Abstract
Integrins mediate cell adhesion and motility on the extracellular matrix, yet they also promote viral attachment and/or entry. Evidence is presented that adenovirus internalization by αv integrins requires activation of phosphoinositide-3-OH kinase (PI3K), whereas αv integrin-mediated cell motility depends on the ERK1/ERK2 mitogen-activated protein kinase pathway. Interaction of adenovirus with αv integrins induced activation of PI3K. Pharmacologic or genetic disruption of endogenous PI3K activity blocked adenovirus internalization and virus-mediated gene delivery yet had no effect on integrin-mediated cell adhesion or motility. Therefore, integrin ligation engages distinct signaling pathways that promote viral endocytosis or cell movement.
Adenovirus entry into host cells depends on αv integrin binding to the penton base viral coat protein (2, 20, 48). A highly mobile protrusion on the adenovirus penton base contains the arginine-glycine-aspartic acid (RGD) sequence which mediates αv integrin binding (42). Integrins are more noted for their ability to mediate cell surface recognition of the extracellular matrix, thereby facilitating adhesion, migration (24), and cell growth and differentiation (28). These interactions have been associated with cell differentiation and tissue development, angiogenesis, wound repair, cancer, and inflammation (22).
A number of cell signaling molecules that are associated with integrin-mediated cellular processes, including adhesion, survival, and motility, have recently been identified (18, 32, 34). For example, the signaling molecule pp125FAK focal adhesion kinase (FAK) (35) is localized to clustered integrins following ligation by extracellular matrix proteins. Engagement (clustering) of integrins by its ligands increases tyrosine phosphorylation and activation of FAK (29). Potential downstream substrates of FAK are the ERK1/ERK2 mitogen-activated protein (MAP) kinases (8, 40) and phosphoinositide-3-OH kinase (PI3K) (7, 17).
Recent studies have demonstrated that ligation of αv and β1 integrins by the extracellular matrix leads to engagement of the ERK1/ERK2 MAP kinase pathway (24). Integrin-mediated regulation of the ERK1/ERK2 MAP kinase pathway results in the activation of myosin light chain kinase and subsequently to phosphorylation of myosin light chains. These molecular events culminate in enhanced cell motility. Cell motility, but not cell adhesion or spreading, can be blocked by ERK antisense oligonucleotides or by the compound PD98059, a specific inhibitor of MEK MAP kinase (24), indicating that the ERK1/ERK2 MAP kinase pathway plays a specific role in cell movement.
PI3K (44) is another downstream effector of FAK. PI3K is a member of a family of lipid kinases comprised of a p85 regulatory subunit and a p110 catalytic subunit. The p85 subunit of PI3K binds directly to phosphorylated FAK (6). The products of PI3K activation, phosphatidylinositol-3,4-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate (PIP3), are increased in the plasma membrane of activated but not quiescent cells and have been proposed to act as second messengers for a number of cell functions (5), including cell cycle progression (9) and cytoskeletal changes underlying the cell plasma membrane (47). PI3K activation also modulates intracellular protein trafficking (41), although a direct role of PI3K in receptor-mediated endocytosis has not been established.
While integrins play an important role in adenovirus entry and in cell migration, the precise mechanisms by which these receptors promote these distinct biological functions are not known. In the studies reported here, we demonstrate that a specific signaling event is involved in the cell entry of a human viral pathogen. Evidence is provided that PI3K is activated upon adenovirus interaction with αv integrins and that this event is required for adenovirus internalization. Surprisingly, activation of ERK1/ERK2 following integrin ligation was necessary for cell migration but not for internalization of adenovirus.
MATERIALS AND METHODS
Cell lines, adenovirus, recombinant proteins, and antibodies.
The human colon carcinoma cell line SW480 and A549 cells were obtained from the American Type Culture Collection (Rockville, Md.). αv integrin-expressing M21-L4 and αv integrin-negative M21-L12 human melanoma cells have been described previously (13). Cells were maintained in Dulbecco modified Eagle (DME) medium supplemented with 10% heat-inactivated fetal calf serum. Murine monoclonal antibodies to FAK, PI3K/p85, and phosphotyrosine were purchased from Transduction Laboratories (Louisville, Ky.). The 9E10 anti-c-myc antibody was obtained from Invitrogen (Carlsbad, Calif.).
Adenovirus type 2 (Ad2) was propagated in human A549 cells and isolated by CsCl density gradient ultracentrifugation as described previously (48). A recombinant adenoviral vector encoding the reporter lacZ gene, Ad5.RSVβgal (43), was propagated in 293 cells. Recombinant Ad2 penton base and fiber proteins were expressed in insect cells by using baculovirus as described previously (48). The proteins were purified to near homogeneity from baculovirus-infected cells by DEAE-Sepharose (Bio-Rad) and Resource Q (Pharmacia) fast protein liquid column chromatography.
Protein phosphorylation and PI3K activation assays.
