Abstract
For many mosquito species, the females must obtain vertebrate blood to complete a gonotrophic cycle. These blood meals are frequently supplemented by feeding on sugary plant nectar, which sustains energy reserves needed for flight, mating, and overall fitness. Our understanding of mosquito nectar foraging behaviors is mostly limited to laboratory experiments and direct field observations, with little research into natural mosquito-host plant relationships done in North America. In this study, we collected nectar-fed female mosquitoes over a 2-year period in Manitoba, Canada, and amplified a fragment of the chloroplast rbcL gene to identify the plant species fed upon. We found that mosquitoes foraged from diverse plant families (e.g., grasses, trees, ornamentals, and legumes), but preferred certain species, most notably soybean and Kentucky blue grass. Moreover, there appeared to be some associations between plant feeding preferences and mosquito species, date of collection, landscape, and geographical region. Overall, this study implemented DNA barcoding to identify nectar sources forage by mosquitoes in the Canadian Prairies.
Keywords: Aedes, Culex, soybean, nectar preferences, sugar feeding
Introduction
Mosquitoes are ubiquitously found, medically important arthropod vectors of disease (Beerntsen et al. 2000). Their vector potential is rooted in a hematophagous lifestyle, as female mosquitoes acquire and transmit pathogens during host blood ingestion (Molaei et al. 2008, Melgarejo-Colmenares et al. 2022). Most species feed on the blood of vertebrates, which provides key nutrients that are required for egg production, including minerals, vitamins, and amino acids (Nasci 1984, Goldstrohm et al. 2003, Zhou et al. 2007). However, sugar feeding also represents an important source of nutrients for both sexes (Barredo and DeGennaro 2020). While males are obligate sugar feeders, females ingest plant sugar throughout their adult life, typically as floral and extrafloral nectar (Foster 1995, 2022). Sugar deprivation has been linked to reductions in energy reserves that can detrimentally impact the fecundity and survival of females (Foster 1995, Fernandes and Briegel 2005, Braks et al. 2006, Chadee et al. 2014). Indeed, plant nectar represents an important and often under-recognized aspect of the life history of female mosquitoes.
Research into the nectar sources foraged by mosquitoes is largely based on laboratory experiments and direct field observations of mosquito plant-feeding behaviors. Olfactory (Foster 1995, Gouagna et al. 2014), gustatory (Kessler et al. 2015), and visual (Peach et al. 2019) cues all appear to be utilized to detect/locate host plants, though the extent by which mosquitoes exhibit plant host specificity is not well established. A broad range of phytochemicals is attractive (i.e., act as semiochemicals) to diverse species, indicating that these dipterans are generalist plant feeders (Lothrop et al. 2012, Nyasembe et al. 2015, Steiner et al. 2018, Foster 2022, Hutcheson et al. 2022). However, many mosquitoes show an inherent preference for nectar-rich plants (Gouagna et al. 2014, Barredo and DeGennaro 2020), may feed disproportionately on particular species (Bowen 1992, Gadawski and Smith 1992, Junnila et al. 2010), and there is evidence of host discrimination based on lipid, glycogen, and protein content (Yu et al. 2018). Although not well studied, they may also become habituated to associating odorant stimuli (Jhumur et al. 2006, Sanford et al. 2013) and visual patterns (Bernáth et al. 2016) with sugar meals. Moreover, males and females show disparities in nectar preferences (Grimstad and DeFoliart 1974, Magnarelli 1979), presumably due to the sexual dimorphism in host plant feeding behaviors and accompanied physiological and metabolic differences (Foster 1995, Lomeli and Dahanukar 2022).
In the Canadian Prairies, Aedes vexans Meigen is the most commonly found species (Baril et al. 2023). It is a competent vector of California serogroup viruses (CSGVs), Rift Valley fever virus, West Nile virus (WNV), and Zika virus (Drebot 2015, Weissmann 2016, O’Donnell et al. 2017, Parry et al. 2020). Ochlerotatus dorsalis Meigen and Culex tarsalis Coquillett are also ubiquitous in the Prairies. Both are capable of transmitting Western equine encephalitis virus (WEEV), WNV, and CSGVs (Wood et al. 1979, Anderson et al. 2015). Other vector species that are less commonly found or associated with specific habitats include Aedes canadensis Theobald, Coquillettidia perturbans Walker, Ochlerotatus triseriatus Say, and Ochlerotatus flavescens Müller (Wood et al. 1979, Berry et al. 1986, McMahon et al. 2008, Anderson et al. 2015, Koloski et al. 2021).
DNA barcoding is a method that has been extensively used for species identification based on the sequence of a short, standardized genetic region (Ankola et al. 2021). In particular, the chloroplast ribulose diphosphate carboxylase (rbcL) gene is an effective barcode for plant species, as it is present in virtually all plant species and contains a region that is highly variable among species (CBOL Plant Working Group 2009, Bell et al. 2017). This approach and gene have been successfully employed to identify the plant/nectar content in the guts of diverse invertebrates (Matheson et al. 2007, Gravendeel et al. 2009, Junnila et al. 2011, Staudacher et al. 2011, Garcia-Robledo, et al. 2013, Kajtoch 2014, Lima et al. 2016, de Vere et al. 2017). To our knowledge, only 2 studies have used DNA barcoding to identify nectar sources in mosquitoes. Nyasembe et al. (2018) barcoded 29 Aedes and Anopheles specimens from Kenya using the trnH-psbA and matK genes, whereas Junnila et al. (2010) sequenced the rbcL of a small number of Anopheles sergentii (22) from Israel. Moreover, virtually nothing is known about the plant-feeding behaviors of mosquitoes in Canada. Consequently, we collected nectar-fed female mosquitoes over a 2-year period in Manitoba to characterize the nectar sources foraged by DNA analysis.
