Abstract
Purpose:
This study presents a method to quantify micro-stiffness variations in experimental arteriovenous fistulae (AVF).
Methods:
AVF created by anastomosing the superficial epigastric vein to the femoral artery in Sprague-Dawley rats were allowed to remodel for 21 days before being harvested and preserved in culture medium. A custom atomic force microscope was used to measure microvascular stiffness (Young’s modulus) in three areas of the AVF: the inflow artery, the juxta-anastomotic area, and the outflow vein. Morphometric measurements and collagen and elastin contents were also determined.
Results:
Atomic force microscopy indentation revealed an increased stiffness in the juxta-anastomotic area of the AVF compared to the outflow vein and inflow artery. The juxta-anastomotic area was also significantly stiffer than the contralateral vein. The lack of elasticity (higher Young’s modulus) of the juxta-anastomotic region was associated with a thicker vascular wall that was rich in collagen but poor in elastin.
Conclusions:
This study demonstrates for the first time the feasibility of using atomic force microscopy to measure local stiffness variations in experimental AVF. This technique could be instrumental in advancing our understanding of how micro-spatial organization of the AVF wall determines the overall biomechanical performance of this type of vascular access.
Keywords: Arteriovenous fistula, Collagen, Elastin, Vascular access, Vascular stiffness
Introduction
The guidelines of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative recommend the arteriovenous fistula (AVF) as the first choice for hemodialysis access due to the better patency rates and lower costs compared to prosthetic arteriovenous grafts (AVG) and central venous catheters (1). Unfortunately, a significant number of AVF never reach the flow rate and luminal diameter necessary to sustain hemodialysis (maturation) requiring additional endovascular or surgical interventions to salvage the vascular access (2). Maturation is the result of profound structural changes in the AVF wall that allow for adequate dilatation and adaptation to the new hemodynamic conditions. One of the most important risk factors for AVF failure is pre-existing vascular stiffness, which compromises proper venous remodeling and favors the development of neointimal hyperplasia (3). In fact, increased venous distensibility, a surrogate marker for vascular elasticity, predicts AVF maturation in hemodialysis patients (4–6).
The mechanisms underlying the biomechanical transformation of a vein into an AVF have been barely explored. It is known that disruption of the vein structure, first by the arterial circulation and then by smooth muscle cell (SMC)-secreted matrix metalloproteinases (MMPs), initiates wall remodeling and luminal expansion of the vessel without compromising hemostasis (7, 8). This vascular process is thought to determine the composition and orientation of collagen and elastin in the extracellular matrix (ECM) and, therefore, the future biomechanical performance of the AVF (9–11). Importantly, vascular remodeling can be modified by pre-existing vasculopathies such as medial fibrosis and arterial calcification, which are frequently found in end-stage renal disease patients (12). Uremia has also been associated with reduced nitric oxide (NO) availability and increased collagen I expression in the vasculature (13, 14). Therefore, the development of new reliable methods with spatial resolution to detect micro-variations in mechanical properties could provide new insights on the pathways that modulate AVF elasticity and their relationship with maturation.
Herein, we present a method that spatially resolves the elastic properties of experimental AVF using atomic force microscopy (AFM) microindentation. Unlike most published studies on vascular access elasticity (10, 11, 15–18), we describe a unique experimental setting that allows for measurements of local Young’s modulus throughout the inflow artery and outflow vein. We also present correlations between mechanical properties and underlying anatomical and pathological features that illustrate how matrix deposition determines AVF stiffness. This method can be extended to experimental AVF from large animals and human samples to study how vascular stiffness varies across space, stenosis progression, and maturation.
