ABSTRACT
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne bunyavirus with high pathogenicity. There has been a gradual increase in the number of reported cases in recent years, with high morbidity and mortality rates. The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway plays an important role in the innate immune defense activated by viral infection; however, the role of the cGAS-STING signaling pathway during SFTSV infection is still unclear. In this study, we investigated the relationship between SFTSV infection and cGAS-STING signaling. We found that SFTSV infection caused the release of mitochondrial DNA into the cytoplasm and inhibits downstream innate immune signaling pathways by activating the cytoplasmic DNA receptor cGAS. We found that the SFTSV envelope glycoprotein Gn was a potent inhibitor of the cGAS-STING pathway and blocked the nuclear accumulation of interferon regulatory factor 3 and p65 to inhibit downstream innate immune signaling. Gn of SFTSV interacted with STING to inhibit STING dimerization and inhibited K27-ubiquitin modification of STING to disrupt the assembly of the STING-TANK-binding kinase 1 complex and downstream signaling. In addition, Gn was found to be involved in inducing STING degradation, further inhibiting the downstream immune response. In conclusion, this study identified the important role of the glycoprotein Gn in the antiviral innate immune response and revealed a novel mechanism of immune escape for SFTSV. Moreover, this study increases the understanding of the pathogenic mechanism of SFTSV and provides new insights for further treatment of SFTS.
IMPORTANCE
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly discovered virus associated with severe hemorrhagic fever in humans. However, the role of the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway during SFTSV infection is still unclear. We found that SFTSV infection inhibits downstream innate immune signaling pathways by activating the cytoplasmic DNA receptor cGAS. In addition, SFTSV Gn blocked the nuclear accumulation of interferon regulatory factor 3 and p65 to inhibit downstream innate immune signaling. Moreover, we determined that Gn of SFTSV inhibited K27-ubiquitin modification of STING to disrupt the assembly of the STING-TANK-binding kinase 1 complex and downstream signaling. We found that the SFTSV envelope glycoprotein Gn is a potent inhibitor of the cGAS-STING pathway. In conclusion, this study highlights the crucial function of the glycoprotein Gn in the antiviral innate immune response and reveals a new method of immune escape of SFTSV.
KEYWORDS: severe fever with thrombocytopenia syndrome virus (SFTSV), cyclic GMP-AMP synthase (cGAS), stimulator of interferon genes (STING), mitochondrial DNA, innate immunity, Gn, infection
INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease caused by the SFTS virus (SFTSV), which was first discovered in China in 2009 (1). SFTSV belongs to the genus Bandavirus of the family Phenuiviridae and order Bunyavirales according to the latest revision of the International Committee on Taxonomy of Viruses in 2020 (2). Ticks are the crucial vector for the transmission of SFTSV to humans, and human-to-human transmission has also been reported, occurring, for example, through contact with a patient’s blood or body fluids (3). In China, SFTS cases are concentrated mainly in Henan, Anhui, and Shandong provinces and show seasonal characteristics, with a high incidence in summer and autumn (4).
Since the discovery of SFTSV, confirmed cases of SFTSV infection have been reported in Korea, Japan, Vietnam, and Myanmar (5). People of all age groups can be infected with SFTSV, and elderly individuals are highly susceptible to infection, with a mortality rate of up to 30% (6). In people infected with SFTSV, the main clinical manifestations are high fever, thrombocytopenia, leukocytopenia, gastrointestinal symptoms, and damage to liver and kidney function, and severe cases may be accompanied by bleeding (7). Currently, there are no effective therapeutics or vaccines available to combat SFTSV infection; thus, SFTSV poses a future threat to global public health. Therefore, it is still important to actively carry out research on SFTSV.
SFTSV is an enveloped, single-stranded, negative-sense RNA virus constituting a spherical particle covered by an envelope consisting of a lipid bilayer with glycoprotein spines. The SFTSV genome consists of three RNA segments: the large (L), medium (M), and small (S) segments. The L segment encodes the RNA-dependent RNA polymerase, which acts as a viral transcriptase/replicase. The two viral surface glycoproteins, Gn and Gc, are encoded by the M segment. The S segment is a double-stranded RNA that is composed of the genes encoding the nonstructural protein NSs and the nuclear protein NP (8). NSs, which has the potential to act as an important virulence factor for SFTSV, impairs the innate antiviral response of host cells. In addition, NSs is related to the cell cycle, viral adsorption, and viral entry (9, 10). NP promotes the encapsulation of SFTSV RNA into the capsid while also promoting SFTSV RNA transcription and replication as well as the assembly of viral particles. Gn and Gc are involved in the processes of SFTSV assembly and attachment to new target cells (11).
Innate immunity is the host’s first line of defense against invading microorganisms. The innate immune response plays an important role in resistance to SFTSV infection. However, findings have shown that viruses can evade the host immune response through a variety of strategies (12). It is conceivable that SFTSV, as an RNA virus, needs to antagonize the RLR pathway (13–16). However, interestingly, it may also be able to antagonize cGAS-STING signaling activity (17), given that accumulating evidence indicates that cGAS-STING activation can also be inhibited by infecting with RNA viruses (18–23). The correlation between SFTSV and the cGAS-STING signaling pathway is unclear; therefore, in the present study, we explored the relationship between SFTSV and the cGAS-STING signaling pathway in depth and evaluated the ability of SFTSV Gn to antagonize the host innate immune response. We discovered that the viral protein Gn inhibits the innate immune signaling pathway and elucidated the specific mechanism and process through which Gn suppresses the cGAS-STING pathway. These findings have implications for SFTSV pathogenesis and reveal potential targets for the development of therapeutic strategies against SFTSV.
RESULTS
Mitochondrial DNA released into the cytosol upon SFTSV infection is captured by cGAS and inhibits the cGAS-STING signaling pathway in the late stages of infection
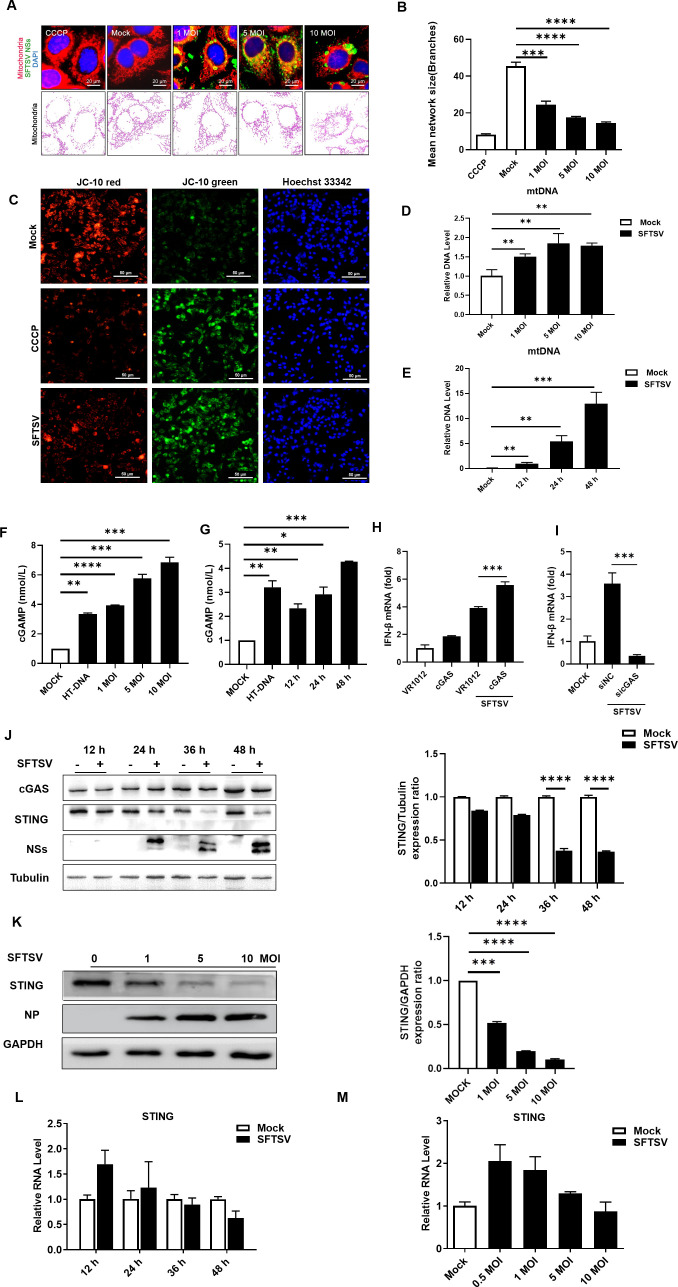
Recent reports have demonstrated that RNA virus infection can cause the release of Mitochondrial DNA (mtDNA) from mitochondria into the cytosol and the consequent priming of innate immune responses via the cytosolic DNA sensor cGAS (24). To further investigate the relationship between SFTSV infection and the cGAS-STING signaling pathway, we first assessed morphological changes in mitochondria after SFTSV infection; to this end, HeLa cells were infected with SFTSV at the multiplicity of infection (MOI) of 0, 1, 5, and 10. Confocal microscopy of the cells after SFTSV infection revealed significant elongation; as the MOI increased, the mitochondrial network was gradually disrupted, and a mesh of highly interconnected, thin, mitochondrial filaments was formed, in contrast with the observations in uninfected cells, indicating that mitochondrial damage occurred after SFTSV infection (Fig. 1A and B). We then assessed whether SFTSV infection led to mitochondrial dysfunction and loss of mitochondrial integrity. Loss of mitochondrial membrane potential is associated with mitochondrial dysfunction (25). Therefore, we measured the mitochondrial membrane potential after SFTSV infection. When the mitochondrial membrane potential is high, JC-10 aggregates in the mitochondrial matrix to form a polymer that emits red fluorescence; when the mitochondrial membrane potential is low, JC-10 cannot aggregate in the mitochondrial matrix and remains a monomer that emits green fluorescence. Treatment with carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was included as the positive control, as previously described (26). The results showed that SFTSV infection induced the loss of mitochondrial membrane potential (Fig. 1C). We next determined whether SFTSV infection can induce the release of mitochondrial DNA into the cytoplasm; to this end, HeLa cells were infected with SFTSV at MOIs of 0, 1, 5, and 10; infected with SFTSV at a constant MOI (MOI = 1) for different durations; or mock-infected. After 36 h, cells were collected, and we evaluated extramitochondrial DNA, with quantitative real-time PCR (qPCR) used to determine the abundance of mtDNA. An analysis of pure cytosolic extracts revealed an increase in cytosolic mtDNA with increasing MOI and increasing duration of SFTSV infection (Fig. 1D and E). Previous work has shown that during SFTSV infection, the viral protein NP interacts with scaffold attachment factor A (SAFA) and recognizes viral genomic RNA to form a ternary complex, which activates STING and thus counteracts SFTSV infection (27). Here, to better demonstrate that the mtDNA released after SFTSV infection directly activates the cGAS-STING pathway, we determined the intracellular cyclic GMP-AMP (cGAMP) content in cells with viral infection. When cGAS is activated, it results in the release of the second messenger cGAMP, which activates the downstream protein STING (28, 29), and the enzyme-linked immunosorbent assay (ELISA) results showed that the mtDNA released after SFTSV infection directly activates the cGAS-STING signaling pathway (Fig. 1F and G).
