ, Zuckerman SL. Diagnostic Algorithms to Study Post-Concussion Syndrome Using Electronic Health Records: Validating a Method to Capture an Important Patient Population. J Neurotrauma. 2019;36:2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdellaoui A, Sanchez-Roige S, Sealock J, Treur JL, Dennis J, Fontanillas P, et al. Phenome-wide investigation of health outcomes associated with genetic predisposition to loneliness. Hum Mol Genet. 2019;28:3853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wise ES, Ladner TR, Song J, Eagle SS, Mocco J, Wergin JE, et al. Race as a predictor of delay from diagnosis to endarterectomy in clinically significant carotid stenosis. J Vasc Surg. 2015;62:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang BQ, Assad TR, O’Leary JM, Xu M, Halliday SJ, D’Amico RW, et al. Racial differences in patients referred for right heart catheterization and risk of pulmonary hypertension. Pulm Circ. 2018;8:2045894018764273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatraman A, Khawaja AM, Gupta S, Hardas S, Deveikis JP, Harrigan MR, et al. Intra-arterial vasodilators for vasospasm following aneurysmal subarachnoid hemorrhage: a meta-analysis. J Neurointerv Surg. 2018;10:380–7. [DOI] [PubMed] [Google Scholar]
- 37.Li K, Barras CD, Chandra RV, Kok HK, Maingard JT, Carter NS, et al. A Review of the Management of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019;126:513–27. [DOI] [PubMed] [Google Scholar]
- 38.Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. [DOI] [PubMed] [Google Scholar]
- 39.Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


