Abstract
Background
The hawthorn has recently been used as a popular herbal medicine in food applications and phytotherapy, especially for the cardiovascular system.
Methods
In this study, phytochemicals were evaluated by LC-ESI-MS, GC-MS, and biological activity, including antioxidant (DPPH test) and antibacterial (broth dilution assay), in different extracts of Crataegus pentagyna fruit, leaf, and root.
Results
Globally, 49 phenolics were tentatively identified using HPLC-ESI-MS/MS in the hydro-methanolic extract of the fruit (major apigenin, caffeoylquinic acid derivative, and 4-O-(3′-O-glucopyranosyl)-caffeoyl quinic acid), 42 in the leaf (major salicylic acid, naringenin-6-C-glucoside, and naringin), and 33 in the root (major naringenin-7-O-neohesperidoside, isovitexin-2″-O-rhamnoside, and 4-O-(3′-O-glucopyranosyl)-caffeoyl quinic acid). The major group compounds analyzed by GC-MS in petroleum ether extracts were hydrocarbons (63.80%) and fatty acids and their derivatives (11.77%) in fruit, hydrocarbons (49.20%) and fatty acids and their derivatives (13.85%) in leaf, and hydrocarbons (53.96%) and terpenes (13.06%) in root. All samples exhibited promising phytochemical profile (total phenol, flavonoid, phenolic acid, and anthocyanin), antioxidant and antibacterial capacities, especially in hydro-methanolic extract of fruit (210.22 ± 0.44 mg GAE/g DE; 79.93 ± 0.54 mg QE/g DE; 194.64 ± 0.32 mg CAE/g DE; 85.37 ± 0.13 mg cyanidin 3-glucoside/100 g FW; DPPH: 15.43 ± 0.65 µg/mL; MIC: 0.15–0.62 µg/mL; and MBC: 0.62–1.25 mg/mL), followed by the leaf and root extracts, respectively. The PCA and heatmap analysis results distinguished metabolite profile differences for samples.
Conclusion
The results of the present work provide scientific support for C. pentagyna as antimicrobial agents and natural antioxidants in human health and food preservation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04430-4.
Keywords: Crataegus pentagyna, HPLC-ESI-MS/MS, GC-MS, PCA, Phenolic compounds, Antioxidant capacity, Antibacterial activity
Introduction
Oxidative stress caused by overproduction of free radicals and reactive oxygen species that results in the development of several diseases such as diabetes, alzheimer’s disease, parkinson’s disease and cardiovascular conditions such as atherosclerosis, stroke and high blood pressure [1, 2]. Also, infectious diseases such as measles, flu, HIV, COVID-19, strep throat and salmonella are disorders caused by organisms such as viruses, bacteria, fungi or parasites [3]. Infectious diseases resistance to antibiotics are 3rd common cause of death worldwide after cardiovascular diseases [4]. So, there is a continuous need for research and investment in the field of new drugs and food preservatives with lower toxicity and higher efficacy. Recently, pharmaceutical and food industries have utilized medicinal plants as sources of bioactive substances, particularly phenolic compounds with a wide range of pharmacological activities [5]. Most research found that phenolic compounds specially flavonoids exert antibacterial activity via damaging microbial cell membranes and inhibiting microbial enzymes and gene expression [6] and antioxidant activity via decreasing enzymatic activity of oxidases [7]. Hawthorn, the common name for more than a thousand species of plants in the genus Crataegus and family Rosaceae (subfamily Maloideae). The genus of Crataegus is primarily found in Asia, Europe, and North America and is recommended by the World Health Organization as a medicinal and food ingredient in several countries. This genus is native to northern temperate regions and consists of 15- to 18-foot-tall trees and deciduous shrubs [8]. Flowers, fruits, leaves, stems, and roots of Crataegus species have been recommended in modern and traditional medicine as cardiotonic, antispasmodic, diuretic, anti-atherosclerotic, and hypotensive agents [9, 10]. In terms of biological activity, proanthocyanidins and flavonoid glycosides are the most important compounds in hawthorn. In the leaves, flowers, and fruits of hawthorn, known phenolic compounds, such as quercetin, isoquercetin, rutin, hyperoside, epicatechin, chlorogenic acid, and protocatechuic acid, may be excellent sources of antioxidants [9–11]. Variations in genetics, maturity of plant organs, collection regions, processing methods, and preharvest and postharvest environmental conditions may influence the chemical compound content of plant organs [12, 13]. In Iran’s flora, Crataegus pentagyna subsp. elburensis is the most common cultivar of hawthorn. This plant is primarily utilized as an antiarrhythmic and cardiovascular disease preventative. The presence of polyphenols, such as flavonoids, phenolic acids, and proanthocyanidins, in the species may be primarily responsible for these effects [14]. Earlier phytochemical investigations of C. pentagyna from different origins have revealed the identification of different compounds such as gallic acid, caffeic acid, and chlorogenic acid in fruit, pulp and seed of Iranian species [15, 16]; hyperoside, rutin, isoquercitrin, sexangularetin-3-O-glucoside, isoorientin, isoorientin-2-O-rhamnoside, isovitexin, orientin, orientin-2-O-rhamnoside, vitexin, and vitexin-2-O-rhamnoside in leaves of Austrian species [17]; coumaric acid, chlorogenic acid, caffeic acid, ferulic acid, quercetin 3-O-glucoside (isoquercetin), quercetin, quercetin 3-O-rutinoside (rutin), (-)-epicatechin, kaempferol 3-O-glucoside, hyperoside, apigenin, cyanidin 3-O-glucoside, luteolin and procyanidins B1 and B2 in fruits, flowers and leaves of Serbian species [18]; and a number of flavonoid aglycones, flavonoid O- and C-glycosides, organic and phenolic acids and proanthocyanidins in leaf, flower and fruit of Romanian species [19]. Previous studies showed that these phytochemicals have been linked to the health-promoting effects of this species, including cardiovascular system influence, antioxidant, anti-cancer, antimicrobial, anti-inflammatory and antihypercholesterolemic activities [9, 20]. As an effective method with high sensitivity and resolution, liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC–ESI-MS/MS) is widely used for plant metabolomics analyses and species discrimination. To our knowledge, there are no reports comparing the chemical profile and biological activities of different morphological parts of C. pentagyna. We investigated the chemical composition, total phenol, total flavonoid, total phenolic acid, total anthocyanin, antioxidant, and antibacterial activities of C. pentagyna fruits, leaves, and roots collected in Golestan province of Iran. Using gas chromatography coupled with mass spectrometry (GC-MS) and LC-ESI-MS/MS techniques, the chemical profile of petroleum ether and hydro-methanolic extracts was determined.
Materials and methods
Chemicals
All chemicals were purchased from Sigma (St. Louis MO, USA), including gallic acid (purity = 97.5%), caffeic acid (98%), quercetin (95%), cyanidin-3-glucoside (≥ 95.5%), butylated hydroxytoluene (BHT) (≥ 99%), hydrochloric acid, sodium hydroxide, sodium molybdate, sodium carbonate, aluminum chloride, sodium acetate, potassium chloride, potassium acetate, Folin–Ciocalteu reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH) (≥ 95%), methanol, petroleum ether, and formic acid (≤ 100%) and etc. Quality of all chemicals used in LC–MS/MS and GC-MS were analytical grade. Aqueous solutions were also prepared using deionized water. Microorganism cultures of Gram-negative Escherichia coli (ATCC 25,922) and Gram-positive Staphylococcus aureus (ATCC 9144) were obtained from Iranian microbial collections, Pasteur Institute of Iran.
Plant samples
The essential factors for growth of plant are water, sunlight, heat, and topographical conditions in the planting region. Research has displayed that the optimum climate conditions for C. pentagyna growing involve 300–400 mm of annual precipitation, 8–12.5 °C of annual mean temperature, 2300–3200 °C of annual cumulative temperature, 2300 h of annual sunshine, and soil pH from 6.0 to 7.0. The highest growth rate occurs in the orchard between the ages of one and five years. Identification of optimal harvesting season is necessary to ensure contents of desired bioactive compounds. Therefore, around 500 g of fruits, leaves, and roots without damage of Crataegus pentagyna Willd. from one plant population were collected from Farsian, Galikesh, Golestan province, Iran (37°16’01.6"N 55°26’09.5"E) in September 2020 (early maturity stage) at the highest content of phenolic compounds [16, 18]. Samples was verified by Dr. Ali Satarian and the voucher number (803,892) was deposited in the Herbarium of Gonbad Kavous University, Gonbad, Iran.
Preparation of plant extracts
Fresh fruits, leaves, and roots were air-dried and ground separately using a mortar. One gram of defatted powder with petroleum ether (3 × 20 mL) was extracted thrice separately in the dark for two hours with water–methanol and water–ethanol 80% (3 × 20 mL). Under reduced pressure, the filtrate extracts were evaporated at 40 °C on a rotary evaporator (Heidolph, Laborota 4000, Schwabach, Germany) to dryness and then freeze-dried. The dried extracts were stored at − 20 °C in the dark until further examination. In addition, the yield of constituents and the weight of dried extracts were determined [18].
Estimation of phenolic profile
Total phenolic content (TPC)
The TPC of the extracts was determined using a modified version of Singleton and Rossi’s (1965) method [21], with gallic acid as the standard. To 500 µL of diluted samples, a mixture of 2.5 mL of Folin–Ciocalteu (0.2 N) reagent and 2 mL of Na2CO3 (75 g/L) was added. The absorbance of the samples was measured at 765 nm following 30 min of incubation at 45 °C. The results were given in milligrams of gallic acid per gram of dry extract (mg GAE/g DE).
Total flavonoid content (TFC)
Using a modified aluminum chloride colorimetric method [22], the TFC content of the samples was determined. In total, 0.5 mL of samples was combined with 0.1 mL of sodium acetate (1 M) and 0.1 mL of AlCl3 (10%) and incubated for 30 min at room temperature. At 415 nm, the absorbance of the reaction mixture was measured. The total flavonoid concentration was calculated as mg of quercetin equivalents (QE) per gram of dry extract.
Determination of total phenolic acid content (TPAC)
The total phenolic acid content of the extracts was determined by spectrophotometry at 474 nm [23]. A total of 1 mL of each extract was combined with 10 g of sodium nitrite and 10 g of sodium molybdate diluted in 100 mL of water, 2 mL of HCl (0.5 M), 3 mL of water, and 2 mL of NaOH (8.5% w/v) in this procedure. The results were reported in terms of mg of caffeic acid (CAE) per g of dry extract (mg CAE/g DE).
Determination of total anthocyanin content
The total anthocyanin concentration was determined using a method previously described [24]. At 530 nm, the absorbance of diluted extracts with 1% HCl in methanol (5:95, v/v) was measured. Using the following equation, the values were expressed as mg malvidin-3-glucoside equivalents per g of dry extract.
The results were expressed in milligrams of cyanidin-3-glucoside per 100 g of fresh weight (mg c3g/100 g FW).
HPLC-PAD analysis
An analytical technique A KNAUER liquid chromatograph system with a photodiode array detector (Smartline PDA 2600) and a quaternary pump (Smartline Pump 1000) has been developed. The dissolved extracts in methanol: water 7:3 (approximately 2 mg/mL) were subjected to a gradient method on a 150 mm length × 4.6 mm inner-diameter C18 amide column (Varian, Darmstadt, Germany) at a flow rate of 1.0 mL/min and injection volume of 20 µL (water as solvent A and methanol as solvent B, including 0.05% trifluoroacetic acid). The elution gradient was 0–10 min with 10% solvent B, 10–35 min with 10–100% B, 35–45 min with 100% B, 45–50 min with 100–10% B, and 50–55 min with 10% B. The system was managed using the software EZ Chrom Elite. For testing the system’s dependability, 4-hydroxymethyl benzoate, uracil, benzophenone, and 4-hydroxy ethyl benzoate were injected into the HPLC [25, 26].
LC-ESI-MS/MS analysis of phenolic compounds
Using a Waters Alliance 2695 HPLC system coupled to a micro mass quattro micro API mass spectrometer with an ion source, the flavonoids and other phenolics compositions of hydro-methanolic extracts from the fruit, leaf, and root of C. pentagyna were determined (ESI). The separation was carried out using a Supelco C18 (15 mm×2.1 mm×3 μm) column with a flow rate of 0.2 mL/min and an injection volume of 10 µL. Using a gradient method (acetonitrile + 0.1% formic acid as solvent A and water + 0.1% formic acid as solvent B), the samples were eluted. The elution gradient was 0–5 min with 10% solvent A, 5–10 min with 10–50% A, 10–16 min with 50% A, 16–20 min with 50–90% A, 20–24 min with 90% A, 24–26 min with 90–10% B, and 26–30 min with 10% A. The mass spectra were acquired using negative ionization and a range of 50 to 2000 m/z. The detection of peaks at 310 °C probe temperature and 3.5 kV probe voltage were monitored. The LC-MS data were analyzed using MZmine version 2.35 software [27].