Materials and methods
Surgeries and tissue harvesting
Arteriovenous fistulae were performed in a total of 14 male, 12-week old Sprague Dawley rats (Harlan, Indianapolis, IN), weighing 280–320 g, by end-to-side anastomosis of the femoral artery to the superficial epigastric vein, as previously described (Fig. 1A) (19). The AVF and corresponding contralateral superficial epigastric veins were collected 21 days after surgery and used for either AFM or histological analysis. Additionally, four femoral arteries and superficial epigastric veins from two unoperated control rats of same age, gender and size as the experimental animals were used to establish baseline elasticity values. Six AVF and contralateral veins were placed in Dulbecco’s Modified Eagle Medium (D1145, Sigma-Aldrich, St. Louis, MO), kept on ice, and delivered to the Atomic Force Microscopy laboratory. The remaining AVF and contralateral veins were formalin-fixed and stored for histopathology and morphometric analysis. The Institutional Animal Care and Use Committee at the University of Miami approved all studies.
Fig. 1 -.
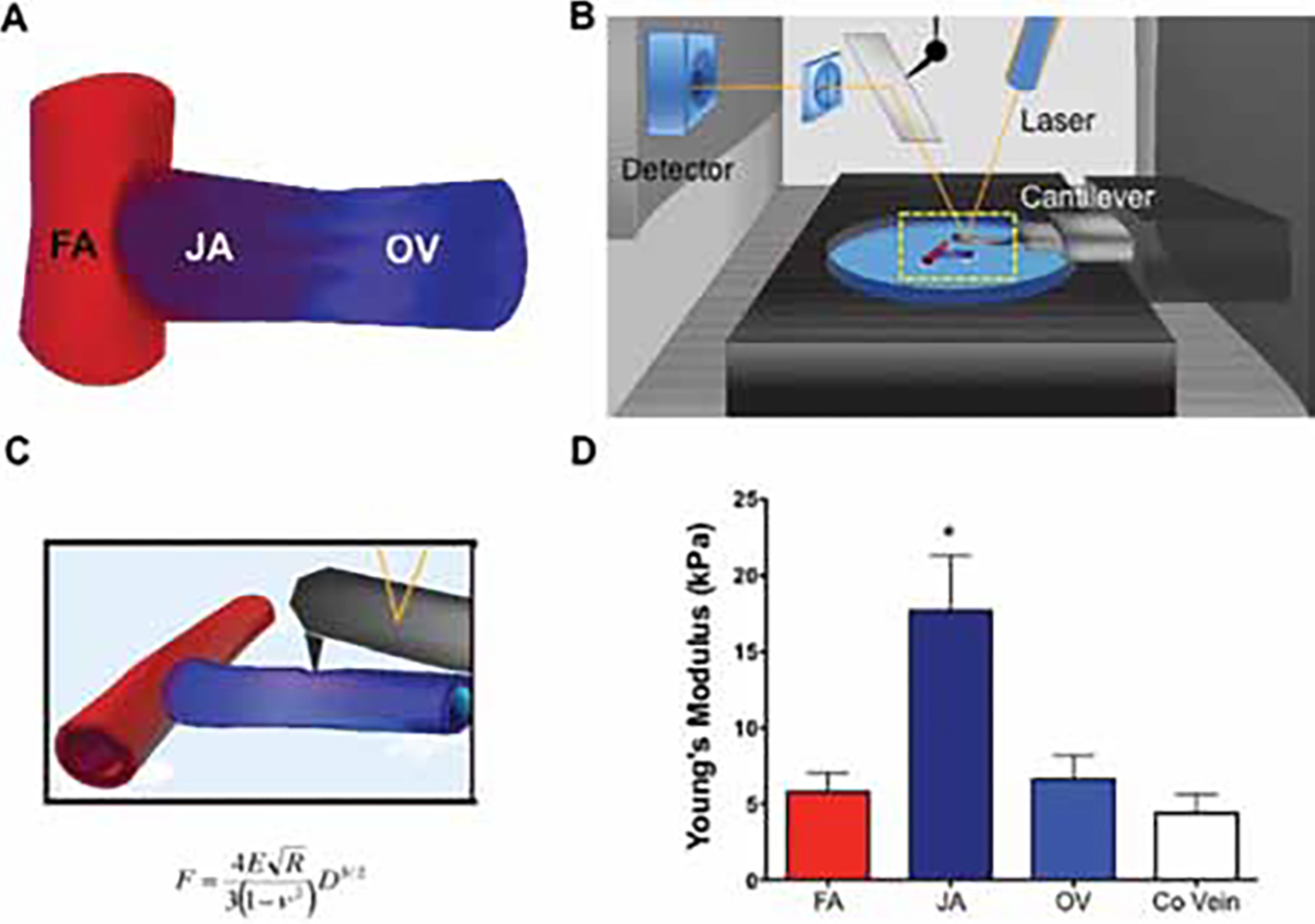
(a) Schematic representation of the femoral artery to superficial epigastric vein arteriovenous fistula (AVF) model in rats. The femoral artery (FA), juxta-anastomotic (JA) zone and outflow vein (OV) are marked. (b) Typical setup of an atomic force microscope used to measure AVF elasticity. The fistula was adhered to a 35 mm petri dish and placed under the cantilever of the microscope. The deflection of the cantilever was measured, representing changes in force applied to the sample and the subsequent indentation. (c) Equation of the Hertz contact mechanical model, where F is the measured force, E is Young’s modulus, ν is Poisson’s ratio (assumed to be 0.49), R is the radius of the spherical indenter, and D is the measured force-indentation. (D) Calculated Young’s modulus of elasticity, a surrogate measure of vascular stiffness, for the three zones of the rat AVF using AFM microindentation (n = 6). Contralateral epigastric veins (Co Vein) served as controls (n = 6). Values are presented as average ± SEM. *p<0.01 as determined by one-way ANOVA with a Newman-Keuls post hoc test.
Histology and morphometric analysis