Materials and Methods
Mosquito Trapping
Collections were carried out between June and August over a 2-year period (2020 and 2021), which was previously described (Baril et al. 2023). In brief, CDC Miniature Light Traps (Model 1012, John W. Hock, Gainesville, FL) with carbon dioxide regulators set at 15 psi (light disabled) were used to sample host-seeking mosquitoes from dawn to dusk. Trapping was carried out twice weekly in 2020 and 2021, from June to August. We operated 24 traps in 8 West Manitoba communities in 2020, with 1 trap per community in 2021. Satellite traps from 9 additional locations in East Manitoba were provided to us by the City of Winnipeg Insect Control Branch. A description of all the sampling sites in which nectar-fed individuals were collected is displayed in Supplementary Table S1.
Nectar-fed individuals (i.e., possessing distended, clear abdomens; Fig. 1) were sorted out and identified to species using applicable mosquito identification keys (Carpenter and LaCasse 1955, Wood et al. 1979, Thielman and Hunter 2007). Specimens were then surface sterilized with 0.5% benzalkonium chloride followed by 70% ethanol and purified water (Yunik et al. 2015). Finally, each sample was placed in individual 1.5 ml tubes coded by species, date, and collection site, and stored at −80 °C until further processing.
Fig. 1.
Representative nectar-fed (left) and non-fed (right) Aedes vexans females collected from Manitoba, Canada. The characteristically clear, distended abdomen can be readily visualized.
DNA Extraction, Sequencing, and Data Analysis
We first dissected out the abdomens (crop, guts) using a new set of sterilized scalpels and forceps for each nectar-fed specimen. The One-4-All Genomic DNA Miniprep Kit (Bio Basic, Markham, ON) was then used to isolate gDNA from nectar-fed individuals. We used the Nanophotometer NP80 (Implen Inc., Westlake Village, CA) to assess DNA quantity and quality. Amplification of the rbcL gene was carried out in 50 μl reactions using Phusion High-Fidelity PCR Master Mix and the following universal primer set (560 bp amplicon size): rbcLa-F: 5’-ATGTCACCACAAACAGAGACTAAAGC-3’ (Kress and Erickson 2007) and rbcLr506: 5’-AGGGGACGACCATACTTGTTCA-3’ (de Vere et al. 2012). Thermocycler (Biometra TOne, Analytik Jena, Germany) conditions consisted of 95 °C for 1 min followed by 35 cycles of 95 °C for 30 s, 51 °C for 30 s, and 68 °C for 1 min, with a final extension at 68 °C for 5 min (Bafeel et al. 2012). We visualized amplicons in 1% agarose gels stained with ethidium bromide using a ChemiDoc Imaging System (Bio-Rad Laboratories, Hercules, CA). In cases where the amplification was unsuccessful, the PCR reactions were redone using a different reverse primer: rbcLa-rev (600 bp amplicon size): 5’-GTAAAATCAAGTCCACCRCG-3’ (Kress et al. 2009) or rbcLr590 (590 bp amplicon size): 5’-AGTCCACCGCGTAGACATTCAT-3’ (de Vere et al. 2012). Amplicons were then sent to Génome Québec Innovation Centre (McGill University, Montreal, QC, Canada) for purification using a Biomek NX robot with a bead solution and Sanger sequencing of one or both the forward and reverse rbcLa strands using the 3730xl DNA Analyzer (Applied Biosystems, Waltham, MA). Resultant sequences were first visualized via their chromatogram and then identified to plant taxon (where possible) using BLASTn, tBLASTx, and the nr database at NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Most sequences that could be resolved to the plant family had sequences similarities > 98% and query coverage of 100.
Statistical Analysis
χ2 (goodness-of-fit) tests were first performed in R (R Core Team 2021) to explore differences in nectar preferences between communities, geographical regions, and collection dates. We computed P values by Monte Carlo simulation (100,000) with a threshold of significance of P < 0.05, no continuity correction, and an effective size (w) of 0.3. The null assumption is that the 2 categorical variables were independent. This analysis was not done between species due to the small sample sizes (<15) with the exception of Ae. vexans. Plant types with expected values of 0 were omitted from a given analysis.
Results
A total of 265,564 mosquitoes were collected from our sampling sites throughout Manitoba, Canada over the 2-year trapping period. A very small number of collected specimens (n = 157) were nectar-fed and thus identified to the species and subjected to DNA barcoding. Of the subset of nectar-fed female mosquitoes, we successfully amplified a fragment of the rbcL gene from 135 specimens (West Manitoba = 75, East Manitoba = 60). The fluid collected from the ~14% that did not amplify may have consisted of water or non-sugar fluids rather than being derived from a plant source. Of those that successfully amplified, 85% (n = 115) generated sequence(s) that could be resolved to specific plant taxon. In nearly all cases, we were able to resolve the nectar source to the family level and to a lesser extent genus and species (Supplementary Table S2). A total of 19 plant families were identified, with Fabaceae (36%) and Poaceae (24%) being the most prevalent (Table 1). Within Fabaceae, 85% (n = 35) of the identified plants were soybean (Glycine max) and this also represented the most commonly foraged plant species overall (30% of the total). It was more challenging to resolve Poaceae to the species level, but based on the geographical location and sequence similarity, 34% (n = 13) of nectar sources identified from that plant family appeared to be Kentucky bluegrass (Poa pratensis). The remaining families detected represented ornamentals (17.4%), trees (12.2%), agricultural crops (7.8%), vines (1.7%), and wetland plants (0.9%). It should be noted that these plant types were subjective and there was some overlap among categories (e.g., soybean could be classified as both a legume and agricultural crop).