GC-MS analysis of essential compounds
Experiments were performed using an Agilent 6890 A mass spectrometer and an Agilent 5973 gas chromatograph. A total of 1 mL of the diluted extracts was injected into the HP5MS column with a length of 30 m, an inner diameter of 0.25 mm, and a thickness of 0.25 μm. As a carrier gas, helium at a flow rate of 1.0 mL/min was utilized. The column temperature was set at 50 °C for 3 min before increasing to 280 °C at a rate of 4 °C/min. The temperature of the GC injector and MS transfer line were set to 250 and 230 °C, respectively. MS detection utilized an ionization energy of 70 eV and a mass range of 50–550 m/z. By comparing their mass fragmentation patterns and retention times with the Wiley 7.0 and NIST libraries, the compound profiles were identified in petroleum ether extracts [28, 29].
Antioxidant activit
DPPH radical-scavenging activity
Modifications were made to the previously reported method [30] to measure antioxidant activity. Briefly, 3 mL of diluted samples with concentrations of 10 to 200 µg/mL were mixed with 1 mL of 100 M methanolic DPPH solution. The absorbance of the mixture was measured at room temperature at 517 nm. DPPH radical scavenging capacity was calculated using the following formula:
% inhibition = [(A0 − At)/A0 × 100].
Where, A0 is the absorbance of the blank (without extracts) and At is the absorbance of the extracts.
The antioxidant capacity of the samples was expressed as IC50. Using the regression equation, the IC50 values were determined as the concentrations of extracts that inhibit 50% of DPPH radicals. In addition, BHT served as a positive control.
Estimation of antibacterial activity
In our laboratory, a Gram-negative (Escherichia coli ATCC 25,922) and a Gram-positive (Staphylococcus aureus ATCC 9144) bacteria were stored at 4 °C and used in this study. Iranian Research Organization for Science and Technology provided the examined bacteria. The microdilution broth technique [31] was used to evaluate the antimicrobial activity of C. pentagyna extracts. Two-fold serial dilutions of extracts in Muller Hinton broth (MHB) were prepared in a microtiter ranging from 0.078 to 20 mg/mL, and one million colony-forming units per milliliter were added to each well. Gentamicin and MHB were used as positive and negative controls, respectively. After 24 h of incubation at 35 °C, MIC was defined as the lowest concentration of the extracts with no visible bacterial growth. Also, MBC was determined as the lowest concentration of the extracts that was resulted in bacterial death.
Statistics
Each experiment was performed three times under the same conditions. The results of each test and analysis were recorded as means and standard deviation. One-way ANOVA test was used to compare variance analyses, and the SAS software (least significant difference (LSD) test) was utilized to compare means (p < 0.05). The chromatograms of fruits, leaves and roots of C. pentagyna were pre-processed using MZmine analysis software package. To perform further statistical analyses was performed through SIMCA software (version 14.1 Umetrics AB, Umea, Sweden), MZmine exported the peak list data for each sample, which included the average retention time (RT), m/z values and peak intensity (height). The exported data were subsequently mean-centered and Pareto-scaled prior to multivariate statistical analysis to enhance the contribution from medium-sized features without inflating the noise from quiet areas of the chromatogram. Principal Component Analysis (PCA) and related biplot and loading plot was used for multidimensional data to identify metabolite differences between samples. Furthermore, heatmap analysis was performed using import-exciting clusters to better show differences between metabolites.
Results and discussion
C. pentagyna extraction yield, quantitative evaluation of phenolic profile, and antioxidant power.
The chemical components of the fruit, leaf, and root of C. pentagyna were fractionated using ether petroleum, 80% aqueous methanol, and ethanol as extraction solvents. The yield of extracts varied between 2.4% and 9.6% for methanolic extracts, between 2.1% and 8.3% for ethanolic extracts, and between 0.9% and 3.6% for petroleum ether extracts. Due to the hydroxyl (-OH) and methoxy (-OCH3) groups in their molecular structures [32], phenolic compounds possess the ability to scavenge free radicals. The phenolic content of hawthorn varies by cultivar, species, geographical location, harvest time, method of chemical determination of phytochemicals, and extraction preparation conditions [33]. There are few studies on the phytochemical content of C. pentagyna compounds. In addition, there have been no reports of hawthorn root. In this study, the phytochemical content (phenol, flavonoid, phenolic acid, and anthocyanin) and antioxidant capacity (DPPH scavenging) of hydro-methanolic and hydro-ethanolic extracts of fruit, leaf, and root were evaluated and compared (Table 1). The results indicated that hydro-methanolic extracts contain more phytochemicals and have more antioxidant capacity than ethanol extracts. Fruit extract contained the highest concentrations of phenolic (210.22 ± 0.44 mg GAE/g DE), total flavonoid (79.93 ± 0.54 mg QE/g DE), total phenolic acid (194.64 ± 0.32 mg CAE/g DE), and total anthocyanin (85.37 ± 0.13 mg cyanidin 3-glucoside/100 g FW) among the tested extracts, followed by leaf and root extracts. The total content of phenol and flavonoid in methanolic extracts of fruit from various regions ranged from 69.12 to 186.72 mg GAE/g and 1.6 to 85.31 mg QE/g dry weight plant, respectively, as reported by other researchers [15, 32, 34–36]. In a different study, the TPC and TFC concentrations in methanolic leaf extracts were 206.94 GAE/g and 57.08 mg (+)-catechin/g, respectively [37, 38]. There was a strong correlation between TPC and DPPH reduction. The phenolic and flavonoid content of fruit, leaf, and root extracts increases their antioxidant activity. The fruit extract with the highest phenolic and flavonoid content exhibited the highest DPPH radical scavenging capacity (IC50 = 15.43 ± 0.65 g/mL), followed by leaf and root extracts (IC50 = 34.67 ± 0.14 g/mL and 60.72 ± 0.32 g/mL, respectively). Moreover, the fruit and leaf extracts exhibited potent activity comparable to the positive control butylate hydroxytoluene (BHT) (IC50 = 49.02 ± 0.2 g/mL), whereas the root extract was less active. Antioxidant activity of fruit and leaf extracts of C. pentaegyna from different regions have been already investigated. For the methanolic fraction of fruit, the IC50 values for DPPH radical scavenging activity range from 17.48 to 341.29 g/mL [32, 34, 36, 39]. Moreover, the DPPH inhibition of C. pentagyna leaf was quantified as 5708 g/mL in hydroacetonic extract and 2.34 M TE/g (micromoles of Trolox equivalents) in ethanolic extract, respectively [18, 37, 40].
Table 1.
Phytochemical screen and antioxidant activity of fruit, leaf, and root of C. pentagyna (TP: total phenol; TF: total flavonoid; TPA: total phenolic acid; TAC: total anthocyanin)
| Analysis | Hydro-Methanolic Extract | Hydro-Ethanolic Extract | ||||
|---|---|---|---|---|---|---|
| Fruit | Leaf | Root | Fruit | Leaf | Root | |
| TP (mg GAE/g) | 210.22 ± 0.44 a | 132.25 ± 0.32 b | 102.46 ± 0.54 c | 189.49 ± 0.19 d | 112.33 ± 0.48 e | 93.19 ± 0.58 f |
| TF (mg QE/g) | 79.93 ± 0.54 a | 67.21 ± 0.54 b | 48.66 ± 0.71 c | 58.9 ± 0.34 d | 39.53 ± 0.58 e | 42.47 ± 0.34 f |
| TPA (mg CAE/g) | 194.64 ± 0.32 a | 98.43 ± 0.76 b | 79.34 ± 0.92 c | 123.21 ± 0.67 d | 86.54 ± 0.39 e | 51.43 ± 0.85 f |
| TAC (mg cyanidin 3-glucoside/100 g FW) | 85.37 ± 0.13 a | 20.45 ± 0.53 b | 1.32 ± 0.13 c | 75.94 ± 0.84 d | 16.45 ± 0.88 e | 0.56 ± 0.44 c |
| DPPH assay, IC50 (µg/mL) | 15.43 ± 0.65 a | 34.67 ± 0.14 b | 60.72 ± 0.32 c | 29.48 ± 0.33 d | 51.32 ± 0.73 e | 70.21 ± 0.12 f |
|
Extraction yield % (w/w) |
9.6 | 6.9 | 2.4 | 8.3 | 6.4 | 2.1 |
Means with different superscript lowercase letters within the same row differ significantly (p < 0.05)
Phenolic characterization using HPLC-PDA and LC/ESI-MS/MS
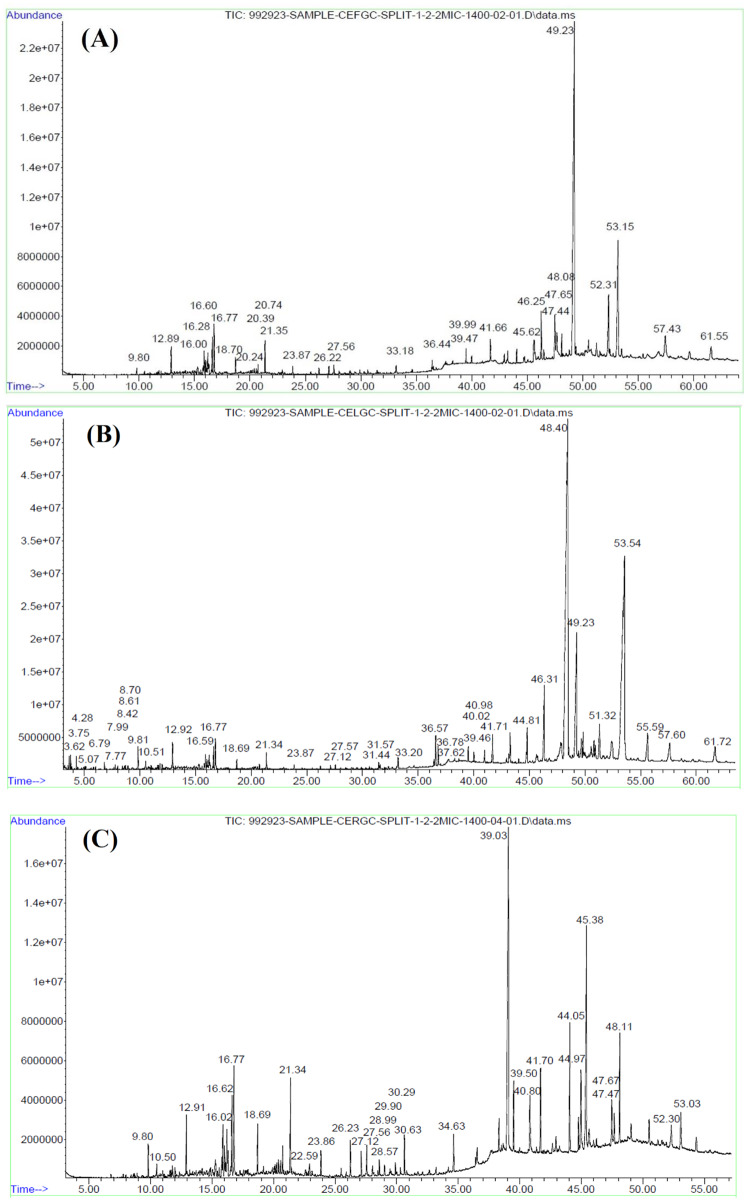
Using HPLC-ESI-MS/MS in negative ionization mode, the phytochemical profile of hydro-methanolic extracts of the fruit, leaf, and root of C. pentagyna tentatively were identified. Fig. S1 A-C depict the HPLC-ESI-MS/MS and HPLC-PDA (280 nm, 330 nm, and 360 nm) fingerprints (Supplementary materials). LC-ESI-MS/MS chromatograms show total ion chromatograms of compounds (TIC). The extracted ion chromatograms (XIC) from total ion chromatograms were processed by MZmine analysis software package which can separate all chromatograms and compounds. Figure 1A-C illustrates the instances of extracted ion chromatograms (XIC). According to the ESI-MS/MS fragmentation pattern, molecular weights, matching mass adducts ([M-H]−, [2M]−, [2 M-H]−, [M-2 H]−,[M-2 H + Na]− and [M-2 H + K]−) and published data, we identified 49, 42, and 33 phenolic compounds from the fruit, leaf, and root, respectively (Table 2) and (Supplementary materials: Table S1 and Fig S3-S5). Other hawthorn species (Crataegus spp.) in which these compounds have been previously identified are listed in Table 2 to compare with obtained results in our study. Phenolic compounds of C. pentagyna have been identified in a limited number of studies. Salmanian et al. [16]. determined the concentrations of gallic acid, caffeic acid, and chlorogenic acid in pulp and seed extracts of C. pentagyna from Iran. In Austrian C. pentagyna leaves, Prinz et al. [17]. isolated and identified hyperoside, rutin, isoquercitrin, sexangularetin-3-O-glucoside, isoorientin, isoorientin-2-O-rhamnoside, isovitexin, orientin, orientin-2-O-rhamnoside, vitexin, and vitexin-2-O-rhamnoside as flavones. In a separate study, Pavlovic et al. [18]. determined phenolics (p-coumaric acid, chlorogenic acid, caffeic acid, ferulic acid, isoquercetin, quercetin, rutin, (-)-epicatechin, kaempferol 3-O-glucoside, hyperoside, apigenin, cyanidin 3-O-glucoside, luteolin, procyanidins B1 and B2) during harvesting period in C. pentagyna fruits, flowers and leaves from Serbia. In addition, Alirezalu et al. [15]. quantified chlorogenic acid, vitexin, hyperoside, rutin, quercetin, and isoquercetin in the Iranian C. pentagyna fruit extract. Moreover, a number of 39 compounds (flavonoid aglycones, flavonoid O- and C-glycosides, organic and phenolic acids and proanthocyanidins) were identified in Romania C. pentagyna leaf, flower and fruit ethyl acetate extracts [19].