Fig 1.
mtDNA released into the cytosol upon SFTSV infection is captured by cGAS and inhibits the cGAS-STING signaling pathway in the late stages of infection. SFTSV infection induces mitochondrial damage to release mtDNA into the cytosol, where it is captured by cGAS, resulting in the inhibition of the cGAS-STING signaling pathway in the late stages of infection. (A, B) Confocal micrographs of mitochondria in HeLa cells infected with SFTSV after 24 h. The results were analyzed via the MiNA workflow, and ImageJ was used to calculate the ratio of cells that contained fragmented mitochondria to total cells. Treatment with CCCP (5 µM) was performed as the positive control. (C) Mitochondrial membrane potential values after SFTSV infection were measured using the JC-10 mitochondrial membrane potential fluorescent probe. CCCP (5 µM) was used as the positive control. (D) Abundance of mtDNA in the cytosol of HeLa cells infected with SFTSV at various MOIs (MOI = 0, 1, 5, and 10). The abundances of specific DNA fragments were quantified by qPCR. (E) Abundance of mtDNA in the cytosol of SFTSV-infected HeLa cells over time (12, 24, and 48 h). The abundances of specific DNA fragments were quantified by qPCR. (F, G) A reagent kit was used to measure the amount of cGAMP released after infection with SFTSV at different MOIs and for different durations. (H) cGAS (200 ng of plasmid) was overexpressed in HeLa cells. The cells were infected with SFTSV, and RNA isolated 48 h postinfection (hpi) was analyzed for interferon (IFN)-β expression by RT-qPCR. A t-test was used for statistical analysis. (I) cGAS was knocked down in HeLa cells. The cells were infected with SFTSV, and RNA isolated 6 hpi was analyzed for IFN-β expression by RT-qPCR. A t-test was used for statistical analysis. (J) HeLa cells were infected with SFTSV at an MOI of 1. The cells were collected at 12, 24, 36, and 48 hpi, and the expression of cGAS and STING was confirmed by Western blotting. The gray values were analyzed by ImageJ and Prism software. (K) HeLa cells were infected with SFTSV (MOI = 0, 1, 5, and 10) and collected 36 h postinfection, and the expression of cGAS and STING was analyzed by Western blotting. The gray values were analyzed by ImageJ and Prism software. (L) HeLa cells were infected with SFTSV at an MOI of 1. The cells were collected 12, 24, 36, and 48 hpi, and the mRNA level of STING was measured via RT-qPCR. (M) HeLa cells were infected with SFTSV (MOI = 0, 0.5, 1, 5, or 10), the cells were collected 48 h postinfection, and the mRNA level of STING was measured via RT-qPCR.
When viruses or other pathogens invade a host cell, nucleic acid sensor molecules in the host cell recognize the nucleic acid molecules derived from the invading pathogen and trigger a downstream signaling process. It has been shown that during SFTSV infection, the innate immune response is the first line of host defense and is dependent mainly on pattern recognition receptors (PRRs). The nucleic acids derived from SFTSV are typical pathogen-associated molecular patterns (PAMPs) that can be specifically targeted by various nucleic acid PRRs. RLRs are important for detecting RNA virus infections via the direct recognition of viral RNA; activated RIG-I recruits the mitochondrial outer membrane protein MAVS and mediates the activation of downstream signaling cascades (30, 31). Moreover, a different type of DNA sensor, cGAS, also exists in cells (32–37); this protein recognizes intracellular mtDNA and is activated and potentially involved in the SFTSV-induced innate immune response (38–41).
Thus, we aimed to prove the direct involvement of mtDNA release in the innate immune response in SFTSV-infected cells. Previous reports have shown that IFN levels first increase and then decrease after SFTSV infection. IFN levels are two to three times greater in infected individuals after 6 h of viral infection than in noninfected cells and subsequently decrease (42). Therefore, we overexpressed the cGAS protein in SFTSV-infected HeLa cells and found that overexpression of cGAS after infection increased IFN-β production following SFTSV infection (Fig. 1H). Moreover, we knocked down the expression of the sensor molecule cGAS that detects intracellular foreign nucleic acids; as expected, cGAS knockdown suppressed IFN-β expression to some extent (Fig. 1I). Thus, cGAS was confirmed to play an important role in activating innate immune responses in host cells during SFTSV infection.
Therefore, the above results suggested that mtDNA released into the cytosol upon SFTSV infection was captured by cGAS and activated cGAS-STING-induced innate immune responses in host cells.
Several studies have shown that viruses can negatively regulate innate immunity by targeting the cGAS-STING proteins and affecting their biological functions (43). We further confirmed whether SFTSV infection exerts a regulatory effect on the cGAS-STING signaling pathway.
Pathogenic DNA is detected via the cGAS-STING signaling axis, which consists of cGAS and the receptor STING, and this process can trigger an innate immune response involving a strong type I interferon response against microbial infections. cGAS catalyzes the conversion of ATP and GTP into the second messenger cGAMP, which is then detected by the cyclic dinucleotide sensor STING, a transmembrane protein at the endoplasmic reticulum (ER). Upon binding to DNA, cGAS undergoes a conformational change to an active state. Ubiquitination controls the process by which cGAMP binding activates STING, which subsequently translocates to the Golgi and activates TANK-binding kinase 1 (TBK1). The interferon regulatory factor 3 (IRF3) transcription factor and additional IKK kinases are subsequently activated upon TBK1 autophosphorylation. IRF3 is translocated into the nucleus, where it induces the production of type I interferons and, subsequently, the expression of interferon-stimulated genes, which together orchestrate antiviral defense mechanisms (44).
We found that there was no significant change in cGAS expression in infected cells compared with uninfected cells in either the early or late phase of SFTSV infection, while STING was downregulated in the late phase of infection but was not significantly downregulated in the early phase of infection (Fig. 1J). Moreover, we found that the expression level of STING decreased with increasing SFTSV MOI (Fig. 1K), indicating that SFTSV infection leads to STING degradation in the late phase. Thus, SFTSV inhibits the cGAS-STING signaling pathway in the late phase. To further confirm these experimental results, we also measured the transcript level of STING by reverse transcription-quantitative (RT-qPCR) and found decreasing trends with increasing duration of SFTSV infection and increasing MOI (Fig. 1L and M). These findings indicated that viral infection decreased the protein and transcript levels of STING in a time-dependent and viral titer-dependent manner. Taken together, these findings suggest that SFTSV infection suppresses the innate immune pathway by targeting the STING protein.
The SFTSV viral protein Gn inhibits the cGAS-STING-mediated innate immune response
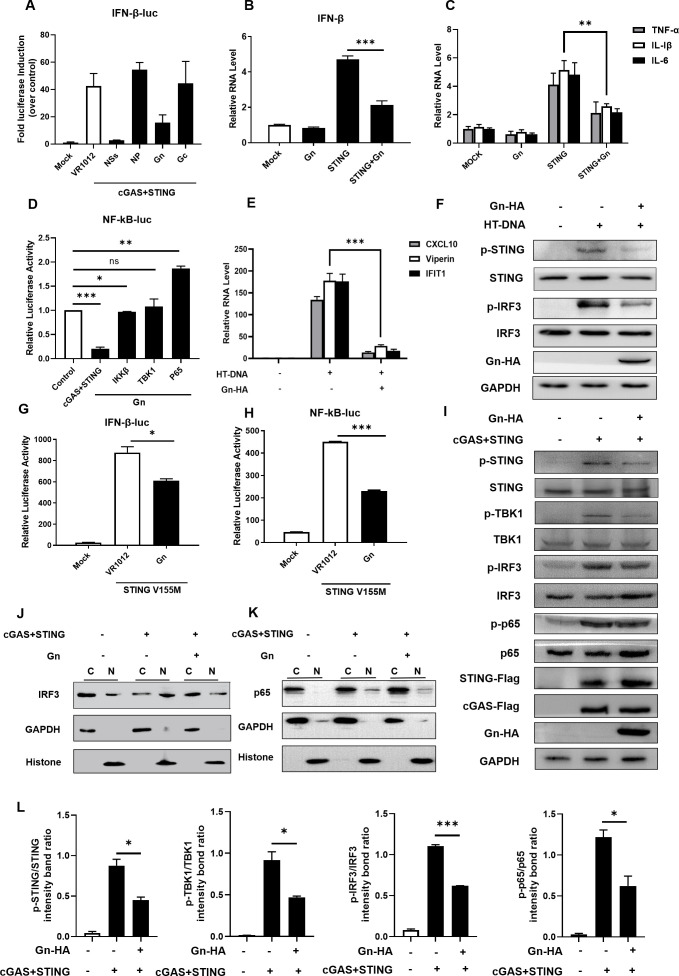
Multiple innate immune escape strategies of SFTSV during host confrontation have been proposed; for example, previous studies have reported that multiple important molecules in innate immune signaling pathways, such as TBK1, IKKε, RIG-1, STAT1, STAT2, IRF3, and IRF7, are sequestered by SFTSV NSs into inclusion bodies to suppress antiviral innate immunity (45–49). Since the modulation of the cGAS-STING function by SFTSV has not yet been reported, we characterized the suppression of cGAS-STING by SFTSV proteins in greater detail. In a luciferase reporter assay, we evaluated innate immune modulation by various viral proteins of SFTSV, and we observed that Gn and NSs could inhibit immune responses induced via the cGAS-STING pathway (Fig. 2A). Previous studies demonstrated that N-glycosylated and correctly folded Gn and Gc form noncovalently linked heterodimers in the ER and that after Gn/Gc dimerization, the glycoproteins exit the ER and translocate to the Golgi apparatus (50). The sites of Gn formation and function are the endoplasmic reticulum and Golgi apparatus, and the biological processes of cGAS-STING also occur in the same locations. Therefore, we selected the viral envelope glycoprotein Gn for subsequent experiments, and to further confirm the abovementioned conclusion, RT-qPCR was performed in HeLa cells to measure the mRNA level of IFN-β and the transcription of NF-κB-dependent genes. Gn was found to inhibit the expression of IFN-β as well as the transcription of NF-κB-dependent genes (Fig. 2B and C). In addition, we found that the viral protein Gn appeared to preferentially inhibit STING because Gn more strongly suppressed the innate immune response induced by cGAS-STING than it suppressed the downstream factors in the cGAS-STING pathway, such as IKKβ, TBK1, and p65 (Fig. 2D).
Fig 2.