Table 1.
Relative proportions of plant families fed upon by female mosquitoes. The nectar sources could be resolved to the family level for 115 specimens
| Plant family | Description | Mosquitoes (%) |
|---|---|---|
| Fabaceae |
 Legume
Legume |
41 (35.7) |
| Poaceae |
 Grass
Grass |
28 (24.3) |
| Musaceae |
 Ornamental Ornamental |
9 (7.8) |
| Solanaceae |
 Agriculture Agriculture |
7 (6.1) |
| Asteraceae |
 Ornamental Ornamental |
6 (5.2) |
| Pinaceae |
 Tree
Tree |
6 (5.2) |
| Oleaceae |
 Tree
Tree |
2 (1.7) |
| Salicaceae |
 Tree
Tree |
2 (1.7) |
| Smilacaceae |
 Vine Vine |
2 (1.7) |
| Ulmaceae |
 Tree
Tree |
2 (1.7) |
| Amaranthaceae |
 Ornamental Ornamental |
2 (1.7) |
| Adoxaceae |
 Ornamental Ornamental |
1 (0.9) |
| Apiaceae |
 Agriculture Agriculture |
1 (0.9) |
| Brassicaceae |
 Agriculture Agriculture |
1 (0.9) |
| Geraniaceae |
 Ornamental Ornamental |
1 (0.9) |
| Juglandaceae |
 Tree
Tree |
1 (0.9) |
| Menyanthaceae |
 Wetland Wetland |
1 (0.9) |
| Philadelpheae |
 Ornamental Ornamental |
1 (0.9) |
| Sapindaceae |
 Tree
Tree |
1 (0.9) |
Evidence of Nectar Preferences for Female Mosquitoes
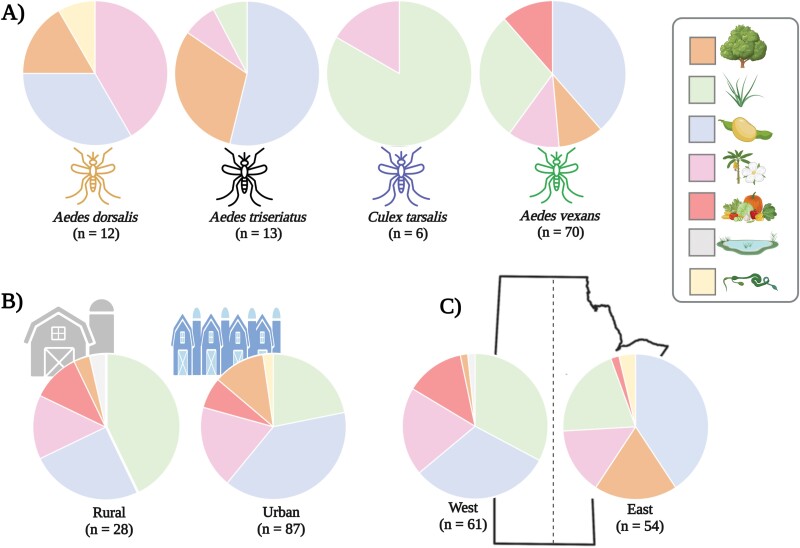
Figure 2 shows the partitioning of nectar sources in positive mosquitoes by species, community (urban or rural), and region of the province (west or east). Although we carried out χ2 tests on the dataset (with the exception of species), it should be emphasized that the relatively small and uneven sample sizes within most comparisons impacted the robustness of the analysis. The majority of nectar-fed mosquitoes captured were Ae. vexans (70), which primarily foraged on legumes and grasses (Fig. 2A). Aedes dorsalis predominately fed on ornamentals and legumes, Aedes triseriatus on legumes and trees, and Cx. tarsalis on grasses. In addition, there were significant differences in nectar preferences between communities (χ2 = 36.5, df = 5, P < 0.0001) and regions (χ2 = 60.4, df = 5, P < 0.0001). Mosquitoes captured within East Manitoba and/or urban areas were more likely to forage legumes and trees, whereas greater proportions of West Manitoba and/or rural mosquitoes were fed on grasses and agricultural crops (Fig. 2B and C). This was consistent when performing the regional statistical analysis with Ae. vexans as the only species included (χ2 = 64.04, df = 5, P < 0.0001). Finally, there were significant temporal differences in nectar-feeding behaviors (χ2 = 68.8, df = 4, P < 0.0001). In June, females foraged more agricultural crops and ornamentals and fewer trees and grasses than in July (Supplementary Figure S1).
Fig. 2.
Partitioning of nectar sources in positive mosquitoes based on A) species, B) community (urban or rural), and C) region of the province (west or east). Each nectar source was first resolved to the plant family and then grouped to the most fitting plant type: legume, grass, ornamental, trees, agricultural crop, vine, or wetland plant. Significance was determined using χ2 goodness-of-fit tests, which indicated differences between communities and regions (P < 0.0001, both comparisons). We did not perform statistical analysis between species due to small sample sizes (<15) with the exception of Aedes vexans. The number of nectar-fed specimens for each contrast is represented in parentheses. This figure was created with Biorender.com (Science Suite Inc., Toronto, ON, Canada).