Fig. 1.
Extracted ion chromatograms (XIC) and corresponding mass adducts in the hydro-methanolic extracts of C. pentagyna. (A) chromatogram XIC of sinapic acid and mass adducts, m/z 223; (B) XIC of Kaempferol-3-O-rutinoside and mass adducts, m/z 285; and (C) XIC of naringenin-7-O-glucoside and mass adducts, m/z 433
Table 2.
Phenolic constituents identified in the hydro-methanolic extracts of the fruit, leaf, and root of C. pentagyna using HPLC-ESI-MS/MS.
| No. | Compound | [M-H]−− (m/z) |
MS2 Fragments | Rt (min)/Intensitya (En)/ Mass error (ppm) |
Used Parts in Literature a | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Fruits | Leaves | Roots | ||||||
| 1 |
Chlorogenic acid glycoside: 4-O-(3′-O-glucopyranosyl)-caffeoyl quinic acid |
515 | 353 |
0.91/7.2E5/ 0.22 |
0.93/2.7E5/ 0.23 |
0.93/4.8E5/ 0.23 |
F, L | [68] |
| 2 | Sinapic acid-O-hexoside | 385 | 223, 208 |
1.1/7.3E4/ 1.21 |
- |
1.3/1.2E5/ 1.17 |
F | [45] |
| 3 | Caffeoylquinic acid derivative | 353 | 191, 179 |
1.3/8.2E5/ 0.27 |
- | - | F ⁎, L ⁎, Fl ⁎ | [18] |
| 4 | Caffeoylquinic acid derivative | 353 | 191, 179 |
1.3/6.9E4/ 0.27 |
1.4/4.4E5 0.25 |
- | F, L ⁎, Fl ⁎ | [12, 18, 33, 79] |
| 5 | Coumaroylquinic acid derivative | 337 | 191 |
1.5/9.7E4/ -0.87 |
- | - | F⁎, Fl⁎, L⁎ | [19, 68, 87] |
| 6 | Caffeoylquinic acid derivative | 353 | 191, 179 | - | - |
1.6/6.3E4/ 0.24 |
F ⁎, L ⁎, Fl ⁎ | [15, 18, 45, 51] |
| 7 | Coumaroylquinic acid derivative | 337 | 191 | - |
1.7/1.1E5/ -0.81 |
1.6/2.0E5/ -0.83 |
F, L | [68] |
| 8 | Coumaroylquinic acid derivative | 337 | 191 | - |
1.7/2.4E5/ -0.82 |
- | F ⁎, L | [18, 68] |
| 9 | Sinapic acid | 223 | 208 | - |
1.9/1.3E5/ 2.54 |
1.8/6.7E4/ 2.57 |
F, Fl | [45] |
| 10 | Protocatechuic acid | 153 | 109 |
2.5/1.5E5/ 0.64 |
- | - | F⁎, Fl⁎, L⁎ | [19, 77] |
| 11 | Caffeic acid | 178 | 178, 135 |
2.9/2.6E5/ -1.9 |
- | - | F ⁎, L ⁎, Fl ⁎ | [18, 19, 45, 71] |
| 12 | Salicylic acid | 137 | 93 |
4.3/1.3 E5/ 1.45 |
4.4/3.0 E6/ 1.39 |
4.3/1.3E5/ 1.45 |
F⁎, Fl⁎, L⁎ | [19, 56] |
| 13 | Hydroxybenzoic acid derivative | 137 | 93 |
4.3/1.2E5 1.35 |
- | - | F⁎, Fl⁎, L⁎ | [19, 45] |
| 14 | Hydroxybenzoic acid derivative | 137 | 93 |
4.3/1.3E5/ 1.35 |
- | - | F | [45] |
| 15 | Ferulic acid | 193 | 178, 149 |
4.7/1.2E5/ -0.31 |
- | - | F ⁎, L ⁎, Fl | [45–47, 57, 77] |
| 16 | Myricetin-3-O-(6″ galloyl) galactoside | 631 | 479, 317 |
5.3/2.0E5/ -1.21 |
5.4/1.6E5/ -1.25 |
5.3/1.6E5/ -1.20 |
F | [56] |
| 17 | Coumaric acid | 163 | 119 | - |
5.3/2.8E5/ 0.89 |
5.4/2.1E5/ 0.84 |
F ⁎, L ⁎, Fl ⁎ | [18, 78] |
| 18 | Quercetin-3-O-rutinoside | 609 | 271, 301 |
5.5/1.0E5 0.45 |
5.5/2.4E5/ 0.43 |
- | L | [55] |
| 19 | Quercetin-3-O-(6″ galloyl) glucoside | 615 | 463, 301 |
6.7/2.5E5/ -0.23 |
6.5/8.2E4/ -0.24 |
6.6/1.9E5/ -0.23 |
L | [56] |
| 20 | Quercetin-7,4′-dimethyl ether-3-O-rutinoside | 637 | 491, 329 |
7.5/1.2E5/ 3.69 |
7.8/1.0E5/ 3.52 |
7.6/1.6E5/ 3.63 |
F | [56] |
| 21 | Quercetin-3-O-rhamnoside | 447 | 301 |
8.4/7.2E4/ -2.71 |
8.2/3.0E5/ -2.63 |
- | L ⁎, Fl ⁎ | [18] |
| 22 |
Orientin or isoorientin glycoside (Orientin-2″-O-rhamnoside) |
593 | 447 |
8.4/1.0E5/ 1.45 |
8.4/1.5E5/ 1.40 |
- | L ⁎, Fl ⁎ | [12, 17] |
| 23 |
Orientin or isoorientin glycoside (Isoorientin-2″-O-rhamnoside) |
593 | 447 |
9.3/4.6E4 1.44 |
- | - | Fl ⁎ | |
| 24 | Vitexin-O-rhamnoside derivative | 577 | 457, 431, 413, 311, 293 | - |
10.9/1.5E5/ 1.87 |
10.7/8.2E4/ 1.81 |
F, L ⁎, Fl ⁎ | [48, 58–65] |
| 25 | Vitexin-O-rhamnoside derivative | 577 | 457, 431, 413, 311, 293 | - |
10.7/1.3E5/ 1.87 |
10.9/5.7E5/ 1.86 |
- | [68] |
| 26 | Vitexin-O-glucoside derivative | 593 | 473, 431, 413, 311, 293 |
10.6/1.2E5/ -2.41 |
- | - | F, L | [58, 60] |
| 27 | Naringenin-7-O-neohesperidoside | 579 | 271 | - |
11.6/3.7E5/ 2.51 |
11.8/6.8E5/ 2.49 |
L | [67] |
| 28 | Vitexin-O-rhamnoside derivative | 577 | 457, 431, 413, 311, 293 | - |
12.1/1.7E5/ 1.88 |
- | L, Fl | [42, 50, 64, 65] |
| 29 | Vitexin-O-glucoside derivative | 593 | 473, 431, 413, 311, 293 |
12.8/5.3E4/ -2.44 |
- | - | L | [51, 64–66] |
| 30 | Myricetin-3-O-galactose | 479 | 317 |
13.9/4.0E4/ -3.15 |
- |
13.8/1.1E5/ -3.18 |
F | [56] |
| 31 | Quercetin-O-glycoside derivative | 463 | 301 |
14.6/8.8E4/ 1.25 |
- |
14.8/1.5E5/ 1.19 |
F, L ⁎, Fl | [12, 15, 18, 43, 46] |
| 32 | Quercetin-O-glycoside derivative | 463 | 301 |
14.6/8.9E4/ 1.26 |
- | - | F ⁎, L ⁎, Fl ⁎, S | [15, 18, 42, 43, 50–54] |
| 33 | Quercetin-O-glycoside derivative | 463 | 301 |
14.7/8.3E4/ 1.26 |
- | - | F, Fl | [1, 47–49] |
| 34 | Epigallocatechin gallate | 457 | 305 |
15.4/9.5E4/ -0.86 |
15.2/1.4E5/ -0.81 |
- | F | [74] |
| 35 | Epicatechin gallate | 441 | 289 |
15.9/9.3E4/ 0.56 |
15.8/1.1E5/ 0.52 |
- | F, L | [17, 49, 65] |
| 36 | Orientin or isoorientin | 447 | 357, 327 |
16.9/8.5E4/ 1.23 |
- | - | L ⁎, Fl ⁎ | [12, 17] |
| 37 | Orientin or isoorientin | 447 | 357, 327 |
16.9/1.1E5/ 1.24 |
- | - | Fl ⁎ | [17] |
| 38 | 8-methoxykaempferol | 315 | 300 |
17.1/2.0E5/ -1.45 |
- | - | Fl | [17, 51] |
| 39 | Eriodictyol-7-glucuronide | 463 | 287 | - |
17.5/7.1E4/ 2.13 |
- | L, Fl | [17, 43] |
| 40 | Luteolin 7-O-glucoside | 447 | 285 |
17.8/7.1E4 0.93 |
17.6/2.1E5/ 0.91 |
- | F ⁎, L ⁎, Fl ⁎ | [12, 17, 18, 42] |
| 41 | Luteolin-7-O-glucuronide | 461 | 267 |
17.8/1.5E5/ 3.02 |
17.5/1.6E5/ 3.10 |
- | L | [43] |
| 42 | Apigenin-8-C-glucoside | 431 | 341, 311 |
19.8/6.8E4/ 1.95 |
19.7/9.0E4/ 1.91 |
19.9/1.1E5/ 1.81 |
F⁎, L⁎, Fl⁎, S | [12, 15, 17, 42, 43, 51, 57] |
| 43 | Naringenin-6-C-glucoside | 433 | 343, 313 |
20.5/3.9E5/ 2.34 |
20.6/5.3E5/ 2.30 |
20.4/9.0E4/ 2.35 |
L | [68] |
| 44 | Naringenin-7-O-glucoside | 433 | 271 |
21.4/2.7E5/ 1.98 |
21.7/1.4 E5/ 1.93 |
21.5/1.4E5/ 1.95 |
L | [68] |
| 45 | Procyanidin B3 7-glucoside | 739 | 721, 450, 435 | - |
22.9/1.6E5/ 2/54 |
- | F | [75] |
| 46 | Myricetin | 317 | 300, 179, 151 |
23.5/1.5E5/ 1.31 |
23.5/8.8E4/ 1.19 |
- | F | [56] |
| 47 | 5-O-methylmyricetin | 331 | 315 |
23.7/1.3E5/ -0.32 |
23.9/1.3E5/ -0.37 |
23.7/1.2E5/ -0.33 |
- | [56] |
| 48 | Quercetin | 301 | 151, 158 |
23.9/5.9E4/ 3.34 |
23.8/1.3E5/ 3.31 |
23.9/1.5E5/ 3.29 |
F⁎, L⁎, Fl | [18, 19, 42, 44, 45] |
| 49 | Catechin or epicatechin monomers | 289 | 271, 245, 137 |
24.2/1.5E5/ 2.23 |
24.3/2.6E5/ 2.23 |
24.3/1.4E5/ 2.30 |
F⁎, L⁎, Fl | [19, 66, 71] |
| 50 | Kaempferol-3-O-rutinoside | 593 | 285 |
24.4/9.0E4/ 0.45 |
- | - | F ⁎, L ⁎ | [18] |
| 51 | Catechin or epicatechin monomers | 289 | 271, 245, 137 |
24.4/7.6E4/ 1.69 |
24.4/1.0E5/ 1.61 |
24.6/1.6E5/ 1.58 |
F ⁎, L ⁎, Fl ⁎ | [1, 18, 72, 73] |
| 52 | Luteolin | 285 | 269 |
24.6/1.3E5/ 3.01 |
24.9/6.7E4/ 3.04 |
24.7/1.0E5/ 3.10 |
L | [42] |
| 53 | Kaempferol | 285 | - |
24.6/2.9E5/ -0.82 |
24.9/7.7E4/ -0.73 |
- | F ⁎, Fl ⁎ | [18, 44] |
| 54 | Eriodictyol | 287 | 153, 163 179 |
24.6/2.7E5/ -2,41 |
24.5/1.7E5/ -2.39 |
24.4/1.0E5/ -2.20 |
Fl | [42] |
| 55 | Hesperetin | 301 | 153, 163, 179 |
24.8/6.5E4/ 0.103 |
24.7/1.2 E5/ 0.101 |
24.8/3.9E4/ 0.10 |
Fl | [42] |
| 56 | Apigenin | 269 | - |
24.9/8.4E5/ 2.92 |
24.8/1.1E5/ 2.84 |
24.9/1.5E5/ 2.92 |
L | [42] |
| 57 | Naringenin | 271 | 152 | - |
26.5/2.0E5/ -0.39 |
26.4/1.0E5 -0.38 |
L | [42, 66] |
| 58 | Procyanidin tetramer-hexoside | 1315 | - | - | - |
26.9/1.0E5/ 1.36 |
F | [75] |
| 59 | Procyanidin C2 | 865 | 713, 576, 425, 289 |
26.9/5.1E4/ 2.78 |
26.8/1.0E5 2.72 |
26.8/1.4E5 2.70 |
F, L, Fl | [75, 76] |
| 60 | Procyanidin pentamer-hexoside | 1605 | - | - |
28.8/7.1E4/ 1.83 |
28.8/1.0E5/ 1.80 |
F | [75] |
| 61 | Procyanidin tetramers | 1154 | - |
28.8/1.6E5/ -0.32 |
28.9/4.4E4/ -0.38 |
28.8/1.2E5/ -0.32 |
F, L, Fl | [75, 76] |
| 62 | B-type procyanidin pentamers | 1442 | 1156 |
29.1/8.5E4 3.24 |
29.3/4.0E4 3.19 |
29.0/9.9E4 3.19 |
F, L, Fl | [75, 76] |
| 63 | B-type procyanidin hexamers | 1731 | 1154, 1156, 1444 |
29.4/1.2E5/ 1.42 |
29.8/3.3E4 1.45 |
29.5/3.9E5/ 1.45 |
F, L, Fl | [75, 76] |
- The MZmine analysis software program, version 2.3, was used to process the data. a Reported parts from other hawthorn species (Crataegus spp.) based on the previous literature sources; fruit (F), leaf (L), flower (Fl), seed (S), and root (R). ⁎ Identified phenolic compounds in different parts of C. pentagyna in previous works
Below is an explanation of the HPLC-ESI-MS/MS identification of phenolics in fruit, leaf, and root, as well as the comparison of literature.
