Abstract
Respiratory syncytial virus (RSV) is the most important cause of bronchiolitis and pneumonia in infants and young children worldwide. As yet, there is no effective vaccine against RSV infection, and previous attempts to develop a formalin-inactivated vaccine resulted in exacerbated disease in recipients subsequently exposed to the virus. In the work described here, a combinatorial solid-phase peptide library was screened with a protective monoclonal antibody (MAb 19) to identify peptide mimics (mimotopes) of a conserved and conformationally-determined epitope of RSV fusion (F) protein. Two sequences identified (S1 [HWYISKPQ] and S2 [HWYDAEVL]) reacted specifically with MAb 19 when they were presented as solid-phase peptides. Furthermore, after amino acid substitution analyses, three sequences derived from S1 (S1S [HWSISKPQ], S1K [KWYISKPQ], and S1P [HPYISKPQ]), presented as multiple antigen peptides (MAPs), also showed strong reactivity with MAb 19. The affinity constants of the binding of MAb 19, determined by surface plasmon resonance analyses, were 1.19 × 109 and 4.93 × 109 M−1 for S1 and S1S, respectively. Immunization of BALB/c mice with these mimotopes, presented as MAPs, resulted in the induction of anti-peptide antibodies that inhibited the binding of MAb 19 to RSV and neutralized viral infection in vitro, with titers equivalent to those in sera from RSV-infected animals. Following RSV challenge of S1S mimotope-immunized mice, a 98.7% reduction in the titer of virus in the lungs was observed. Furthermore, there was a greatly reduced cell infiltration in the lungs of immunized mice compared to that in controls. These results indicate the potential of peptide mimotopes to protect against RSV infection without exacerbating pulmonary pathology.
Respiratory syncytial virus (RSV) is the major cause of serious lower respiratory tract illness in infants and immunosuppressed individuals worldwide and is estimated to be responsible for 65 million infections and 1 million deaths annually (12, 19). Although the severity of disease declines with repeated infection, previous infection with RSV does not prevent illness in subsequent infections, and it is apparent that immunity is incomplete. Furthermore, attempts to develop a vaccine against RSV have encountered a series of problems. In the late 1960s, a formalin-inactivated vaccine not only failed to protect infants against RSV but also induced exacerbated disease during a subsequent epidemic (5, 19). Retrospective analysis of the sera demonstrated that the inactivated vaccine induced high anti-F (fusion) protein antibody titers, but with poor neutralizing activity, suggesting that the inactivation treatment had denatured or modified epitopes which were the target for neutralizing antibodies (19, 25). Live attenuated vaccines were also investigated as candidates; however, these were either poorly immunogenic (overattenuated) or genetically unstable (5, 45). Although new attenuated vaccines have given encouraging results in animal models (10, 11), there is an urgent need for alternative approaches to the development of an effective vaccine.
Studies with experimental animals have provided evidence that RSV-specific neutralizing antibodies can prevent infection in the lungs when administered prophylactically (29). Intravenous administration of pooled human immunoglobulin G (IgG) containing a high titer of neutralizing antibodies prevented serious RSV lower respiratory tract illness in high-risk children (18). Both the F and G (attachment) glycoproteins of RSV play a major role in eliciting this humoral immunity. The conserved F glycoprotein induces protective immune responses (8, 36), and passive immunization with neutralizing anti-F monoclonal antibodies (MAbs) or recombinant Fab protects small animals from RSV infection (12, 38, 39). Based on the results of these studies, a neutralizing and protective MAb (MAb 19) has been reshaped and humanized (as MAb RSHZ19) and has been demonstrated to have protective properties superior to those of anti-RSV polyclonal antibodies in a rodent model (46). Its safety and efficacy have recently been assessed in human volunteers (13).
The immunochemical characterization of the epitopes recognized by these protective antibodies is critical for the development of a vaccine against RSV, but the conformational constraints associated with the protective epitopes in RSV F protein make it unlikely that they will be identified from analysis of the primary amino acid sequence. Indeed, earlier attempts in our laboratory and elsewhere with synthetic peptides representing continuous sequences of the F protein (amino acids [aa] 205 to 225, 221 to 237, 261 to 273, 215 to 275, 417 to 438, and 481 to 491) failed to generate protective humoral responses (4, 23, 32, 42). The use of peptides (3), antigen fragments (24), and antibody escape mutants (39) has confirmed the involvement of discontinuous residues within the F protein in epitopes recognized by protective MAbs (39). Thus, the use of alternative approaches for the identification of these epitopes is essential.
Solid-phase and bacteriophage combinatorial peptide libraries (22, 31) have been used for identification of peptide mimics (mimotopes) of ligands, and the identification of peptide sequences that mimic the conformational structure of protective epitopes would have great potential for the development of a synthetic peptide vaccine. Recently, using a solid-phase combinatorial peptide library, we identified 8-mer peptides that mimic an epitope recognized by a monoclonal anti-measles virus F protein antibody. The mimotopes identified by this approach did not bear any primary sequence relationship to sequences in the viral protein but mimicked its conformation. When used as an immunogen, one of the mimotopes induced virus-neutralizing and protective antibody responses (35). Phage-displayed peptide libraries have been used to delineate sequences which mimic a discontinuous epitope of hepatitis B surface antigen (7), the secondary structure of a neutralizing epitope of human immunodeficiency virus type 1 (15), and peptides that reacted with MAbs specific for polysaccharide antigens (43).
In this paper, we describe the identification of mimotopes of a protective epitope of RSV F protein, as defined by the protective MAb 19, following the screening of a solid-phase combinatorial peptide library. One of these mimotopes induced a virus-neutralizing antibody response equivalent to that in RSV-immunized animals, which significantly reduced the viral load in RSV-challenged mice. These findings indicate the potential of synthetic peptide mimotopes for the development of a novel vaccine against RSV.
MATERIALS AND METHODS
Screening of the solid-phase peptide library.
A solid-phase combinatorial peptide library was synthesized on Novasyn TG resin (Novabiochem, Nottingham, United Kingdom) and represents 14 × 106 combinations of 8-mer peptides (containing all natural amino acids except cysteine) per gram of resin (35). The library was screened with the neutralizing and protective monoclonal anti-RSV F protein antibody MAb 19 (39). Beads which stained dark brown at the lowest MAb 19 concentration were selected, treated with trifluoroacetic acid to remove bound antibodies, and microsequenced (Protein Sequencing Unit, Medical Research Council Centre, Cambridge, United Kingdom).