Discussion
The objective of our study was to provide insights into the nectar foraging behaviors of female mosquitoes in the Canadian Prairies. Our 2 consecutive years of trappings throughout southern Manitoba during the active season (June–August) yielded 135 nectar-fed females, which were subjected to DNA barcoding to identify the source plants. Laboratory experiments have uncovered possible sugar sources and preferences of mosquitoes (for review, see Foster 1995, Barredo and DeGennaro 2020); however, validation of these findings under natural conditions has almost exclusively relied on direct observations in the field (Foster 1995, 2022). One notable exception used DNA barcoding to provide clear and unbiased detection of the host plant preferences of Anopheles sergenti in the semi-desert south Jordan Valley of Israel (Junnila et al. 2010). No such studies have been undertaken in North America, and field investigations into natural mosquito-host plant relationships have been mostly limited to ground orchids and catchfly flowers (Stoutamire 1968, Thien and Utech 1970, Brantjes and Leemans 1976, Lahondère et al. 2020).
Our results suggest that female mosquitoes of the Canadian Prairies forage from a relatively small number of plant families that are varied (e.g., trees, grasses, ornamentals, and legumes). Although plants within Fabaceae and Poaceae represented the majority of the nectar sources, this is largely attributed to strong preferences for particular species within these families. Indeed, 42% of barcoded mosquitoes fed on nectar from soybean or Kentucky blue grass. This is consistent with field studies indicating that even mosquito species with diverse plant diets show preferential feeding on certain species (Sandholm and Price 1962, Grimstad and DeFoliart 1974, Magnarelli 1977, 1978, Gadawski and Smith 1992). Soybean fields represent an abundant nectar source, as a single plant produces 200–800 flowers (van Schaik and Probst 1958) and each flower can yield 0.5 µl of nectar with sugar concentrations up to 45% (Erickson 1984). To this end, the legume is a common and preferred nectar source of honeybees (Apis mellifera L.) in Midwestern United States of America (Lin et al. 2022). Soybean is widely cultivated in Manitoba, Canada, with more than 1.1 million acres sown in 2020 (SOY Canada 2020). This includes nearly 80% of legumes produced in the province. In our study, the ubiquitous use of soybean as a nectar source in both rural and urban areas was particularly surprising, given the latter collection sites were consistently > 2 km away from a field. Mosquito species in the genera Culex and Aedes typically have flight capacities above this threshold (Verdonschot and Besse-Lototskaya 2014) and their dispersal could be aided by wind (Service 1997). Nonetheless, there appears to be at least some degree of nectar preferences exhibited by Prairie mosquitoes for soybean. Feeding upon Kentucky blue grass may be indicative of more opportunistic foraging, as the grass is an aggressive invasive species found throughout the sampling region (Palit et al. 2021).
In addition to preferential feeding on certain plant species, several other notable trends could be teased out of our dataset. Most of the nectar-fed mosquitoes captured were Ae. vexans, which was expected given it is the most pervasive species in the Canadian Prairies (Brust and Ellis 1976, Baril et al. 2023). While the sample sizes were considerably smaller for the other barcoded species, it appears that their preferred nectar sources differed and may be associated with their life history. For instance, Ae. triseriatus had the highest proportion of sugar meals derived from trees. Colloquially known as the eastern tree hole mosquito due to its propensity to oviposit in standing water found in tree holes of hardwood forests (Barker et al. 2003, Koloski et al. 2021), its feeding preferences may represent opportunistic behaviors. Similarly, mosquitoes from West Manitoba foraged more grasses and agricultural crops than those from the East Manitoba, which seems to correspond to the ecoregions within the province (Smith et al. 1998). Future studies are needed that associate the mosquito nectar sources with characteristics of the flora surrounding the trapping site (e.g., plant species abundance, distance from the site, and average crown expansion) in order to better determine the extent by which mosquito plant feeding behaviors are opportunistic and selective.
There are other considerations pertaining to mosquito nectar foraging associated with our study. We collected and barcoded only female mosquitoes, and given the extensive metabolic and physiological differences between sexes (Foster 1995, Lomeli and Dahanukar 2022) it is plausible that they exhibit divergent plant feeding behaviors. There is some evidence that the sucrose:hexose ratio of a plant dictates sexual dimorphism, where males favor sucrose-rich nectar and females prefer hexose-rich nectar (Grimstad and DeFoliart 1974, Magnarelli 1979, Baker and Baker 1983). Future work is needed to determine whether differences exist between sexes in their preferred nectar sources. The capacity of mosquitoes to play important roles in plant pollination is also not fully established. Although there is considerable evidence of pollination by mosquitoes (e.g., Coleman 1934, Brantjes and Leemans 1976, Peach and Gries 2016, Lahondère et al. 2020), it is rarely investigated with unequivocal evidence (Foster 2022). Moreover, it is difficult to ascertain whether a given mosquito species is an essential pollinator for a specific plant species (Foster 2022). Soybean are self-pollinating plants, though biotic pollinators are capable of pollinating its flowers and increasing crop productivity to some extent (Milfont et al. 2013, Cunha et al. 2023). Kentucky blue grass is an aggressive invasive species that is predominately pollinated by wind and can in fact detrimentally impact pollinator diversity (Pei et al. 2023). Given the 2 commonly identified nectar sources do not rely on pollinators and others that are not intended to be pollinated (e.g., ornamentals), the contribution of mosquitoes to plant pollination may not be too significant, at least in the Canadian Prairies.