The SFTSV viral protein Gn inhibits the cGAS-STING-mediated innate immune response. SFTSV Gn inhibited the cGAS-STING-mediated downstream innate immune response. (A) HEK293T cells were cotransfected with IFN-β Luc and the cGAS-STING, NSs, NP, Gn, or Gc expression vector. A luciferase assay and Western blot analysis were performed 36 h after transfection. (B) HeLa cells were transfected with the VR1012, Gn (200 ng), STING (200 ng), or STING and Gn plasmids. Cellular RNA was extracted 36 h after transfection, and the RNA level of IFN-β was measured by RT-qPCR. (C) HeLa cells were transfected with VR1012, Gn (200 ng), STING (200 ng), or STING and Gn plasmids. Cellular RNA was extracted 36 h after transfection, and the RNA levels of TNF-α, IL-1β, and IL-6 were measured by RT-qPCR. (D) Comparison of Gn-mediated inhibition of NF-κB signaling induced by cGAS (200 ng), STING (200 ng), IKKβ (200 ng), TBK1 (200 ng), and p65 (200 ng). HEK293T cells were cotransfected with the NF-κB-Luc (200 ng) and cGAS (200 ng), STING (200 ng), IKKβ (200 ng), TBK1 (200 ng), or p65 (200 ng) expression vectors in the presence or absence of the Gn expression vector. The values in cells transfected with cGAS (200 ng), STING (200 ng), IKKβ (200 ng), TBK1 (200 ng), or p65 (200 ng) alone were set to 1, as appropriate. (E) HeLa cells were transfected with VR1012, HT-DNA, or HT-DNA and Gn (200 ng) for 36 h, after which the RNA levels of CXCL10, viperin, and IFIT1 were measured via RT-qPCR. A t-test was used for statistical analysis. (F) The degree of phosphorylation of STING and IRF3 was evaluated. (G, H) HEK293T cells were transfected with the IFN-β Luc/NF-κB Luc reporter plasmid and pRL-SV40, VR1012, STING V155M, or STING V155M and Gn. Transactivation of the luciferase reporter was evaluated 36 h after transfection. (I) HeLa cells were cotransfected with STING-Flag (200 ng), cGAS-Flag (200 ng), Gn-HA (200 ng), or the control vector. After 36 h, the cells were harvested, and the protein expression levels were analyzed by immunoblotting. (J, K) Gn inhibits the nuclear translocation of IRF3 and p65. HEK293T cells were transfected with the control vector, STING-Flag (200 ng), cGAS-Flag (200 ng), or STING-Flag and cGAS-Flag plus Gn-HA (200 ng), as indicated. The cells were harvested, total cell lysates were prepared, and the nuclear (N) and cytoplasmic (C) fractions were separated 36 h after transfection. The indicated proteins were analyzed by immunoblotting using anti-p65 and anti-IRF3 antibodies. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH and histone were used as controls and detected using anti-GAPDH and anti-histone antibodies, respectively. (L) Degradation levels of p-STING, p-TBK1, p-IRF3, and p-p65 in (I) were quantified using ImageJ and Prism, respectively.
HT-DNA is a double-stranded DNA analog that binds and activates the cGAS protein, which in turn activates the downstream immune pathway (51). We examined the effect of Gn on the HT-DNA-activated cGAS-STING immune pathway as an activator and found that Gn inhibited the transcription of CXCL10, viperin, and IFIT1 (Fig. 2E). We found that Gn inhibited the increases in the protein levels of p-STING and p-IRF3 induced by HT-DNA, as determined by immunoblotting (Fig. 2F). In addition, STING V155M is a constitutively activated STING mutant that has been shown in multiple experiments to activate the downstream IFN-β and NF-κB signaling pathways at sustained high levels (52). We used this mutant to examine the effect of the Gn protein on cGAS-STING-mediated immune pathway activation. Gn inhibited the function of the STING mutant V155M in the absence of cGAS (Fig. 2G and H). We further examined the effect of Gn on downstream effectors of the cGAS-STING signaling pathway and IRF3 and p65 translocation from the cytoplasm into nuclei.
cGAS-STING and SFTSV Gn were ectopically overexpressed in HeLa cells, and we observed by examining the protein levels of p-STING, p-IRF3, p-TBK1, and p-p65 that Gn inhibited the phosphorylation of STING, IRF3, TBK1, and p65 activated by cGAS-STING, suggesting that Gn blocks the relevant intermediate steps of cGAS-STING signaling (Fig. 2I). And by quantitative analysis, we could find decreased levels of p-STING, p-IRF3, p-TBK1, and p-p65 (Fig. 2L). Further nuclear–cytoplasmic fractionation experiments showed that Gn inhibited the nuclear accumulation of P65 and IRF3, which is activated by cGAS-STING signaling activity (Fig. 2J and K). In addition, the inhibition of IRF3 and p65 phosphorylation by Gn also indicated that the nuclear translocation of IRF3 and p65 was inhibited (53–55).
Taken together, the above results suggest that Gn can inhibit innate immune signaling induced by cGAS-STING and suppress the production of the downstream inflammatory factors.
Gn interacts with STING and affects STING dimerization
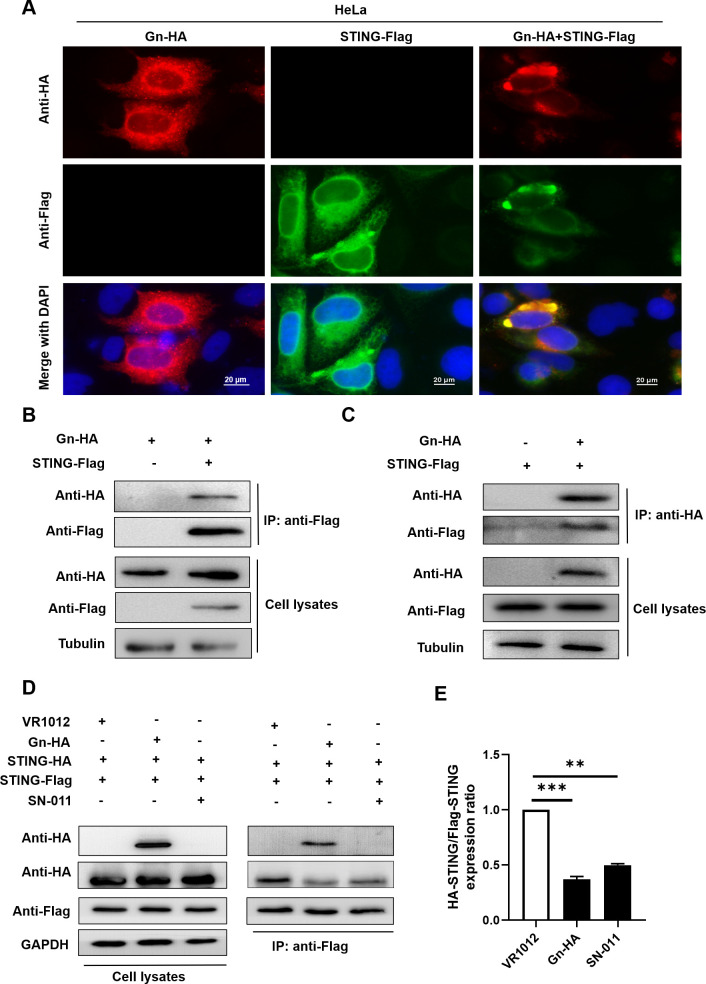
The above results confirmed that the cGAS-STING signaling pathway is inhibited in the late phase of SFTSV infection and that the Gn glycoprotein plays an important role in inhibiting the innate immune signaling pathway activated by cGAS-STING. To further investigate the specific mechanism by which Gn suppresses innate immunity, we first investigated the interaction between Gn and STING. We performed immunofluorescence experiments in HeLa cells coexpressing Gn and STING and revealed that Gn colocalized with STING (Fig. 3A). Moreover, a coimmunoprecipitation (co-IP) assay showed that exogenous STING and Gn were mutually interacted (Fig. 3B and C).
Fig 3.
Gn interacts with STING and affects STING dimerization. Gn interacts with STING and affects STING dimerization. (A) Colocalization of intracellular Gn and STING. HeLa cells were transfected with STING-Flag alone, Gn-HA alone, or STING-Flag plus Gn-HA, as shown. 4',6-diamidino-2-phenylindole (DAPI) staining was performed to visualize nuclei. (B, C) Identification of the Gn–STING interaction by coimmunoprecipitation. HEK293T cells were transfected with Gn and the STING-Flag or control vector as indicated. Cell lysates were prepared and subjected to immunoprecipitation using anti-Flag/anti-HA beads 48 h after transfection. Proteins in the precipitate samples were separated by SDS-PAGE; an anti-HA antibody was used to detect Gn-HA, and an anti-Flag antibody was used to detect STING-Flag. (D) Gn affects STING dimerization. HEK293T cells were cotransfected with STING-Flag, STING-HA, and Gn-HA or the control vector. SN-011 was used as the positive control. HeLa cells were pretreated with SN-011 for 5 h (2 µM). After 48 h, the cells were harvested, and protein expression levels were measured by immunoblotting with anti-Flag, anti-HA, or anti-GAPDH antibodies. (E) STING dimerization was decreased in the presence of Gn as described in (D); binding was quantified using ImageJ software. The means and standard deviations are presented.
Next, we further explored the functions of Gn upon interaction with STING. Following activation by second messenger binding, STING undergoes conformational changes in addition to dimerization and translocates from the endoplasmic reticulum to the perinuclear region, further providing a platform for recruiting molecules such as TBK1 (56). We, therefore, further investigated whether the process of STING dimerization is influenced by Gn. We used SN-011, a potent and selective STING inhibitor that inhibits STING oligomerization and impairs STING recruitment to TBK1, as a positive control for co-IP (57). We transfected HEK293T cells with two differently tagged STING proteins, STING-Flag and STING-HA, and transfected them with the control plasmid VR1012 or the Gn expression plasmid for co-IP. We found that Gn interacted with STING dimers and that Gn inhibited STING dimerization relative to the observations in control cells (Fig. 3D and E).
Gn inhibits the K27-linked ubiquitination of STING and suppresses its recruitment of TBK1
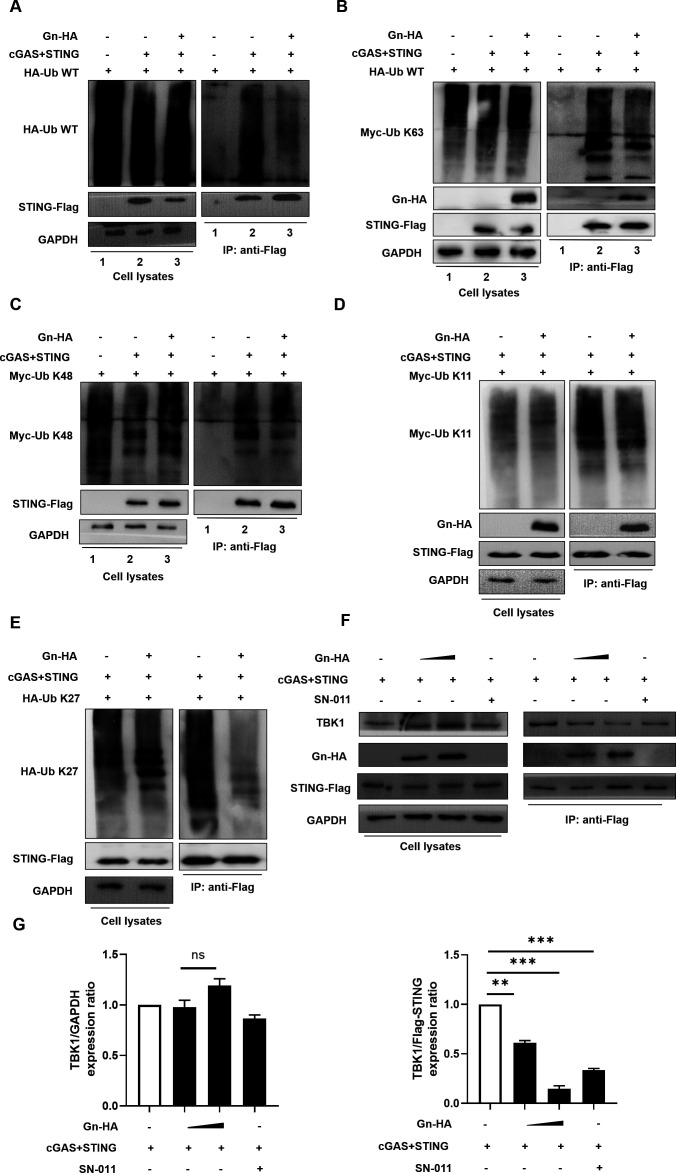
In the process of cGAS-STING signaling, when STING translocates from the ER to the Golgi after binding to the second messenger, its level is regulated by ubiquitination; the regulation of STING by posttranslational modifications is becoming increasingly clear, especially its regulation by ubiquitination, which is involved in specific biological processes such as the activation, translocation, and degradation of STING (44). A recent study on SARS-CoV-2 showed that STING-mediated NF-κB signaling requires K63-linked ubiquitination of STING, a modification that is critical for its downstream protein binding (23). SARS-CoV-2 3CL binds to STING and specifically inhibits this modification; therefore, we sought to further investigate whether the SFTSV envelope glycoprotein Gn affects the ubiquitination of STING. We found that the level of wild-type ubiquitin chains on STING was reduced in the experimental group of cells with Gn overexpression (Fig. 4A), indicating that Gn negatively regulates the ubiquitination of STING.