Epithelial cells were starved for serum by culturing for 20 h in DME medium lacking fetal calf serum. The cells were then incubated with adenovirus at a multiplicity of infection (MOI) of 200 or with 1.0 μg of recombinant penton base or fiber per ml for various times at 37°C. A total of 2 × 106 cells per sample were then disrupted on ice for 20 min by the addition of 0.5 ml of lysis buffer containing 1% Nonidet P-40, 100 mM each NaF and Na4P2O7, 1 mM Na3VO4, 2 mM EGTA, 15 mM MgCl2, and protease inhibitors (1 μg of aprotinin per ml, 1 μg of leupeptin per ml, and 1 mM phenylmethylsulfonyl fluoride). Cell debris and nuclei were removed by centrifugation at 10,000 × g for 20 min at 4°C, and the cell lysate was then immunoprecipitated with an antiphosphotyrosine monoclonal antibody coupled to protein G-Sepharose beads (Pierce) for 2 h at 4°C. The Sepharose beads containing the immune complexes were then washed once each with ice-chilled lysis buffer, 0.5 M NaCl, and 0.5 M LiCl and twice with phosphate-buffered saline (PBS) and then boiled in sodium dodecyl sulfate-gel sample buffer. The samples were separated on a sodium dodecyl sulfate–7% polyacrylamide gel and transferred to a nitrocellulose membrane (Immobilon-P; Millipore). The membrane (blot) was then incubated with anti-FAK or anti-p85/PI3K antibodies at the concentrations recommended by the manufacturer in PBS-containing 5% nonfat dry milk, followed by incubation with a 1:1,000 dilution of goat anti-mouse immunoglobulin conjugated to horseradish peroxidase (KPL Laboratories, Gaithersburg, Md.). The blot was then developed by the use of enhanced chemiluminescence (Amersham Corp.).
To analyze PI3K activation, protein G-Sepharose beads containing antiphosphotyrosine immune complexes were washed once with kinase buffer (30 mM Tris-HCl [pH 7.4] containing 125 mM NaCl, 15 mM MgCl2, and 200 μM adenosine) and then resuspended in 60 μl of kinase buffer containing 10 μCi of [γ-32P]ATP, 20 μM ATP, and 0.5 mg of the substrate phosphatidylinositol 4,5-bisphosphate [PI(4,5)-bisphosphate; Sigma, St. Louis, Mo.] per ml. The kinase reactions were allowed to continue for 10 min at 22°C, and the reaction product, PIP3, was extracted with CHCl3–methanol–1 M HCl (1:1:1), washed with methanol-H2O, and then analyzed on thin-layer-chromatography silica gel plates (E. Merck, Darmstadt, Germany) by using isopropanol–2 M acetic acid (4:1) as the separation solvent. The production of PIP3 was quantitated by phosphorimager densitometry (Molecular Dynamics, Sunnyvale, Calif.).
Pharmacologic studies.
Human epithelial cells were preincubated with various concentrations of wortmannin, ML7, LY294002 (Calbiochem, San Diego, Calif.), or PD98059 (Parke-Davis) or in serum-free medium containing a 1:1,000 dilution of dimethyl sulfoxide (control). The cells were then infected at an MOI of 10 PFU with Ad5.RSVβgal (43). Gene delivery was quantitated by measuring the expression of β-galactosidase activity 24 to 48 h postinfection as previously described (21). The percentage of adenovirus and transferrin internalized was determined by measuring the amount of ligand that was resistant to trypsin treatment (48). Briefly, 2 × 106 epithelial cells were incubated with 125I-labeled Ad2 at an MOI of 10 PFU or with 40 ng of labeled transferrin at 4°C for 60 min. The cells were then warmed to 37°C for various lengths of time and transferred to ice. Uninternalized ligand was removed by the addition of 1 mg of trypsin per ml and incubation for 5 min at 37°C. The trypsinized cells were then washed twice with ice-cold PBS, and the radioactivity of the cell pellets was counted with a gamma counter. The percentage of internalized ligand was determined by dividing the number of counts in the cell pellets by the total number of counts in the cells incubated with ligand at 4°C. The amount of nonspecific binding, which was subtracted from the total, was determined by the addition of a 100-fold excess of unlabeled ligand.
Effect of a regulatory domain of p85/PI3K on virus cell entry and cell adhesion or motility.
Epithelial cells were grown to a confluency of 30% and then transiently transfected (LipofectAMINE; GIBCO/BRL) with a cDNA plasmid encoding the p85/iSH2 domain of PI3K (pRC/CMV/p85iSH2) (38) or with a control plasmid (pcDNA3; Invitrogen). Under these conditions, approximately 50 to 70% of the cells were effectively transduced. The cells were assayed 48 h posttransfection for the expression of the p85/iSH2 protein by Western blotting by using an anti-c-myc tag antibody. To examine disregulation of endogenous PI3K, the cells were transfected with a plasmid encoding iSH2 or a control plasmid. The transfected cells were incubated 48 h later, with or without purified adenovirus, and then cell lysates were prepared and immunoprecipitated with a p85 monoclonal antibody specific for the N-SH3 domain of p85 (Upstate Biotech Inc., Lake Placid, N.Y.). Kinase reactions were performed with the immune complexes by using PI(4,5)-bisphosphate as a substrate, as described above.