Intima-media and luminal areas were measured on hematoxylin and eosin (H&E) stained sections taken at 2–3 mm (juxta-anastomotic zone) and 4–7 mm (outflow vein) from the anastomosis site. All morphometric measurements were performed on digital images using the Image Pro Plus software (Media Cybernetics, Inc., Bethesda, MD). Total medial collagen and elastin deposition were measured in RGB digital images of three consecutive Masson’s trichrome or Elastic-Van Gieson (EVG) stained sections after color separation using the Adobe Photoshop software (Adobe Systems, Inc., San Jose, CA). The amount of collagen and elastin was expressed as the ratio of colored pixels per total pixels in the media.
Atomic force microscopy
Elasticity testing was conducted using a custom-built atomic force microscopy (AFM) system (Figs. 1B and C) (20–22). The AFM cantilever (5 μm diameter borosilicate glass particle, silicon nitride cantilever, 0.12 N/m, Novascan Technologies, Ames, IA) was lowered onto the samples using a piezoelectric mechanism (60 μm maximal expansion, P-841.40, Physik Instrumente, Germany). The contact interaction between the cantilever and sample was recorded using a custom code based in IGOR Pro (WaveMetrics, Lake Oswego, OR). The recorded cantilever deflection-indentation curves were used to derive the sample’s force-indentation curves, after factoring out the cantilever deflection on a hard surface and incorporating the spring constant. According to the Hertz contact mechanical model (23), the force-indentation relation is a function of the effective Young’s modulus of elasticity:
where is the measured force, is Young’s modulus, is Poisson’s ratio (assumed to be 0.49), is the radius of the spherical indenter, and is the measured indentation. A custom curve-fitting MATLAB program was used to analyze the force-indentation curves with the Hertz model for spherical indenters. Multiple measurements were taken along the length of the fistula, targeting the same positions specified above for histology sections. All experiments were performed with samples submerged in DMEM at room temperature.
Statistical analysis
Results were expressed as mean ± standard error of the mean (SEM). Multi-group comparisons were conducted using one-way ANOVA with a Newman-Keuls post hoc test. Statistics were calculated with Prism 5 (GraphPad Software, La Jolla, CA). Results are considered statistically significant when p<0.05.