Fig 4.
Gn inhibits the K27-linked ubiquitination of STING and suppresses its recruitment of TBK1. Gn inhibits the K27-linked ubiquitination of STING and its recruitment of TBK1. (A) Gn inhibits the modification of STING with WT-Ub. HEK293T cells were transfected with HA-Ub WT alone or cotransfected with STING-Flag, cGAS-Flag, and HA-Ub WT in the presence or absence of Gn. Cell lysates were prepared and subjected to immunoprecipitation with anti-Flag beads 48 h after transfection. Precipitated samples were prepared and reacted with an anti-Flag antibody to detect Flag-STING and an anti-HA antibody to detect HA-Ub WT and Gn-HA. GAPDH was used as the loading control. (B–E) Gn inhibits the K27-linked ubiquitination of STING. HEK293T cells were transfected with Myc-Ub K63/Myc-Ub K48/Myc-Ub K27/HA-Ub K63 alone or cotransfected with STING-Flag, cGAS-Flag, or Myc-Ub K63/Myc-Ub K48/Myc-Ub K27/HA-Ub K63 in the presence or absence of Gn. Cell lysates were prepared and subjected to immunoprecipitation with anti-Flag beads 48 h after transfection. Precipitated samples were prepared and reacted with an anti-Flag antibody to detect Flag-STING and an anti-Myc/anti-HA antibody to detect Myc-Ub K63/Myc-Ub K48/Myc-Ub K27/HA-Ub K63 and Gn-HA. GAPDH was used as the loading control. (F) Gn disrupts the interaction between STING and endogenous TBK1. HEK293T cells were transfected with cGAS-STING in the presence or absence of Gn. Cell lysates were prepared and subjected to immunoprecipitation using anti-Flag beads 36 h after transfection. Precipitated samples were prepared and reacted with an anti-Flag antibody to detect STING-Flag and an anti-TBK1 antibody to detect endogenous proteins. GAPDH was used as the loading control. SN-011 was used as the positive control. HeLa cells were pretreated with SN-011 for 5 h (2 µM). (G) STING-TBK1 binding was attenuated in the presence of Gn as described in (F); binding was quantified using ImageJ software. The means and standard deviations are presented.
Different ubiquitination modifications have different effects on STING. K63-linked ubiquitination was shown to affect the oligomerization of STING and its translocation from the endoplasmic reticulum to the Golgi apparatus (58); K48-linked ubiquitination was shown to be involved in the regulation of STING protein stability (59); K11-linked ubiquitination, which competed for K48-linked ubiquitination, stabilized the STING protein in the early stage of viral infection (60); and the K27-linked ubiquitin chain was found to function as a platform for STING molecules to recruit the downstream mediator TBK1, thus allowing its translocation to the region containing perinuclear vesicles (61). To clarify the type of ubiquitination of STING affected by Gn, we cotransfected plasmids expressing Gn, cGAS-STING, and HA-tagged wild-type Ub or Ub mutants with only one unmodified lysine residue (K63, K48, K11, or K27). As demonstrated in the results, Gn significantly inhibited the K27-linked ubiquitination of STING (Fig. 4E) but had no significant effect on the K63-linked, K48-linked, or K11-linked ubiquitination of STING (Fig. 4B through D). These results indicated that Gn inhibited the K27-linked ubiquitination of STING and potentially affected the downstream signaling of STING. K27-linked ubiquitination of STING could affect its ability to recruit the protein kinase TBK1, and we further designed co-IP experiments to verify whether this function is regulated by Gn. We also used SN-011 as the positive control. As expected, the interaction between STING and TBK1 was diminished by the presence of Gn (Fig. 4F and G). This finding suggests that SFTSV Gn affects the ability of STING to recruit the downstream mediator TBK1 by inhibiting the K27-linked ubiquitination of STING.
SFTSV degrades STING via the autophagy pathway
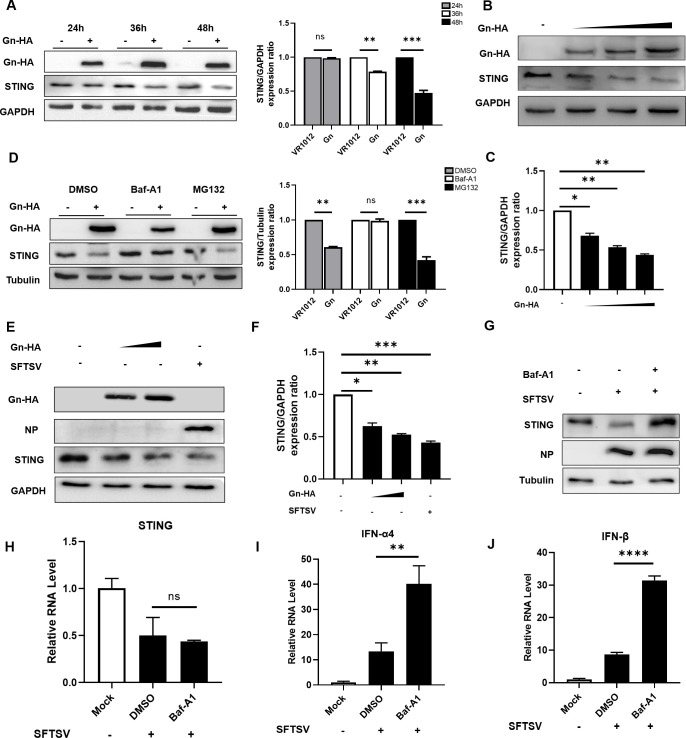
As mentioned previously, STING can be degraded in the late phase of SFTSV infection (Fig. 1E). We found that Gn can also mediate the degradation of STING; thus, to investigate the degradation mechanism and pathway of STING in SFTSV infection, we conducted a more detailed investigation. We found that Gn expression resulted in the degradation of STING in a time-dependent manner upon transfection of Gn (Fig. 5A). In addition, Gn expression resulted in the degradation of STING in a concentration-dependent manner (Fig. 5B and C). Previous studies demonstrated that the pathways for the degradation of intracellular proteins include mainly the autophagy pathway and the proteasome-dependent ubiquitin-mediated degradation pathway, and we used the autophagy inhibitor Bafilomycin A1 (Baf-A1) and the proteasome inhibitor MG132 to determine the degradation pathway of STING. The results showed that Gn was likely to be involved in the degradation of STING via the autophagy pathway (Fig. 5D). We again evaluated the degradation of endogenous STING by overexpressing Gn and infecting the cells with SFTSV under physiological infection conditions, as well as in the presence of the autophagy inhibitor Baf-A1, which reversed the reduction in the STING level induced by SFTSV infection (Fig. 5E through G).
Fig 5.
SFTSV degrades STING via the autophagy pathway. (A) HeLa cells were transfected with VR1012 or Gn-HA (1,000 ng), and the cells were collected at 24, 36, and 48 h for immunoblotting to measure the expression of STING. (B) HeLa cells were transfected with 250, 500, or 1,000 ng of the Gn-HA plasmid. After 48 h, the cells were collected, and protein expression levels were measured by immunoblotting with anti-STING, anti-HA, or anti-GAPDH antibodies. (C) Quantitative analysis using ImageJ software. The averages and standard deviations are shown. (D) HeLa cells were transfected with VR1012 or Gn-HA. The cells were pretreated by adding Baf-A1 to the medium (final concentration, 20 nM) 12 h before transfection, and this medium was changed to a medium containing Baf-A1 at a final concentration of 20 nM after transfection. (E, F) With SFTSV-infected cells as a positive control group, the dose-dependent effect of Gn on endogenous STING expression was evaluated. (G) HeLa cells were infected with SFTSV, and Baf-A1 was added as described in (D). After 48 h, the cells were harvested, and protein expression levels were measured by immunoblotting. (H–J) HeLa cells were treated as described in (A). After 48 h, the cells were collected, and the mRNA levels of STING, IFN-α4, and IFN-β were measured by RT-qPCR.
To further demonstrate that the increase in STING expression in the Baf-A1-treated group occurred at the protein level rather than the mRNA level, we measured the mRNA levels of STING and type I IFN by RT-qPCR. Consistent with the previous findings, SFTSV infection suppressed the mRNA expression of STING, which was not altered after the addition of the autophagy inhibitor, indicating that the restoration of STING occurred at the protein level (Fig. 5H). Infection with SFTSV activated type I IFN expression, which further activated the downstream innate immune response after the addition of the autophagy inhibitor Baf-A1 (Fig. 5I and J).
The above results show that autophagy inhibitors can rescue STING degradation at the protein level and that SFTSV Gn may also be involved in the degradation of STING. Additionally, the overexpressed STING protein can activate the innate immune response.