In conclusion, we characterized the plants fed upon by female mosquitoes in Manitoba, Canada via DNA barcoding. This represents some of the first DNA-based evidence of nectar feeding behaviors of mosquitoes in Canada. Female mosquitoes foraged from a relatively small number of plant families that are varied, with soybean as their preferred nectar source. Nevertheless, nectar-fed mosquito species appeared to have different foraging preferences, which may be influenced by a variety of factors (e.g., landscape and geographical region). Future research is needed to determine the extent by which these preferences are opportunistic/selective, differ between sexes, and play roles in plant pollination. For instance, semifield experiments with a selection of plants provided could better disentangle plant-specific preferences. Moreover, studies aimed at discerning the precise source of the plant fluids in barcoded individuals (e.g., floral nectar, extra-floral nectar, plant sap/phloem, and aphid honeydew) would also be of interest.
Supplementary Material
Acknowledgments
We thank Cole Baril, Milah Mikkelsen, and Jessica Sparrow for assistance with mosquito collections. The authors also thank the City of Winnipeg Insect Control Branch for assistance with mosquito collections and Manitoba Public Health for use of trapping equipment.
Contributor Information
Bryan J Cassone, Department of Biology, Brandon University, Brandon, MB R7A 6A9, Canada.
Ben G Pilling, Department of Biology, Brandon University, Brandon, MB R7A 6A9, Canada.
Ana Borrego-Benjumea, Department of Biology, Brandon University, Brandon, MB R7A 6A9, Canada.
Christophe M R LeMoine, Department of Biology, Brandon University, Brandon, MB R7A 6A9, Canada.
Funding
This work was supported by the Public Health Agency of Canada (PHAC) and Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants Program. Both grants were awarded to Bryan Cassone.
Author Contributions
Bryan Cassone (Conceptualization [lead], Data curation [lead], Formal analysis [lead], Funding acquisition [lead], Investigation [equal], Project administration [lead], Resources [lead], Supervision [lead], Writing—original draft [lead]), Ben Pilling (Methodology [equal], Writing—review & editing [equal]), Ana Borrego-Benjumea (Methodology [equal], Writing—review & editing [lead]), and Christophe LeMoine (Formal analysis [supporting], Writing—review & editing [equal])
References
- Anderson JF, Main AJ, Armstrong PM, Andreadis TG, Ferrandino FJ.. Arboviruses in North Dakota, 2003–2006. Am J Trop Med Hyg. 2015:92(2):377–393. 10.4269/ajtmh.14-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankola K, Mahadevegowda L, Melichar T, Boregowda MH.. Advances in animal genomics. In: Mondal S, Singh RL, editors. DNA barcoding: nucleotide signature for identification and authentication of livestock. Cambridge (MA): Academic Press; 2021. p. 299–308. [Google Scholar]
- Bafeel SO, Arif IA, Bakir MA, Al Homaidan AA, Al Farhan AH, Khan HA.. DNA barcoding of arid wild plants using rbcL gene sequences. Genet Mol Res. 2012:11(3):1934–1941. 10.4238/2012.July.19.12 [DOI] [PubMed] [Google Scholar]
- Baril C, Pilling BG, Mikkelsen MJ, Sparrow JM, Duncan CAM, Koloski CW, LaZerte SE, Cassone BJ.. The influence of weather on the population dynamics of common mosquito vector species in the Canadian Prairies. Parasit Vectors. 2023:16(1):153. 10.1186/s13071-023-05760-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HG, Baker I.. The biology of nectaries. In: Bentley B, Elias T, editors. A brief historical review of the chemistry of floral nectar. New York (NY): Columbia University Press; 1983. p. 126–152. [Google Scholar]
- Barker CM, Paulson SL, Cantrell S, Davis BS.. Habitat preferences and phenology of Ochlerotatus triseriatus and Aedes albopictus (Diptera: Culicidae) in southwestern Virginia. J Med Entomol. 2003:40(4):403–410. 10.1603/0022-2585-40.4.403 [DOI] [PubMed] [Google Scholar]
- Barredo E, DeGennaro M.. Not just from blood: mosquito nutrient acquisition from nectar sources. Trends Parasitol. 2020:36(5):473–484. 10.1016/j.pt.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Beerntsen BT, James AA, Christensen BM.. Genetics of mosquito vector competence. Microbiol Mol Biol Rev. 2000:64(1):115–137. 10.1128/MMBR.64.1.115-137.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KL, Loeffler VM, Brosi BJ.. An rbcL reference library to aid in the identification of plant species mixtures by DNA metabarcoding. Appl Plant Sci. 2017:5(3):1600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernáth B, Anstett V, Guerin PM.. Anopheles gambiae females readily learn to associate complex visual cues with the quality of sugar sources. J Insect Physiol. 2016:95:8–16. 10.1016/j.jinsphys.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Berry RL, Parsons MA, Lalonde-Weigert BJ, Lebio J, Stegmiller H, Bear GT.. Aedes canadensis, a vector of La Crosse virus (California serogroup) in Ohio. J Am Mosq Control Assoc. 1986:2(1):73–78. [PubMed] [Google Scholar]