Results
Surgical outcomes
A total of 14 AVF were created by surgically anastomosing the rat superficial epigastric vein to the nearby femoral artery. Three out of the 14 AVF (21%) were excluded from the study due to evident thrombosis at the time of harvesting. Six AVF were submitted to the Atomic Force Microscopy laboratory for Young’s modulus determinations. The remaining five were used to monitor venous wall and luminal area changes after remodeling.
Vascular stiffness using AFM
To obtain a complete understanding of how changes in vascular microstructure influence AVF mechanical properties, the Young’s modulus of elasticity was calculated in the inflow artery, the juxta-anastomotic area and outflow vein of six experimental AVF as well as in their corresponding contralateral veins (Tab. I, Fig. 1D). Baseline values of vascular elasticity were obtained from four femoral arteries and superficial epigastric veins of unoperated animals. Interestingly, the vascular wall in the juxta-anastomotic region of experimental AVF was up to nine times stiffer (higher Young’s modulus values) than in the contralateral vein (Tab. I). In addition, the former area was also approximately six times stiffer than the inflow artery or outflow vein (17.7 ± 7.2 vs. 5.8 ± 2.4 and 6.6 ± 2.7 kPa, respectively). The average baseline value in control arteries from unoperated animals (5.9 ± 0.9 kPa) was equivalent to the one obtained in the inflow artery of the AVF (5.8 ± 2.4 kPa) demonstrating no increase in arterial stiffness during remodeling. The average baseline Young’s modulus value in control veins was slightly smaller than in contralateral veins of operated animals without reaching statistical significance (2.9 ± 0.4 vs. 4.4 ± 1.8 kPa, p = 0.07).
TABLE I-.
Calculated Young's modulus of elasticity for the three zones along the length of rat arteriovenous fistulae using AFM microindentation
| Animal | Inflow artery | Juxta anastomotic area | Outflow vein | Contralateral vein |
|---|---|---|---|---|
|
| ||||
| 1 | 3.0 | 6.5 | 3.1 | 1.0 |
| 2 | 8.3 | 17.9 | 12.7 | 2.0 |
| 3 | 6.2 | 15.1 | 9.1 | 5.7 |
| 4 | 10.5 | 33.8 | 7.8 | 3.9 |
| 5 | 4.1 | 17.0 | 3.7 | 9.7 |
| 6 | 2.6 | 15.9 | 3.2 | 4.1 |
| Average | 5.8 | 17.7* | 6.6 | 4.4 |
| SEM | 2.4 | 7.2 | 2.7 | 1.8 |
p<0.01 determined by One-Way ANOVA with a Newman-Keuls post hoc test.
Vascular wall characteristics
Collagen and elastin deposition were quantified in the three AVF zones and the contralateral epigastric veins (Fig. 2A–D). Medial collagen deposition increased from 2.7 ± 0.5% in the contralateral vein to 18.4 ± 6.8% in the juxta-anastomotic area (Fig. 2E). Further down the fistula, collagen deposition decreased to 9.4 ± 4.6% in the outflow vein. In contrast, elastin content was significantly lower in the juxta-anastomotic area compared to the inflow artery and contralateral vein (0.009 ± 0.009 vs. 9.1 ± 6.4 and 4.7 ± 1.1%, respectively), and similarly reduced in the outflow vein (0.2 ± 0.1%) (Fig. 2F). In terms of morphometric changes, the average intima-media area in the juxta-anastomotic region and outflow vein increased compared to the contralateral vein (0.15 ± 0.03 and 0.06 ± 0.01 vs. 0.03 ± 0.01 mm2) (Tab. II). The augmented vascular wall area allows for stable luminal enlargement without compromising hemostasis. The average luminal area in the juxta-anastomotic region was 62 times larger than in the contralateral veins (0.62 ± 0.11 vs. 0.01 ± 0.009 mm2).
Fig. 2 -.