The C-terminus of SFTSV Gn is required for the Gn-STING interaction
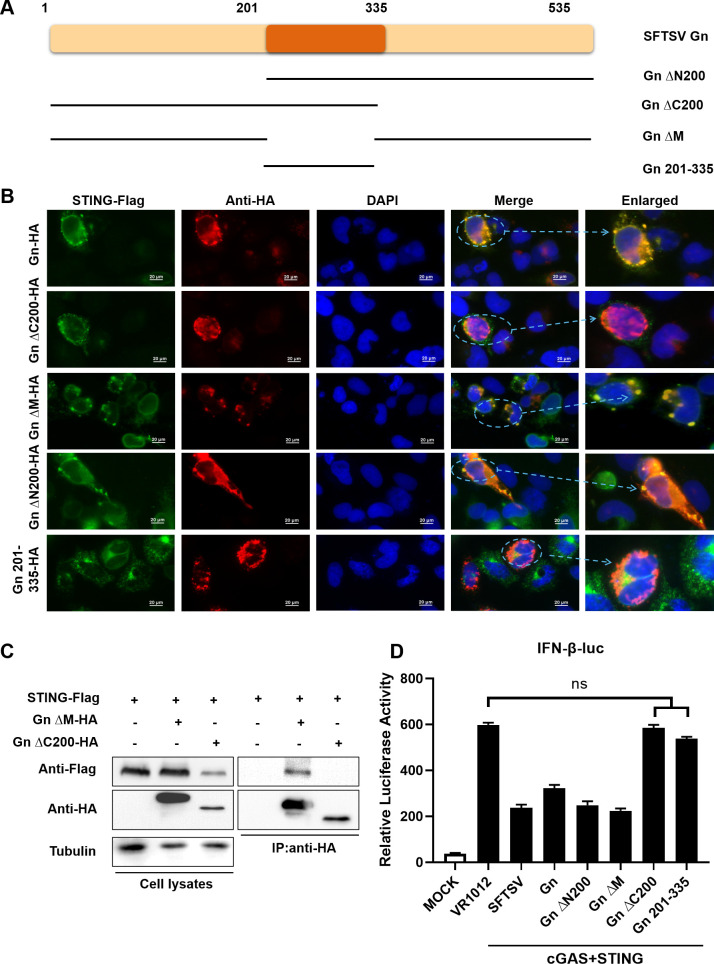
The above findings suggested that Gn of SFTSV affects various regulatory processes of STING through its interaction with STING, including affecting the dimerization and inhibiting the K27-linked ubiquitination of STING, thereby affecting the ability of STING to recruit TBK1 and possibly promoting the degradation of STING via the autophagy pathway. To further determine the sites of the Gn–STING interaction, plasmids encoding several HA-tagged truncation mutants of Gn were constructed.
The SFTSV Gn glycoprotein is composed of 535 amino acids, and the Gn-ΔN200, Gn-ΔC200, Gn-ΔM, and Gn 201–335 mutants were generated by the deletion of 200 amino acids at its N-terminal, C-terminal, and two intermediate regions, respectively (Fig. 6A). The interactions of Gn-ΔN200 and Gn-ΔM with STING were also confirmed by their intracellular colocalization, while Gn-ΔC200 and Gn 201–335 did not colocalize with STING (Fig. 6B), as revealed by immunofluorescence analysis. The C-terminus of Gn is required for the Gn–STING interaction, as indicated by co-IP experiments (Fig. 6C). We then found that the ability of Gn-ΔC200 and Gn 201–335 to inhibit IFN-β promoter activity was lost, while Gn-ΔN200 and Gn-ΔM could still inhibit IFN-β promoter activity (Fig. 6D). Taken together, these results indicate that the interaction between Gn and STING and the ability of the resulting complex to inhibit the downstream cGAS-STING pathway are controlled by the C-terminal region containing amino acids 1–200.
Fig 6.
The C-terminus of SFTSV Gn is required for the Gn-STING interaction. STING interacts with C-terminal fragments of Gn. (A) Various Gn truncation mutants. (B) Colocalization of the Gn truncation mutants and STING. HeLa cells were transfected with HA-Gn and STING-Flag alone or with HA-Gn-ΔN200/HA-Gn-ΔC200/HA-Gn-ΔM/HA-Gn 201–335 and STING-Flag, as shown. DAPI staining was performed to visualize nuclei. After 24 h, the cells were harvested, and colocalization was observed via immunofluorescence staining. (C) Identification of STING–Gn truncation mutant interactions by coimmunoprecipitation. HEK293T cells were transfected with STING-Flag and HA-Gn-ΔC200/HA-Gn-ΔM or the control vector as indicated. Cell lysates were prepared and subjected to immunoprecipitation using anti-HA beads 48 h after transfection. Representative immunoblot results are shown. (D) HEK293T cells were transfected with the IFN-β Luc reporter plasmid and pRL-SV40 plasmid simultaneously with the STING and Gn or Gn truncation plasmids. Cells were collected 36 h after transfection, and IFN-β promoter activity was evaluated via a luciferase reporter assay.
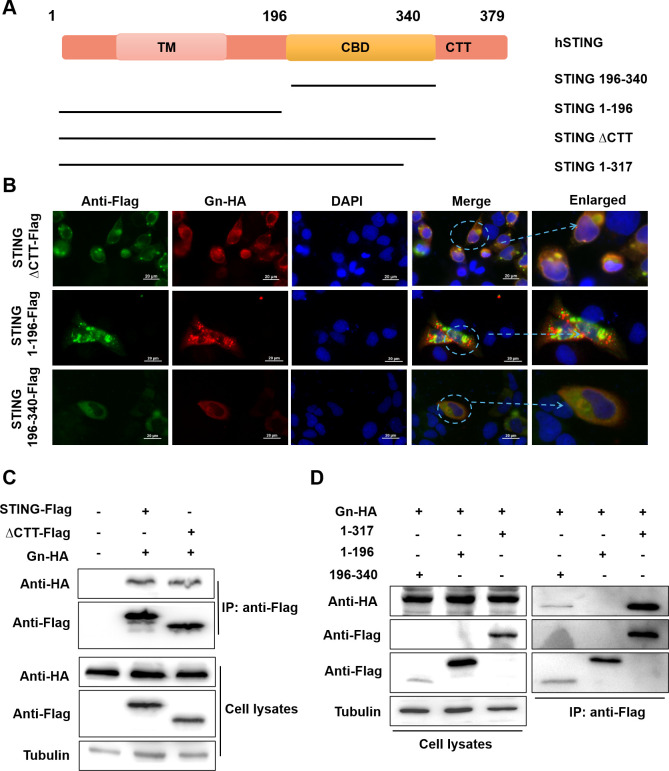
Amino acids 196–317 of STING are required for the STING–Gn interaction
The C-terminal structural domain (CTT) of STING is a key functional site for STING-mediated activation of downstream interferon gene expression (62). To identify the region where STING interacts with Gn, we designed four mutants: STINGΔCTT, STING 1–196, STING 196–340, and STING 1–317 (Fig. 7A). Immunofluorescence experiments revealed that STING-ΔCTT and STING 196–340 still colocalized with Gn, but STING 1–196 did not colocalize with Gn (Fig. 7B). We further confirmed by a coimmunoprecipitation assay that the interaction site between STING and Gn was located in the region containing amino acids 196–317 of STING (Fig. 7B and C).
Fig 7.
Amino acids 196–317 of STING are required for the STING–Gn interaction. Gn interacts with the CDN-binding domain (CBD) of STING. (A) Schematic of the STING functional domains and various STING truncation mutants. (B) Colocalization of Gn and STING truncation mutants. HeLa cells were sttransfected with STING-ΔCTT-Flag/STING 1–196-Flag/STING 196–340-Flag and Gn-HA as shown. DAPI staining was performed to visualize nuclei. After 24 h, the cells were harvested, and colocalization was observed via immunofluorescence staining. (C, D) Identification of Gn–STING truncation mutant interactions by coimmunoprecipitation. HEK293T cells were transfected with HA-Gn and STING-Flag/STING-ΔCTT-Flag/STING 1–317-Flag/STING 1–196-Flag/STING 196–340-Flag or the control vector as indicated. Cell lysates were prepared and subjected to immunoprecipitation using anti-Flag beads 48 h after transfection. Representative immunoblot results are shown.
The above results demonstrated that the 200-amino acid domain at the C-terminus of Gn is the site at which the SFTSV Gn glycoprotein interacts with STING and that deletion of this structural domain resulted in loss of the ability of Gn to inhibit the STING downstream signaling pathway. In addition, we found that amino acids 196–317 of STING were required for the STING–Gn interaction. These findings showed that amino acids 196–340 of STING are the key structural domains for its binding to second messengers and dimerization.
SFTSV Gn enhances viral replication
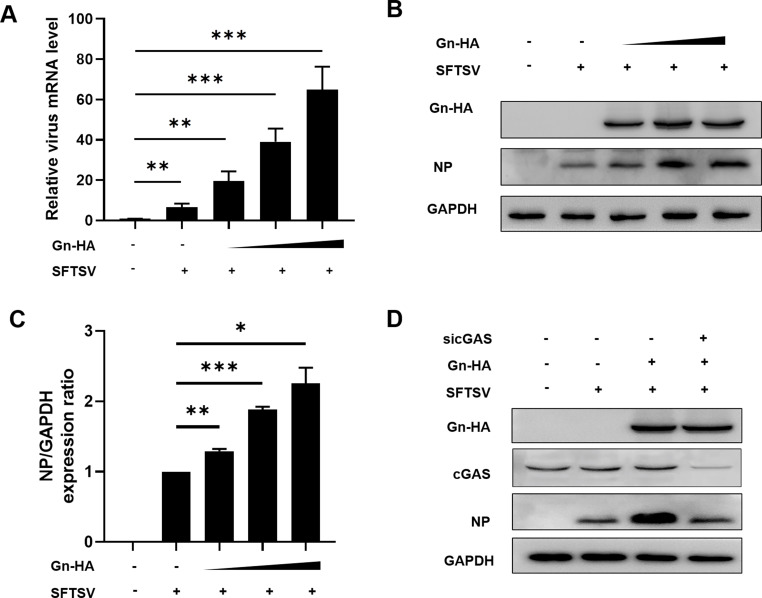
Considering these results, we hypothesized that SFTSV can achieve immune escape through the inhibition of the cGAS-STING signaling pathway via the viral protein Gn, which further affects its replication level. Therefore, we explored the effect of Gn on viral replication at the transcriptional and translational levels. After overexpressing Gn in a dose-dependent manner in HeLa cells, the cells were infected with the virus and harvested 48 h later, and qRT-PCR assays were performed. We found that Gn enhanced viral transcription at the transcriptional level (Fig. 8A). After the same transfection and infection steps, immunoblot analysis was performed, and we found that Gn similarly enhanced SFTSV replication at the translational level and that this increase occurred in a Gn dose-dependent manner (Fig. 8B and C). Therefore, we further performed cGAS knockdown experiments to explore whether the effect of Gn overexpression on viral replication would be eliminated by cGAS knockdown, and immunoblot analysis showed that overexpression of Gn did not enhance SFTSV replication when cGAS was knocked down (Fig. 8D). In summary, Gn can enhance SFTSV replication by regulating the cGAS-STING signaling pathway.
Fig 8.
SFTSV Gn enhances viral replication. (A, B) HeLa cells overexpressing Gn (250, 500, and 1,000 ng) were infected with SFTSV, and Gn was found to enhance viral replication. (C) Quantitative analysis using ImageJ software. The averages and standard deviations are shown. (D) Gn was overexpressed (500 ng) and cGAS was knocked down in virally infected cells; after 48 h, the impact on viral replication was evaluated.
DISCUSSION
With the improvements in healthcare, the morbidity and mortality rate of fever with thrombocytopenia syndrome has decreased from 12% to 5% (63), but in recent years, the incidence of SFTSV infection has been increasing annually in high-incidence provinces, and SFTSV has started to become endemic in multiple locations worldwide, affecting global public health and posing a significant threat (9, 10, 45). During the innate immune response to SFTSV, there is a complex relationship between host immune defense and viral infection. Upon SFTSV infection, PAMPs are recognized by cellular PRRs, and numerous signaling cascades are activated, leading to the production of IFNs (64). Moreover, bunyaviruses have developed strategies to circumvent this antiviral response by inhibiting IFN production or interfering with the IFN-mediated response (65). It was found that SFTSV NSs specifically traps TRIM25 in viral inclusion bodies and blocks TRIM25-mediated K63-linked ubiquitination of RIG-I, contributing to the inhibition of RLR-mediated antiviral signaling in the initial phase (30).