The transfected cells were subsequently analyzed for adenovirus internalization and transferrin uptake or for their susceptibility to adenovirus-mediated gene delivery as described above. Binding of soluble penton base to control or p85/iSH2-transfected cells was measured by flow cytometry. The cells were incubated with 0.5 μg of biotinylated penton base per ml for 60 min at 4°C, washed with medium, and then incubated for 15 min at 4°C with a 1:500 dilution of fluorescein isothiocyanate (FITC)-streptavidin (KPL Laboratories). After final washing in Hanks balanced salt solution–1 mM CaCl2 containing 1% fetal calf serum, the cells were analyzed by flow cytometry (FACScan II).
Cell adhesion assays were performed as described previously (49) with 48-well non-tissue-culture-treated cluster plates (Costar) coated with 2 μg of recombinant penton base or fibronectin per ml. Cell migration assays were performed with modified Boyden chambers (6.5-mm-diameter, 8-μm-pore-size, tissue-culture-treated Transwells; Costar) as previously described (24). Briefly, the underside of the membrane of the upper chambers was coated overnight with 1 μg of recombinant penton base or fibronectin in PBS at 4°C. The wells were rinsed with PBS and then placed in the lower chambers containing DME medium without fetal calf serum. A total of 5 × 104 to 1 × 105 SW480 or A549 human epithelial cells were placed into the upper chamber and then allowed to migrate to the underside of the top chamber for 6 h at 37°C. The nonmigrating cells on the top of the membrane filter were removed with a cotton swab, while the migratory cells that were attached to the bottom of the filter were fixed with 0.5% paraformaldehyde and then stained with 0.1% crystal violet in 0.1 M borate buffer (pH 9.0)–2% ethanol. Migratory cells were then counted by light microscopy. Nonspecific adhesion or migration was determined by counting cells that had migrated on membrane filters coated with 1% bovine serum albumin.
RESULTS
Adenovirus promotes phosphorylation of FAK and PI3K.
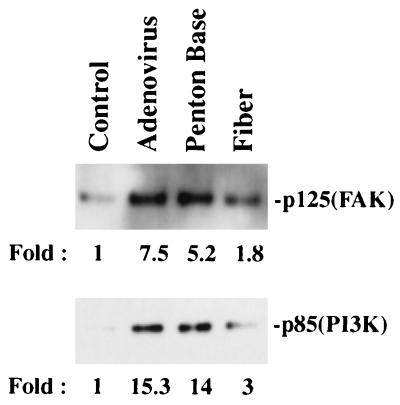
Early signaling events that result from ligand engagement of cell integrins include phosphorylation of the 125-kDa FAK, p85/PI3K, and ERK1/ERK2 MAP kinases (27). We therefore examined whether there was an increase in direct or indirect phosphorylation of these molecules by adenovirus interaction with αv integrins. Incubation of SW480 cells with purified adenovirus particles or with recombinant penton base protein caused a five- to sevenfold increase in phosphotyrosine-associated FAK compared to that of control cells incubated with medium alone (Fig. 1). In contrast, binding of the adenovirus fiber protein to cells caused only a minimal increase in phosphorylation (1.8-fold). In parallel studies, we also examined phosphotyrosine-associated PI3K and ERK1/ERK2, downstream effectors of FAK. Adenovirus and recombinant penton base interaction with cells caused 14- and 15-fold increases, respectively, in phosphotyrosine-associated p85/PI3K, while binding of the fiber protein to cells caused only a minimal increase in phosphorylation (Fig. 1). No significant change in phosphorylation of ERK1/ERK2 MAP kinases was observed in SW480 cells incubated with adenovirus (data not shown). These studies indicate that the interaction of the adenovirus penton base with αv integrins is the principal means by which cell signaling events are initiated by adenovirus.
FIG. 1.
Phosphorylation events occurring during adenovirus interaction with cells. SW480 cells were incubated with adenovirus at an MOI of 200 PFU or with 1 μg of recombinant penton base or fiber for 10 min at 37°C. Cell lysates were then immunoprecipitated with an antiphosphotyrosine antibody followed by Western blotting with an anti-FAK (upper panel) or anti-p85/PI3K antibody (lower panel) as described in Materials and Methods. The protein bands were quantitated by densitometry. Control cell samples were incubated in medium alone.
Adenovirus interaction with host cells promotes activation of the PI3K catalytic subunit.