(a-D) Collagen deposition in the rat femoral-epigastric fistula. Sections from the femoral artery (a), juxta-anastomotic area (b), outflow vein (c), and contralateral vein (D) were Masson’s trichrome stained to demonstrate the increased medial collagen deposition (blue color) associated with the remodeling of the AVF wall. (e-F) Deposed collagen (e) and elastin (F) in the wall of the femoral artery (FA), juxta-anastomotic area (JA), outflow vein (OV), and contralateral vein (Co Vein) measured as a percentage of the medial wall area (n = 5). Values are presented as average ± SEM. *p<0.01 as determined by one-way ANOVA with a NewmanKeuls post hoc test. M = Media; A = Adventitia.
TABLE II -.
Vascular wall area and lumen area of the main three segments of the rat arteriovenous fistula and the corresponding contralateral vein
| Intima-media area (mm2) | Lumen area (mm2) | |
|---|---|---|
|
| ||
| Inflow artery | 0.08 ± 0.01 | 0.55 ± 0.04 |
| Juxta anastomotic area | 0.15 + 0.03* | 0.62 ± 0.11 |
| Outflow vein | 0.06 ± 0.01 | 0.58 ± 0.06 |
| Contralateral vein | 0.03 ± 0.002 | 0.01 ± 0.009* |
p<0.01 determined by One-Way ANOVA with a Newman-Keuls post hoc test.
Discussion
Poor distensibility in both the pre-fistula artery and vein has been associated with non-maturation (3, 4, 6). The existing techniques to study the biomechanical properties of AVF lack the spatial resolution necessary to identity those areas within the vascular access that are more prone to failure. In this study, we present a new AFM-based method with the ability to quantify micro-variations in Young’s modulus in experimental AVF models. We validate this technique by demonstrating that increased stiffness in the juxta-anastomotic region of the AVF is associated with excessive collagen accumulation, lower elastin levels, and increased intima-media area.
AFM determines the biomechanical properties of a vascular access using the principle of nanoindentation. The AFM cantilever tip can be lowered onto the sample until it makes contact, applying a small force (24). The cantilever deflection resulting from this interaction positively correlates with the existing pockets of stiffness within the vascular access. This technique is widely used in the field of cell mechanics, but we now have expanded it to the tissue level by using AFM cantilevers modified with glass microspheres that increase the surface contact area.
While validating the use of AFM to measure ex-vivo AVF vascular elasticity, we have found that stiffness in the juxta-anastomotic region is likely due to an increased deposition of collagen in the vascular wall combined with a markedly reduced elastin content. The resulting fibrosis can account for the observed susceptibility of this AVF region to thrombosis and intimal hyperplasia (25, 26). This pathological remodeling is usually associated with disturbed flow patterns frequently found in the juxta-anastomotic area of end-to-side AVF (27–29). The disturbed flow causes rapid endothelial denudation and up-regulation of inflammatory cytokines such as MCP-1, ET-1, TGF-β and IL-8, which contribute to SMC proliferation and collagen deposition (30, 31). The lack of endothelium also creates a NO-poor environment that prevents MMP activation to degrade the excess collagen (32). Of note, the juxta-anastomotic area experiences most of the surgical trauma in the form of crush injury, kinking or spasm during vein mobilization. These physical insults likely trigger vascular repair mechanisms that can also exacerbate vascular fibrosis (32).
The various hemodynamic conditions and microvascular environments that occur along the length of the AVF suggest that distinct remodeling processes are at play in different AVF regions. Therefore, the main advantage of the AFM methodology is that for the first time we have a tool that allows the localization of pockets of stiffness within the AVF that, combined with experimental models, will facilitate the study of the mechanisms leading to site-specific vascular fibrosis. The tourniquet, warm water immersion and tension-based techniques used in patients to measure distensibility only provide macro information on the biomechanical properties of the wall (6, 33, 34). In addition, they cannot be adapted to animal models due to the small size and extreme fragility of experimental AVF, or because most are created using non-superficial arteries and veins where it is difficult to assess induced dilation.