To date, studies on the role of the bunyavirus glycoprotein Gn in innate immune signaling are rare (66). Related studies have reported that the pathogenic NY-1 hantavirus G1 cytoplasmic tail regulates RIG-I- and TBK-1-induced interferon responses and disrupts the TRAF3-TBK1 complex (67). TULV Gn-T regulates IFN induction via unique interactions with cellular TBK1 complexes (68). In the present study, we found that the SFTSV glycoprotein Gn can interact with STING and disrupt the formation of the STING-TBK1 complex, thereby regulating the interferon signaling pathway.
We found that SFTSV infection caused the release of mitochondrial DNA into the cytoplasm and inhibited the downstream innate immune signaling pathway by activating the cytoplasmic DNA receptor cGAS (Fig. 1A and B). However, the STING protein was degraded in the late phase of SFTSV infection, suggesting that this signaling pathway was inhibited (Fig. 1J and K). In addition, we observed that SFTSV Gn was a potent inhibitor of the cGAS-STING pathway, as it inhibited the phosphorylation of STING, TBK1, IRF3, and p65 activated by cGAS-STING and blocked the nuclear accumulation of IRF3 and p65 to inhibit downstream signaling (Fig. 2). In this study, we also discovered that NSs can inhibit immune responses induced via the cGAS-STING pathway; however, subsequent research on NSs is needed.
The SFTSV glycoproteins Gn and Gc facilitate viral entry into host cells and are known to play crucial roles in viral assembly, viral particle synthesis, and virion incorporation. These proteins are decorated with high-mannose-subtype N-linked glycans in the ER, which can be converted into hybrid and complex forms when Gn and Gc are translocated into the Golgi apparatus (69, 70). During this process, these proteins might colocalize with STING translocated from the ER to the Golgi apparatus. We found that there was an actual interaction between Gn and STING and that this interaction inhibited STING dimerization (Fig. 3).
It has been reported that STING is regulated by multiple ubiquitination modifications. Through our study, we found that Gn of SFTSV affects K27-linked ubiquitination of STING and thus suppresses its recruitment of TBK1, which explains the inhibition of IFN production by Gn (Fig. 4E and F).
We also found that SFTSV results in the degradation of the STING via the autophagy pathway and that degradation of STING was observed when the Gn protein was overexpressed (Fig. 5A and B); however, the exact mechanism involved remains to be investigated. According to previous reports, the African swine fever virus L83L protein negatively regulates the cGAS-STING-mediated IFN-I pathway by recruiting Tollip to promote the autophagic degradation of STING (71). In addition, the SVCV nucleoprotein (N protein) negatively regulates cellular IFN production by degrading STING via the autophagy‒lysosomal pathway (72). These findings led us to propose additional research directions for subsequent experiments. Additionally, through our study, we found that SFTSV Gn can mediate the immune escape of SFTSV and enhance its replication by inhibiting the cGAS-STING signaling pathway (Fig. 8).
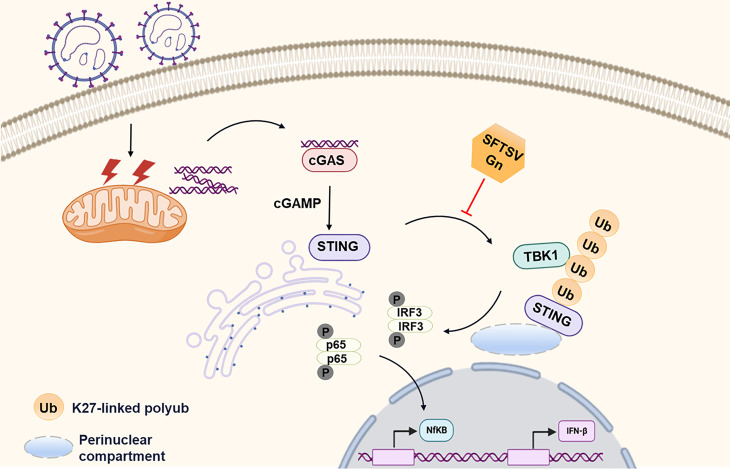
In summary, accumulating evidence suggests that SFTSV, in general, can trigger cGAS-STING signaling (17). SFTSV Gn inhibits K27-linked ubiquitination of STING to disrupt the assembly of the STING-TBK1 complex and downstream signaling (Fig. 9). The findings from this present study enrich the knowledge of the ability of RNA viruses to antagonize cGAS-STING-mediated innate immune responses, increase the understanding of SFTSV infection, and may help in identifying new antiviral targets, which are essential for developing specific therapies.
Fig 9.
Proposed model showing the mechanism by which SFTSV Gn inhibits the cGAS-STING signaling pathway. SFTSV infection causes the release of mitochondrial DNA into the cytoplasm and the activation of the cytoplasmic DNA receptor cGAS. SFTSV Gn is a potent inhibitor of the cGAS-STING pathway and blocks the nuclear accumulation of IRF3 and p65 to inhibit downstream signaling. Gn interacts with STING to inhibit STING dimerization, and Gn of SFTSV inhibits K27-linked ubiquitination of STING to disrupt the assembly of the STING-TBK1 complex and downstream signaling.
MATERIALS AND METHODS
Viruses, cell lines, and plasmids
The SFTSV strain HB29 (GenBank accession nos. NC_018136, NC_018138, and NC_018137) was obtained from Dexin Li (Chinese Center for Disease Control and Prevention). The experiments were performed with HeLa cervical carcinoma cells (HeLa cells), African green monkey kidney epithelial cells (Vero cells), and HEK293T human embryonic kidney 293 cells (HEK293T cells). All the cell lines were purchased from the American Type Culture Collection.
VR1012 was a gift from Professor Wenyan Zhang. IFN-β Luc and NF-κB Luc were acquired from Promega (Madison, WI, USA), and pcDNA3.1(+) was acquired from Miaoling Biology. SFTSV-NSs-HA, SFTSV-NP-HA, SFTSV-Gn-HA, SFTSV-Gc-HA, TBK1-V5, Gn truncated plasmids (ΔN200, ΔM, ΔC200, and 201–335), HA-Ub-WT, Myc-Ub-K63, Myc-Ub-K11, HA-Ub-K27, and Myc-Ub-K48 were all stored in our laboratory. The pcDNA3.1(+) -cGAS-Flag, pcDNA3.1(+) -STING-Flag, and the corresponding truncation plasmids (1–196, 1–317, 196–340, ΔCTT, and V155M) were constructed by the author. PCR primers are listed in Table S1.
Antibodies and reagents
Anti-β-tubulin mouse mAb (M9003), anti-GAPDH mouse mAb (KM9002T), and anti-Flag-Tag mouse mAb (KM8002) were obtained from Tianjin Sungene Biotech. Anti-p65 rabbit mAb (# 8242S), anti-IRF-3 (D83B9) rabbit mAb (# 4302S), anti-TBK1/NAK (D1B4) rabbit mAb (# 3504S), anti-phospho-STING (Ser366) rabbit mAb (# 50907), and anti-phospho-TBK1/NAK (Ser172) rabbit mAb (# 5483) were purchased from Cell Signaling Technology. Anti-TMEM173/STING polyclonal antibody (19851-1-AP), anti-cGAS polyclonal antibody (29958-1-AP), anti-histone-H3 antibody (17168-1-AP), and anti-DYKDDDDK tag rabbit polyclonal antibody (20543-1-AP) were purchased from Proteintech. Anti-mouse HA-Tag mAb (AE008) and anti-phospho-IRF3-S386 rabbit mAb (AP0995) were purchased from Abclonal. Anti-NSs rabbit mAb and anti-NP rabbit mAb were purchased from China CDC. SN-011 (HY-145010), MG132 (HY-13259), and BafA1 (HY-100558) were purchased from MCE. CCCP (C6700) and JC-10 assay kit (CA1310) were purchased from Solarbio. Human cGAMP ELISA detection kit (MM-60090H1) was purchased from MEIMIAN.
RT-qPCR
Total RNA was extracted using the QIAzol lysis reagent. RNA was reverse-transcribed into cDNA using TransScript First-Strand cDNA Synthesis SuperMix (catalog no. AT301; Transgen Biotech). S segment-specific primers were used to determine the quantities of viral mRNAs, which were normalized to GAPDH mRNA. The TaqMan Probe and SYBR Green SuperMix were used for the RT-qPCR amplifications. Three technical replicates were performed for each biological sample. The reactions were performed under the following conditions: 94°C for 30 s, followed by 40 cycles of 94°C for 5 s and 60°C for 30 s, followed by a dissociation protocol. The target sequences were amplified using primer pairs. qPCR primers are listed in Table S2.
Western blotting
Cells were homogenized with lysis buffer; the samples were separated on 10% SDS-PAGE and transferred onto nitrocellulose membranes (cat. no. 66485; Pall Corporation). After blocking with 5% nonfat milk for 30 min, the membranes were probed with primary antibodies and followed by secondary antibody labeled with horseradish peroxidase (HRP).
RNA interference
Specific siRNAs (Table S3) were purchased from Synbio Technologies. Cells in 24-well plates were transfected with siRNA (100 nM) using Lipo2000 according to the manufacturer’s instructions.
Coimmunoprecipitation
HEK293T cells were transfected with various combinations of plasmids. After 48 h, the cells were lysed in lysis buffer, and the lysates were incubated with anti-HA affinity matrix or anti-Flag affinity gel at 4°C for 6 h. After six to eight washes, the immunoprecipitates were boiled in loading buffer and analyzed by Western blotting.
Immunofluorescence
HeLa cells were transfected with Gn-HA plasmids, STING-Flag plasmids, Gn truncation plasmids, or STING truncation plasmids. After 36 h, the cells were fixed with paraformaldehyde (4%) for 15 min, permeabilized with 0.5% Triton X-100 for 10 min, blocked with 1% bovine serum albumin for 20 min, and then incubated with specific antibodies. DAPI was used to counterstain nuclei, and the cells were observed using a confocal laser scanning microscopy system.
Enzyme-linked immunosorbent assay
Take out the required Flat noodles were taken out from the aluminum foil bag after 20 min of room-temperature balance, and the remaining Flat noodles were sealed with a self-sealing bag and put back at 4°C. Standard and sample wells were set, with different concentrations of standard added to each standard well (μL). The sample to be tested was added to the sample well first (μL). Another sample diluent of 40 µL was added. No blank holes were added. Except for blank wells, 100 detection antibodies labeled with HRP were added to each well of the standard and sample wells (μL). The reaction pores were sealed with a sealing film and incubated in a 37°C water bath or constant-temperature incubator for 60 min. The liquid was discarded and pat dry on absorbent paper, each hole was filled with washing solution and let stand for 1 min, the washing solution was shaken off and pat dry on absorbent paper, and this process was repeated five times. The board can also be washed with a washing machine. Fifty substrates A and B were added to each well (μL). It was incubated at 37°C in the dark for 15 min. Fifty microliters of termination solution was added to each well, and within 15 min, the OD values of each well at a wavelength of 450 nm were measured.