Flavonoids
Flavonoids containing two benzene rings and one oxygenated ring were the most abundant phenols found in hawthorn in this study. According to Table 2, the aerial parts and roots of C. pentagyna contain 63 phenolic compounds. In mass spectrometry, all O-glycosides, including glucose or galactose (162 Daltons), rhamnose (146 Daltons), pentose—xylose or arabinose (132 Daltons), and disaccharide structures—rutinose or neohesperidose, lost their sugar moiety (308 Daltons). Due to cross-ring cleavages of sugar residues, C-glycosides exhibited fragments at m/z [M-H-18]−−, [M-H-60]−, [M-H-90]−, [M-H-120]−, [M-H-180]−, and [M-H-210]− for pentosyl residues and [M-H-74]− and [M-H-104]− for deoxyhexosyl residues [41].
Identification of luteolin derivatives
Compounds 50, 40, and 41 were identified as luteolin 7-O-glucoside, luteolin-7-O-glucuronide, and luteolin, respectively. Previous studies have identified compound 41 in C. microphylla leaves [42]; compound 50 from fruits, leaves, and flowers of C. microphylla [12, 17, 18, 42]; and compound 40 from leaves of C. macrocarpa [43].
Identification of orientin derivatives
Compounds 36 and 37 were characterized as orientin or isoorientin; and compounds 22 and and 23 as orientin or isoorientin glycoside derivatives (orientin-2″-O-rhamnoside and isoorientin-2″-O-rhamnoside. Previous studies have identified compound orientin from leaves and flowers of C. monogyna and C. pentagyna; compound isoorientin from flowers of C. monogyna and C. pentagyna; compound orientin-2″-O-rhamnoside from leaves and flowers of C. pentagyna; and compound isoorientin-2″-O-rhamnoside from flowers of C. pentagyna [12, 17].
Identification of quercetin derivatives
Compounds 48 was identified as quercetin aglycone. Compounds 31, 33 and 32 were identified as quercetin-O-glycoside derivatives (quercetin 3-O-glucoside (isoquercitrin), quercetin 4′-O-glucoside (spiraeoside) or quercetin 3-O-galactoside (hyperoside); and compounds 18, 21, 20 and 19 as quercetin-3-O-rutinoside, quercetin-3-O-rhamnoside, quercetin 7,4′-dimethyl ether-3-O-rutinoside and quercetin-3-O-(6″ galloyl) glucoside, respectively. Previous studies have identified compound 48 from fruits and leaves of C. pentagyna, C. monogyna, and C. oxyacantha, fruits of C. pinnatifida, C. germanica, C. cuneata, and C. brettschneideri, flowers and leaves of C. microphylla, leaves of C. scabrifolia and C. pinnatifid, and flowers of C. azarolus [18, 19, 42, 44, 45]; isoquercitrin from leaves of C. pentagyna, flowers of C. monogyna, C. macrocarpa, C. rhipidophylla, C. laevigata, and C. azarolus, and fruits of C. scabrifolia [12, 15, 18, 43, 46]; spiraeoside from fruits and flowers of C. monogyna and C. azarolus [1, 47–49]; hyperoside from fruits, leaves, and flowers of C. pentagyna, seeds and fruits of C. microphylla, C. macrocarpa, and C. oxyacantha, and leaves of C. pinnatifida [12, 15, 18, 42, 43, 50–54]; compound 18 from leaves of C. scabrifolia [55]; compound 21 from leaves and flowers of C. pentagyna [18]; and compound 20 from fruits of C. monogyna [56]; and compound 19 from leaves of C. monogyna [56].
Identification of kaempferol derivatives
Compounds 53, 50 and 38 were assigned as kaempferol aglycone, kaempferol-3-O-rutinoside and 8-methoxykaempferol (sexangularetin), respectively. Previous studies have identified compound 53 from fruits and flowers of C. pentagyna [18, 44]; compound 50 from fruits and leaves of C. pentagyna [18]; and compound 38 from flowers of C. maximowiczii [51].
Identification of apigenin derivatives
Compounds 56 and 42 were identified as apigenin aglycone and apigenin 8-C-glucoside (vitexin). Also, compounds 24, 25, 28 were detected as vitexin-O-rhamnoside derivatives (vitexin 2″-O-rhamnoside, isovitexin 2″-O-rhamnoside or vitexin-4′- O-rhamnoside); and compounds 29 and 26 as vitexin-O- glucoside (vitexin 4′-O-glucoside or vitexin-2″-O-glucoside), respectively. Previous studies have identified compound 56 from leaves of C. microphylla [42]; compound 42 from fruits, leaves, and flowers of C. pentagyna, C. monogyna, C. microphylla, and C. macrocarpa, and leaves C. pinnatifida [12, 15, 17, 42, 43, 51, 57]; vitexin-2″-O-rhamnoside from leaves and flowers of C. pentagyna, fruits, leaves, and flowers of C. monogyna and C. pinnatifida, leaves of C. microphylla, C. aronia, C. pseudoheterophylla, C. scabrifolia, and C. cuneata, flowers of C. macrocarpa, C. rhipidophylla, and C. laevigata, and fruits and leaves of C. pinnatifida [48, 58–65]; vitexin-4′-O-rhamnoside from leaves of C. oxyacantha and leaves and flowers of C. microphylla [42, 50, 64, 65]; vitexin-4′-O-glucoside from leaves of C. scabrifolia and C. cuneata, and fruits and leaves of C. pinnatifida [51, 64–66]; and vitexin-2″-O-glucoside from leaves of C. pinnatifida [58, 60].
Identification of eriodictyol derivatives
Compounds 54, 55 and 39 were assigned as eriodictyol aglycon, hesperetin and eriodictyol-7-glucuronide, respectively. Previous studies have identified compounds 54 and 55 from flowers of C. microphylla [42] and compound 39 from leaves and flowers of C. macrocarpa [43].
Identification of naringenin derivatives
Compounds 57, 27, 43 and 44 were suggested to be naringenin aglycon, naringenin 7-O-neohesperidoside (naringin), naringenin-6-C-glucoside and naringenin 7-O-glucoside, respectively. Previous studies have identified compound 57 from leaves of C. microphylla [42, 66]; compound 27 from leaves of C. oxyacantha [67]; and compounds 43 and 44 from leaves of C. monogyna and C. laevigata [68].
Identification of catechins, proanthocyanidins, and their derivatives
There are two major categories of procyanidins (A-type and B-type). (Epi)catechin units linked through C4 to C8 or C4 to C6 are called B-type procyanidins, while those with an additional bond (C2-O-C7) are called A-type. In the negative ion mode, there are three characteristic fragmentation routes, including retro Diels–Alder (RDA) [M-152-H]−, heterocyclic ring fission (HRF) [M-125-H]−, and quinine methide (QM) reaction (cleavage of the interflavan bond), with [M-289-H]− or [M-287-H]− as fragment ions of procyanidins [69]. Due to the degree of polymerization [70], procyanidins may exist as several isomers with the same molecular weight and mass spectrometry. In the present study, samples of C. pentagyna contained B-types and their derivatives (Table 2).
Compounds 49, 51 were detected as catechin or epicatechin monomers. In addition, compounds 35, 34, 59, 61, 62, 63, 45, 58 and 60 were identified as epicatechin gallate, epigallocatechin gallate, B-type trimer (procyanidin C2), B-type procyanidin tetramer, B-type pentamer, B-type procyanidin hexamer, procyanidin dimer hexosides (procyanidin B3 7-glucoside), procyanidin tetramer glycoside and procyanidin pentamer glycoside, respectively. Previous studies have identified catechin from fruits and leaves of C. pubescens [66, 71]; epicatechin from fruits, leaves, and flowers of C. pentagyna, callus of C. monogyna, fruits and leaves of C. oxyacantha, and fruits of C. pubescens [1, 18, 19, 72, 73]; compound 59 from fruits and leaves of C. oxyacantha [49, 65]; compound 61 from fruits of C. orientalis [74] and compounds 45, 58 and 60–63 from fruits of C. pinnatifida [75, 76].
Identification of myricetin derivatives
Compound 16, 30, 46 and 47 were identified as myricetin-3-O-(6″-galloyl) galactoside, myricetin-3-O-galactose, myricetin and 5-O-methylmyricetin, respectively. Previous studies have identified these compounds from fruits of C. monogyna [56].
Phenolic acids
Free and conjugated phenolic acids are classified into two groups: hydroxybenzoic acids and hydroxycinnamic acids. Increasing interest in the profile of phenolic acids is due to their possible health benefits and antioxidant activity. In mass spectrometry, phenolic acids and their glycoside derivatives are distinguished by the loss of an ion at m/z -162 Da (glucose or galactose), followed by the loss of ions at m/z -18 Da (hydroxyl), -15 Da (methyl), and − 44 Da (carbon dioxide). In this study, a total of 16 phenolic acids were identified.
Identification of hydroxybenzoic acids and their glycosidic derivatives
Compound 10 and 12 were identified as protocatechuic acid and salicylic acid. Also, compounds 13 and 14 can be tentatively assigned as hydroxybenzoic acid derivatives. Previous studies have identified compound 12 from fruits of C. monogyna; compound 10 from fruits of C. germanica and fruits, leaves and flowers of C. pentagyna [19, 77]; 3-hydroxybenzoic acid and 4-hydroxybenzoic acid from fruits of C. germanica [45, 56, 68]; and hydroxybenzoic acid, hydroxybenzoic acid hexoside from fruits, leaves and flowers of C. pentagyna [19].