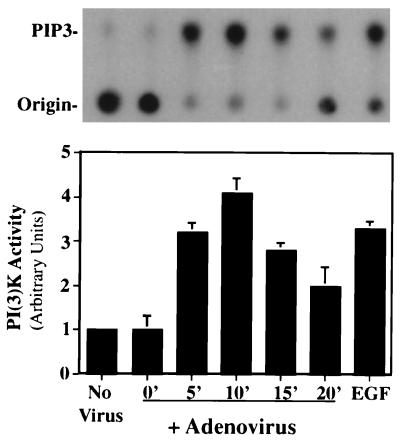
Previous studies have suggested that the interaction between FAK and PI3K, specifically, the phosphorylation of FAK at tyrosine 397, allows binding of phosphorylated p85, the regulatory subunit of PI3K (6). This may represent a mechanism by which the iSH2 domain of p85 is free to interact with, and subsequently activate, the p110 catalytic subunit. To determine whether adenovirus interaction with cells leads to activation of the p110 subunit of PI3K, cells were incubated with adenovirus at 4 or 37°C for various times. Cell lysates were then immunoprecipitated with an antiphosphotyrosine monoclonal antibody, and the amount of PI3K activity present in the immune complexes was analyzed by the addition of [γ-32P]ATP and a specific substrate, PI(4,5)-bisphosphate. Basal levels of PI3K activity were detected in cells incubated with adenovirus at 4°C or in cells incubated at 37°C without virus (Fig. 2). In contrast, a rapid increase in PI3K activity was detected during adenovirus interaction with cells at 37°C that was similar to that elicited by a known PI3K activator, epidermal growth factor (EGF) (Fig. 2). These findings indicate that initial signaling events in adenovirus interaction with cells also result in PI3K activation.
FIG. 2.
PI3K activation during adenovirus entry into cells. SW480 cells were incubated with adenovirus at an MOI of 200 PFU for various times at 37°C, and cell extracts were immunoprecipitated with an antiphosphotyrosine antibody. The kinase reaction was performed with the immune precipitates with [γ-32P]ATP and PI(4,5)-bisphosphate as a substrate. Following solvent extractions and washes, the reaction product, PIP3, was detected by thin-layer chromatography (top) and quantitated by phosphorimager densitometry (bottom). Control cell samples were incubated in medium without adenovirus. Cells were also incubated with EGF for 10 min at 37°C as a positive control for PI3K activation. The data are representative of four experiments.
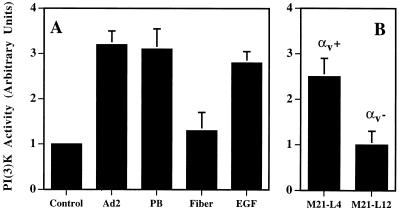
To further investigate the interactions required for activation of PI3K, we next examined the effects of isolated viral capsid protein(s) on PI3K activation. Soluble recombinant penton base caused a three- to fourfold increase in PI3K activation similar to that induced by intact virus particles (Fig. 3A). In contrast, recombinant adenovirus fiber protein failed to significantly stimulate PI3K activation. These results provide evidence that Ad2 interaction with cells via the penton base binding to αv integrins stimulates PI3K activation. To confirm this, we compared the relative ability of Ad2 to induce PI3K activation in αv integrin-expressing M21-L4 human melanoma cells with that in αv integrin-negative M21-L12 cells (Fig. 3B). Both cell types support equivalent levels of Ad2 binding via the fiber protein, but only M21-L4 cells interact specifically with the penton base protein and, thus, show viral internalization and infection (48). Adenovirus stimulated PI3K activation by two- to threefold in M21-L4 (αv integrin-positive) cells but did not promote activation in M21-L12 (αv integrin-negative) cells (Fig. 3B). This was not due to a deficiency of PI3K in the M21-L12 cells, since EGF treatment activated PI3K to similar levels as it did in M21-L4 cells (data not shown). These findings provide further evidence that the interaction of the penton base protein with αv integrins, an event that promotes adenovirus internalization, specifically mediates PI3K activation.
FIG. 3.
Adenovirus penton base interaction with αv integrins promotes PI3K activation. (A) SW480 epithelial cells were incubated with Ad2, recombinant penton base (PB), fiber proteins, or EGF for 10 min at 37°C and then analyzed for PI3K activation as described in the legend to Fig. 2. The results represent the average of duplicate samples (+ standard deviation) and are representative of at least three separate experiments. (B) αv integrin-expressing M21-L4 cells or αv integrin-negative M21-L12 cells were incubated with adenovirus particles for 10 min at 37°C prior to analyzing cell lysates for PI3K activation by using phosphatidylinositol as a substrate.
PI3K-specific pharmacologic agents inhibit adenovirus cell entry.