- Bowen MF. Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) females. J Med Entomol. 1992:29(5):843–849. 10.1093/jmedent/29.5.843 [DOI] [PubMed] [Google Scholar]
- Braks MAH, Juliano SA, Lounibos LP.. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Med Vet Entomol. 2006:20(1):53–59. 10.1111/j.1365-2915.2006.00612.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantjes NBM, Leemans JAAM.. Silene otites (Caryophyllaceae) pollinated by nocturnal lepidoptera and mosquitoes. Acta Botanica Neerlandica. 1976:25(4):281–295. 10.1111/j.1438-8677.1976.tb00240.x [DOI] [Google Scholar]
- Brust RA, Ellis RA.. Mosquito surveys in Manitoba during 1975. Can J Public Health. 1976:67(Suppl 1):47–53. [PubMed] [Google Scholar]
- Carpenter SJ, LaCasse WJ.. Mosquitoes of North America (North of Mexico). Berkeley (CA): University of California Press; 1955. [Google Scholar]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc Natl Acad Sci U S A. 2009:106:12794–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee DD, Sutherland JM, Gilles JRL.. Diel sugar feeding and reproductive behaviours of Aedes aegypti mosquitoes in Trinidad: with implications for mass release of sterile mosquitoes. Acta Trop. 2014:132(Suppl):S86–S90. 10.1016/j.actatropica.2013.09.019 [DOI] [PubMed] [Google Scholar]
- Coleman E. Pollination of Pterostylis acuminata R. BR. and Pterostylis falcata Rogers. Vic Nat. 1934:50:248–252. [Google Scholar]
- Cunha NL, Chacoff NP, Sáez A, et al. Soybean dependence on biotic pollination decreases with latitude—data and computer code. Agric Ecosyst Environ. 2023:347:108376. [Google Scholar]
- de Vere N, Rich TCG, Ford CR, Trinder SA, Long C, Moore CW, Satterthwaite D, Davies H, Allainguillaume J, Ronca S, et al. DNA barcoding the native flowering plants and conifers of wales. PLoS One. 2012:7(6):e37945. 10.1371/journal.pone.0037945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vere N, Jones LE, Gilmore T, Moscrop J, Lowe A, Smith D, Hegarty MJ, Creer S, Ford CR.. Using DNA metabarcoding to investigate honey bee foraging reveals limited flower use despite high floral availability. Sci Rep. 2017:7:42838. 10.1038/srep42838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebot M. Emerging mosquito-borne bunyaviruses in Canada. Can Commun Dis Rep. 2015:41(6):117–123. 10.14745/ccdr.v41i06a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson EH. Soybean pollination and honey production—a research progress report. Am Bee J. 1984:124:775–779. [Google Scholar]
- Fernandes L, Briegel H.. Reproductive physiology of Anopheles gambiae and Anopheles atroparvus. J Vector Ecol. 2005:30(1):11–26. [PubMed] [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995:40:443–474. 10.1146/annurev.en.40.010195.002303 [DOI] [PubMed] [Google Scholar]
- Foster WA. Sensory ecology of disease vectors. In: Ignell R, Lazzari CR, Lorenzo MG, Hill SR, editors. Behavioural ecology of plant-mosquito relations. The Netherlands: Wageningen Academic Publishers; 2022. p. 171–234. [Google Scholar]
- Gadawski RM, Smith SM.. Nectar sources and age structure in a population of Aedes provocans (Diptera: Culicidae). J Med Entomol. 1992:29(5):879–886. 10.1093/jmedent/29.5.879 [DOI] [PubMed] [Google Scholar]
- Garcia-Robledo C, Erickson DL, Staines CL, Erwin TL, Kress WJ.. Tropical plant-herbivore networks: reconstructing species interactions using DNA barcodes. PLoS One. 2013:8(1):e52967. 10.1371/journal.pone.0052967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm DA, Pennington JE, Wells MA.. The role of hemolymph proline as a nitrogen sink during blood meal digestion by the mosquito Aedes aegypti. J Insect Physiol. 2003:49(2):115–121. 10.1016/s0022-1910(02)00267-6 [DOI] [PubMed] [Google Scholar]
- Gouagna LC, Kerampran R, Lebon C, Brengues C, Toty C, Wilkinson DA, Boyer S, Fontenille D.. Sugar-source preference, sugar intake and relative nutritional benefits in Anopheles arabiensis males. Acta Trop. 2014:132(Suppl):S70–S79. 10.1016/j.actatropica.2013.09.022 [DOI] [PubMed] [Google Scholar]
- Gravendeel B, Eurlings M, Heijerman T.. Use of DNA barcoding for host plant identification. Entomol Ber. 2009:69:30–35. [Google Scholar]
- Grimstad PR, DeFoliart GR.. Nectar sources of Wisconsin mosquitoes. J Med Entomol. 1974:11(3):331–341. 10.1093/jmedent/11.3.331 [DOI] [PubMed] [Google Scholar]
- Hutcheson RP, Ebrahimi B, Njiru BN, Foster WA, Jany W.. Differences between Aedes aegypti and Aedes albopictus in attraction to several release-rates and ratios of a 3-part phytochemical blend in a mesocosm. J Med Entomol. 2022:59(2):440–445. 10.1093/jme/tjab195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhumur US, Dötterl S, Jürgens A.. Naïve and conditioned responses of Culex pipiens pipiens biotype molestus (Diptera: Culicidae) to flower odors. J Med Entomol. 2006:43(6):1164–1170. 10.1093/jmedent/43.6.1164 [DOI] [PubMed] [Google Scholar]