The AFM method herein described to measure vascular stiffness is limited to ex-vivo experimental conditions and is not amenable to perioperative patient evaluation. It also requires proper handling of tissues to ensure reproducibility. However, once these technical skills are acquired, this technique is highly reproducible and allows for area-specific biomechanical analysis of vascular and other elastic tissues. We believe that the spatial resolution of this method will bring important contributions to the branch of biomechanics in vascular biology and disease.
Disclosures
Financial support: Supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-098511 to RIV-P, FA and LHS.
Footnotes
Conflict of interest: None to declare.
References
- 1.Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol. 2007;2(5):1043–1053. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jaishi AA, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(3):464–478. [DOI] [PubMed] [Google Scholar]
- 3.Kheda MF, Brenner LE, Patel MJ, et al. Influence of arterial elasticity and vessel dilatation on arteriovenous fistula maturation: a prospective cohort study. Nephrol Dial Transplant. 2010;25(2):525–531. [DOI] [PubMed] [Google Scholar]
- 4.Kim JT, Chang WH, Oh TY, Jeong YK. Venous distensibility as a key factor in the success of arteriovenous fistulas at the wrist. Ann Vasc Surg. 2011;25(8):1094–1098. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden J, Lameris TW, van den Meiracker AH, de Smet AA, Blankestijn PJ, van den Dorpel MA. Forearm venous distensibility predicts successful arteriovenous fistula. Am J Kidney Dis. 2006;47(6):1013–1019. [DOI] [PubMed] [Google Scholar]
- 6.Kim MH, Kim YK, Jun KW, et al. Clinical Importance of Intraoperative Cephalic Vein Distensibility as a Predictor of Radiocephalic Arteriovenous Fistula Maturation. Semin Dial. 2015; 28(6):E64–E70. [DOI] [PubMed] [Google Scholar]
- 7.Berceli SA, Jiang Z, Klingman NV, et al. Differential expression and activity of matrix metalloproteinases during flow-modulated vein graft remodeling. J Vasc Surg. 2004;39(5):1084–1090. [DOI] [PubMed] [Google Scholar]
- 8.Chung AW, Rauniyar P, Luo H, Hsiang YN, van Breemen C, Okon EB. Pressure distention compared with pharmacologic relaxation in vein grafting upregulates matrix metalloproteinase-2 and -9. J Vasc Surg. 2005;42(4):747–756. [DOI] [PubMed] [Google Scholar]
- 9.Wong CY, Rothuizen TC, de Vries MR, et al. Elastin is a key regulator of outward remodeling in arteriovenous fistulas. Eur J Vasc Endovasc Surg. 2015;49(4):480–486. [DOI] [PubMed] [Google Scholar]
- 10.Sassani SG, Theofani A, Tsangaris S, Sokolis DP. Time-course of venous wall biomechanical adaptation in pressure and flow-overload: assessment by a microstructure-based material model. J Biomech. 2013;46(14):2451–2462. [DOI] [PubMed] [Google Scholar]
- 11.Kritharis EP, Kakisis JD, Giagini AT, et al. Biomechanical, morphological and zero-stress state characterization of jugular vein remodeling in arteriovenous fistulas for hemodialysis. Biorheology. 2010;47(5–6):297–319 [DOI] [PubMed] [Google Scholar]
- 12.Paulson WD. Does vascular elasticity affect arteriovenous fistula maturation? Open Urol Nephrol J. 2014;7(Suppl 1: M4):26–32. [Google Scholar]
- 13.Ketteler M, Ritz E. Renal failure: a state of nitric oxide deficiency? Kidney Int. 2000;58(3):1356–1357. [DOI] [PubMed] [Google Scholar]
- 14.Bai Y, Zhang J, Xu J, Cui L, Zhang H, Zhang S. Alteration of type I collagen in the radial artery of patients with end-stage renal disease. Am J Med Sci. 2015;349(4):292–297. [DOI] [PubMed] [Google Scholar]
- 15.Hasson JE, Megerman J, Abbott WM. Increased compliance near vascular anastomoses. J Vasc Surg. 1985;2(3):419–423. [PubMed] [Google Scholar]