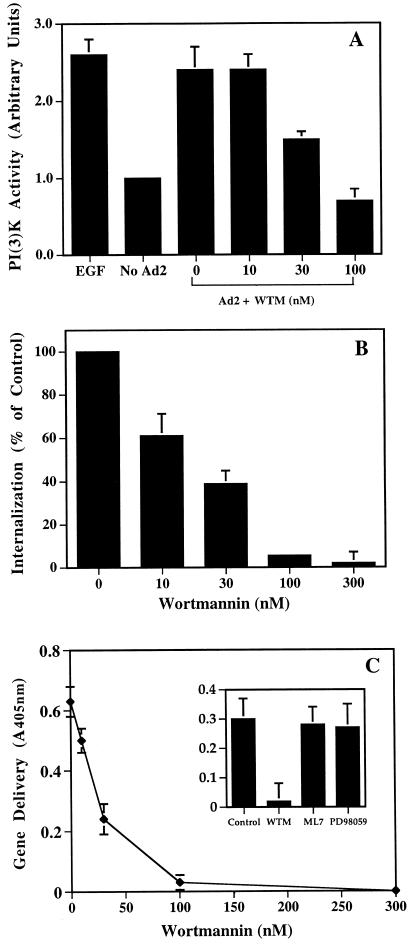
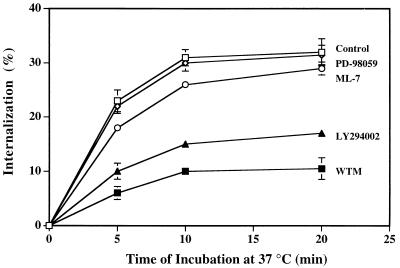
To establish whether PI3K activation was required for adenovirus uptake, cells were treated with pharmacologic agents that inhibit PI3K activation at low concentrations and then analyzed for their ability to support adenovirus internalization and virus-mediated gene delivery. Wortmannin, a fungal metabolite that is a potent inhibitor of PI3K activity (45), caused dose-dependent inhibition of adenovirus-mediated PI3K activation (Fig. 4A). Similar concentrations of wortmannin inhibited both adenovirus internalization and virus infection as measured by virus-mediated gene delivery (Fig. 4B and C). The 50% inhibitory concentration was approximately 20 nM, an amount previously shown to inhibit other PI3K-mediated cell functions (1, 23). Adenovirus internalization was also inhibited by LY294002 (46) (Fig. 5), another PI3K-directed compound which has a different mode of action than wortmannin. Importantly, adenovirus internalization or gene delivery was not inhibited by ML7, an inhibitor of myosin light chain kinase (39). Further, PD98059, a selective and potent inhibitor of the ERK1/ERK2-dependent MAP kinase pathway, had no effect (33) (Fig. 4C and 5). Together, these studies indicate that adenovirus uptake and virus infection are specifically associated with PI3K activation but not MAP kinase or myosin light chain kinase signaling.
FIG. 4.
Effect of wortmannin on adenovirus-induced PI3K activation (A), virus internalization (B), and gene delivery (C). SW480 cells were treated with various concentrations of wortmannin for 10 min at 37°C and then incubated with adenovirus particles for an additional 10 min at 37°C. PI3K activation was analyzed as described in the legend to Fig. 2 by using PI(4,5)-bisphosphate as a substrate. Cells were also treated with various concentrations of wortmannin at 4°C for 10 min followed by preincubation with 125I-labeled adenovirus for 30 min at 4°C. After removing unbound virus by washing, the cells were resuspended in PBS and then warmed to 37°C for 10 min. Wortmannin was present throughout the internalization process. Internalization was measured by resistance to trypsin digestion as described in Materials and Methods. In separate studies to analyze virus infection, SW480 cells were pretreated with various amounts of wortmannin or with a 2 μM concentration of the myosin light chain kinase inhibitor ML7 or with 20 μM of the ERK1/ERK2 MAP kinase inhibitor PD98059 (C, inset). β-Galactosidase activity in virally transduced cells was measured 48 h postinfection by use of a colorimetric assay (A600). The data are the average of duplicate samples (± standard deviation) and are representative of two experiments.
FIG. 5.
Effect of different lipid and protein kinase inhibitors on adenovirus internalization. SW480 cells were pretreated in suspension for 10 min at 4°C with 100 nM wortmannin (WTM) or were treated with 100 μM LY294002, 2 μM ML7, or 20 μM PD98095 or in medium alone (control) for 2 h at 37°C before detachment. The cells were then incubated with 125I-labeled adenovirus for 30 min at 4°C, washed, and warmed to 37°C for various times prior to assaying for virus internalization as measured by resistance to trypsin treatment.
Genetic disregulation of endogenous PI3K inhibits adenovirus cell entry but not primary integrin functions.