Identification of hydroxycinnamic acids and their glycosidic derivatives
Compounds 17, 15, 11, 9, 2 and 1 were determined as coumaric acid, ferulic acid, caffeic acid, sinapic acid and sinapic acid-O-hexoside and chlorogenic acid glycoside derivative, respectively. Also, compounds 6, 3 and 4 can be tentatively identified as caffeoylquinic acid derivatives (chlorogenic acid, cryptochlorogenic acid or cryptochlorogenic acid); and compounds 7, 8, and 5 as coumaroylquinic acid derivatives (3-O-p- coumaroylquinic acid, 4-O-p- coumaroylquinic acid or 5-O-p- coumaroylquinic acid). Previous studies have identified compound 17 from fruits, leaves, and flowers of C. pinnatifida [18, 78]; compound 15 from fruits, leaves, and flowers of C. pinnatifida and C. monogyna, fruits and leaves of C. scabrifolia and C. pinnatifida, leaves of C. cuneata, fruits of C. brettschneideri, leaves and flowers of C. laevigata, and flowers of C. azarolus [12, 45–47, 57, 77]; compound 11 from fruits, leaves, and flowers of C. pinnatifida and C. pentagyna, fruits and leaves of C. oxyacantha, and fruits of C. germanica [18, 19, 45, 71]; compound 9 from fruits and flowers of C. monogyna and fruits of C. germanica [45]; compound 2 from fruits and flowers of C. monogyna and fruits of C. germanica [45]; chlorogenic acid from fruits, leaves, and flowers of C. pinnatifida and C. pentagyna, callus of C. monogyna, leaves of C. pinnatifida, and fruits of C. germanica and C. pubescens [15, 18, 19, 45, 51]; cryptochlorogenic acid and neochlorogenic acid from fruits, leaves, and flowers of C. pinnatifida and C. pentagyna [12, 18, 19, 33, 79]; 3-O-p-coumaroylquinic acid and 5-O-p-coumaroylquinic acid from fruits, leaves, and flowers of C. monogyna and C. pentagyna [19, 68]; 4-O-p-coumaroylquinic acid from fruits of C. pentagyna [18, 68] and 4-O-(3′-O-glucopyranosyl)-caffeoyl quinic acid from leaves and fruits of C. monogyna and C. laevigata [68].
Comparison between phenolic compounds in different parts of C. pentagyna based on ion intensity.
The phenolics and their bioactivity are influenced by agronomic, climatic, genomic, processing, and harvesting factors [18]. The relative intensity (En) values indicated that apigenin (8.4E5), one of the caffeoylquinic acid derivatives (8.2E5), 4-O-(3′-O-glucopyranosyl)-caffeoyl quinic acid (7.2E5), and naringenin-6-C-glucoside (3.9E5) are the major phenolic compounds in fruit; salicylic acid (3.0E6), naringenin-6-C-glucoside (5.3E5), naringin (3.7E5), and one of the caffeoylquinic acid derivatives (4.4E5) in leaf; and naringenin-7-O-neohesperidoside (6.8E5), isovitexin-2″-O-rhamnoside (5.7E5), 4-O-(3′-O-glucopyranosyl)-caffeoyl quinic acid (4.8E5), and B-type procyanidin hexamers (3.9E5) in root. Previous studies on C. pentagyna indicated that hyperoside, isoquercetin and chlorogenic acid were other main phenolics in the fruit extracts; isoorientin, isoquercitrin, 8-methoxykaempferol-3-O-glucoside, (-)-epicatechin, neochlorogenic and chlorogenic acid, and procyanidin B2 in leaf and flower; and gallic acid and chlorogenic acid in pulp and peel [15, 17, 18]. LC–MS/MS indicated that fruit, leaf, and root of C. pentagyna have a similar profile. Meanwhile, orientin, isoorientin, isoorientin-2″-O-rhamnoside, quercetin-4′-O-glucoside, quercetin-3-O-galactoside, kaempferol-3-O-rutinoside, vitexin-4′-O-glucoside, vitexin-2″-O-glucoside, one of the hydroxybenzoic acid derivatives, protocatechuic acid, ferulic acid, caffeic acid, and one of the caffeoylquinic acid derivatives have been observed only in fruit; vitexin-4′-rhamnoside, eriodictyol-7-glucuronide, procyanidin B3 7-glucoside, and coumaroylquinic acid derivative only in leaf; and one of the caffeoylquinic acid derivatives only in root.
GC-MS analysis of the petroleum ether extracts
Currently, there are no reports on GC-MS analysis of C. pentagyna extracts. In this study, for the first time, GC-MS was used to investigate the chemical composition of various petroleum ether extracts (Fig. 2). As shown in Table 3, the analysis of extracts identified 28, 42, and 25 chemical compounds belonging to different chemical families, representing 91.13%, 95.71%, and 89.67%, respectively, of the relative area in the fruit, leaf, and root extracts. The fruit extract consists of hydrocarbons (63.80%), fatty acids and their derivatives (11.77%), steroids (9.54%), and terpenes (2.051%), with nonacosane (50.74%), tetratetracontane (7.22%) and γ-sitosterol (6.11%). Hydrocarbons (49.20%), fatty acids and their derivatives (13.85%), steroids (13.04%) and terpenes (10.95%) and predominated in the leaf extract, with nonacosane (26.29%), squalene (10.29%), γ-sitosterol (7.30%) and oleyl palmitoleate (6.53%). In addition, the root extract contained hydrocarbons (53.96%), terpenes (13.06%), steroids (6.83%) and fatty acids and their derivatives (6.70%) with squalene (10.15%), bis(2-ethylhexyl) adipate (9.65%) and tributyl acetylcitrate (6.42%). Several studies have documented the antimicrobial and antioxidant effects of nonpolar extracts [80–82].
Fig. 2.
Gas ion chromatogram of C. pentagyna extracts: (A) fruit; (B) leaf; (C) root
Table 3.
Chemical composition of petroleum ether extracts from C. pentagyna fruit, leaf, and root analyzed by GC-MS.
| Compounds | Molecular Formula | Classification | RT (min) | Percentage % | ||||
|---|---|---|---|---|---|---|---|---|
| Fruit | Leaf | Root | Fruit | Leaf | Root | |||
| 2-Methylheptane | C8H18 | Alkane hydrocarbon | - | 3.628 | - | - | 0.779% | - |
| 3-Methylheptane | C8H18 | Alkane hydrocarbon | - | 3.758 | - | - |
0.824 % |
- |
| Octane | C8H18 | Alkane hydrocarbon | - | 4.283 | - | - |
0.662 % |
- |
| 2,6-Dimethylheptane | C9H20 | Alkane hydrocarbon | - | 4.915 | - | - |
0.128 % |
- |
| 1,2-Dimethylcyclohexane | C8H16 | Cyclo alkane | - | 4.953 | - | - |
0.102 % |
- |
| 2,5-Dimethylheptane | C9H20 | Alkane hydrocarbon | - | 5.074 | - | - |
0.280 % |
- |
| 1,1,3-Trimethylcyclohexane | C9H18 | Cyclo alkane | - | 5.141 | - | - |
0.160 % |
- |
| 2,3,5-Trimethylhexane | C9H20 | Alkane hydrocarbon | - | 5.600 | - | - |
0.091 % |
- |
| 2-Methyloctane | C9H20 | Alkane hydrocarbon | - | 5.819 | - | - |
0.218 % |
- |
| 2,5-Dimethylheptane | C9H20 | Alkane hydrocarbon | - | 6.015 | - | - |
0.179 % |
- |
| Nonane | C9H20 | Alkane hydrocarbon | - | 6.790 | - | - |
0.446 % |
- |
| 2,6-Dimethyloctane | C10H22 | Alkane hydrocarbon | - | 7.776 | - | - |
0.353 % |
- |
| 3-Ethyl-2-methylheptane | C10H22 | Alkane hydrocarbon | - | 7.994 | - | - |
0.309 % |
- |
| 3,7,11-Trimethyl-1-dodecanol | C15H32O | Alkane hydrocarbon | - | 8.423 | - | - |
0.292 % |
- |
| 4-Methylnonane | C10H22 | Alkane hydrocarbon | - | 8.619 | - | - |
0.380 % |
- |
| 2-Methylnonane | C10H22 | Alkane hydrocarbon | - | 8.709 | - | - |
0.240 % |
- |
| Decane | C10H22 | Alkane hydrocarbon | 9.803 | 9.816 | 9.809 | 0.290% |
1.574 % |
1.665% |
| 4-Methyldecane | C11H24 | Alkane hydrocarbon | - | 10.518 | 10.509 | - |
0.675 % |
0.751% |
| Undecane | C11H24 | Alkane hydrocarbon | 12.890 | 12.925 | 12.911 | 1.189% |
2.341 % |
3.310% |
| 2,6-Dimethyldecalin | C12H22 | Polycyclic hydrocarbon | 16.006 | - | 16.020 | 0.873% | - | 2.313% |
| Decahydro-1,6-dimethylnaphthalene | C12H22 | Polycyclic hydrocarbon | 16.285 | - | - | 0.609% | - | - |
| 2,6-Dimethyldecalin | C12H22 | Polycyclic hydrocarbon | 16.608 | 16.599 | 16.622 | 1.842% |
2.170 % |
5.222% |
| 1,5-Dimethyldecahydronaphthalene | C12H22 | Polycyclic hydrocarbon | 16.774 | 16.779 | 16.787 | 3.163% |
3.011 % |
9.014% |
| Tridecane | C13H28 | Alkane hydrocarbon | 18.701 | 18.699 | 18.707 | 0.845% |
0.756 % |
3.218% |
| Geranyl isovalerate | C15H26O2 | Fatty ester | 20.244 | - | - | 0.286% | - | - |
| 2,6,10-Trimethyltetradecane | C17H36 | Isoprenoid hydrocarbon | 20.395 | - | - | 0.4312% | - | - |
| Farnesan | C15H32 | Sesquiterpene | 20.741 | - | 20.732 | 0.479% | - | 1.823% |
| Tetradecane | C14H30 | Alkane hydrocarbon | 21.351 | 21.349 | 21.357 | 1.565% |
1.196 % |
6.375% |
| 2-Methyl-1-hexadecanol | C17H36O | Fatty alcohol | - | - | 22.591 | - | - | 0.888% |
| Pentadecane | C15H32 | Alkane hydrocarbon | 23.873 | 23.863 | 23.871 | 0.4280% |
0.346 % |
1.482% |
| Hexadecane | C16H34 | Alkane hydrocarbon | 26.229 | - | 26.235 |
0.298 % |
- | 2.207% |
| 8-Amino-6-methoxy-4-methyl-5-[n-nonoxy]-quinoline | C10H10N2O | Heterocyclic aromatic derivatives | - | 27.122 | - | - |
0.277 % |
- |
| Benzene, 1,3,5-tris(1-methylpropyl)- | C18H30 | Benzene derivatives | 27.569 | 27.574 | 27.567 | 0.493% |
0.462 % |
2.316% |
| 11-Eicosenoic acid | C20H38O2 | Fatty Acid | - | - | 28.576 | - | - | 1.256% |
| 3α-Methyl-24-noroleana-4(23),12-diene | C30H48 | Triterpene | - | - | 28.997 | - | - | 1.089% |
| Naphthalene, 1,2,3,4-tetrahydro-1-isopropyl-1,2,4,4,7-pentamethyl- | C18H28 | Polycyclic aromatic hydrocarbon | - | - | 29.908 | - | - | 0.899% |
| Octadecane | C18H38 | Alkane hydrocarbon | - | - | 30.638 | - | - | 2.385% |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | Diterpene | - | 31.443 | - | - |
0.656 % |
- |
| 6,10,14-Trimethylpentadecan-2-one | C18H36O | Sesquiterpenoid | - | 31.586 | - | - |
0.415 % |
- |
| 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | C17H24O3 | Lactone | 33.185 | - | - | 0.814% | - | - |
| Methyl palmitate | C17H34O2 | Fatty acid ester | - | 33.205 | - | - |
1.581 % |
- |
| Eicosane | C20H42 | Alkane hydrocarbon | - | - | 34.636 | - | - | 2.643% |
| Methyl linolelaidate | C19H34O2 | Fatty acid ester | 36.442 | - | - |
0.72 % |
- | - |
| Methyl linolenate | C19H32O2 | Fatty acid ester | - | 36.570 | - | - |
2.904 % |
- |
| Oleic Acid | C18H34O2 | Fatty acid | - | 37.623 | - | - |
2.834 % |
- |
| Tributyl acetylcitrate | C20H34O8 | Citrates (tricarboxylic acid) | 39.470 | - | 39.506 | 0.924% | - | 6.424% |
| Oleic acid, 3-(octadecyloxy)propyl ester | C39H76O3 | Fatty acid ester | 39.997 | - | - | 0.628% | - | - |