Peptide synthesis. (i) Linear free peptides.
The mimotope sequences were synthesized as linear free peptides by automated solid-phase synthesis (9050 Pep synthesizer; Milligen Division, Millipore, Bedford, Mass.) with 9-fluorenylmethoxycarbonyl (Fmoc) chemistry and TGA resin (Novabiochem). The purity of the peptides was assessed by reverse-phase high-pressure liquid chromatography and mass spectrometry. The T-helper (Th) epitope of measles virus F protein (aa 288 to 302), employed for the immunogenicity studies (27), was synthesized as indicated above. This sequence does not have any sequence similarity to RSV protein sequences.
(ii) Resin-bound peptides.
The mimotope sequences were also synthesized as resin-bound peptides by the same procedure as described for the free peptides but with Novasyn TG resin so that, after deprotection of the side chains with trifluoroacetic acid and scavengers, the peptides remained attached to the resin. Sequences were confirmed by microsequencing.
(iii) SPOTs peptides on derivatized cellulose membrane.
The mimotope sequences which were reactive as resin-bound peptides were also synthesized on a 96-SPOTs activated cellulose membrane by the SPOTs method (Genosys, Cambridge, United Kingdom), in which the peptides remain linked to the membrane and are thus very similar to those linked to the TG resin.
(iv) MAPs.
Mimotope sequences reactive as TG resin-bound peptides and SPOTs were synthesized as tetramer multiple-antigen peptides (MAPs) with (Fmoc)4-Lys2-Lys-Cys-βAla-resin (Novabiochem) (14, 37). S1S-MAP was also synthesized colinearly with one copy of the measles virus Th epitope as (HWSISKPQ)4-Lys2-Lys-Cys-Lys-Lys–Th (S1S-MAP–Th). The purity of the MAPs was assessed by C18 and C8 reverse-phase high-pressure liquid chromatography and by amino acid analysis.
Virus.
RSV (A2 strain) stock was grown in HEp-2 cells. The titer of RSV stock was estimated by a plaque assay and expressed as the log10 of the reciprocal of the dilution giving 1 PFU (17).
Animals, immunization schedules, and RSV challenge.
Inbred female BALB/c (H-2d) mice were purchased from the Medical Research Council, Mill Hill, London, United Kingdom.
The optimal dose of MAPs injected intraperitoneally (i.p.) was found to be 25 μg/mouse, as in previous studies (14). Groups of 6- to 7-week-old BALB/c mice were coimmunized i.p. either with 25 μg of MAPs and 8 μg of Th in Freund’s complete adjuvant (FCA) (molar ratio, 1:1; ∼5 nmol) (33, 35) or with 33 μg of MAP-Th in FCA. The mice were boosted 3 weeks later with the same dose of peptides in Freund’s incomplete adjuvant, and sera were collected weekly. Further groups of mice received a second boost (5 nmol in Freund’s incomplete adjuvant) 6 weeks after the first boost. Groups of mice immunized i.p. with MAP plus Th in FCA, MAP-Th in FCA, Th in FCA, or saline were challenged intranasally (i.n.) with RSV (106 PFU/50 μl) 4 to 5 weeks after the boost or 3 weeks after a second boost. A separate group was infected i.n. with RSV (106 PFU) 9 days prior to challenge as a positive control. For challenge of passively immunized animals, groups of four 14- to 15-week-old mice received the following: (i) i.p., either 400 μl of pooled S1S-MAP–Th antiserum (anti-RSV IgG log10 titer, 3.21), 100 μl (340 μg) of MAb 19 ascites (anti-RSV IgG log10 titer, 6.20), or 400 μl of normal mouse serum; and (ii) i.n., 60 μl of protein G-purified IgG (2 mg/ml) either from anti-S1S-MAP–Th antiserum (anti-RSV IgG log10 titer, 3.36) or from normal mouse serum (28).
Four days following challenge the mice were killed and their lungs were removed (40), and RSV titers were estimated by a plaque assay and expressed as log10 PFU/g of lung tissue (17, 21). RSV plaques were confirmed, after methanol fixation, by immunostaining with a polyclonal anti-RSV antibody-peroxidase conjugate (Biogenesis, Poole, United Kingdom).
Histology.
For histological analysis, lungs were removed 7 days postchallenge and fixed, and sections were stained with hematoxylin and eosin (40).
Immunochemical assays. (i) Peptide enzyme-linked immunosorbent assay (ELISA).
Immunolon IV 96-well microplates (Nunc, Roskilde, Denmark) were coated with 50 μl of peptide at 5 μg/ml in sodium bicarbonate buffer (pH 9.6) overnight at 4°C, washed with water, and blocked with 200 μl of phosphate-buffered saline (PBS)–bovine serum albumin (BSA) 2.5% per well for 2 h at 37°C. After being washed, the plates were incubated with twofold serial dilutions of biotinylated MAb or polyclonal mouse serum in PBS-Tween (PBS-T)–BSA 2.5% (diluting buffer) for 2 h at 37°C and washed.
For biotinylated MAb, plates were incubated for 1 h at 37°C with a streptavidin-peroxidase conjugate (Boehringer, Mannheim, Germany) at 1/5,000 in diluting buffer. For polyclonal sera, plates were incubated with a rabbit anti-mouse IgG (heavy and light chains)–peroxidase conjugate (Nordic, Tillburg, The Netherlands) at 1/2,000 in diluting buffer for 1 h at 37°C.
Unbound conjugate was removed by washing, and enzyme activity was detected by the addition of 100 μl of substrate solution (0.04% o-phenylenediamine–0.004% hydrogen peroxide in citrate-phosphate buffer). After 10 min at room temperature the enzymatic reaction was stopped with 50 μl of 2 M H2SO4. IgG titers were expressed as log10 of the reciprocal of the dilution giving an absorbance at 492 nm of 0.2.
(ii) Reactivity of MAb 19 with resin-bound peptides.
The binding of MAbs to resin-bound peptides was performed as in the screening of the library and was quantified with a soluble substrate (o-phenylenediamine–hydrogen peroxide).
(iii) Reactivity of MAb 19 in the SPOTs ELISA.