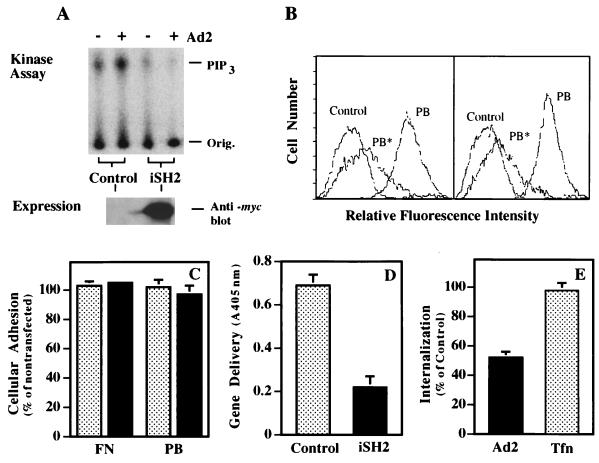
To provide further evidence that PI3K is required for adenovirus uptake, cells were transfected with the iSH2 domain of p85 to disrupt the interaction of p110 with endogenous p85 (19). Transfection of SW480 cells with the iSH2 domain inhibited adenovirus-mediated activation of endogenous PI3K, as indicated by decreased formation of PIP3 (Fig. 6A). Cells transfected with the iSH2 domain of p85 retained the ability to bind soluble, recombinant penton base at levels similar to that of mock-transfected cells, and binding was inhibited to a similar degree by the addition of unlabeled competitor (Fig. 6B). In addition, both mock- and iSH2-transfected cells showed identical levels of adhesion to fibronectin or penton base (Fig. 6C). In contrast, iSH2-transfected cells supported lower amounts of adenoviral gene delivery, as well as decreased levels of virus internalization (Fig. 6D and E). These results are in agreement with the previously generated pharmacological data, suggesting that PI3K activity is required for viral infection. Importantly, these results suggest that the p85 regulatory domain provides a context for p110 activity and that iSH2-p110 interaction alone is not sufficient to facilitate viral uptake. Further, the effect of iSH2 appears to be specific for the integrin-mediated uptake of adenovirus, since iSH2-expressing cells do not exhibit altered transferrin internalization relative to that of mock-transfected cells (Fig. 6E).
FIG. 6.
Expression of the iSH2 domain of the p85 subunit of PI3K inhibits adenovirus internalization and gene delivery. SW480 cells were transfected with a control plasmid lacking a foreign gene or with a plasmid encoding p85/iSH2. The transfection efficiency was approximately 50 to 70%, as judged by delivery of the lacZ-containing plasmid pcDNA3. (A) The cells were assayed 48 h posttransfection for adenovirus-induced PI3K activation as described in the legend to Fig. 2 and for expression of the iSH2 protein by immunoblotting with an anti-c-myc tag antibody. Orig., origin of sample application. (B) Transfected cells were also analyzed for binding of soluble penton base by flow cytometry (Control, cells incubated with FITC-streptavidin alone; PB, cells incubated with biotinylated penton base followed by incubation with FITC-streptavidin; PB*, cells incubated with unlabeled penton base followed by incubation with biotinylated penton base and FITC-streptavidin). (C to E) Control transfected (stippled bars) or p85/iSH2-transfected (solid bars) cells were also assayed for their ability to adhere to plastic tissue culture wells coated with immobilized penton base (PB) or fibronectin (FN) (C) and for susceptibility to adenovirus-mediated gene delivery (D) and virus internalization (E). Tfn, transferrin. The data are the average of duplicate samples (+ standard deviation) and are representative of two experiments.
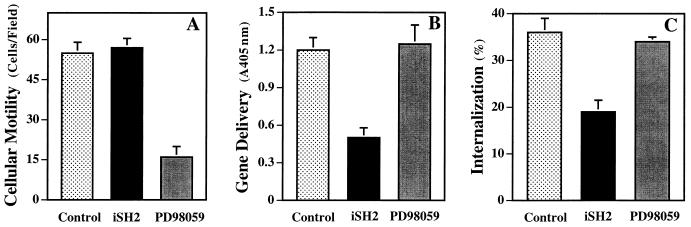
Although primary integrin function (i.e., ligation) did not appear to be affected by expression of the iSH2 domain of p85, it remained possible that other downstream events mediated by integrins were also downregulated as a result of iSH2 transfection. For example, migration and endocytosis have been suggested to utilize several common elements (3, 26). To examine the role of PI3K on cell movement and virus uptake, we investigated A549 cells which, unlike SW480 cells, are capable of good cell movement, as well as being highly susceptible to adenovirus infection. The expression of the iSH2 domain in A549 cells did not influence migration (haptotaxis) (Fig. 7A) but did inhibit adenovirus gene delivery (Fig. 7B), as well as virus internalization (Fig. 7C). Additionally, the MAP kinase inhibitor PD98059, which failed to block adenovirus cell entry (Fig. 3), was a potent inhibitor of cellular migration (Fig. 7A), as was previously shown by Klemke et al. (24). Together, these results suggest a specific role for PI3K activation in integrin-mediated adenovirus internalization and further illustrate that different signaling molecules are involved in distinct integrin-mediated cellular processes.
FIG. 7.
Effect of transient expression of p85/iSH2 on cell motility. A549 cells were transfected with p85/iSH2 or a control plasmid and examined 48 h posttransfection for cell migration on fibronectin-coated membrane filters (A), for adenovirus-mediated gene delivery (B), and for virus internalization (C). In parallel studies, A549 cells were treated with the MAP kinase inhibitor PD98059 prior to assaying cell functions.