- 16.Hofstra L, Bergmans DC, Leunissen KM, et al. Anastomotic intimal hyperplasia in prosthetic arteriovenous fistulas for hemodialysis is associated with initial high flow velocity and not with mismatch in elastic properties. J Am Soc Nephrol. 1995;6(6):1625–1633. [DOI] [PubMed] [Google Scholar]
- 17.Hofstra L, Bergmans DC, Hoeks AP, Kitslaar PJ, Leunissen KM, Tordoir JH. Mismatch in elastic properties around anastomoses of interposition grafts for hemodialysis access. J Am Soc Nephrol. 1994;5(5):1243–1250 [DOI] [PubMed] [Google Scholar]
- 18.Wesly RL, Vaishnav RN, Fuchs JC, Patel DJ, Greenfield JC Jr. Static linear and nonlinear elastic properties of normal and arterialized venous tissue in dog and man. Circ Res. 1975;37(4): 509–520. [DOI] [PubMed] [Google Scholar]
- 19.Globerman AS, Chaouat M, Shlomai Z, Galun E, Zeira E, Zamir G. Efficient transgene expression from naked DNA delivered into an arterio-venous fistula model for kidney dialysis. J Gene Med. 2011;13(11):611–621. [DOI] [PubMed] [Google Scholar]
- 20.Ziebarth NM, Arrieta E, Feuer WJ, Moy VT, Manns F, Parel JM. Primate lens capsule elasticity assessed using Atomic Force Microscopy. Exp Eye Res. 2011;92(6):490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dias J, Diakonis VF, Kankariya VP, Yoo SH, Ziebarth NM. Anterior and posterior corneal stroma elasticity after corneal collagen crosslinking treatment. Exp Eye Res. 2013;116:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias JM, Ziebarth NM. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp Eye Res. 2013;115:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertz H The contact of elastic solids. Journal für die Reine und Angewandte Mathematik. 1881;92:156. [Google Scholar]
- 24.Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56(9):930–933. [DOI] [PubMed] [Google Scholar]
- 25.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17(4):1112–1127. [DOI] [PubMed] [Google Scholar]
- 26.Badero OJ, Salifu MO, Wasse H, Work J. Frequency of swing-segment stenosis in referred dialysis patients with angiographically documented lesions. Am J Kidney Dis. 2008;51(1):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ene-Iordache B, Remuzzi A. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrol Dial Transplant. 2012;27(1):358–368. [DOI] [PubMed] [Google Scholar]
- 28.Roy-Chaudhury P, Arend L, Zhang J, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50(5):782–790. [DOI] [PubMed] [Google Scholar]
- 29.Remuzzi A, Ene-Iordache B. Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis. Clin J Am Soc Nephrol. 2013; 8(12):2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones GT, van Rij AM, Packer SG, Walker RJ, Stehbens WE. Venous endothelial changes in therapeutic arteriovenous fistulae. Atherosclerosis. 1998;137(1):149–156. [DOI] [PubMed] [Google Scholar]
- 31.Lu DY, Chen EY, Wong DJ, et al. Vein graft adaptation and fistula maturation in the arterial environment. J Surg Res. 2014;188(1):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon BS. Why don’t fistulas mature? Kidney Int. 2006;70(8): 1413–1422. [DOI] [PubMed] [Google Scholar]
- 33.Planken RN, Keuter XH, Kessels AG, Hoeks AP, Leiner T, Tordoir JH. Forearm cephalic vein cross-sectional area changes at incremental congestion pressures: towards a standardized and reproducible vein mapping protocol. J Vasc Surg. 2006;44(2):353–358. [DOI] [PubMed] [Google Scholar]
- 34.van Bemmelen PS, Kelly P, Blebea J. Improvement in the visualization of superficial arm veins being evaluated for access and bypass. J Vasc Surg. 2005;42(5):957–962. [DOI] [PubMed] [Google Scholar]