After synthesis of the SPOTs, the membrane was washed three times with TBS-T (Tris-buffered saline–0.1% Tween) and blocked with TBS-casein 1%. After washing with TBS-T, MAb 19 was incubated at a concentration of 1.25 μg/ml in TBS-casein 1% for 3 h at room temperature, and positive reactivities were identified after successive incubations with secondary antibody and substrates.
(iv) RSV ELISA.
RSV was purified by sucrose gradient centrifugation (10), and Immulon IV 96-well microplates were coated with 50 μl of RSV at 5 μg/ml (total protein concentration) in PBS, washed with water, blocked with PBS-BSA 2.5% for 2 h at 37°C, and washed with PBS-T. For the indirect ELISA, the plates were incubated with twofold serial dilutions of polyclonal sera overnight at 4°C. The plates were then washed and developed as described for the peptide ELISA.
For the competition ELISA, 50 μl of serial dilutions of polyclonal sera and 50 μl of biotinylated MAb 19 (125 ng/ml) were incubated overnight at 4°C. After being washed, bound MAb 19 was reacted with streptavidin-peroxidase conjugate (1/5,000; 1 h at 37°C). The plates were developed and read as described above. The inhibition titer was expressed as the log2 of the reciprocal dilution giving a 50% inhibition of MAb 19 binding.
(v) Surface plasmon resonance interaction analysis with BIAcore.
A biosensor (BIAcore) was used to quantify the interaction of MAb 19 with the mimotopes (30). MAPs were monobiotinylated at the cysteine residue with biotin-maleimide (BMCC, Pierce, United Kingdom), and streptavidin (Sigma) was immobilized onto the CM5 sensorchip. The monobiotinylated MAPs were allowed to react with the streptavidin-immobilized chips, and the association (ka) and dissociation (kd) rate constants and affinity constants (K = ka/kd) of the interaction of MAb 19 with the immobilized MAPs were determined over a range of MAb 19 concentrations (16.6 to 64.8 nM). The kinetic parameters were determined from the sensorgrams with BIAanalysis version 2.1 software (Pharmacia).
Virus neutralization.
RSV (50 PFU) was mixed with a range of dilutions of heat-inactivated sera in minimal essential medium containing 1% fetal calf serum and 25 mM HEPES for 1 h at room temperature. The virus-serum mixtures were transferred to HEp2 cell monolayers for 3 h at 37°C, after which the virus-serum mixtures were discarded, and 100 μl of a carboxymethyl cellulose overlay was added. The plates were observed daily for cytopathic effect, and up to 4 days later, the number of plaques was enumerated. Neutralization titers were expressed as the log2 of the reciprocal of the serum dilution which reduced the plaque numbers to 50% of the mean normal mouse serum control value.
RESULTS
Identification of mimotopes and their reactivity with MAb 19.
Five peptides were selected from the solid-phase peptide library with MAb 19 (Table 1), and Swiss-Prot analysis showed that the sequences have no homology with the primary amino acid sequence of RSV F protein. When the sequences were synthesized as free peptides, none reacted with MAb 19 in a peptide-based ELISA (Table 1), nor were they able to inhibit the binding of MAb 19 to RSV in the fluid phase (data not shown). The sequences were therefore resynthesized as peptide-TG resin complexes (identical to those employed in the solid-phase library), and all (except S5) reacted specifically with MAb 19. In particular, MAb 19 reacted strongly with two sequences (S1 [HWYISKPQ] and S2 [HWYDAEVL]) presented as resin-bound peptides (Table 1). However, when the sequences were presented as SPOTs peptides, MAb 19 bound strongly to S1 but not to S2. In an attempt to maximize the binding of the mimotopes by MAb 19, single-amino-acid substitutions at each position of the 8-mer peptides S1 and S2 were performed and the reactivities of the substituted peptides were analyzed. Some substitutions resulted in improved reactivity with MAb 19 (i.e., S1K, S1P, and S1S), but others abolished recognition by MAb 19 (for example, replacement of Y by F at position 3 in peptide S1; this shows the importance of the hydroxyl group for optimal binding, since Y can be replaced successfully by S [peptide S1S]) (Table 1). These results indicate that the presentation of the sequences as C-terminal linked peptides and as multiple copies at high density (as in the solid-phase library) appears to be critical for their recognition by MAb 19. Peptides S1, S1K, S1P, S1S, and S2 were therefore synthesized as tetrameric MAPs (S1-MAP, S1K-MAP, S1P-MAP, S1S-MAP, and S2-MAP) for use as antigens and immunogens. The binding of MAb 19 to S1-MAP in a peptide ELISA was specifically inhibited by the addition of increasing amounts of sucrose gradient-purified RSV but was not inhibited by monomeric S1 (Table 2). Similar binding curves were obtained with S1K-, S1P-, and S1S-MAPs, but no binding with S2-MAP was demonstrated (Table 2). Affinity constants for the interaction of MAb 19 with S1-MAP and S1S-MAP in the 109 M−1 range were determined by surface plasmon resonance, and more than a fourfold difference between the affinity of MAb 19 for S1S-MAP and that for S1-MAP was observed (Table 2).
TABLE 1.
Reactivity of mimotopes with MAb 19a
| Mimotope | Sequence | Reactivity as:
|
||
|---|---|---|---|---|
| Free peptide | Resin-bound peptide | SPOTs peptide | ||
| S1 | HWYISKPQ | <0.10 | 1.25 | +++ |
| S2 | HWYDAEVL | <0.10 | 1.43 | + |
| S3 | YWYEEPIX | <0.10 | 0.54 | + |
| S4 | MRRTHLQR | <0.10 | 0.27 | ± |
| S5 | TRRAYYPK | <0.10 | <0.10 | − |
| S1Kb | KWYISKPQ | ND | ND | ++++ |
| S1Pb | HPYISKPQ | ND | ND | ++++ |
| S1Sb | HWSISKPQ | ND | ND | +++++ |
Sequences were identified by microsequencing of five positive beads (X, undetermined amino acid). The reactivity with MAb 19 (at a concentration of 1.25 μg/ml) was tested with the sequences presented as follows. (i) Free peptides: ELISA plates were coated with the corresponding sequences and tested in a peptide ELISA. The results are expressed as corrected optical densities (ODs) (OD for MAb 19 − OD for control MAb). (ii) Resin-bound peptides: sequences were synthesized as TG resin-bound peptides and tested in a modified ELISA protocol. The results are expressed as corrected ODs. (iii) SPOTs peptides: sequences were synthesized as peptides linked to nitrocellulose and tested in SPOTs ELISA. The results are expressed as arbitrary units, representing the color intensity of the SPOTs. None of the SPOTs peptides reacted with a control MAb.