- Junnila A, Müller GC, Schlein Y.. Species identification of plant tissues from the gut of An. sergentii by DNA analysis. Acta Trop. 2010:115(3):227–233. 10.1016/j.actatropica.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Junnila A, Muller GC, Schlein Y.. Identification of plant tissues from the gut of Phlebotomus papatasi by DNA analysis. Acta Trop. 2011:117(1):14–18. 10.1016/j.actatropica.2010.08.019 [DOI] [PubMed] [Google Scholar]
- Kajtoch LA. DNA metabarcoding study of a polyphagous beetle dietary diversity: the utility of barcodes and sequencing techniques. Folia Biol. 2014:62(3):223–234. 10.3409/fb62_3.223 [DOI] [PubMed] [Google Scholar]
- Kessler S, Vlimant M, Guerin PM.. Sugar-sensitive neurone responses and sugar feeding preferences influence lifespan and biting behaviours of the Afrotropical malaria mosquito, Anopheles gambiae. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015:201(3):317–329. 10.1007/s00359-015-0978-7 [DOI] [PubMed] [Google Scholar]
- Koloski CW, Drahun I, Cassone BJ.. Occurrence of the mosquito Aedes triseriatus (Diptera: Culicidae) beyond its most northwestern range limits in Manitoba, Canada. J Med Entomol. 2021:58(4):1958–1961. 10.1093/jme/tjab021 [DOI] [PubMed] [Google Scholar]
- Kress WJ, Erickson DL.. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS One. 2007:2(6):e508. 10.1371/journal.pone.0000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress WJ, Erickson DL, Andrew Jones F, et al. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc Natl Acad Sci U S A. 2009:106:18621–18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahondère C, Vinauger C, Okuboa RP, et al. The olfactory basis of orchid pollination by mosquitoes. Proc Natl Acad Sci U S A. 2020:117:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LHG, Mesquita MR, Skrip L, et al. DNA barcode for the identification of the sand fly Lutzomyia longipalpis plant feeding preferences in a tropical urban environment. Sci Rep. 2016:6:29742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Suresh S, Matcham E, Monagan P, Curtis H, Richardson RT, Johnson RM.. Soybean is a common nectar source for honey bees (Hymenoptera: Apidae) in a Midwestern agricultural landscape. J Econ Entomol. 2022:115(6):1846–1851. 10.1093/jee/toac140 [DOI] [PubMed] [Google Scholar]
- Lomeli AM, Dahanukar AA.. Sensory ecology of disease vectors. In: Ignell R, Lazzari CR, Lorenzo MG, Hill SR, editors. Host-plant feeding in mosquitoes. The Netherlands: Wageningen Academic Publishers; 2022. p. 449–468. [Google Scholar]
- Lothrop HD, Wheeler SS, Fang Y, Reisen WK.. Use of scented sugar bait stations to track mosquito-borne arbovirus transmission in California. J Med Entomol. 2012:49(6):1466–1472. 10.1603/me12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA. Nectar feeding by Aedes sollicitqns and its relation to gonotrophic activity. Environ Entomol. 1977:6(2):237–242. 10.1093/ee/6.2.237 [DOI] [Google Scholar]
- Magnarelli LA. Bionomics of the salt-marsh mosquito, Aedes cantator (Diptera: Culicidae). Environ Entomol. 1978:7(4):512–517. 10.1093/ee/7.4.512 [DOI] [Google Scholar]
- Magnarelli LA. Diurnal nectar feeding of Aedes cantator and A. solicitans (Diptera: Culicidae). Environ Entomol. 1979:8(5):949–955. 10.1093/ee/8.5.949 [DOI] [Google Scholar]
- Matheson CD, Muller GC, Junnila A, Vernon K, Hausmann A, Miller M, Greenblatt C, Schlein Y.. A PCR method for detection of plant meals from the guts of insects. Org Divers Evol. 2007:7(4):294–303. 10.1016/j.ode.2006.09.002 [DOI] [Google Scholar]
- McMahon TJ, Galloway TD, Anderson RA.. Tires as larval habitats for mosquitoes (Diptera: Culicidae) in southern Manitoba, Canada. J Vector Ecol. 2008:33(1):198–204. 10.3376/1081-1710(2008)33[198:talhfm]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Melgarejo-Colmenares K, Cardo MV, Vezzani D.. Blood feeding habits of mosquitoes: hardly a bite in South America. Parasitol Res. 2022:121(7):1829–1852. 10.1007/s00436-022-07537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milfont M, Rocha EEM, Lima AON, Freitas BM.. Higher soybean production using honeybee and wild pollinators, a sustainable alternative to pesticides and autopollination. Environ Chem Lett. 2013:11:335–341. [Google Scholar]
- Molaei G, Andreadis T, Armstrong P, Diuk-Wasser M.. Host-feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J Med Entomol. 2008:45:1143–1151. [DOI] [PubMed] [Google Scholar]
- Nasci RS. Variations in the blood-feeding patterns of Aedes vexans and Aedes trivittatus (Diptera: Culicidae). J Med Entomol. 1984:21(1):95–99. 10.1093/jmedent/21.1.95 [DOI] [PubMed] [Google Scholar]