Separation and extraction of mtDNA
HEK293T cells were plated in six-well plates. The cells were infected with SFTSV for different times beginning 12 h after seeding (12, 24, and 48 h) or infected with SFTSV at different MOIs. The cells were collected, 300 µL of digitalis saponin separation buffer was added to resuspend the cell precipitate, and the suspension was placed on a 4°C rotator for 10 min to complete lysis. The suspension was subjected to three cycles of centrifugation at 1,000 × g for 3 min and centrifuged at 17,000 × g for 10 min. Then, 80 µL of SDS loading buffer was added to the precipitate, and 17 µL of 4× loading buffer was added to 50 µL of the supernatant as the cytosolic fraction. The remainder of the supernatant was transferred to a new centrifuge tube and prepared for the extraction of cytoplasmic DNA. According to the instructions of the cell genome DNA extraction kit, 20 µL of proteinase K was added to the solution obtained in the previous step, after which the solution was mixed for the extraction of total DNA. The abundances of specific DNA fragments were quantified by qPCR; the primer sequences are shown in Table S2.
Cytoplasmic and nuclear fractionation
HEK293T cells were transfected with plasmids encoding Gn-HA and cGAS/STING-FLAG or with an empty vector and the Gn-HA plasmid. After 48 h, the cells were collected and suspended in 100 µL of cytoplasmic lysis buffer [containing protease inhibitor cocktail (1:1,000) and dithiothreitol (1:2,000)]. The cells were lysed on ice for 10 min and then disrupted by aspirating and expelling the solution 200 times with a 200-µL pipette to lyse any remaining intact cells. Next, the mixture was centrifuged at 4°C and 5,000 × g for 20 min, after which the supernatant (the cytoplasmic lysate) was removed for later analysis. The precipitate was washed five times with phosphate-buffered saline, after which 50 µL of nuclear lysis buffer and 4 µL of SDS loading buffer were added. The samples were analyzed by Western blotting.
Dual-luciferase reporter system
HEK293T cells were plated in 24-well plates and transfected the following day. After 36 h, the cells were collected and treated. Luciferase signals were detected with a Centro XS3 LB 960 microplate luminometer (Berthold Technologies, Germany) according to the instructions of the TransDetect Double-Luciferase Reporter Assay Kit (cat. no. FR201; Transgene Biotech).
Statistical analysis
All results were analyzed with GraphPad Prism and are presented as means ± standard deviations. Statistical significance was determined using the two-tailed Student unpaired t-test (*P< 0.05; **P<0.01; ***P<0.001).
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (32170144).
Y.J. performed the conceptualization, investigation, methodology, validation, formal analysis, data curation, and writing—original draft. F.L. performed the conceptualization, investigation, methodology, validation, formal analysis, and data curation. Z.L. performed the methodology, formal analysis, resources, and data curation. S.L. performed the methodology, formal analysis, resources, and data curation. M.H. performed the methodology, formal analysis, resources, and data curation. X.G. performed the validation, formal analysis, resources, and data curation. X.S. performed the validation, formal analysis, resources, and data curation. Z.W. performed the conceptualization, methodology, supervision, and project administration. T.W. performed the conceptualization, methodology, supervision, project administration, and funding acquisition.
Contributor Information
Zhiyun Wang, Email: zhiyun_wang@tju.edu.cn.
Tao Wang, Email: wangtaobio@tju.edu.cn.
Mark T. Heise, University of North Carolina, Chapel Hill, North Carolina, USA
DATA AVAILABILITY
All data generated or analyzed during this study are included in the manuscript and supplemental material.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jvi.01815-23.
Tables S1 to S3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Yu X-J, Liang M-F, Zhang S-Y, Liu Y, Li J-D, Sun Y-L, Zhang L, Zhang Q-F, Popov VL, Li C, et al. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. doi: 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuhn J-O, Adkins S-O, Alioto D-O, Alkhovsky S-O, Amarasinghe G-O, Anthony SJ, Avšič-Županc T-O, Ayllón M-O, Bahl J-O, Balkema-Buschmann A-O, et al. 2020. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X, Liu Y, Zhao L, Li B, Yu H, Wen H, Yu X-J. 2013. An emerging hemorrhagic fever in China caused by a novel bunyavirus SFTSV. Sci China Life Sci 56:697–700. doi: 10.1007/s11427-013-4518-9 [DOI] [PubMed] [Google Scholar]
- 4. Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, Song Y, Guo D. 2017. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin 32:51–62. doi: 10.1007/s12250-016-3931-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, et al. 2014. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209:816–827. doi: 10.1093/infdis/jit603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fang X, Hu J, Peng Z, Dai Q, Liu W, Liang S, Li Z, Zhang N, Bao C. 2021. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome bunyavirus human-to-human transmission. PLoS Negl Trop Dis 15:e0009037. doi: 10.1371/journal.pntd.0009037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang F, Wu Y, Jiao J, Wang J, Ge Z. 2020. Risk factors and clinical characteristics of severe fever with thrombocytopenia syndrome. Int J Gen Med 13:1661–1667. doi: 10.2147/IJGM.S292735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bopp NE, Kaiser JA, Strother AE, Barrett ADT, Beasley DWC, Benassi V, Milligan GN, Preziosi MP, Reece LM. 2020. Baseline mapping of severe fever with thrombocytopenia syndrome virology, epidemiology and vaccine research and development. NPJ Vaccines 5:111. doi: 10.1038/s41541-020-00257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu S, Liu H, Kang J, Xu L, Zhang K, Li X, Hou W, Wang Z, Wang T. 2020. The severe fever with thrombocytopenia syndrome virus NSs protein interacts with CDK1 to induce G(2) cell cycle arrest and positively regulate viral replication. J Virol 94:e01575-19. doi: 10.1128/JVI.01575-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Liu S, Liu Z, Gao X, Xu L, Huang M, Su Y, Wang Z, Wang T. 2022. Dabie bandavirus nonstructural protein interacts with actin to induce F-actin rearrangement and inhibit viral adsorption and entry. J Virol 96:e0078822. doi: 10.1128/jvi.00788-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo N, Li M, Xu M, Shi C, Shi X, Ni R, Chen Y, Zheng L, Tu Y, Hu D, Yu C, Li Q, Lu Y. 2023. Research progress of fever with thrombocytopenia syndrome. Intensive Care Res:1–10. doi: 10.1007/s44231-023-00035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Chen T, Li R, Xu Y, Xiong Y. 2023. Recent advances in the study of the immune escape mechanism of SFTSV and its therapeutic agents. Viruses 15:940. doi: 10.3390/v15040940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada S, Shimojima M, Narita R, Tsukamoto Y, Kato H, Saijo M, Fujita T. 2018. RIG-I-like receptor and toll-like receptor signaling pathways cause aberrant production of inflammatory cytokines/chemokines in a severe fever with thrombocytopenia syndrome virus infection mouse model. J Virol 92:e02246-17. doi: 10.1128/JVI.02246-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JH, Shim YR, Seo W, Kim MH, Choi WM, Kim HH, Kim YE, Yang K, Ryu T, Jeong JM, Choi HG, Eun HS, Kim SH, Mun H, Yoon JH, Jeong WI. 2020. Mitochondrial double‐stranded RNA in exosome promotes Interleukin‐17 production through toll‐like receptor 3 in alcohol‐associated liver injury. Hepatology 72:609–625. doi: 10.1002/hep.31041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fitzgerald KA, Kagan JC. 2020. Toll-like receptors and the control of immunity. Cell 180:1044–1066. doi: 10.1016/j.cell.2020.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. doi: 10.1038/nri3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394. doi: 10.1126/science.1244040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moriyama M, Koshiba T, Ichinohe T. 2019. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat Commun 10:4624. doi: 10.1038/s41467-019-12632-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aguirre S, Luthra P, Sanchez-Aparicio MT, Maestre AM, Patel J, Lamothe F, Fredericks AC, Tripathi S, Zhu T, Pintado-Silva J, Webb LG, Bernal-Rubio D, Solovyov A, Greenbaum B, Simon V, Basler CF, Mulder LCF, García-Sastre A, Fernandez-Sesma A. 2017. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat Microbiol 2:17037. doi: 10.1038/nmicrobiol.2017.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng Y, Liu Q, Wu Y, Ma L, Zhang Z, Liu T, Jin S, She Y, Li YP, Cui J. 2018. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J 37:e99347. doi: 10.15252/embj.201899347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu Z, Li X, Yang M, Huang J, Fang Q, Jia J, Li Z, Gu Y, Chen T, Cao X. 2021. TRIM41 is required to innate antiviral response by polyubiquitinating BCL10 and recruiting NEMO. Signal Transduct Target Ther 6:90. doi: 10.1038/s41392-021-00477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han L, Zhuang MW, Deng J, Zheng Y, Zhang J, Nan ML, Zhang XJ, Gao C, Wang PH. 2021. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. J Med Virol 93:5376–5389. doi: 10.1002/jmv.27050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rui Y, Su J, Shen S, Hu Y, Huang D, Zheng W, Lou M, Shi Y, Wang M, Chen S, Zhao N, Dong Q, Cai Y, Xu R, Zheng S, Yu XF. 2021. Unique and complementary suppression of cGAS-STING and RNA sensing- triggered innate immune responses by SARS-CoV-2 proteins. Signal Transduct Target Ther 6:123. doi: 10.1038/s41392-021-00515-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Webb LG, Fernandez-Sesma A. 2022. RNA viruses and the cGAS-STING pathway: reframing our understanding of innate immune sensing. Curr Opin Virol 53:101206. doi: 10.1016/j.coviro.2022.101206 [DOI] [PubMed] [Google Scholar]
- 25. Li S, Li H, Zhang Y-L, Xin Q-L, Guan Z-Q, Chen X, Zhang X-A, Li X-K, Xiao G-F, Lozach P-Y, Cui J, Liu W, Zhang L-K, Peng K. 2020. SFTSV infection induces BAK/BAX-dependent mitochondrial DNA release to trigger NLRP3 Inflammasome activation. Cell Rep 30:4370–4385. doi: 10.1016/j.celrep.2020.02.105 [DOI] [PubMed] [Google Scholar]
- 26. Soutar MPM, Kempthorne L, Annuario E, Luft C, Wray S, Ketteler R, Ludtmann MHR, Plun-Favreau H. 2019. FBS/BSA media concentration determines CCCP's ability to depolarize mitochondria and activate PINK1-PRKN mitophagy. Autophagy 15:2002–2011. doi: 10.1080/15548627.2019.1603549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu BY, Yu XJ, Zhou CM. 2021. SAFA initiates innate immunity against cytoplasmic RNA virus SFTSV infection. PLoS Pathog 17:e1010070. doi: 10.1371/journal.ppat.1010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ge Z, Ding S. 2022. Regulation of cGAS/STING signaling and corresponding immune escape strategies of viruses. Front Cell Infect Microbiol 12:954581. doi: 10.3389/fcimb.2022.954581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, Hopfner KP, Ludwig J, Hornung V. 2013. cGAS produces a 2'-5'-linked cyclic dinucleotide second messenger that activates STING. Nature 498:380–384. doi: 10.1038/nature12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Min YQ, Ning YJ, Wang H, Deng F. 2020. A RIG-I-like receptor directs antiviral responses to a bunyavirus and is antagonized by virus-induced blockade of TRIM25-mediated ubiquitination. J Biol Chem 295:9691–9711. doi: 10.1074/jbc.RA120.013973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khalil J, Kato H, Fujita T. 2021. The role of non-structural protein NSs in the pathogenesis of severe fever with thrombocytopenia syndrome. Viruses 13:876. doi: 10.3390/v13050876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, Huang DCS, Kile BT. 2014. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159:1549–1562. doi: 10.1016/j.cell.2014.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andreeva L, Hiller B, Kostrewa D, Lässig C, de Oliveira Mann CC, Jan Drexler D, Maiser A, Gaidt M, Leonhardt H, Hornung V, Hopfner K-P. 2017. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature 549:394–398. doi: 10.1038/nature23890 [DOI] [PubMed] [Google Scholar]