Amino acid substitution in the sequence of S1 (HWYISKPQ) analyzed by the SPOTs ELISA method. Only the substitutions resulting in an increase in MAb 19 (1.25 μg/ml) reactivity are presented (bold and underlined). ND, not determined.
TABLE 2.
Reactivity of MAb 19 with S1-, S1S-, and S2-MAP constructs and respective equilibrium constants
| MAP construct | ELISA resulta | Inhibition by monomeric peptideb | Inhibition by RSV (%)c | Biosensor K (M−1)d |
|---|---|---|---|---|
| S1-MAP | 1.15 | NS | 75.3 | 1.19 × 109 (± 0.23) |
| S1S-MAP | 1.57 | NS | 68.8 | 4.93 × 109 (± 0.31) |
| S2-MAP | <0.1 | NA | NA | NB |
ELISA results are presented as absorbance obtained with MAb 19 at 30 ng/ml.
Inhibition with the corresponding monomeric peptide was performed with a concentration of S1 or S1S of 0.5 mg/ml in the liquid phase. NS, not significant; NA, not applicable.
Inhibition with sucrose gradient-purified RSV was obtained with a concentration of 30 μg/ml (total protein concentration) in the liquid phase; no inhibition was detected when the same concentration of measles virus was used.
Results are the means of three different experiments (± standard deviations). NB, no detectable binding.
Induction of specific anti-RSV antibody responses.
To assess the immunogenicity of the RSV mimotopes presented as MAP constructs, BALB/c mice were coimmunized with the constructs and a Th epitope (molar ratio, 1:1) from measles virus F protein (aa 288 to 302) (27), and peak anti-peptide antibody titers (log10 titer range, 4.52 to 5.83) were observed 4 to 5 weeks after the boost. Mice immunized with MAP constructs in the absence of the Th epitope did not induce detectable anti-RSV antibodies (data not shown). Sera obtained from mice coimmunized with either the S1-, S1K-, S1P-, S1S-, or S2-MAP construct and the Th peptide (optimal dose, 25 μg; ∼5 nmol/animal) at the peak of antibody response were tested for their abilities to cross-react with RSV, to compete with MAb 19, and to neutralize viral infection in vitro (Table 3). Although all mimotopes tested induced antibodies cross-reactive with RSV, S1S-MAP generated the highest titer (log10 titer, 3.20 ± 0.12). Furthermore, S1S-MAP induced the highest titers of antibody that specifically inhibited the binding of MAb 19 to RSV and neutralized the virus in vitro (Table 3). Although antibodies induced by S2-MAP reacted weakly with RSV, they had no detectable MAb 19-inhibiting nor RSV-neutralizing activity. S1S-MAP colinearly synthesized with one copy of the Th epitope (S1S-MAP–Th) induced slightly lower anti-RSV antibody titers than those obtained with S1S-MAP, but they were more efficient at inhibition of MAb 19 binding and neutralization of RSV in vitro. These anti-S1S-MAP–Th antibodies had MAb 19-inhibiting titers and neutralizing titers similar to those in sera from mice naturally infected with RSV, which had high levels of anti-RSV antibodies (log10 titer, 4.51 ± 0.40). Immunization with the Th epitope alone did not induce RSV-specific antibodies. S1S-MAP (coimmunized with the Th epitope) and S1S-MAP–Th were therefore selected as immunogens to assess their effectiveness at inducing a protective antibody response against RSV challenge in vivo.
TABLE 3.
Induction of RSV-specific neutralizing antibody responses in mice following immunization with mimotopesa
| Immunization or infection | No. of mice | Anti-RSV IgG titer (log10) | MAb 19 inhibition titer (log2) | RSV neutralization titer (log2) |
|---|---|---|---|---|
| S1-MAP + Th | 3 | 2.72 ± 0.10 | 5.32 ± 2.82b | 4.82 ± 2.02b |
| S1K-MAP + Th | 3 | 2.46 ± 0.22 | 5.19 ± 2.64b | 4.32c |
| S1P-MAP + Th | 3 | 2.62 ± 0.11 | 3.82 ± 0.12b | 4.32c |
| S1S-MAP + Th | 5 | 3.20 ± 0.12 | 5.81 ± 1.07 | 5.66 ± 1.15 |
| S2-MAP + Th | 3 | 2.31 ± 0.13 | <3.32 | <3.32 |
| S1S-MAP–Th | 5 | 3.02 ± 0.19 | 6.24 ± 0.93 | 7.99 ± 1.53 |
| Th | 5 | <1.90 | <3.32 | <3.32 |
| RSV infected | 3 | 4.51 ± 0.40 | 5.72 ± 0.27 | 7.66 ± 0.58 |
BALB/c mice were immunized with either MAP + Th, MAP-Th, or Th (i.p.; 5 nmol/animal) and boosted 3 weeks later. Sera were collected 5 weeks following the boost. For the RSV-infected group, the mice were infected (i.n.) twice at 2-week intervals with live RSV (106 PFU) and sera were collected 3 weeks later. The results are presented as means ± 1 standard deviation.
Only two animals had significant MAb 19-inhibiting or RSV-neutralizing titers.
Only one animal had a significant RSV-neutralizing titer.
Reduction of viral load in the murine model of RSV infection.