| 17-Pentatriacontene | C35H70 | Unsaturated hydrocarbon | - | 40.025 | 47.674 | - |
1.412 % |
8.667% |
| 4,8,12,16-Tetramethylheptadecan-4-olide | C21H40O2 | Lactone | - | 40.981 | - | - |
0.961 % |
- |
| Dioctyl adipate | C22H42O4 | Adipate (dicarboxylic acids) | 41.668 | 41.711 | - | 1.822% |
1.411 % |
- |
| Bis(2-ethylhexyl) adipate | C22H42O4 | Adipate (dicarboxylic acids) | - | - | 41.704 | - | - | 9.659% |
| Diisooctyl phthalate | C24H38O4 | Phthalate ester | - | - | 44.053 | - | - | 4.805% |
| Hexacosane | C26H54 | Alkane hydrocarbon | - | 44.812 | - | - |
2.700 % |
- |
| Docosyl heptanoate | C29H58O2 | Fatty esters | 45.628 | - | - | 2.501% | - | - |
| Heptacosane | C27H56 | Alkane hydrocarbon | 46.253 | 46.310 | - | 3.065% |
4.490 % |
- |
| Erucamide | C22H43NO | Fatty amide | 47.442 | - | 47.470 | 3.591% | - | 4.583% |
| Oleic acid, 3-(octadecyloxy)propyl ester | C39H76O3 | Fatty acid ester | 47.653 | - | - | 4.046% | - | - |
| Lycopersene | C40H66 | Tetraterpene | 48.089 | - | - | 1.561% | - | - |
| Squalene | C30H50 | Triterpene | - | 48.403 | 48.118 | - |
10.298 % |
10.156% |
| Nonacosane | C29H60 | Alkane hydrocarbon | 49.234 | 49.239 | - | 50.747% |
26.295 % |
- |
| Oleyl palmitoleate | C34H64O2 | Fatty esters | - | 51.324 | - | - |
6.537 % |
- |
| Stigmast-5-en-3-ol, oleate | C29H50O | Steroid | - | - | 52.303 | - | - | 6.83% |
| Tetratetracontane | C44H90 | Alkane hydrocarbon | 52.313 | - | - | 7.223% | - | - |
| Triacontane-1,30-diol | C30H62O2 | Alcohol | - | 55.592 | - | - |
6.186 % |
- |
| γ-Sitosterol | C29H50O | Steroid | 57.431 | 57.602 | - | 6.117% |
7.308 % |
- |
| Pollinasterol | C29H48O | Steroid | 61.557 | - | - | 3.430% | - | - |
| β-Sitosterol acetate | C31H52O2 | Steroid | - | 61.720 | - | - |
5.740 % |
- |
| Major Grouped Compounds | Fruits | Leaves | Roots | |||||
| Terpenes | 2.051% | 10.954% | 13.06% | |||||
| Fatty acids, Fatty acid esters, Fatty amids | 11.77% | 13.85% | 6.7% | |||||
| Steroids | 9.54% | 13.04% | 6.83% | |||||
| Hydrocarbons | 63.80% | 49.20% | 53.96% | |||||
| Miscellaneous | 3.97% | 5.7% | 6.99% | |||||
| Total Identified% | 91.13% | 95.71% | 89.67% | |||||
Antimicrobial activities
Table 4 provides a summary of the antibacterial activities of petroleum ether and hydro-methanolic extracts of C. pentagyna fruit, leaf, and root against two pathogenic bacteria (Staphylococcus aureus and Escherichia coli). All examined extracts inhibited bacterial growth with MIC and MBC values ranging from 0.15 to 5.12 mg/mL and 0.15 to 10.12 mg/mL, respectively. The activity of the petroleum ether extract of leaf was higher than that of the root and fruit extracts (MICs 1.25–5 mg/mL). The presence of terpenes and flavonoids explains the high antibacterial activity in petroleum ether and hydro-methanol extracts, respectively [83]. . few studies have been conducted on the crude extracts of C. pentagyna to date. Salmanian et al. [16] examined the antimicrobial activity of seed and pulp extracts against four clinical pathogens. These extracts inhibited bacterial strains, with MICs and MBCs ranging between 2.5 and 40 mg/mL and 5 and > 40 mg/mL, respectively. In the study of Safapour et al. [84], C. pentagyna fruit extract demonstrated potent antibacterial activity, especially against Gram-negative bacteria. In a separate study, fruit acetonic extract exhibited the highest antibacterial activity against Bacillus subtilis (MBC = 2.5 mg/mL) [85]. These antibacterial effects may be attributable to the flavonoid content of the extracts, consistent with our findings. Terpenoids are known for their antibacterial effect and aromatic qualities. Non-polar constituents are more soluble in non-polar solvents and polar constituents are more soluble in polar solvents, so there was a different antibacterial effect between solvents. Polar and non-polar solvents have high capacity to dissolve active antimicrobial compounds than the medium polar solvent. Terpenoids are fat soluble, so these phytochemicals can be attracted to the petroleum ether solvent as non-polar solvent [86].
Table 4.
MICs and MBCs (mean ± SD) (mg/mL) of C. pentagyna petroleum ether (EP) and hydro-methanol (HM) extracts against pathogenic bacteria (mg/mL)
| Plant part | S. aureus | E.coli | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |||||
| EP | HM | EP | HM | EP | HM | EP | HM | |
| Fruit | 2.52 ± 2.11 a | 0.15 ± 1.32 b | 5.25 ± 2.14 c | 0.62 ± 3.14 d | 5.12 ± 1.42 c | 0.62 ± 2.94 d | 10.12 ± 2.76 f | 1.25 ± 3.12 g |
| Leaf | 0.31 ± 1.09 a | 0.31 ± 1.45 a | 0.62 ± 1.32 b | 2.52 ± 3.52 c |
0.31 ± 1.15 a |
0.15 ± 2.31 d | 1.25 ± 2.77 e | 0.31 ± 2.34 a |
| Root | 1.25 ± 1.21 a | 1.25 ± 3.16 a | 5.78 ± 1.38 b | 2.52 ± 2.41 c | 2.58 ± 1.56 c | 2.52 ± 1.11 c | 5.56 ± 2.86 b | 5.25 ± 3.32 d |
| Gentamicin | 0.00229 ± 1.51 | 0.03196 ± 1.85 | 0.01651 ± 1.15 | 0.12668 ± 2.41 | ||||
Means with different superscript lowercase letters within the same row differ significantly (p < 0.05)
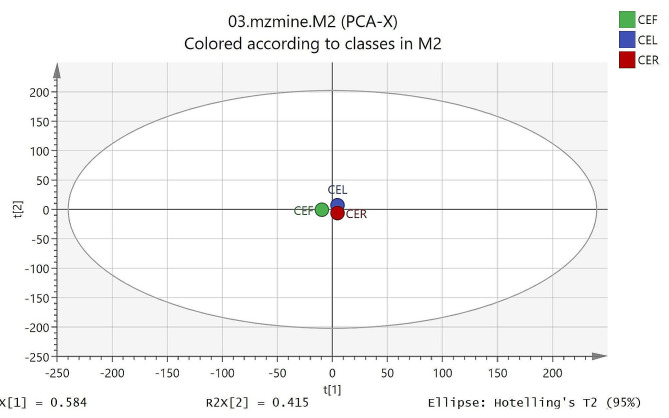
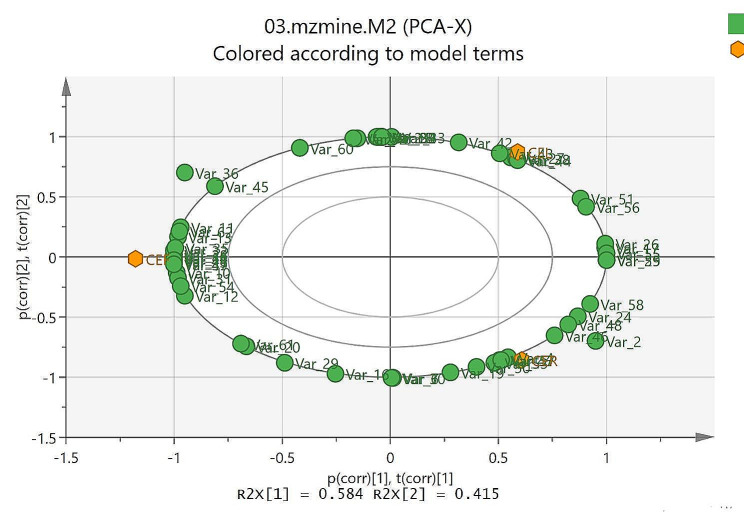
Principle component analysis (PCA) and heatmap analysis
The PCA model as the most common multivariate data analysis is an unsupervised method to reduce the dimensionality of huge multivariate data sets. As shown in Fig. 3 samples (CEF: fruit, CEL: leaf and CER: root) revealed a clear difference in the PCA model, indicating differences in metabolic fingerprints of samples. The resulting values of R2X(cum) and Q2(cum) of 0.96 and 0.98, respectively, indicating a good fitness, discrimination, and predictability of the PCA model. The loading plot (Fig. 4) and bipolot (Fig. 5) are useful for identifying the variable responsible for similarities and differences between samples. The assignment of these variables led to the identification of compounds that are responsible for separation in the score plot. The variables of salicylic acid, luteolin, catechin, eriodictyol, quercetin, hesperetin, 5-O-methylmyricetin, apigenin-8-C-glucoside, naringenin-6-C-glucoside, naringenin-7-O-glucoside, chlorogenic acid glycoside, quercetin-3-O-(6″ galloyl) glucoside, myricetin-3-O-(6″ galloyl) galactoside, quercetin-7,4′-dimethyl ether-3-O-rutinoside, procyanidin C2, procyanidin tetramers, B-type procyanidin pentamers and B-type procyanidin hexamers were identified for all the samples; caffeoylquinic acid derivative, quercetin-3-O-rutinoside, quercetin-3-O-rhamnoside, orientin glycoside, epigallocatechin gallate, epicatechin gallate, luteolin 7-O-glucoside, luteolin-7-O-glucuronide, myricetin and kaempferol for fruit and leaf; sinapic acid-O-hexoside, myricetin-3-O-galactose and quercetin-O-glycoside derivative for fruir and root; and coumaroylquinic acid derivative, sinapic acid, coumaric acid, vitexin-O-rhamnoside derivative, naringenin-7-O-neohesperidoside, naringenin and procyanidin pentamer-hexoside for leaf and root. Also, caffeoylquinic acid derivative, coumaroylquinic acid derivative, protocatechuic acid, caffeic acid, hydroxybenzoic acid derivative, ferulic acid, orientin glycoside, vitexin-O-glucoside derivative, quercetin-O-glycoside derivative, orientin, 8-methoxykaempferol and kaempferol-3-O-rutinoside were found only in fruit; coumaroylquinic acid derivative, eriodictyol-7-glucuronide and procyanidin B3 7-glucoside in leaf; and caffeoylquinic acid derivative and procyanidin tetramer-hexoside in root. The heatmap analysis was presented in Fig. S2 to better show differences between metabolites. (Supplementary materials). The metabolites of Table 2 were displayed in the heat map as variables 1–62. The major metabolites based on the relative abundance in heat map included some phenolic compounds including variables 1,3,5, 10–15, 23, 27,31,32, 35–37, 41,45, 49,54, 61 and 62 in fruit sample (CEF); variables 8,9, 17,18, 21,22, 26,27, 32, 33, 38–40, 42–44, 51,52 and 56 in leaf (CEL); and variables 4, 6, 19, 24, 30, 46–48, 50, 53, 57 and 58 in root (CER). As shown in the heat map, both CEF and CEL sample contains several metabolites with significantly higher abundance, whereas, less metabolites with high abundance were found in the sample of CER.
Fig. 3.
Principal component analysis (PCA) score plot of samples including CEF, CEL and CER
Fig. 4.
Principal component analysis (PCA) loading plot of samples including CEF, CEL and CER
Fig. 5.
Principal component analysis (PCA) biplot of samples including CEF, CEL and CER
Conclusions
In this study, the compound profiles in petroleum ether and hydro-methanolic extracts of C. pentagyna fruit, leaf, and root tentatively were identified and characterized. In addition, the chemical profile of hawthorn root was discovered for the first time. The fruit hydro-methanolic extract exhibited the highest levels of antioxidant and antibacterial activity, followed by the leaf and root extracts. Antibacterial and antioxidant activities of C. pentagyna extracts were attributed to the major phenolics and terpenes detected by HPLC-MS/MS and GC-MS. Using LC-ESI-MS, it was possible to characterize 62 compounds including mainly flavone apigenin, phenolic acid salicylic acid and flavanone naringin in fruit, leaf and root, respectively. Also, bioactive compounds such as alkane nonacosane in fruit and leaf extracts and triterpene squalene in root extract were identified using GC-MS as major components. The results of the PCA and heatmap analysis distinguished metabolite profile differences for fruit, leaf and root samples into well-defined groups. To confirm their potential as phytotherapeutic agents, it is suggested that further research should be conducted, including the purification of the main compounds and the investigation of their biological activities and mechanisms of action.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the support of this work by the University of Gonbad Kavous Research Council.