DISCUSSION
Although receptors for attachment and internalization of adenovirus have recently been identified, little information exists on the precise mechanisms by which these receptors promote the entry process. The studies reported here reveal an important role for PI3K in αv integrin-mediated signaling events associated with adenovirus internalization and infection of human epithelial cells.
Binding of the adenovirus penton base to αv integrins induces phosphorylation of several signaling proteins, including FAK and PI3K (Fig. 1). FAK but not p85/PI3K has been previously shown to be phosphorylated during integrin ligation by extracellular matrix proteins. A low level of FAK and p85/PI3K phosphorylation was induced by interaction of the fiber protein with its receptor; however, further studies indicated that fiber binding was not required for PI3K activation (Fig. 3). It is not known whether FAK phosphorylation is required for adenovirus cell entry; however, overexpression in epithelial cells of a mutant FAK that cannot be phosphorylated (Tyr397) did not alter virus uptake or gene delivery (data not shown). Since Tyr397 has been shown to be a site for PI3K binding to FAK (6) and the penton base can activate PI3K in lymphoid-derived cells that lack FAK (data not shown), this suggested that FAK was probably not required for adenovirus uptake. Therefore, we were compelled to investigate whether alternative effector molecules such as PI3K were involved in virus uptake and infection.
Our studies demonstrated an increase in phosphotyrosine-associated p85/PI3K under conditions that permit virus entry (Fig. 1) and that this also leads to activation of the lipid kinase (Fig. 2). Moreover, we showed that PI3K activation is dependent on the interaction of the penton base with αv integrins rather than the binding of fiber to its receptor (Fig. 3).
In addition to facilitating virus internalization, the penton base also promotes adenovirus-mediated membrane permeabilization and virus-mediated gene delivery in vivo. It is therefore possible that PI3K activation also occurs during adenovirus disruption of the cell endosome; however, we observed that PI3K can be activated by a temperature-sensitive mutant adenovirus, ts1 (11), that fails to penetrate cell endosomes (data not shown). The ts1 mutant internalizes normally (16) but lacks a functional cysteine protease required for endosome penetration and subsequent uncoating (11). Therefore, PI3K activation is associated with virus uptake rather than virus penetration of cell endosomes.
A crucial requirement for PI3K activation for adenovirus infection was indicated by pharmacologic studies using two separate inhibitors of PI3K, wortmannin and LY294002. The concentration of wortmannin required to inhibit virus-induced PI3K activation was also similar to that needed to inhibit virus uptake and gene delivery (Fig. 3). In confirmation of the pharmacologic studies, transfection of cells with a regulatory domain of the p85 subunit of PI3K (iSH2) (38) inhibited adenovirus uptake and gene delivery (Fig. 6), while transferrin uptake was unaffected (Fig. 6C), suggesting that distinct pathways exist for internalization of different ligands. In contrast, adenovirus attachment to cell surface αv integrins did not promote ERK1/ERK2 activity. iSH2 expression and pharmacologic inhibitors of myosin light chain kinase or the ERK1/ERK2 MAP kinase pathway did not affect virus uptake or gene delivery; however, these inhibitors blocked αv integrin-mediated cell migration (24) (Fig. 7). Although adenovirus infection has been previously reported to activate the Raf/MAP kinase pathway (4), this pathway is apparently not required for virus cell entry or infection. The findings reported here indicate that different signaling pathways regulate virus uptake and cell motility.
The exact signaling pathway involved in PI3K-mediated adenovirus uptake has yet to be fully elucidated; however, previous studies have indicated that entry of Listeria monocytogenes into cells also requires PI3K activation (23). Certain cell signaling processes lead to the reorganization of the actin cytoskeleton (10). Interestingly, adenovirus entry (36), as well as uptake of influenza virus into polarized epithelial cells (14), has also been reported to require an intact actin cytoskeleton. These findings suggest that recruitment of the actin cytoskeleton may be an important feature of receptor-mediated virus internalization. FAK and PI3K, as well as other signaling molecules, associate with the actin cytoskeleton shortly after integrin clustering (35, 37). PI3K has been proposed to modulate the reorganization of the actin cytoskeleton via interactions with the small GTPase protein, Rac (32). The interactions of these signaling molecules may, therefore, facilitate adenovirus cell entry by promoting actin polymerization. The actin cytoskeleton may provide the mechanical force necessary to internalize adenovirus-containing vesicles as has been demonstrated with other ligands in macrophages (1) and yeast cells (30, 31). PI3K signaling could also stimulate adenovirus uptake by interacting with dynamin (15), a GTPase protein required for endocytic vesicle formation (12). The small GTPases Rac1 and RhoA have also been shown to modulate transferrin internalization by regulating endosome formation (25). However, our studies and those of others have shown that transferrin uptake does not require PI3K activation or recruitment of the actin cytoskeleton (25), thus suggesting that distinct biochemical pathways exist for receptor-mediated endocytosis. While further studies are needed to fully characterize these endocytic pathways, an increased understanding of the signaling events involved in αv integrin-mediated adenovirus internalization provides insight into the cellular mechanisms of endocytosis and cell motility, as well as the development of more effective strategies for adenovirus gene therapy.