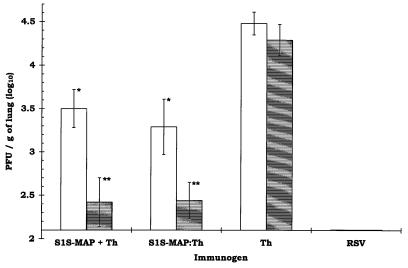
Groups of BALB/c mice were coimmunized with either S1S-MAP and the Th epitope, S1S-MAP–Th, or the Th epitope alone as described above; additional groups received a second boost 6 weeks after the first. Four to 5 weeks after the last boost, the mice were challenged with RSV i.n. (106 PFU/50 μl). A control group of naive mice was infected i.n. with RSV (106 PFU/50 μl) 9 days prior to challenge. Four days following challenge, at the peak of RSV infection in the lungs of BALB/c mice (40), the levels of virus recoverable from the lungs of the mice were determined. In groups immunized with S1S-MAP plus the Th epitope or with S1S-MAP–Th, there was a significant reduction in the titer of RSV recoverable from the lungs compared to that from controls immunized with the Th peptide alone (Fig. 1). The viral load in mice receiving two boosts was reduced by 77-fold in comparison to the viral load in Th-immunized animals (a reduction in titer of log10 1.85 to 1.89). Furthermore, prior to RSV challenge, the mice that received two boosts had higher anti-RSV antibody titers than animals that received one boost (Fig. 1). The control mice infected with RSV 9 days prior to challenge had no detectable virus in their lungs and were considered totally protected (8, 40).
FIG. 1.
Reduction of RSV infection in the lungs of mice following immunization with S1S-MAP. The animals were challenged i.n. with RSV (106 PFU/50 μl) 4 to 5 weeks after the last boost. The lungs were removed 4 days later (at the peak of the viral load in the lungs) for quantification of viral titers. The results are expressed as log10 RSV PFU/g of lung tissue ± 1 standard deviation. The lowest level of virus detectable in this assay was 2.10 log10 PFU/g of lung tissue (12 PFU/animal). The animals in the RSV group (the positive control for protection) were infected with RSV (106 PFU/50 μl) 9 days prior to rechallenge with RSV. The open bars represent BALB/c mice (5 animals/group) immunized i.p. and boosted 3 weeks later with S1S-MAP plus Th, S1S-MAP–Th, or Th (5 nmol/animal). Anti-RSV IgG log10 titers for each group prior to challenge were as follows: S1S-MAP plus Th, 3.29 ± 0.13; S1S-MAP–Th, 3.01 ± 0.11; Th, <1.90. The shaded bars represent BALB/c mice (4 animals/group) immunized following the schedule described above but which received a second boost 6 weeks later. Anti-RSV IgG log10 titers for each group prior to challenge were as follows: S1S-MAP plus Th, 3.61 ± 0.20; S1S-MAP–Th, 3.38 ± 0.15; Th, <1.90. A significant reduction in RSV titers was observed in the lungs of all animals immunized with S1S-MAP plus Th and S1S-MAP–Th in comparison to that in the Th control group (∗, P < 0.01; ∗∗, P < 0.001 [Student t test]). Over five experiments, no significant differences were observed in RSV titers recovered from the lungs of Th-immunized and mock-immunized animals (P > 0.2 by one-way analysis of variance).
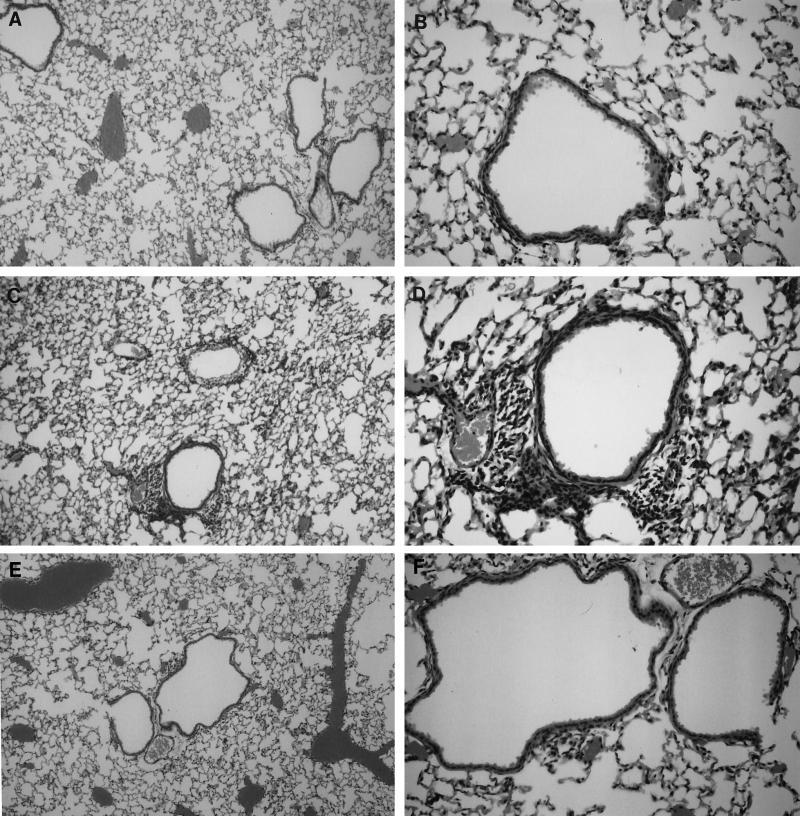
Histological evaluation of hematoxylin-and-eosin-stained lung sections 7 days after challenge revealed greatly reduced mononuclear cell infiltration in peribronchial and perivascular areas in mice immunized with S1S-MAP compared to that in control (Th-immunized) animals (Fig. 2) and similar to that observed in RSV-immunized and -challenged mice.
FIG. 2.
Sections of lung tissue stained with hemotoxylin and eosin showing reduced cellular infiltration in mice immunized with S1S-MAP plus Th constructs (A and B) compared to that in control mice immunized with Th alone (C and D) following challenge with RSV. As controls, sections from RSV-immunized and challenged mice showing no infiltration are also shown (E and F). The histological analyses were performed 7 days after challenge with RSV (106 PFU/50 μl). Magnification, ×100 (A, C, and E) and ×250 (B, D, and F).
The demonstration that S1S-MAP induced antibodies which cross-reacted with RSV, inhibited MAb 19 binding, and neutralized RSV in vitro suggested that these antibody responses were responsible for the observed reduction in viral load in vivo. Accordingly, groups of 14- to 15-week-old BALB/c mice were passively immunized i.p. with 400 μl of pooled anti-S1S-MAP antiserum or with 400 μl of normal mouse serum. As a control, one group of mice received 100 μl of MAb 19 ascites. Additionally, two other groups of mice received i.n. 120 μg of purified IgG either from S1S-MAP–Th-immunized mice or from normal mouse serum. The animals were challenged 24 h later, and titers of virus recoverable from the lungs were determined. Significantly lower levels of virus were recovered from mice which received pooled anti-S1S-MAP–Th antiserum (3.23 ± 0.15 log10 PFU/g) than from mice receiving normal mouse serum (4.32 ± 0.10 log10 PFU/g; P = 0.002). A similar reduction of viral load was observed in mice passively transferred i.n. with purified IgG from anti-S1S-MAP–Th antiserum (3.12 ± 0.28 log10 PFU/g) compared to the load in animals that received normal IgG (4.08 ± 0.13 log10 PFU/g; P = 0.005). However, these reductions were lower than those observed following passive transfer of 340 μg of MAb 19 (<2.1 log10 PFU/g) and following active immunization and may be a result of the transfer of an insufficient amount of specific anti-S1S-MAP antibodies.