Abbreviations
- LC–ESI-MS/MS
liquid chromatography coupled with electrospray ionization tandem mass spectrometry
- GC-MS
gas chromatography coupled with mass spectrometry
- EP
ether petroleum
- HM
hydro-methanol
- TP
total phenol
- TF
total flavonoid
- TPA
total phenolic acid
- TAC
total anthocyanin
- RT
retention time
- MHB
Muller Hinton broth
- BHT
butylate hydroxytoluene
- RDA
retro Diels–Alder
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- XIC
extracted ion chromatograms
- TIC
total ion chromatogram
- PCA
principal component analysis
- LSD
least significant difference
Author contributions
A. T: Conceptualization (equal); Funding acquisition (equal); Writing—original draft (lead); Software (equal); S.E: Funding acquisition (equal); Software (equal); Methodology (equal); R.M: Funding acquisition (equal); Investigation (equal);M. M: Investigation (equal); Methodology (equal).;All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All experimental protocols were approved by the University of Gonbad Kavous, Iran.
All methods were performed in accordance with the relevant guidelines and regulations.
The plant samples used in this study were collected from Farsian, Galikesh, Golestan province, Iran. Identification of this species was confirmed by Dr. Ali Satarian from the Biology department, Science College, Gonbade Kavous university, Gonbad Kavous, Iran. The voucher specimen (803892) has been deposited at Dr. Ali Satarian’s laboratory for reference.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bahorun T, Aumjaud E, Ramphul H, Rycha M, Luximon-Ramma A, Trotin F, et al. Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts. Mol Nutr Food Res. 2003;47(3):191–8. doi: 10.1002/food.200390045. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Shivaprasad HN, Mulukuri NS, Chandrasekar SB, Baheti AM, Pawar AT. Role of natural products in infectious diseases. Viral, parasitic, bacterial, and fungal in fections. Academic; 2023. pp. 757–70.
- 4.Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. J Healthc. 2023;11(13):1946. doi: 10.3390/healthcare11131946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–7. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- 6.Pathak D, Mazumder A. Potential of flavonoids as Promising Phytotherapeutic agents to combat Multidrug-resistant infections. Curr Pharm Biotechnol. 2024 doi: 10.2174/0113892010271172231108190233. [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Rodríguez P, Baquero LP, Larrota HR. Flavonoids: potential therapeutic agents by their antioxidant capacity. Bioact Compd. Woodhead Publishing; 2019. pp. 265–88.
- 8.Venskutonis PR. Phytochemical composition and bioactivities of hawthorn (Crataegus spp.): review of recent research advances. J food Bioact. 2018;4:69–87. doi: 10.31665/JFB.2018.4163. [DOI] [Google Scholar]
- 9.Nazhand A, Lucarini M, Durazzo A, Zaccardelli M, Cristarella S, Souto SB et al. Hawthorn (Crataegus spp.): An Updated Overview on Its Beneficial Properties. Forests; 2020, 11(5): 564.
- 10.Tassell MC, Kingston R, Gilroy D, Lehane M, Furey A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn Rev. 2010;4(7):32–41. doi: 10.4103/0973-7847.65324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holubarsch CJ, Colucci WS, Eha J. Benefit-risk assessment of Crataegus extract WS 1442: an evidence-based review. Am J Cardiovasc Drugs. 2018;18:25–36. doi: 10.1007/s40256-017-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B, Liu P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J Sci Food Agric. 2012;92(8):1578–90. doi: 10.1002/jsfa.5671. [DOI] [PubMed] [Google Scholar]
- 13.Cloud A, Vilcins D, McEwen B. The effect of hawthorn (Crataegus spp.) on blood pressure: a systematic review. Adv Integr Med. 2019;7(3):167–75. doi: 10.1016/j.aimed.2019.09.002. [DOI] [Google Scholar]
- 14.Kumar D, Arya V, Bhat ZA, Khan NA, Prasad DN. The genus Crataegus: Chemical and pharmacological perspectives. Rev Bras Farmacogn. 2012;22(5):1187–200. doi: 10.1590/S0102-695X2012005000094. [DOI] [Google Scholar]
- 15.Alirezalu A, Ahmadi N, Salehi P, Sonboli A, Alirezalu K, Khaneghah AM, et al. Physicochemical characterization, antioxidant activity, and phenolic compounds of Hawthorn (Crataegus spp.) fruits species for potential use in food applications. Foods. 2020;9(4):436. doi: 10.3390/foods9040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmanian S, Sadeghi Mahoonak A, Alami M, Ghorbani M. Phenolic content, antiradical, antioxidant, and antibacterial properties of hawthorn (Crataegus elbursensis) seed and pulp extract. J Agric Sci Technol. 2014;16(2):343–54. [Google Scholar]
- 17.Prinz S, Ringl A, Huefner A, Pemp E, Kopp B. 4′′′-Acetylvitexin-2 ″-O-rhamnoside, isoorientin, orientin, and 8-methoxykaempferol-3-O-glucoside as markers for the differentiation of Crataegus monogyna and Crataegus pentagyna from Crataegus laevigata (Rosaceae) Chem Biodivers. 2007;4(12):2920–31. doi: 10.1002/cbdv.200790241. [DOI] [PubMed] [Google Scholar]
- 18.Pavlovic J, Mitic S, Mitic M, Kocic G, Pavlovic A, Tosic S. Variation in the phenolic compounds profile and antioxidant activity in different parts of hawthorn (Crataegus pentagyna Willd.) During harvest periods. Pol J Food Nutr Sci. 2019;69(4):367–78. doi: 10.31883/pjfns/112019. [DOI] [Google Scholar]
- 19.Bujor A, Miron A, Luca SV, Skalicka-Wozniak K, Silion M, Trifan A, et al. Vasorelaxant effects of Crataegus pentagyna: links with arginase inhibition and phenolic profile. J Ethnopharmacol. 2020;252:112559. doi: 10.1016/j.jep.2020.112559. [DOI] [PubMed] [Google Scholar]
- 20.Cui M, Cheng L, Zhou Z, Zhu Z, Liu Y, Li C, et al. Traditional uses, phytochemistry, pharmacology, and safety concerns of hawthorn (Crataegus Genus): a comprehensive review. J Ethnopharmacol. 2023;319:117229. doi: 10.1016/j.jep.2023.117229. [DOI] [PubMed] [Google Scholar]
- 21.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–58. doi: 10.5344/ajev.1965.16.3.144. [DOI] [Google Scholar]
- 22.Celep E, Aydın A, Yesilada E. A comparative study on the in vitro antioxidant potentials of three edible fruits: Cornelian cherry, Japanese persimmon and cherry laurel. Food Chem Toxicol. 2012;50(9):3329–35. doi: 10.1016/j.fct.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Mihailovic V, Kreft S, Benkovic ET, Ivanovic N, Stankovic MS. Chemical profile, antioxidant activity and stability in stimulated gastrointestinal tract model system of three Verbascum species. Ind Crops Prod. 2016;89:141–51. doi: 10.1016/j.indcrop.2016.04.075. [DOI] [Google Scholar]
- 24.Connor AM, Luby JJ, Tong CB. Variability in antioxidant activity in blueberry and correlations among different antioxidant activity assays. J Am Soc Hortic Sci. 2002;127(2):238–44. doi: 10.21273/JASHS.127.2.238. [DOI] [Google Scholar]
- 25.Mahmoodi S, Taleghani A, Akbari R, Mokaber-Esfahani M. Rhamnus pallasii subsp. sintenisii fruit, leaf, bark and root: phytochemical profiles and biological activities. Arab J Chem. 2022;15(7):103924. doi: 10.1016/j.arabjc.2022.103924. [DOI] [Google Scholar]
- 26.Shour S, Iranshahy M, Pham N, Quinn RJ, Iranshahi M. Dereplication of cytotoxic compounds from different parts of Sophora pachycarpa using an integrated method of HPLC, LC-MS and 1H-NMR techniques. Nat Prod Res. 2017;31(11):1270–6. doi: 10.1080/14786419.2016.1239095. [DOI] [PubMed] [Google Scholar]
- 27.Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010;11(1):395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Hameed ESS, Bazaid SA, Al Zahrani O, El-Halmouch Y, El-Sayed MM, El-Wakil E. Chemical composition of volatile components, antimicrobial and anticancer activity of n-hexane extract and essential oil from Trachyspermum ammi L. seeds. Orient J Chem. 2014;30(4):1653–62. doi: 10.13005/ojc/300425. [DOI] [Google Scholar]
- 29.Gholamalipour Alamdari E, Taleghani A. New bioactive compounds characterized by liquid chromatography–mass spectrometry and gas chromatography–mass spectrometry in hydro-methanol and petroleum ether extracts of Prosopis farcta (Banks & Sol.) JF Macbr weed. J Mass Spectrom. 2022;57(9):e4884. doi: 10.1002/jms.4884. [DOI] [PubMed] [Google Scholar]
- 30.Celep E, Aydın A, Kırmızıbekmez H, Yesilada E. Appraisal of in vitro and in vivo antioxidant activity potential of cornelian cherry leaves. Food Chem Toxicol. 2013;62:448–55. doi: 10.1016/j.fct.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 31.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Microbiol Infect. 2003;9:1–7. doi: 10.1046/j.1469-0691.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 32.Ebrahimzadeh MA, Enayatifard R, Khalili M, Ghaffarloo M, Saeedi M, Charati JY. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran J Pharm Sci. 2014;13(3):1041–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Jurikova T, Sochor J, Rop O, Mlcek J, Balla S, Szekeres L, Adam V, Kizek R. Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida bunge) fruits. Molecules. 2012;17(12):14490–509. doi: 10.3390/molecules171214490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebrahimzadeh M, Bahramian F. Antioxidant activity of Crataegus Pentaegyna Subsp. elburensis fruits extracts used in Traditional Medicine in Iran. Pak J Biol Sci. 2009;12(5):413–9. doi: 10.3923/pjbs.2009.413.419. [DOI] [PubMed] [Google Scholar]
- 35.Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008;7(18):3188–92. [Google Scholar]
- 36.Rabiei K, Bekhradnia S, Nabavi S, Nabavi S, Ebrahimzadeh M. Antioxidant activity of polyphenol and ultrasonic extracts from fruits of Crataegus pentagyna subsp. elburensis. Nat Prod Res. 2012;26(24):2353–7. doi: 10.1080/14786419.2012.658799. [DOI] [PubMed] [Google Scholar]
- 37.Bujor A, Ochiuz L, Sha’at M, Stoleriu I, Iliuţa SM, Luca SV, Miron A. Chemical, antioxidant and in vitro permeation and penetration studies of extracts obtained from Viburnum opulus and Crataegus pentagyna. Farmacia. 2020;68(4):672–8. doi: 10.31925/farmacia.2020.4.12. [DOI] [Google Scholar]
- 38.Bedreag CFG, Trifan A, Bucur LA, Arcus M, Tebrencu C, Miron A, Costache II. Chemical and antioxidant studies on Crataegus pentagyna leaves and flowers. Rom Biotechnol Lett. 2014;19(6):9859. [Google Scholar]
- 39.Diao WR, Hu QP, Zhang H, Xu JG. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill) Food Control. 2014;35(1):109–16. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- 40.Bujor O, Stefanache C, Volf I, Danila D. Antioxidant capacity of Crataegus monogyna and Crataegus pentagyna leaves and fruits harvested from the Danube Delta. Planta Med. 2016;82(S 01):S1–381. [Google Scholar]
- 41.Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 2004;39(1):1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 42.Melikoglu G, Bitis L, MeriCli AH. Flavonoids of Crataegus microphylla. Nat Prod Res. 2004;18(3):211–3. doi: 10.1080/14786410310001620673. [DOI] [PubMed] [Google Scholar]
- 43.Ringl A, Prinz S, Huefner A, Kurzmann M, Kopp B. Chemosystematic Value of flavonoids from Crataegus x macrocarpa (Rosaceae) with special emphasis on (R)-and (S)-Eriodictyol-7-O-glucuronide and Luteolin-7-O-glucuronide. Chem Biodivers. 2007;4(2):154–62. doi: 10.1002/cbdv.200790020. [DOI] [PubMed] [Google Scholar]
- 44.Sadeghi-Kiakhani M, Tehrani-Bagha AR, Safapour S, Eshaghloo-Galugahi S, Etezad SM. Ultrasound-assisted extraction of natural dyes from Hawthorn fruits for dyeing polyamide fabric and study its fastness, antimicrobial, and antioxidant properties. Environ Dev Sustain. 2020;23:9163–80. doi: 10.1007/s10668-020-01017-0. [DOI] [Google Scholar]
- 45.Gruz J, Ayaz FA, Torun H, Strnad M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011;124(1):271–7. doi: 10.1016/j.foodchem.2010.06.030. [DOI] [Google Scholar]
- 46.Cui T, Li JZ, Kayahara H, Ma L, Wu LX, Nakamura K. Quantification of the polyphenols and triterpene acids in Chinese hawthorn fruit by high-performance liquid chromatography. J Agric Food Chem. 2006;54(13):4574–81. doi: 10.1021/jf060310m. [DOI] [PubMed] [Google Scholar]
- 47.Bahri-Sahloul R, Ammar S, Fredj R, Saguem S, Grec S, Trotin F, Skhiri FH. Polyphenol contents and antioxidant activities of extracts from flowers of two Crataegus azarolus L. varieties. Pak J Biol Sci. 2009;12(9):660–8. doi: 10.3923/pjbs.2009.660.668. [DOI] [PubMed] [Google Scholar]
- 48.Alirezalu A, Salehi P, Ahmadi N, Sonboli A, Aceto S, Hatami Maleki H, Ayyari M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int J Food Prop. 2018;21(1):452–70. doi: 10.1080/10942912.2018.1446146. [DOI] [Google Scholar]
- 49.Liu P. Composition of hawthorn (Crataegus spp.) fruits and leaves and emblic leafflower (Phyllanthus emblica) fruits. Ph.D. Thesis, Department of Biochemistry and Food Chemistry and Functional Foods Forum, University of Turku, Turku, Finland, 2012.