ACKNOWLEDGMENTS
We thank Alex Toker and Lew Cantley for providing the p85/iSH2 plasmid, David Schlaepfer for the FAK constructs, and Gary Bokoch for helpful discussions. We also thank Joseph Weber for providing the ts1 mutant adenovirus and Catalina Hope and Joan Gausepohl for preparation of the manuscript.
This work was supported by NIH grants HL54352 and EY11431.
REFERENCES
- 1.Araki N, Johnson M T, Swanson J A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bretscher M S. Cells can use their transferrin receptors for locomotion. EMBO J. 1992;11:383–389. doi: 10.1002/j.1460-2075.1992.tb05066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter C L, Cantley L C. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen H-C, Appeddu P A, Isoda H, Guan J-L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 7.Chen H-C, Guan J-L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 9.Chou M M, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 10.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 11.Cotten M, Weber J M. The adenovirus protease is required for virus entry into host cells. Virology. 1995;213:494–502. doi: 10.1006/viro.1995.0022. [DOI] [PubMed] [Google Scholar]
- 12.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felding-Habermann B, Mueller B M, Romerdahl C A, Cheresh D A. Involvement of integrin αv gene expression in human melanoma tumorigenicity. J Clin Invest. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb T A, Ivanov I E, Adesnik M, Sabatini D D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–709. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gout I, Dhand R, Hiles I D, Fry M J, Panayotou G, Das P, Truong O, Totty N F, Hsuan J, Booker G W, Campbell I D, Waterfield M D. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. [PubMed] [Google Scholar]
- 16.Greber U F, Webster P, Helenius A, Weber J. The role of the adenovirus protease in virus entry into cells. EMBO J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- 17.Guinebault C, Payrastre B, Racaud-Sultan C, Mazarguil H, Breton M, Mauco G, Plantavid M, Chap H. Integrin-dependent translocation of phosphoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85α with actin filaments and focal adhesion kinase. J Cell Biol. 1995;129:831–842. doi: 10.1083/jcb.129.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 19.Hu Q, Klippel A, Muslin A J, Fantl W J, Williams L T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Endo R I, Nemerow G R. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 23.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 24.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamaze C, Chuang T-H, Terlecky L J, Bokoch G M, Schmid S L. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1995;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- 26.Lawson M A, Maxfield F R. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 27.Lewis J M, Cheresh D A, Schwartz M A. Protein kinase C regulates αvβ5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meredith J E, Jr, Winitz S, McArthur Lewis J, Hess S, Ren X-D, Renshaw M W, Schwartz M A. The regulation of growth and intracellular signaling by integrins. Endocr Rev. 1996;17:207–220. doi: 10.1210/edrv-17-3-207. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto S, Akiyama S K, Yamada K M. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 30.Moreau V, Madania A, Martin R P, Winsor B. The Saccharomyces cerevisiae actin-related protein Arp2 is involved in the actin cytoskeleton. J Cell Biol. 1996;1:117–132. doi: 10.1083/jcb.134.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 33.Pang L, Sawada T, Decker S J, Saltiel A R. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 34.Parsons J T. Integrin-mediated signaling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- 35.Parsons J T, Schaller M D, Hildebrand J, Leu T-H, Richardson A, Otey C. Focal adhesion kinase: structure and signaling. J Cell Sci Suppl. 1994;18:109–113. doi: 10.1242/jcs.1994.supplement_18.16. [DOI] [PubMed] [Google Scholar]
- 36.Patterson S, Russell W C. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J Gen Virol. 1983;64:1091–1099. doi: 10.1099/0022-1317-64-5-1091. [DOI] [PubMed] [Google Scholar]
- 37.Plopper G E, McNamee H P, Dike L E, Bojanowski K, Ingber D E. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rameh L E, Chen C-S, Cantley L C. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- 40.Schlaepfer D D, Hanks S K, Hunter T, vanderGeer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd P R, Reaves B J, Davidson H W. Phosphoinositide 3-kinases and membrane traffic. Trends Cell Biol. 1996;6:92–97. doi: 10.1016/0962-8924(96)80998-6. [DOI] [PubMed] [Google Scholar]
- 42.Stewart P L, Chiu C, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow G. Localization of an integrin binding motif (RGD) on adenovirus type 2 particles by cryo-electron microscopy. EMBO J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toker A, Cantley L C. Signalling through the lipid products of phosphoinositide. Nature. 1997;12:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 45.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 46.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase 2-(4-morpholinyl)-8--phenyl-4H-1benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 47.Wennström S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 48.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 49.Wilkins J A, Li A, Ni H, Stupack D G, Shen C. Control of β1 integrin function. J Biol Chem. 1996;271:3046–3051. [PubMed] [Google Scholar]