DISCUSSION
The monoclonal anti-RSV antibody (MAb 19) which recognizes a conformational epitope on the F protein of both human RSV (A and B subtypes) and bovine RSV strains has been shown to be protective following passive transfer to experimental animals (38, 39). Furthermore, this MAb has been reshaped and humanized and has been used therapeutically in human volunteers (13). In the work described in this paper, a solid-phase combinatorial peptide library was used to identify peptides that mimic the epitope recognized by this neutralizing and protective MAb. When used as an immunogen, one of these mimotopes induced virus-neutralizing antibody responses and reduced viral load following challenge of mice with RSV.
Five peptide sequences were identified following screening of the library with MAb 19, but there was no demonstrable binding of MAb 19 to these sequences when they were presented as free peptides. However, when the sequences were synthesized as resin-bound peptides (identical to those presented in the solid-phase library) or as SPOTs peptides, MAb 19 demonstrated strong specific binding to two sequences: S1 (HWYISKPQ) and S2 (HWYDAEVL).
Since the solid-phase library we used contained only a fraction of the theoretical number of possible 8-mer peptides (2.6 × 1010), the SPOTs method was used for amino acid substitution analysis in an attempt to generate peptides with increased reactivity with MAb 19. Three modified sequences derived from S1 showed enhanced reactivity with MAb 19: S1K (KWYISKPQ), S1P (HPYISKPQ), and S1S (HWSISKPQ). These results indicate that once a mimotope has been identified from a combinatorial library, it will be possible to enhance its ability to interact with its corresponding antibody by making appropriate amino acid substitutions and thus making its conformation more like that of the epitope. It is important to note that the sequences identified by this type of approach do not necessarily have any similarity to the primary amino acid sequence of the F protein of RSV, but they mimic the conformation of the epitope (6, 15, 35).
The ability of MAb 19 to bind to these sequences was highly dependent on the way in which they were presented as antigens to the antibody, and the similarity of the precise spatial arrangements and densities of the sequences to those in the solid-phase library was critical for optimal binding (6, 26, 35). Since the monomeric free peptides did not inhibit the binding of MAb 19 to the corresponding MAPs or to RSV, it is likely that more than one copy of the sequence is involved in mimicking the epitope. The manner of peptide presentation is also likely to be important for immunogenicity, since none of the mimotopes used as immunogens in the form of free peptides was able to induce anti-RSV antibody responses in vivo. MAb 19 reacted specifically with S1-MAP (and derivative sequences of S1), with affinity constants of the order of 109 M−1. However, presentation of S2 as a MAP failed to mimic the epitope for MAb 19. Coimmunization of mice with the S1-, S1P-, S1K-, and S1S-MAP constructs and a Th peptide induced anti-peptide antibodies which inhibited the binding of MAb 19 to RSV and neutralized the virus in vitro, with the S1S-MAP construct being particularly effective. However, anti-peptide antibodies induced following coimmunization of mice with the S2-MAP construct and the Th epitope had no demonstrable RSV-neutralizing activity and did not inhibit MAb 19 binding to the virus.
There is a considerable literature showing that the biological activity of antibodies of high affinity is superior to that of lower-affinity antibodies (34), and we have previously shown that coimmunization induces antibodies of lower affinity than those induced following immunization with a chimeric immunogen in which the Th-cell epitope was covalently coupled to the B-cell epitope (33). S1S-MAP was therefore covalently coupled to the Th epitope (S1S-MAP–Th), and immunization with this chimeric construct induced anti-peptide antibodies that had MAb 19-inhibiting and RSV-neutralizing activities which were greater than those in sera from coimmunized animals and, interestingly, similar to those in sera from RSV-infected mice (Table 3).
The abilities of the antibody responses induced to confer protection were assessed in the well-established BALB/c mouse model of RSV infection (17, 40). A maximum 1.89 log10 (77-fold) reduction in viral load compared to those of control groups was observed in mice receiving two booster immunizations with the S1S-MAP constructs. Indeed, these animals had on average only 25 PFU detectable per pair of lungs. The reduction in viral load in S1S-MAP-immunized mice was not as great as that in RSV-immunized mice despite similar levels of neutralizing antibody in both groups. It is likely that immunization with RSV induced other protective immune mechanisms, such as cytotoxic T-lymphocyte (CTL) responses, which contributed to the enhanced protection observed compared to that seen with antibody alone.
The results of histological analyses were consistent with lung viral titers in that mononuclear-cell infiltration in the peribronchial and perivascular areas of the lungs of RSV-challenged S1S-MAP-immunized mice was greatly reduced compared to that in infected control animals (40). The reduction in virus titer is similar to that observed in mice immunized with purified F protein either by the mucosal or the parenteral route, which still had detectable virus after challenge (in nasal or lung lavages), or a 1.7 log10 reduction in titer (20, 44), although in our studies, we used three immunizations in Freund’s adjuvant. They are also consistent with other studies in which immunization with F protein incorporated into immune-stimulating complexes or with vaccinia viruses expressing F protein (vF), which induces both humoral and cellular immune responses, resulted in more than a 2 log10 reduction in viral titer (8, 41). In contrast to the enhanced pulmonary pathology seen in mice immunized with purified F protein or with vF and challenged with RSV, there was little or no inflammatory response in the lungs of mice immunized with S1S-MAP under our immunization schedule. However, we cannot exclude the possibility that under different conditions, enhancement of pathology may occur. T cells appear to be responsible for the development of lesions in RSV-infected mice (2, 16), and T cells mediate the exacerbated pathology seen in mice immunized with formalin-inactivated RSV (9) or with recombinant vaccinia viruses expressing RSV proteins (1). The minimal cellular response in the lungs of RSV-challenged mice coimmunized with S1S-MAP and the measles Th epitope suggests that, not surprisingly, RSV-specific T cells were not primed by the measles Th peptide. The possibility that replacement of the measles Th epitope with a Th from RSV will prime mice for increased pulmonary pathology is being investigated.