- 50.Verma S, Jain V, Verma D, Khamesra R. Crataegus oxyacantha-A cardioprotective herb. J Herb Med Toxicol. 2007;1(1):65–71. [Google Scholar]
- 51.Lu M, Hu JY, Song ZY, Jiao J, Mu FS, Ruan X, Gai QY, Qiao Q, Zu YG, Fu YJ. Optimization of ultrasound-assisted extraction (UAE) of phenolic compounds from Crataegus pinnatifida leaves and evaluation of antioxidant activities of extracts. RSC Adv. 2015;5(83):67532–40. doi: 10.1039/C5RA07445B. [DOI] [Google Scholar]
- 52.Mraihi F, Fadhil H, Trabelsi-Ayadi M, Cherif JK. Chemical characterization by HPLC-DAD-ESI/MS of flavonoids from hawthorn fruits and their inhibition of human tumor growth. J New Sci. 2015;3:840–6. [Google Scholar]
- 53.Orhan I, Ozçelik B, Kartal M, Ozdeveci B, Duman H. HPLC quantification of vitexine-2″-O-rhamnoside and hyperoside in three Crataegus species and their antimicrobial and antiviral activities. Chromatographia. 2007;66(Suppl 1):153–7. doi: 10.1365/s10337-007-0283-x. [DOI] [Google Scholar]
- 54.Bykov VI, Glyzin VI. Flavonoids of the genus Crataegus. Chem Nat Compd. 1972;8:672–3. doi: 10.1007/BF00564349. [DOI] [Google Scholar]
- 55.Si J, Gao G, Chen D. Chemical constituents of the leaves of Crataegus Scabrifolia. (Franch) Rehd China J Chin Mater Med. 1998;23(7):422–48. [PubMed] [Google Scholar]
- 56.Simirgiotis MJ. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean Peumo (Cryptocarya Alba) fruits and comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules. 2013;18(2):2061–80. doi: 10.3390/molecules18022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu RH, Yu BY, Qiu SX, Zheng D. Comparative analysis of eight major polyphenolic components in leaves of Crataegus L. by HPLC. Chin J Nat Med. 2005;3(3):162–7. [Google Scholar]
- 58.Wang CH, Wang YX, Liu HJ. Validation and application by HPLC for simultaneous determination of vitexin-2″-O-glucoside, vitexin-2″-O-rhamnoside, rutin, vitexin, and hyperoside. J Pharm Anal. 2011;1(4):291–6. doi: 10.1016/j.jpha.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martino E, Collina S, Rossi D, Bazzoni D, Gaggeri R, Bracco F, Azzolina O. Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin-2′′-O-rhamnoside from Crataegus monogyna Jacq.(hawthorn) Phytochem Anal. 2008;19(6):534–40. doi: 10.1002/pca.1081. [DOI] [PubMed] [Google Scholar]
- 60.Cheng S, Qiu F, Huang J, He J. Simultaneous determination of vitexin-2′′-O-glucoside, vitexin-2′′-O-rhamnoside, rutin, and hyperoside in the extract of hawthorn (Crataegus pinnatifida Bge.) Leaves by RP-HPLC with ultraviolet photodiode array detection. J Sep Sci. 2007;30(5):717–21. doi: 10.1002/jssc.200600353. [DOI] [PubMed] [Google Scholar]
- 61.Kirakosyan A, Seymour E, Kaufman PB, Warber S, Bolling S, Chang SC. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J Agric Food Chem. 2003;51(14):3973–6. doi: 10.1021/jf030096r. [DOI] [PubMed] [Google Scholar]
- 62.Han F, Guo Y, Gu H, Li F, Hu B, Yang L. Application of alkyl polyglycoside surfactant in ultrasonic-assisted extraction followed by macroporous resin enrichment for the separation of vitexin-2″-O-rhamnoside and vitexin from Crataegus pinnatifida leaves. J Chromatogr B. 2016;1012–1013:69–78. doi: 10.1016/j.jchromb.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Ying X, Wang R, Xu J, Zhang W, Li H, Zhang C, Li F. HPLC determination of eight polyphenols in the leaves of Crataegus pinnatifida Bge. Var. Major. J Chromatogr Sci. 2009;47(3):201–5. doi: 10.1093/chromsci/47.3.201. [DOI] [PubMed] [Google Scholar]
- 64.Urbonaviciute A, Jakstas V, Kornysova O, Janulis V, Maruska A. Capillary electrophoretic analysis of flavonoids in single-styled hawthorn (Crataegus monogyna Jacq.) Ethanolic extracts. J Chromatogr A. 2006;1112(1–2):339–44. doi: 10.1016/j.chroma.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 65.Kartnig T, Kogl G, Heydel B. Production of flavonoids in cell cultures of Crataegus monogyna. Planta Med. 1993;59(06):537–8. doi: 10.1055/s-2006-959756. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Xu M, Yu C, Zhang G, Tang X. Simultaneous determination of vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside, rutin and vitexin from hawthorn leaves flavonoids in rat plasma by UPLC–ESI-MS/MS. J Chromatogr B. 2010;878(21):1837–44. doi: 10.1016/j.jchromb.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 67.Li H, Song F, Xing J, Tsao R, Liu Z, Liu S. Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS. J Am Soc Mass Spectrom. 2009;20(8):1496–503. doi: 10.1016/j.jasms.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Karar ME, Kuhnert N, UPLC-ESI-Q-TOF-MS /MS characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen) J Chem Biol Ther. 2015;1(102):2572–0406. [Google Scholar]
- 69.Salminen JP, Karonen M, Lempa K, Liimatainen J, Sinkkonen J, Lukkarinen M, Pihlaja K. Characterisation of proanthocyanidin aglycones and glycosides from rose hips by high-performance liquid chromatography–mass spectrometry, and their rapid quantification together with vitamin C. J Chromatogr A. 2005;1077(2):170–80. doi: 10.1016/j.chroma.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 70.Karonen M. Plant Proanthocyanidins. Characterization and Quantification by Degradation Methods. HPLC-DAD/ESI-MS and NMR. Annales Universitatis Turkuensis. Ph.D. Dissertation, University of Turku, Turku, Finland. 2007. p. 53.
- 71.Demiray S, Pintado ME, Castro PML. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. Int J Pharm Pharm Sci. 2009;3(6):74–9. [Google Scholar]
- 72.Gonzalez-Jimenez FE, Salazar-Montoya JA, Calva-Calva G, Ramos-Ramírez EG. Phytochemical characterization, in vitro antioxidant activity, and quantitative analysis by micellar electrokinetic chromatography of hawthorn (Crataegus pubescens) fruit. J Food Qual. 2018; 2018: 11.
- 73.Benabderrahmane W, Lores M, Benaissa O, Lamas JP, de Miguel T, Amrani A, et al. Polyphenolic content and bioactivities of Crataegus oxyacantha L. (Rosaceae) Nat Prod Res. 2019;35(4):627–32. doi: 10.1080/14786419.2019.1582044. [DOI] [PubMed] [Google Scholar]
- 74.Coklar H, Akbulut M, Kilinc S, Yildirim A, Alhassan I. Effect of freeze, oven and microwave pretreated oven drying on color, browning index, phenolic compounds and antioxidant activity of hawthorn (Crataegus orientalis) fruit. Not Bot Horti Agrobot Cluj-Napoca. 2018;46(2):449–56. doi: 10.15835/nbha46211027. [DOI] [Google Scholar]
- 75.Liu P, Yang B, Kallio H. Characterization of phenolic compounds in Chinese hawthorn (Crataegus pinnatifida Bge. Var. Major) fruit by high performance liquid chromatography–electrospray ionization mass spectrometry. Food Chem. 2010;121(4):1188–97. doi: 10.1016/j.foodchem.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Svedstrom U, Vuorela H, Kostiainen R, Huovinen K, Laakso I, Hiltunen R. High-performance liquid chromatographic determination of oligomeric procyanidins from dimers up to the hexamer in hawthorn. J Chromatogr A. 2002;968(1–2):53–60. doi: 10.1016/S0021-9673(02)01000-2. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z, Chang Q, Zhu M, Huang Y, Ho WK, Chen ZY. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem. 2001;12(3):144–52. doi: 10.1016/S0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 78.Mraihi F, Hidalgo M, de Pascual-Teresa S, Trabelsi-Ayadi M, Cherif JK. Wild grown red and yellow hawthorn fruits from Tunisia as source of antioxidants. Arab J Chem. 2015;8(4):570–8. doi: 10.1016/j.arabjc.2014.11.045. [DOI] [Google Scholar]
- 79.Wyspianska D, Kucharska AZ, Sokoł-Letowska A, Kolniak-Ostek J. Physico-chemical, antioxidant, and anti-inflammatory properties and stability of hawthorn (Crataegus monogyna Jacq.) Procyanidins microcapsules with inulin and maltodextrin. J Sci Food Agric. 2017;97(2):669–78. doi: 10.1002/jsfa.7787. [DOI] [PubMed] [Google Scholar]
- 80.Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biochem Res. 2010;4(7):191–5. [Google Scholar]
- 81.Abad MJ, Bedoya LM, Apaza L, Bermejo P. The Artemisia L. Genus: a review of bioactive essential oils. Molecules. 2012;17(3):2542–66. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dickson RA, Houghton PJ, Hylands PJ. Antibacterial and antioxidant cassane diterpenoids from Caesalpinia Benthamiana. Phytochem. 2007;68(10):1436–41. doi: 10.1016/j.phytochem.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 83.Negi PS. Plant extracts for the control of bacterial growth: efficacy, stability and safety issues for food application. Int J Food Microbiol. 2012;156(1):7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Safapour S, Sadeghi-Kiakhani M, Eshaghloo-Galugahi S, Extraction Dyeing, and antibacterial properties of Crataegus elbursensis fruit natural dye on wool yarn. Fibers Polym. 2018;19:1428–34. doi: 10.1007/s12221-018-7643-z. [DOI] [Google Scholar]
- 85.Alami M, Ghorban M. Evaluation of total phenolic, flavonoid, anthocyanin compounds, antibacterial and antioxidant activity of hawthorn (Crataegus Elbursensis) fruit acetonic extract. J Rafsanjan Univ Med Sci. 2014;13(1):53–66. [Google Scholar]
- 86.Saha P, Rahman FI, Hussain F, Rahman SM, Rahman MM. Antimicrobial diterpenes: recent development from natural sources. Front Pharmacol. 2022;12:820312. doi: 10.3389/fphar.2021.820312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuczkowiak U, Petereit F, Nahrstedt A. Hydroxycinnamic acid derivatives obtained from a commercial Crataegus Extract and from authentic Crataegus spp. Sci Pharm. 2014;82(4):835–46. doi: 10.3797/scipharm.1404-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.