- 34. Herzner AM, Hagmann CA, Goldeck M, Wolter S, Kübler K, Wittmann S, Gramberg T, Andreeva L, Hopfner KP, Mertens C, Zillinger T, Jin T, Xiao TS, Bartok E, Coch C, Ackermann D, Hornung V, Ludwig J, Barchet W, Hartmann G, Schlee M. 2015. Sequence-specific activation of the DNA sensor cGAS by Y-form DNA structures as found in primary HIV-1 cDNA. Nat Immunol 16:1025–1033. doi: 10.1038/ni.3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. 2013. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 51:226–235. doi: 10.1016/j.molcel.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. 2013. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503:530–534. doi: 10.1038/nature12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792. doi: 10.1038/nature08476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang C, Shang G, Gui X, Zhang X, Bai XC, Chen ZJ. 2019. Structural basis of STING binding with and phosphorylation by TBK1. Nature 567:394–398. doi: 10.1038/s41586-019-1000-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka Y, Chen ZJ. 2012. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal 5:ra20. doi: 10.1126/scisignal.2002521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. González-Navajas JM, Lee J, David M, Raz E. 2012. Immunomodulatory functions of type I interferons. Nat Rev Immunol 12:125–135. doi: 10.1038/nri3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma F, Li B, Liu S, Iyer SS, Yu Y, Wu A, Cheng G. 2015. Positive feedback regulation of type I IFN production by the IFN-inducible DNA sensor cGAS. J Immunol 194:1545–1554. doi: 10.4049/jimmunol.1402066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hong Y, Bai M, Qi X, Li C, Liang M, Li D, Cardona CJ, Xing Z. 2019. Suppression of the IFN-α and -β induction through sequestering IRF7 into viral inclusion bodies by nonstructural protein NSs in severe fever with thrombocytopenia syndrome bunyavirus infection. J Immunol 202:841–856. doi: 10.4049/jimmunol.1800576 [DOI] [PubMed] [Google Scholar]
- 43. Cheng Z, Dai T, He X, Zhang Z, Xie F, Wang S, Zhang L, Zhou F. 2020. The interactions between cGAS-STING pathway and pathogens. Signal Transduct Target Ther 5:91. doi: 10.1038/s41392-020-0198-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hopfner KP, Hornung V. 2020. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol 21:501–521. doi: 10.1038/s41580-020-0244-x [DOI] [PubMed] [Google Scholar]
- 45. Qu B, Qi X, Wu X, Liang M, Li C, Cardona CJ, Xu W, Tang F, Li Z, Wu B, Powell K, Wegner M, Li D, Xing Z. 2012. Suppression of the interferon and NF-κB responses by severe fever with thrombocytopenia syndrome virus. J Virol 86:8388–8401. doi: 10.1128/JVI.00612-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu Q, Gu T, Su LY, Jiao L, Qiao X, Xu M, Xie T, Yang LX, Yu D, Xu L, Chen C, Yao YG. 2021. GSNOR facilitates antiviral innate immunity by restricting TBK1 cysteine S-nitrosation. Redox Biol 47:102172. doi: 10.1016/j.redox.2021.102172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ding Z, Fang L, Jing H, Zeng S, Wang D, Liu L, Zhang H, Luo R, Chen H, Xiao S. 2014. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J Virol 88:8936–8945. doi: 10.1128/JVI.00700-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sui L, Zhao Y, Wang W, Wu P, Wang Z, Yu Y, Hou Z, Tan G, Liu Q. 2021. SARS-CoV-2 membrane protein inhibits type I interferon production through ubiquitin-mediated degradation of TBK1. Front Immunol 12:662989. doi: 10.3389/fimmu.2021.662989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Visser LJ, Aloise C, Swatek KN, Medina GN, Olek KM, Rabouw HH, de Groot RJ, Langereis MA, de Los Santos T, Komander D, Skern T, van Kuppeveld FJM. 2020. Dissecting distinct proteolytic activities of FMDV Lpro implicates cleavage and degradation of RLR signaling proteins, not its deISGylase/DUB activity, in type I interferon suppression. PLoS Pathog 16:e1008702. doi: 10.1371/journal.ppat.1008702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spiegel M, Plegge T, Pöhlmann S. 2016. The role of phlebovirus glycoproteins in viral entry, assembly and release. Viruses 8:202. doi: 10.3390/v8070202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Y, Yang W, Wen Y, Niu Q, Yang J, Guan G, Yin H, Zheng H, Li D, Liu Z. 2022. [The E248R protein of African swine fever virus inhibits the cGAS-STING-mediated innate immunity]. Sheng Wu Gong Cheng Xue Bao 38:1837–1846. doi: 10.13345/j.cjb.210397 [DOI] [PubMed] [Google Scholar]
- 52. Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, Tenbrock K, Wittkowski H, Jones OY, Kuehn HS, et al. 2014. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 371:507–518. doi: 10.1056/NEJMoa1312625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hiscott J. 2007. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem 282:15325–15329. doi: 10.1074/jbc.R700002200 [DOI] [PubMed] [Google Scholar]
- 54. Petro TM. 2020. IFN regulatory factor 3 in health and disease. J Immunol 205:1981–1989. doi: 10.4049/jimmunol.2000462 [DOI] [PubMed] [Google Scholar]
- 55. Chattopadhyay S, Veleeparambil M, Poddar D, Abdulkhalek S, Bandyopadhyay SK, Fensterl V, Sen GC. 2015. EGFR kinase activity is required for TLR4 signaling and the septic shock response. EMBO Rep 16:1535–1547. doi: 10.15252/embr.201540337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shang G, Zhang C, Chen ZJ, Bai XC, Zhang X. 2019. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature 567:389–393. doi: 10.1038/s41586-019-0998-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hong Z, Mei J, Li C, Bai G, Maimaiti M, Hu H, Yu W, Sun L, Zhang L, Cheng D, Liao Y, Li S, You Y, Sun H, Huang J, Liu X, Lieberman J, Wang C. 2021. STING inhibitors target the cyclic dinucleotide binding pocket. Proc Natl Acad Sci U S A 118:e2105465118. doi: 10.1073/pnas.2105465118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang ZD, Xiong TC, Yao SQ, Wei MC, Chen M, Lin D, Zhong B. 2020. RNF115 plays dual roles in innate antiviral responses by catalyzing distinct ubiquitination of MAVS and MITA. Nat Commun 11:5536. doi: 10.1038/s41467-020-19318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo Y, Jiang F, Kong L, Wu H, Zhang H, Chen X, Zhao J, Cai B, Li Y, Ma C, Yi F, Zhang L, Liu B, Zheng Y, Zhang L, Gao C. 2021. OTUD5 promotes innate antiviral and antitumor immunity through deubiquitinating and stabilizing STING. Cell Mol Immunol 18:1945–1955. doi: 10.1038/s41423-020-00531-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qin Y, Zhou MT, Hu MM, Hu YH, Zhang J, Guo L, Zhong B, Shu HB. 2014. RNF26 temporally regulates virus-triggered type I interferon induction by two distinct mechanisms. PLoS Pathog 10:e1004358. doi: 10.1371/journal.ppat.1004358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Q, Liu X, Cui Y, Tang Y, Chen W, Li S, Yu H, Pan Y, Wang C. 2014. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41:919–933. doi: 10.1016/j.immuni.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 62. Wu J, Chen YJ, Dobbs N, Sakai T, Liou J, Miner JJ, Yan N. 2019. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med 216:867–883. doi: 10.1084/jem.20182192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen QL, Zhu MT, Chen N, Yang D, Yin WW, Mu D, Li Y, Zhang YP, Zainawudong Y. 2022. Epidemiological characteristics of severe fever with thtrombocytopenia syndrome in China, 2011-2021. Zhonghua Liu Xing Bing Xue Za Zhi 43:852–859. doi: 10.3760/cma.j.cn112338-20220325-00228 [DOI] [PubMed] [Google Scholar]
- 64. Ning YJ, Feng K, Min YQ, Cao WC, Wang M, Deng F, Hu Z, Wang H. 2015. Disruption of type I interferon signaling by the nonstructural protein of severe fever with thrombocytopenia syndrome virus via the hijacking of STAT2 and STAT1 into inclusion bodies. J Virol 89:4227–4236. doi: 10.1128/JVI.00154-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu X, Qi X, Qu B, Zhang Z, Liang M, Li C, Cardona CJ, Li D, Xing Z. 2014. Evasion of antiviral immunity through sequestering of TBK1/IKKε/IRF3 into viral inclusion bodies. J Virol 88:3067–3076. doi: 10.1128/JVI.03510-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun Y, Qi Y, Liu C, Gao W, Chen P, Fu L, Peng B, Wang H, Jing Z, Zhong G, Li W. 2014. Nonmuscle myosin heavy chain IIA is a critical factor contributing to the efficiency of early infection of severe fever with thrombocytopenia syndrome virus. J Virol 88:237–248. doi: 10.1128/JVI.02141-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alff PJ, Gavrilovskaya IN, Gorbunova E, Endriss K, Chong Y, Geimonen E, Sen N, Reich NC, Mackow ER. 2006. The pathogenic NY-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J Virol 80:9676–9686. doi: 10.1128/JVI.00508-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Matthys V, Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. 2011. The C-terminal 42 residues of the Tula virus Gn protein regulate interferon induction. J Virol 85:4752–4760. doi: 10.1128/JVI.01945-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Plegge T, Hofmann-Winkler H, Spiegel M, Pöhlmann S. 2016. Evidence that processing of the severe fever with thrombocytopenia syndrome virus Gn/Gc polyprotein is critical for viral infectivity and requires an internal Gc signal peptide. PLoS One 11:e0166013. doi: 10.1371/journal.pone.0166013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Overby AK, Popov V, Neve EPA, Pettersson RF. 2006. Generation and analysis of infectious virus-like particles of Uukuniemi virus (bunyaviridae): a useful system for studying bunyaviral packaging and budding. J Virol 80:10428–10435. doi: 10.1128/JVI.01362-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cheng M, Kanyema MM, Sun Y, Zhao W, Lu Y, Wang J, Li X, Shi C, Wang J, Wang N, Yang W, Jiang Y, Huang H, Yang G, Zeng Y, Wang C, Cao X. 2023. African swine fever virus L83L negatively regulates the cGAS-STING-mediated IFN-I pathway by recruiting tollip to promote STING autophagic degradation. J Virol 97:e0192322. doi: 10.1128/jvi.01923-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang XL, Li ZC, Zhang C, Jiang JY, Han KJ, Tong JF, Yang XL, Chen DD, Lu LF, Li S. 2023. Spring viremia of carp virus N protein negatively regulates IFN induction through autophagy-lysosome-dependent degradation of STING. J Immunol 210:72–81. doi: 10.4049/jimmunol.2200477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3.
Data Availability Statement
All data generated or analyzed during this study are included in the manuscript and supplemental material.