RSV-specific cytotoxic T cells induced following i.n. immunization with a chimeric peptide consisting of a fusion peptide from measles virus F protein and a CTL epitope from the M2 protein of RSV resulted in a reduction in virus load following challenge (21). The possibility that immunization with a mixture of synthetic peptides to induce RSV-specific anti-mimotope antibody and CTL responses will result in a more effective protection against infection is currently being studied. Nevertheless, the results presented in this report highlight the potential of synthetic peptide mimotopes for the induction of protective antibody responses to RSV and suggest that this approach could be used for the development of a new vaccine against this virus.
ACKNOWLEDGMENTS
This work was supported by grants from the British Lung Foundation and the Medical Research Council (ROPAs scheme).
We thank Paul Hobby (King’s College, Randall Institute, London, United Kingdom) for help and advice for the BIAcore analyses, Carolynne Stanley for expert technical assistance, and Maggie Long for the synthesis of peptides.
REFERENCES
- 1.Alwan W H, Kozlowska W J, Openshaw P J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan W H, Record F M, Openshaw P J. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbiza J, Taylor G, Lopez J A, Furze J, Wyld S, Whyte P, Stott E J, Wertz G, Sullender W, Trudel M. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73:2225–2234. doi: 10.1099/0022-1317-73-9-2225. [DOI] [PubMed] [Google Scholar]
- 4.Bourgeois C, Corvaisier C, Bour J B, Kohli E, Pothier P. Use of synthetic peptides to locate neutralizing antigenic domains on the fusion protein of respiratory syncytial virus. J Gen Virol. 1991;72:1051–1058. doi: 10.1099/0022-1317-72-5-1051. [DOI] [PubMed] [Google Scholar]
- 5.Chanock R M, Parrott R H, Connors M, Collins P L, Murphy B R. Serious respiratory tract disease caused by respiratory syncytial virus: prospects for improved therapy and effective immunization. Pediatrics. 1992;90:137–143. [PubMed] [Google Scholar]
- 6.Chargelegue D, Obeid O E, Shaw D M, Denbury A N, Hobby P, Hsu S C, Steward M W. Peptide mimics of conformationally constrained epitopes of RSV fusion protein. Immunol Lett. 1997;57:15–17. doi: 10.1016/s0165-2478(97)00045-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y C, Delbrook K, Dealwis C, Mimms L, Mushahwar I K, Mandecki W. Discontinuous epitopes of hepatitis B surface antigen derived from a filamentous phage peptide library. Proc Natl Acad Sci USA. 1996;93:1997–2001. doi: 10.1073/pnas.93.5.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connors M, Collins P L, Firestone C-Y, Murphy B R. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors M, Kulkarni A B, Firestone C Y, Holmes K L, Morse H C, Sotnikov A V, Murphy B R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe J E J, Bui P T, Davis A R, Chanock R M, Murphy B R. A further attenuated derivative of a cold-passaged temperature-sensitive mutant of human respiratory syncytial virus retains immunogenicity and protective efficacy against wild-type challenge in seronegative chimpanzees. Vaccine. 1994;12:783–790. doi: 10.1016/0264-410x(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 11.Crowe J E J, Bui P T, Firestone C Y, Connors M, Elkins W R, Chanock R M, Murphy B R. Live subgroup B respiratory syncytial virus vaccines that are attenuated, genetically stable, and immunogenic in rodents and nonhuman primates. J Infect Dis. 1996;173:829–839. doi: 10.1093/infdis/173.4.829. [DOI] [PubMed] [Google Scholar]
- 12.Crowe J E J, Murphy B R, Chanock R M, Williamson R A, Barbas C F, Burton D R. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci USA. 1994;91:1386–1390. doi: 10.1073/pnas.91.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everitt D E, Davis C B, Thompson K, DiCicco R, Ilson B, Demuth S G, Herzyk D J, Jorkasky D K. The pharmacokinetics, antigenicity, and fusion-inhibition activity of RSHZ19, a humanized monoclonal antibody to respiratory syncytial virus, in healthy volunteers. J Infect Dis. 1996;174:463–469. doi: 10.1093/infdis/174.3.463. [DOI] [PubMed] [Google Scholar]
- 14.Francis M J, Hastings G Z, Brown F, McDermed J, Lu Y A, Tam J P. Immunological evaluation of the multiple antigen peptide (MAP) system using the major immunogenic site of foot-and-mouth disease virus. Immunology. 1991;73:249–254. [PMC free article] [PubMed] [Google Scholar]
- 15.Gershoni J M, Stern B, Denisova G. Combinatorial libraries, epitope structure and the prediction of protein conformations. Immunol Today. 1997;18:108–110. doi: 10.1016/s0167-5699(97)01024-4. [DOI] [PubMed] [Google Scholar]
- 16.Graham B S, Bunton L A, Wright P F, Karzon D T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham B S, Perkins M D, Wright P F, Karzon D T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 18.Groothuis J R, Simoes E A, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner H C, Fulton D R, Welliver R C the Respiratory Syncytial Virus Immune Globulin Study Group. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 19.Hall C B. Prospects for a respiratory syncytial virus vaccine. Science. 1994;265:1393–1394. doi: 10.1126/science.7915433. [DOI] [PubMed] [Google Scholar]
- 20.Hancock G E, Speelman D J, Frenchick P J, Mineo-Kuhn M M, Baggs R B, Hahn D J. Formulation of the purified fusion protein of respiratory syncytial virus with the saponin QS-21 induces protective immune responses in BALB/c mice that are similar to those generated by experimental infection. Vaccine. 1995;13:391–400. doi: 10.1016/0264-410x(95)98263-a. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, S. C., D. Chargelegue, and M. W. Steward. Reduction of respiratory syncytial virus titer in the lungs of mice after intranasal immunization with a chimeric peptide consisting of a single CTL epitope linked to a fusion peptide. Virology, in press. [DOI] [PubMed]