- Nyasembe VO, Tchouassi DP, Mbogo CM, Sole CL, Pirk C, Torto B.. Linalool oxide: generalist plant based lure for mosquito disease vectors. Parasit Vectors. 2015:8:581. 10.1186/s13071-015-1184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Tchouassi DP, Pirk CWW, Sole CL, Torto B.. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Negl Trop Dis. 2018:12(2):e0006185. 10.1371/journal.pntd.0006185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KL, Bixby MA, Morin KJ, Bradley DS, Vaughan JA.. Potential of a northern population of Aedes vexans (Diptera: Culicidae) to transmit Zika virus. J Med Entomol. 2017:54(5):1354–1359. 10.1093/jme/tjx087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palit R, Gramig G, DeKeyser ES.. Kentucky bluegrass invasion in the Northern Great Plains and prospective management approaches to mitigate its spread. Plants (Basel) 2021:10(4):817. 10.3390/plants10040817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry R, Naccache F, Ndiaye EH, Fall G, Castelli I, Lühken R, Medlock J, Cull B, Hesson JC, Montarsi F, et al. Identification and RNAi profile of a novel iflavirus infecting Senegalese Aedes vexans arabiensis mosquitoes. Viruses. 2020:12(4):440. 10.3390/v12040440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach DAH, Gries G.. Nectar thieves or invited pollinators? A case study of tansy flowers and common house mosquitoes. Arthropod Plant Interact. 2016:10(6):497–506. 10.1007/s11829-016-9445-9 [DOI] [Google Scholar]
- Peach DAH, Ko E, Blake AJ, Gries G.. Ultraviolet inflorescence cues enhance attractiveness of inflorescence odour to Culex pipiens mosquitoes. PLoS One. 2019:14(6):e0217484. 10.1371/journal.pone.0217484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei CK, Hovick TJ, Limb RF, Harmon JP, Geaumont BA.. Invasive grass and litter accumulation constrain bee and plant diversity in altered grasslands. Global Ecol Conserv. 2023:41:e02352. 10.1016/j.gecco.2022.e02352 [DOI] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2021. https://www.R-project.org [Google Scholar]
- Sandholm HA, Price RD.. Field observations on the nectar-feeding habits of some Minnesota mosquitoes. Mosq News. 1962:22:346–349. [Google Scholar]
- Sanford MR, Olson JK, Lewis WJ, Tomberlin JK.. The effect of sucrose concentration on olfactory-based associative learning in Culex quinquefasciatus. J Insect Behav. 2013:26:494–513. [Google Scholar]
- Service MW. Mosquito (Diptera: Culicidae) dispersal—the long and short of it. J Med Entomol. 1997:34(6):579–588. 10.1093/jmedent/34.6.579 [DOI] [PubMed] [Google Scholar]
- Smith RE, Veldhuis H, Mills GF, et al. Terrestrial ecozones, ecoregions, and ecodistricts of Manitoba: an ecological stratification of Manitoba’s natural landscapes. Technical bulletin; 1998-9E. Canada: Agriculture and Agri-Food Canada, Research Branch. [Google Scholar]
- SOY Canada. 2020. Canadian soybean seeded acres (1980 to current). https://soycanada.ca/industry/statistics/seeded-area-acres/ [accessed June 2023].
- Staudacher K, Wallinger C, Schallhart N, Traugott M.. Detecting ingested plant DNA in soil-living insect larvae. Soil Biol Biochem. 2011:43(2):346–350. 10.1016/j.soilbio.2010.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner CD, Riemersma KK, Stuart JB, Singapuri A, Lothrop HD, Coffey LL.. Scented sugar baits enhance detection of St. Louis Encephalitis and West Nile viruses in mosquitoes in suburban California. J Med Entomol. 2018:55(5):1307–1318. 10.1093/jme/tjy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoutamire WP. Mosquito pollination of Habenaria obtusata (Orchidaceae). Mich Bot. 1968:7:203–212. [Google Scholar]
- Thielman AC, Hunter FF.. Photographic key to the adult female mosquitoes (Diptera: Culicidae) of Canada. Can J Arthropod Identif. 2007:4:14. [Google Scholar]
- Thien LB, Utech F.. The mode of pollination in Habenaria obtusata (Orchidaceae). Am J Bot. 1970:57(9):1031–1035. 10.1002/j.1537-2197.1970.tb09905.x [DOI] [Google Scholar]
- van Schaik PH, Probst AH.. Effects of some environmental factors on flower production and reproductive efficiency in soybeans. Agron J. 1958:50(4):192–197. 10.2134/agronj1958.00021962005000040007x [DOI] [Google Scholar]
- Verdonschot PFM, Besse-Lototskaya AA.. Flight distance of mosquitoes (Culicidae): a metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica. 2014:45:69–79. [Google Scholar]
- Weissmann M. Mosquito of the month: Aedes vexans—the inland floodwater mosquito; 2016. https://www.vdci.net/blog/mosquito-of-the-month-aedes-vexans-the-inland-floodwater-mosquito [accessed 14 July 2023].
- Wood DM, Dang PT, Ellis RA.. The insects and arachnids of Canada, Part 6. The mosquitoes of Canada: Diptera: Culicidae. Hull, Quebec, Canada: Agriculture Canada Research Branch; 1979. [Google Scholar]
- Yu B-T, Hu Y, Ding Y-M, Tian J-X, Mo J-C.. Feeding on different attractive flowering plants affects the energy reserves of Culex pipiens pallens adults. Parasitol Res. 2018:117(1):67–73. 10.1007/s00436-017-5664-y [DOI] [PubMed] [Google Scholar]
- Yunik MEM, Galloway TD, Lindsay RL.. Assessment of prevalence and distribution of spotted fever group Rickettsiae in Manitoba, Canada, in the American dog tick, Dermacentor variabilis (Acari: Ixodidae). Vector Borne Zoonotic Dis. 2015:15:103–108. [DOI] [PubMed] [Google Scholar]
- Zhou G, Kohlhepp P, Geiser D, del Carmen Frasquillo M, Vazquez-Moreno L, Winzerling JJ.. Fate of blood meal iron in mosquitos. J Insect Physiol. 2007:53:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.