- 22.Lam K S, Salmon S E, Hersh E M, Hruby V J, Kazmierski W M, Knapp R J. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 23.Lopez J A, Andreu D, Carreno C, Whyte P, Taylor G, Melero J A. Conformational constraints of conserved neutralizing epitopes from a major antigenic area of human respiratory syncytial virus fusion glycoprotein. J Gen Virol. 1993;74:2567–2577. doi: 10.1099/0022-1317-74-12-2567. [DOI] [PubMed] [Google Scholar]
- 24.Lounsbach G R, Bourgeois C, West W H, Robinson J W, Carter M J, Toms G L. Binding of neutralizing monoclonal antibodies to regions of the fusion protein of respiratory syncytial virus expressed in Escherichia coli. J Gen Virol. 1993;74:2559–2565. doi: 10.1099/0022-1317-74-12-2559. [DOI] [PubMed] [Google Scholar]
- 25.Murphy B R, Prince G A, Walsh E E, Kim H W, Parrott R H, Hemming V G, Rodriguez W J, Chanock R M. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nardin E H, Oliveira G A, Calvo-Calle J M, Nussenzweig R S. The use of multiple antigen peptides in the analysis and induction of protective immune responses against infectious diseases. Adv Immunol. 1995;60:105–149. doi: 10.1016/s0065-2776(08)60585-4. [DOI] [PubMed] [Google Scholar]
- 27.Obeid O E, Partidos C D, Howard C R, Steward M W. Protection against morbillivirus-induced encephalitis by immunization with a rationally designed synthetic peptide vaccine containing B- and T-cell epitopes from the fusion protein of measles virus. J Virol. 1995;69:1420–1428. doi: 10.1128/jvi.69.3.1420-1428.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power U F, Plotnicky-Gilquin H, Huss T, Robert A, Trudel M, Stahl S, Uhlen M, Nguyen T N, Binz H. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology. 1997;230:155–166. doi: 10.1006/viro.1997.8465. [DOI] [PubMed] [Google Scholar]
- 29.Prince G A, Hemming V G, Horswood R L, Chanock R M. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3:193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 30.Saunal H, Van-Regenmortel M H. Kinetic and functional mapping of viral epitopes using biosensor technology. Virology. 1995;213:462–471. doi: 10.1006/viro.1995.0019. [DOI] [PubMed] [Google Scholar]
- 31.Scott J K, Smith G P. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 32.Shaw D M. The immunogenicity and protective capacity of synthetic peptide vaccines comprising epitopes from the surface glycoproteins of respiratory syncytial virus. Ph.D. dissertation. London, United Kingdom: London University; 1994. [Google Scholar]
- 33.Shaw D M, Stanley C M, Partidos C D, Steward M W. Influence of the T-helper epitope on the titre and affinity of antibodies to B-cell epitopes after co-immunization. Mol Immunol. 1993;30:961–968. doi: 10.1016/0161-5890(93)90121-q. [DOI] [PubMed] [Google Scholar]
- 34.Steward M W, Chargelegue D. Overview. Antibody affinity: measurement and biological significance. In: Weir D M, Herzenberg L A, Blackwell C C, editors. Handbook of experimental immunology. 5th ed. Oxford, United Kingdom: Blackwell Science; 1997. pp. 38.1–38.37. [Google Scholar]
- 35.Steward M W, Stanley C M, Obeid O E. A mimotope from a solid-phase peptide library induces a measles virus-neutralizing and protective antibody response. J Virol. 1995;69:7668–7673. doi: 10.1128/jvi.69.12.7668-7673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stott E J, Taylor G, Ball L A, Anderson K, Young K K, King A M, Wertz G W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61:3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tam J P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, G. The role of antibody in controlling and/or clearing virus infections, p. 18–35. In G. L. Ada (ed.), Strategies in vaccine design—1994. R. G. Landes Co., London, United Kingdom.
- 39.Taylor G, Stott E J, Furze J, Ford J, Sopp P. Protective epitopes on the fusion protein of respiratory syncytial virus recognized by murine and bovine monoclonal antibodies. J Gen Virol. 1992;73:2217–2223. doi: 10.1099/0022-1317-73-9-2217. [DOI] [PubMed] [Google Scholar]
- 40.Taylor G, Stott E J, Hughes M, Collins A P. Respiratory syncytial virus infection in mice. Infect Immun. 1984;43:649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trudel M, Nadon F, Seguin C, Brault S, Lusignan Y, Lemieux S. Initiation of cytotoxic T-cell response and protection of Balb/c mice by vaccination with an experimental ISCOMs respiratory syncytial virus subunit vaccine. Vaccine. 1992;10:107–112. doi: 10.1016/0264-410x(92)90026-g. [DOI] [PubMed] [Google Scholar]
- 42.Trudel M, Stott E J, Taylor G, Oth D, Mercier G, Nadon F, Seguin C, Simard C, Lacroix M. Synthetic peptides corresponding to the F protein of RSV stimulate murine B and T cells but fail to confer protection. Arch Virol. 1991;117:59–71. doi: 10.1007/BF01310492. [DOI] [PubMed] [Google Scholar]
- 43.Valadon P, Nussbaum G, Boyd L F, Margulies D H, Scharff M D. Peptide libraries define the fine specificity of anti-polysaccharide antibodies to Cryptococcus neoformans. J Mol Biol. 1996;261:11–22. doi: 10.1006/jmbi.1996.0438. [DOI] [PubMed] [Google Scholar]
- 44.Walsh E E. Humoral, mucosal, and cellular immune response to topical immunization with a subunit respiratory syncytial virus vaccine. J Infect Dis. 1994;170:345–350. doi: 10.1093/infdis/170.2.345. [DOI] [PubMed] [Google Scholar]
- 45.Wertz G W, Sullender M W. Approaches to immunization against respiratory syncytial virus. In: Eltis R W, editor. Vaccines: new approaches to immunological problems—1992. Boston, Mass: Butterworth-Heinemann; 1992. pp. 151–176. [DOI] [PubMed] [Google Scholar]
- 46.Wyde P R, Moore D K, Hepburn T, Silverman C L, Porter T G, Gross M, Taylor G, Demuth S G, Dillon S B. Evaluation of the protective efficacy of reshaped human monoclonal antibody RSHZ19 against respiratory syncytial virus in cotton rats. Pediatr Res. 1995;38:543–550. doi: 10.1203/00006450-199510000-00012. [DOI] [PubMed] [Google Scholar]